High-efficiency blue photoluminescence in the Cs2NaInCl6:Sb3+ double perovskite phosphor†
Matthew B.
Gray
 a,
Shruti
Hariyani
a,
Shruti
Hariyani
 b,
T. Amanda
Strom
c,
Jackson D.
Majher
b,
T. Amanda
Strom
c,
Jackson D.
Majher
 a,
Jakoah
Brgoch
a,
Jakoah
Brgoch
 b and
Patrick M.
Woodward
b and
Patrick M.
Woodward
 *a
*a
aDepartment of Chemistry and Biochemistry, The Ohio State University, 100 W. 18th Avenue, Columbus, Ohio 43210, USA. E-mail: woodward.55@osu.edu
bDepartment of Chemistry, University of Houston, 3585 Cullen Boulevard, Houston, Texas 77204, USA
cDepartment of Materials Science, UC Santa Barbara, 2066C Materials Research Lab, Santa Barbara, California 93106, USA
First published on 14th April 2020
Abstract
In this paper, the photoluminescent properties of a lead-free double perovskite Cs2NaInCl6 doped with Sb3+ are explored. The host crystal structure is a cubic double perovskite with Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry, a = 10.53344(4) Å, and rock salt ordering of Na+ and In3+. It is a wide bandgap compound (Eg ≈ 5.1 eV), and substitution with Sb3+ leads to strong absorption in the UV due to localized 5s2 → 5s15p1 transitions on Sb3+ centers. Radiative relaxation back to the 5s2 ground state, via a 3P1 → 1S0 transition, leads to intense blue luminescence, centered at 445 nm, with a photoluminescent quantum yield of 79%. The Stokes shift of 0.94 eV is roughly 33% smaller than it is in the related vacancy ordered double perovskite Cs2SnCl6. The reduction in Stokes shift is likely due to a change in coordination number of Sb3+ from 6-coordinate in Cs2NaInCl6 to 5-coordinate in Cs2SnCl6. In addition to the high quantum yield, Cs2NaInCl6:Sb3+ exhibits excellent air/moisture stability and can be prepared from solution; these characteristics make it a promising blue phosphor for applications involving near-UV excitation.
m symmetry, a = 10.53344(4) Å, and rock salt ordering of Na+ and In3+. It is a wide bandgap compound (Eg ≈ 5.1 eV), and substitution with Sb3+ leads to strong absorption in the UV due to localized 5s2 → 5s15p1 transitions on Sb3+ centers. Radiative relaxation back to the 5s2 ground state, via a 3P1 → 1S0 transition, leads to intense blue luminescence, centered at 445 nm, with a photoluminescent quantum yield of 79%. The Stokes shift of 0.94 eV is roughly 33% smaller than it is in the related vacancy ordered double perovskite Cs2SnCl6. The reduction in Stokes shift is likely due to a change in coordination number of Sb3+ from 6-coordinate in Cs2NaInCl6 to 5-coordinate in Cs2SnCl6. In addition to the high quantum yield, Cs2NaInCl6:Sb3+ exhibits excellent air/moisture stability and can be prepared from solution; these characteristics make it a promising blue phosphor for applications involving near-UV excitation.
Introduction
Main group ions with an ns2np0 configuration, like Sb3+ and Bi3+, are classic activator ions used in a variety of phosphors and scintillators.1 The magnitude of the luminescent Stokes shift for these ions can vary greatly depending on the degree of reorganization that occurs in the excited state, which in turn depends upon the structure of the host. When an activator ion is placed on a site that is compressed with respect to its preferred environment, reorganization of the excited state is suppressed, leading to a small Stokes shift. Conversely, if it is placed on a large site, relaxation of the coordination sphere of the activator ion in the excited state can be extensive, resulting in a large Stokes shift. Chloride double perovskites, with the general formula Cs2MM’Cl6 are favorable phosphor host structures for a variety of luminescent centers, including Mn2+, Yb3+, Eu3+, and Cr3+.2–8 For example, in a pair of recent reports, Tang et al. substituted Sb3+ and Bi3+ into the vacancy-ordered double perovskite Cs2SnCl6, and observed photoluminescence (PL).9,10 Incorporating up to ∼1 mol% of Sb3+ produced orange-red light (λmax = 602 nm) with a maximum photoluminescent quantum yield (PLQY) of 37% whereas doping with Bi3+ at levels that approach 7 mol% resulted in a blue emission (λmax = 455 nm) with a PLQY as high as 80%. Nanocrystals of Cs2SnCl6:Sb3+ have also been prepared that show dual emission: a low temperature blue emission (λmax = 438 nm) that disappears upon warming to room temperature and an orange-red emission at 615 nm that is only present when Sb3+ is introduced.11 The quantum efficiency of the nanocrystals is relatively low (PLQY = 8.3%).The PL characteristics of Sb3+ and Bi3+ are known to be highly sensitive to their crystallographic environment, but when these ions are doped into Cs2SnCl6, the coordination environment is unclear. The aliovalent doping of the trivalent ions for Sn4+ requires a compensating charged defect. Recent DFT calculations have suggested the likeliest defect is a chloride vacancy.10 If the Sb3+/Bi3+ ion and the chloride vacancy are located in the same octahedron, it will reduce the coordination environment around the dopant ion from a 6-coordinate octahedron to a 5-coordinate square pyramid. To better understand the structure–property relationships in this class of materials, it would be highly desirable to study a compound where the trivalent ion coordination environment is unambiguous. Cs2M+M3+Cl6 double perovskites are an obvious choice because an isovalent substitution for M3+ does not require a compensating defect.
Studies of double perovskite hosts have been difficult because many of these compounds have intrinsic moisture instabilities.12,13 For example, the double perovskites Cs2NaMCl6:Sb3+ and Cs2NaMBr6:Sb3+ (M = Sc, Y, La) are incredibly hygroscopic, complicating characterization and limiting potential applications.12,13 Attempts by Blasse et al. to synthesize Cs2NaSbX6 (X = Cl, Br) were performed under ultra-dry conditions, but measurements indicated the presence of the thermodynamically stable Cs3Sb2X9 phases. While this phase is non-luminescent it could provide a non-radiative decay pathway. It seems that the incorporation of significant amounts of Sb3+ rapidly destabilizes most Cs2NaMX6 (X = Cl, Br) systems, which necessitates the investigation of alternative host structures.14,15 Developing a chemically stable double perovskite with an isovalent doping site for the Sb3+ cation would alleviate the need for charge compensating chloride vacancy defects, allowing comparative analysis of the effect of coordination number on the photoluminescent properties. This goal led us to study the optical properties of Sb3+ ions doped into the lead-free halide double perovskite host Cs2NaInCl6. Herein, we show that Cs2NaInCl6:Sb3+ is not only useful as a model compound, it is a promising rare-earth free blue phosphor.
Experimental
Cs2NaInCl6:Sb3+ was synthesized by precipitation from an HCl(aq) solution. For a typical synthesis, 20.0 mL of concentrated HCl(aq) (Fisher Scientific, 37%) and 2.0 mL of phosphinic acid (H3PO2, Sigma-Aldrich, 50 wt% in H2O) were heated to 80 °C. Caution should be taken when heating, as phosphinic acid can undergo autoignition upon decomposition into phosphine gas, which occurs near 110 °C. To this solution, 1.00 mmol of In2O3 (Alfa Aesar, 99.994%), 2.00 mmol of NaCl (GFS Chemicals, 99%), and varying amounts (0.001–1.00 mmol) of Sb2O3 (Acros Organics, 99+%) were added. The solution was heated and stirred to allow the reagents to dissolve. Next, 4.00 mmol of CsCl (Acros Organics, 99+%) was added, immediately triggering the precipitation of Cs2NaInCl6:Sb3+. This reaction can be described by eqn (1):| 4CsCl + 2NaCl + In2O3 + 6HCl → 2Cs2NaInCl6 + 3H2O. | (1) |
The precipitate was then filtered using a porous fritted funnel, washed several times with neat ethanol (Decon Labs Inc., 200 proof), and dried overnight via vacuum filtration.
Powder X-ray diffraction (PXRD) data were collected on a Bruker D8 Advance powder diffractometer (40 kV, 40 mA, sealed Cu X-ray tube) equipped with a Lynxeye XE-T position-sensitive detector. The data were collected with an incident beam monochromator (Johansson type SiO2-crystal) that selects only Cu Kα1 radiation (λ = 1.5406 Å). Rietveld refinements of laboratory PXRD data were carried out using the TOPAS-Academic (Version 6) software package to determine the crystal structure. Thermogravimetric analysis (TGA) was performed on a Thermogravimetric Analyzer TGA Q50. Samples were heated under a nitrogen stream of 50 mL per minute with a heating rate of 25 °C per minute between 25 °C and 900 °C.
UV-visible diffuse reflectance spectroscopy (DRS) data were collected from 178–890 nm with an Ocean Optics USB4000 spectrometer equipped with a Toshiba TCD1304AP (3648-element linear silicon CCD array). The spectrometer was used with an Ocean Optics DH-2000-BAL deuterium and halogen UV-vis-NIR light source and a 400 μm R400-7-ANGLE-VIS reflectance probe. The detector was calibrated using a Spectralon Diffuse Reflectance Standard.
Steady-state photoluminescence (PL) data were obtained using a Jovin Horiba FluoroMax4 (xenon source, 0.5 nm excitation and emission slit widths, 1 nm step size) equipped with a solid-state sample holder. Luminescent data was analyzed using the FluorEssence (v3.5) software powered by Origin. Temperature-dependent emission spectra were collected by mixing the samples with an optically transparent silicone resin (GE Silicones, RTV615) and depositing the combination onto a quartz slide (Chemglass). Then, using a Janis cryostat (VPF-100) for a temperature-controlled environment from 300–600 K, the emission was measured in 20 K increments (λex = 340 nm). Photoluminescent lifetime data was collected using a Horiba DeltaFlex System with a NanoLED N-330 nm (λex = 330 nm). Internal photoluminescent quantum yield (PLQY) measurements were performed with a Jovin Horiba FluoroMax4 equipped with a Quanta-φ integrating sphere (15 cm) and a PTFE sample cup. BaSO4 powder dispersed in a silicone resin was used as the blank reference sample. All samples and the blank were excited at 335 nm (λmax) and the absorbance and luminescence signals integrated from 325–345 nm and 370–570 nm, respectively. Radiometric, sphere, and dark count corrections were applied during data acquisition, while corrections for filters and integration time differences were applied in the FluorEssence™ analysis package for Quantum Yield (FluorEssence v3.8.0.60, Origin v8.6001). Additional details are available in the ESI.†
A white light-emitting phosphor-converted light emitting diode (pc-LED) device incorporating the Cs2NaInCl6:Sb3+ phosphor was mixed with lab-made red-emitting Sr2Si5N8:Eu2+ and commercially available green-emitting β-SiAlON:Eu2+ in the same silicon resin mentioned above and cured in a custom brass mold to form a phosphor cap. This cap was placed on a 370 nm LED driven by a 20 mA current, and an AvaSphere-50-IRRAD spectrophotometer was used to obtain the pc-LED luminescence spectrum and performance characteristics.
Results and discussion
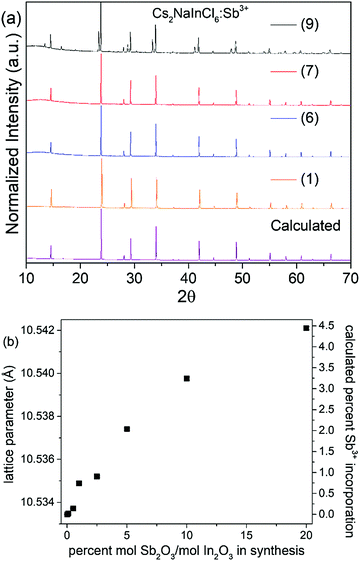
Solution precipitation yields single-phase samples characteristic of the double perovskite crystal structure with sharp diffraction peaks (Fig. 1). Analysis indicates that all compounds have Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m crystal symmetry (Fig. S1, S2 and Table S1, ESI†). The In3+ and Na+ sites are fully ordered in Cs2NaInCl6, evidenced by the presence of a strong (111) peak at ∼14.5° 2θ. The rock salt ordering of octahedral-site cations can be imagined as a three dimensional checkerboard ordering of Na+ and In3+ cations separated by bridging chloride ions. Rietveld refinement yielded bond distances of: d(Cs–Cl) = 3.7271(1) Å, d(Na–Cl) = 2.782(4) Å, and d(In–Cl) = 2.485(4) Å. Sb3+ incorporation does not change the average crystal structure, aside from a slight increase in the lattice parameter. Indeed, the lattice parameters increase to 10.54211(8) Å for the highest concentration of Sb3+ substitution achieved in a phase-pure sample compared to 10.53344(4) Å in the undoped Cs2NaInCl6 sample (Table 1). Although the 6-coordinate Shannon radius of Sb3+ (0.90 Å) is slightly smaller than In3+ (0.94 Å), the increase in lattice parameter with increased Sb3+ content is consistent with the observation that chloride double perovskites with Sb3+ have larger lattice parameters than their In3+ analogs.15,16 As shown in previous reports, the amount of Sb3+ incorporated into the structure is much smaller than the Sb/In ratio in solution prior to precipitation. This may be related to the hygroscopicity of the Sb3+ end-member, Cs2NaSbCl6.12–14 Since the full Cs2NaIn1−xSbxCl6 solid solution cannot be prepared, the amount of Sb3+ incorporated into the Cs2NaInCl6:Sb3+ phosphor was estimated using the lattice parameter changes found in the Cs2AgIn1−xSbxCl6 system.15 In that report, the lattice parameter increased by 0.195 Å as x increased from 0 to 1. If we assume a similar linear increase in the lattice parameter of the Cs2NaIn1−xSbxCl6 solid solution, we can estimate the Sb3+ content in these samples (Table 1).
m crystal symmetry (Fig. S1, S2 and Table S1, ESI†). The In3+ and Na+ sites are fully ordered in Cs2NaInCl6, evidenced by the presence of a strong (111) peak at ∼14.5° 2θ. The rock salt ordering of octahedral-site cations can be imagined as a three dimensional checkerboard ordering of Na+ and In3+ cations separated by bridging chloride ions. Rietveld refinement yielded bond distances of: d(Cs–Cl) = 3.7271(1) Å, d(Na–Cl) = 2.782(4) Å, and d(In–Cl) = 2.485(4) Å. Sb3+ incorporation does not change the average crystal structure, aside from a slight increase in the lattice parameter. Indeed, the lattice parameters increase to 10.54211(8) Å for the highest concentration of Sb3+ substitution achieved in a phase-pure sample compared to 10.53344(4) Å in the undoped Cs2NaInCl6 sample (Table 1). Although the 6-coordinate Shannon radius of Sb3+ (0.90 Å) is slightly smaller than In3+ (0.94 Å), the increase in lattice parameter with increased Sb3+ content is consistent with the observation that chloride double perovskites with Sb3+ have larger lattice parameters than their In3+ analogs.15,16 As shown in previous reports, the amount of Sb3+ incorporated into the structure is much smaller than the Sb/In ratio in solution prior to precipitation. This may be related to the hygroscopicity of the Sb3+ end-member, Cs2NaSbCl6.12–14 Since the full Cs2NaIn1−xSbxCl6 solid solution cannot be prepared, the amount of Sb3+ incorporated into the Cs2NaInCl6:Sb3+ phosphor was estimated using the lattice parameter changes found in the Cs2AgIn1−xSbxCl6 system.15 In that report, the lattice parameter increased by 0.195 Å as x increased from 0 to 1. If we assume a similar linear increase in the lattice parameter of the Cs2NaIn1−xSbxCl6 solid solution, we can estimate the Sb3+ content in these samples (Table 1).
| Sample | [Sb3+]/[In3+] in solution (%) | Lattice parameter (Å) | Nominal Sb content, x |
|---|---|---|---|
| (1) | 0.0 | 10.53344(4) | 0.0 |
| (2) | 0.1 | 10.53347(4) | 0.0002 |
| (3) | 0.5 | 10.53372(3) | 0.0014 |
| (4) | 1.0 | 10.53489(4) | 0.0074 |
| (5) | 2.5 | 10.5352(4) | 0.009 |
| (6) | 5.0 | 10.53741(5) | 0.020 |
| (7) | 10.0 | 10.53976(7) | 0.032 |
| (8) | 20.0 | 10.54211(8) | 0.044 |
| (9) | 50.0 | 10.5489(3) | 0.079 |
The product remains phase pure until reaching sample (9), where peaks arising from Cs3Sb2Cl9 appear in the powder diffraction patterns shown in Fig. 1. Analyzing the thermal stability of the samples by TGA indicates the samples are thermally stable up to 550 °C, as shown in Fig. S3 (ESI†). Increasing the incorporation of Sb3+ does not significantly impact the thermal stability; if anything, the thermal stability is slightly enhanced by antimony doping. The samples also did not show any signs of degradation when stored under ambient conditions (room temperature, in air), as evidenced by the lack of changes in the PXRD patterns and optical measurements taken over multiple weeks, (see Fig. S4 and Table S2, ESI†).
The optical properties of the phase-pure samples (1–8) were studied by first analyzing the UV-visible diffuse reflectance spectra (Fig. S5, ESI†). After converting into pseudo-absorbance via the Kubelka–Munk function,17 provided in eqn (2),
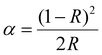 | (2) |
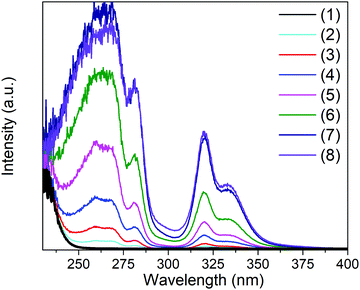 | ||
| Fig. 2 Pseudo-absorbance obtained from Kubelka–Munk transformation of the diffuse reflectance data of Cs2NaInCl6:Sb3+ for samples with various concentrations of Sb3+. | ||
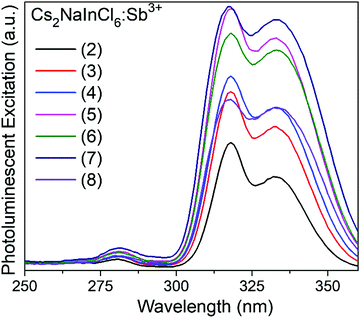
Analyzing the photoluminescent excitation spectra provides more information on the electronic structure across this series of compounds (see Fig. 3). The two excitation maxima at 317 nm and 333 nm, which correspond to the Jahn–Teller split 5s2 → 5s1p1 transitions of the [SbCl6]3− octahedra, lead to the same broad emission. These correspond nicely with the two main absorption features observed in the Kubelka–Munk pseudo-absorption spectra (Fig. 2). The weak excitation observed at 280 nm corresponds to the 1S0 → 3P2 transition. Interestingly the relative intensities of the three peaks are quite different in the absorbance and excitation spectra. Not surprisingly, the efficiency of the emission is much higher when both the excitation and emission are associated with the 1S0 → 3P1 transitions. The 1S0 → 1P1 transition, which produces a strong absorbance at ∼260 nm, does not lead to emission. This may be related to its proximity in energy to the band edge, which could allow for thermal excitation into the conduction band and subsequent energy migration. It is also possible that a rapid, radiative return to the ground state (fluorescence) with minimal Stokes shift occurs before crossing over to the triplet excited state.
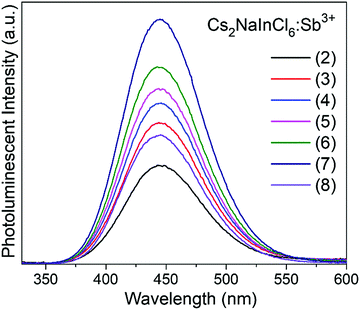
Cs2NaInCl6 doped with Sb3+ exhibits bright blue photoluminescence (PL), centered at 445 nm with a full-width-at-half-maximum (FWHM) of ∼80 nm (0.51 eV) (Fig. 4). As observed in other double perovskites doped with Sb3+, this blue emission can be attributed to local Sb3+ excited state relaxation via a 3P1 → 1S0 pathway. The magnitude of the Stokes shift is indicative of the extent of an excited state reorganization of the [SbCl6]3− octahedra.12 The relatively small Stokes shift (0.94 eV) observed in this compound follows a trend observed in other Sb3+-doped double perovskites, which show an increasing Stokes shift with increasing ionic radii of the 6-coordinate 3+ cation (Table 2).19 The exception is Cs2SnCl6:Sb3+, where the Stokes shift does not follow the trend extrapolated from the double perovskites. We hypothesize that this is due to the Sb3+ adopting a 5-coordinate environment ([SbCl5]2−), which is not surprising given the attraction between the negatively charged 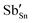 and positively charged
and positively charged 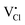 defects. The stereoactive lone pair of Sb3+ makes the square pyramidal geometry more likely than trigonal bipyramidal. The asymmetric coordination environment allows for larger reorganization of the excited state, and thus, a larger Stokes shift.
defects. The stereoactive lone pair of Sb3+ makes the square pyramidal geometry more likely than trigonal bipyramidal. The asymmetric coordination environment allows for larger reorganization of the excited state, and thus, a larger Stokes shift.
Analyzing the optical properties of Cs2NaInCl6:Sb3+ reveals an increase in the Stokes shift as the radius of the trivalent ion that Sb3+ replaces increases. While the extent of structural relaxation around the Sb3+ dopant is not known, it is reasonable to assume that the antimony ion has more freedom to relax in the excited state as the lattice parameter of the host double perovskite increases, thereby lowering its energy and red shifting the ensuing emission.1 The emission position in Cs2NaInCl6:Sb3+ is also independent of the excitation wavelength, indicating that the emission arises from a consistent radiative decay process (Fig. S7, ESI†). The emission characteristics of Cs2NaInCl6:Sb3+ are similar to the industry standard blue-emitting phosphor, BaMgAl10O17:Eu2+ (BAM:Eu2+), which has a 0.90 eV Stokes shift (340 nm excitation, 452 nm emission, FWHM = 55 nm).20 However, the BAM:Eu2+ phosphor deteriorates over extended usage due to oxidation of the luminescent center from Eu2+ to Eu3+.21 Not only is this degradation mechanism not operative in the Cs2NaInCl6:Sb3+ system, the absence of rare-earth ions is an attractive feature.
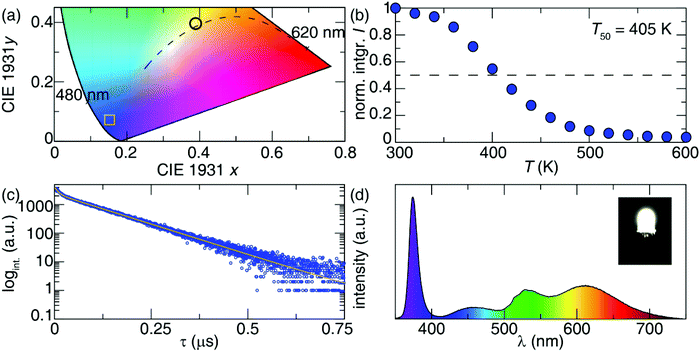
The maximum emission intensity was observed for the sample (7), corresponding to an approximately 3% substitution of Sb3+ for In3+. The slight decrease in luminescence intensity for sample (8) (estimated [Sb3+] ≈ 4%), suggests concentration quenching begins to play a role for the higher Sb3+ contents. The lower emission intensity of sample (9) can also be attributed in part to the presence of Cs3Sb2Cl9. This may give rise to a non-radiative deactivation pathway,22 not to mention the expected decrease in emission because a non-negligible part of the sample has formed a non-emissive phase. The CIE coordinate diagram (Fig. 5a) for Cs2NaInCl6:Sb3+ corroborates the expected blue emission, with CIE coordinates of (0.148, 0.067). The PLQY of this compound was determined to be 79(5)% for sample (6), an impressive efficiency for a phosphor prepared from solution (Fig. S8 and Table S3, ESI†).
The thermal stability of the photoluminescence was explored by collecting temperature-dependent PL measurements in 20 K intervals between 300 K and 600 K. These measurements indicate the temperature at which the photoluminescence intensity drops to 50% of its room-temperature value (T50) is 405 K, as shown in Fig. 5b. Minimal shifts were also seen in the emission maximum with temperature, indicating excellent color stability (Fig. S9, ESI†). This is consistent with locally excited systems since the shape of the [SbCl6]3− octahedra will not significantly change with temperature.
Photoluminescent lifetime measurements were conducted to further understand the decay mechanism. The data plotted in Fig. 5c for sample (6) were fit to a biexponential, following eqn (3), resulting in a lifetime of τ1 = 0.090 μs accounting for 46% of the decay, and a longer lifetime τ2 = 1.016 μs accounting for the rest.
| I = I0 + A1e−t/τ1 + A2e−t/τ2 | (3) |
These lifetime values are reasonably close to those reported for Cs2SnCl6:Sb3+ system, which had τ1 = 0.154 μs and τ2 = 0.821 μs, where the two lifetimes were attributed to 3P1 → 3P0 and 3P1 → 1S0 transitions, respectively.9
To explore the potential of this phosphor for use in lighting applications, a prototype pc-LED was fabricated by combining a UV-LED chip (λex = 370 nm) with a mixture of the Cs2NaInCl6:Sb3+, a lab prepared red-emitting Sr2Si5N8:Eu2+ and a commercially available green-emitting β-SiAlON:Eu2+. The device was driven by a 20 mA current to yield the corresponding emission spectrum plotted in Fig. 5d. The full-spectrum warm white light produced using Cs2NaInCl6:Sb3+ possess excellent color quality with a color rendering index (Ra) of 90.6, a low correlated color temperature (CCT) of 3972.6 K, and CIE coordinates of (0.3890, 0.4009). To highlight the capability of Cs2NaInCl6:Sb3+ as a blue-emitting phosphor a pc-LED with BaMgAl10O17:Eu2+, Sr2Si5N8:Eu2+ and β-SiAlON:Eu2+ was also fabricated. The luminescence spectrum and CIE coordinates of the pc-LED can be seen in Fig. S10 (ESI†).23 The CIE coordinates of the BAM:Eu2+ containing pc-LED closely resembles that of the pc-LED composed of Cs2NaInCl6:Sb3+, indicating a similar broad band spectrum can be produced from both blue-emitters. The resulting Ra and CCT of the pc-LED using the BaMgAl10O17:Eu2+ synthesized here is 95.3 and 4479.2 K. The Ra of the BAM:Eu2+ containing pc-LED is slightly better; however, the CCT of this device is significantly higher than the pc-LED using Cs2NaInCl6:Sb3+. The nearly 500 K difference in CCT highlights the ability of Cs2NaInCl6:Sb3+ to be used in conjunction with UV-LEDs to produce a broad spectrum, warm white light.
Based on the results reported here, it could be imagined that a variety of moisture stable double perovskites could successfully host the Sb3+ activator in a 6-coordinate environment. To this end, Sb3+ was doped into Cs2NaBiCl6 and Cs2AgInCl6 hosts using modifications of previously reported synthesis methods.5,24,25 The samples were each irradiated with light from a broadband Ultra-Violet Products UVSL-25 Mineralight Lamp (365 longwave, 254 shortwave) excitation source to test for luminescence. However, no luminescence is observed at room temperature in Cs2NaBiCl6 samples doped with Sb3+. The lack of observed luminescence in the Bi3+ containing system may arise from the close alignment of the energy levels of Sb3+ and Bi3+. These orbitals tend to hybridize efficiently in double perovskites, as demonstrated by the pronounced band gap bowing effect seen in Cs2AgBi1−xSbxBr6 and Cs2AgBi1−xSbxCl6 solid solutions.26,27 This energetic alignment allows for facile energy transfer between the Sb3+ activator and the host, which can enhance concentration quenching. In Cs2AgInCl6:Sb3+, trace (<1%) amounts of Sb3+ lead to an intense yellow-white luminescence, reminiscent of Cs2Ag0.60Na0.40InCl6:Bi3+ or Cs2AgIn1−xBixCl6 phosphors.24,28 It appears as though self-trapped excitonic emission leads to the broad photoluminescence observed in both systems. These alternative hosts, while non-toxic and stable, do not have the correct electronic structure to localize the excited state on Sb3+-dopants, illustrating the importance of the host structure in the design of new phosphors.
During the review process, another paper describing photoluminescence in Cs2NaInCl6:Sb3+ appeared in the literature.29 The photophysical properties reported in both studies are generally in good agreement with each other.
Conclusions
Bright blue luminescence (λmax = 445 nm, FWHM ≈ 0.51 eV) with an internal PLQY of 79(5)% is observed when Sb3+ ions are doped into the ordered double perovskite Cs2NaInCl6 host. In samples precipitated from HCl(aq) solution, approximately 5% of the In3+ ions can be replaced with Sb3+, but the PL starts to decrease when the Sb-content gets larger than ∼3%. When compared to Cs2SnCl6:Sb3+ phosphors where the Sb3+ ions are likely 5-coordinate, the Stokes shift in Cs2NaInCl6:Sb3+ is smaller (0.94 eV vs. 1.38 eV) leading to blue emission rather than orange-red, and the quantum efficiency is more than doubled (79% vs. 37%). The emission characteristics of Cs2NaInCl6:Sb3+ phosphors are comparable to the commercial blue phosphor, BaMgAl10O17:Eu2+ (BAM:Eu2+), which makes it an attractive, rare-earth free alternative to commercial blue phosphors.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Funding was provided by the National Science Foundation under award number DMR 1610631 (M. B. G., J. D. M., and P. M. W.), DMR 1847701 (S. H. and J. B.) and CER 1911311 (S. H. and J. B.). S. H. and J. B. also thank the Welch Foundation (E-1981) and the Texas Center for Superconductivity at the University of Houston (TcSUH) for supporting this work. PLQY measurements in the MRL Shared Experimental Facilities by A. S. are supported by the MRSEC Program of the NSF under award no. DMR 1720256; a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org). The authors would like to thank David Liu for artistic contributions.References
-
G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, 1994 Search PubMed
.
- P. A. Tanner, C. K. Duan, G. Jia and B. M. Cheng, Luminescence of the Elpasolite Series MI2MIIMCl6 (MI = Cs, Rb; MII = Li, Na; M = Lu, Y, Sc, In) Doped with Europium Using Synchrotron Radiation Excitation, J. Solid State Chem., 2012, 188, 105 CrossRef CAS
.
- N. Chen, T. Cai, W. Li, K. Hills-Kimball, H. Yang, M. Que, Y. Nagaoka, Z. Liu, D. Yang, A. Dong, C. Y. Xu, R. Zia and O. Chen, Yb-and Mn-Doped Lead-Free Double-Perovskite Cs2AgBiX6 (X = Cl−, Br−) Nanocrystals, ACS Appl. Energy Mater., 2019, 11, 16855 CrossRef CAS PubMed
.
- Y. Mahor, W. J. Mir, A. Nag and W. J. Mir, Synthesis and Near Infrared Emission of Yb Doped Cs2AgInCl6 Double Perovskite Microcrystals and Nanocrystals, J. Phys. Chem. C, 2019, 123, 15787 CrossRef CAS
.
- J. D. Majher, M. B. Gray, T. A. Strom and P. M. Woodward, Cs2NaBiCl6:Mn2+: A New Orange-Red Halide Double Perovskite Phosphor, Chem. Mater., 2019, 31, 1738 CrossRef CAS
.
- R. Knochenmuss, C. Reber, M. V. Rajasekharan and H. U. Güdel, Broadband Near-Infrared Luminescence of Cr3+ in the Elpasolite Lattices Cs2NaInCl6, Cs2NaYCl6, and Cs2NaYBr6, J. Chem. Phys., 1986, 85, 4280 CrossRef CAS
.
- F. Zhao, Z. Song, J. Zhao and Q. Liu, Double Perovskite Cs2AgInCl6:Cr3+: A Broadband and Near-Infrared Luminescent Materials, Inorg. Chem. Front., 2019, 6, 3621 RSC
.
- G. D. Boyd, H. Kasper, J. H. McFee, L. Bernstein, S. C. Abrahams, F. Lissalde, H. U. Güdel and T. R. Snellgrove, Jahn-Teller Effect in the 4T2g State of Chromium(III) in Dicesium Sodium Indium(III) Hexachloride, Inorg. Chem., 1978, 17, 1617 CrossRef
.
- J. Li, Z. Tan, M. Hu, C. Chen, J. Luo, S. Li, L. Gao, Z. Xiao, G. Niu and J. Tang, Antimony Doped Cs2SnCl6 with Bright and Stable Emission, Front. Optoelectron., 2019, 12, 352 CrossRef
.
- Z. Tan, J. Li, C. Zhang, Z. Li, Q. Hu, Z. Xiao, T. Kamiya, H. Hosono, G. Niu, E. Lifshitz, Y. Cheng and J. Tang, Highly Efficient Blue-Emitting Bi-Doped Cs2SnCl6 Perovskite Variant: Photoluminescence Induced by Impurity Doping, Adv. Funct. Mater., 2018, 28, 1801131 CrossRef
.
- Y. Jing, Y. Liu, J. Zhao and Z. Xia, Sb3+ Doping-Induced Triplet Self-Trapped Excitons Emission in Lead-Free Cs2SnCl6 Nanocrystals, J. Phys. Chem. Lett., 2019, 10, 7439 CrossRef CAS PubMed
.
- E. W. J. L. Oomen, W. M. A. Smit and G. Blasse, On the Luminescence of Sb3+ in Cs2NaMCl6 (with M = Sc, Y, La): A Model System for the Study of Trivalent s2 Ions, J. Phys. C: Solid State Phys., 1986, 19, 3263 CrossRef CAS
.
- E. W. J. L. Oomen, G. J. Dirksen, W. M. A. Smit and G. Blasse, On the Luminescence of Sb3+ in Cs2NaMBr6, J. Phys. C: Solid State Phys., 1987, 20, 1161 CrossRef CAS
.
- E. W. J. L. Oomen, W. M. A. Smit and G. Blasse, The Luminescence of Cs2NaSbCl6 and Cs2NaSbBr6: A Transition from a Localized to a Delocalized Excited State, Chem. Phys. Lett., 1987, 138, 23 CrossRef CAS
.
- T. T. Tran, J. R. Panella, J. R. Chamorro, J. R. Morey and T. M. McQueen, Designing Indirect–Direct Bandgap Transitions in Double Perovskites, Mater. Horiz., 2017, 4, 688 RSC
.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, A32, 751 CrossRef CAS
.
- M. Kubelka, The Kubelka–Munk Theory of Reflectance, Zeit. Für Tekn. Physik., 1931, 593–602 Search PubMed
.
- E. T. McClure, PhD thesis, Ohio State University, 2019 Search PubMed
.
- X. Wang, W. Meng, W. Liao, J. Wang, R. G. Xiong and Y. Yan, Atomistic Mechanism of Broadband Emission in Metal Halide Perovskites, J. Phys. Chem. Lett., 2019, 10, 501 CrossRef PubMed
.
- A. C. Duke, S. Hariyani and J. Brgoch, Ba3Y2B6O15:Ce3+ A High Symmetry, Narrow-Emitting Blue Phosphor for Wide-Gamut White Lighting, Chem. Mater., 2018, 30, 2668 CrossRef CAS
.
- S. Oshio, Mechanism of Luminance Decrease in BaMgAl10O17:Eu2+ Phosphor by Oxidation, J. Electrochem. Soc., 1998, 145, 3903 CrossRef CAS
.
- C. W. M. Timmermans, S. O. Cholakh and G. Blasse, The Luminescence of Cs3Bi2Cl9 and Cs3Sb2Cl9, J. Solid State Chem., 1983, 46, 222 CrossRef CAS
.
- J. Zhong, Y. Zhuo, S. Hariyani, W. Zhao, J. Wen and J. Brgoch, Closing the Cyan-Gap toward Full Spectrum LED Lighting with NaMgBO3:Ce3+, Chem. Mater., 2020, 32, 882 CrossRef CAS
.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, L. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. Sargent and J. Tang, Efficient and Stable Emission of Warm-White Light from Lead-Free Halide Double Perovskites, Nature, 2018, 563, 541 CrossRef CAS PubMed
.
- M. B. Gray, E. T. McClure and P. M. Woodward, Cs2AgBiBr6−xClx Solid Solutions-Band Gap Engineering with Halide Double Perovskites, J. Mater. Chem. C, 2019, 7, 9686 RSC
.
- K. Z. Du, W. Meng, X. Wang, Y. Yan and D. B. Mitzi, Bandgap Engineering of Lead-Free Double Perovskite Cs2AgBiBr6 through Trivalent Metal Alloying, Angew. Chem., Int. Ed., 2017, 56, 8158 CrossRef CAS PubMed
.
- B. Yang, F. Hong, J. Chen, Y. Tang, L. Yang, Y. Sang, X. Xia, J. Guo, H. He, S. Yang, W. Deng and K. Han, Colloidal Synthesis and Charge-Carrier Dynamics of Cs2AgSb1−yBiyX6 (X: Br, Cl; 0 ≤ y ≤ 1) Double Perovskite Nanocrystals, Angew. Chem., Int. Ed., 2018, 58, 2278 CrossRef PubMed
.
- M. B. Gray, J. D. Majher, T. A. Strom and P. M. Woodward, Broadband white emission in Cs2AgIn1−xBixCl6 phosphors, Inorg. Chem., 2019, 58, 13403 CrossRef CAS PubMed
.
- R. Zeng, L. Zhang, Y. Xue, B. Ke, Z. Zhao, D. Huang, Q. Wei, W. Zhou and B. Zou, Highly Efficient Blue Emission from Self-Trapped Excitons in Stable Sb3+-Doped Cs2NaInCl6 Double Perovskites, J. Phys. Chem. Lett., 2020, 11, 2053 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tc01037e |
| This journal is © The Royal Society of Chemistry 2020 |