Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineered mesoporous silica-based nanoparticles as smart chemotherapy nanodevice for bortezomib administration†
M.
De Santo
a,
A.
Giovinazzo
b,
M.
Fava
a,
E.
Mazzotta
c,
I. E.
De Napoli
b,
M.
Greco
a,
A.
Comandé
a,
A.
Nigro
a,
P.
Argurio
 b,
I.
Perrotta
d,
M.
Davoli
d,
A.
Tagarelli
e,
R.
Elliani
e,
T.
Granato
b,
G.
Nicolini
c,
A.
Chiorazzi
c,
S.
Semperboni
c,
E.
Ballarini
c,
C.
Crocamo
c,
G.
Cavaletti
c,
D.
Lombardo
f,
D.
Sisci
a,
C.
Morelli‡
a,
A.
Leggio‡
b,
I.
Perrotta
d,
M.
Davoli
d,
A.
Tagarelli
e,
R.
Elliani
e,
T.
Granato
b,
G.
Nicolini
c,
A.
Chiorazzi
c,
S.
Semperboni
c,
E.
Ballarini
c,
C.
Crocamo
c,
G.
Cavaletti
c,
D.
Lombardo
f,
D.
Sisci
a,
C.
Morelli‡
a,
A.
Leggio‡
 *a and
L.
Pasqua‡
*a and
L.
Pasqua‡
 *b
*b
aDepartment of Pharmacy, Health and Nutritional Sciences, University of Calabria, via P. Bucci, 87036 Arcavacata di Rende, CS, Italy. E-mail: antonella.leggio@unical.it
bDepartment of Environmental Engineering University of Calabria, via P. Bucci, cubo 44/A, 87036, Arcavacata di Rende, CS, Italy. E-mail: l.pasqua@unical.it; Fax: +39 0984 496655; Tel: +39 0984 496642
cExperimental Neurology Unit, School of Medicine and Surgery and Milan Center for Neuroscience, University of Milano-Bicocca, via Cadore 48, 20900 Monza, Italy
dDepartment of Biology, Ecology and Earth Sciences, Centre for Microscopy and Microanalysis (CM2), Transmission Electron Microscopy Laboratory, University of Calabria, via P. Bucci, 87036 Arcavacata di Rende, CS, Italy
eDepartment of Chemistry and Chemical Technologies, University of Calabria, via P. Bucci, 87036 Arcavacata di Rende, CS, Italy
fInstitute for Chemical-Physical Processes, National Research Council, 98158 Messina, Italy
First published on 9th November 2022
Abstract
Adverse reactions, toxicity, and poor compliance from patients still represent major challenges for conventional chemotherapy treatments. Localized drug delivery would ideally improve therapeutic efficacy, minimizing the side effects. An MSU-type mesoporous silica-based nanodevice (FOL-MSN-BTZ), able to selectively deliver the antineoplastic drug bortezomib (BTZ) to folate receptor over-expressing multiple myeloma (FR+ MM) cells is described. The receptor-specific ligand, folic acid, grafted on the external surface of the nanosystem, allows tumor recognition and cell internalization, while BTZ, mainly linked to the pore internal surface through a covalent pH-sensitive bond, is released in an acidic tumor environment. A detailed investigation showed that only the fine balancing of different functionalities of the nanodevice around the external and internal surfaces of MSN particles shows the absence of toxicity towards healthy cells in vitro and negligible BTZ-release at physiological pH, which are suitable features for applicative purposes in the engineering of therapies. After complete characterization in vitro, an accurate suspendability assessment, which considered the sedimentation process that reduces the particle amount and, consequently, drug content in the suspensions, allowed the development of an injectable formulation of FOL-MSN-BTZ that showed higher antitumor efficacy and an overall tendency to lower toxicity in a MM mice model compared to the conventional bortezomib chemotherapy.
Introduction
The main challenge of modern anticancer medicine is to exclusively address drugs to cancer tissue without affecting normal tissues, thus reducing side effects and maximizing therapeutic efficacy. This ambitious project embraces materials science, chemistry, biology, pharmacology, and medicine, resulting in the engineering of smart nanosystems, which offer unparalleled opportunities to treat various diseases such as cancer.The wide potentialities in the functionalization of material surfaces allow the versatile developments of tailor-made nanostructured platforms for several biomedical applications, modulating biological response, and improving biocompatibility, therapeutic performance, and selectivity toward specific targets.1,2 Inorganic materials appear as promising platforms to meet technical needs for the development of nanodevices for nanomedicine applications. In this context, mesoporous silica nanoparticles (MSNs) have been broadly tested as starting architectures for biomedical applications.3,4
Their solid framework, nanostructured through different organic functionalities, provides hybrid organic–inorganic nanodevices able to interact with biological structures,5 triggering cell internalization6 and drug release, as a response to several stimuli.7
MSNs have several advantages, such as high stability, good biocompatibility, regularly sized pores and tunable pore diameter in the range of 15–100 Å, large loading capacity, and ease of surface functionalization.8,9 The availability of two different functionalizable surfaces, one internal and the other external to the pores, makes them desirable options for encapsulating therapeutic/diagnostic (theranostic) agents (e.g., drugs, miRNA, siRNA, proteins, enzymes, DNA, as well as probes for imaging applications) to be delivered to the desired target (e.g. tumors)10,11 These features endorse MSN exploitation in the field of personalized medicine.12
Moreover, silica is classified by FDA as “Generally Recognized as Safe”, and it is used as a food additive, in pharmaceutical formulations and cosmetics.13,14
In 2007, we pioneered the preferential functionalization of the external surface of MSN, removal of the structure-directing agent, and drug loading of pores.15 The mesoporous silica, obtained from double-phase emulsions,16 externally derivatized with FOL, fluoresceine isothiocyanate and cisplatin-loaded, showed receptor-mediated uptake and cell killing in FR+ MM cells, without uptake in FR-negative (FR−) cells.17
Here, we describe the development and optimization process of an MSU-type mesoporous silica-based nanodevice, functionalized with folic acid and bearing the anticancer drug bortezomib linked through a pH-sensitive bond. Bortezomib is a synthetic compound approved by the US FDA for multiple myeloma (MM) patient treatment.18
The choice of using folic acid as a targeting function comes from the well-documented evidence that the folate receptor is highly expressed in tumor cells, including MM cells, compared with normal cells. Moreover, folic acid has been largely acknowledged as an effective targeting function to be exploited in drug-delivery nanosystems.19
The overall system engineered in order to be recognized and internalized by FR+ MM cells provides drug release when triggered in the acidic tumor microenvironment20 and/or by the low pH21 of the endosomal vesicles during MSN cell internalization.
An accurate optimization process for the device, based on drug release at different pH and toxicity on healthy cells, also supported by a preliminary in vitro study,22 provided a prototype showing striking selectivity towards FR+ cancer cells without toxicity toward FR− healthy cells. Finally, a careful study of the suspensions revealed the best formulation to administer to myeloma-bearing mice. The obtained in vivo results showed improvement in the therapeutic efficacy, lower bortezomib toxicity when administered through the nanodevices, trend to drug accumulation in tumors, and lower drug deposits in normal tissues if compared with conventional bortezomib chemotherapy.
Results
The device: design, development, and characterization
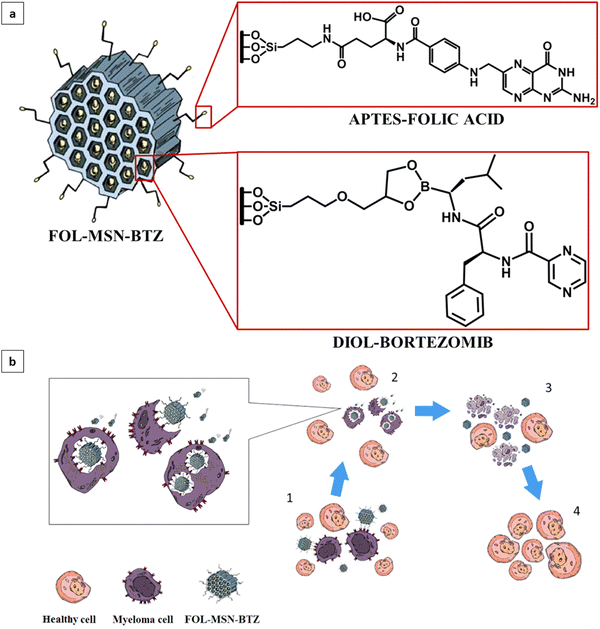
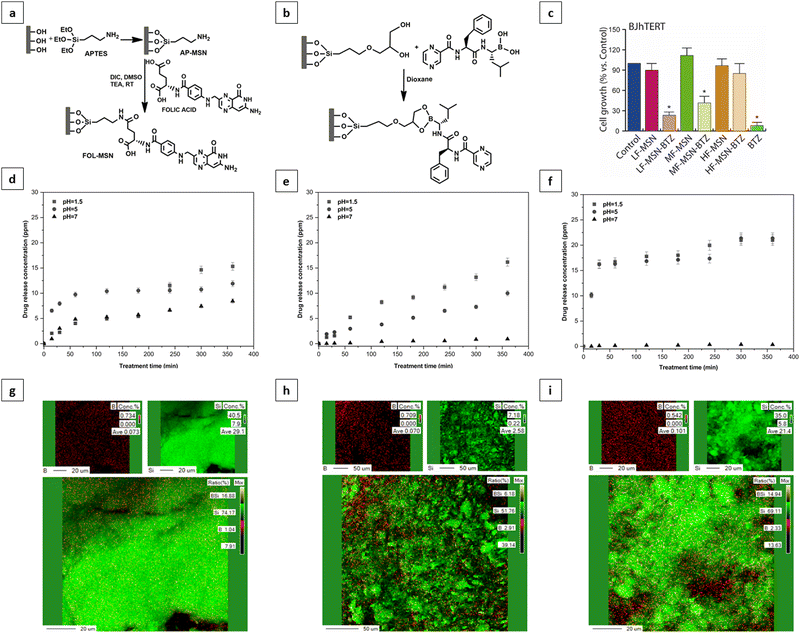
The FOL-MSN-BTZ prototype was designed with the aim of improving the performance of conventional BTZ-based chemotherapies, increasing tumor selectivity, and reducing drug diffusion and deposit in healthy tissues.It is a totally engineered device (Fig. 1a) able to release bortezomib as a response to a pH stimulus received only in the acidic microenvironment of the tumor (Fig. 1b). Fig. 1a shows the representation of FOL-MSN-BTZ with evidence of functionalization structural details. The targeting ligand, folic acid is covalently bonded, via an amide bond, to an aminopropyl group preferentially linked to the external surface of the nanoparticles while bortezomib forms, with a diol linker mainly anchored to the internal pore silica surfaces, a pH-sensitive cyclic boronate ester (Fig. 1). The as-synthesized materials were functionalized before solvent extraction of the polyethylene glycol (PEG)-based surfactant thus protecting the internal silica surface of the pores and, at the same time, preferentially addressing the aminosilane-modifying agent on the external surface of the mesoporous particles. According to this specific synthetic protocol, as previously reported, a considerable pore volume was recovered after the surfactant extraction from the PEG-templated folic acid-functionalized hybrid mesoporous silica. Folic acid was covalently linked on the external surface in a way that it neither blocked the pore entrances nor substantially filled the pores, allowing a relevant drug loading. Aminopropyl-functionalized particles (AP-MSN) were prepared by covalent grafting of (3-aminopropyl)triethoxysilane (APTES) on the MSN surface. Folic acid-functionalized nanoparticles (FOL-MSN) were then obtained by amide bond formation between the amino group of AP-MSN and folic acid carboxylic function (Fig. 2a). After the surfactant removal, the subsequent synthesis steps concerned BTZ-prodrug grafting through a pH-sensitive bond on the silica pore wall surface (Fig. 2b). The nanostructure FOL-MSN-BTZ was developed and successively optimized until negligible drug release at neutral pH was obtained. The optimized composition showed a lack of toxicity of FOL-MSN-BTZ in vitro towards healthy cells at physiological pH.
The drug release as a function of time from three different FOL-MSN-BTZ mesoporous silica compositions was studied. The analysis was performed using HPLC at different pH values to test the pH-sensitive behavior of the device. The composition indicated as Low Folic (LF) showed significant toxicity towards healthy cells in vitro (Fig. 2c) corresponding to a bortezomib release, as evidenced in Fig. 2d. Other two different samples, Medium Folic (MF) and High Folic (HF) were developed for the purpose of reaching the ideal nanostructure composition that is characterized, as mentioned, by lack of toxicity on healthy cells and negligible bortezomib release at neutral pH. Fig. 2e shows that very small amounts of bortezomib were still released at pH 7 from the MF composition. The best performance was reached with the HF composition, as shown in Fig. 2c and f. The increase in the folic acid content on the external surface of the mesoporous silica particle produces a continuous folic acid coverture that prevents the covalent grafting of the diol linker and consequently of the BTZ prodrug on the external surface. Our hypothesis is that the BTZ prodrug grafted on the external surface would be less protected and more easily hydrolysable also due to the catalytic role of silica's external surface that could lead to faster cleavage of the bond between BTZ and silica nanoparticles by water molecules even at neutral pH.
This hypothesis was confirmed using energy dispersive X-ray analysis (EDAX) carried out on the surface of LF, MF, and HF samples (Fig. 2g–i). The B/Si elemental ratios are 0.027 for LF (Fig. 2g), 0.0047 for MF (Fig. 2h), and 0.0025 for the HF prototypes (Fig. 2i). Corresponding polychromatic elemental maps of B and Si are reported in the energy-dispersive X-ray analysis provided in the ESI,† Results and discussion. The decrease of bortezomib prodrug content on the external surface of the particles is related to the increase of the folic acid content, which results, at the same time, in a reduction of the drug release at neutral pH and toxicity to normal cells. Hereafter, we will refer to FOL-MSN-BTZ to indicate the optimized prototype (composition HF-MSN-BTZ) that exhibits an optimal drug release profile.
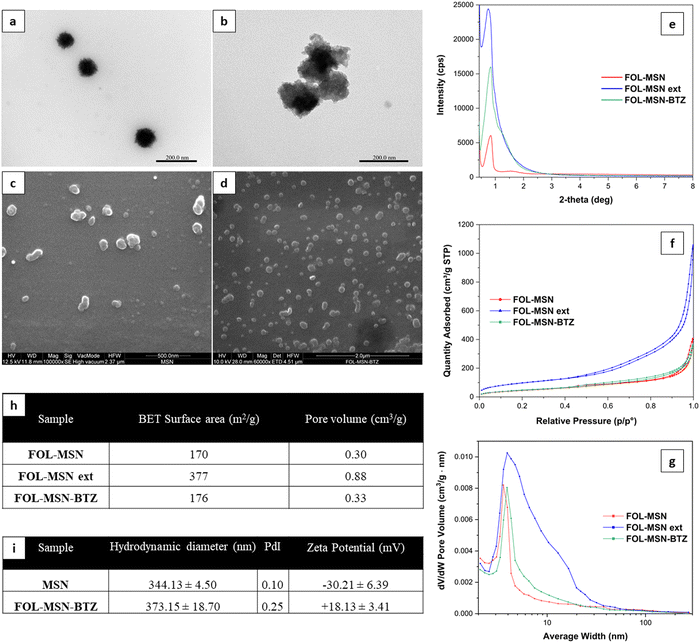
Transmission electron microscopy (TEM) micrographs (Fig. 3a and b) show that both the samples, MSN (Fig. 3a) and FOL-MSN-BTZ (Fig. 3b), exhibit a porous texture in adherence with materials of the MSU family, with dimensions of primary particles in the range between 80–120 nm.
Scanning electron microscopy (SEM) micrographs (Fig. 3c and d) show that this synthesis and successive modification procedures yielded nanoscaled particles without a regular morphology appearing also as aggregates of up to 300 nm.
All MSN samples highlighted a broad single reflection arising from the lack of long-range crystallographic order (Fig. 3e). This behavior is due to disorder in the assembly of the surfactant-templated channels in adherence to the patterns observed for the MSU materials.23
Fig. 3f and g show nitrogen adsorption-desorption isotherms at 77 K and pore size distributions of FOL-MSN, FOL-MSN ext (surfactant-free FOL-MSN), and FOL-MSN-BTZ. The similar patterns observed for FOL-MSN and FOL-MSN-BTZ are due to the pore filling by surfactant micelles and BTZ prodrug, respectively. The FOL-MSN ext sample exhibited a higher pore volume due to surfactant extraction. Pore volume values shown (Fig. 3h) reflect pore size distributions (Fig. 3g). DLS characterization data (Fig. 3i) show a hydrodynamic diameter of around 344 and 373 nm for MSN and FOL-MSN-BTZ, respectively, assigned to the aggregates observed in SEM and TEM micrographs (Fig. 3b–d). Zeta potential values of MSN and FOL-MSN-BTZ are −30.2 ± 6.39 mV and 18.1 ± 3.41 mV respectively (Fig. 3i). The changes observed are related to the successful functionalization of the nanoparticles' surface24 (refer to zeta potential analysis in the ESI,† Results and discussion for details).
Solid-state 29Si and 13C NMR analysis of FOL-MSN-BTZ (Fig. S1, ESI†) confirmed the conjugation of the organic ligands and BTZ to the silica nanostructure. 13C NMR spectrum shows characteristic resonances that can be associated with the carbon atoms of the alkyl chains linked to the silicon25 and the carbon atoms of FOL and BTZ.
FOL-MSN-BTZ selectively kills FR+ cancer cells
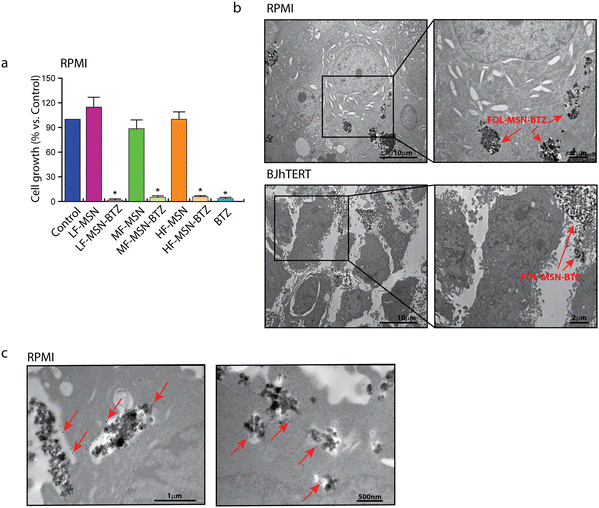
The obtained three different synthetic compositions of FOL-MSN-BTZ (LF-MSN-BTZ, MF-MSN-BTZ, HF-MSN-BTZ) were also tested on the human FRα−/FRβ+ MM RPMI cell lines (Fig. 4a). Interestingly, the specificity towards FR+ cells increased proportionally to the increase in the content of FOL on MSNs. Therefore, while LF-MSN-BTZ showed similar toxicities on both FR+ RPMI (Fig. 4a) and normal FR− BJhTERT cells (Fig. 2c), MF-MSN-BTZ and HF-MSN-BTZ gradually showed increased selectivity towards FR+ RPMI cells. In particular, HF-MSN-BTZ did not show any significant toxicity on FR− BJhTERT cells. As expected, from our previous results,17,21 the vehicle alone (LF-MSN, MF-MSN, and HF-MSN) was not toxic to both normal or cancer cells (Fig. 2c). The effect of HF-MSN-BTZ and the corresponding precursor HF-MSN (i.e. FOL-MSN-BTZ and FOL-MSN, respectively) on cell proliferation was evaluated on FR− cell lines, and FR+ RPMI MM cells being BTZ the treatment of choice for this type of cancer. Strikingly, FOL-MSN-BTZ was able to selectively induce death or inhibit proliferation of FR+ tumor cells, but not in FR− normal cells, while free BTZ was not sign selective and resulted toxic for all cell lines tested, independently on their FR expression. These results fit very well with our TEM observations on RPMI and BJhTERT cells treated with FOL-MSN-BTZ, which showed how MSNs are able to enter FR+ RPMI only and not FR− BJhTERT cells, where they remained confined in the intercellular spaces (Fig. 4b). Immunogold labelling experiments on RPMI confirmed that FOL-MSN-BTZ uptake occurs through the FR-mediated endocytosis (Fig. 4c).In vivo administration of the smart chemotherapy: biocompatibilty and antitumor efficacy
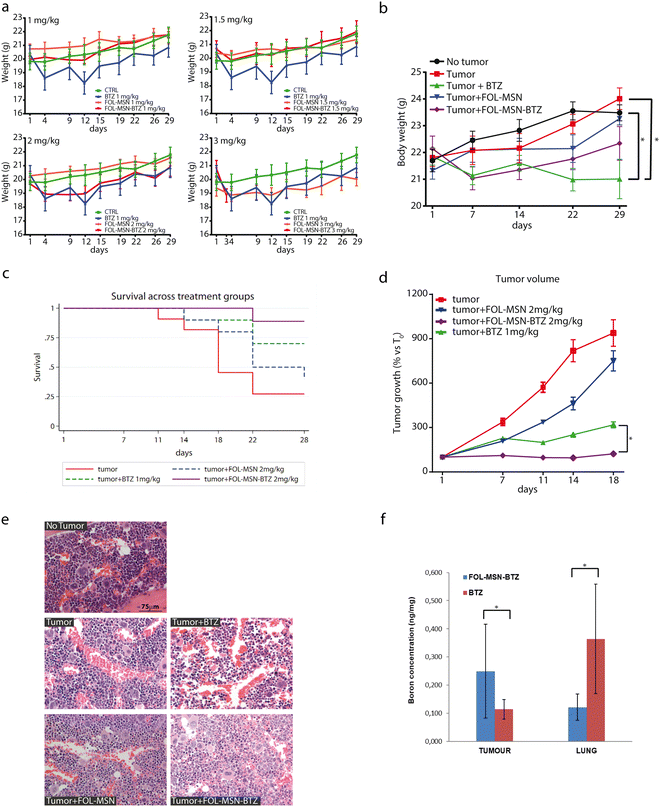
Due to the sedimentation process of the particles that reduce their concentration in the suspensions and consequently the concentration of the drug, we have studied in detail the FOL-MSN and FOL-MSN-BTZ suspensions with the aim of developing the proper in vivo administration protocol. The protocol should take into account that the real drug amount that the mice were receiving was lower than the nominal concentration of the suspensions (see below: Materials and methods, in vivo studies, and smart chemotherapy administration). Thus, the stability of the suspensions obtained according to the developed protocol was monitored in the time interval immediately preceding the injection. The stability analysis results show that, although a linear decrease in stability was observed, the correct administration of the selected doses was ensured in the first 10 minutes. The biodistribution and fate of MSNs were correlated to their physicochemical properties and to the medium in which they were suspended.26 The evolution of nanoparticles over time and fate in vivo remains undefined.27 (ESI,† Results and discussion).A repeated dose range-finding toxicity study (No Observed Adverse Event Level, NOAEL) for FOL-MSN and FOL-MSN-BTZ was performed on healthy mice (Fig. 5a) to assess the most efficacious concentration of the nanodevice to be employed in the subsequent in vivo efficacy study. FOL-MSN showed outstanding tolerability at all tested doses, throughout the treatment period (Fig. 5a). In fact, it did not cause any significant reduction in body weight, or signs of general toxicity, in treated animals compared to controls. Moreover, mice well tolerated FOL-MSN-BTZ up to the dose of 2 mg kg−1 (i.e. MSNs bearing an amount of BTZ equal to 2 mg kg−1 (BTZ EQ), see Tables 1 and 2), but did not tolerate the highest dose (3 mg kg−1 BTZ EQ), thus, for ethical reasons, these animals were sacrificed after the first administration. However, since the highest dose of the vehicle FOL-MSN (3 mg kg−1 BTZ EQ) was tolerated, we can conclude that the toxicity of FOL-MSN-BTZ 3 mg kg−1 (BTZ EQ), was due to the activity of BTZ itself and not to the nature of the vehicle (Fig. 5a), confirming, also in vivo, the safety and biocompatibility of the nanocarrier.
| Sample | Measured % (BTZ sample/BTZ tot) | Theoretical % (BTZ sample/BTZ tot) | BTZ concentration (mg mL−1) | Sample recovery (%) | BTZ dose in 0.2 mL (mg) |
|---|---|---|---|---|---|
| FOL-MSN-BTZ1 | 32.2 | 50 | 0.13 | 64.4 | 0.026 |
| FOL-MSN-BTZ2 | 36.8 | 50 | 0.15 | 73.6 | 0.030 |
| FOL-MSN-BTZ3 | 29.9 | 50 | 0.14 | 59.8 | 0.028 |
| Avg. | 22.9 | 0.14 | 65.9 | 0.028 | |
| St. dev. | 3.5 | 0.01 | 5.8 | 0.002 |
This result leads to the first important conclusion: animals tolerated a double dose of BTZ (2 mg kg−1 BTZ EQ) when the drug was delivered through the MSN platform if compared to the free drug formulation, for which the assessed maximum-tolerated dose was 1 mg kg−1 BTZ.18 Therefore, we expect that our pH-triggerable DDS, by protecting a double dose of the drug from premature release, will improve the therapeutic efficacy of BTZ towards the tumor.
Once identified FOL-MSN-BTZ 2 mg kg−1 (BTZ EQ) as the highest tolerated dose with no adverse observable events, we evaluated the efficacy of the nanodevice using an in vivo female SCID mice subcutaneous tumor (RPMI 8226 cells) model. Briefly, the mice were treated intravenously once a week for 5 weeks with FOL-MSN-BTZ 2 mg kg−1 (BTZ EQ), FOL-MSN 2 mg kg−1 (BTZ EQ), and BTZ 1 mg kg−1 used as the reference drug.28 Our results showed that all the mice well-tolerated the MSN treatments since no significant body weight loss was observed throughout the experiment. Otherwise, mice treated with BTZ 1 mg kg−1 showed a significant reduction (p < 0.05) in body weight compared with the untreated no-tumor group as well as with the untreated tumor-bearing group at the end of the treatment (Fig. 5b).
Survival analysis indicated a significant mortality rate in the untreated animals, as compared to treated animals (p = 0.013), especially considering FOL-MSN-BTZ (Fig. 5c) while the median survival time for the untreated group was 18 days. Indeed, during the experiment, it was necessary to sacrifice some untreated animals for ethical issues (Table S3, ESI†), thus the sample size of the untreated group progressively decreased. For this reason, and in order to have an adequate number of animals for each group, we performed the statistical analyses up to the 18th day (refer antitumor efficacy in the ESI,† Results and discussion for details). Mice treated with free BTZ showed only a slight increase in tumour volume during the whole experiment, confirming the anti-neoplastic effect of the drug (Fig. 5d). Notably, FOL-MSN-BTZ was able to completely stop the tumour growth as soon as after the first administration and throughout the treatment period. These data strongly show the higher efficacy of our delivery system compared to the free BTZ (Fig. 5d).
It is also worth mentioning that, although not statistically significant, the average tumor volumes in the FOL-MSN treated group were smaller than those in the untreated control animals at all time points. This intrinsic antitumor effect of FOL-targeted MSNs on tumor mass has already been observed by other authors29 and could be referred to as FOL-MSN accumulation at the tumor site, supposedly due to FR recognition30 and to the enhanced permeability and retention (EPR) effect.31 This result could also be due to the beneficial effect of both folic acid and mesoporous silica vehicles on fostering the immune system response.32
Two days after the last administration, mice were sacrificed and blood samples were analyzed. No statistically significant differences were noticed among treated and not treated animals in all the hematochemical parameters (Fig. S6a, ESI†). The leukocyte formula showed a statistically significant increase (p < 0.01) in granulocyte counts in tumor-bearing mice treated with FOL-MSN-BTZ 2 mg kg−1 (BTZ EQ) compared to mice without tumor or tumor untreated animals. Such an increase is clearly due to the drug, which, very likely, concentrates in the tumor site triggering sustained immunogenic cell death (ICD). In fact, bortezomib, by increasing ROS and ER stress, is one of the few chemotherapeutic drugs that have been recognized as an ICD inducer.33,34 ICD is associated with the chronic release and/or exposure of damage-associated molecular patterns (DAMPs) by some dying apoptotic cells (e.g. tumor cells). DAMPs act as danger signals, eliciting immunostimulatory effects, including the recruitment and activation of macrophages, neutrophils, and other immune cells,35 thus promoting immune-mediated elimination of tumor cells. This hypothesis would justify the increase in granulocyte count in FOL-MSN-BTZ treated mice (Fig. S6b, ESI†).
Tumor-bearing mice treated with BTZ and FOL-MSN-BTZ showed hepatic toxicity (GPT/ALT increase) compared to tumor not treated animals and mice without tumors (Fig. S6c, ESI†).
Nevertheless, the histological analysis of the liver tissue sections did not show any sign of injury in all the treatment groups, including FOL-MSN-BTZ (Fig. S7, ESI†). However, this effect is not surprising, considering the detoxification function of the liver. In fact, in large clinical trials of BTZ, elevations in serum aminotransferase levels were common, occurring in ∼10% of patients, but the effect is transitory and normal values are restored after the treatment cycles.36
Renal functionality was not affected by any treatment as confirmed by renal marker values (Fig. S6d, ESI†) and histological analysis (Fig. S7, ESI†). Mild hypoplasia was solely observed in the bone marrow of BTZ-treated mice (Fig. 5e), while no pathological alterations were noticed in the organs explanted from all the other experimental groups.
Moreover, boron and silicon, coming from BTZ and silica nanoparticles, respectively, have been dosed by ICP-MS in the tissues obtained from different organs 48 h after the last administration. Fig. 5f and Fig. S8 in the ESI,† present all the statistically significant data obtained.
The spleen, sternum, bladder, uterus, heart, and brain were collected and analyzed, for boron and silicon content. The obtained results have not been reported since no statistical analysis could be conducted due to the very low or even undetectable Si and B content found in these tissues.
As depicted in Fig. 5f, FOL-MSN-BTZ displayed a slightly higher accumulation in tumor tissue compared to free BTZ and this trend well fits with the higher in vivo antitumor efficacy of the developed platform, very likely due to its targeting capacity. Noteworthy, FOL-MSN-BTZ distribution in lungs was lower than the free BTZ, and this could represent a signal of the general lower diffusion and accumulation of the drug, if administered through FOL-MSN-BTZ nanodevice, in the different tissues of the organism, in comparison to the free drug but also to the excretion of FOL-MSN-BTZ that carries the drug away in the inactive form.
Higher silicon accumulation was detected in the liver tissue of mice treated with FOL-MSN-BTZ compared to the same tissue of mice treated with free BTZ, probably due to the role of the liver as the primary organ of the nanoparticle detoxification37 (Fig. S8, ESI†). Conversely, no significant difference (*p > 0.05) in silicon accumulation was recorded in the kidney of untreated mice (CTR TUM), treated with FOL-MSN-BTZ, and FOL-MSN. Silicon detected in tissues of untreated mice (CTR TUM) and treated with free BTZ is related to the natural presence of this element in various organs as reported in the literature.38
Conclusions
Here, we described a totally engineered approach to the development of an MSN-based nanodevice able to provide the smart administration of a chemotherapeutic agent. The system is engineered to be internalized by myeloma cells and to release BTZ only at a slightly acid pH, maintaining the drug in an inactive form at physiological pH and also in the case of direct excretion. Furthermore, it is designed to leave healthy cells unaffected. The fine balancing of the different functionalities of the nanodevice around the external and internal surface of the MSN particles was investigated with the aim of identifying an optimized nanostructure owning the most suitable features for target therapy applications.The obtained nanodevices were engineered as injectable suspensions, which allowed the delivery of up to double the maximum administered dose of bortezomib in a MM animal model. This expectedly leads to a clear gain in therapeutic efficacy compared to the free drug. Noteworthily, the higher antitumor efficacy is not accompanied by higher toxicity, but rather a trend towards lower toxicity was observed. Indeed, compared to free bortezomib, FOL-MSN-BTZ-treated animals tended to live longer, did not significantly lose weight, and did not show marrow aplasia. Moreover, BTZ delivered through the nanodevice preferentially accumulates in tumors (very likely the reason for the higher efficacy of our system) and much less in other tissues.
The evidence gathered here shows the striking specificity of FOL-MSN-BTZ toward FR-expressing MM cells, a significantly higher in vivo antitumor efficacy, and a better safety profile compared to conventional bortezomib formulations. Our data suggest that FOL-MSN-BTZ represents a great opportunity for the future exploitation of MSNs-based strategies in the therapeutic management of multiple myeloma.
Materials and methods
Chemicals and reagents
Reagents were commercially available with analytical grade and used as purchased without further purification. Solvents were purified according to well-known laboratory methods and freshly distilled before use. Triton X-100, neutral polyoxyethylene (10) octylphenyl ether, tetraethylorthosilicate (TEOS), (3-aminopropyl)-triethoxysilane (APTES), folic acid (FOL), diisopropylcarbodiimide (DIC), water (HPLC grade), acetone (HPLC grade) were purchased from MERCK/Sigma-Aldrich (Milan, Italy). Bortezomib was purchased from LC Laboratories, Woburn, MA. Ethanol, diethyl ether, 1,4-dioxane, dimethylformamide (DMF), tetrahydrofuran (THF), trifluoroacetic acid (TFA), and acetic acid were obtained from VWR. Dimethyl sulfoxide (DMSO) and cyclohexane were purchased from Merck and triethylamine from Carlo Erba. Ultrapure water was distilled using the MilliQ® water, Millipore.Instruments and general experimental details
Thermogravimetric analysis (TGA) was carried out using a Netzsch STA 409 instrument between 293.15 K and 1123.15 K at a ramp of 10 K min−1 in the air with a flow rate of 10 mL min−1. The zeta potential values were determined using the Zeta-sizer ZS (Malvern Instruments Ltd, Malvern, U.K.) at 298.15 ± 0.1 K. The size and distribution of MSNs were determined, at 298.15 ± 0.1 K using Dynamic Light Scattering (DLS) analysis using a 90 Plus Particle Size Analyzer (Brookhaven Instruments Corporation, New York, USA).NMR spectra were obtained at 300 K on a Bruker spectrometer Avance II 400 MHz.
29Si {1H} CP-MAS NMR spectra were recorded at 300 K on a Bruker spectrometer Avance II 400 MHz (9.4 T), operating at 79.4 MHz for 29Si nuclide with a rotation rate of magic-angle of 6 KHz, 1000 scans, contact time 8 ms, a proton pulse of 5.1 ms and delay time of 5 s. Optimization of the Hartmann–Hahn condition was performed using a standard sample of Q8M8 (Si[CH3)3]8Si8O20). 13C{1H} CP-MAS NMR spectra were obtained at 300 K and 100.63 MHz for nuclide 13C with a rotation magic angle rate of 6 MHz, 4096 scans, a contact time of 2 ms, a proton pulse of 5.1 ms and a delay time of 3 s. Optimization of the Hartmann–Hahn condition was performed using a standard adamantine sample. All the samples were pressed using a zirconia rotator of 4 mm with Kel-F caps.
Determination of boron content was performed using atomic absorption spectroscopy on an Analytik.
Jena AG contrAA 700 – High-Resolution Continuum Source Atomic Absorption Spectrometer. HPLC analyses for the release tests were performed on a Jasco HPLC analyzer using a flow rate of 1 mL min−1. A 30/70% v/v of a solution of acetonitrile/water was used as the mobile phase. A UV-VIS wavelength of 270 nm was chosen to acquire the HPLC chromatograms.
Bortezomib release from FOL-MSN-BTZ was monitored, as a function of time, after keeping the suspension in physiological solutions at pH 7 typical of a haematic environment, pH 5 typical of intracellular organelles of cancer cells and pH 1.5, a strong acid pH, which is useful for the evaluation of the amount of bortezomib not hydrolysed at pH 5 that could be considered retained in the nanoparticles.
Synthesis of FOL-MSN-BTZ
The surfactant Triton X-100 (21 g) was dissolved in ultrapure water (230 g) for about four hours at room temperature. In order to create two phases, along the vessel, it was slowly added to a solution of TEOS (22 g) in cyclohexane (9.8 g) (molar composition TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexane
cyclohexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Triton X-100
Triton X-100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O was 1
H2O was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.08
1.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.32
0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120, respectively).
120, respectively).
The synthesis was carried out at room temperature. The upper phase was removed and the resulting precipitate was collected by filtration and washed three times with ultrapure water. Finally, the sample was dried in the oven at 343.15 K for 24 h thus a white powder was obtained.
Three different FOL-MSN samples were synthesized using different FOL/AP-MSN ratios, LF-FOL-MSN, MF-FOL-MSN, and HF-FOL-MSN equal to 0.11, 0.12, and 0.14 g g−1, respectively. Specifically, AP-MSN (1) was employed as starting material for LF-FOL-MSN, while AP-MSN (2) for MF-FOL-MSN and HF-FOL-MSN.
For the functionalization process, folic acid was used in combination with triethylamine (TEA) and N,N′-diisopropylcarbodiimide (DIC) in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6
1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.15 mmol, respectively.
10.15 mmol, respectively.
Folic acid was completely dissolved in DMSO (0.04 g mL−1). After that, TEA, DIC, and finally AP-MSN were added. The so-obtained suspension was stirred at room temperature for 40 hours. Finally, the mixture was filtered and washed with dimethylformamide (DMF), dioxane, diethyl ether, and ultrapure water (once for each solvent). The resultant yellow powder (5 g) was dried and stored in sealed containers protected from light. Subsequently, the surfactant within the pores was removed at room temperature using 1 g of material in 0.33 L of ultrapure water. The number of extractions required to reach a complete surfactant removal was established by monitoring (by TGA) the total mass loss of the sample subjected to subsequent extraction and filtration steps until a constant value was reached. Then, the resulting FOL-MSN was washed with 1,4-dioxane and dried at 318.15 K overnight.
For the FOL-MSN-BTZ preparation, bortezomib was loaded using a FOL-MSN-DIOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTZ molar ratio of 1
BTZ molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 on the basis of the 1,2-diol linker content on FOL-MSN determined by TGA.
1.5 on the basis of the 1,2-diol linker content on FOL-MSN determined by TGA.
The reaction was carried out under an inert atmosphere. FOL-MSN-DIOL was suspended in dry 1,4-dioxane and then BTZ was added. The reaction mixture was gently stirred at room temperature for 24 h; then the nanoparticles were filtered and washed three times with dry dioxane and three times with dry dichloromethane. The recovered liquid phase was concentrated under reduced pressure and used for a second drug loading cycle. The second drug loading procedure was performed by adding 10% of the initially used amount of the drug to the solution resulting from the first drug loading cycle. The reaction was left for 24 h at room temperature under gentle stirring (30 rpm). The final product (FOL-MSN-BTZ) was filtered and washed as previously described. The sample was then stored in sealed containers at 253.15 K to preserve the integrity of the drug.
The sample was treated as described below:
(1) dried at low temperature in a water bath (temperature heating plate 323.15 K – bath temperature 311.15 K); (2) transferred into plastic containers; (3) treated with 0.6 mL HF (complete dissolution of the MSN powder was observed); (4) treated with 0.1 mL HNO3; (5) the addition of 6.3 mL of ultrapure water (MilliQ-test 1) to reach the final 7 mL sample volume required for the spectrophotometric analysis. The obtained samples were analyzed by atomic absorption spectroscopy (contrAA® 700, Analytikjena, Germany).
The same technique and procedure were employed for the determination of the drug amount in the suspensions (see below: Smart chemotherapy administration).
Cell culture and treatments
Human FR+ MM RPMI-8226 (RPMI) and FR− normal foreskin fibroblast BJhTERT were purchased from ATCC where they were authenticated. Cells were stored according to the supplier's instructions and used within 6 months after frozen aliquot resuscitations. RPMI cells were cultured in RPMI-1640 medium and BJhTERT in Dulbecco's Modified Eagle's Medium (DMEM), both containing 10% Fetal Bovine Serum (FBS), 100 IU mL−1 penicillin/streptomycin (pen/strep) and 0.2 mM L-glutamine. All culture media and additives were obtained from Gibco™ (Life Technologies, Monza MB, Italy). Trypsin–EDTA solution 10×, formaldehyde, EtOH, tween80, and NP-40 were obtained from MERCK/Sigma-Aldrich (Milan, Italy). Mycoplasma negativity was tested monthly (PlasmoTest, Invivogen). For the cell treatment, a ratio of 1 μg MSNs/105 cells was used based on titration experiments. Free BTZ was added as a positive control in amounts corresponding to the percentage of BTZ carried by FOL-MSN-BTZ.Cell proliferation assays
MSN effect on cell proliferation was assessed by the trypan blue exclusion assay. Cells were seeded in triplicates for each condition, synchronized in serum-free media (SFM) for 24 h, and then treated for 1 h with MSNs. Cells were then switched to fresh growing medium plus 1% FBS and counted after 72 h. Cell viability was determined by Countess® II Automated Cell Counter (Invitrogen, Life Technology, IT), according to the supplier's instructions.Transmission electron microscopy (TEM) and electron immunocytochemistry
For conventional TEM analysis and electron immunocytochemistry, cells were treated as described for growth experiments and harvested after 1 h of treatment to detect MSN uptake. All samples were routinely fixed, dehydrated, and resin-embedded using heat polymerization.For indirect immunolabeling, grids were floated on drops of 1% bovine serum albumin (BSA) in PBS containing 0.02-M glycine at RT for 30 minutes to reduce nonspecific binding. Sections were then incubated with a rabbit polyclonal antibody against FR-β (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) (Invitrogen, cat#PA5-45768) at 277.15 K overnight. The grids were then transferred to 50 μL drops of secondary antibody conjugated to 10 nm gold particles for 1 h, at RT. Observations were performed under a Jeol JEM-1400 Plus electron microscope (Jeol Ltd, Tokyo, Japan) operating at 80 kV.
10) (Invitrogen, cat#PA5-45768) at 277.15 K overnight. The grids were then transferred to 50 μL drops of secondary antibody conjugated to 10 nm gold particles for 1 h, at RT. Observations were performed under a Jeol JEM-1400 Plus electron microscope (Jeol Ltd, Tokyo, Japan) operating at 80 kV.
The results presented in Table 1 suggest that the BTZ content in the liquid phase is 65.9% (±5.8%) of the expected dose of FOL-MSN-BTZ resuspended with magnetic stirring for 13 minutes and immediately collected, without sedimentation.
| BTZ EQ concentration (mg kg−1) | FOL-MSN-BTZ concentration (mg kg−1) | FOL-MSN concentration (mg kg−1) |
|---|---|---|
| 1.0 | 14.6 | 13.1 |
| 1.5 | 21.9 | 19.6 |
| 2.0 | 29.2 | 26.2 |
| 2.5 | 36.5 | 32.7 |
| 3.0 | 43.8 | 39.3 |
Animals and ethical statements
10 weeks old female Balb/cOlaHsd mice and five weeks old female SCID mice (C.B-17/IcrHanHsd-Prkdcscid) were purchased from Envigo (Bresso, Italy).All mice were housed under a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light
12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle with food and water available ad libitum.
dark cycle with food and water available ad libitum.
Animal studies were reviewed and approved by the Ethics Committee of the University of Milano-Bicocca and the Ministry of Health (approval numbers 919/2015-PR del 27/07/2015). The accreditation number of the laboratory used for animal studies is 08/2015-UT of 21/05/2015. The care and husbandry of animals were in conformity with the institutional guidelines in compliance with national (D.L.vo no. 26/2014) and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, Dec.12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
NOAEL (no observed adverse event level)
NAOEL was conducted on 10 groups of female Balb/c mice (6 mice/group), receiving MSN-FOL and FOL-MSN-BTZ, at increasing concentrations corresponding to doses 1, 1.5, 2, and 3 mg kg−1 of free BTZ, as follows:(1) Not treated mice (controls);
(2) Free BTZ (1 mg kg−1);
(3) FOL-MSN-BTZ (1 mg kg−1),
4) MSN-FOL (1 mg kg−1);
(5) FOL-MSN-BTZ (1.5 mg kg−1);
6) MSN-FOL (1.5 mg kg−1),
(7) FOL-MSN-BTZ (2 mg kg−1),
(8) MSN-FOL (2 mg kg−1);
(9) FOL-MSN-BTZ (3 mg kg−1),
(10) MSN-FOL (3 mg kg−1).
BTZ was administered at a final concentration of 1 mg kg−1, chosen on the basis of previous results.18
The drug was dissolved in 5% EtOH and dissolved in a warm bath (37 °C). 5% tween80 and saline were then added and the obtained solution was sonicated by immersion until complete clarification. FOL-MSN-BTZ and MSN-FOL were suspended in saline solution with a magnetic stirrer for 15 minutes and were tested at the concentrations of 1, 1,5, 2, and 3 mg kg−1. 10 mL kg−1 of all treatments were administered intravenously once a week for 5 weeks (1qwx5). Corresponding amounts of saline solution were fed to control mice.
In vivo antitumor efficacy
RPMI 8226 cancer cells were subcutaneously implanted in the left hip of 7 weeks old animals (10 × 106 cells per animal in 200 μl PBS). When the tumor reached 200–500 mg size (about 30–40 days after implant), mice were divided into 5 groups, randomized on tumor size:(1) No tumor (n = 7);
(2) Untreated tumor (n = 11);
(3) Tumor + BTZ 1 mg kg−1 (n = 10);
(4) Tumor + FOL-MSN 2 mg kg−1 (n = 10);
(5) Tumor + FOL-MSN-BTZ 2 mg kg−1 (n = 9).
Based on NOAEL data (Fig. 5a), FOL-MSN and FOL-MSN-BTZ were used at 2 mg kg−1, while BTZ was at 1 mg kg−1.26 BTZ was dissolved in 5% EtOH in a warm bath (310.15 K). 5% tween80 and saline were then added and the obtained solution was sonicated by immersion until complete clarification. FOL-MSN-BTZ and FOL-MSN were suspended in saline solution with a magnetic stirrer for 15 minutes and were tested at the concentrations of 2 mg kg−1.
All the treatments were administered intravenously at 10 mL kg−1 once a week for 5 weeks (1qwx5). Corresponding amounts of saline solutions were fed to control mice.
Animal general conditions were recorded daily until sacrifice. Changes in their appearance (decreased grooming, disheveled fur, piloerection, exaggerated kyphosis), behavior (decreased nesting), and activity (decreased exploring) were monitored daily. Mice's weight and tumor size were measured twice a week. The growth of subcutaneous tumors was measured using a Vernier caliper. The length (L) and width (W) of the tumors was measured and their volumes were calculated using the formula (L × W2)/2. Animals were sacrificed when tumor volume exceeded about 10% of their body weight.
Hematological, hematochemical, and histological analysis
48 h after the last administration, the mice were sacrificed and blood was analyzed for the hematochemical and hematological parameters.48 h after the last administration, 3 mice/group were sacrificed and the main organs were collected for histological examinations. Some of the animals were sacrificed for ethical reasons at the end of the experiment, in case abnormal tumor growth occurred (Table S3, ESI†).
Once collected, the target organs were washed with 0.1 M PBS, pH 7.4, fixed by immersion in 10% buffered formalin O/N at room temperature, embedded in paraffin (Embedding Center Leica EG1160, Leica Biosystem, Wetzlar, Germany), cross-sectioned at 3 μm thickness by a rotary microtome, mounted on slides, stained using hematoxylin and eosin (HE) and observed under a light microscope (Nikon Eclipse 50i, Melville, NY, USA). Representative images were captured with a digital camera (Nikon Digital Sight DS-2Mv).
MSN biodistribution
Metal concentrations in tissues were measured by inductively coupled plasma-mass spectrometry (ICP-MS) analysis. The determinations were carried out utilizing an Elan DRC-e ICP-MS instrument (PerkinElmer SCIEX, Canada), and the sample delivery system consisted of a PerkinElmer autosampler model AS-93 Plus with a peristaltic pump and a cross-flow nebulizer with a Scott type spray chamber. The ICP torch was standard (Fassel-type torch) with a platinum injector. A solution containing Rh, Mg, Pb, Ba, and Ce (10 μg L−1, Merck) was used to optimize the instrument in terms of sensitivity, resolution, and mass calibration.An Anton Paar Multiwave 3000 with programmable power control (maximum power 1400 W) and rotor XF100 (operating pressure up to 120 bar maximum; operating temperature, 533.15 K maximum; construction material, PTFE-TFM for the liner) was used for the microwave digestion of samples. The digestion was achieved by adding 4 mL of HNO3 and 2 mL H2O2 into vessels containing 100 mg of tissue with a power ramp from 0 to 600 W in 15 min, a 10 min hold step at 600 W, and a cooling step for 10 min. After digestion, the extracts were quantitatively transferred to a graduated polypropylene test tube, diluted with ultrapure water to 50 mL, and then analyzed by ICP-MS.
Single element solutions of B and Si (100 mg L−1, Merck) were used for the preparation of aqueous calibration standard solutions after appropriate dilution. For the quantitative analysis, external calibration curves were built on seven different concentrations in a calibration range of 0.2–500 μg L−1.
The comparison between the boron concentration values detected in tumour tissues of mice treated with free BTZ and FOL-MSN-BTZ was made by applying the non-parametric Mann–Whitney U test. The same statistical approach was applied to compare the boron concentrations found in lung tissue.
Body weight and hematochemical/haematological parameters were statistically analyzed using a 2-step approach involving a nonparametric One-way ANOVA test and the Kruskall–Wallis post-test (significance level set at p < 0.05). Data analysis was carried out using GraphPad 4.0 software (GraphPad Software, Inc., San Diego, California).
Author contributions
L. P. and A. L. formulated the conception and the aim of the work, L. P., A. L., and C. M. provided the acquisition of the financial support for the research and oversaw it, and wrote and critically revised the manuscript. M. D. S., A. G., I. E. D. N., and M. G. performed MSNs synthesis and physicochemical characterization, A. C. prepared intermediates and substrate for MSN synthesis, E. M. conducted DLS analysis, I. P. and M. D. carried out TEM and SEM analysis respectively. T. G. developed the HPLC methodology to study bortezomib release from engineered nanoparticles at different pH. P. A. executed the AAF analysis and interpreted the data. A. T. performed ICP-MS analysis with the contribution of R. E. and conducted the statistical analysis of the acquired data. A. N. and M. F. conducted the in vitro studies, D. S. conducted the statistical analysis of the in vitro and in vivo data, and helped draft the manuscript. G. N. performed conceptualization and supervision of the in vivo studies, A. C. and S. S. conducted the in vivo studies, E. B. carried out the histopathological studies, C. C. conducted biostatistical analysis, G. C. supervised, analyzed, interpreted, and drafted the data of animal studies. L. P., A. L., and C. M. performed analysis and interpretation of the data, drafting the final manuscript with the contribution of M. D. S., A. G., E. M., D. S., G. C., and D. L. performed the final manuscript curation. All authors read and approved the manuscript before submission.Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
NanoSiliCal Devices, a company founded by the authors L. P., A. L., and C. M., a spinoff of the University of Calabria, owns the Patent EP 3 288 955 B1 that claims, among other things, the prototype FOL-MSN-BTZ.Acknowledgements
The research activities were funded by NanoSiliCal Devices's private financial support and related co-funded by Calabria National Operating Programme (NOP) 2007–2013 Project “TalentLab Spinoff” (FESR funds, Axis I), Calabria NOP 2014–2020 Projects “Research and Innovation” (ERDF-ESF funds, Axis I, Action 1.1.2), “R&D” (Axis I, Action 1.2.2), NOP “Research and Innovation” (Axis I, Action I.1) and by EU Horizon 2020-Research and Innovation Framework Program-SME-Instrument Phase I.References
- A. Rifai, N. Tran, P. Reineck, A. Elbourne, E. Mayes, A. Sarker, C. Dekiwadia, E. P. Ivanova, R. J. Crawford, T. Ohshima, B. C. Gibson, A. D. Greentree, E. Pirogova and K. Fox, Engineering the interface: nanodiamond coating on 3D-printed titanium promotes mammalian cell growth and inhibits Staphylococcus aureus colonization, ACS Appl. Mater. Interfaces, 2019, 11, 24588–24597 CrossRef CAS PubMed.
- E. Rivero-Buceta, C. Vidaurre-Agut, C. D. Vera-Donoso, J. M. Benlloch, V. Moreno-Manzano and P. Botella, PSMA-targeted mesoporous silica nanoparticles for selective intracellular delivery of docetaxel in prostate cancer cells, ACS Omega, 2019, 4, 1281–1291 CrossRef CAS.
- A. Nigro, M. Pellegrino, M. Greco, A. Comandè, D. Sisci, L. Pasqua, A. Leggio and C. Morelli, Dealing with skin and blood-brain barriers: The unconventional challenges of mesoporous silica nanoparticles, Pharmaceutics, 2018, 10, 250 CrossRef CAS PubMed.
- C. Chircov, A. Spoială, C. Păun, L. Crăciun, D. Ficai, A. Ficai, E. Andronescu and Ş. C. Turcules, Mesoporous silica platforms with potential applications in release and adsorption of active agents, Molecules, 2020, 25, 3814 CrossRef CAS PubMed.
- R. Mout, D. F. Moyano, S. Rana and V. M. Rotello, Surface functionalization of nanoparticles for nanomedicine, Chem. Soc. Rev., 2012, 41, 2539–2544 RSC.
- R. Huang, Y. W. Shen, Y. Y. Guan, Y. X. Jiang, Y. Wu, K. Rahman, L. J. Zhang, H. J. Liu and X. Luan, Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology, Acta Biomater., 2020, 116, 1–15 CrossRef CAS PubMed.
- R. Salve, P. Kumar, W. Ngamcherdtrakul, V. Gajbhiye and W. Yantasee, Stimuli-responsive mesoporous silica nanoparticles: A custom-tailored next generation approach in cargo delivery, Mater. Sci. Eng., 2021, 124, 112084 CrossRef CAS PubMed.
- M. Vallet-Regí, I. Colilla and M. Izquierdo-Barba, Manzano, Mesoporous silica nanoparticles for drug delivery: Current insights, Molecules, 2018, 23, 1–19 Search PubMed.
- A. M. Mebert, C. J. Baglole, M. F. Desimone and D. Maysinger, Nanoengineered silica: Properties, applications and toxicity, Food Chem. Toxicol., 2017, 109, 753–770 CrossRef CAS PubMed.
- W. X. Mai and H. Meng, Mesoporous silica nanoparticles: a multifunctional nano therapeutic system, Integr. Biol., 2013, 5, 19–28 CrossRef CAS PubMed.
- F. Tang, L. Li and D. Chen, Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- W. H. Chen, G. F. Luo, W. X. Qiu, Q. Lei, L. H. Liu, S. B. Wang and X. Z. Zhang, Mesoporous silica-based versatile theranostic nanoplatform constructed by layer-by-layer assembly for excellent photodynamic/chemo therapy, Biomaterials, 2017, 117, 54–65 CrossRef CAS.
- J. G. Croissant, Y. Fatieiev and N. M. Khashab, Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles, Adv. Mater., 2018, 29, 1604634 CrossRef.
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm .
- L. Pasqua, F. Testa, R. Aiello, S. Cundari and J. B. Nagy, Preparation of bifunctional hybrid mesoporous silica potentially useful for drug targeting, Microporous Mesoporous Mater., 2007, 103, 166–173 CrossRef CAS.
- L. Pasqua, F. Testa and R. Aiello, Interfacial polycondensation of mesoporous silica particles at the nanometer scale, Stud. Surf. Sci. Catal., 2005, 158A, 557–564 CrossRef.
- C. Morelli, P. Maris, D. Sisci, E. Perrotta, E. Brunelli, I. Perrotta, M. L. Panno, A. Tagarelli, C. Versace, M. F. Casula, F. Testa, S. Andò, J. B. Nagy and L. Pasqua, PEG-templated mesoporous silica nanoparticles exclusively target cancer cells, Nanoscale, 2011, 3, 3198–3200 RSC.
- C. Meregalli, V. A. Carozzi, B. Sala, A. Chiorazzi, A. Canta, N. Oggioni, V. Rodriguez-Menendez, E. Ballarini, C. Ceresa, G. Nicolini, L. Crippa, M. Orciani, G. Cavaletti and P. Marmiroli, Age-related changes in the function and structure of the peripheral sensory pathway in mice, J. Biol. Regul. Homeostatic Agents, 2015, 29, 115 CAS.
- L. Pasqua, A. Leggio, D. Sisci, S. Andò and C. Morelli, Mesoporous silica nanoparticles in cancer therapy: Relevance of the targeting function, Minireviews Med. Chem., 2016, 16, 743–753 CrossRef CAS PubMed.
- J. S. Burns and G. Manda, Metabolic pathways of the Warburg effect in health and disease: perspectives of choice, chain or chance, Int. J. Mol. Sci., 2017, 18, 2755 CrossRef PubMed.
- C. Wang, T. Zhao, Y. Li, G. Huang, M. A. White and J. Gao, Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes, Adv. Drug Delivery Rev., 2017, 113, 87–96 CrossRef CAS.
- A. Nigro, L. Frattaruolo, M. Fava, I. De Napoli, M. Greco, A. Comandè, M. De Santo, M. Pellegrino, E. Ricci, F. Giordano, I. Perrotta, A. Leggio, L. Pasqua, D. Sisci, A. R. Cappello and C. Morelli, Bortezomib-loaded mesoporous silica nanoparticles selectively alter metabolism and induce death in multiple myeloma cells, Cancers, 2020, 12, 2709–2728 CrossRef CAS.
- S. A. Bagshaw, E. Prouzet and T. J. Pinnavaia, Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactants, Science, 1995, 269, 1242–1244 CrossRef PubMed.
- M. Chen, J. Hu, L. Wang, Y. Li, C. Zhu, C. Chen, M. Shi, Z. Ju, X. Cao and Z. Zhang, Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy, Sci. Rep., 2020, 10, 1–12 CrossRef.
- A. Marchetti, J. Yin, Y. Su and X. Kong, Solid-state NMR in the field of drug delivery: State of the art and new perspectives, Magn. Reson. Lett., 2021, 1, 28–70 CrossRef CAS.
- G. Croissant, Y. Fatieiev, A. Almalik and N. M. Khashab, Mesoporous silica and organosilica nanoparticles: physical chemistry, biosafety, delivery strategies, and biomedical applications, Adv. Healthcare Mater., 2018, 7, 1700831 CrossRef.
- N. Feliu, D. Docter, M. Heine, P. Del Pino, S. Ashraf, J. Kolosnjaj-Tabi, P. Macchiarini, P. Nielsen, D. Alloyeau, F. Gazeau, R. H. Stauber and W. J. Parak, In vivo degeneration and the fate of inorganic nanoparticles, Chem. Soc. Rev., 2016, 45, 2440–2457 RSC.
- C. Meregalli, V. A. Carozzi, B. Sala, A. Chiorazzi, A. Canta, N. Oggioni, V. Rodriguez-Menendez, E. Ballarini, C. Ceresa, G. Nicolini, L. Crippa, M. Orciani, G. Cavaletti and P. Marmiroli, Bortezomib-induced peripheral neurotoxicity in human multiple myeloma-bearing mice, J. Biol. Regul. Homeostatic Agents, 2015, 29, 115–124 CAS.
- W. Cheng, J. Nie, L. Xu, C. Liang, Y. Peng, G. Liu, T. Wang, L. Mei, L. Huang and X. Zeng, pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy, ACS Appl. Mater. Interfaces, 2017, 9, 18462–18473 CrossRef CAS PubMed.
- B. Frigerio, C. Bizzoni, G. Jansen, C. P. Leamon, G. J. Peters, P. S. Low, L. H. Matherly and M. Figini, Folate receptors and transporters: biological role and diagnostic/therapeutic targets in cancer and other diseases, J. Exp. Clin. Cancer Res., 2019, 38, 125 CrossRef PubMed.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review, J. Controlled Release, 2000, 65, 271–274 CrossRef CAS.
- K. T. Mody, A. Popat, D. Mahony, A. S. Cavallaro, C. Yu and N. Mitter, Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery, Nanoscale, 2013, 5, 5167–5179 RSC.
- R. Spisek, A. Charalambous, A. Mazumder, D. H. Vesole, S. Jagannath and M. V. Dhodapkar, Bortezomib enhances dendritic cell (DC)–mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications, Am. J. Hematol., 2007, 109, 4839–4845 CAS.
- A. Serrano-del Valle, A. Anel, J. Naval and I. Marzo, Immunogenic cell death and immunotherapy of multiple myeloma, Front. Cell Dev. Biol., 2019, 7, 50 CrossRef PubMed.
- S. Nagata and M. Tanaka, Programmed cell death and the immune system, Nat. Rev. Immunol., 2017, 17, 333–340 CrossRef CAS PubMed.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. https://www.ncbi.nlm.nih.gov/books/NBK548027/?report=reader#_NBK548027_pubdet_.
- Y. N. Zhang, W. Poon, A. J. Tavares, I. D. McGilvray and W. C. Chan, Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination, J. Controlled Release, 2016, 240, 332 CrossRef CAS PubMed.
- N. P. Zaksas, S. E. Soboleva and G. A. Nevinsky, Twenty Element Concentrations in Human Organs Determined by Two-Jet Plasma Atomic Emission Spectrometry, Sci. World J., 2019, 2019, 9782635 Search PubMed.
- N. Garofalo, A. Comandé, I. Perrotta, M. Davoli, G. Niceforo and L. Pasqua, Synthesis and Characterization of Large Pore MSU-Type Mesoporous Silica, Adv. Sci. Lett., 2017, 23, 6026–6028 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01009g |
| ‡ Equally share the last authorship. |
| This journal is © the Partner Organisations 2023 |