A highly efficient and thermally stable broadband Cr3+-activated double borate phosphor for near-infrared light-emitting diodes†
Decai
Huang
ab,
Haomiao
Zhu
 *ab,
Zhonghua
Deng
a,
Hongyi
Yang
ab,
Jie
Hu
ab,
Sisi
Liang
ab,
Dejian
Chen
ab,
En
Ma
ab and
Wang
Guo
*ab,
Zhonghua
Deng
a,
Hongyi
Yang
ab,
Jie
Hu
ab,
Sisi
Liang
ab,
Dejian
Chen
ab,
En
Ma
ab and
Wang
Guo
 a
a
aCAS Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Key Laboratory of Nanomaterials Fujian Institute of Research on the Structure of Matter Chinese Academy of Sciences, Fuzhou, Fujian 350002, China
bXiamen Institute of Rare Earth Materials, Haixi Institute, Chinese Academy of Sciences, Xiamen, Fujian 361021, China
First published on 10th November 2020
Abstract
Highly efficient and thermally stable broadband near-infrared (NIR) phosphors are urgently required for phosphor-converted light-emitting diodes (pc-LEDs), which are compact, low cost and promising for applications such as food analysis and agricultural science. Herein, a NIR phosphor, GdAl3(BO3)4:Cr3+ (GAB:Cr3+), with high emission efficiency and excellent thermal stability is presented. Under blue light excitation, GAB:Cr3+ shows a broad emission band covering 650–1000 nm with a full width at half maximum of ∼140 nm. Particularly, the 1.0 at% Cr3+ doped sample exhibits a photoluminescence (PL) quantum yield as high as 91%, and no decline of the PL intensity is observed even when the temperature is raised from 298 to 423 K. By combining the GAB:Cr3+ phosphor with a commercial blue LED chip, a high-performance NIR pc-LED is fabricated with a NIR radiant flux of 81 mW under a drive current of 350 mA. Furthermore, an aging test was performed on this device at 85 °C and 85% humidity for 480 h, and the radiant flux retained up to 95% of its initial value, which is comparable to the commercial phosphors. These results suggest that GAB:Cr3+ is a promising candidate for broadband pc-LEDs in NIR spectroscopy applications.
1. Introduction
Near-infrared (NIR) spectroscopy, which utilizes electromagnetic radiation in the spectral range of 650–1400 nm as a real-time monitoring and non-destructive analytical tool, has been widely studied for its promising applications in remote sensing, biological recognition, in vivo bio-imaging, medicine, agricultural science, food processing, etc.1–5 Particularly, in recent years, the fast technical progress of optoelectronic components and detectors (Si, InGaAs or colloidal quantum dots) has made it possible to fabricate compact and low-cost NIR spectrometers, which enable the application of NIR spectroscopy in various commercial fields with huge markets.6–8 For instance, cell phones integrated with Si-based cameras are anticipated to be used to analyse the contents of water, sugar or pesticides in foodstuffs.9–11 There are two basic analytical principles for NIR spectroscopy. One is measuring the NIR photoluminescence (PL) emitted by the analytes under excitation. The other is based on the reflection, transmission or scattering of NIR-light that contains characteristic absorption information of the analytes. In the second case, a compact and highly efficient NIR light source with a broad emission band, which can cover the frequencies of the stretching vibrations of O–H, C–H and N–H bonds, is highly desired.12 Although the current available commercial light sources, such as the tungsten halogen lamp and Nichrome/Globar heaters, can generate broadband emission in the NIR spectral region, they suffer from a short service life, poor efficiency, high operating temperature and large size. Meanwhile, compact NIR diode chips usually exhibit relatively narrow emission bands, thus limiting their application in some fields such as food analysis. In contrast, NIR phosphor-converted LEDs (pc-LEDs), which comprise blue diodes and NIR phosphors, can offer remarkable advantages such as compactness, a long service life, high efficiency, customized spectral design and low-cost mass production. To achieve this goal, it is essential to explore new high-performance NIR phosphors that can be excited efficiently by blue light.Up to now, lanthanide and transition metal ion, typically Eu2+, Ni2+ and Cr3+, doped inorganic materials have been reported as potential candidates for broadband emissive NIR phosphors.13–16 Particularly, Cr3+-doped phosphors have attracted special attention because of their broad excitation bands in the blue or orange-red spectral regions, which match well with the emissions of low-cost commercial diode chips (especially blue diodes). The emission of Cr3+ ions with an octahedral coordination commonly originates from 2E → 4A2 or 4T2 → 4A2 transitions, depending on the crystal field strength of the host crystal.17–19 Generally, with decreasing crystal field strength, the emission of Cr3+ ions transforms gradually from sharp lines (the spin-forbidden 2E → 4A2 transition) to a broad band (the spin-allowed 4T2 → 4A2 transition). In other words, the emission can be tuned finely through choosing appropriate host materials for the Cr3+ ion. This unique spectral feature makes Cr3+ a promising active ion for generating broadband NIR emission.
As a commercial NIR phosphor, several rigorous requirements must be met, including a high internal PL QY (usually >90%), strong absorption in a specific light region (blue is preferred), small PL thermal quenching at high temperature and excellent chemical stability. Up to now, most of the reported Cr3+-activated broadband NIR phosphors have employed the garnet-type hosts X3M2A3O12 (X = La, Lu, Y, Gd; M = Zr, Hf, Ga; A = Al, Si, Ge),20–25 gallogermanate (La3Ga5GeO14 and ZnGa2O4),26–28 double-perovskite oxides (NaScSi2O6, LiInSi2O6, La2MgTiO6 and La2MgZrO6),29–35 all inorganic halide perovskites Cs2AgInCl6,36 fluoride A3MF6 (A = K, Na; M = Al, Ga), etc.37 They usually demonstrate broad emission bands in the spectral range of 650–1400 nm. Unfortunately, most of these NIR phosphors exhibit relatively low internal PL QYs (<90%) and poor thermal quenching properties (30–90% of the initial PL intensities can be sustained at 150 °C), thus hindering their further commercial application.38,39 Therefore, exploring novel NIR phosphors with extremely high PL QYs and excellent PL thermal quenching properties is a great challenge.
Double borates with a general formula of RAl3(BO3)4 (where R = Rare earth), which are isostructural to the mineral huntite CaMg3(CO3)4, were firstly reported by Ballman in 1962.40,41 As a member of the double borate family, GdAl3(BO3)4 crystal and its lanthanide doped counterparts have proved to be promising non-linear optical and laser crystals due to their high non-linear optical coefficients, high mechanical strength, excellent chemical and thermal stability, etc.42–46 B. Malysa et al. reported the temperature dependent properties of Cr3+-doped Gd/YAl3(BO3)4 materials.47 However, an in-depth investigation of the energy level structure, decay behaviour, device performance and application of Cr3+-activated GdAl3(BO3)4 as a NIR phosphor is still lacking. Herein, we present the synthesis, optical properties and device performance of a broadband NIR phosphor with the formula of GdAl3(BO3)4:Cr3+ (abbreviated as GAB:Cr3+ hereafter), which exhibits an extremely high internal PL QY of up to 91% and excellent luminescence thermal stability (PL intensity at 423 K retains ∼100% of the initial intensity at room temperature, RT). By employing the efficient GAB:Cr3+ as a NIR phosphor, a high-power NIR pc-LED with a radiant flux of 81 mW under a drive current of 350 mA has been produced. These results prove that GAB:Cr3+ is a promising NIR phosphor for commercial applications.
2. Experimental
2.1. Materials and preparation
Raw materials of Gd2O3 (99.99%), Al2O3 (99.99%), Cr2O3 (99.95%) and H3BO3 (AR, >99.5%) were used to prepare the GAB:Cr3+ phosphor. The samples were synthesized by the traditional solid-state reaction method. Stoichiometric amounts of the raw materials were weighed according to the formula of GdAl3(BO3)4:Cr3+, except a 10 wt% excess of H3BO3 was added to compensate for the volatilization of B2O3 during calcining. The chemicals were mixed thoroughly by grinding in an agate mortar and then put into a corundum crucible and preheated at 500 °C for 2 h in a muffle furnace. After being reground, the powder mixtures were sintered again at 1100 °C for 6 h. Finally, the products were cooled down to RT in the furnace.2.2. Characterization
XRD patterns of the samples were recorded by an X-ray diffractometer (Miniflag 600, Rigaku) at an interval of 0.02° with a scanning speed of 7° min−1 and Cu Kα1 radiation (λ = 0.154187 nm). Rietveld refinement was performed in the range of 5–110° by the GSAS program based on XRD patterns collected using an X-ray diffractometer (X'Pert Pro, Panalytical B.V., Cu Kα, 40 kV, 40 mA) with a count time of 15 s per step and a step size of 0.0163°. The morphologies of the samples were analyzed using field-emission scanning electron microscopy (Thermo Scientific, Apreo S) equipped with an energy dispersive spectroscopy (EDS) analyzer. Diffuse reflection spectra were recorded by a UV-vis-NIR spectrophotometer (Agilent, Cary 5000) using BaSO4 as the reference. The excitation and emission spectra, and PL decay curves were measured using a spectrometer (FLS 980, Edinburgh Instruments) equipped with both continuous (450 W) and microsecond pulsed xenon (Xe) lamps. For the temperature dependence experiments at 8–298 K, the samples were mounted on an optical cryostat (8–350 K, DE202, Advanced Research Systems). To investigate the PL thermal stability at 298–573 K, the samples were placed on a thermal stage (77–873 K, THMS 600, Linkam Scientific Instruments) and a fiber coupled spectrometer (QE 65000, Ocean Optics) was used to record the emission spectra of the sample under excitation by 426 nm light. For PL QY measurement, the samples were put inside an optical integrating sphere coupled to the FLS 980 spectrometer. A standard tungsten halogen lamp was used to calibrate the optical response of the instrument. The EPR measurement was carried out on an EPR spectrometer (Bruker-BioSpin, E 500) with an operating range of the X-band frequencies at RT.3. Results and discussion
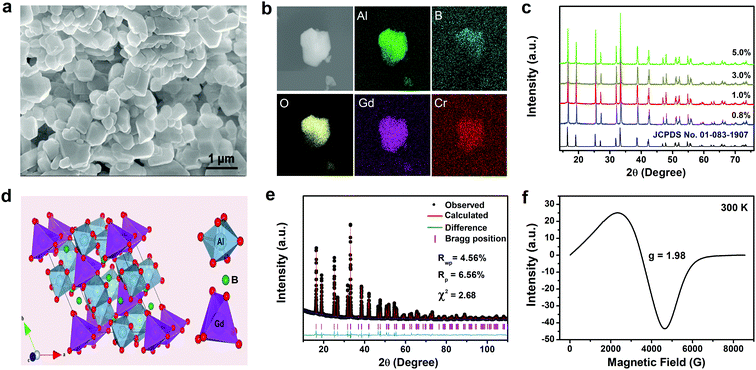
GAB:Cr3+ phosphors with Cr3+ doping concentrations of 0.8–5.0 at% were synthesized by the high-temperature solid-state reaction method. All of the samples exhibit a light cyan tint under natural light illumination. From the SEM image of the 1.0 at% sample, we can see that the phosphor particles exhibit a hexagonal shape with clear edges and corners and a diameter of about 1 μm (Fig. 1a). The elemental mapping results show a uniform distribution of the Gd, Al, B, O and Cr elements (Fig. 1b). EDS analysis gave a molar ratio of Gd, Al, B and O of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, which is consistent with the molecular formula of GAB (Fig. S1 in the ESI†). The powder X-ray diffraction (XRD) patterns of the samples together with that of the pure GAB crystal are compared in Fig. 1c. All the diffraction peaks of the GAB:Cr3+ samples can be indexed to the R32 space group of the trigonal GAB phase (JCPDS No. 01-083-1907) and no other impurity phases were identified. Fig. 1d displays the crystal structure of GAB, in which both Gd3+ and Al3+ ions are surrounded by six oxygen ions. The coordination polyhedron around the Gd3+ ion approximates to a trigonal prism with a local site symmetry of D3, while that around the Al3+ ion is a distorted octahedron with a local site symmetry of C2.
12, which is consistent with the molecular formula of GAB (Fig. S1 in the ESI†). The powder X-ray diffraction (XRD) patterns of the samples together with that of the pure GAB crystal are compared in Fig. 1c. All the diffraction peaks of the GAB:Cr3+ samples can be indexed to the R32 space group of the trigonal GAB phase (JCPDS No. 01-083-1907) and no other impurity phases were identified. Fig. 1d displays the crystal structure of GAB, in which both Gd3+ and Al3+ ions are surrounded by six oxygen ions. The coordination polyhedron around the Gd3+ ion approximates to a trigonal prism with a local site symmetry of D3, while that around the Al3+ ion is a distorted octahedron with a local site symmetry of C2.
As mentioned above, the local coordination environment has a strong influence on the spectral features of Cr3+ ions. When Cr3+ ions are doped into the GAB host, they are most likely to occupy the Al3+ site because of the same valence and similar ionic radius of the Cr3+ (0.62 Å, coordination number = 6) and Al3+ (0.535 Å, coordination number = 6) ions. To verify this, Rietveld structural refinement on the diffraction pattern of the GAB:Cr3+ (1.0 at%) sample was carried out using the GSAS program (Fig. 1e). The refinement result is well convergent, with low residual factors of Rp = 6.56%, Rwp = 4.56% and χ2 = 2.68, when the Cr3+ ions are assumed to occupy the crystallographic site of Al3+ ions. The cell parameters of the GAB:Cr3+ (1.0 at%) sample are slightly larger than that of the pure GAB crystal due to the slightly larger ionic radius of Cr3+ than Al3+ (Table S1 in ESI†). In contrast, when Cr3+ ions are assumed to locate at the Gd3+ site for Rietveld refinement, higher residual factors of Rp = 8.26%, Rwp = 11.87% and χ2 = 8.68 were obtained, which means less reliability of the fitting result. In addition, we carried out electron paramagnetic resonance (EPR) measurement, which is a powerful technique to obtain valuable information about the ground state of paramagnetic ions and their interaction with the surrounding ions, on the GAB:Cr3+ crystal at 298 K (Fig. 1f). As we can see, there is only one wide EPR line with a resonance signal at g = 1.98, which is assigned to the Cr3+–Cr3+ exchange coupled pairs. It is known that the spin–spin interaction between distant Cr3+ neighbors and inhomogeneity of the crystal field can lead to broadening of the EPR lines. If the Cr3+ ion (S = 3/2) is located at a site with high symmetry, only one narrow EPR line should be observed.48 Hence, the wide EPR line in Fig. 1f should originate from Cr3+ ions situated at the Al3+ site of a distorted [AlO6] octahedron rather than the Gd3+ site of [GdO6]. In summary, all of these results confirm that Cr3+ ions locate at the crystallographic site of the Al3+ ions.
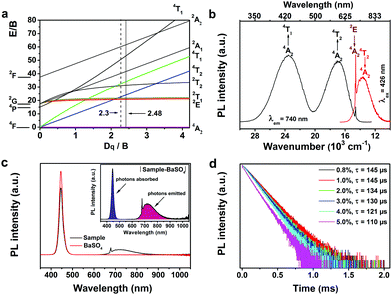
The energy levels and spectral features of Cr3+ ions in an octahedral crystal field can be well illustrated by the Tanabe–Sugano (Fig. 2a) and configurational coordinate diagrams (Fig. S2 in ESI†). They clearly show that the energy state positions depend on the ratio of crystal field strength Dq and Racah parameter B. Especially, there is a crossover point between the 2E and 4T2 states with the value of Dq/B ≈ 2.3. When the value of Dq/B is less than 2.3, the 4T2 state is the lowest excited state, whereas for larger values of Dq/B, the lowest excited state is 2E. Therefore, the observed emission spectra of the Cr3+ ions could be attributed to the spin-forbidden 2E → 4A2 transition with sharp lines, the spin-allowed broadband 4T2 → 4A2 transition or a superposition of both transitions, depending on the crystal field strength of the host crystal. Fig. 2b displays the PL and PL excitation spectra of the synthesized GAB:Cr3+ phosphor. Obviously, the sharp line emission peaks at 680 and 685 nm are due to the 2E → 4A2 transition, while the broad emission band centered at about 740 nm (FWHM of ∼140 nm) originates from the 4T2 → 4A2 transition. The coexistence of these two transitions suggests that the 4T2 state locates close to the 2E state. The PL excitation spectrum is composed of two broad excitation bands, which are centered at 426 nm (4A2 → 4T1) and 598 nm (4A2 → 4T2), respectively. Additionally, a sharp and weak excitation band with its peak at 685 nm, which is ascribed to the transition from the 4A2 ground state to the 2E excited state, can be observed at the long-wavelength shoulder of the 598 nm excitation band. It is worth mentioning that the 426 nm band is suitable for commercial blue diode chip excitation. The crystal field strength parameter Dq and two Racah parameters B and C can be calculated from the energies of 4T1, 4T2 and 2E, which were estimated to be 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470, 16
470, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730, and 14
730, and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 598 cm−1 from the excitation and emission bands, respectively. Then, the values of B, C and Dq/B were calculated to be 675 cm−1, 3197 cm−1 and 2.48, respectively (see the Discussion, ESI†). The value of Dq/B in the GAB host is slightly larger than that of the crossover point (Dq/B ≈ 2.3), indicating that the 2E state locates lower than the 4T2 state in energy (Fig. S2, ESI†), the 4T2 state is thermally populated at RT, and emissions will be observed from both 4T2 and 2E states.
598 cm−1 from the excitation and emission bands, respectively. Then, the values of B, C and Dq/B were calculated to be 675 cm−1, 3197 cm−1 and 2.48, respectively (see the Discussion, ESI†). The value of Dq/B in the GAB host is slightly larger than that of the crossover point (Dq/B ≈ 2.3), indicating that the 2E state locates lower than the 4T2 state in energy (Fig. S2, ESI†), the 4T2 state is thermally populated at RT, and emissions will be observed from both 4T2 and 2E states.
The PL QY is an important parameter to characterize the emission efficiency of phosphors. For commercial phosphors, the internal PL QYs are usually larger than 90%. Unfortunately, the PL QY of most reported NIR phosphors cannot meet this standard. We measured the PL QYs of the synthesized GAB:Cr3+ phosphors with different Cr3+ doping concentrations using an integrating sphere at RT (Fig. 2c and Table 1). The 1.0 at% Cr3+-doped sample shows an extremely high internal PL QY of 91%, which has never been obtained for Cr3+-activated double borates and is comparable to that of previous reported broadband NIR phosphors (Table S2, ESI†). Nevertheless, the external PL QY and absorption efficiency of the phosphor should be improved in future for commercial application. With increasing Cr3+ doping concentration (Fig. S3a (ESI†) and Table 1), the absorption efficiency increases continuously but the internal PL QY decreases gradually due to the concentration quenching effect. The PL intensities of the samples also exhibit the same tendency (Fig. S3b and c in ESI†). Furthermore, PL dynamic measurement at RT by monitoring the emission at 685 nm showed that the PL lifetime decreased from 144 to 110 μs with increasing Cr3+ concentration, owing to the increased non-radiative transition probability for the samples with higher Cr3+ doping concentrations. It should be noted that the monitored emission at 685 nm is contributed by both 2E and 4T2 states, which are thermally coupled, and the measured PL lifetimes are nearly the same for both transitions.
| Cr3+ (at%) | 0.6 | 0.8 | 1.0 | 1.2 | 1.4 | 2.0 | 3.0 | 4.0 | 5.0 |
|---|---|---|---|---|---|---|---|---|---|
| IQY (%) | 88 | 89 | 91 | 87 | 87 | 80 | 74 | 65 | 58 |
| EQY (%) | 17 | 20 | 23 | 23 | 24 | 26 | 28 | 26 | 25 |
| Abs. (%) | 19 | 22 | 25 | 26 | 28 | 33 | 38 | 40 | 43 |
| Lifetime (μs) | 145 | 145 | 145 | 144 | 143 | 134 | 130 | 121 | 110 |
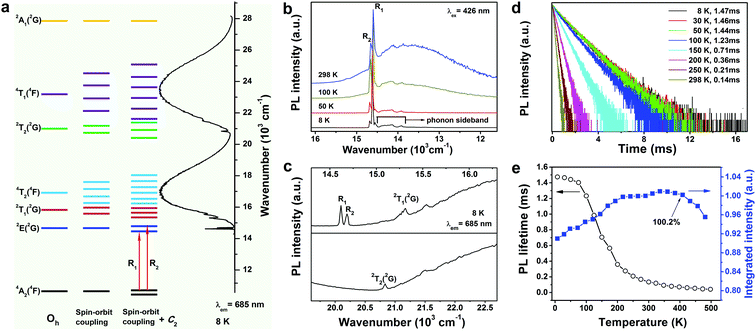
To further reveal the electronic structure of the Cr3+ ions in the GAB host, we measured the PL spectra of the 1.0 at% sample in the temperature range of 8–298 K. Particularly, measuring the spectra at the extremely low temperature of 8 K can minimize thermal broadening of the spectra, which is helpful for the precise assignment of energy levels. As we discussed above, the Cr3+ ions occupy the Al3+ site with a local symmetry of C2 in the GAB host. Thus, the energy splitting of Cr3+ ions in GAB can be deduced, as shown in Fig. 3a. We can find that both 2E and 4A2 states split into two energy levels, which indicates that a maximum of four sharp zero-phonon emission lines (ZPLs) could be observed for the 2E → 4A2 transition. Generally, the emissions from the lower and upper sublevels of 2E are denoted as ZPLs of R1 and R2, respectively. Nevertheless, as shown in Fig. 3b, only two sharp lines at around 680 and 685 nm were detected in the temperature range of 8–298 K. The most likely reason for this is that the splitting of the ground 4A2 state is too small and beyond the spectral resolution of the spectrometer. For instance, the observed energy level splitting of 4A2 in ruby is as small as 0.39 cm−1.49 With decreasing temperature, the ∼680 nm emission peak decreased gradually in intensity due to thermal de-population from the upper sublevel of the 2E state. Hence, these two sharp emission lines at 680 and 685 nm can be unquestionably assigned as the R1 and R2 ZPLs of the 2E → 4A2 transition, respectively. Meanwhile, the broad emission band centered at 740 nm (4T2 → 4A2) also decreased gradually in intensity with decreasing temperature. This experimental observation is in good agreement with the above prediction made based on the calculated value of Dq/B, i.e., that the 4T2 state locates at a slightly higher energy position than 2E, thus thermal de-population from the 4T2 state occurred at lower temperature. In the PL spectrum recorded at 8 K, besides the ZPLs, there are several relatively weak emission lines distributed in the spectral range of 687–720 nm, which are phonon-coupled vibronic transitions (that is, phonon sidebands) of 2E → 4A2. We further measured the PL excitation spectrum of the 1.0 at% sample at 8 K (Fig. 3a and c) and it is composed of two broad excitation bands (4A2 → 4T2, 4T1) and several sharp excitation lines located at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619 (ZPL R1), 14
619 (ZPL R1), 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684 (ZPL R2), 15
684 (ZPL R2), 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256, 15
256, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280, 15
280, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315, and 20
315, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 cm−1, respectively. The 15
833 cm−1, respectively. The 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256, 15
256, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280, and 15
280, and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 cm−1 peaks can be assigned to the 4A2 → 2T1 transition while the 20
315 cm−1 peaks can be assigned to the 4A2 → 2T1 transition while the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 cm−1 peak was ascribed to the 4A2 → 2T2 transition. All these spectral data are in accordance with the previous conclusion that Cr3+ ions are situated at the crystallographic site of Al3+ ions with C2 local symmetry.
833 cm−1 peak was ascribed to the 4A2 → 2T2 transition. All these spectral data are in accordance with the previous conclusion that Cr3+ ions are situated at the crystallographic site of Al3+ ions with C2 local symmetry.
The temperature dependent PL dynamics are pivotal for understanding the luminescence mechanism of Cr3+ ions in the GAB host. In theory, the excited electrons are dominantly populated in the 2E state at extremely low temperature, thus the measured PL decay curve mainly demonstrates the decay dynamics of the 2E → 4A2 transition, which has a small radiative transition probability due to its spin-forbidden nature. With increasing temperature, the 4T2 state is increasingly thermally populated and the measured PL decay curve represents the decay process for the combination of 2E → 4A2 and 4T2 → 4A2 transitions. Therefore, the measured PL lifetime should decay faster with increasing temperature since the 4T2 → 4A2 transition is spin-allowed with a much larger radiative transition probability. Fig. 3d displays the PL decay curves of the 1.0 at% sample measured at 8–298 K by monitoring the emission at 685 nm. All the curves exhibit a single exponential decay behavior and the fitted PL lifetime decreases dramatically from 1.47 to 0.14 ms when the temperature increases from 8 to 298 K, which is in accordance with the theoretical prediction. Note that the PL lifetime decreases significantly faster when the temperature increases over 100 K, which indicates that the 4T2 → 4A2 transition dominates the emission above 100 K.
The operating temperature of a NIR pc-LED is usually much higher than RT, hence it is important to investigate the PL thermal stability of the GAB:Cr3+ phosphor at higher temperatures. We further measured the temperature dependent PL spectra of the 1.0 at% sample at 298–498 K (see Fig. S3d in the ESI†), and the plot of integrated PL intensity vs. temperature is shown in Fig. 3e together with those data for 8–298 K. Generally, the remaining PL intensity at 423 K with respect to that at RT is used to evaluate the PL thermal stability of phosphors. This value for the 1.0 at% sample is about 100%, which proves the outstanding PL thermal stability of the GAB:Cr3+ phosphor (Table S2 in ESI†). This phenomenon is frequently observed in phosphors activated by Mn4+ or Cr3+ ions, which have the same 3d3 electronic configuration. Two factors are mainly responsible for the observed increase of PL intensity. Firstly, the transition probability of the vibronic absorption of 4A2 → 4T2 is temperature-dependent and proportional to coth(ħω/2κT), where ħω is the energy of the coupled vibronic mode and κ is the Boltzmann constant.49 This means that the absorption of the excitation light will increase with increasing temperature.50 Secondly, as discussed above, the population of the 4T2 state will increase at higher temperature, and the radiative transition probability of the spin-allowed 4T2 → 4A2 transition is much larger than that of the spin-forbidden 2E → 4A2 transition. Therefore, the total radiative transition probability will increase with increasing temperature. These two combined effects will lead to the increase of PL intensity in a certain temperature range in which the nonradiative transition probability is non-significant. Nevertheless, when the temperature is further increased, the nonradiative transition probability will increase drastically and result in a decrease of PL intensity, as observed in the GAB:Cr3+ sample when the temperature is higher than 348 K. The activation energy for the thermal quenching process can be calculated according to the single-barrier model:51
IT/I0 = [1 + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) exp(−ΔEq/κT)]−1 exp(−ΔEq/κT)]−1 | (1) |
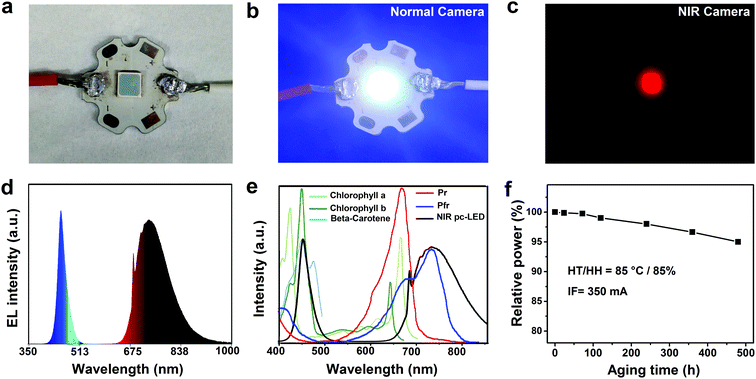
In order to evaluate the device performance of the synthesized phosphors, a NIR pc-LED was fabricated using the GAB:Cr3+ (1.0 at%) phosphor combined with a commercial blue InGaN chip (Fig. 4a–c). The electroluminescence (EL) spectrum of the NIR pc-LED is composed of a blue emission band centered at 450 nm (blue diode) and a NIR emission band at 650–1000 nm originating from the GAB:Cr3+ phosphor (Fig. 4d). The radiant flux of the pc-LED device in the spectral range of 650–1000 nm reached 81 mW under a drive current of 350 mA, which is among the highest reported to date.39,54–56 It is worth noting that the EL spectrum overlaps well with the absorption spectra of common plant pigments (Fig. 4e), including chlorophyll b and beta-carotene (450 nm), and phytochrome PFR (730 nm), suggesting that this NIR pc-LED can be used as an efficient plant lighting device when combining GAB:Cr3+ with a Mn4+ activated phosphor (emission peak at 660 nm, such as Mg4GeO6:Mn4+). It is well-known that LED brightness will decrease during long term operation, especially in a high temperature and high humidity environment such as an indoor greenhouse. However, few studies have investigated the moisture-resistant performance of NIR phosphors. To assess this, we carried out an aging test on the fabricated NIR pc-LED at 85 °C and 85% humidity. The radiant flux of this LED retained 95% (77 mW) of its initial value after aging for 480 h, which is comparable to that of commercial phosphors (such as YAG:Ce3+, Eu2+-doped nitride and Mn4+-doped fluoride),57,58 demonstrating the good stability of the GAB:Cr3+ phosphor.
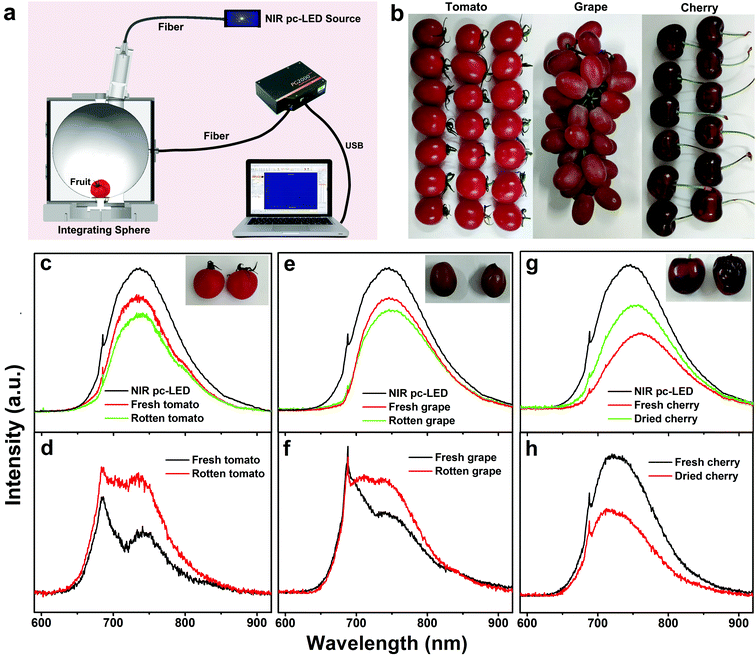
To demonstrate the application of the broadband NIR pc-LED in food analysis, we tried to detect the freshness of different kinds of fruit by measuring their NIR absorption. The fruit samples were put inside an integrating sphere equipped with the fabricated NIR pc-LED, and a fiber coupled spectrometer was used to record the reflected NIR spectra (Fig. 5a). Three kinds of fruit, tomato, grape and cherry, were used for the experiment (Fig. 5b). For tomato and grape, both fresh and rotten samples were chosen for the measurements. Meanwhile, for the cherry sample, a cherry was dried at 70 °C for 1 h in an oven to evaporate part of its water content for comparison. For each kind of fruit, the reflection spectra were recorded with and without the fruit in the integrating sphere, respectively (Fig. 5c, e and g). As we can see, the intensity of the reflection spectra decreased when the fruit was put inside the integrating sphere due to the absorption of the fruit in the NIR region, which is attributed to the third (730 nm) overtones of O–H stretching in water, the fourth (714 nm) overtones of arene stretching in soluble solids, and the third (910 nm) and fourth (746 nm) overtones of C–H stretching in soluble sugar (sucrose, glucose, and fructose).10,59 Thus, the number of absorbed NIR photons by the fruit can be obtained by subtracting the spectra recorded without the fruit from those recorded with the fruit. We found that the rotten tomato and grape exhibit stronger absorption because the decomposition of the fiber and saccharides results in more alcohol and water in the rotten fruit (Fig. 5d and f).60 By contrast, the absorption of the dried cherry is weaker than that of the fresh one since a large amount of water in the pulp has been evaporated during the baking process (Fig. 5h). Moreover, the characteristic absorptions of these three fruits are distinct, thus making it possible to identify them using the NIR spectral technique. All of the results indicate that broadband NIR spectroscopy is a promising technology in nondestructive food analysis.
4. Conclusions
In this work, we presented a highly efficient and thermally stable phosphor, GAB:Cr3+, with a broad NIR emission band (FWHM of ∼140 nm) in the spectral range of 650–1000 nm. Structural and spectral studies revealed that Cr3+ ions occupy the octahedrally coordinated Al3+ sites with a local symmetry of C2. The phosphor shows two broad absorption bands centered at 426 and 598 nm, which are perfect for commercial blue or red diode chip excitation. Most importantly, the GAB:Cr3+ (1.0 at%) phosphor exhibits an extremely high PL QY of up to 91%, which is superior to most other NIR phosphors. Furthermore, the sample shows excellent PL thermal stability, i.e., the PL intensity at 423 K is about 100% of the PL intensity at RT. A NIR pc-LED was fabricated by combining the synthesized GAB:Cr3+ phosphor with a commercial blue diode chip, which generated a NIR radiant flux as high as 81 mW under a forward current of 350 mA. Particularly, the radiant flux of this LED remained at 95% of its initial value after being aged at 85 °C and 85% humidity for 480 h. These results show the great promise of GAB:Cr3+ as a commercial phosphor for NIR pc-LEDs in NIR spectroscopy applications such as plant lighting and food analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Priority Research Platform Project of Xiamen (No. 3502ZCQ20171002), Xiamen Municipal Bureau of Science and Technology (No. 3502Z20182020), and the Natural Science Foundation of China (No. 11904363).References
- A. Guelpa, F. Marini, A. du Plessis, R. Slabbert and M. Manley, Food Control, 2017, 73, 1388–1396 CrossRef CAS.
- M. Ferrari and V. Quaresima, NeuroImage, 2012, 63, 921–935 CrossRef.
- G. N. A. De Guzman, M. H. Fang, C. H. Liang, Z. Bao, S. F. Hu and R. S. Liu, J. Lumin., 2020, 219, 116944 CrossRef CAS.
- Y. Gu, Z. Guo, W. Yuan, M. Kong, Y. Liu, Y. Liu, Y. Gao, W. Feng, F. Wang and J. Zhou, Nat. Photonics, 2019, 13, 525–531 CrossRef CAS.
- C. Dincer, R. Bruch, E. Costa-Rama, M. T. Fernandez-Abedul, A. Merkoci, A. Manz, G. A. Urban and F. Guder, Adv. Mater., 2019, 31, e1806739 CrossRef.
- R. Filippo, E. Taralli and M. Rajteri, Sensors, 2017, 17, 1672–1685 CrossRef.
- Z. Pan, Y. Lu and F. Liu, Nat. Mater., 2012, 11, 58–63 CrossRef CAS.
- S. Kawano, T. Fujiwara and M. Iwamoto, J. Jpn. Soc. Hortic. Sci., 1993, 62, 465–470 CrossRef CAS.
- L. Pan, R. Lu, Q. Zhu, J. M. McGrath and K. Tu, Postharvest Biol. Technol., 2015, 102, 42–50 CrossRef CAS.
- M. Halper, The future of quality control, https://www.osram.com/os/press/press-releases/oslon-black-sfh-4736-new-broadband-infrared-led-helps-famers-plan-the-best-time-for-harvest.jsp, 2018, accessed 12 December 2018.
- B. M. Nicolaï, K. Beullens, E. Bobelyn, A. Peirs, W. Saeys, K. I. Theron and J. Lammertyn, Postharvest Biol. Technol., 2007, 46, 99–118 CrossRef.
- J. Qiao, G. Zhou, Y. Zhou, Q. Zhang and Z. Xia, Nat. Commun., 2019, 10, 5267 CrossRef.
- Z. Gao, Y. Liu, J. Ren, Z. Fang, X. Lu, E. Lewis, G. Farrell, J. Yang and P. Wang, Sci. Rep., 2017, 7, 1783 CrossRef.
- G. Bai, M. K. Tsang and J. Hao, Adv. Funct. Mater., 2016, 26, 6330–6350 CrossRef CAS.
- Z. Tang, Q. Zhang, Y. Cao, Y. Li and Y. Wang, Chem. Eng. J., 2020, 388, 124231 CrossRef CAS.
- M. H. Fang, G. N. A. De Guzman, Z. Bao, N. Majewska, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, C. W. Yang, K. M. Lu, H. S. Sheu, S. F. Hu and R. S. Liu, J. Mater. Chem. C, 2020, 8, 11013–11017 RSC.
- S. Adachi, ECS J. Solid State Sci. Technol., 2019, 8, R164–R168 CrossRef CAS.
- S. Liu, Z. Wang, H. Cai, Z. Song and Q. Liu, Inorg. Chem. Front., 2020, 7, 1467–1473 RSC.
- M. Mao, T. Zhou, H. Zeng, L. Wang, F. Huang, X. Tang and R. Xie, J. Mater. Chem. C, 2020, 8, 1981–1988 RSC.
- L. Zhang, D. Wang, Z. Hao, X. Zhang, G. H. Pan, H. Wu and J. Zhang, Adv. Opt. Mater., 2019, 7, 1900185 CrossRef.
- B. Malysa, A. Meijerink and T. Jüstel, J. Lumin., 2018, 202, 523–531 CrossRef CAS.
- H. Lin, G. Bai, T. Yu, M.-K. Tsang, Q. Zhang and J. Hao, Adv. Opt. Mater., 2017, 5, 1700227 CrossRef.
- L. Yao, Q. Shao, X. Xu, Y. Dong, C. Liang, J. He and J. Jiang, Ceram. Int., 2019, 45, 14249–14255 CrossRef CAS.
- C. Liu, Z. Xia, M. S. Molokeev, Q. Liu and H. Guo, J. Am. Ceram. Soc., 2015, 98, 1870–1876 CrossRef CAS.
- Z. Jia, C. Yuan, Y. Liu, X. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Light: Sci. Appl., 2020, 9, 86–95 CrossRef CAS.
- V. Rajendran, M. H. Fang, G. N. D. Guzman, T. Lesniewski, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, Y. S. Lin, K. M. Lu, C.-M. Lin, H. Chang, S. F. Hu and R. S. Liu, ACS Energy Lett., 2018, 3, 2679–2684 CrossRef CAS.
- T. Gao, W. Zhuang, R. Liu, Y. Liu, C. Yan, J. Tian, G. Chen, X. Chen, Y. Zheng and L. Wang, J. Am. Ceram. Soc., 2020, 103, 202–213 CrossRef CAS.
- W. Huang, C. Cheng, Z. Bao, C. Yang, K. Lu, C. Kang, C. Lin and R. S. Liu, Angew. Chem., Int. Ed., 2019, 58, 2069–2072 CrossRef CAS.
- D. Xu, X. Wu, Q. Zhang, W. Li, T. Wang, L. Cao and J. Meng, J. Alloys Compd., 2018, 731, 156–161 CrossRef CAS.
- W. Li, T. Chen, W. Xia, X. Yang and S. Xiao, J. Lumin., 2018, 194, 547–550 CrossRef CAS.
- K. L. A. R. V. Deun, J. Mater. Chem. C, 2018, 6, 7302 RSC.
- X. Xu, Q. Shao, L. Yao, Y. Dong and J. Jiang, Chem. Eng. J., 2020, 383, 123108 CrossRef CAS.
- Q. Shao, H. Ding, L. Yao, J. Xu, C. Liang, Z. Li, Y. Dong and J. Jiang, Opt. Lett., 2018, 43, 5251 CrossRef CAS.
- H. Zeng, T. Zhou, L. Wang and R. J. Xie, Chem. Mater., 2019, 31, 5245–5253 CrossRef CAS.
- D. Yu, Y. Zhou, C. Ma, J. H. Melman, K. M. Baroudi, M. LaCapra and R. E. Riman, ACS Appl. Electron. Mater., 2019, 1, 2325–2333 CrossRef CAS.
- F. Zhao, Z. Song, J. Zhao and Q. Liu, Inorg. Chem. Front., 2019, 6, 3621–3628 RSC.
- L. Zhang, J. Zhang, Z. Hao, H. Wu, G. Pan, H. Wu and X. Zhang, Chin. J. Lumin., 2019, 40, 1449–1459 CrossRef.
- M. H. Fang, P. Y. Huang, Z. Bao, N. Majewska, T. Leśniewski, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, C. W. Yang, K. M. Lu, H. S. Sheu and R. S. Liu, Chem. Mater., 2020, 32, 2166–2171 CrossRef CAS.
- G. Liu, M. S. Molokeev, B. Lei and Z. Xia, J. Mater. Chem. C, 2020, 8, 9322–9328 RSC.
- A. A. Ballman, Am. Mineral., 1962, 47, 1380–1383 CAS.
- G. Kuz’micheva, I. Kaurova, V. Rybakov and V. Podbel'skiy, Crystals, 2019, 9, 100 CrossRef.
- J. He, S. Zhang, J. Zhou, J. Zhong, H. Liang, S. Sun, Y. Huang and Y. Tao, Opt. Mater., 2015, 39, 81–85 CrossRef CAS.
- Y. W. Xiaoxia Li, J. Lumin., 2007, 122–123, 1000–1002 Search PubMed.
- K. N. Gorbachenya, V. E. Kisel, A. S. Yasukevich, V. V. Maltsev, N. I. Leonyuk and N. V. Kuleshov, Opt. Lett., 2013, 38, 2446 CrossRef CAS.
- G. Wang, T. P. J. Han, H. G. Gallagher and B. Henderson, Appl. Phys. Lett., 1995, 67, 3906–3908 CrossRef.
- G. F. Wang, Structure, Growth, Nonlinear Optics, and Laser Properties of RX3(BO3)4 (R = Y, Gd, La; X = AlSc), Springer, Berlin, Heidelberg, 2012, vol. 144, pp. 105–119 Search PubMed.
- B. Malysa, A. Meijerink and T. Jüstel, J. Lumin., 2016, 171, 246–253 CrossRef CAS.
- A. D. Prokhorov, E. E. Zubov, A. A. Prokhorov, L. F. Chernush, R. Minyakaev, V. P. Dyakonov and H. Szymczak, Phys. Status Solidi B, 2013, 250, 1331–1338 CrossRef CAS.
- B. Henderson and G. F. Imbusch, Optical spectroscopy of inorganic solids, Oxford University Press, 2006 Search PubMed.
- H. Zhu, C. C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R. S. Liu and X. Chen, Nat. Commun., 2014, 5, 4312 CrossRef CAS.
- M. Back, E. Trave, J. Ueda and S. Tanabe, Chem. Mater., 2016, 28, 8347–8356 CrossRef CAS.
- S. S. Wang, W. T. Chen, Y. Li, J. Wang, H. S. Sheu and R. S. Liu, J. Am. Chem. Soc., 2013, 135, 12504–12507 CrossRef CAS.
- L. Zhang, J. Zhang, X. Zhang, Z. Hao, H. Zhao and Y. Luo, ACS Appl. Mater. Interfaces, 2013, 5, 12839–12846 CrossRef CAS.
- L. Yao, Q. Shao, S. Han, C. Liang, J. He and J. Jiang, Chem. Mater., 2020, 32, 2430–2439 CrossRef CAS.
- X. Zhou, W. Geng, J. Li, Y. Wang, J. Ding and Y. Wang, Adv. Opt. Mater., 2020, 8, 1902003 CrossRef CAS.
- E. T. Basore, W. Xiao, X. Liu, J. Wu and J. Qiu, Adv. Opt. Mater., 2020, 8, 2000296 CrossRef CAS.
- Y. T. Tsai, H. D. Nguyen, A. Lazarowska, S. Mahlik, M. Grinberg and R. S. Liu, Angew. Chem., Int. Ed., 2016, 55, 9652–9656 CrossRef CAS.
- D. Huang, H. Zhu, Z. Deng, Q. Zou, H. Lu, X. Yi, W. Guo, C. Lu and X. Chen, Angew. Chem., Int. Ed., 2019, 58, 3843–3847 CrossRef CAS.
- E. J. N. Marques, S. T. de Freitas, M. F. Pimentel and C. Pasquini, Food Chem., 2016, 197, 1207–1214 CrossRef CAS.
- C. J. Clark, V. A. McGlone and R. B. Jordan, Postharvest Biol. Technol., 2003, 28, 87–96 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tc04803h |
| This journal is © The Royal Society of Chemistry 2021 |