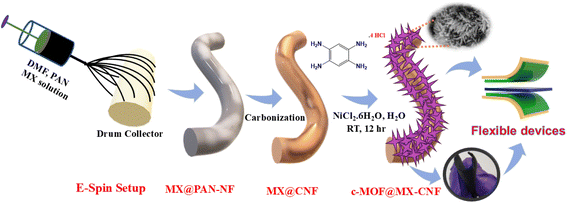
A π–d conjugated metal–organic framework decorated on a MXene-carbon nanofiber as a self-standing electrode for flexible supercapacitors†
Zahir
Abbas
a and
Shaikh M.
Mobin
 *ab
*ab
aDepartment of Chemistry, Indian Institute of Technology Indore, Simrol, Khandwa Road, Indore, 453552, India. E-mail: xray@iiti.ac.in
bCentre for Advanced Electronics (CAE), Indian Institute of Technology Indore, Simrol, Khandwa Road, Indore, 453552, India
First published on 12th November 2024
Abstract
Flexible electrode materials have gained significant breakthroughs recently due to their freestanding nature and long-term stability. The integration of MXene into carbon nanofiber leads to improved conductivity and stability. Herein, we employed an electrospinning technique to prepare self-standing MXene (Ti3C2Tx) carbon nanofiber (MX-CNF), onto which a one-dimensional π–d conjugated conductive metal–organic framework (c-MOF) is uniformly coated, exhibiting outstanding properties. The enhanced specific capacitance and conductivity is due to π–d mode of electron transfer in c-MOF on MX-CNF leads to improved conductivity. The obtained composite material achieved a specific capacitance of 1076 F g−1 with an excellent rate capability and superior cycling retention of 86.4% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles owing to its self-standing nature and ultra-stability. The electrode materials show better conductivity, hydrophilicity, and flexibility. A fabricated flexible asymmetric energy storage device achieved an energy density of 45.7 W h kg−1 with outstanding cycling stability. The flexible device was tested for different bending angles, maintaining its flexibility and ensuring no deformation occurred. The CV curves retains its orignal shapes at different bending angles. This work offers a new avenue for utilizing 1D conductive MOF on 2D material-based conductive nanofibers for flexible energy storage systems.
000 cycles owing to its self-standing nature and ultra-stability. The electrode materials show better conductivity, hydrophilicity, and flexibility. A fabricated flexible asymmetric energy storage device achieved an energy density of 45.7 W h kg−1 with outstanding cycling stability. The flexible device was tested for different bending angles, maintaining its flexibility and ensuring no deformation occurred. The CV curves retains its orignal shapes at different bending angles. This work offers a new avenue for utilizing 1D conductive MOF on 2D material-based conductive nanofibers for flexible energy storage systems.
1. Introduction
The need for wearable smart devices has emerged in the global flexible electronics industry, particularly in stretchable and fiber-based supercapacitors.1,2 To date, various electronic devices have been developed for wearable applications that can withstand in a variety of deformations, including stretching, bending, and twisting.3 This includes biomedical sensors and wearable energy storage devices.4 Owing to their beneficial characteristics, which include high power density, quick charge–discharge rates, extended cycle life, lightweight, and simple integrability, flexible supercapacitors can deliver the necessary energy and withstand the numerous deformations required for smart applications.5,6 Over the years, the conventional approach has been used to prepare flexible electrode materials via a different route that is not cost-effective, exhibiting low flexibility and poor electrochemical performance.7 In the past decade, CNF has been used as a flexible electrode material in flexible devices; however, the conductivity and flexibility of CNF are not satisfactory.8,9 To prepare a variety of permeable and highly charge-storing stretchable electrodes, it has been determined that adding fillers such as metal nanoparticles and 2D materials to the host electrospinning polymer solution would be an effective approach.10,11 Among these, 2D-MXene is a capable electrode material for energy storage because of its hydrophilicity and electrical conductivity.12 However, manufactured MX nanosheets are susceptible to restacking when formulating flexible film-based electrode materials. MX self-oxidation poses a significant obstacle to its use as a flexible electrode.13 To prevent MX from restacking and self-oxidation, carbon materials, particularly graphene and carbon nanotubes, are mixed with MX nanosheets to address restacking issues while improving electrical conductivity and surface functions.14 The integration of MX-CNF enhances both the conductivity and hydrophilicity of the composite. These properties are crucial for applications requiring efficient ion transport, such as in fast-charging devices, and make the material more competitive compared to other flexible electrodes with lower conductivity.However, the primary barrier to obtaining efficient electrochemical performance is the thickening of the electrolytes caused by the slow ion diffusion and degradation of the electrode materials over long-term cycling stability.15 The prudent design of a hierarchical integrated hybrid structure is necessary to satisfy the aforementioned requirements and overcome the obstacle.16,17 Some of the electroactive materials such as MOFs,18 COFs hybrid,19 MOF@MXene/CNF derived MX-PCNF,20 and MOF-derived carbon, etc. have already been explored.21 Unfortunately, most traditional MOFs have limited electrical conductivity, which severely restricts their application, especially when used as electrode materials. Unfortunately, several problems, such as accumulations happening in pristine MOFs, and relatively low cycle performance, restrict the direct application of MOF in supercapacitors and show mediocre performance.22,23 c-MOFs have attracted much consideration in research studies due to improved intrinsic conductivity and ion transport.24 Some of the explored 1D and 2D conductive MOFs for energy storage include Ni3(HITP)2 2D conductive MOF for energy storage applications.25 Cai et al. prepared 1D π–d conjugated conductive MOF for electrochromic energy storage with a high gravimetric capacity,26 In addition, Sang et al. explored π–d conductive MOFs for zinc air batteries.27 Nevertheless, 2D conductive MOF in a powder state cannot be directly applied as a flexible electrode.28 Some of the reports explored 2D conductive MOF on MXene to create MXene/MOF composites.29 Researchers have recently investigated freestanding, flexible electrodes having porous carbon nanofiber produced from MOF embedded with MXene, however, the MOF-derived process framework starts to collapse at higher temperatures.30 In addition to the pristine MOFs and 2D conductive MOFs, 1D conductive MOFs have been explored by researchers in energy storage because it has the 1D vertically aligned structure further enhances the density of redox-active centers and improves the charge carrier pathway.31,32
By taking into consideration all the parameters in terms of freestanding flexible electrodes, sustainability, and efficient electrochemical performance. We explored a unique composition of one-dimensional π–d conductive MOF over an extremely flexible and electroconductive MXene-carbon nanofiber mat (MX-CNF). As far as we are aware, no report is present for the utilization of 1D π–d conductive MOF-based nanoarrays on 2D MXene embedded 1D carbon nanofiber (1D–2D–1D) hybrid structure nanofiber mat as a binder-free, self-auxiliary supercapacitor electrode. In this process, firstly the MXene-PAN nanofiber is prepared by electrospinning, followed by stabilization and carbonization to create a 1D-2D hybrid structure; in situ approach via direct growth of 1D π–d conjugated MOF on the MX-CNF structure. We further explored the energy storage performance and for practical applicability asymmetric supercapacitor device was tested at different bending and twisting angles, which demonstrated stable energy storage performance under different circumstances and also efficient cycling stability. This work offers an alternative strategy to achieve π–d conjugated MOFs and COF-based flexible fiber as support and conductive freestanding electrodes for next-generation energy storage devices. In the future perspective in terms of integration of these flexible electrodes can be explored in various hybrid energy systems such as supercapacitor integration with piezoelectric and triboelectric energy harvesting.
2. Results and discussion
The preparation procedure of freestanding, flexible c-MOF@MX-CNF-based electrode materials is depicted in Scheme 1. Initially, MXene was dispersed in polyacrylonitrile/DMF solution via ultrasonication followed by electrospinning to prepare an MX-PAN nanofiber mat. The as-spun MX-PAN nanofiber mat was stabilized at 280 °C followed by carbonization at 800 °C under an inert atmosphere to obtain MX-CNF. However, c-MOF@MX-CNF electrode materials were prepared via an in situ approach of c-MOF on MX-CNF to prepare a self-standing electrode for flexible energy storage devices. The synthesis of c-MOF was carried out using 1,2,4,5-benzene tetramine (BTA) as an organic ligand with Ni2+ ions in the presence of ammonia solution. In this process, the –NH– species are formed by deprotonating –NH2 from the BTA ligand coordination with Ni2+ ions in a square planar.27 This results in the formation of 1D Ni-BTA bonds with a π–π/π–d conjugated structure that has an abundance of delocalized electrons. The synthesis of conductive nanowire-shaped Ni-BTA MOF on MX-CNF leads to a promising 1D-MOF on 1D fibers structure, making it a unique freestanding electrode for flexible supercapacitors.X-ray diffraction (XRD) was used to determine the phase purity of the prepared materials. The XRD pattern of the Ti3C2Tx-MXene peak at 10° shifted towards a lower angle at 7° with a broadening of the peak, which is the evidence of selective etching of the Al layer of the Ti3AlC2 MAX phase. The removal was further confirmed from the whole elimination of the strong (104) peak at 39° from the MAX phase of Al, which illustrates the successful preparation of MXenes, as depicted in the XRD plot, Fig. S1.†12,30 The XRD analysis of c-MOF and c-MOF@MX-CNF was performed. The XRD plot of c-MOF shows three prominent peaks at 2θ = 20.6, 23.9, and 29.3, exhibiting the preparation of c-MOF with improved crystallinity.26 While the XRD pattern of c-MOF@MX-CNF having MX-CNF and c-MOF showed all the peaks present in the composite but with slightly lower intensity of c-MOF, as shown in Fig. 1a. Furthermore, in the Raman spectra of MX-CNF, and c-MOF@MX-CNF, the peak at ∼620 cm−1 determines the M–N bond, which confirms the M–NH–C bond coordination structure of –NH–C. The peaks at 251, 415, and 634 cm−1 are similar to those from the previous reports.20,33 Furthermore, a peak at 415 cm−1 corresponds to oxygen vibrations. The peak at around 634 cm−1 corresponds to the Eg vibration of the carbon atom in the OH-terminated structure, as depicted in Fig. 1b. These corresponding peaks are of lower intensities. The additional two bands correspond to carbon, the G band corresponds to graphitic carbon and the D band is related to defective carbon. Whereas, ID/IG for MX-CNF is 1.18, and for the c-MOF@MX-CNF is 1.1, which signifies the degree of graphitization.7,12 X-ray photoelectron spectroscopy (XPS) was used to determine the elemental composition and bonding state, which illustrates the presence of C, N, O, Ni, and Ti, confirming the formation of c-MOF on MX-CNF via room temperature synthesis. The elements analysis of c-MOF@MX-CNF, in XPS are consistent with the EDS analysis. The percentage of carbon is the highest as expected due to carbonized nanofiber and carbon in the MOF ligand. The high-resolution XPS spectrum of Ni 2p is deconvoluted into Ni 2p3/2 and Ni 2p1/2 at 857 eV and 875 eV, respectively. Additionally, the deconvoluted two satellite peaks at 862.3 eV and 879.5 eV are shown in Fig. 1c.34 The high-resolution N 1s XPS spectra are deconvoluted into distinguished peaks such as pyridinic-N, pyrrolic-N, and graphitic-N with a binding energy of 398.2 eV, 399.8 eV, and 401 eV, respectively. It confirms the formation of N-doped carbon during the carbonization process of PAN nanofiber. The deconvoluted spectra of –C–NH–M were assigned as C–N with a peak position at 399.6 eV, which confirms the bonding of conductive ligands with metal ions. However, there was a coexistence of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peak with pyridinic-N at 398 eV. It signifies the successful formation of c-MOF with MX-CNF composites. Pyridinc and oxidic nitrogen helps to increase the conductivity of the carbon network, which is crucial for electrochemical performance in energy storage, Fig. 1d.35 Furthermore, the high-resolution C 1s spectra are also deconvoluted into four peaks, which are shown in Fig. 1e. The deconvoluted peaks of C–Ti, C–C, C–N, and C
N peak with pyridinic-N at 398 eV. It signifies the successful formation of c-MOF with MX-CNF composites. Pyridinc and oxidic nitrogen helps to increase the conductivity of the carbon network, which is crucial for electrochemical performance in energy storage, Fig. 1d.35 Furthermore, the high-resolution C 1s spectra are also deconvoluted into four peaks, which are shown in Fig. 1e. The deconvoluted peaks of C–Ti, C–C, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities have binding energies of 282.3 eV, 284.3 eV, 286.5 eV and 288 eV, respectively. The O 1s profile in Fig. S2† has four distinct peaks of O–Ti, O2−, C–O, and H2O/OH− with peak positions of 529.6 eV, 530.9, 531.8 and 533 eV, respectively. This indicates that the external terminating functionalities and partially oxidized are well preserved in the fiber.12Fig. 1f shows the XPS spectra of Ti 2p that is deconvoluted into Ti–C, Ti3+, Ti–O, and satellite peaks of Ti with peaks positions at 456.8 eV, 457.9 eV, 458.2 eV, and 464 eV, respectively, which illustrates the successful interaction of MXene with MOFs.30 Thus, the overall results indicate the N-doping and integration of MXene with CNF and successful implantation for the room temperature growth of c-MOF to make a composite of c-MOF@MX-CNF.
O functionalities have binding energies of 282.3 eV, 284.3 eV, 286.5 eV and 288 eV, respectively. The O 1s profile in Fig. S2† has four distinct peaks of O–Ti, O2−, C–O, and H2O/OH− with peak positions of 529.6 eV, 530.9, 531.8 and 533 eV, respectively. This indicates that the external terminating functionalities and partially oxidized are well preserved in the fiber.12Fig. 1f shows the XPS spectra of Ti 2p that is deconvoluted into Ti–C, Ti3+, Ti–O, and satellite peaks of Ti with peaks positions at 456.8 eV, 457.9 eV, 458.2 eV, and 464 eV, respectively, which illustrates the successful interaction of MXene with MOFs.30 Thus, the overall results indicate the N-doping and integration of MXene with CNF and successful implantation for the room temperature growth of c-MOF to make a composite of c-MOF@MX-CNF.
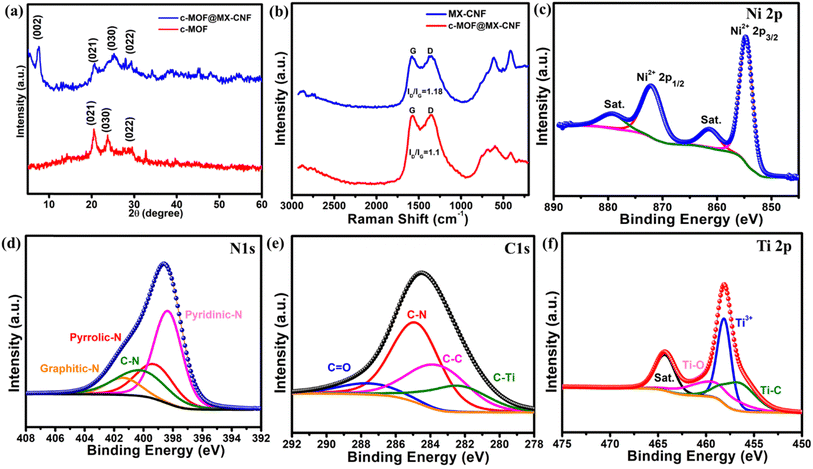 | ||
| Fig. 1 (a) PXRD analysis of c-MOF and c-MOF@MX-CNF (b) Raman analysis of MX-CNF and c-MOF@MX-CNF; XPS deconvoluted spectra of c-MOF@MX-CNF, and (c) Ni 2p (d) N 1s (e) C 1s (f) Ti 2p. | ||
Four probe direct current methods were used to determine the electrical conductivity of the electrode materials such as c-MOF and c-MOF@MX-CNF at 273 K. The I/V plot determines the ohmic behavior. The measured electrical conductivities of c-MOF and c-MOF@MX-CNF are 1.9 × 10−5 S cm−1 and 1.8 × 10−3 S cm−1, respectively, as depicted in Fig. S3a and b.† The improvement in the conductivity is due to 1D c-MOF along with 2D-MXene embedded in the 1D-CNF. This makes a unique kind of (1D–2D–1D) hybrid structure. To determine morphological analysis, field emission scanning electron microscopy (FE-SEM) was performed for CNF, MX-CNF, c-MOF, and c-MOF@MX-CNF. The morphology of MXene shows its sheet-like morphology, as depicted in Fig. S4.† As shown in Fig. 2a, pure CNF shows an average diameter of 282 nm and uniform growth of nanofiber. For the MX-CNF, MXene is completely embedded in CNF, confirming the formation of the MX-CNF mat with different magnifications (Fig. 2b and c).
 | ||
| Fig. 2 (a) SEM image of CNF (b and c) SEM images of MX-CNF (d) c-MOF, and (e and f) SEM and HR-TEM of c-MOF@MX-CNF. | ||
MX-CNF mat showed better flexibility with higher hydrophilic properties along with the increase in the diameter of the fiber. Fig. 2d shows the SEM image of c-MOF having needle-shaped morphology. In the c-MOF@MX-CNF composite structure, c-MOF particles are completely embedded in MX-CNF with a uniform growth on the surface. This verifies the formation of c-MOF on the nanofiber, as depicted in Fig. 2e. To illustrate the presence of different elements in the hybrid material, FESEM-EDS/EDX was performed with a surface scan in the electron image of c-MOF@MX-CNF, which indicated the uniform dispersion of all elements. The presence of carbon (57.88%), nitrogen (21.46%), oxygen (8.01%), titanium (8.26%), and nickel (4.38%) verified the presence of c-MOF on MX-CNF along with the percentage content as shown in Fig. S5.† As expected carbon is present in higher content due to carbonized CNF and also carbon present in the ligands. Nitrogen is doped in the carbon framework from polyacrylonitrile (PAN) nanofiber and additionally, nitrogen is present in the ligand of MOFs. Therefore, EDS results are consistent with XPS analysis. To further determine the growth of c-MOF on MX-CNF, we performed high-resolution transmission electron microscopy (HR-TEM) analysis; Fig. 2f. shows that needle/rod-like c-MOF uniformly grows on the surface of MX-CNF. This confirms the formation of hybrid and flexible porous electrode material. The elemental mapping of c-MOF@MX-CNF is depicted in Fig. S6a,† which shows the electron image. The brighter region of carbon indicates a higher percentage content of carbon in the mapping. Additionally, other elements are also present in EDS mapping analysis, as depicted in Fig S6b–f.† The hydrophilicity of the obtained materials was confirmed via water contact angle measurement, the contact angle for pure carbon nanofiber was 76.82°, whereas, the contact angle measurement for MX-CNF was 30.58°, indicating better hydrophilicity as depicted in Fig. S7a and b.† FTIR was performed to determine the functionality of the obtained materials. In the FTIR spectra of c-MOF, MX-CNF, and c-MOF@MX-CNF, a small peak band at 530 cm−1 corresponds to the Ti–C bond of Ti3C2Tx. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration peaks are seen at 1580 cm−1, whereas, the peak at 1460 cm−1 corresponds to the C–H in-plane vibration of CNF and the peak at 1336 cm−1 belongs to the stretching mode of N–H. The Ti–O bond was observed at 630 cm−1, confirming the formation of all the materials, as shown in Fig. S8.† To, determine the porosity and texture properties of the electrode material such as c-MOF@MX-CNF and MX-CNF, the BET surface area was measured by the N2 adsorption–desorption test. The obtained surface areas were 81 m2 g−1 and 18.2 m2 g−1, respectively. The BET plot illustrated the higher surface area of the MX-CNF structure confirmed its porous nature. Whereas the composite structure surface area was decreased it confirmed that the c-MOF was completely compacted on the MX-CNF structure, as shown in Fig. S9a and b.† The porosity developed during the carbonization process and the uniformly arranged hierarchical hybrid structure of c-MOF was formed. The porous nature is crucial for contributing to enhanced electrochemical performance. The high surface area and abundant pores are favorable for the passage of hydrated ions and the enhancement of the electrochemical performance. The obtained electrode materials have higher flexibility and outstanding properties such as conductivity, and stability. Based on these parameters, we have explored it for electrochemical properties.
N vibration peaks are seen at 1580 cm−1, whereas, the peak at 1460 cm−1 corresponds to the C–H in-plane vibration of CNF and the peak at 1336 cm−1 belongs to the stretching mode of N–H. The Ti–O bond was observed at 630 cm−1, confirming the formation of all the materials, as shown in Fig. S8.† To, determine the porosity and texture properties of the electrode material such as c-MOF@MX-CNF and MX-CNF, the BET surface area was measured by the N2 adsorption–desorption test. The obtained surface areas were 81 m2 g−1 and 18.2 m2 g−1, respectively. The BET plot illustrated the higher surface area of the MX-CNF structure confirmed its porous nature. Whereas the composite structure surface area was decreased it confirmed that the c-MOF was completely compacted on the MX-CNF structure, as shown in Fig. S9a and b.† The porosity developed during the carbonization process and the uniformly arranged hierarchical hybrid structure of c-MOF was formed. The porous nature is crucial for contributing to enhanced electrochemical performance. The high surface area and abundant pores are favorable for the passage of hydrated ions and the enhancement of the electrochemical performance. The obtained electrode materials have higher flexibility and outstanding properties such as conductivity, and stability. Based on these parameters, we have explored it for electrochemical properties.
2.1 Electrochemical performance
Inspired by the homogeneous and extremely porous structure, substantial surface area, and appropriate hybrid structure of conductive 1D c-MOF and conductive MXene nanofiber, we endeavored to evaluate the electrochemical efficacy of the prepared electrodes for supercapacitors. The improved ability of the substance to store charge is facilitated by MXene's intrinsic conductivity and alleviating restacking. Larger ions from electrolytes are unable to pass through the fiber layers of MXenes arranged horizontally. Under these conditions, electrochemical adsorption is used to store energy rather than intercalation.20 The c-MOF@MX-CNF self-supporting binder and additives-free electrode were studied for three-electrode configuration by using 2 M KOH as the electrolyte. At a scan rate of 100 mV s−1 with an optimized voltage window of 0 to 0.5 V cyclic voltammetry was recorded for c-MOF, MX-CNF, and c-MOF@MX-CNF, as depicted in Fig. 3a.The appearance of rectangular two redox peaks for c-MOF@MX-CNF and c-MOF for both electrode materials is due to faradaic activity, which is suggestive of the presence of multiple redox peaks. Remarkably, the CV profile of the c-MOF grown on conductive MX-CNF shows a large area under the CV curves, as compared to its counterparts such as c-MOF and MX-CNF. The higher area is directly linked to improved capacitance performance as compared to other electrodes. Detailed CV analysis was performed for all the samples at different scan rates. The CV curve for c-MOF@MX-CNF is displayed in Fig. 3b at scan rates ranging from 5 mV s−1 to 100 mV s−1. As observed with the increase in scan rates the corresponding anodic and cathodic peaks slightly shift towards the positive and negative side. The shift determines the polarisation of the electrode material, enhancing ion and electron transfer rates, a trend consistent with the other electrode materials. The reversible oxidation-reduction occurs between the π–d mode of electron transport, which leads to the one-electron redox mechanism of c-MOF over nickel ions as (Ni2+/Ni3+) and the two-electron redox mechanism over reversible rearrangement of organic ligands such as (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–N). However, the MX-CNF hierarchical porous architecture offers numerous, conductive, and interconnected transport pathways for quick electron transfer. As a result, the c-MOF@MX-CNF electrode shows highly pseudocapacitive redox-active behavior. The CV curves at various scan rates show approximately rectangular shapes with broad redox peaks, indicating that pseudocapacitive and electric double-layer capacitor (EDLC)-type electrodes contributed to the overall capacitance. To further explore the in-detail charge storage mechanism of the c-MOF@MX-CNF cathode, the capacitive and diffusive contributions are discussed in the ESI† provided in Fig. S10a–c† along with a detailed description. The supposed increment of redox reactions and the symmetrical shape of CV curves indicate the electrode materials' rate capability and electrochemical reversibility. The nature of the CV curves of c-MOF@MX-CNF is different from those of c-MOF and MX-CNF due to EDLC behavior from MX-carbon nanofiber and pseudocapacitive from redox-active c-MOF. Fig. S11a† shows the CV curves at different scan rates of the c-MOF, it illustrates the appearance of highly redox-active anodic and cathodic peaks, it signifies that the c-MOF is pseudocapacitive in nature and the sharp redox peaks are still maintained at higher scan rates of 100 mV s−1, it signifies the fast charge transfer and lower internal resistance. The CV curves of MX-CNF at diverse scan rates signify EDLC behaviors owing to high carbonaceous content in the electrode materials as shown in Fig. S12a.†
N/C–N). However, the MX-CNF hierarchical porous architecture offers numerous, conductive, and interconnected transport pathways for quick electron transfer. As a result, the c-MOF@MX-CNF electrode shows highly pseudocapacitive redox-active behavior. The CV curves at various scan rates show approximately rectangular shapes with broad redox peaks, indicating that pseudocapacitive and electric double-layer capacitor (EDLC)-type electrodes contributed to the overall capacitance. To further explore the in-detail charge storage mechanism of the c-MOF@MX-CNF cathode, the capacitive and diffusive contributions are discussed in the ESI† provided in Fig. S10a–c† along with a detailed description. The supposed increment of redox reactions and the symmetrical shape of CV curves indicate the electrode materials' rate capability and electrochemical reversibility. The nature of the CV curves of c-MOF@MX-CNF is different from those of c-MOF and MX-CNF due to EDLC behavior from MX-carbon nanofiber and pseudocapacitive from redox-active c-MOF. Fig. S11a† shows the CV curves at different scan rates of the c-MOF, it illustrates the appearance of highly redox-active anodic and cathodic peaks, it signifies that the c-MOF is pseudocapacitive in nature and the sharp redox peaks are still maintained at higher scan rates of 100 mV s−1, it signifies the fast charge transfer and lower internal resistance. The CV curves of MX-CNF at diverse scan rates signify EDLC behaviors owing to high carbonaceous content in the electrode materials as shown in Fig. S12a.†
Fig. 3c shows the galvanostatic charge–discharge curve recorded for all the electrode materials such as c-MOF, MX-CNF, and c-MOF@MX-CNF for comparison at 1 A g−1. The calculated specific capacitance of c-MOF@MX-CNF was 1076 F g−1, for c-MOF it was 300 F g−1 and for MX-CNF it was 175 F g−1. The higher specific capacitance of c-MOF@MX-CNF is due to the combined effect of MX-CNF and c-MOF. The obtained electrochemical performance is superior to that from the recent literature for the flexible electrode, as depicted in Table S1.† The shape of the GCD curve in the case of pure c-MOF is non-symmetrical due to the pseudocapacitive influence and the c-MOF@MX-CNF composites are also nonsymmetrical and there is no change in the GCD pattern even at higher current densities. The pattern of MX-CNF is symmetrical and it signifies the EDLC behaviour. From the aforementioned GCD curves of c-MOF@MX-CNF, the calculated specific capacitance values were 1076, 1048, 985, 948, 900, 728, 622, 595 and 440 F g−1 at various current densities of 1, 1.5, 2, 3, 5, 7, 10, 15 and 20 A g−1, respectively, as depicted in Fig. 3d. The bar plot diagram shows the comparison of specific capacitance vs. current densities, as shown in Fig. 3e. The GCD plots of c-MOF are depicted in Fig. S11b† and the GCD plots of MX-CNF are shown in Fig. S12b.† The enhanced efficiency is accredited to the following reasons, the conductive 1D nanofiber and the growth of redox-active π–d conjugated conductive MOF, which leads to the improvement in the electrochemical performance. (i) The interconnected conductive MX-nanofiber-based multiple electron transport pathways for rapid electron transfer. (ii) Whereas, the imine species (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in Ni-BTA is the redox-active center for the electron transport pathway. The d-orbital of the metal atom and π orbital of the ligand leads to the π–d electron transfer system, suggesting the conversion of –C
N) in Ni-BTA is the redox-active center for the electron transport pathway. The d-orbital of the metal atom and π orbital of the ligand leads to the π–d electron transfer system, suggesting the conversion of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N to C–N bonds during the discharging process.35 The MXene fibrous network provides a conductive channel for electron transfer and improves its stability. Whereas, the CNF adds flexibility and structural support, enabling a freestanding, flexible electrode. This leads to the formation of heterojunction of c-MOF@MX-CNF with improved performance and stability. Cycling stability is another important parameter in determining the efficacy of electrode materials. The electrode materials were tested for 15
N to C–N bonds during the discharging process.35 The MXene fibrous network provides a conductive channel for electron transfer and improves its stability. Whereas, the CNF adds flexibility and structural support, enabling a freestanding, flexible electrode. This leads to the formation of heterojunction of c-MOF@MX-CNF with improved performance and stability. Cycling stability is another important parameter in determining the efficacy of electrode materials. The electrode materials were tested for 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles at a current density of 15 A g−1. The competent outcome shows that the electrode material retains 86.4% capacitance of the initial cycles. The improved stability and durability of the cathode materials are accredited to the following reasons. A carbonaceous network of nanofiber integrated with conductive MX provides stable support to the c-MOF during the cycling test and helps to withstand electrochemical conditions. The cycling stability plot is displayed in Fig. 3f, and the first and last five GCD cycles are displayed in the inset of the same figure. To further test the stability of the c-MOF@MX-CNF electrode material in an alkaline medium, we performed post-electrochemical characterization such as PXRD, SEM, and FTIR analysis. It was observed that there is no deformation occurring in the crystallinity of c-MOF and only the intensity in the composite structure was decreased. The morphology in the composite structure was retained and confirmed via SEM analysis. Furthermore, there was no change in the functional group of the c-MOF@MX-CNF, which confirmed its structural and material stability in an alkaline environment, as depicted in Fig. S13a–c.†
000 GCD cycles at a current density of 15 A g−1. The competent outcome shows that the electrode material retains 86.4% capacitance of the initial cycles. The improved stability and durability of the cathode materials are accredited to the following reasons. A carbonaceous network of nanofiber integrated with conductive MX provides stable support to the c-MOF during the cycling test and helps to withstand electrochemical conditions. The cycling stability plot is displayed in Fig. 3f, and the first and last five GCD cycles are displayed in the inset of the same figure. To further test the stability of the c-MOF@MX-CNF electrode material in an alkaline medium, we performed post-electrochemical characterization such as PXRD, SEM, and FTIR analysis. It was observed that there is no deformation occurring in the crystallinity of c-MOF and only the intensity in the composite structure was decreased. The morphology in the composite structure was retained and confirmed via SEM analysis. Furthermore, there was no change in the functional group of the c-MOF@MX-CNF, which confirmed its structural and material stability in an alkaline environment, as depicted in Fig. S13a–c.†
To comprehend the ion diffusion and charge transfer process, all the electrode materials were analyzed using electrochemical impedance spectroscopy (EIS). The electrical conductivity and reaction kinetics were ascertained using the EIS measurements. The obtained Nyquist plot shows the high-frequency X-axis is assigned ohmic (uncompensated) resistance (Rs), which is often caused by the electrolyte's ionic resistance. The charge transfer resistance (Rct), which is established by the electrical resistance of the electrode material and the contact resistance of the electrode/electrolyte interface, is correlated with the semicircle that is generated from the high to the low-frequency zone. The straight line generated at the low-frequency region represents the Warburg impedance. The diameter of the semicircle represents charge transfer resistance (Rct) at the electrode/electrolyte contact. All EIS spectra with a semicircle at high frequency and a straight line at low frequency are simulated by equivalent circles, as depicted in Fig. S14.† The Rct value of all the electrode materials is determined and the corresponding Rct is 1.3 Ohm for c-MOF@MX-CNF, 2.5 ohm for MX-CNF, and 4.3 ohm for c-MOF. The lower the resistance value, the higher the electrical conductivity and good charge transfer kinetics, and the smaller the semicircle. Based on these findings, the MXene-integrated electrode material in the system appears to have high conductivity and charge transfer kinetics, additionally, conductive MOF is present in the composite structure. Therefore, the electrode gave an exceptional electrochemical performance in the three-electrode systems as a self-standing electrode. We explored it for two electrode systems, to determine its practical applicability in flexible supercapacitor devices.
The optimized CV curve at different scan rates. The optimized voltage for the CV curve is from 0 to 1 V and similarly up to 1.4 V as depicted in Fig. 4b. GCD is another important parameter to optimize the device performance. The working voltage window is up to 0 to 1.4 V, polarization was observed beyond that due to oxygen evolution reaction occurring on the electrode surface. As a result, 1.4 V was fixed as a stable voltage window for a flexible device as depicted in Fig. 4c. CV was analyzed for ASC flexible devices at different scan rates varying from 5 to 100 mV s−1. The sigmoidal shape of the CV pattern remains even with the increase in scan rates and the enclosed area increased with the increase in scan rates, confirming the high rate and kinetic reversibility performance of the flexible device. The plot of CV curves at various scan rates is shown in Fig. 4d. It exhibits a miniature redox because the c-MOF decorated on MX-CNF has redox properties, this signifies both pseudocapacitive and EDLC behavior of the electrode material. GCD was analyzed at different current densities varied from 1 A g−1 to 20 A g−1, as presented in Fig. 4e. The calculated specific capacitance of flexible devices for various current densities at 1 A g−1 is 163.5 F g−1. Stability is another crucial factor to consider for device performance. We tested the cycling stability of the c-MOF@MX-CNF//AC ASC device for 8000 GCD cycles. The device exhibits excellent cycling retention of 80.4%. The achieved retention is best among the reported flexible devices. The stability plot is depicted in Fig. 4f. and the inset has the first 5 and last 5 cycles.
CV analysis was conducted to evaluate the flexibility of the c-MOF@MX-CNF//AC ASC device at various bending angles, including 45°, 90°, 130°, and 180°. These results prove the promising device's exceptional flexibility and stability, as shown in Fig. 5a. There was no change in the CV curves at all angles indicating its excellent flexibility at all angles as an excellent electrode material for flexible energy storage devices. In addition, two devices, c-MOF@MX-CNF//AC ASC were connected in series and could be used to illuminate colored LEDs, as per the photographs shown along with the representation of device bending in Fig. S15a–d.† Furthermore, the excellent flexibility and better electrochemical performance were supported by EIS measurement. Fig. S16† shows the Nyquist plot to determine the resistance of the flexible device. The lower Rct value of the ASC device is 2.3 ohm, indicating the lower internal resistance and improved conductivity and electrochemical performance of the device. Fig. 5b shows the Rangone plot of the c-MOF@MX-CNF//AC device. The energy density and power density were calculated using eqn (S2) and (S3)† and the achieved energy density (ED) and power density (PD) of 45.7 W h kg−1 and 719 W kg−1, respectively. Thus, these results elucidate that the obtained ED is superior to some of the recent literature-based nanofiber-based flexible devices, for example, NiO-embedded@PCNFs-1 ED, 43.1 W h kg−1, PD 412.5 W kg−1.36 CNF@Ni-CAT 18.67 ED W h kg−1, PD 297.12 W kg−1.37 Ni–Fe–OH@PCNFs-1//Fe2O3/NPC@PCNFs ED 44.3 W h kg−1, PD 907 W kg−1.38 MOF-derived CNT@HCNF-1.5 ED 20.13 W h kg−1, PD 499.8 W kg−1.39 Fe2O3/NPC@Fe3C/EPCNFs ED 21.6 W h kg−1, PD 499.05 W kg−1.40 Ni–Fe–O/NPC@PCNFs-400 ED 41.3 W h kg−1, PD 892.2 W kg−1.41 Therefore, this unexceptional performance of flexible MX-CNF-based substrate and π–d c-MOF on it shows an exceptional electrode material for futuristic smart and flexible devices.
3. Conclusions
In summary, we demonstrated the 1D porous architecture of nanofibers integrated with conductive MXene to form a 1D–2D architecture and π–d c-MOF decorated on it to make it a unique composition structure, c-MOF grows on it during the room temperature synthesis. This synthesis route provides a significant advancement in preparing sustainable supercapacitor electrodes. The prepared flexible film serves as a self-standing electrode in the flexible device. The as-obtained c-MOF@MX-CNF works as an electrode in supercapacitor studies, without utilizing binder and additives. Electrode materials achieved the best output performance in terms of specific capacitance with improved stability. Flexible devices have better flexibility at different angles and there is no deformation in the CV pattern of the device even at 90° and 180°. Furthermore, the ASC device delivers an energy density of 45.7 W h kg−1. Our finding demonstrates that the (1D–2D–1D architecture) provides a new avenue for utilizing conductive MOFs on conductive fabrics for improved performance and as an electrochemically stable electrode material in flexible devices. Using this strategy, other 2D materials-based nanofibers such as graphadyine and metal chalcogenides etc. with c-MOF-based flexible electrodes can be utilized in futuristic smart fabric-based wearable devices.4. Experimental section
4.1 Synthesis of MXene
In a synthesis of MXene, the reported protocol for the synthesis involves etching of LiF/HCl followed by exfoliation. 1.56 g of LiF was added to 20 ml of 9 M HCl in a Teflon-lined container and 1 g of Ti3AlC2 was added slowly; during the addition process it was placed under an ice bath then it was heated at 35 °C along with stirring for 24 h. The resulting suspension was washed with DI water until the pH of the dispersion became ∼6. The obtained MXene was collected and redispersed in DI water and sonicated for 1 h under inert conditions to obtain the Ti3C2TX MXene nanosheets. The synthesis process was carried out according to a slightly modified literature protocol.12,304.2 Preparation of MX-CNF mat
In order to create a uniform viscous solution, 600 mg of polyacrylonitrile (PAN) polymer was electrospun in 5 ml dimethyl formamide while being continuously stirred at room temperature for 10 hours. Then, 20 wt% of MXene was added to the above solution, sonicated for 2 h, and then stirred at room temperature for 5 h to obtain an MXene/PAN solution. The obtained solution was loaded into a 5 ml syringe with optimized parameters of voltage 17 V at a flow rate of solution of 1 ml h−1. The distance between the tip to the collector was 12 cm. We used a roller collector; after collecting the MXene/PAN nanofiber mat, it was further thermally treated and first oxidized in air at 280 °C for 2 h at a heating rate of 5 °C min−1. In the second step, the fiber mat was carbonized at 800 °C for 4 h under an inert N2 atmosphere carefully to obtain the carbonized MXene-carbon nanofiber (MX-CNF).4.3 Preparation of c-MOF on MX-CNF mat
In the synthesis of c-MOF on MX-CNF nanosheets, 0.1 mmol of 1,2,4,5-benzene tetramine (BTA·4HCl) was dissolved in 5 ml DI water and stirred until complete dissolution to form the solution A. Then, 0.1 mmol of NiCl2·6H2O was dissolved in another 5 ml of DI water as solution B. Solution B was slowly added to solution A to obtain a dark purple mixture, followed by the addition of a self-standing mat of MX-CNF (2 × 2). Afterward, 0.5 ml of ammonia solution was added and stirred for 10 h at room temperature to obtain a light brown powder coated on MX-CNF. The obtained self-standing nanofiber was thoroughly washed with acetone to remove any unreacted impurities/precursor materials. Further, the nanofiber mat was dried to obtain a π–d c-MOF decorated MX-CNF. It was characterized and utilized as a self-standing electrode for flexible devices in supercapacitors.Data availability
Original data are available upon request and can be obtained by contacting the corresponding author of the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. M. M. thanks to SERB-DST, New Delhi, India (Project CRG/2020/001769), NTTM, Ministry of Textile, New Delhi India, and IIT Indore for financial support. Z. A. thanks CSIR for providing fellowship. The authors acknowledge Sophisticated Instrumentation Centre, IIT Indore for all of the characterization facilities. We acknowledge CSIR-AMPRI Bhopal for HR-TEM analysis, and MNIT Jaipur for Raman analysis. IIT Mandi for XPS analysis. We thank Prof. Preeti A. Bhobe, Department of Physics, IIT Indore, for providing the facility to record electrical conductivity.References
- D. D. Khumujam, T. Kshetri, T. I. Singh, N. H. Kim and J. H. Lee, Adv. Funct. Mater., 2023, 33, 2302388 CrossRef.
- M. Jiang, D. Jiang, X. Cao, J. Wang, Y. Sun, M. Zhang and J. Liu, Adv. Funct. Mater., 2024, 34, 2312692 CrossRef.
- X. Zhang, H. Lin, H. Shang, J. Xu, J. Zhu and W. Huang, SusMat, 2021, 1, 105–126 CrossRef.
- Z. Gao, Y. Zhou, J. Zhang, J. Foroughi, S. Peng, R. H. Baughman, Z. L. Wang and C. H. Wang, Adv. Mater., 2024, 36, 2404492 CrossRef CAS.
- Z. Yan, S. Luo, Q. Li, Z.-S. Wu and S. Liu, Advanced Science, 2024, 11, 2302172 CrossRef CAS PubMed.
- J. Zhu, S. Han, Q. Wu, J. Zhang, A. Chen, B. Huang, J. Huang and L. Guan, ACS Appl. Energy Mater., 2023, 6, 4157–4167 CrossRef CAS.
- T. Kshetri, D. D. Khumujam, T. I. Singh, Y. S. Lee, N. H. Kim and J. H. Lee, Chem. Eng. J., 2022, 437, 135338 CrossRef CAS.
- B.-H. Xiao, K. Xiao, J.-X. Li, C.-F. Xiao, S. Cao and Z.-Q. Liu, Chem. Sci., 2024, 15, 11229–11266 RSC.
- X. Fan, W. Nie, H. Tsai, N. Wang, H. Huang, Y. Cheng, R. Wen, L. Ma, F. Yan and Y. Xia, Adv. Sci., 2019, 6, 1900813 CrossRef CAS.
- D. Ji, Y. Lin, X. Guo, B. Ramasubramanian, R. Wang, N. Radacsi, R. Jose, X. Qin and S. Ramakrishna, Nat. Rev. Methods Primers, 2024, 4, 1–21 CrossRef.
- J. Xue, T. Wu, Y. Dai and Y. Xia, Chem. Rev., 2019, 119, 5298–5415 CrossRef PubMed.
- Z. Wang, D. Zhang, Y. Guo, H. Jiang, D. Wang, J. Cheng, P. K. Chu, H. Yan and Y. Luo, Chem. Commun., 2023, 59, 14309–14312 RSC.
- S. A. Kadam, K. P. Kadam and N. R. Pradhan, J. Mater. Chem. A, 2024, 12, 17992–18046 RSC.
- K. Khan, A. Khan Tareen, M. Iqbal, I. Hussain, A. Mahmood, U. Khan, M. Farooq Khan, H. Zhang and Z. Xie, J. Mater. Chem. A, 2023, 11, 19764–19811 RSC.
- X. Zhou, Y. Zhou, L. Yu, L. Qi, K.-S. Oh, P. Hu, S.-Y. Lee and C. Chen, Chem. Soc. Rev., 2024, 53, 5291–5337 RSC.
- D. P. Chatterjee and A. K. Nandi, J. Mater. Chem. A, 2021, 9, 15880–15918 RSC.
- N. Hussain, Z. Abbas, K. Nabeela and S. M. Mobin, J. Mater. Chem. A, 2024, 12, 17642–17650 RSC.
- Z. Abbas, N. Hussain, S. Kumar and S. M. Mobin, Nanoscale, 2024, 16, 868–878 RSC.
- K. Nabeela, R. Deka, Z. Abbas, P. Kumar, M. Saraf and S. M. Mobin, Cryst. Growth Des., 2023, 23, 3057–3078 CrossRef.
- I. Pathak, D. Acharya, K. Chhetri, P. C. Lohani, S. Subedi, A. Muthurasu, T. Kim, T. H. Ko, B. Dahal and H. Y. Kim, J. Mater. Chem. A, 2023, 11, 5001–5014 RSC.
- Z. Abbas, N. Hussain, I. Ahmed and S. M. Mobin, Inorg. Chem., 2023, 62, 8835–8845 CrossRef.
- G. Song, Y. Shi, S. Jiang and H. Pang, Adv. Funct. Mater., 2023, 33, 2303121 CrossRef.
- T. Deng, W. Zhang, O. Arcelus, D. Wang, X. Shi, X. Zhang, J. Carrasco, T. Rojo and W. Zheng, Commun. Chem., 2018, 1, 1–9 CrossRef.
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef PubMed.
- M. Stodolka, J. Y. Choi, X. Fang, C. Lu, H. T. B. Pham, B. Check and J. Park, ACS Mater. Lett., 2024, 6, 49–55 CrossRef.
- G. Cai, P. Cui, W. Shi, S. Morris, S. N. Lou, J. Chen, J.-H. Ciou, V. K. Paidi, K.-S. Lee, S. Li and P. S. Lee, Advanced Science, 2020, 7, 1903109 CrossRef PubMed.
- Z. Sang, J. Liu, X. Zhang, L. Yin, F. Hou and J. Liang, ACS Nano, 2023, 17, 3077–3087 CrossRef PubMed.
- J. Liu, Y. Chen, X. Feng and R. Dong, Small Struct., 2022, 3, 2100210 CrossRef.
- S. Bibi, S. S. A. Shah, M. A. Nazir, M. H. Helal, S. M. El-Bahy, Z. M. El-Bahy, S. Ullah, M. A. Wattoo and A. ur Rehman, Adv. Sustainable Syst., 2024, 8, 2400011 CrossRef.
- I. Pathak, D. Acharya, K. Chhetri, P. Chandra Lohani, T. Hoon Ko, A. Muthurasu, S. Subedi, T. Kim, S. Saidin, B. Dahal and H. Yong Kim, Chem. Eng. J., 2023, 469, 143388 CrossRef.
- S. Shang, C. Du, Y. Liu, M. Liu, X. Wang, W. Gao, Y. Zou, J. Dong, Y. Liu and J. Chen, Nat. Commun., 2022, 13, 7599 CrossRef PubMed.
- D. Yang, Z. Liang, P. Tang, C. Zhang, M. Tang, Q. Li, J. J. Biendicho, J. Li, M. Heggen, R. E. Dunin-Borkowski, M. Xu, J. Llorca, J. Arbiol, J. R. Morante, S.-L. Chou and A. Cabot, Adv. Mater., 2022, 34, 2108835 CrossRef.
- S. Kumar, M. A. Rehman, S. Lee, M. Kim, H. Hong, J.-Y. Park and Y. Seo, Sci. Rep., 2021, 11, 649 CrossRef.
- D. Yang, Z. Liang, P. Tang, C. Zhang, M. Tang, Q. Li, J. J. Biendicho, J. Li, M. Heggen, R. E. Dunin-Borkowski, M. Xu, J. Llorca, J. Arbiol, J. R. Morante, S.-L. Chou and A. Cabot, Adv. Mater., 2022, 34, 2108835 CrossRef.
- Y. Huang, M. Gao, Y. Fu, J. Li, F. Wang, S. Yang, M. Wang, Z. Qian, X. Lu, P. Zhang and R. Wang, Energy Storage Mater., 2024, 70, 103522 CrossRef.
- Y. Li, G. Zhu, X. Xu, L. Chen, T. Lu, J. P. Hill, L. Pan and Y. Yamauchi, Small Struct., 2022, 3, 2200015 CrossRef.
- S. Zhao, H. Wu, Y. Li, Q. Li, J. Zhou, X. Yu, H. Chen, K. Tao and L. Han, Inorg. Chem. Front., 2019, 6, 1824–1830 RSC.
- D. Acharya, I. Pathak, A. Muthurasu, R. M. Bhattarai, T. Kim, T. H. Ko, S. Saidin, K. Chhetri and H. Y. Kim, J. Energy Storage, 2023, 63, 106992 CrossRef.
- T. Kim, S. Subedi, B. Dahal, K. Chhetri, T. Mukhiya, A. Muthurasu, J. Gautam, P. C. Lohani, D. Acharya, I. Pathak, S.-H. Chae, T. H. Ko and H. Y. Kim, Advanced Science, 2022, 9, 2200650 CrossRef.
- D. Acharya, A. Muthurasu, T. H. Ko, R. M. Bhattarai, T. Kim, S.-H. Chae, S. Saidin, K. Chhetri and H. Y. Kim, ACS Appl. Energy Mater., 2023, 6, 9196–9206 CrossRef.
- D. Acharya, I. Pathak, B. Dahal, P. C. Lohani, R. M. Bhattarai, A. Muthurasu, T. Kim, T. H. Ko, K. Chhetri and H. Y. Kim, Carbon, 2023, 201, 12–23 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06232a |
| This journal is © The Royal Society of Chemistry 2024 |