Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon dots: a promising path towards environmental sustainability
Ajith Manayil
Parambil
 *ab and
Paulraj
Rajamani
*ab and
Paulraj
Rajamani
 *a
*a
aSchool of Environmental Sciences, Jawaharlal Nehru University (JNU), New Delhi, 110067, India. E-mail: ajithmptnr@gmail.com; paulrajr@mail.jnu.ac.in
bNanotechnology Centre, Centre for Energy and Environmental Technologies, VSB-Technical University of Ostrava, Ostrava Poruba, 70800, Czech Republic
First published on 4th October 2024
Abstract
Carbon dots (CDs) have received a lot of interest in recent years because of their unique features and wide range of uses, especially in environmental research. Several reviews have already addressed different aspects of CDs, including production, optical characteristics, and applications in bioimaging and drug administration. However, there is a significant void in the research regarding CDs' full environmental potential, particularly in addressing environmental deterioration through monitoring and rehabilitation. This article separates itself by concentrating on the significance of co-formed molecules in modifying CD properties, as well as the importance of purifying methods for optimal environmental performance. Previous assessments have typically neglected how co-formed compounds during synthesis can have a significant impact on CD surface chemistry, solubility, and photoluminescence properties. This perspective delves into how tailoring the synthesis and purification of CDs can optimize them for environmental applications. The article then looks into the promising future of CDs for environmental monitoring and remediation. Their distinguishing characteristics make them appropriate for sensing applications such as fluorescence-based detection, colorimetric sensing, and electrochemical sensing. Furthermore, CDs have the potential to accelerate the breakdown of organic pollutants, hence increasing the effectiveness of environmental restoration efforts. Their vast surface area and variable surface chemistry enable the effective sorptive removal of organic and inorganic contaminants from water. Integrating CDs with membrane filtration systems improves pollutant removal efficiency. Then, we investigated the mechanisms behind the antibacterial properties of CDs. By extensively studying these issues, this paper intends to demonstrate the revolutionary potential of CDs in building a more ecologically friendly and sustainable future.
Environmental significanceThe worsening environmental crisis demands urgent action towards sustainable practices. This article discusses the great potential of carbon dots (CDs), a revolutionary class of nanoparticles, for environmental applications. Using sophisticated sensor techniques, we look at how to construct CDs for peak performance and their critical function in environmental monitoring. Furthermore, the research studies CD-based environmental remediation options such as adsorption, pollutant degradation, improved membrane filtration, and antimicrobial activity. This article thoroughly investigates these diverse functionalities and highlights carbon dots' transformational potential in crafting a more sustainable future, which reflects the current trend of leveraging nanotechnology for sustainable solutions. This forward-looking perspective highlights a solid argument for the continued discovery and development of CD-based technologies to tackle environmental concerns and pave the way for a cleaner and healthier lifestyle. |
1. Introduction
The specter of environmental deterioration is extremely serious and calls for a paradigm change in favor of sustainable solutions. A remarkable nanomaterial, carbon dots (CDs), has come to light as a ray of hope at this crucial time. These tiny, fluorescent nanoparticles provide a ground-breaking method for addressing various environmental issues. Their unique qualities have enormous potential for environmental monitoring, remediation, and, eventually, a more environmentally friendly future.1–3 This perspective article delves into the exciting world of CDs for environmental applications.We start by investigating how co-formed small molecules affect CD attributes. By adjusting the synthesis procedure and the co-formation of small molecules, the intrinsic characteristics of CDs may be precisely adjusted. This can significantly impact their surface chemistry, photoluminescence, and solubility. These small molecules, frequently leftovers from precursor materials, can alter CDs' surface states and electrical characteristics, improving their functionalities.4 We then examine the vital function of purification in guaranteeing peak performance. The remaining starting materials and impurities might be unwelcome guests quenching CDs' fluorescence and diminishing their applicability in sensing applications. For this reason, careful purification methods are necessary to ensure the consistency and quality of CDs used in environmental monitoring.
Then, we will see the prospects of CDs in environmental monitoring and remediation. The field of environmental applications has demonstrated the great potential of CDs. Due to their exclusive properties, they are ideal for sensing applications such as fluorescence-based detection, and colorimetric, and electrochemical sensing.5 Moreover, CDs can stimulate the breakdown of organic contaminants, improving the effectiveness of environmental restoration procedures. The efficient adsorption of organic pollutants and heavy metals from water is made possible by their large surface area and functionalizable surface chemistry. The removal effectiveness of contaminants is further increased when these materials are integrated into membrane filtering systems.
The intrinsic and extrinsic limitations of CDs, such as poor QY, complex processing, time-consuming purification, and environmental challenges, pose considerable hurdles for their use in environmental monitoring and remediation. However, these difficulties may be addressed using a variety of tactics, including surface passivation, heteroatom doping, immobilization on support matrices, magnetic coupling, and improvement of synthesis and purification procedures. By addressing these challenges, CDs can become more effective and widely used in environmental applications, providing powerful tools for pollution detection, water treatment, and sensing. By thoroughly investigating these facets, our goal is to highlight how CDs may significantly impact the development of a more environmentally friendly and sustainable future.
2. Understanding carbon dots
2.1. Influence of co-formed small molecules and purification strategies
Since its accidental discovery in 2004, when single-walled carbon nanotube purification was underway, CDs have captured the attention of scientists with their distinct characteristics and wide range of uses.6 These nanoparticles, typically less than 10 nanometers in size, were first thought to be an impurity, but when it was discovered that they showed brilliant photoluminescence, curiosity about their possible applications grew. Since CDs are made of carbon, they are safe for the environment. They can be produced from a range of organic resources, including food waste, making them compatible with sustainable development objectives. Their synthesis is relatively simple, often involving hydrothermal, microwave, or electrochemical methods, which are cost-effective and environmentally benign.7 Due to their exceptional chemical stability, biocompatibility, and adjustable fluorescence, CDs have become a viable material for future technological developments in a variety of areas, including environmental engineering.8The co-formation of small molecules greatly influences the characteristics of CDs during their synthesis.9 The small molecules changing the surface chemistry of the dots can all impact the dots' solubility, stability, and photoluminescence activity. For instance, nitrogen-doped (N-CDs) or sulfur-doped (S-CDs) CDs are produced during synthesis when nitrogen or sulfur atoms co-form with CDs. By serving as PL centers, these co-formed heteroatoms can improve the quantum yield and emission tunability of the CDs. Because of nitrogen's capacity to donate electrons, N-CDs frequently exhibit improved tunability and increased photoluminescence in the visible spectrum, making them ideal for optical sensing applications. In the same way, during CD synthesis, surface functional groups including amine (–NH3), hydroxyl (–OH), and carboxyl (–COOH) groups frequently co-form, particularly if citric acid or ethylenediamine are utilized as precursors. The hydrophilicity, solubility, and chemical reactivity of CDs are improved by these groups. Also, surface functionalization enhances CDs' capacity for chemical sensing since these functional groups can interact with organic contaminants like pesticides or heavy metal ions like Pb2+ and Hg2+. As a result, CDs are great choices for environmental monitoring. The small molecules' interactions with the carbon core can also modify the CDs' energy levels and electron distribution, adjusting their optical and electrical characteristics. During pyrolytic synthesis, co-forming CDs with graphene-like domains or sp2 hybridized carbon structures can greatly increase their electron mobility, improving their suitability for electrochemical sensing and catalytic applications. With increased electron mobility, CDs may detect contaminants electrochemically more effectively—for example, by spotting dangerous metals or pesticide traces in water—and can react more quickly and sensitively. Thus, synthesizing highly specialized nanomaterials is made possible by the co-formation of small molecules, which is essential for customizing the characteristics of CDs for particular applications.
Purification strategies are essential for managing the impact of these co-formed small molecules.10 Unreacted precursors or byproducts (such as organic solvents or salts) may stay in the solution during synthesis. For example, residual carbonaceous by-products in citric acid-derived CDs might affect the optical and chemical characteristics of the CDs. CDs made by chemical or thermal synthesis frequently have by-products of non-fluorescent or amorphous carbon. These byproducts have the potential to reduce the CDs' total quantum yield, which would result in weak fluorescence. Over time, aggregation or precipitation may occur as a result of contaminants affecting the colloidal stability of CDs during their production. For applications in watery settings, including environmental sensing or water purification, this problem is especially crucial.
In one case, it has been reported that red, green, and blue fluorescent CDs can be produced by solvothermal synthesis of CDs from three structural isomers of a single benzene diamine precursor.11 Fast single-molecule diffusion of reaction products revealed fluorescence dominated by small molecules, but identifying the small molecules was challenging. Ultrafiltration, chromatography, and dialysis are frequently used techniques to eliminate undesirable small molecules and provide a more homogeneous result. Successful purification can remove fluorescent contaminants and stabilize surface states, resulting in CDs with more consistent characteristics and better quantum yields.12 On the other hand, inadequate purification can lead to CDs with heterogeneous characteristics, where the stability and effectiveness of photoluminescence might be adversely affected by the presence of unreacted precursors and by-products. Therefore, optimizing purification techniques to customize CDs for particular applications and guarantee their dependability and performance in real-world applications is crucial.
2.2. Engineering strategies for tailored environmental application
The efficiency of CDs in environmental applications is directly linked to their physicochemical features, which are primarily influenced by precursor selection and synthetic procedures—known as pre-synthetic control. While pre-synthetic control sets the fundamental properties of CDs, post-synthetic techniques give additional chances to fine-tune and improve their functionality (Fig. 1).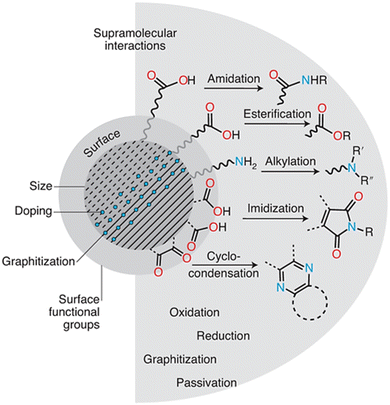 | ||
| Fig. 1 Strategies for tailored environmental application of CDs. Reproduced with permission.13 Copyright 2022, Nature. | ||
Precursor type and reaction temperature are two parameters that affect the CD size and graphitization degree. For example, utilizing lower temperatures or smaller precursor molecules usually results in smaller CDs with less graphitization (more disordered carbon structure). On the other hand, larger precursors and higher temperatures typically result in more graphitized, larger CDs. The types and functionalities of precursors significantly impact the surface chemistry of CDs. The functionalities of amine (–NH2), carboxyl (–COOH), and hydroxyl (–OH) groups in precursors will be translated onto the CD surface. These surface groups determine the kinds of supramolecular interactions CDs can have with contaminants, which is why these groups are important in environmental applications. Because they have a lot of hydroxyl and carboxyl groups, CDs made from citric acid can create hydrogen bonds with other contaminants, including nitrogen or oxygen atoms. This makes it easier for impurities to adsorb onto the CD surface, which helps remove them from water.
Exploiting CDs' surface chemistry has resulted in the creation of multifunctional CD-based materials. CDs functionalized with metal ions, such as silver, have improved antibacterial activity, making them useful in water purification. Surface engineering enables CDs to be utilized in environmental sensing, where certain functional groups on the CD surface interact with contaminants to produce very sensitive and selective detection techniques. Furthermore, the presence of functional groups such as carboxyl, hydroxyl, and amine groups on the surface of CDs enhances supramolecular interactions such as hydrogen bonding, electrostatic interactions, and π–π stacking.16 These interactions are essential for pollutant adsorption and catalytic degradation of organic contaminants. For example, a post-synthetic amination procedure on hydroxylated CDs can introduce amine groups. These amine groups can then form electrostatic interactions with metal ions, allowing for the selective capture of certain metals from water.
3. Current advances and prospects of carbon dots in environmental applications
Current research on CDs highlights their transformational potential in environmental applications, including sensing and remediation. CDs' extraordinary features include adjustable photoluminescence, large surface area, chemical stability, and biocompatibility. These characteristics make them ideal for solving environmental problems.3.1. Sensing of contaminants
CD fluorescence occurs via transitions between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) (Fig. 2A).3,17 When CDs absorb photons, electrons from the HOMO are excited to the LUMO. This is followed by non-radiative relaxation, in which electrons lose energy due to vibrations or interactions with their environment. Finally, the electrons return to the HOMO and emit the remaining energy as fluorescent light. The energy difference between the HOMO and LUMO determines the exact wavelengths (colours) of the emitted fluorescence, which may be affected by the CDs' size, shape, and surface functional groups.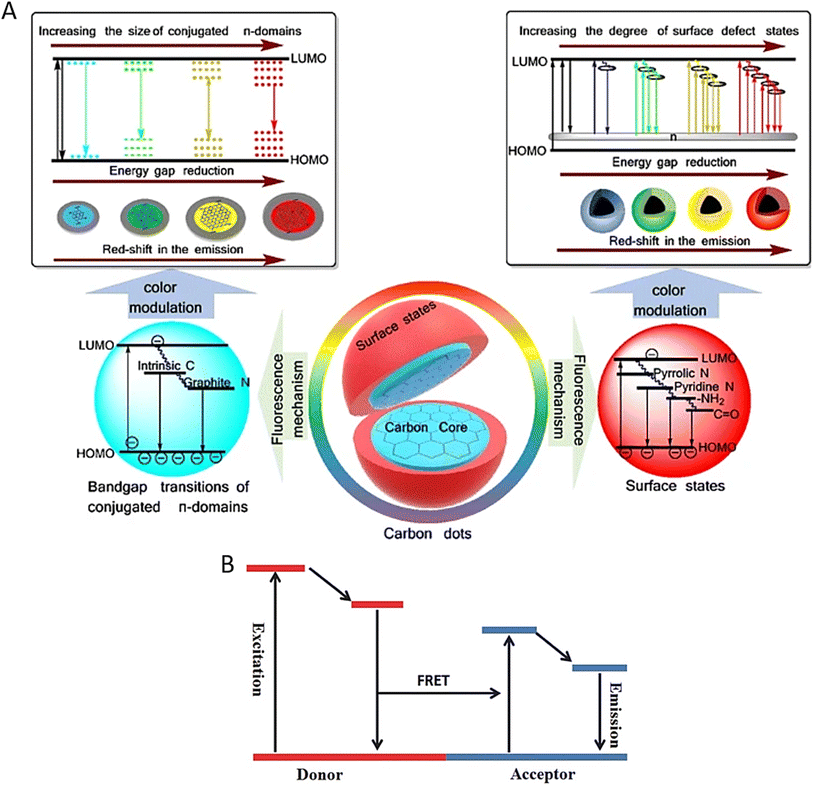 | ||
| Fig. 2 (A) Fluorescence mechanism of CDs. Reproduced with permission.17 Copyright 2019, Springer. (B) Mechanism of Förster Resonance Energy Transfer (FRET). Reproduced with permission.19 Copyright 2017, Wiley. | ||
CD-based sensing relies on their excellent fluorescence capabilities. By precisely adjusting CDs' surface chemistry and size, researchers may produce fluorescence responses tailored to distinct pollutants. This selectivity is achieved mainly via selective binding and Förster Resonance Energy Transfer (FRET).8,18 FRET occurs when donor–acceptor energy transfer is based on a long-range dipole–dipole interaction.19Fig. 2B shows the mechanism of FRET. CDs can be functionalized with ligands with a strong affinity for specific contaminants. When these ligands attach to CDs, they affect their electronic structure, resulting in fluorescence intensity or wavelength variations. CDs can operate as energy donors in FRET, transmitting excitation energy to pollutant molecules (acceptors) when they come into close contact. The presence of a contaminant hinders this energy transfer, resulting in decreased CD fluorescence. CD-based fluorescence sensing was initially investigated in 2012 by Dong et al., who showed the detection of Cu2+ ions using citric acid-branched polyethylenimine-derived CDs.20 Since then, CDs have been effectively used to detect various contaminants, including heavy metals, herbicides, organic pollutants, oil components such as polycyclic aromatic hydrocarbons (PAHs) and even explosives.15,21
Although fluorescence sensing is highly sensitive, it is prone to interference from background fluorescence in ambient materials. Researchers are investigating new sensing technologies that use CDs to fix this issue. CDs can be inserted into electrodes in electrochemical sensing, altering their electrical characteristics when in contact with certain pollutants. Electrochemical methods can monitor these changes, resulting in a reliable and label-free sensing strategy.22 Colorimetric sensing involves CDs exhibiting apparent colour changes when they come into contact with specific contaminants. This visual readout provides a simple, user-friendly sensing approach ideal for outdoor applications.23,24 Research into these alternate sense modalities is a fast-expanding area of study. The development of new electrode materials and chromogenic CD capabilities promises to expand the CD sensing toolset in the future significantly.
The potential for CD-based sensing is huge. These sensors provide various benefits over conventional methods, including increased sensitivity, selectivity, convenience, and cost-effectiveness. Additionally, CDs are biocompatible and easily adapted for specialized sensing applications. However, hurdles such as specificity, sensitivity, and long-term stability remain. Identifying individual pollutants in complicated environmental samples is an ongoing research priority. While progress has been achieved, more sensitivity is required to identify low-concentration contaminants. Additional research is needed to determine the long-term durability of CD fluorescence under environmental conditions. Despite these obstacles, the future of CD-based sensing seems promising.
Wearable sensors and portable detection devices may benefit from CDs' special features, allowing on-site, real-time environmental pollutant monitoring. As research advances, combining CDs' optical and electrical capabilities with sophisticated data processing and analysis techniques will likely result in more robust and adaptable environmental sensing technologies, such as logic gates and device fabrications.16 The ongoing development of innovative CD synthesis processes and sensing algorithms offers enormous potential for creating extremely sensitive, selective, and field-deployable sensors for environmental monitoring.
3.2. Remediation of contaminants
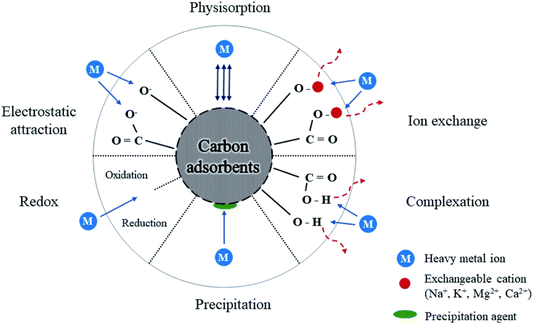 | ||
| Fig. 3 Adsorption mechanism of carbon adsorbents. Reproduced with permission.27 Copyright 2022, RSC. | ||
The mid-2010s observed the emergence of early CD-based adsorption research, with studies concentrating on removing heavy metals, including lead, copper, and mercury.24,28,29 Since then, various studies have emerged showing how effective CDs are at adsorbing various pollutants. Metal ion selectivity on CDs can be accomplished by modifying their surface functional groups, which play an important role in metal ion interaction. CDs have surface groups such as –COOH, –OH, and –NH2 that bind strongly to certain metal ions. The selective adsorption process is primarily driven by electrostatic interactions, coordination bonding, and complexation, in which metal ions are drawn to certain functional sites based on their charge and size. For instance, –COOH groups can bind to Pb2+ ions by coordination, while –NH2 groups may preferentially engage with Hg2+ ions. Furthermore, by altering the pH of the solution, the charge state of CDs and metal ions may be altered, increasing selectivity. Surface engineering, which involves functionalizing CDs with ion-specific ligands or chelating agents, can further increase selectivity by establishing tailored binding sites for certain metals. These techniques have potential for environmental applications in heavy metal detection and removal.
When hydrophobic and hydrophilic surface groups are added to CDs, they can adsorb oil molecules from tainted water. While the hydrophilic sections promote water dispersibility and aid in the efficient removal of oil under aqueous conditions, the hydrophobic regions interact with the oil. For the cleaning of oil spills, this makes CDs very helpful. CDs effectively adsorb other organic contaminants such as dyes, medicines, insecticides, and polycyclic aromatic hydrocarbons (PAHs) through the above-mentioned mechanisms. CDs have also demonstrated encouraging outcomes in eliminating inorganic contaminants from water, such as fluoride, iron, arsenic, and other metals.
The following areas are the focus of current CD-based adsorption research trends. Improving adsorption capacity: techniques like pore engineering and surface modification with specific functional groups are being investigated to increase the quantity of binding sites and adsorption affinity for the pollutants of interest. Selectivity enhancement: researchers are experimenting with surface functions and creating composite materials with additional adsorbents to obtain selective adsorption of certain pollutants from complicated mixtures. Development of hybrid materials: hybrid materials comprise CDs combined with polymers, metal nanoparticles, and other nanostructures to provide multipurpose adsorbents. Creating green synthesis techniques with natural precursors to make CDs is known as sustainable synthesis. Reusability and regeneration: creating techniques for effectively regenerating used CDs is essential for their long-term viability and practical use.
There is much promise for CD-based adsorption in the future. Highly effective and selective contamination removal will be made possible by creating next-generation CDs with regulated sizes, porosities, and surface functions. More studies are needed on scalable synthesis and economical regeneration techniques to take this technology from the lab to practical applications.
Research began to investigate the photocatalytic properties of CDs in 2010–2015. According to Li et al. (2010), CDs may be used to degrade dyes in the presence of visible light photocatalytically.30 During this time, CDs were investigated as stand-alone photocatalysts and were integrated with conventional semiconductors. Significant progress was achieved in the creation of composite materials based on CDs. Wang et al. (2015) used CD/TiO2 composites to exhibit improved photocatalytic degradation of organic contaminants.31 The emphasis moved to comprehending the underlying mechanics and increasing photocatalytic efficiency. The mechanism of photocatalytic degradation is given in Fig. 4. The creation of multifunctional CDs and sustainable synthesis techniques has received much attention lately. The green synthesis of CDs from biomass for the breakdown of pharmaceutical pollutants is described by Awfa et al. (2019).32 The integration of CDs with other materials for improved photocatalytic performance is a prominent trend.33
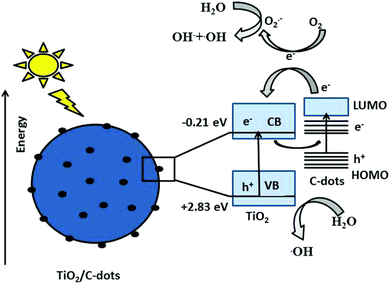 | ||
| Fig. 4 The mechanism of photocatalytic degradation by CD/TiO2. Reproduced with permission.34 Copyright 2018, RSC. | ||
Future studies will probably concentrate on controlling CDs' size, shape, and surface functionalization more effectively in order to increase their photocatalytic effectiveness. It will be essential to comprehend how these elements relate to photocatalytic activity. Integrating CDs with advanced oxidation processes (AOPs) can potentially enhance the efficiency of decomposing resistant organic pollutants. Investigating these integrations will be a fruitful field of study. The basic technique includes employing CDs as catalysts or co-catalysts to create reactive oxygen species (ROS), such as hydroxyl radicals (˙OH) and superoxide radicals (˙O2−), which are critical in the degradation of persistent organic pollutants (POPs). CDs, with their tailored surface features and high electron transport capabilities, may accelerate photocatalytic and Fenton-like processes in AOPs. Functionalizing CDs with certain surface groups can improve their capacity to collect light and create ROS under UV or visible light, increasing the degradation efficiency of contaminants such as dyes, insecticides, and medicines.
However, extended exposure to ROS may cause CDs to oxidize and degrade during AOPs. This deterioration has the potential to reduce CDs' long-term durability and reusability in AOP applications. To mitigate this, CDs can be surface passivated or hybridized with other stable materials (for example, metal oxides) to shield them from oxidative degradation while retaining their catalytic characteristics. It is imperative to tackle obstacles associated with CD-based photocatalysts' industrial manufacturing and marketing. Creating scalable and reasonably priced synthesis techniques will be essential for their broad acceptance.
Regarding photocatalysts, CDs are a promising class that can help break down organic pollutants. Research in material design, composite formation, and mechanism elucidation is still ongoing, and it has great promise to yield scalable, sustainable, and effective CD-based environmental remediation systems.
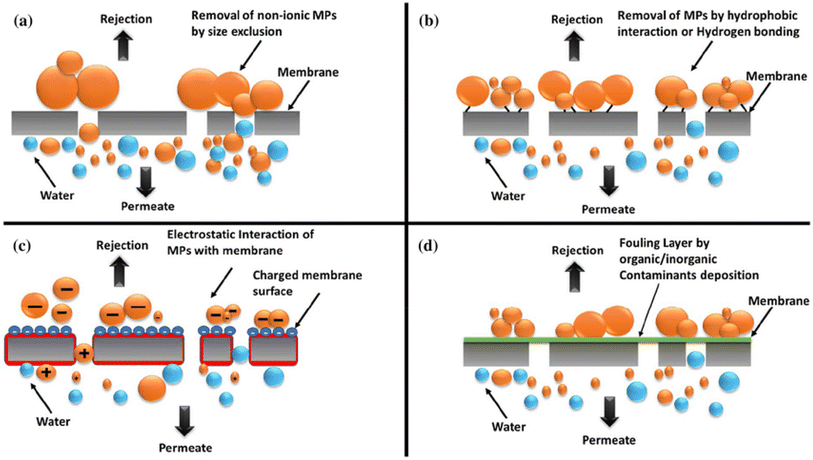 | ||
| Fig. 5 Membrane filtration mechanisms—(a) size exclusion, (b) hydrophobicity, (c) electrostatic interaction, and (d) adsorption. Reproduced with permission.35 Copyright 2020, Elsevier. | ||
Initial research centered on integrating CDs into polymer nanocomposites to improve membrane characteristics such as hydrophilicity and porosity. Current research focuses on CD surface functionalization to improve pollutant selectivity and adsorption capability.8 Understanding the complicated interplay of CDs, contaminants, and membranes is a challenge. Future studies should concentrate on refining CD characteristics for particular pollutants, creating scalable and economical processes for fabricating CD-incorporated membranes, and assessing the stability and reusability of CD-based membranes over the long run. All things considered, CDs show potential for increasing membrane filtration efficiency and selectivity; nevertheless, before they are widely used, further study is required to solve existing limitations.
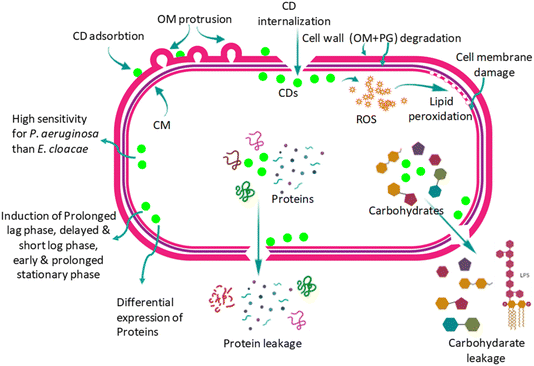 | ||
| Fig. 6 Mechanisms of antimicrobial activity. Reproduced with permission.36 Copyright 2023, RSC. | ||
Biogenic CDs provide an environmentally acceptable method of applying antimicrobials from natural precursors like bacteria and plant extracts. Because these CDs include bioactive functional groups inherited from their natural origins, they frequently have inherent antibacterial properties.36,37 Surface modification and functionalization are being used to improve CDs' antibacterial activity and specificity. Researchers are looking at several metals and non-metal doping methods to increase ROS production and CDs' photothermal effects. Furthermore, there is a lot of interest in creating synergistic therapies by combining CDs with other antimicrobial drugs and other nanomaterials.38,39
Prospects for future research on CDs in antimicrobial applications include the creation of multifunctional CDs that combine diagnostic and antibacterial properties. Theranostic platforms for real-time infection monitoring and therapy may result from this. Furthermore, the cost-effectiveness and scalability of the synthesis of biogenic CDs provide potential for extensive uses in environmental sanitation and healthcare. Overall, CDs show potential as next-generation antimicrobials, but more study is needed to overcome present limitations before broad application.
4. Challenges and potential solutions
4.1. Intrinsic challenges
Surface passivation, which involves adding functional groups or polymers to the surface of CDs, is one of the most successful methods for improving QY. This reduces surface imperfections and improves electron–hole recombination, resulting in greater PL. For example, covering CDs with polyethylene glycol (PEG) has been proven to greatly enhance QY. Adding heteroatoms such as nitrogen, sulfur, or phosphorus to the CD structure can also increase QY. Nitrogen-doped CDs (N-CDs) have been extensively studied for their improved optical characteristics and are more useful for environmental sensing due to their increased luminosity.
To solve the handling problem, CDs can be immobilized on a support matrix like silica, graphene, or polymer-based materials. This enhances their effective density and facilitates recovery by filtering or centrifugation. For example, CDs embedded in silica or graphene oxide sheets have been effectively employed for heavy metal adsorption in water treatment applications. Another new technique is to connect CDs with magnetic nanoparticles (e.g., Fe3O4), allowing for simple separation via magnetic fields. This strategy makes it easier to retrieve CDs after they've been used for environmental remediation jobs like contaminant adsorption.
Advances in synthetic processes, such as hydrothermal or microwave-assisted synthesis, can aid in producing CDs with more uniform size distributions and fewer contaminants, decreasing the need for significant purification. These approaches also provide scalability for large-scale manufacturing. Using solvents or surfactants to selectively precipitate CDs in specific size ranges can aid in improving fractionation. This approach has been used to separate CDs with more consistent optical and chemical characteristics, which are necessary for accurate environmental monitoring and cleanup. Electrophoresis is another way for separating CDs based on size and charge, and it can give more control over the fractionation process. This approach has been used to distinguish several forms of carbon nanomaterials, including CDs, according to their electrophoretic mobility.
4.2. Synthesis challenges
The existing CD synthesis methods provide a significant challenge. These procedures frequently provide insufficient control over the size, surface chemistry, and functional groups of the resultant dots. This discrepancy causes variances in their effectiveness as sensors or remedial agents. Imagine a CD that detects lead in water. If its surface qualities aren't precisely engineered, it may not be sensitive enough to detect low levels of lead, perhaps leading to undetected contamination incidents. Researchers are continuously looking into “green” synthesis methods that use natural precursors such as plants or biowaste. These technologies provide better control over size and surface chemistry, resulting in more reliable CDs. Furthermore, creating standardized synthesis techniques would assure uniformity among research organizations, allowing for more valid comparisons and advances in the area.4.3. Sensing challenges
When detecting certain pollutants, some CDs interfere with other environmental toxins. For example, a CD designated for mercury might react with iron in the water sample, resulting in a false positive for mercury. This lack of accuracy may result in incorrect judgments of environmental pollution. Surface modification involving particular functional groups can improve selectivity. By tailoring the surface chemistry, CDs can interact more strongly with the target pollutant while limiting interference from other pollutants. Consider a CD intended to detect lead with a surface tailored to bind closely to lead ions. This would reduce interactions with other metal ions in the water, resulting in more accurate detection of lead pollution. Advanced detection approaches, such as integrating CDs with various sensing materials, can enhance selectivity. Researchers can construct extremely selective environmental sensors by combining CDs with specialized antibodies or enzymes that target the relevant contaminant.4.4. Environmental challenges
Another major issue is the uncertain long-term environmental and health impact of CDs. Since they are nanoparticles, their behavior in natural systems is unknown. We need to learn how they interact with species and ecosystems. For example, will they accumulate in the food chain? Will they degrade naturally in the environment, or will they endure for a long time? Researchers are working on making biodegradable CDs, or those that can be readily removed from the environment after usage. This might entail creating CDs that degrade spontaneously under sunlight or inserting specialized enzymes that cause their disintegration. Furthermore, life cycle evaluations to analyze the environmental effect of CD manufacturing and disposal are critical. Understanding the whole life cycle of CDs allows researchers to design more sustainable production techniques and ensure safe disposal after usage.4.5. Cost-effectiveness and scalability
Scalability and cost-effectiveness are significant issues in the large-scale manufacture of CDs for widespread environmental use. CDs have intriguing features in the laboratory, but their commercial feasibility is hampered by high manufacturing costs, energy-intensive synthesis methods, and difficult purifying processes. Common approaches, such as hydrothermal and pyrolysis methods, may need costly precursors, extensive reaction durations, and post-synthesis purification, restricting scalability. To solve these issues, researchers are looking at low-cost raw sources such biomass, waste products, and cheap organic molecules as CD precursors. Furthermore, improving synthesis processes to decrease energy consumption, reaction time, and solvent usage can boost scalability. Microwave-assisted synthesis and continuous flow systems, for example, are being studied for their potential to improve production efficiency. Moreover, cost-saving purifying methods like dialysis or inexpensive filtration might be employed to streamline processes. Large-scale CD manufacture can become more feasible by resolving these problems, opening the door for their wider application in environmental sensing, remediation, and other fields.By solving these issues, CDs have the potential to transform environmental monitoring and repair. With continuous study and development, CDs have the potential to be a significant weapon for a cleaner, healthier earth. They can enable real-time, on-site monitoring of contaminants, enabling more efficient and focused cleanup activities. Furthermore, their capacity for pollutant degradation makes them an attractive option for clearing up existing contamination. As researchers overcome these hurdles and realize CDs' full potential, a new age of environmental preservation may emerge.
5. Conclusion
Environmental deterioration necessitates inventive solutions. CDs, with their distinct features, provide a ray of hope. This article looks at the possibility of CDs for environmental monitoring and remediation. We look at how to modify CD qualities using co-formed molecules and the importance of purification for maximum performance. We then demonstrate the tremendous potential of CDs in environmental monitoring using various sensing approaches. Furthermore, their capacity to digest toxins and improve membrane filtering systems demonstrates their promise for environmental remediation. We then investigated the mechanisms behind CDs' antibacterial properties. By looking into these issues, this essay highlights how CDs may immensely contribute to a more sustainable future.Data availability
No primary research results, software, or code have been included, and no new data were generated or analyzed as part of this perspective article.Conflicts of interest
There are no conflicts to declare.References
- N. Gong, X. Ma, X. Ye, Q. Zhou, X. Chen, X. Tan, S. Yao, S. Huo, T. Zhang, S. Chen, X. Teng, X. Hu, J. Yu, Y. Gan, H. Jiang, J. Li and X.-J. Liang, Nat. Nanotechnol., 2019, 14, 379–387 CrossRef CAS.
- L. Wang, W. Li, L. Yin, Y. Liu, H. Guo, J. Lai, Y. Han, G. Li, M. Li, J. Zhang, R. Vajtai, P. M. Ajayan and M. Wu, Sci. Adv., 2020, 6, eabb6772 CrossRef CAS.
- Q. Zhang, R. Wang, B. Feng, X. Zhong and K. (Ken) Ostrikov, Nat. Commun., 2021, 12, 6856 CrossRef CAS PubMed.
- J. B. Essner, J. A. Kist, L. Polo-Parada and G. A. Baker, Chem. Mater., 2018, 30, 1878–1887 CrossRef CAS.
- P. D. Howes, R. Chandrawati and M. M. Stevens, Science, 2014, 346, 1247390 CrossRef PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- A. M. Parambil, S. Pardhiya and P. Rajamani, Small, 2022, 18, 2105579 CrossRef.
- Z. Bian, A. Wallum, A. Mehmood, E. Gomez, Z. Wang, S. Pandit, S. Nie, S. Link, B. G. Levine and M. Gruebele, ACS Nano, 2023, 17, 22788–22799 CrossRef CAS.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2015, 54, 5360–5363 CrossRef CAS PubMed.
- Y. Song, S. Zhu, S. Zhang, Y. Fu, L. Wang, X. Zhao and B. Yang, J. Mater. Chem. C, 2015, 3, 5976–5984 RSC.
- L. Đorđević, F. Arcudi, M. Cacioppo and M. Prato, Nat. Nanotechnol., 2022, 17, 112–130 CrossRef PubMed.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- M. P. Ajith, S. Pardhiya, A. K. Prabhakar and P. Rajamani, Chemosphere, 2022, 303, 135090 CrossRef CAS PubMed.
- A. Manayil Parambil, M. Nabeel Mattath, P. Rajamani, P. V. Pham, G. Kumar and V. K. Ponnusamy, Chem. Eng. J., 2023, 463, 142354 CrossRef CAS.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, Microchim. Acta, 2019, 186, 583 CrossRef.
- W. Zhang, W. Li, Y. Song, Q. Xu and H. Xu, Biosens. Bioelectron., 2024, 255, 116244 CrossRef CAS.
- W. Wu, X. Shao, J. Zhao and M. Wu, Advanced Science, 2017, 4, 1700113 CrossRef.
- Y. Dong, R. Wang, H. Li, J. Shao, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 2810–2815 CrossRef CAS.
- E. Priyadarshini, A. M. Parambil, P. Rajamani, V. K. Ponnusamy and Y.-H. Chen, Environ. Res., 2023, 225, 115577 CrossRef CAS.
- C. Long, Z. Jiang, J. Shangguan, T. Qing, P. Zhang and B. Feng, Chem. Eng. J., 2021, 406, 126848 CrossRef CAS.
- B. Liu, J. Zhuang and G. Wei, Environ. Sci.: Nano, 2020, 7, 2195–2213 RSC.
- M. P. Ajith, E. Priyadarshini and P. Rajamani, Bioresour. Technol., 2020, 314, 123786 CrossRef CAS PubMed.
- M. P. Ajith and R. Paulraj, in Nanomaterials for Advanced Technologies, eds. J. K. Katiyar, V. Panwar and N. Ahlawat, Springer Nature, Singapore, 2022, pp. 127–140 Search PubMed.
- M. P. Ajith and P. Rajamani, J. Nanosci. Nanotechnol., 2021, 2, 88–91 Search PubMed.
- Y. Fei and Y. H. Hu, J. Mater. Chem. A, 2022, 10, 1047–1085 RSC.
- L. Wang, C. Cheng, S. Tapas, J. Lei, M. Matsuoka, J. Zhang and F. Zhang, J. Mater. Chem. A, 2015, 3, 13357–13364 RSC.
- M. Zhang, Q. Yao, C. Lu, Z. Li and W. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 20225–20233 CrossRef CAS.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S.-T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- W. Wang, Y. Ni and Z. Xu, J. Alloys Compd., 2015, 622, 303–308 CrossRef CAS.
- D. Awfa, M. Ateia, M. Fujii and C. Yoshimura, J. Water Proc. Eng., 2019, 31, 100836 CrossRef.
- Y. Cheng, L. Deng, D. Wang, X. Wang, C. Ji and Y.-H. Zhou, Environ. Sci.: Adv., 2023, 2, 495–507 CAS.
- S. Sharma, A. Umar, S. K. Mehta, A. O. Ibhadon and S. K. Kansal, New J. Chem., 2018, 42, 7445–7456 RSC.
- N. K. Khanzada, M. U. Farid, J. A. Kharraz, J. Choi, C. Y. Tang, L. D. Nghiem, A. Jang and A. K. An, J. Membr. Sci., 2020, 598, 117672 CrossRef CAS.
- A. M. Parambil, A. Prasad, A. K. Tomar, I. Ghosh and P. Rajamani, J. Mater. Chem. B, 2023, 12, 202–221 RSC.
- P. Li, L. Sun, S. Xue, D. Qu, L. An, X. Wang and Z. Sun, SmartMat, 2022, 3, 226–248 CrossRef CAS.
- S. Raina, A. Thakur, A. Sharma, D. Pooja and A. P. Minhas, Mater. Lett., 2020, 262, 127122 CrossRef CAS.
- M. A. Miller, Sci. Transl. Med., 2017, 9, eaao0969 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |