Optical study of Te8 ring clusters: comparison with density functional theory and a step towards materials design using nanoporous zeolite space
Vladimir
Poborchii
 *a and
Dmitrij
Rappoport
*a and
Dmitrij
Rappoport
 b
b
aNational Institute of Advanced Industrial Science and Technology, Tsukuba 305-8565, Japan. E-mail: Vladimir.poborchii@gmail.com
bDepartment of Chemistry, 1102 Natural Sciences 2, University of California, Irvine, CA 92697-2025, USA
First published on 13th April 2024
Abstract
The Te8 ring molecule (cluster) is poorly investigated due to the lack of experimental data. Here, we report an experimental and theoretical study of a regular array of oriented Te8 rings formed in the ∼1.14 nm diameter cavities of zeolite LTA, which are arranged in a cubic lattice with a spacing of ∼1.2 nm. Single crystals of LTA with encapsulated tellurium (LTA-Te) were studied using Raman spectroscopy (RS) and optical absorption spectroscopy (OAS). The experimental LTA-Te spectra were found to be in agreement with those calculated using density functional theory (PBE0 hybrid functional and def2-TZVP basis sets) for the crown-shaped Te8 ring molecule with D4d symmetry. Using polarization–orientation RS, we show that the Te8 rings are oriented by their major axes along the 4-fold axes of cubic LTA. We also show that the site symmetry of Te8 in LTA-Te is lower than D4d. Te8 bond-bending modes are well described in the harmonic approximation, while bond-stretching modes are mixed due to the reduced ring symmetry and, probably, anharmonicity. Importantly, OAS data of LTA-Te display dependence on the Te8 concentration, implying the interaction of the rings from neighbouring LTA cavities with the generation of the valence and conduction electron bands of such a cluster crystal.
Introduction
Tellurium (Te) belongs to the same group of the periodic table as oxygen (O), sulphur (S) and selenium (Se). Since its discovery at the end of the 18th century, Te was widely used in metal alloys. Nowadays, Te and its compounds have become a real frontier of science and technology. They have found important applications in electronics, optoelectronics, photonics, phase-change materials, topological insulators, Li–Te batteries, thermoelectrics and solar cells.1–6 However, elemental Te is mainly known in its chain form analogous to crystalline trigonal tellurium (t-Te) while it is nearly unknown in the ring form.Zeolites provide a unique opportunity to form and accommodate uniform guest species in their cavities/channels where the species are oriented due to the crystalline nature of zeolites.7–13 For example, only zeolites allowed obtaining polarized Raman spectra (RS) and optical absorption spectra (OAS) of isolated Te chains and rings.7–11 Moreover, fabrication of such regular high-density Te species arrays can be considered as an important direction in the development of new functional materials, so-called cluster crystals.14–16
Here, we study Te8 rings regularly arranged in the large cavities of zeolite LTA. Importantly, we are using the polarization–orientation RS examination of zeolite single crystals. On one hand, this method can be considered as an alternative to X-ray diffraction (XRD) for the structural study of zeolite-confined clusters. On the other hand, it provides very useful information about the cluster properties and their interaction with zeolites. We also study the OAS spectra of Te8 and demonstrate the effect of the interaction between Te8 rings in neighboring cavities, which is important for the fabrication of real cluster crystals.
The idea of Te8 ring formation in LTA cavities was proposed in ref. 17 where the only observed RS feature at ∼168 cm−1 was assigned to the ring with an argument that its frequency is different from the dominant trigonal tellurium (t-Te) band frequency at ∼120.5 cm−1.18,19 However, this observation was not confirmed later. In ref. 20, the RS of LTA-Te microcrystalline powder was re-examined. A strong band at ∼182 cm−1 and a weaker one at ∼45 cm−1 were observed and attributed rather to Te12 than to Te8 since Te8 requires a relatively strong symmetric bond-bending mode band at around 60 cm−1, which was not clearly detected due to laser-induced band broadening, as was found later. The LTA-Te band at ∼62 cm−1 accompanied by ∼182 cm−1 and ∼45 cm−1 bands was observed in ref. 21. This was a rather strong argument in favor of Te8 ring formation in LTA cavities. The observed bands at ∼45, ∼62 and ∼182 cm−1 look similar to the bending E2 and A1 mode bands and stretching A1 mode bands of S8 (∼153, ∼222, ∼480 cm−1) and Se8 (∼76, 111, 267 cm−1) rings.21 The RS of another zeolite AFI with encapsulated tellurium (AFI-Te) showed similar bands at 41–47 cm−1, 65 cm−1 and 184 cm−1.7 The bands were attributed to the Te8 ring. However, no polarized RS study of LTA-Te single crystals has been performed yet, which could provide crucial evidence for Te8 ring formation. Here, we present this evidence. Importantly, no other species but Te8 was found in LTA-Te. This is beneficial for realizing the idea of cluster crystal formation.
Experimental and theoretical methods
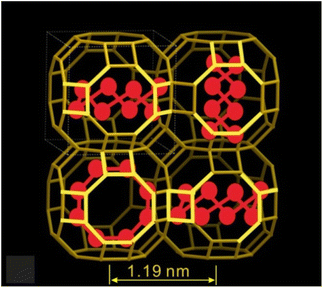
Synthetic zeolite LTA with the composition Na12Al12Si12O48 was used in this work. The crystal growth procedure for LTA is described in ref. 22. The sizes of the cubic LTA crystals were ∼20 μm along the edge of the cube. LTA has nearly spherical-shaped large cavities with a diameter of ∼1.14 nm, which are connected through narrow windows of ∼0.42 nm diameter (Fig. 1). The LTA cavities are arranged in a simple cubic lattice. The zeolite structures are accessible at the International Zeolite Association website https://www.iza-structure.org/IZA-SC/ftc_table.php.Dehydration of zeolites in a vacuum was performed in Pyrex ampoules for several hours at t ∼ 550 °C. The design of the ampoules made it possible to add crystalline Te powder to the zeolite after dehydration without exposing the dehydrated samples to air. The subsequent adsorption of the Te vapour was carried out at t ∼ 550 °C for a few days until the brownish colour of the sample was saturated.
RS spectra of the LTA-Te single crystals were studied using a Renishaw micro-Raman spectrometer equipped with Semrock edge-filters and an Olympus micro-objective lens allowing an ∼1 μm focused laser probe size. A 633 nm wavelength line of the He–Ne laser and a 785 nm line of the light-emitting diode laser were used for RS excitation. Weak absorption of Te8 at these wavelengths is beneficial for Te8 compared to the non-absorbing LTA matrix for obtaining the cluster Raman signal with nearly no contribution of LTA. An additional measurement with 561 nm laser diode line excitation was performed using a Nanofinder-30 Raman/AFM system (Tokyo Instruments Inc.) specially designed for the maximal RS detection sensitivity around this wavelength, which allowed LTA-Te Raman measurement at relatively high Te8 absorption and very low laser excitation power in order not to strongly heat or destroy the clusters. Moreover, a Nanofinder-30 (Tokyo Instruments Inc.) was equipped with a set of three volume Bragg grating notch filters (OptiGrate Co), allowing the detection of the Raman signal with frequencies down to ∼6 cm−1. UV-visible OAS was performed using a Carl Zeiss micro-optical spectrometer with a light probe size of ∼5 μm. The samples were intentionally broken to minimize their optical density in the ultraviolet (UV) spectral range and placed in glycerol between two cover glasses to minimize surface light scattering.
The Te8 RS, infrared (IR) spectra and OAS were simulated using density functional theory (DFT).23–26 The structure and vibrational frequencies of Te8 were computed using the hybrid PBE0 exchange–correlation functional26 and the resolution-of-identity approximation for the Coulomb part (RI-J).27 The def2-TZVP basis sets28 together with the corresponding auxiliary basis sets29 and small-core effective core potentials (ECPs)30 were employed for Te. RS intensities were obtained in the double harmonic approximation using analytical derivatives of static electronic polarizabilities31 and analytical energy Hessians.32,33 Calculations of static polarizability derivatives used the PBE0 functional and augmented def2-TZVPD basis sets.34 IR spectra were computed in the double harmonic approximation from the analytical dipole and energy Hessians.32,33 The vibrational frequencies were not scaled in this work. OAS was performed with time-dependent DFT (TDDFT)35 using the PBE0 functional with the RI-J approximation36 and def2-TZVPD basis sets. All calculations were performed with the Turbomole program package, version 7.5.23,37
Structure and Raman and absorption spectra of single Te8 rings via DFT calculations
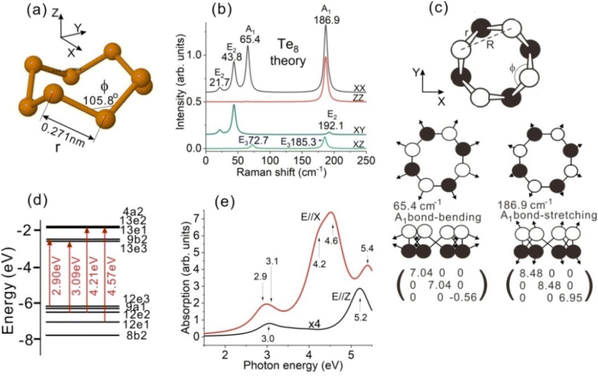
The calculated Te8 (D4d point group symmetry) ring structure is shown in Fig. 2(a). The bond length of Te8 is r = 0.271 nm and the second neighbour distance is R = 0.432 nm, while its bond and dihedral angles are 105.8° and 101.2°, respectively. The obtained structure parameters are in agreement with previous DFT calculations.38–42 The ring Z axis is directed along its 4-fold axis, while the X and Y axes lie in the molecule plane normal to the Z axis (Fig. 2(a)).Theoretical point group analysis for the vibration modes of Te8 is similar to that made for the S8 molecule with a similar crown shape.43 The Te8 vibration irreducible representations (symmetries) with calculated frequencies and IR/Raman activities are shown in Table 1. The E1 symmetry corresponds to the IR activity for the light polarized in the ring plane (E//X or E//Y), while the B2 symmetry corresponds to the E//Z IR activity. Calculated Te8 Raman intensities for different polarization configurations are equal to their squared Raman tensor components (XX, ZZ, XY, XZ) for each mode, which are determined by the corresponding atomic displacements and related molecular polarizability derivatives. The corresponding values in Table 1 are given in atomic units of 10−2e2a0/Eh, where e, a0 and Eh are the electron charge, Bohr radius and Hartree energy, respectively.
| Symmetry | Frequency (cm−1) | IR intensity (km mol−1) | Raman activity XX | Raman activity ZZ | Raman activity XY | Raman activity XZ |
|---|---|---|---|---|---|---|
| E2 | 21.7 | 0 | −0.49 | 0 | −2.08 | 0 |
| 21.7 | 0 | −2.08 | 0 | 0.49 | 0 | |
| E2 | 43.8 | 0 | −4.38 | 0 | −3.36 | 0 |
| 43.8 | 0 | 3.36 | 0 | −4.38 | 0 | |
| E1 | 56.9 | 0.524 | 0 | 0 | 0 | 0 |
| 56.9 | 0.524 | 0 | 0 | 0 | 0 | |
| A1 | 65.4 | 0 | −7.04 | 0.56 | 0 | 0 |
| B2 | 68.6 | 0.128 | 0 | 0 | 0 | 0 |
| E3 | 72.7 | 0 | 0 | 0 | 0 | −1.86 |
| 72.7 | 0 | 0 | 0 | 0 | −0.86 | |
| B1 | 179.9 | 0 | 0 | 0 | 0 | 0 |
| E3 | 185.3 | 0 | 0 | 0 | 0 | −3.54 |
| 185.3 | 0 | 0 | 0 | 0 | −0.17 | |
| A1 | 186.9 | 0 | −8.48 | −6.95 | 0 | 0 |
| E1 | 188.1 | 1.29 | 0 | 0 | 0 | 0 |
| 188.1 | 1.29 | 0 | 0 | 0 | 0 | |
| E2 | 192.1 | 0 | 1.21 | 0 | −1.05 | 0 |
| 192.1 | 0 | −1.05 | 0 | −1.21 | 0 |
The calculated RS spectra of Te8 for different polarization configurations, corresponding to the Raman tensor components from Table 1, are shown in Fig. 2(b). Raman bands are represented as Lorentz curves with the amplitudes corresponding to the calculated Raman activities and half-widths of 5 cm−1 for all bands.
The dominant band in the calculated RS spectra of Te8, displaying comparable XX and ZZ activities, corresponds to the A1 symmetric bond-stretching mode at a frequency of 186.9 cm−1. In contrast, the A1 bond-bending mode at 65.4 cm−1 displays high XX activity and nearly negligible ZZ activity. Fig. 2(c) shows atomic displacements and Raman tensors of these two A1 modes, which explain the difference in their ZZ activities. One can consider the A1 bond-stretching as a breathing mode since both in-plane and off-plane Te8 dimensions expand and contract the in-phase, while the A1 bond bending mode is an important instrument for Te8 ring orientation determination.
The E2 bond-bending mode at 43.8 cm−1 is rather active in the XX and XY configurations, while inactive in the ZZ configuration. Similar but weaker activity is displayed by the E2 torsion and stretching modes at 21.7 cm−1 and 192.1 cm−1, respectively. The E3 bond-bending mode at 72.7 cm−1 and the bond-stretching mode at 185.3 cm−1 dominate in the XZ configuration spectrum.
In addition to the Te8 Raman-active modes, there are several inactive modes: E1 bond-bending at 56.9 cm−1, B2 bond-bending at 68.6 cm−1, B1 bond stretching at 179.9 cm−1 and E1 bond stretching at 188.1 cm−1, which can become active when the ring D4d symmetry is reduced. As we mentioned above, the E1 and B2 modes are IR-active.
The calculated partial molecular orbital (MO) diagram of Te8 including the highest occupied MOs and the lowest unoccupied MOs is presented in Fig. 2(d). These MOs consist of combinations of Te 5p orbitals, as determined by Mulliken population analysis. The occupied orbitals correspond to Te lone pairs, while the unoccupied orbitals have antibonding character with respect to Te–Te bonds. The largest MO contributions to the allowed electronic transitions with photon energies <4 eV and the largest oscillator strengths are shown in red. It should be noted that the electron transition energies take into account excitonic effects. Therefore, the transition energies are less than the orbital energy differences of the dominant MO contributions. All these four transitions have E1 symmetry, implying the polarization of light E//X.
There are also E//Z-allowed transitions of B2 symmetry. However, their oscillator strengths appeared to be much lower than those of the strongest E//X transitions (Table 2). The calculated OAS spectra of Te8 for polarizations of light E//X and E//Z are shown in Fig. 2(e). Allowed electronic transitions (E1 for E//X and B2 for E//Z) with photon energies of up to 5.5 eV were computed. Due to the difference in the oscillator strengths, the E//X absorption is stronger than the E//Z one. The E//X absorption is mainly represented by transitions at photon energies of ∼2.9, ∼3.1, ∼4.2, ∼4.6 and ∼5.4 eV, while the E//Z one is determined by transitions at photon energies of ∼3, ∼3.6, ∼4 and ∼5.2 eV. The corresponding absorption bands are approximated by the Lorentz curves with half-widths of ∼0.6 eV.
| Symmetry | Photon energy (eV) | Oscillator strength |
|---|---|---|
| E1 | 2.90 | 0.104 |
| B2 | 3.03 | 0.016 |
| E1 | 3.09 | 0.053 |
| B2 | 3.45 | 0.003 |
| E1 | 3.53 | 0.001 |
| B2 | 3.61 | 0.003 |
| E1 | 3.89 | 0.012 |
| B2 | 3.96 | 0.004 |
| E1 | 4.11 | 0.006 |
| E1 | 4.21 | 0.357 |
| E1 | 4.35 | 0.002 |
| E1 | 4.57 | 0.525 |
| B2 | 5.21 | 0.071 |
| E1 | 5.42 | 0.333 |
| B2 | 5.77 | 0.008 |
| E1 | 5.82 | 0.229 |
| B2 | 5.94 | 0.002 |
Experimental polarized Raman spectra of LTA-Te single crystals
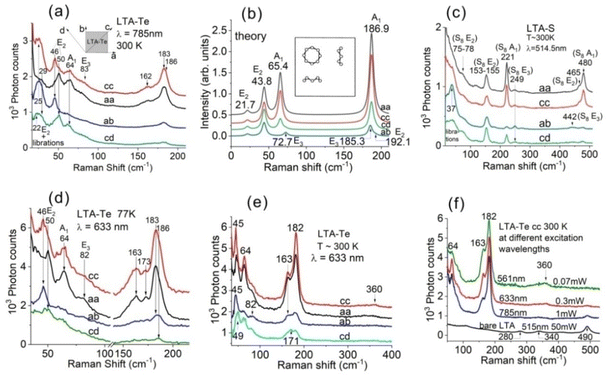
Using Te vapor adsorption, we obtained LTA-Te samples with Te loading densities of ∼3 and ∼8 atoms per cavity (at. per cav.). Unlike the RS of LTA-Se with different loading densities showing changes in the Se8/Se12 intensity ratio,44 LTA-Te RS spectra did not show any qualitative dependence on the Te loading density except an increase in the Raman signal of the same bands with an increase in the Te density. This is a strong argument in favour of the formation of only one stable Te cluster in the LTA cavities.RS spectra of LTA-Te with a loading density of ∼8 at. per cav. at an excitation wavelength of 785 nm are shown in Fig. 3(a). We performed measurements in four different polarization configurations: aa, cc, ab and cd (see the inset in Fig. 3(a)) similar to the polarization–orientation Raman study of LTA with sulphur LTA-S12 and LTA with selenium LTA-Se.12,44,45 Experimentally, we rotated the LTA-Te crystals in the ab plane with incident and scattered light polarizations (1) parallel and (2) perpendicular to each other. The procedure was described in detail earlier.12 Theoretical RS spectra of LTA-Te for the aa, cc, ab and cd polarization configurations were obtained via summation of the Raman responses of three Te8 rings in their three possible orientations in LTA crystals [Fig. 3(b)].
The experimental LTA-Te RS spectra display rather good correspondence with the calculated ones. Indeed, the bands at 162 cm−1 and 183 cm−1 can be assigned to the Te8 E3 and A1 bond-stretching modes similar to the 442 cm−1 and 480 cm−1 bands of S8 in LTA-S [Fig. 3(c)]. The correspondence between the experiment and theory looks especially good for the A1 mode frequency (experimental: ∼183 cm−1vs. theoretical: 186.9 cm−1) and intensity (the strongest band in the spectrum). At first glance, the polarization dependence of its intensity also looks reasonable: the band is strong in aa and cc polarization configurations, while it is weak in ab and cd configurations as expected for the A1 modes. Actually, as we have shown below, the situation is more complicated for the bond stretching modes due to a possible mode mixing effect but, in a rough approximation, the assignment of the 183 cm−1 band to the A1 bond-stretching mode looks reasonable.
Polarization dependencies of the LTA-Te bands in the Te8 bond-bending mode region appear to be qualitatively similar to those of the bond-bending modes of S8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 [Fig. 3(c)] and Se8
12 [Fig. 3(c)] and Se8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12,44,45 rings in LTA. Indeed, the 46–50 cm−1 band polarization–orientation dependence is similar to that of the E2 band at 74–76 cm−1 or 77–79 cm−1 of Se8
12,44,45 rings in LTA. Indeed, the 46–50 cm−1 band polarization–orientation dependence is similar to that of the E2 band at 74–76 cm−1 or 77–79 cm−1 of Se8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 and the E2 band at 153–155 cm−1 of S8 [Fig. 3(c)]. The calculated Te8 E2 bond-bending mode frequency of 43.8 cm−1 is in good agreement with the observed 46–50 cm−1 band frequencies of LTA-Te. The splitting of the degenerate E2 mode indicates structural distortion of Te8 in LTA and therefore a reduced symmetry compared to the initial D4d symmetry of the ring. For example, a slight reduction of the Te8 symmetry from D4d to C4v would split the E2 mode into B1 and B2 modes, active in XX and XY polarization configurations, respectively. This is exactly what we experimentally observed. Interestingly, the splitting of the Te8 E2 mode in LTA is very strong, ∼8.3%, in contrast to the weaker splitting of the corresponding E2 modes of S8, ∼ 1.3%, and Se8, ∼ 2.6%.
45 and the E2 band at 153–155 cm−1 of S8 [Fig. 3(c)]. The calculated Te8 E2 bond-bending mode frequency of 43.8 cm−1 is in good agreement with the observed 46–50 cm−1 band frequencies of LTA-Te. The splitting of the degenerate E2 mode indicates structural distortion of Te8 in LTA and therefore a reduced symmetry compared to the initial D4d symmetry of the ring. For example, a slight reduction of the Te8 symmetry from D4d to C4v would split the E2 mode into B1 and B2 modes, active in XX and XY polarization configurations, respectively. This is exactly what we experimentally observed. Interestingly, the splitting of the Te8 E2 mode in LTA is very strong, ∼8.3%, in contrast to the weaker splitting of the corresponding E2 modes of S8, ∼ 1.3%, and Se8, ∼ 2.6%.
The LTA-Te 64 cm−1 band displays polarization dependence similar to the A1 symmetric bond-bending mode band of S8 at ∼221 cm−1 [Fig. 3(c)] and the analogous band of Se8 at ∼112 cm−1.12,44,45 The frequency of 64 cm−1 is very close to the calculated frequency of 65.4 cm−1 of the Te8 A1 bond-bending mode [Fig. 2(b); 3(b)]. The experimental 64 cm−1 band is rather strong in the aa and cc polarization configurations and it is still not weak in the cd configuration, while the band completely disappears in the ab configuration. This is a consequence of the 2-dimensional character of the Raman tensor of this mode with only two strong diagonal tensor components in contrast to the rather 3-dimensional tensor of the A1 bond-stretching mode [Fig. 2(c)]. Correspondingly, the single free-standing Te8 A1 bond-bending mode band is strong in the XX polarization configuration and negligibly weak in the ZZ configuration with the zero XY and XZ activities [Fig. 2(b)]. Theoretical calculation of this mode activity in the RS spectra of LTA-Te [Fig. 3(b)] is also in very good agreement with the experiment, namely high aa activity and no ab activity. This is a clear indication of the orientation of the Te8 4-fold axis along the 4-fold axis of LTA.
The experimental LTA-Te ∼ 83 cm−1 band looks similar to the 248 cm−1 E3 bond-bending mode band of S8 [Fig. 3(c)]. It can be attributed to the E3 bond-bending mode of Te8 with a calculated frequency of 72.7 cm−1. The ∼29 cm−1 band of LTA-Te can be attributed to the E2 torsional mode of Te8 with a calculated frequency of 21.7 cm−1. A broad ∼25 cm−1 band, probably, originates from the superposition of the second component of the E2 torsional mode and the ring librations in the LTA cavity.
As we mentioned above, the bands of LTA-Te in the bond-stretching mode region require more detailed consideration. Fig. 3(d) shows LTA-Te RS in this region taken at temperature T ∼ 77 K and an excitation wavelength of 633 nm with better resolved bands due to their temperature-induced narrowing. Fig. 3(e) shows room temperature LTA-Te RS at the same excitation wavelength in a wider spectral range. Four bands at ∼163 cm−1, ∼173 cm−1, and ∼183 cm−1 and its shoulder at ∼186 cm−1 can be observed in Fig. 3(d). The shoulder is, probably, associated with the Te8 E2 bond-stretching mode (theoretical frequency of ∼192 cm−1). Taking into account our preliminary assignment of the 182–183 cm−1 and 162–163 cm−1 bands to the Te8 A1 and E3 modes, respectively, we have to attribute the 171–173 cm−1 band to the forbidden B1 (theoretical frequency of ∼180 cm−1) mode which can be activated due to the reduced symmetry of Te8 in the LTA cavity, and we found that the bands in the bond-stretching mode region can be mixed.
The band at 182–183 cm−1 shows slightly stronger activity in the cc polarization configuration than that in the aa configuration, which is clearly seen in the room temperature RS of LTA-Te obtained by excitation with 633 nm light, as shown in Fig. 3(e). This can be associated with the mode mixing due to the reduced symmetry of the Te8 rings. Interestingly, the 2nd order Raman band of Te8 can be recognized in the cc configuration spectrum at ∼360 cm−1 [Fig. 3(e)]. An even stronger 2nd order Raman band can be clearly seen at an excitation wavelength of 561 nm [Fig. 3(f), green curve] due to the resonant Raman enhancement. The band is very broad covering the spectral range from ∼300 cm−1 to ∼375 cm−1. Importantly, LTA bands do not contribute to the 2nd order Raman band of Te8 since even the strongest LTA band at ∼490 cm−1 [Fig. 3(f), black curve] does not contribute to LTA-Te RS obtained by excitation with 561 nm light.
Summarizing this section, the polarization dependence of the A1 bond-bending mode Raman band of Te8 indicates the orientation of the ring by its 4-fold axis along the LTA 4-fold axis, while that of the E2 bond-bending mode suggests a noticeable symmetry reduction of the ring from the ideal D4d point group symmetry. Te8 bond-stretching modes are mixed due to the ring symmetry reduction. The Raman bands at 162–163 cm−1 and 171–173 cm−1, probably, originate from the E3 mode and formerly inactive B1 mode while the Raman bands at 182–183 cm−1 and 186 cm−1 originate from the A1 and E2 modes of Te8. The originally inactive E1 bond-stretching mode may also contribute to the observed Raman bands.
Optical absorption spectra of LTA-Te single crystals
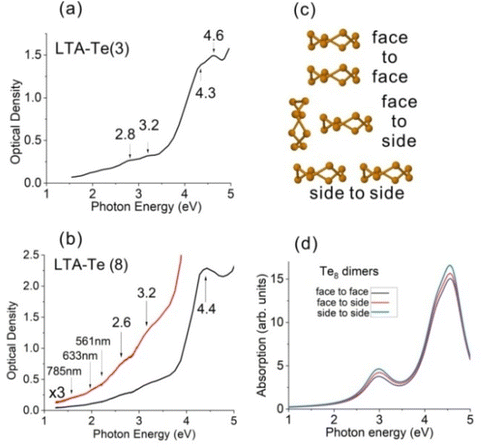
Fig. 4(a and b) show the OAS of LTA-Te with the loading densities of ∼3 atoms per cavity [LTA-Te(3)] and ∼8 atoms per cavity [LTA-Te(8)]. Absorption bands at ∼2.8 eV, 3.2 eV, 4.3 eV and 4.6 eV are observed in the OAS of LTA-Te(3), while absorption bands at ∼2.6 eV, 3.2 eV and 4.4 eV are observed in the OAS of LTA-Te(8). The spectra indicate that the energy band gap decreases when the Te loading density increases. Moreover, LTA-Te(8) bands look slightly broader than those of LTA-Te(3). In particular, the absorption band doublet at ∼4.3 eV and 4.6 eV, resolved in LTA-Te(3), looks like a single band at ∼4.4 eV.First, the observed experimental OAS spectra of LTA-Te [Fig. 4(a and b)] appeared to be in nearly perfect agreement with the theoretical OAS spectra of Te8 [Fig. 2(e)]. The agreement with theoretical OAS for a single Te8 ring is especially good for LTA-Te(3) with approximately two rings per five cavities. We have to note that surface light scattering, which increases with the photon energy, contributes to the experimental spectra. In contrast, theoretical spectra do not include this effect and do not include high-energy transitions (>5.5 eV in Fig. 2(e)). This may produce an impression that the experimental and theoretical spectra are different. However, actually, they are very similar.
Secondly, differences between the OAS of LTA-Te(3) and LTA-Te(8) indicate a noticeable interaction between the Te8 rings in neighbouring LTA cavities, in particular, for LTA-Te(8) with one ring per cavity. In Fig. 4(b), we show the spectral positions of the Raman excitation laser lines, clearly indicating rather strong absorption of LTA-Te(8) at a wavelength of 561 nm corresponding to the resonant Raman effect [Fig. 3(f)]. To avoid laser-induced heating/destruction of Te8, we used a very low 561 nm laser power of ∼0.07 mW. At weaker LTA-Te absorption corresponding to wavelengths of 785 nm and 633 nm [Fig. 4(b)], we were able to use higher laser powers of ∼1 mW and ∼0.3 mW, respectively [Fig. 3(f)].
In order to examine a possible effect of the ring interaction on the OAS spectrum of LTA-Te, we theoretically considered three possible Te8 ring dimers with a separation of ∼1.2 nm between the ring centres [Fig. 4(c)]. The corresponding calculated dimer OAS spectra are shown in Fig. 4(d). The effect of the ring interaction is indicated by the absorption enhancement with an increase in the ring interaction, which is the weakest in the “face-to-face” configuration and the strongest in the “side-to-side” configuration. Due to the inter-ring interaction, the unoccupied MOs of the Te8 rings organize conduction bands of the LTA-Te cluster crystals, while the highest occupied MOs organize its valence band. The conduction and valence bands should be rather narrow. Therefore, a variety of non-linear electric conductance effects predicted for narrow semiconductor superlattice mini-bands46 can be expected in the LTA-Te electric properties. A negative differential electric resistance of LTA-Te observed in ref. 47 confirms this.
Te8 interaction with LTA and its librations vs. temperature
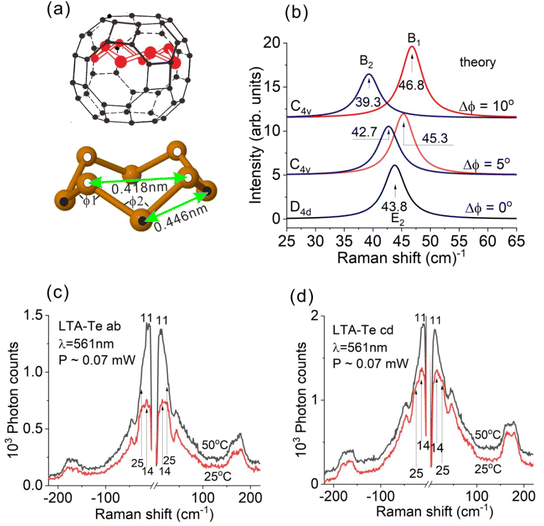
According to the calculated Te8 structure [Fig. 2(a)], the diameter of the ring, including the van der Waals radius of ∼0.2 nm of the Te atom, is ∼1 nm, which is slightly smaller than the LTA large cavity diameter. Therefore, one can expect that the Te8 ring is slightly shifted from the cavity center along the 4-fold LTA axis similar to the S8 and Se8 rings12,44,45 but not as much as in the cases of S8 and Se8. This should cause Te8 symmetry reduction from D4d at least to C4v [Fig. 5(a)]. We imposed the bond angle changes in order to simulate such structural distortion. The angles of type ϕ1 were enlarged while those of type ϕ2 were reduced. The second nearest neighbor distances R = 0.418 nm and 0.446 nm in Fig. 5(a) correspond to Δϕ = ϕ1 − ϕ2 = 5° (R = 0.432 nm for undisturbed Te8).The experimentally observed splitting of the E2 bond-bending mode [Fig. 3(a, d and e)] is, definitely, a sign of D4d symmetry reduction. It is important to verify whether the observed E2 mode splitting fits the ring structure distortion shown in Fig. 5(a) or not. Due to the Te8 symmetry reduction from D4d to C4v, the E2 bond-bending mode splits into B1 and B2 modes, displaying XX and XY Raman activities, respectively. We performed calculations of the B1 and B2 mode frequencies and intensities as a function of the distortion Δϕ. The results are shown in Fig. 5(b). The B1 mode frequency increases while the B2 mode frequency decreases with Δϕ. This corresponds well to our experimental observation for the E2 bond-bending mode with the LTA-Te aa-active component frequency higher than that of the ab-active component. Thus, we conclude that the Te8 structural distortion shown in Fig. 5(a) reasonably describes the experimentally observed splitting of the E2 bond-bending mode of Te8. However, even stronger symmetry reduction of Te8 is possible.
As we mentioned in the Introduction, Raman polarization–orientation spectroscopy in application to the zeolite-confined cluster structure study can be considered in some sense as an alternative to XRD. Indeed, using Raman polarization–orientation spectroscopy, we can determine a type of cluster (molecule) and its orientation in a zeolite. Then we can suggest its possible location in the zeolite when we know its size. However, we can only guess what sort of interaction occurs between the Te atoms and the zeolite. For understanding this point, the XRD data would be very useful. However, a structural analysis of LTA-Te by XRD is rather difficult because Te is distributed statistically inside the cavities. The resulting scattering power of Te sites with partial occupancy is comparable to those of Na+ cations and water molecules filling spaces not occupied by Te.
The ∼20 micron size of LTA-Te is insufficient for single-crystal XRD, while powder XRD of zeolites with clusters is, definitely, not effective for the examination of the cluster structure. It shows that the zeolite filling with clusters or molecules causes just changes in the relative intensities of the XRD peaks like, for example, in ref. 48 and its ESI, where a number of different types of zeolites, including LTA, with/without sulphur clusters were examined using powder XRD. In contrast, XRD analysis of ∼65 micron size LTA single crystals containing sulphur clearly showed double S8 ring cluster formation in each large cavity,49 which is in agreement with the published RS results12,50,51 and this work [Fig. 3(c)].
An XRD study of ∼60 micron size LTA-Te single crystals was performed in ref. 52. The XRD analysis suggested the interaction of Te atoms with the zeolite oxygen atoms and cations, while it showed low occupancy and high temperature factors for Te atomic sites. Therefore, the authors could not make any univocal conclusion about the Te cluster structure and they proposed several options including Te8 rings.
Using the RS of LTA-Te, the interaction between the Te8 ring and the zeolite can be probed via observation of the Te ring librations vs. temperature. Taking into account the S8 libration frequency of ∼37 cm−1 [Fig. 3(c)] and assuming approximately the same van der Waals interaction force with LTA for both S8 and Te8, we can roughly estimate the expected Te8 room temperature libration frequency as ∼18.5 cm−1 using the square root of the Te and S mass ratio, which is ∼2.
In the LTA-Te RS spectra obtained by excitation with 785 nm light, Te8 librations, probably, contribute to the broad ∼22–25 cm−1 band [Fig. 3(a)]. Instrumental limitations did not allow RS detection at frequencies <20 cm−1 at this excitation wavelength. In contrast, such measurements were available at 561 nm light excitation. A weak point of this wavelength is that the corresponding Te8 absorption and light-induced heating are not negligible.
Fig. 5(c and d) show the Stokes and anti-Stokes low-frequency RS of LTA-Te in ab and cd polarization configurations at ∼25 °C and ∼50 °C temperatures of the heat-controlling table. We roughly estimated the laser-induced heating from the Stokes/anti-Stokes intensity ratio as ∼20–30 °C, the value that should be added to the heating table temperature. In the RS taken at ∼25 °C table temperature, one can see a ∼25 cm−1 band in combination with a ∼14 cm−1 band for both configurations. The last band, definitely, originates from the Te8 librations. With the increase of the table temperature to ∼50 °C, it shifts to a lower frequency of ∼11 cm−1 and displays significant enhancement. This corresponds to the intensification of librations and the reduction of the ring interaction with LTA. The position of the ∼25 cm−1 feature remains unchanged, confirming its origin from the internal torsional E2 mode of Te8.
Thus, the Te8 libration amplitudes strongly increase with a moderate increase in the LTA temperature. This corresponds to a rather weak van der Waals interaction of Te8 with LTA. At room temperature, the libration amplitudes are, probably, quite strong as well. This explains high temperature factors observed for Te sites,52 which complicates the XRD analysis of LTA-Te. In contrast, this effect does not cause any trouble for polarization–orientation Raman spectroscopy, which appears to be a very fruitful method of zeolite-confined cluster study providing information on the cluster structure with distortions, orientation and interaction with zeolites.
Conclusions
In summary, the electronic and vibrational properties of Te8 rings were studied experimentally using Raman and absorption optical spectroscopy and theoretically using density functional theory. A good agreement is obtained between (1) the theoretical optical absorption and Raman spectra of isolated Te8 and (2) those of Te8 formed in the large cavities of the zeolite LTA. Owing to zeolite utilization, this is a big step in the experimental study of Te8, which is limited by the fabrication of exotic compounds like Cs3Te22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 or cluster registration in molecular beams.54 Polarization–orientation Raman spectra of LTA-Te show that the rings are oriented by their major axes along the 4-fold axes of LTA. The observed splitting of the Te8 bond-bending modes suggests the reduction of the ring D4d symmetry in the LTA cavity. The bond-stretching modes demonstrate mixing due to the reduced symmetry of the ring and, probably, the essential anharmonicity of the vibrations. Even moderate heating of LTA-Te causes significant intensification of Te8 librations in the LTA cavities, which corresponds to a rather weak van der Waals interaction between the ring and the zeolite. Experimental optical absorption spectra of LTA-Te display dependence on the Te loading density due to the electronic interaction of Te8 from neighbouring cavities, which increases with an increase in the loading density. The effect is confirmed using DFT calculations of the absorption spectra of Te8 dimers. The observed Te8 ring–ring interaction is beneficial for the formation of cluster crystals with narrow electron bands and predicted non-linear electric properties. On the other hand, our results can be interesting in the sense of relationships with other inorganic 8-membered ring clusters such as Pd8.55
53 or cluster registration in molecular beams.54 Polarization–orientation Raman spectra of LTA-Te show that the rings are oriented by their major axes along the 4-fold axes of LTA. The observed splitting of the Te8 bond-bending modes suggests the reduction of the ring D4d symmetry in the LTA cavity. The bond-stretching modes demonstrate mixing due to the reduced symmetry of the ring and, probably, the essential anharmonicity of the vibrations. Even moderate heating of LTA-Te causes significant intensification of Te8 librations in the LTA cavities, which corresponds to a rather weak van der Waals interaction between the ring and the zeolite. Experimental optical absorption spectra of LTA-Te display dependence on the Te loading density due to the electronic interaction of Te8 from neighbouring cavities, which increases with an increase in the loading density. The effect is confirmed using DFT calculations of the absorption spectra of Te8 dimers. The observed Te8 ring–ring interaction is beneficial for the formation of cluster crystals with narrow electron bands and predicted non-linear electric properties. On the other hand, our results can be interesting in the sense of relationships with other inorganic 8-membered ring clusters such as Pd8.55
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank V. P. Petranovskii for LTA crystal synthesis and A. V. Fokin for the Te vapour adsorption procedure.References
- T. Chivers and R. S. Laitinen, Tellurium: A Maverick Among the Chalcogens, Chem. Soc. Rev., 2015, 44, 1725–1739, 10.1039/C4CS00434E.
- Y. Liu, J. Wang, Y. Xu, Y. Zhu, D. Bigio and C. Wan, Lithium-Tellurium Batteries Based on Tellurium/Porous Carbon Composite, J. Mater. Chem. A, 2014, 2, 12201–12207, 10.1039/C4TA02075H.
- T. Cao, R. Wang, R. E. Simpson and G. Li, Photonic Ge-Sb-Te phase change metamaterials and their applications, Prog. Quantum Electron., 2020, 74, 100299, DOI:10.1016/j.pquantelec.2020.100299.
- M. Z. Hasan and C. L. Kane, Topological Insulators, Rev. Mod. Phys., 2010, 82, 3045–3067, DOI:10.1103/RevModPhys.82.3045.
- S. Lin, W. Li, Z. Chen, J. Shen, B. Ge and Y. Pei, Tellurium as a high-performance elemental thermoelectric, Nat. Commun., 2016, 7, 10287, DOI:10.1038/ncomms10287.
- W. K. Metzger, S. Grover, D. Lu, E. Colegrove, J. Moseley, C. L. Perkins, X. Li, R. Mallick, W. Zhang, R. Malik, J. Kephart, C.-S. Jiang, D. Kuciauskas, D. S. Albin, M. M. Al-Jassim, G. Xiong and M. Gloeckler, Exceeding 20% efficiency with in situ group V doping in polycrystalline CdTe solar cells, Nat. Energy, 2019, 4, 837–845, DOI:10.1038/s41560-019-0446-7.
- V. V. Poborchii, A. V. Fokin and A. A. Shklyaev, Optical properties of extreme tellurium nanowires formed in subnanometer-diameter channels, Nanoscale Adv., 2023, 5, 220–227, 10.1039/d2na00590e.
- V. N. Bogomolov, S. G. Romanov, S. V. Kholodkevich and L. S. Agroskin, The absorption spectra of single selenium and tellurium chains in dielectric matrix channels, Solid State Commun., 1983, 47, 181–182, DOI:10.1016/0038-1098(83)90704-4.
- V. V. Poborchii, Polarized Raman and optical absorption spectra of the mordenite single crystals containing sulphur, selenium or tellurium confined in the one-dimensional nanochannels, Chem. Phys. Lett., 1996, 251, 230–234, DOI:10.1016/0009-2614(96)00045-0.
- S.-I. Inoue, N. Koshizaki and T. Kodaira, Formation of Te nanowires in zeolite AFI and their polarized absorption spectra, Int. J. Mod. Phys. B, 2005, 19, 2817–2822, DOI:10.1142/s0217979205031754.
- T. Kodaira and T. Ikeda, The selective adsorption of tellurium in the aluminosilicate regions of AFI- and MOR-type microporous crystals, Dalton Trans., 2014, 43, 13979–13987, 10.1039/c4dt01028k.
- V. V. Poborchii, Raman microprobe polarization measurements as a tool for studying the structure and orientation of molecules and clusters incorporated into cubic zeolites: S8 and Se12 rings in zeolite A, J. Chem. Phys., 2001, 114, 2707–2717, DOI:10.1063/1.1339268.
- V. N. Bogomolov, V. V. Poborchii and S. V. Kholodkevich, Size effects in the vibrational spectrum of 10 Å selenium particles, JETP Lett., 1985, 42, 517–520 Search PubMed.
- V. N. Bogomolov, Liquids in ultrathin channels (Filament and cluster crystals), Sov. Phys. Usp., 1978, 21, 77–83, DOI:10.1070/PU1978v021n01ABEH005510.
- G. D. Stucky and J. E. MacDougall, Quantum Confinement and Host/Guest Chemistry: Probing a New Dimension, Science, 1990, 247, 669–678, DOI:10.1126/science.247.4943.669.
- G. A. Ozin, Nanochemistry: Synthesis in diminishing dimensions, Adv. Mater., 1992, 4, 612–649, DOI:10.1002/adma.19920041003.
- V. N. Bogomolov, A. I. Zadorozhnii, V. I. Petranovskii, A. V. Fokin and S. V. Kholodkevich, Identification of a new tellurium modification – a Te8 ring in small-diameter clusters, JETP Lett., 1979, 29, 373–375 Search PubMed.
- A. S. Pine and G. Dresselhaus, Raman Spectra and Lattice Dynamics of Tellurium, Phys. Rev. B: Solid State, 1971, 4, 356–371, DOI:10.1103/PhysRevB.4.356.
- V. V. Poborchii, V. A. Sachkov, A. A. Shklyaev, A. V. Fokin and P. I. Geshev, Photonic and phononic properties of oriented 5 nm diameter tellurium nanowires, J. Phys. Chem. Solids, 2024, 185, 111806, DOI:10.1016/j.jpcs.2023.111806.
- V. V. Poborchii, M. S. Ivanova, V. P. Petranovskii, Y. A. Barnakov, A. Kasuya and Y. Nishina, Raman and absorption spectra of the zeolites A and X containing selenium and tellurium in the nanopores, Mater. Sci. Eng., A, 1996, 217/218, 129–134, DOI:10.1016/S0921-5093(96)10365-8.
- V. V. Poborchii, Raman spectra of sulfur, selenium or tellurium clusters confined in nano-cavities of zeolite A, Solid State Commun., 1998, 107, 513–518, DOI:10.1016/S0038-1098(98)00205-1.
- V. P. Petranovskii, Y. Kiyozumi, N. Kikuchi, H. Hayamisu, Y. Sugi and F. Mizukami, The influence of mixed organic additives on the zeolite A and X crystal growth, Stud. Surf. Sci. Catal., 1997, 105, 149–156 CrossRef.
- S. G. Balasubramani, et al., TURBOMOLE: Modular program suite for ab initio, quantum-chemical and condensedmatter simulations, J. Chem. Phys., 2020, 152, 184107, DOI:10.1063/5.0004635.
- D. Rappoport and F. Furche, Lagrangian approach to molecular vibrational Raman intensities using time-dependent hybrid density functional theory, J. Chem. Phys., 2007, 126, 201104, DOI:10.1063/1.2744026.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868, DOI:10.1103/PhysRevLett.77.3865.
- J. P. Perdew, M. Ernzerhof and K. Burke, Rationale for mixing exact exchange with density functional approximations, J. Chem. Phys., 1996, 105, 9982–9985, DOI:10.1063/1.472933.
- K. Eichkorn, O. Treutler, H. Öhm, M. Häser and R. Ahlrichs, Auxiliary Basis Sets to Approximate Coulomb Potentials, Chem. Phys. Lett., 1995, 242, 652–660, DOI:10.1016/0009-2614(95)00838-u.
- F. Weigend and R. Ahlrichs, Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305, 10.1039/b508541a.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065, 10.1039/B515623H.
- K. A. Peterson, D. Figgen, E. Goll, H. Stoll and M. Dolg, Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post- d Group 16–18 Elements, J. Chem. Phys., 2003, 119, 11113–11123, DOI:10.1063/1.1622924.
- D. Rappoport and F. Furche, Lagrangian Approach to Molecular Vibrational Raman Intensities Using Time-Dependent Hybrid Density Functional Theory, J. Chem. Phys., 2007, 126, 201104, DOI:10.1063/1.2744026.
- P. Deglmann, F. Furche and R. Ahlrichs, An Efficient Implementation of Second Analytical Derivatives for Density Functional Methods, Chem. Phys. Lett., 2002, 362, 511–518, DOI:10.1016/s0009-2614(02)01084-9.
- P. Deglmann, K. May, F. Furche and R. Ahlrichs, Nuclear Second Analytical Derivative Calculations Using Auxiliary Basis Set Expansions, Chem. Phys. Lett., 2004, 384, 103–107, DOI:10.1016/j.cplett.2003.11.080.
- D. Rappoport and F. Furche, Property-Optimized Gaussian Basis Sets for Molecular Response Calculations, J. Chem. Phys., 2010, 133, 134105, DOI:10.1063/1.3484283.
- R. Bauernschmitt and R. Ahlrichs, Treatment of Electronic Excitations within the Adiabatic Approximation of Time Dependent Density Functional Theory, Chem. Phys. Lett., 1996, 256, 454–464, DOI:10.1016/0009-2614(96)00440-x.
- R. Bauernschmitt, M. Häser, O. Treutler and R. Ahlrichs, Calculation of Excitation Energies within Time-Dependent Density Functional Theory Using Auxiliary Basis Set Expansions, Chem. Phys. Lett., 1997, 264, 573–578, DOI:10.1016/s0009-2614(96)01343-7.
- Y. J. Franzke, C. Holzer, J. H. Andersen, T. Begušić, F. Bruder, S. Coriani, F. D. Sala, E. Fabiano, D. A. Fedotov, S. Fürst, S. Gillhuber, R. Grotjahn, M. Kaupp, M. Kehry, M. Krstić, F. Mack, S. Majumdar, B. D. Nguyen, S. M. Parker, F. Pauly, A. Pausch, E. Perlt, G. S. Phun, A. Rajabi, D. Rappoport, B. Samal, T. Schrader, M. Sharma, E. Tapavicza, R. S. Treß, V. Voora, A. Wodyński, J. M. Yu, B. Zerulla, F. Furche, C. Hättig, M. Sierka, D. P. Tew and F. Weigend, TURBOMOLE: Today and Tomorrow, J. Chem. Theory Comput., 2023, 19, 6859–6890, DOI:10.1021/acs.jctc.3c00347.
- B. C. Pan, Geometric structures, electronic properties, and vibrational frequencies of small tellurium clusters, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 085407, DOI:10.1103/PhysRevB.65.085407.
- J. Akola and R. O. Jones, Structure and dynamics in amorphous tellurium and Ten, clusters: A density functional study, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 134103, DOI:10.1103/PhysRevB.85.134103.
- H. H. Abdallah, Revisiting the Tellurium Clusters (Ten; n = 2-8) using Ab initio Methods, Can. J. Phys., 2020, 98, 57–64, DOI:10.1139/cjp-2019-0015.
- V. S. Ghemud, H. A. S. Gol and A. Kshirsagar, How different are Te clusters - a first-principles study, J. Nanopart. Res., 2018, 20, 177, DOI:10.1007/s11051-018-4274-7.
- T. Sharma, R. Sharma, R. A. Tamboli and D. G. Kanhere, Ab initio investigation of structural and electronic properties of selenium and tellurium clusters, Eur. Phys. J. B, 2019, 92, 51, DOI:10.1140/epjb/e2019-90491-5.
- D. W. Scott, J. P. McCollough and F. H. Kruse, Vibrational Assignment and Force Constants of S8 from a Normal-Coordinate Treatment, J. Mol. Spectrosc., 1964, 13, 313–320, DOI:10.1016/0022-2852(64)90079-7.
- V. V. Poborchii and A. V. Fokin, Raman and optical absorption spectra of oriented Se8 and Se12 rings formed in zeolites: Dependence on the Se loading density, Microporous Mesoporous Mater., 2022, 338, 111954, DOI:10.1016/j.micromeso.2022.111954.
- V. V. Poborchii, V. P. Petranovskii, I. A. Glukhov and A. A. Fotiadi, Optical study of oriented double-Se8-ring clusters and luminescent Se2− anions in LTA at extremely high selenium loading density, Microporous Mesoporous Mater., 2023, 348, 112395, DOI:10.1016/j.micromeso.2022.112395.
- V. V. Bryksin and P. Kleinert, Microscopic theory of high-field miniband transport in semiconductor superlattices, J. Phys.: Condens.Matter, 1997, 9, 7403–7418, DOI:10.1088/0953-8984/9/35/014.
- Z. K. Tang and X.-R. Wang, Nonresonant electron tunneling in cluster superlattice of tellurium in zeolite, Appl. Phys. Lett., 1996, 68, 3449–3451, DOI:10.1063/1.115789.
- E. Han, Y.-G. Kim, H.-M. Yang, I.-H. Yoon and M. Choi, Synergy between Zeolite Framework and Encapsulated Sulfur for Enhanced Ion-Exchange Selectivity to Radioactive Cesium, Chem. Mater., 2018, 30, 5777–5785, DOI:10.1021/acs.chemmater.8b02782.
- K. Seff, The Crystal Structure of a Sulfur Sorption Complex of Zeolite 4A, J. Phys. Chem., 1972, 76, 2601–2605, DOI:10.1021/j100662a023.
- V. V. Poborchii, V. Petranovskii, I. A. Glukhov and A. A. Fotiadi, Single crystal polarization-orientation Raman spectroscopy of zeolite LTA with confined S3− anions - High dielectric constant nanoporous material, Mater. Chem. Phys., 2024, 316, 129103, DOI:10.1016/j.matchemphys.2024.129103.
- V. N. Bogomolov, V. P. Petranovskii, V. V. Poborchii and S. V. Kholodkevich, Absorption, Raman and ESR spectra and the dielectric permittivity of NaA-S cluster crystals, Sov. Phys. Solid State, 1983, 25, 1415–1417 Search PubMed.
- W. T. Lim, J. S. Park, S. H. Lee, K. J. Jung and N. H. Heo, Synthesis of Tellurium Sorption Complexes in Fully Dehydrated and Fully Ca2+-exchanged Zeolites A and X and their Single-crystal Structures, Bull. Korean Chem. Soc., 2009, 30, 1274–1284, DOI:10.5012/bkcs.2009.30.6.1274.
- W. S. Sheldrick and M. Wachhold, Discrete crown-shaped Te8 rings in Cs3Te22, Angew. Chem., Int. Ed. Engl., 1995, 34, 450–451, DOI:10.1002/anie.199504501.
- J. Becker, K. Rademann and F. Hensel, Electronic structure of selenium- and tellurium-clusters, Z. Phys. D: At., Mol. Clusters, 1991, 19, 233–235, DOI:10.1007/BF01448300.
- P. An, R. Anumula, C. Cui, Y. Liu, F. Zhan, Y. Tao and Z. Luo, A facile method to synthesize water-soluble Pd8 nanoclusters unraveling the catalytic mechanism of p-nitrophenol to p-aminophenol, Nano Res., 2019, 12, 2589–2596, DOI:10.1007/s12274-019-2491-8.
| This journal is © The Royal Society of Chemistry 2024 |