Degradation of antibiotic fosfomycin by peroxymonosulfate/ferrate and simultaneous phosphate removal with in situ formed ferric nanoparticles
Wen
Jiang
a,
Wenhao
Lao
a,
Zhe
Xu
a,
Yiping
Feng
 *a and
Jiuli
Ruan
*b
*a and
Jiuli
Ruan
*b
aGuangdong Key Laboratory of Environmental Catalysis and Health Risk Control, School of Environmental Science and Engineering, Institute of Environmental Health and Pollution Control, Guangdong University of Technology, Guangzhou 510006, China. E-mail: ypfeng@gdut.edu.cn
bState key Laboratory of Environment Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China. E-mail: ruanjiuli1987@163.com
First published on 20th November 2025
Abstract
This study systematically investigates the efficient degradation of the antibiotic fosfomycin (FOS) by a ferrate/peroxymonosulfate (Fe(VI)/PMS) system and simultaneous phosphate removal via in situ formed ferric nanoparticles. The Fe(VI)/PMS system achieved complete FOS degradation within 10 min, with a pseudo-first-order rate constant (0.25 min−1) significantly higher than that of Fe(VI) alone (0.03 min−1) or Fe(VI)/peroxodisulfate (0.18 min−1). Reactive species including SO4˙−, HO˙, and high-valent iron species (Fe(V)/Fe(IV)) were identified as key contributors, with HO˙ playing a dominant role. Optimal conditions included 200 μM PMS, 100 μM Fe(VI), and pH 5.0–7.0. Natural water matrices (e.g., river water and wastewater effluent) slightly inhibited degradation, while seawater enhanced FOS degradation efficiency. The degradation pathways of FOS involve oxidation, bond cleavage, and coupling reactions, with by-products showing reduced toxicity. Notably, the in situ formed ferric nanoparticles effectively removed released phosphate via co-precipitation, and post-treatment solutions exhibited negligible toxicity towards E. coli. This study highlights the use of Fe(VI)/PMS as a promising strategy for FOS remediation with simultaneous nutrient control.
Environmental significanceThe sustainable and efficient green degradation of fosfomycin (FOS) was achieved using an Fe(VI)/PMS oxidation system. The degradation efficiency and reaction kinetics of FOS were systematically investigated under different experimental conditions. The reactive species involved in the process were identified, and the degradation pathways were elucidated based on by-product identification. These findings reveal a synergistic degradation mechanism involving both free radical species (e.g., SO4˙− and HO˙) and high-valent iron intermediates Fe(V)/Fe(IV), providing a mechanistic foundation for FOS degradation in the Fe(VI)/PMS system. Overall, this study enhances the understanding of Fe(VI)/PMS-based remediation strategies for FOS-contaminated water and contributes to the development of integrated approaches that combine pharmaceutical degradation with nutrient control. |
1. Introduction
Antibiotics have found extensive applications in both human and veterinary medicine for the treatment of bacterial infections, leading to substantially enhanced outcomes in public health and animal well-being. However, their inappropriate use, improper disposal, and incomplete metabolism have led to their widespread occurrence across diverse environmental matrices, which in turn poses significant risks to ecological stability and human health. A critical concern is the rising antibiotic resistance development, driven by the persistent exposure of microorganisms to residual antibiotics.1Fosfomycin (1R-2S-epoxypropyl phosphonic acid, FOS) is a broad-spectrum antibiotic that was first identified more than 50 years ago. In recent years, it has reemerged as a promising option for combating highly drug-resistant and multidrug-resistant pathogens.2 Its clinical applications primarily include treating infections caused by susceptible Gram-negative bacteria such as those affecting the digestive tract, skin, soft tissues, and urinary tract.3 Yet, this widespread administration, combined with the fact that conventional wastewater treatment methods fail to completely remove FOS, has led to its frequent detection in aquatic ecosystems, both in its original form and as metabolites.4 This presence causes potential dangers to both the environment and human health.3,5 Thus, there is an urgent need to develop effective technologies for degrading FOS and neutralizing its bacterial toxicity to reduce these hazards.
Chemical oxidation, particularly advanced oxidation processes (AOPs) that rely on reactive radicals such as sulfate radicals (SO4˙−) or hydroxyl radicals (HO˙), has demonstrated effectiveness in breaking down persistent organic compounds into CO2, H2O, and inorganic ions.6,7 The reaction mechanisms of AOPs can be mainly categorized into two pathways. The radical pathway generated reactive oxygen species (ROS) such as sulfate radicals (SO4˙−) or hydroxyl radicals (HO˙) through oxidant activation.8 In addition, it was found the non-radical pathway involving reactive species such as singlet oxygen (1O2) and high-valent metal complexes contributes to selective oxidation under complex aqueous environments.9 Among various AOP activation strategies, transition metal-based activation is widely adopted for its ambient temperature/pressure requirements, avoiding the high energy costs of ultraviolet (UV)/thermal methods.8,10 Notably, AOP performance is strongly influenced by water matrices. For example, inorganic anions (e.g., Cl− and HCO3−) may scavenge radicals or shift to non-radical pathways, while natural organic matter (NOM) competes for reactive species.11,12 Similarly, the UV/persulfate (PS) system has been explored for FOS degradation,13 but its practical application is limited by the high energy demands. Importantly, current research into the oxidation of FOS is still in its early stages, with key uncertainties remaining regarding the degradation rates, the production of by-products, changes in toxicity, and the concurrent removal of related pollutants (e.g., phosphate, given FOS's phosphonic acid component).
Within the realm of emerging AOPs, processes based on Fe(VI) have attracted considerable attention. This is because Fe(VI) functions both as a powerful oxidizing agent and a disinfectant, able to convert organic micropollutants into less harmful substances. Additionally, Fe(VI) breaks down into ferric (Fe(III)) compounds (e.g., Fe(OH)3, and Fe2O3), which can act as coagulants for removing heavy metals and inorganic anions.14,15 For instance, studies have shown that Fe(VI) can effectively eliminate p-arsanilic acid, while As(V) released during this process is simultaneously removed by iron (oxyhydr)oxides formed in situ through surface adsorption.16 However, Fe(VI) alone reacts slowly with certain persistent pollutants, restricting its efficiency in practical settings. To overcome this limit, combining Fe(VI) with peroxodisulfate (PDS) or peroxymonosulfate (PMS), the widely used oxidants that can be activated to generate HO˙ and SO4˙−, has emerged as a viable approach.17
Recent investigations have revealed that the Fe(VI)/PMS system enhances the degradation of various contaminants including atrazine,18 fluoroquinolones,19 sulfamethoxazole,20 and diclofenac21 through the synergistic production of reactive species (HO˙, SO4˙−, and high-valent iron species like Fe(V)/Fe(IV)). Given that PMS exhibits a lower LUMO energy than that of PDS, which in turn endows it with stronger electron-accepting capacity, coupled with its acidic nature that promotes Fe(VI) conversion to reactive Fe(III)/Fe(II), it can be proposed that Fe(VI)/PMS will outperform both Fe(VI) alone and the Fe(VI)/PDS system in the degradation of FOS. Moreover, the in situ formation of Fe(III) nanoparticles from this system simultaneously enables phosphate co-precipitation and toxicity reduction. Despite these advancements, the Fe(VI)/PMS system has not yet been applied to FOS degradation, nor has its ability to simultaneously remove phosphate (via iron(III) nanoparticles formed in situ) been examined. Phosphate, a vital nutrient in aquatic environments, can exacerbate eutrophication. Therefore, its removal alongside FOS would address a dual environmental concern.22 Furthermore, critical factors such as pH, operational parameters, the toxicity of by-products, and the role of in situ formed Fe(III) nanoparticles in phosphate sequestration have not been investigated for FOS treatment.
This study aims to address these knowledge gaps by systematically examining the Fe(VI)/PMS system for FOS degradation and concurrent phosphate removal. Specifically, the objectives are: (1) to assess FOS oxidation efficiency under varying conditions (pH, Fe(VI)/PMS ratios, initial concentrations); (2) to identify reactive species and clarify degradation mechanisms through by-product analysis; (3) to evaluate changes in bacterial toxicity to ensure the elimination of resistance gene induction; and (4) to characterize in situ formed Fe(III) nanoparticles and their role in phosphate removal. The findings of this research will contribute to a comprehensive understanding of the Fe(VI)/PMS system for sustainable FOS remediation, connecting laboratory-based research with practical water treatment applications.
2. Materials and methods
2.1. Chemicals and reagents
Fosfomycin sodium salt (FOS, with a purity exceeding 98%), methyl phenyl sulfoxide (PMSO, 98% purity), potassium peroxodisulfate (PDS, K2S2O8, 99.5% purity), potassium peroxymonosulfate (PMS, KHSO5·0.5KHSO4·0.5K2SO4, with at least 47% KHSO5 basis), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO, C6H11NO, 97.0%), and humic acid (HA) were purchased from Aladdin (Shanghai, China). Analytical grade sodium sulfite (Na2SO4, ≥99%), sodium nitrate (NaNO3, ≥99%), sodium bicarbonate (NaHCO3, ≥99.8%), and sodium chloride (NaCl, ≥99.5%) were obtained from Shanghai Titanchem Co., Ltd (Shanghai, China). 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) was sourced from Sigma Aldrich Ltd Co. (Shanghai, China). Potassium ferrate (K2FeO4, >95%) was synthesized by a wet chemical method.23 HPLC-grade formic acid and methanol were provided by Merck (Darmstadt, Germany). Deionized (DI) water, prepared using a Millipore Milli-Q system (>18.2 MΩ cm−1), was utilized for preparing solutions. All other solvents and reagents were of analytical grade. A stock solution of FOS (1.0 mM) was prepared in DI water and stored at 4 °C for subsequent use.2.2. Degradation experiments
To prevent any interference resulting from possible side reactions between hydroxyl and sulfate radicals and buffer species (such as PO43− and CO32−), the experiments were conducted without the use of any buffer solutions. The pH changes throughout the reactions were monitored. Unless otherwise specified, all reactions were conducted in 30 mL brown glass test vials sealed with Teflon-lined silicone septa and used a constant-temperature water bath to maintain 25.0 ± 0.2 °C. First, a 40 μM solution of FOS was introduced into the vial. Then 0.01 M H2SO4 or NaOH was used to adjust the solution's pH, and no further pH adjustment was made during the degradation process. Subsequently, the required amounts of Fe(VI) and PDS/PMS were added to initiate the reaction. At specific time intervals, 1.0 mL of the aqueous sample was withdrawn and immediately quenched by adding 20 μL of 2.0 mM Na2S2O3 solution. Na2S2O3 was added in excess relative to the reaction system (at a molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), thereby ensuring that subsequent reactions were completely quenched and that the FOS concentration in the quenched sample remained unchanged. After filtration through a 0.22 μm membrane filter (Anpel, Shanghai, China), the remaining FOS was quantified for further analyses.
1), thereby ensuring that subsequent reactions were completely quenched and that the FOS concentration in the quenched sample remained unchanged. After filtration through a 0.22 μm membrane filter (Anpel, Shanghai, China), the remaining FOS was quantified for further analyses.
The impacts of Fe(VI) concentrations ranging from 0 to 400 μM and PMS dosages from 0 to 200 μM on the degradation of FOS were evaluated separately at a pH of 7.0. The effect of pH on the degradation of FOS by Fe(VI)/PMS oxidation systems was studied at 25 °C with pH values varying from 5.0 to 9.0. The influences of typical natural water components on the oxidation of FOS, including different inorganic anions (such as Cl−, SO42−, NO3−, and HCO3−) and HA, a representative of dissolved organic matter, were also investigated. All experiments were repeated three times, and the average values were reported.
In this study, natural water samples were also used to explore the oxidation efficiency of FOS in natural waters. These samples included seawater collected from the South China Sea in Huizhou, municipal wastewater effluent from an activated sludge sewage treatment plant (STP) in Guangzhou, river water from the Pearl River in Guangzhou, and tap water from the pipeline in our laboratory. The collected water samples were transported to the laboratory within 12 hours and stored at 4 °C for later use. Before use, all collected waters were passed through a 0.22 μm membrane filter (ANPEL, Shanghai, China) to remove particulate matter. The relevant physicochemical parameters of the water samples were characterized, which are presented in Table S1 of the SI. All experiments were conducted in triplicate, and the error bar represented the calculated standard deviation.
2.3. Analysis methods
The detection of residual FOS was accomplished through analysis by liquid chromatography-triple quadrupole mass spectrometry (LC-MS/MS, TSQ Quantiva, Thermo Scientific), as described in Text S1. The concentration of released phosphate ions (PO43−) in the reaction solutions was determined by the Mo–Sb antispectrophotometer method.24 The total organic carbon (TOC) content was measured using a TOC analyzer (Shimadzu, TOC-L CPH, Japan). The Fe(VI) concentration was determined by the direct spectrophotometric method with ABTS, as reported in previous research.25 The residual concentration of PMS was measured using the iodometric titration method.26 To identify the transformation products of FOS degradation, a sample of the reaction solution was taken after 10 min of treatment, which enabled the collection of the greatest variety of transformation products. Product characterizations were performed using an HPLC system coupled with a hybrid quadrupole-orbitrap mass spectrometer (UPLC-Q-Exactive Orbitrap-MS, Thermo, Bremen, Germany) (Text S2).The precipitate was collected by centrifugation at 8000 rpm for ten min. The obtained solid was washed with DI water, then dried at 60 °C, and used for the following characterizations. The morphology of the settled particles from Fe(VI)/PMS treatment was observed by scanning electron microscopy (SEM, Hitachi SU8220, Japan). Energy-dispersive spectroscopy (EDS) mapping was performed using the same instrument during SEM measurements to observe the elemental composition and distribution in the settled particles.27 The elemental composition was analyzed using an X-ray photoelectron spectrometer (XPS, Thermo VG ESCALAB 250) with non-monochromatic Al Kα X-ray as the excitation source and the C 1s peak at 284.8 eV referenced for calibration.28 The chemical compositions of the obtained precipitate were analyzed using an X-ray diffractometer (XRD, Bruker D8) with Cu Kα radiation.29 The ROS generated in the Fe(VI)/PMS system were examined by electron spin resonance (EPR, EMXplus-10/12) in the case of DMPO as a spin trap for SO4˙− and HO˙.30
2.4. Toxicity assessment
In this research, E. coli (ATCC25922, obtained from the Beijing Microbiological Culture Collection Center) was employed to assess the toxicity of FOS and its transformation products. Before the tests, a bacterial suspension (1.0 × 108 CFU mL−1) was prepared following the method described in Text S3 of the SI. The toxicity tests were carried out for 8 h at 37 °C using a shaker incubator at 150 rpm.13,31 The cell density, which was interchangeable with the optical absorbance at 600 nm, was used to represent the bacterial survival. The growth inhibition assay was monitored throughout the exposure periods of 0, 1, 2, 3, 4, 5, and 8 h. All treatments were performed in triplicate.3. Results and discussion
3.1. Catalytic degradation of FOS
The degradation of FOS was explored through multiple oxidation processes, namely the Fe(VI), PMS, PDS, Fe(VI)/PDS, and Fe(VI)/PMS processes. In Fig. 1a, the removal rates of FOS in diverse systems including PDS, PMS, Fe(VI), Fe(VI)/PDS, and Fe(VI)/PMS are presented. When using PDS and PMS individually, the removal of FOS was minimal during the 30 minute treatment. Conversely, Fe(VI) alone led to approximately 40% removal of FOS. The Fe(VI)/PDS and Fe(VI)/PMS systems were capable of removing 98% and 100% of FOS, respectively. Fe(VI)/PMS demonstrated a higher efficiency than Fe(VI)/PDS, achieving complete removal of FOS within 10 minutes. Evidently, the combination of Fe(VI) with PMS or PDS exerted a synergistic effect. Despite PDS having a relatively high redox potential, Fe(VI)/PMS was more effective.Fig. S1 depicts the reduction in Fe(VI) levels during the treatment of FOS using Fe(VI)-only, Fe(VI)/PDS, and Fe(VI)/PMS systems. For comparison, the self-decay of Fe(VI) in the absence of FOS was also monitored. When FOS was removed using Fe(VI) alone, the consumption of Fe(VI) was greater than in the control case. Subsequently, upon the addition of PDS and PMS, a notable acceleration in the decay of Fe(VI) was observed. Moreover, the decay of Fe(VI) was more rapid with the addition of PMS compared to PS. It can be inferred that PDS and PMS facilitated the decay of Fe(VI) by reacting with the subsequently-formed Fe3+ to generate SO4˙− and HO˙. Additionally, depending on the pH, Fe3+ could aggregate to form Fe(OH)3, enabling the coagulation process to remove certain organic substances from aqueous solutions. Fig. S2 illustrates the consumption of 200 μM PDS and PMS during the removal of FOS. Evidently, without Fe(VI) catalysis, the consumption of PDS and PMS was negligible. In contrast, when combined with Fe(VI), there was a substantial decrease in the concentrations of PDS and PMS. In essence, the addition of PDS and PMS to the solution enhanced the decay of Fe(VI), while the presence of Fe(VI) in the medium accelerated the consumption of PDS and PMS.
The degradation of FOS in the Fe(VI), Fe(VI)/PDS, and Fe(VI)/PMS systems could be accurately modeled using the pseudo-first-order kinetics model (Fig. 1a). The pseudo-first-order rate constants, as shown in Fig. 1b, were 0.03, 0.18, and 0.25 min−1 for the Fe(VI), Fe(VI)/PDS, and Fe(VI)/PMS systems, respectively. The Fe(VI)/PMS system's enhanced oxidation potential towards FOS might stem from PMS's superior electron-accepting ability compared to PDS. This could be attributed to PMS having the lowest-energy unoccupied molecular orbital (LUMO) relative to PDS.32 Moreover, the acidic effect resulting from the addition of PMS facilitated the greater degradation of FOS.18
The relative significance of ROS generated in the Fe(VI)/PMS system according to eqn (1)–(6) was monitored using EPR experiments, as shown in Fig. 1. According to Fig. 1e, a group of three characteristic peaks with an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 appeared in the EPR spectrum, which conformed to the signal of DMPO–HO˙. The DMPO–X adduct with seven-line peaks at an intensity ratio of 1
1 appeared in the EPR spectrum, which conformed to the signal of DMPO–HO˙. The DMPO–X adduct with seven-line peaks at an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed, which was ascribed to the DMPO oxidation by SO4˙− and HO˙ (Fig. 1f), indicating the generation of SO4˙− and HO˙ from the Fe(VI)/PMS system.33 The contribution of the generated ROS on FOS degradation was then elucidated using chemical scavengers. Fig. 1c illustrates the removal of FOS in the presence of PMSO, methanol (MeOH), and tert-butyl alcohol (TBA). In this research, MeOH and TBA served as radical scavengers to identify the predominant reactive radicals (HO˙ and SO4˙−). MeOH, which contains α-hydrogen, reacts rapidly with both HO˙ and SO4˙−, with rate constants of kSO4˙− = 0.9–1.3 × 107 M−1 s−1 and kHO˙ = 0.8–1.0 × 109 M−1 s−1. In contrast, TBA, lacking α-hydrogen, reacts much more swiftly with HO˙ (kHO˙ = 3.8–7.6 × 108 M−1 s−1) than SO4˙− (kSO4˙− = 4–9.1 × 105 M−1 s−1), signifying that TBA can be employed as a scavenger for HO˙.34,35 PMSO can react with SO4˙−, HO˙, and high-valent iron species such as Fe(V).17,36 The addition of all three chemical scavengers diminished ROS-mediated degradation, highlighting the essential involvement of HO˙, SO4˙−, and high-valent iron species in the FOS degradation process. Fig. 1d shows that upon adding MeOH, TBA, and PMSO, the observed rate constant (kobs) of FOS decreased from 0.2543 to 0.0369, 0.0427, and 0.0252 min−1, respectively. These results indicated that the generation of HO˙ was the most critical factor for the efficient degradation of FOS, while SO4˙−, high-valent iron species, i.e., Fe(V), Fe(IV), and other potential reactive species, also contributed to the oxidation of FOS.32 Overall, these findings suggest that employing combined oxidant systems like Fe(VI)/PDS or Fe(VI)/PMS holds promise for enhancing the degradation of FOS in water treatment procedures.
1 was observed, which was ascribed to the DMPO oxidation by SO4˙− and HO˙ (Fig. 1f), indicating the generation of SO4˙− and HO˙ from the Fe(VI)/PMS system.33 The contribution of the generated ROS on FOS degradation was then elucidated using chemical scavengers. Fig. 1c illustrates the removal of FOS in the presence of PMSO, methanol (MeOH), and tert-butyl alcohol (TBA). In this research, MeOH and TBA served as radical scavengers to identify the predominant reactive radicals (HO˙ and SO4˙−). MeOH, which contains α-hydrogen, reacts rapidly with both HO˙ and SO4˙−, with rate constants of kSO4˙− = 0.9–1.3 × 107 M−1 s−1 and kHO˙ = 0.8–1.0 × 109 M−1 s−1. In contrast, TBA, lacking α-hydrogen, reacts much more swiftly with HO˙ (kHO˙ = 3.8–7.6 × 108 M−1 s−1) than SO4˙− (kSO4˙− = 4–9.1 × 105 M−1 s−1), signifying that TBA can be employed as a scavenger for HO˙.34,35 PMSO can react with SO4˙−, HO˙, and high-valent iron species such as Fe(V).17,36 The addition of all three chemical scavengers diminished ROS-mediated degradation, highlighting the essential involvement of HO˙, SO4˙−, and high-valent iron species in the FOS degradation process. Fig. 1d shows that upon adding MeOH, TBA, and PMSO, the observed rate constant (kobs) of FOS decreased from 0.2543 to 0.0369, 0.0427, and 0.0252 min−1, respectively. These results indicated that the generation of HO˙ was the most critical factor for the efficient degradation of FOS, while SO4˙−, high-valent iron species, i.e., Fe(V), Fe(IV), and other potential reactive species, also contributed to the oxidation of FOS.32 Overall, these findings suggest that employing combined oxidant systems like Fe(VI)/PDS or Fe(VI)/PMS holds promise for enhancing the degradation of FOS in water treatment procedures.
3.2. Effects of PMS and Fe(VI) dosage on FOS degradation
Fig. 2 illustrates the impacts of PMS and Fe(VI) dosages on the degradation of FOS. As depicted in Fig. 2a, the degradation of FOS by the Fe(VI)/PMS system was enhanced as the PMS dosage increased. When 100 μM of PMS was present, FOS was completely removed within 30 minutes. The degradation of FOS in Fe(VI)/PMS systems with different PMS dosages could be precisely described by the pseudo-first-order kinetics model (Fig. 1a). The observed rate constants (kobs) for FOS degradation at different PMS concentrations are presented in Fig. 2b. Within the investigated range (0–400 μM), kobs first increased with the increase in PMS concentration from 0 to 200 μM but then decreased with the further increase in PMS concentration to 400 μM. Although as the PMS concentration increases, more active species will be generated, followed by the self-quenching of the generated radicals and the consumption of the radicals by excessive PMS (eqn (3)).37–39As the Fe(VI) dosage increased from 50 to 400 μM, the degradation efficiency of FOS significantly improved, as shown in Fig. 2c. Notably, with 400 μM of Fe(VI), complete degradation of FOS was accomplished within 15 minutes. Increased Fe(VI) concentration led to an increase in the kobs value of FOS, as shown in Fig. 2d. There are likely two factors contributing to the rapid and efficient degradation of FOS. First, Fe(VI) can directly oxidize FOS by targeting its alkyl-chain moieties.40 Second, iron oxides/hydroxides or Fe(III) ions (eqn (7)–(13)), which are produced from the reduction of Fe(VI), may activate PMS, thereby accelerating the degradation of FOS.18
| 3HSO5− + H2O → 2O2˙− + 3SO42− + 5H+ | (1) |
| O2˙− + HSO5− → SO4˙− + OH− + O2 | (2) |
| HSO5− + SO4˙− → HSO5˙− + SO42− | (3) |
| SO4˙− + SO4˙− → S2O82− | (4) |
| SO4˙− + OH− → ˙OH + SO42− | (5) |
| SO4˙− + H2O → ˙OH + H+ + SO42− | (6) |
| FeO42− + H2O → FeO32− + H2O2 | (7) |
| FeO32− + H2O2 + 2H+ → Fe(OH)2 + O2 + H2O | (8) |
| FeO32− + Fe(OH)2 + 3H2O → Fe(OH)3 + 2OH− | (9) |
| FeO42− + 8H+ + 3e− → Fe3+ + 4H2O | (10) |
| FeO52− + 4H2O + 3e− → Fe(OH)3 + 5OH− | (11) |
| HSO5− + Fe3+ → Fe2+ + SO5˙− + H+ | (12) |
| HSO5− + Fe2+ → Fe3+ + SO4˙− + OH− | (13) |
3.3. Effects of water matrix on FOS oxidation
3.4. Proposed reaction pathways
The UPLC-Q-TOF was used to analyze the transformation products of FOS. Structural assignments of the transformation products were determined through product ion scans, taking into account their MS/MS spectra and fragmentation patterns. The possible structures of FOS transformation products and their corresponding fragmentation ions are provided in Table S3.The detailed pathways and bond cleavages of FOS are proposed, as shown in Fig. 5. First, HO˙ attacks the C atom of the epoxy groups, forming a carbon-centered radical I1 and releasing phosphate.51,52 Additionally, Fe(V) and Fe(IV) species facilitate the generation of carbon-centered radicals during the pollutant transformation.53 Afterward, HO˙ quickly attacked the carbon-centered radical, producing the hydroxylation intermediate P1–74. Then, after acidic hydrolysis of the epoxy groups on P1–74, P2–76 was generated, and further oxidation of P2–76 will generate P3–90. This acidic hydrolysis of epoxy groups was confirmed by ofloxacin degradation by Fe(VI)/PMS, where proton-driven epoxy ring opening is a key step for intermediate product formation.54 In addition, SO4˙− and HO˙ could abstract a hydrogen atom from three sites on the FOS molecule, forming carbon-centered radicals (I2). The hydrogen abstraction is regulated by spin state-dependent reactivity because the spin state affects the selectivity of radical attack on target molecules.55 On the one hand, I2 will be transformed into P4–154 by acidic hydrolysis of the epoxy groups. On the other hand, I2 will be further attacked by HO˙ to form P5–152. Then, SO4˙− and HO˙ abstract the hydrogen atoms from the degradation products P1–74, P2–76, P4–154, and P5–152, causing the production of many C-centered and C–O radicals I3, as well as the release of phosphate. C–O cross-coupling reactions will occur between these radical intermediates and, finally, result in the formation of abundant dimerization products like P6–150, and further coupling reactions will result in the generation of P7–298. Meanwhile, further attack by SO4˙− and HO˙ could also lead to the mineralization of FOS, which ultimately resulted in the formation of CO2 and H2O,56 as demonstrated by the TOC removal rate in Fig. S4. Overall, the transformation pathways of FOS by PMS-Fe(VI) primarily involved oxidation, bond cleavage and coupling reactions.
3.5. Release of phosphate and co-precipitation
The release of phosphate in the degradation of FOS through Fe(VI) and Fe(VI)/PMS treatment was further examined. UV/PMS treatment was also performed to examine the role of ferrate on phosphate co-precipitation. As displayed in Fig. S5a, FOS was rapidly degraded by UV/PMS and Fe(VI)/PMS systems. After 60 min of treatment, FOS was completely removed by UV/PMS and Fe(VI)/PMS systems, while ca. 50% FOS was removed by sole Fe(VI) treatment. Fig. S5b shows that phosphate accumulated in the solution with the degradation of FOS in all processes. The incremental concentration of phosphate was observed in the first 15 min, and then reached a plateau where the accumulation of phosphate was retarded. Although the removal of FOS by UV/PMS and Fe(VI)/PMS is similar, the phosphate accumulation in solution is different. The phosphate in the solution of FOS by UV/PMS treatment is highest among the three systems, which reached to be 8.71 μM after 60 min. However, the maximum phosphate of FOS in solution was 2.23 and 1.14 μM for the Fe(VI) and Fe(VI)/PMS processes, respectively, indicating the co-precipitation of phosphate during these treatments. Previous studies have demonstrated that Fe(III) precipitation and flocculation were effective for the removal of phosphate. The above-mentioned results further demonstrated that the Fe(VI)/PMS system was essential to enhance the final TP removal during precipitation.We further characterized the solid samples after co-precipitation in terms of SEM and elemental analysis. As shown in the SEM images (Fig. 6a and b), except for Fe and O, the elements P and C were found in the floc particles. The SEM-EDS image in Fig. S6 also indicated that P was captured in the formed floc particles. The XRD pattern of the formed floc particles is shown in Fig. S7. Notably, the XRD pattern clearly shows that the material has five characteristic peaks at 18°, 35°, 43°, 57°, and 63°, representing the (111), (311), (400), (511), and (440) crystal faces of γ-Fe2O3.
 | ||
| Fig. 6 (a) SEM and (b) EDX mapping images of the precipitation. (c) XPS C 1s, O 1s, Fe 2p, and P 2p spectrum peak fitting of the obtained floc particles. | ||
The XPS spectra displayed in Fig. S8 indicated Fe, P, C, and O peaks of the floc particles. XPS peak fitting for C 1s, O 1s, P 2p, and Fe 2p spectra is shown in Fig. 6c. The Fe 2p3/2 region was divided into two peaks, where the peak at 710.58 eV belongs to Fe(II) and the peak at 712.58 eV is assigned to Fe(III) in tetrahedral sites. Similarly, the Fe 2p1/2 peak was fitted to two peaks, with 724.28 eV (Fe(II)) and 726.78 eV (Fe(III)) in tetrahedral sites. A satellite peak in Fe 2p was observed at 719.28 eV, arising from Fe3+/Fe2+charge transfer.57 Collectively, the Fe 2p spectrum confirms that Fe(VI) in the reaction system was reduced to Fe(II)/Fe(III)-based oxides or hydroxides (e.g., γ-Fe2O3, γ-FeOOH).
The C 1s spectrum was divided into four peaks. The peak at 292.68 eV belongs to carbon with strong electron-withdrawing groups, e.g., polyfluoro-substituted carbon in FOS or carbonate impurities. The peak at 288.48 eV corresponded to carboxyl carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) from oxidative degradation intermediates like carboxylic acids. The peak at 286.08 eV was attributed to carbon bonded to heteroatoms (e.g., C–F and C–N in FOS, or C–O in oxygenated intermediates). The peak at 284.58 eV was ascribed to saturated carbon (C–C/C
O) from oxidative degradation intermediates like carboxylic acids. The peak at 286.08 eV was attributed to carbon bonded to heteroatoms (e.g., C–F and C–N in FOS, or C–O in oxygenated intermediates). The peak at 284.58 eV was ascribed to saturated carbon (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), possibly originating from carbon impurities or unreacted alkyl moieties. The C 1s spectrum demonstrates that FOS in the reaction system underwent oxidative degradation, generating oxygenated intermediates and cleavage of C–F and C–N bonds.30 For the O 1s spectrum, deconvolution revealed two main components, i.e., lattice oxygen (529.88 eV) and adsorbed oxygen/hydroxyl oxygen (531.28–532.58 eV). The peaks at 529.88 and 531.28 eV correspond to Fe–O and Fe–OH originating from iron hydroxides and oxides. The presence of the 531 eV peak in the O 1s spectra confirms the existence of hydroxide bonding, verifying again iron hydroxide formation.58 Lattice oxygen corresponds to the intrinsic oxygen in iron (hydr)oxides, whereas adsorbed oxygen/hydroxyl oxygen originates from surface-adsorbed H2O, OH−, or organic oxygen-containing groups. In the P 2p spectrum, a characteristic peak at 133.18 eV matches the typical binding energy of phosphate. This indicates that phosphate release during FOS degradation underwent co-precipitation/adsorption with Fe(III) (hydro)oxides, forming iron phosphate compounds. These results confirm phosphate fixation via Fe–O–P coordination.
C), possibly originating from carbon impurities or unreacted alkyl moieties. The C 1s spectrum demonstrates that FOS in the reaction system underwent oxidative degradation, generating oxygenated intermediates and cleavage of C–F and C–N bonds.30 For the O 1s spectrum, deconvolution revealed two main components, i.e., lattice oxygen (529.88 eV) and adsorbed oxygen/hydroxyl oxygen (531.28–532.58 eV). The peaks at 529.88 and 531.28 eV correspond to Fe–O and Fe–OH originating from iron hydroxides and oxides. The presence of the 531 eV peak in the O 1s spectra confirms the existence of hydroxide bonding, verifying again iron hydroxide formation.58 Lattice oxygen corresponds to the intrinsic oxygen in iron (hydr)oxides, whereas adsorbed oxygen/hydroxyl oxygen originates from surface-adsorbed H2O, OH−, or organic oxygen-containing groups. In the P 2p spectrum, a characteristic peak at 133.18 eV matches the typical binding energy of phosphate. This indicates that phosphate release during FOS degradation underwent co-precipitation/adsorption with Fe(III) (hydro)oxides, forming iron phosphate compounds. These results confirm phosphate fixation via Fe–O–P coordination.
Overall, the above-mentioned results suggest that, in addition to inorganic phosphate, phosphorus in organic form or organic intermediates without phosphorus might be adsorbed by the formed floc particles. Both inorganic phosphate and organic phosphorus could be removed by coagulation through the in situ generation of Fe(III), which contributed to the final phosphate removal. The long-term stability of the formed floc particles was evaluated via leaching experiments,59 which demonstrated that the concentrations of leached Fe3+ and PO43− were both below the detection limit. This indicates that the complex solid phase was stable, achieving sustained phosphorus chelation while eliminating the risk of secondary pollution, thereby maintaining phosphorus mass balance and ultimately confirming the long-term stability of the system.
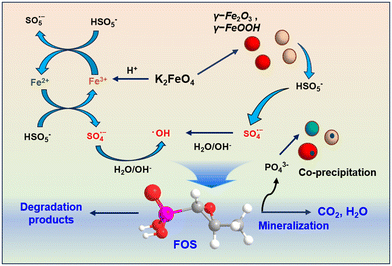
On the basis of the above-mentioned results, the mechanism of PMS activation by Fe(VI) is proposed and illustrated in Scheme 1. On the one hand, PMS provided an acidic environment, where Fe(VI) was rapidly reduced to Fe(III) in solution. Fe(III) could react with PMS to produce Fe(II). Subsequently, Fe(II) could activate PMS to produce SO4˙−. On the other hand, the self-decomposition of Fe(VI) and the coagulation of Fe(III) ions led to the formation of γ-Fe2O3 or γ-FeOOH particles.43 SO4˙− could be produced by the exposed active sites of γ-Fe2O3 or γ-FeOOH particles for PMS activation. Finally, SO4˙− could react with H2O to generate HO˙. These radicals resulted in the degradation of FOS and the release of inorganic phosphate. During this process, the released inorganic phosphate tends to form complexes through the in situ generation of Fe(III) or adsorbs onto γ-Fe2O3 or γ-FeOOH particles, leading to final phosphorus coagulation removal.
3.6. Toxicity evaluation
Biological toxicity has become an increasingly significant indicator in assessing the performance of wastewater treatment.60 Herein, E. coli was employed as the model Gram-negative and Gram-positive bacteria to assess how toxicity changed following treatment. As shown in Fig. S9. It was found that the presence of only 10 μM of FOS inactivated approximately 90% of E. coli within 8 h, which reflected that the antibiotics are highly toxic to E. coli and can completely inhibit their growth. Meanwhile, the sole PMS at 100 μM did not affect the bacterial activity compared with the sole FOS group. However, the sole Fe(VI) at 100 μM is able to alleviate FOS toxicity to E. coli. For example, after 100 μM Fe(VI) was treated for 30 min, the survival rate of E. coli with 10 μM FOS increased to 28%.Then, we tested the toxicity of the Fe(VI)/PMS-treated FOS solution at different treatment times. Obviously, the Fe(VI)/PMS process drastically alleviated the toxicity of FOS at 10 μM (Fig. S9). For example, after 2, 5, 10, and 30 minutes of treatment, the survival rate of E. coli with 10 μM FOS increased from 9.5% to 42.3%, 71.8%, 119.1%, and 120.5%, respectively. When the treatment was prolonged to more than 20 minutes, no negative influence of 10 μM FOS on E. coli was found. As discussed in the context, a great proportion of FOS was degraded within 30 min, and the treated solution therefore mainly comprised the degradation products of FOS. The negligible sterilization of the treated solution evidenced that the products formed in the Fe(VI)/PMS system possess a much lower toxicity than that of FOS. Overall, treatment with Fe(VI)/PMS technique led to rapid degradation of FOS, and the associated toxicity was reduced to a large extent. This finding highlights the effects of antibiotic degradation products on the emergence of bacterial resistance and emphasizes the significance of taking these effects into account when evaluating the environmental risks associated with antibiotics.
4. Conclusions
The Fe(VI)/PMS system demonstrates exceptional performance in degrading FOS and synchronously removing phosphate, addressing dual environmental concerns of antibiotic persistence and eutrophication risk. The results revealed that Fe(VI)/PMS synergistically enhances FOS degradation, achieving complete removal within 10 min under optimal conditions (100 μM PMS, 200 μM Fe(VI), pH 5.0–7.0), outperforming single-oxidant or Fe(VI)/PDS systems. The FOS degradation pseudo-first-order rate constant (kobs) was determined to be 0.25 min−1, much higher than that of Fe(VI) alone (0.03 min−1) and Fe(VI)/PDS (0.18 min−1). Reactive species SO4˙−, HO˙, and high-valent iron collectively drive FOS oxidation, with HO˙ as the primary contributor based on EPR and scavenger measurements. FOS degradation involves oxidation, C–P bond cleavage, and radical coupling, generating less toxic by-products and ultimately undergoing mineralization to CO2 and H2O. In situ formed ferric nanoparticles (e.g., γ-Fe2O3 or γ-FeOOH particles) efficiently sequester released phosphate via adsorption and co-precipitation. The system effectively reduces FOS toxicity to E. coli and performs well in real-water matrices. These results indicate that Fe(VI)/PMS is a sustainable technology for FOS-contaminated water treatment. Future work needs to investigate the interference mechanisms of complex matrices (e.g., high-organic industrial wastewater and high-salt aquaculture water) on FOS degradation and optimize process parameters to enhance the anti-interference ability.Author contributions
Yiping Feng and Jiuli Ruan conceived and supervised the project. Wen Jiang, Wenhao Lao, and Ze Xu conducted the experiments and analyzed the data. All the authors discussed and wrote the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
All data included in this study are available from the corresponding author upon request. Supplementary information (SI): including detailed experimental procedures for FOS quantification via LC-MS/MS, identification of FOS degradation products using UPLC-Q-Exactive Orbitrap-MS, and toxicity assessment with Escherichia coli; characterization protocols for SEM-EDS, XRD, and XPS analyses of formed floc particles; radical interconversion reaction schemes; and supplementary figures illustrating Fe(VI) decay dynamics, persulfate residual changes, pH and TOC variations during the reaction, phosphate release/precipitation behavior, and E. coli. survival rates. The file also provides complete properties of various water samples, mobile phase gradient elution parameters for FOS analysis, and structural and spectral data of identified FOS degradation products. See DOI: https://doi.org/10.1039/d5en00701a.Acknowledgements
This study was supported by the National Natural Science Foundation of China (21707019, 22176130), the Natural Science Foundation of Guangdong Province (2021A1515010019), and the Research Fund Program of Guangdong Provincial Engineering Research Center of Intelligent Low-Carbon Pollution Prevention and Digital Technology/SCNU (NAN'AN) Green and Low-Carbon Innovation Center (2024K04).References
- J. L. Martínez, Antibiotics and antibiotic resistance genes in natural environments, Science, 2008, 321(5887), 365–367 CrossRef PubMed.
- S. Sastry and Y. Doi, Fosfomycin: Resurgence of an old companion, J. Infect. Chemother., 2016, 22(5), 273–280 CrossRef CAS PubMed.
- K. Zurfluh, A. Treier, K. Schmitt and R. Stephan, Mobile fosfomycin resistance genes in enterobacteriaceae—an increasing threat, MicrobiologyOpen, 2020, 9(12), e1135 CrossRef CAS PubMed.
- G. Qiu, Y. Song, P. Zeng, S. Xiao and L. Duan, Phosphorus recovery from fosfomycin pharmaceutical wastewater by wet air oxidation and phosphate crystallization, Chemosphere, 2011, 84(2), 241–246 CrossRef CAS PubMed.
- R. Gothwal and T. Shashidhar, Antibiotic pollution in the environment: A review, Clean: Soil, Air, Water, 2015, 43(4), 479–489 CAS.
- Y.-H. Guan, J. Ma, Y.-M. Ren, Y.-L. Liu, J.-Y. Xiao, L. Lin and C. Zhang, Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals, Water Res., 2013, 47(14), 5431–5438 CrossRef CAS PubMed.
- T. Zhang, H. Zhu and J.-P. Croué, Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism, Environ. Sci. Technol., 2013, 47(6), 2784–2791 CrossRef CAS PubMed.
- H. Li, H. Lin, S. Raza, Z. Zhao, S. Chen, Y. Wang, Q. Zeng, C. Chen, W. Yu and L. Shen, Molecular insights in outstanding performance of Ca2+ bridging MXene/sodium alginate composite membranes, Water Res., 2025, 286, 124296 CrossRef CAS.
- M. Xie, W. Yang, X. Zhou, B. Wang, J. Liu, X. Xu, L. Shen, H. Zhang, M. Zhang, W. Yu, J. Teng, H. Lin, L. Zhao and B. Li, Multiscale thermodynamic insights into membrane fouling control by biochar-activated peroxymonosulfate pretreatment: Synergy of oxidation and adsorption, Water Res., 2026, 288, 124564 CrossRef CAS PubMed.
- X. Zhang, M. Feng, R. Qu, H. Liu, L. Wang and Z. Wang, Catalytic degradation of diethyl phthalate in aqueous solution by persulfate activated with nano-scaled magnetic CuFe2O4/MWCNTs, Chem. Eng. J., 2016, 301, 1–11 CrossRef CAS.
- J. Sun, M. Yan, G. Tao, R. Su, X. Xiao, Q. Wu, F. Chen, X.-L. Wu and H. Lin, A single-atom manganese nanozyme mediated membrane reactor for water decontamination, Water Res., 2025, 268, 122627 CrossRef CAS PubMed.
- H. Wang, Y. Cao, B. Li, L. Shen, X.-L. Wu, R. Li and H. Lin, Photothermal nano-confinement reactor with bimetallic sites for enhanced peroxymonosulfate activation in antibiotic degradation, Water Res., 2025, 268, 122623 CrossRef CAS PubMed.
- J. Wu, Z. Xu, K. Yao, Z. Wang, R. Li, L. Zuo, G. Liu and Y. Feng, Efficient degradation and detoxification of antibiotic fosfomycin by UV irradiation in the presence of persulfate, Sci. Total Environ., 2023, 905, 167249 CrossRef CAS PubMed.
- Y. Jiang, J. E. Goodwill, J. E. Tobiason and D. A. Reckhow, Effect of different solutes, natural organic matter, and particulate Fe (III) on ferrate (VI) decomposition in aqueous solutions, Environ. Sci. Technol., 2015, 49(5), 2841–2848 CrossRef CAS PubMed.
- A. A. Dar, B. Pan, J. Qin, Q. Zhu, E. Lichtfouse, M. Usman and C. Wang, Sustainable ferrate oxidation: Reaction chemistry, mechanisms and removal of pollutants in wastewater, Environ. Pollut., 2021, 290, 117957 CrossRef CAS PubMed.
- T. Yang, L. Wang, Y. Liu, J. Jiang, Z. Huang, S.-Y. Pang, H. Cheng, D. Gao and J. Ma, Removal of organoarsenic with ferrate and ferrate resultant nanoparticles: Oxidation and adsorption, Environ. Sci. Technol., 2018, 52(22), 13325–13335 CrossRef CAS PubMed.
- Z. Wang, W. Qiu, S. Pang, Y. Zhou, Y. Gao, C. Guan and J. Jiang, Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment, Chem. Eng. J., 2019, 371, 842–847 CrossRef CAS.
- S. Wu, H. Li, X. Li, H. He and C. Yang, Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants, Chem. Eng. J., 2018, 353, 533–541 CrossRef CAS.
- Z. Wang, Y. Du, Y. Jiang, J. Li, C. He, Y. Liu, Z. Xiong and B. Lai, Fe(V) Dominates the toxicity increase pathway during Ferrate(VI) degradation of fluoroquinolone antibiotics, ACS ES T Eng., 2023, 3(6), 883–893 CrossRef CAS.
- X. Zhang, X. Zhu, H. Li, C. Wang and T. Zhang, Combination of peroxymonosulfate and Fe(VI) for enhanced degradation of sulfamethoxazole: The overlooked roles of high-valent iron species, Chem. Eng. J., 2023, 453, 139742 CrossRef CAS.
- X. Huang, C. Tian, Y. Fu, C. Hu, G. Amy and C. Liu, Enhanced diclofenac abatement by ferrate in the presence of montmorillonite: Trapping in-situ formed Fe(III), J. Environ. Chem. Eng., 2024, 12(4), 113160 CrossRef CAS.
- R. P. Kralchevska, R. Prucek, J. Kolařík, J. Tuček, L. Machala, J. Filip, V. K. Sharma and R. Zbořil, Remarkable efficiency of phosphate removal: Ferrate(VI)-induced in situ sorption on core-shell nanoparticles, Water Res., 2016, 103, 83–91 CrossRef CAS PubMed.
- J. Hu, Z. Li, A. Zhang, S. Mao, I. R. Jenkinson and W. Tao, Using a strong chemical oxidant, potassium ferrate (K2FeO4), in waste activated sludge treatment: A review, Environ. Res., 2020, 188, 109764 CrossRef CAS PubMed.
- Q. Song, Y. Feng, G. Liu and W. Lv, Degradation of the flame retardant triphenyl phosphate by ferrous ion-activated hydrogen peroxide and persulfate: Kinetics, pathways, and mechanisms, Chem. Eng. J., 2019, 361, 929–936 CrossRef CAS.
- Y. Lee, J. Yoon and U. von Gunten, Spectrophotometric determination of ferrate (Fe(VI)) in water by ABTS, Water Res., 2005, 39(10), 1946–1953 CrossRef CAS PubMed.
- Y. Liu, L. Wang, Y. Dong, W. Peng, Y. Fu, Q. Li, Q. Fan, Y. Wang and Z. Wang, Current analytical methods for the determination of persulfate in aqueous solutions: A historical review, Chem. Eng. J., 2021, 416, 129143 CrossRef CAS.
- J. Wang, H. Wang, L. Shen, R. Li and H. Lin, A sustainable solution for organic pollutant degradation: Novel polyethersulfone/carbon cloth/FeOCl composite membranes with electric field-assisted persulfate activation, Water Res., 2023, 244, 120530 CrossRef CAS PubMed.
- M. Wu, M. Zhang, L. Shen, X. Wang, D. Ying, H. Lin, R. Li, Y. Xu and H. Hong, High propensity of membrane fouling and the underlying mechanisms in a membrane bioreactor during occurrence of sludge bulking, Water Res., 2023, 229, 119456 CrossRef CAS PubMed.
- L. Xiong, W. Yu, C. Ji, L. Liu, J. Teng, C. Chen, H. Lin and L. Shen, Bio-derived carboxymethyl cellulose-graft-gallic acid antiscalant with remarkable antibacterial activity for reverse osmosis scaling and biofouling control, Water Res., 2025, 281, 123716 CrossRef CAS PubMed.
- C. Wang, X. Wang, W. Wu, Y. Zong, Z. Zhang, Y. Li, S. Qin, F. Wang, X. Niu and L. Ding, A biochar-based catalyst prepared by potassium ferrate oxidation coupled high-temperature pyrolysis and its application for the activation of peroxymonosulfate towards the degradation of norfloxacin: Performance and mechanism insight, Sep. Purif. Technol., 2023, 323, 124302 CrossRef CAS.
- M. Li, L. Zhu and D. Lin, Toxicity of ZnO Nanoparticles to escherichia coli: Mechanism and the influence of medium components, Environ. Sci. Technol., 2011, 45(5), 1977–1983 CrossRef CAS.
- O. Dinc, S. Wacławek, R. R. Solís and D. D. Dionysiou, Synergistic oxidative removal of sulfamethoxazole using ferrate(VI) and peroxymonosulfate, Chem. Eng. J., 2024, 488, 151085 CrossRef CAS.
- C. Chen, L. Liu, Y. Li, L. Zhou and Y. Lan, Efficient degradation of roxarsone and simultaneous in-situ adsorption of secondary inorganic arsenic by a combination of Co3O4-Y2O3 and peroxymonosulfate, J. Hazard. Mater., 2021, 407, 124559 CrossRef CAS PubMed.
- G. P. Anipsitakis and D. D. Dionysiou, Radical generation by the interaction of transition metals with common oxidants, Environ. Sci. Technol., 2004, 38(13), 3705–3712 CrossRef CAS PubMed.
- Y. Ji, Y. Fan, K. Liu, D. Kong and J. Lu, Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds, Water Res., 2015, 87, 1–9 CrossRef CAS PubMed.
- R. Pi, Z. Yang, J. Chai, Y. Qi, X. Sun and Y. Zhou, Peroxysulfur species-mediated enhanced oxidation of micropollutants by ferrate(VI): Peroxymonosulfate versus peroxydisulfate, J. Hazard. Mater., 2024, 475, 134871 CrossRef CAS PubMed.
- M. Nie, Y. Yang, Z. Zhang, C. Yan, X. Wang, H. Li and W. Dong, Degradation of chloramphenicol by thermally activated persulfate in aqueous solution, Chem. Eng. J., 2014, 246, 373–382 CrossRef CAS.
- Y. Fan, Y. Ji, D. Kong, J. Lu and Q. Zhou, Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process, J. Hazard. Mater., 2015, 300, 39–47 CrossRef CAS PubMed.
- M. Wang, Q. Wang, Y. Cai, R. Yuan, F. Wang, Y. Qian, Z. Chen, B. Zhou and H. Chen, Efficient degradation and defluorination of perfluorobutyric acid under UV irradiation in the presence of persulfate, J. Cleaner Prod., 2021, 327, 129472 CrossRef CAS.
- B. Tian, N. Wu, X. Pan, Z. wang, C. Yan, V. K. Sharma and R. Qu, Ferrate(VI) oxidation of bisphenol E–kinetics, removal performance, and dihydroxylation mechanism, Water Res., 2022, 210, 118025 CrossRef CAS PubMed.
- F. Dong, C. Fu, M. Feng, D. Wang, S. Song, C. Li, E. Lichtfouse, J. Li, Q. Lin and V. K. Sharma, Simultaneous generation of free radicals, Fe(IV) and Fe(V) by ferrate activation: A review, Chem. Eng. J., 2024, 481, 148669 CrossRef CAS.
- T. Kamachi, T. Kouno and K. Yoshizawa, Participation of multioxidants in the pH dependence of the reactivity of ferrate(VI), J. Org. Chem., 2005, 70(11), 4380–4388 CrossRef CAS PubMed.
- Y. Gao, Y. Zhou, S.-Y. Pang, Z. Wang, Y.-M. Shen and J. Jiang, Quantitative evaluation of relative contribution of high-valent iron species and sulfate radical in Fe(VI) enhanced oxidation processes via sulfur reducing agents activation, Chem. Eng. J., 2020, 387, 124077 CrossRef CAS.
- X. Liu, Y. Liu, S. Lu, Z. Wang, Y. Wang, G. Zhang, X. Guo, W. Guo, T. Zhang and B. Xi, Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism, Chem. Eng. J., 2020, 385, 123987 CrossRef CAS.
- A. Ghauch, A. Baalbaki, M. Amasha, R. El Asmar and O. Tantawi, Contribution of persulfate in UV-254nm activated systems for complete degradation of chloramphenicol antibiotic in water, Chem. Eng. J., 2017, 317, 1012–1025 CrossRef CAS.
- D. Deng, L. Peng, M. Guan and Y. Kang, Impact of activation methods on persulfate oxidation of methyl tert-butyl ether, J. Hazard. Mater., 2014, 264, 521–528 CrossRef CAS PubMed.
- Q. Yi, J. Tan, W. Liu, H. Lu, M. Xing and J. Zhang, Peroxymonosulfate activation by three-dimensional cobalt hydroxide/graphene oxide hydrogel for wastewater treatment through an automated process, Chem. Eng. J., 2020, 400, 125965 CrossRef CAS.
- Y. Feng, Q. Song, W. Lv and G. Liu, Degradation of ketoprofen by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices, Chemosphere, 2017, 189, 643–651 CrossRef CAS PubMed.
- Y. Xiao, L. Zhang, W. Zhang, K.-Y. Lim, R. D. Webster and T.-T. Lim, Comparative evaluation of iodoacids removal by UV/persulfate and UV/H2O2 processes, Water Res., 2016, 102, 629–639 CrossRef CAS PubMed.
- A. Ghauch, A. M. Tuqan and N. Kibbi, Naproxen abatement by thermally activated persulfate in aqueous systems, Chem. Eng. J., 2015, 279, 861–873 CrossRef CAS.
- S. Sun, S. Wang, Y. Ye and B. Pan, Highly Efficient Removal of Phosphonates from Water by a Combined Fe(III)/UV/Co-Precipitation Process, Water Res., 2019, 153, 21–28 CrossRef CAS PubMed.
- K. A. Barrett and M. B. McBride, Oxidative degradation of glyphosate and aminomethylphosphonate by manganese oxide, Environ. Sci. Technol., 2005, 39(23), 9223–9228 CrossRef CAS PubMed.
- X.-N. Zhao, Z.-S. Huang, Y.-L. Liu, H.-T. Gu, Z. Gao, C. Cui, J. Ma and L. Wang, Roles of iron (V) and iron (IV) species in ferrate-triggered oxidation of phenolic pollutants and their transformation induced by phenoxyl radical, Water Res., 2025, 274, 123133 CrossRef CAS PubMed.
- Q. Guo, Z. Xu, W. Chu, Y. Zhou, X. Gao and C. Ye, Exploring the degradation of ofloxacin in sewer overflows by Fe(VI)/PMS, Fe(VI)/PDS, and Fe(VI)/SPC: Overlooked synergistic effect of oxidation and in-situ coagulation, J. Hazard. Mater., 2025, 488, 137333 CrossRef CAS PubMed.
- B. Zhang, X. Li, K. Akiyama, P. A. Bingham and S. Kubuki, Elucidating the mechanistic origin of a spin state-dependent FeNx–C catalyst toward organic contaminant oxidation via peroxymonosulfate activation, Environ. Sci. Technol., 2022, 56(2), 1321–1330 CrossRef CAS PubMed.
- L. Wu, L. Wang, Y.-L. Liu, X.-N. Zhao and J. Ma, VUV activated Fe(VI) by promoting the generation of intermediate valent iron and hydroxyl radicals, Environ. Sci. Technol., 2024, 58(45), 20256–20266 CrossRef CAS PubMed.
- Q. Zhong, J. Liu, J. Wang, Y. Li, J. Li and G. Zhang, Efficient degradation of organic pollutants by activated peroxymonosulfate over TiO2@C decorated Mg–Fe layered double oxides: Degradation pathways and mechanism, Chemosphere, 2022, 300, 134564 CrossRef CAS PubMed.
- S. Maiti, S. Choung, K. Maiti, M. T. Curnan, J. Hur and J. W. Han, Engineering active sites into iron hydroxide/Pt-based nanocatalysts to enrich the oxygen reduction reaction, ACS Appl. Mater. Interfaces, 2025, 17(28), 40517–40526 CrossRef CAS PubMed.
- H. Ai, K. A. Clavier, B. E. Watts, S. A. Gale and T. G. Townsend, The efficacy of pH-dependent leaching tests to provide a reasonable estimate of post-carbonation leaching, J. Hazard. Mater., 2019, 373, 204–211 CrossRef CAS PubMed.
- R. Brandes, B. Newton, M. Owens and E. Southerland, Technical support document for water quality-based toxics control, Bytes, 1991, 505 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |