Cyclometalated Ir(III) complexes towards blue-emissive dopant for organic light-emitting diodes: fundamentals of photophysics and designing strategies
Sunhee
Lee
and
Won-Sik
Han
 *
*
Department of Chemistry, Seoul Women's University, 621 Hwarang-ro, Seoul 01797, Republic of Korea. E-mail: wshan@swu.ac.kr
First published on 7th May 2020
Abstract
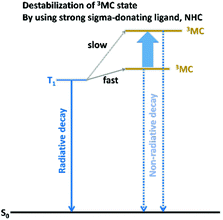
The main difficulties hindering development of a deep-blue phosphorescent cyclometalated Ir(III) complex are insufficient colour purity, i.e., failure to achieve ideal Commission Internationale de L'Eclairage (CIE) coordinates of (0.14, 0.09), and insufficient emission efficiency and stability. The latter problem is due to the highly energetic and hot excited states of these complexes, which yield faster decomposition. Therefore, control of the excited-state properties of cyclometalated Ir(III) complexes through systematic chemical modification of the ligands is being extensively investigated, with the aim of developing efficient and stable blue phosphorescent materials. The most common strategies towards achievement of a blue phosphorescent cyclometalated Ir(III) complex involve (1) substitution of electron-withdrawing F atoms at the cyclometalating ligands that stabilise the HOMO orbitals and (2) use of a heteroleptic system with electron-rich ancillary ligands bearing a 5-membered ring heterocycle to increase the LUMO energy level. However, the C–F bonds on the cyclometalating ligands have been found to be inherently unstable during device operation; thus, other types of electron-withdrawing groups (e.g., the cyano, trifluoromethyl, and sulfonyl groups) have been applied. Along with phosphorescence colour tuning to blue, the influence of the ligand structure on the photoluminescence quantum yield (PLQY) is also being intensively investigated. Two major PLQY lowering mechanisms for blue emissive Ir(III) complexes have been identified: (1) the vibronic-coupled non-radiative decay process and (2) crossing from the emissive state to an upper non-emissive 3MC excited state. To enhance the PLQY, mechanism (1) can be suppressed by employing rigid ligand frameworks to restrict intramolecular motion, whereas mechanism (2) can be prevented by destabilising the 3MC state using strong σ donor ligands such as N-heterocyclic carbenes. This review summarises the fundamental photophysics of cyclometalated Ir(III) complexes and surveys design strategies for efficient blue phosphorescent Ir(III) complexes, to provide a guide for future research in this field.
1. Introduction
Organic light-emitting diodes (OLEDs) have attracted considerable interest in the display field in recent decades because of their various advantages, such as their light weight, high contrast ratio, wide viewing angle, improved energy efficiency, and excellent design versatility. The OLED working principle involves operation using two kinds of radiative relaxation process, i.e., fluorescence and phosphorescence, which originate from singlet and triplet excited states, respectively. In theory, utilisation of triplet excitons along with singlet excitons yields 100% efficiency;1,2 therefore, intensive research has been performed on materials that can achieve high triplet excited states with high photoluminescence quantum yield (PLQY). It is well-known that the triplet excited state can be generated efficiently via heavy-atom-induced spin–orbit coupling. Among the transition metal complexes, cyclometalated Ir(III) complexes have been deemed the most efficient because of their highly efficient populations of triplet excited states, which induce radiative decay processes.3 Indeed, almost 100% internal quantum efficiency for conversion of electric energy to photons has been achieved.2,4–12In this regard, cyclometalated Ir(III) complexes play an essential role in OLED applications such as flat panel displays and solid-state lighting.1,13–15 Previous studies have established the structure–property relationships within cyclometalated Ir(III) complexes that enable high-efficiency phosphorescence emission with full spectral coverage spanning the near ultraviolet (UV) to near infrared. However, phosphorescence tuning over the entire visible spectrum remains a challenge. The design and synthesis of deep-blue-emitting cyclometalated Ir(III) complexes is particularly difficult. Although high external quantum efficiencies (EQEs) exceeding 30% have been achieved for green and red OLED devices,16–20 high EQEs for deep-blue OLED devices have rarely been reported.21–24
The main difficulties in developing deep-blue-emissive Ir(III) complexes are the following: (1) they lack sufficient colour purity for the ideal Commission Internationale de L'Eclairage (CIE) coordinates of (0.14, 0.09), (2) they have insufficient emission efficiency, and (3) attainment of an appropriate host and carrier transport materials with sufficient triplet energy levels is challenging. (Note: The materials for the host and carrier transport materials are not discussed in this review.) These difficulties are due to the highly energetic excited states of blue phosphors, which can yield faster decomposition than those of green and red phosphors.25–28 In addition, the upper-lying excited states of blue-emissive Ir(III) complexes can induce several degradation processes, for example, triplet–triplet annihilation,29,30 triplet–polaron annihilation,31 and polaron–polaron annihilation.32
Note that review articles of blue phosphorescent cyclometalated Ir(III) complexes for applications in OLEDs have been well presented previously.33–50 In this review, we will discusses the current research status regarding development of cyclometalated Ir(III) complexes towards a blue-emissive dopant for OLEDs. We summarise the fundamental photophysics of cyclometalated Ir(III) complexes, considering radiative and non-radiative (NR) decay processes (section 2). We then discuss the structure–property relationship dependence on the ancillary ligand (section 3). We hope this review will be helpful to OLED researchers working to design efficient deep-blue phosphorescent Ir(III) complexes.
2. Fundamental photophysics of cyclometalated Ir(III) complexes
2.1. General molecular photophysics upon excitation
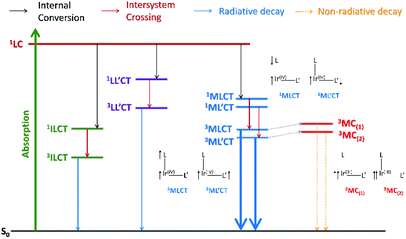
When a molecule absorbs energy (e.g., photons), the electrons in the ground state become excited and decays along multiple photophysical pathways. These processes can be presented schematically as an energy diagram known as a ‘Jablonski diagram’,51 as depicted in Fig. 1. The main processes can be classified as photoabsorption, vibrational relaxation, internal conversion (IC), fluorescence, intersystem crossing (ISC), phosphorescence, and photochemical processes, and summarised as follows.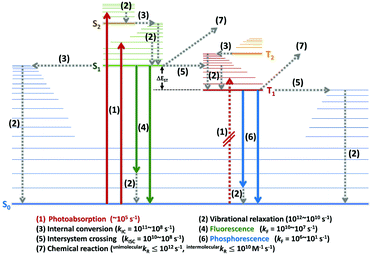 | ||
| Fig. 1 Modified Jablonski diagram with time scales for photophysical processes in molecular system. Bold and dotted lines indicate radiative and non-radiative (NR) decay processes, respectively. | ||
(1) Photoabsorption: As a molecule absorbs photons, an electron is excited from the singlet ground state (S0), to the lowest (S1) or higher singlet excited states (Sn). The transition rate is very fast, at >1015 s−1. In general, a transition from S0 to the lowest triplet state (T1) has low probability because the electron spin is parallel to the ground-state spin; thus, this transition is called ‘forbidden’. However, this forbidden transition can be observed under specific conditions, for example, for internal and external heavy-atom effects with very low extinction coefficients (ε), with for which εmax = 10−1–10−2.
(2) Vibrational relaxation: Immediately after excitation to S1 or Sn, the molecule population distribution in the Franck–Condon state of the higher excited-vibrational states (v′ ≥ 1) relaxes to a less energetic vibrational state (v′ = 0) through vibrational energy transfer. This process also occurs immediately after IC and intersystem crossing. The vibrational relaxation rate is in the range of a few picoseconds, at >1012 s−1.
(3) IC: This process involves a change in the electronic states, for example, Sn to Sn−1, which have energetically degenerate vibrational states. Therefore, there is no energy change and this process is radiationless. The IC range is <108 s−1 and depends on the energy gap between the Sn and Sn−1 states according to the ‘Energy Gap Law’.
(4) Fluorescence: This process is a radiative transition from S1 to S0, as allowed by the selection rules, at a rate of ≤109 s−1. As the IC rate from Sn to Sn−1 is very high (in the picoseconds range), most fluorescence occurs from the lowest S1 state; this is called ‘Kasha's Rule’.
(5) ISC: This process is similar to IC. However, although the molecular spin state remains the same for IC, the ISC process requires a change in spin state. The latter process involves transitions from S1 to the higher excited-vibrational states of T1 and Tn, followed by relaxation to less energetic vibrational states (similar to IC). The ISC rate is in the range of 1012 s−1 and depends on the degree of spin–orbit coupling and the ‘El-Sayed Rule’. The transition from T1 to S0 is also an ISC process, but with a lower transition rate (≤106 s−1) than the S1 → T1 transition according to the “Energy Gap Law” (the energy gap between the T1 and S0 states is usually larger than that between the S1 and T1 states).
(6) Phosphorescence: This process is another radiative transition and involves transition from the T1 to S0 states. As the direct formation of T1 from S0 is a forbidden transition, the T1 state is usually generated via the following sequence: excitation from S0 to Sn → rapid IC from Sn to S1 → ISC from S1 to T1. The phosphorescence rate for organic compounds is approximately 103 s−1 but is much higher for organometallic complexes that possess heavy metals, being in the range of >106 s−1.
(7) Photochemical processes: The energy or electron in the excited state can participate in chemical reactions. In general, these processes occur from the S1 and T1 states. In the case of the unimolecular reaction process, the reaction rate is in the range of ≤1012 s−1. For the intermolecular (bimolecular) reaction process, the reaction rate depends on the solvent temperature (T) and viscosity (≤1010 M−1 s−1).
In particular, triplet-related processes are a major focus in the context of cyclometalated Ir(III) complexes for OLED application, as the maximum internal quantum efficiency for conversion of electric energy to photons can be achieved through phosphorescence.
2.2. General photophysics in the cyclometalated Ir(III) complex
As an example, Fig. 2 shows the absorption spectrum of the well-known cyclometalated homoleptic Ir(III) complex, fac-tris[2-phenylpyridine]iridium(III), fac-Ir(ppy)3, (1). The absorption spectrum involves strong 1LC spin-allowed π–π* transitions at <300 nm and spin-allowed d–π* transitions (1MLCT) in the 320–430 nm range. The >435 nm absorptions are attributable to a spin-forbidden 3MLCT transition with very low ε; this is a consequence of the heavy-atom effect yielding a strong SOC.52 However, it should be noted that the lowest energy state of 1 is a hybrid state with 3MLCT and 3LC characteristics (vide infra).
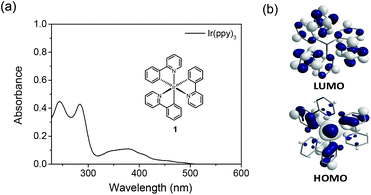 | ||
| Fig. 2 (a) Absorption spectrum in 10 μM solution of dichloromethane (inset: chemical structure) and (b) orbital contributions of HOMO and LUMO of 1. | ||
These assignments for the transitions of 1 are supported by theoretical calculations.53–55 According to those calculations, the molecular orbitals mainly participating in generation of the lowest triplet state are from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). As shown in Fig. 2b, the HOMO is delocalised over the Ir t2g orbital and the π orbitals of the phenyl ring in the 2-ppy ligand, whereas the LUMO exclusively involves the π* orbitals of the pyridine ring in the 2-ppy. Therefore, one of the causes of the S0 → T1 transition is charge transfer from the 5d Ir metal orbital to the ppy ligands. Further studies involving time-dependent density functional theory (TD-DFT) calculations have indicated that the lowest triplet state is in a hybrid form of the MLCT and LC transition states.56,57 In the triplet manifold, the dominant LC state may be lowest. This is because the singlet–triplet splitting from the electron-exchange interaction is much smaller for MLCT than LC π–π*, as the orbitals in the MLCT excited state have greater spatial extension. The optimised structure of the lowest triplet energy state indicates broken C3 symmetry and excitation localised on a single ligand, supporting hybridisation in the triplet excited states. More detailed analysis based on multi-configurational self-consistent field orbitals and second-order configurational interactions have been performed to study the different radiative and NR processes of fac-1 and mer-1.58 Heteropletic59,60 and bis-tridentate61 Ir(III) complexes have also yielded similar calculation results.
Yersin et al. investigated the detailed properties of the emitting triplet state of 1, including the corresponding radiative and NR transitions.52,66 The lowest triplet state can be divided into three substates: I, II, and III. For most organometallic complexes containing transition metal ions, the transition between the lowest substate I and S0 is forbidden, but the other transitions from II and III to S0 are permitted.68–71 The same results were found for 1.66 Indeed, three emissive triplet substates, I–III, of 1 in tetrahydrofuran (THF) solution were identified from the temperature-dependent emission spectra (1.2 K ≤ T ≤ 300 K) and classified as substates of a 3MLCT state. Through further combined analysis with application of high magnetic fields, the energy differences between the substates were successfully estimated, i.e., ΔEII–I = 13.5 cm−1 and ΔEIII–I = 83.5 cm−1, as depicted in Fig. 3. This relatively large total zero-field splitting (ZFS) indicates that the lowest triplet state of 1 is a 3MLCT state. The same assignments were concluded from theoretical calculations.53,56,72
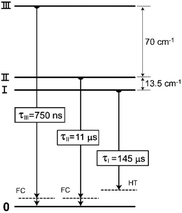 | ||
| Fig. 3 Energy levels for three lowest triplet substates, I, II, and III, and decay times of 1. Adapted with permission from ref. 66. Copyright 2003, Elsevier. | ||
Heteroleptic Ir(III) complexes possess more complex photophysical processes than those of a homolectic system, because the presence of the ancillary ligand (L′) induces additional transitions such as L′C and ML′CT. This is in addition to the ligand-to-ligand charge transfer (LL′CT) transition that occurs between a main ligand and ancillary ligand, as shown in Fig. 4.
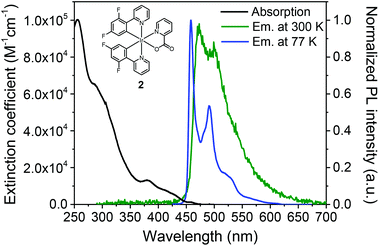
The most well-known blue-emissive heteroleptic Ir(III) is likely a bis[2-(4,6-difluorophenyl)pyridinato-C2,N](picolinato)iridium(III), commonly known as FIrpic (2).73Fig. 5 shows typical emission spectra of 2 at RT and 77 K. The RT emission spectrum exhibits blue phosphorescence peaks at 471 and 495 nm with fine vibronic structure, which are virtually independent of the solvent polarity. These observations indicate that the emitting lowest triplet state has a predominantly 3LC character with a minor 3MLCT contribution. Thus, if the cyclometalated Ir(III) complex emission has a non-vibronic structure and the emission changes depending on the solvent polarity, the lowest triplet state has a predominantly 3MLCT character. At low temperature (77 K), the emission spectrum of 2 shows narrower and highly structured emission bands at 461 and 495 nm, indicating that the 3LC character is increased at this temperature. A small thermally induced Stokes shift (ΔES) with of 10 nm also indicates that the nature of the lowest triplet state in 2 is based on the 3LC state. In general, small and large ΔES indicate 3LC and 3MLCT excited states, respectively.74–76 The PLQY of 2 in dilute solution was initially reported to be approximately 0.6. Note, however, that this value was later corrected to higher values of >0.90.77–79 Accordingly, the radiative- and NR constants of 2 were estimated to be kr = 5 × 105 s−1 and knr = 0.5 × 105 s−1, respectively.
Temperature-independent decay processes occur via direct crossing between the two potential energy surfaces of the triplet and ground states. These are vibronic-coupled NR decay process, as studied in detail by Samuel et al.80,81 Those researchers suggested that stronger vibrational coupling yields an increased NR decay rate and decreased PLQY. They estimated the vibrational coupling values using the Huang–Rhys factor, SM, which roughly quantifies the structural distortion (ΔQ) of the excited state relative to the ground state.82,83 If SM = 0, the geometries of the excited and ground states should be identical, and only a sharp peak corresponding to the 0–0 transition should be observed. With increased ΔQ and, hence, appearance of the 0–1 transition, the SM value can be estimated using the intensity ratios of the 0–0 and 0–1 vibrational peaks: SM = (I0–1/I0–0). Thus, higher SM indicates increased vibrational coupling between the potential surfaces of the triplet excited state and the ground state; this yields lower PLQY as the transition probability is increased with increasing overlap between the initial and final states.
Samuel et al. also found that the experimental phosphorescence lifetime (τP) value of 4 (Fig. 6) in 2-methyl-tetrahydrofuran (2-MeTHF) increases from 0.9 μs at 300 K to 2.2 μs at 200 K and to 3.2 μs at 77 K.81 An Arrhenius plot showing the temperature-dependence of τP gives activation energy of 0.27 eV (2178 cm−1). In the case of 5, τP changes from 0.15 μs at 290 K to 3 μs at 200 K, but is constant (3 μs) at ≤200 K. The temperature-dependence of τP gives an activation energy of 0.67 eV (5404 cm−1). On polymethyl methacrylate (PMMA) solid films, the emission decays of 3–5 (Fig. 6) reveal bi-exponential behaviour. The longer component for 5 increases from 1.35 μs at 310 K to 3.7 μs at 77 K, and the temperature dependence of τP gives an activation energy of 0.13 eV (1049 cm−1); this is much smaller than that for the homogeneous solution in 2-MeTHF, as shown in Fig. 6. As the activation energies differ considerably between homogeneous solutions and solid films, they suggested that a vibronic-coupled NR decay mechanism could play an important role in lowering the PLQY. Such NR decay processes can be suppressed by employing rigid ligand frameworks.
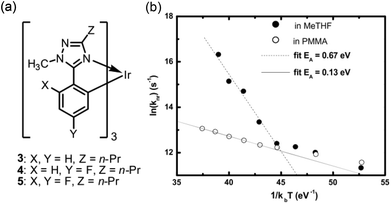 | ||
| Fig. 6 (a) Chemical structures of 3–5 and (b) Arrhenius (activation energy) plot of 5 dissolved in 2-MeTHF or solid PMMA host. Adapted with permission from ref. 81. Copyright 2008, Elsevier. | ||
We next consider, temperature-dependent NR decay processes, which occur for crossing from the emissive state to an upper non-emissive 3MC excited state. As the non-emissive 3MC states of cyclometalated Ir(III) complexes are high-energy, green and red phosphorescent Ir(III) complexes cannot access this state at RT. However, the upper-lying 3MLCT states of blue phosphorescent Ir(III) complexes can easily access this non-emissive 3MC state. In particular, Thompson et al. reported the generation of the 3MC state in blue phosphorescent Ir(III) complexes, 6–12.84 The temperature-dependences of τP in 2-MeTHF reveal two different lower- and higher-temperature regions. These can be analysed according to the Boltzmann model eqn (1):
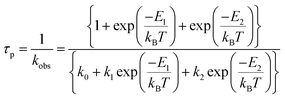 | (1) |
where, k0 is the decay rate from the lowest-energy triplet substate (TI), k1 and k2 are pre-exponential factors (decay rate constants), E1 and E2 are the activation energies for NR decay, kB is the Boltzmann constant, and T is temperature in kelvin. In the lower-temperature region, k1 = 105–106 s−1 and E1 = 40–120 cm−1 were obtained; the latter corresponds to the ZFS energies between substates I and III. The NR decay at low temperatures proceeds from the non-emissive substate III populated from the emissive substate I. In the higher-temperature region, E2 values of 4700 ± 100 (6 and 7), 3400 ± 100 (8 and 9), and 1600 ± 100 cm−1 (10–12) were obtained, along with k2 values of 4 × 1014 (6), 3.4 × 1013 (7), 5 × 1012 (8), 6.1 × 1012 (4), 1.2 × 1012 (10), 2.6 × 1011 (11), and 3.7 × 109 s−1 (12). The NR decay in the high-temperature region was attributed to crossing from the emissive lowest triplet state to the non-emissive 3MC state. The argument for this mechanism is supported by (E0–0 + E2) = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680–27
680–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 cm−1 for 6–11, respectively (corresponding to 74–76 kcal mol−1), which are close to the dissociation energies of Ir–ligand bonds. The large k2 values, which are associated with high-frequency vibrations accompanied by bond breaking, also support this interpretation. Based on the ‘Energy-Gap Law’, the knr value for vibration-coupled deactivation has been estimated as 4 × 103 s−1, sufficiently low for a minor impact of vibration-coupled decay on the net NR decay. Accordingly, the authors concluded that NR decay rates can be decreased and PLQY can be improved by increasing the energy separation between the emissive and non-emissive states. In addition, on the basis of a DFT calculation for 10, they propose a change from the 6-coordinated lowest triplet state to the 3MC state with a 5-coordinated trigonal bipyramid structure accompanied by rupturing of one Ir–N bond, as shown in Fig. 7. Later, structural change in the excited state is supported by theoretical calculations, which showed a lengthened Ir–N bond up to 2.70 Å in the 3MC state due to the strong σ-antibonding interactions between the metal and the N atom in the ppy ligand.85
370 cm−1 for 6–11, respectively (corresponding to 74–76 kcal mol−1), which are close to the dissociation energies of Ir–ligand bonds. The large k2 values, which are associated with high-frequency vibrations accompanied by bond breaking, also support this interpretation. Based on the ‘Energy-Gap Law’, the knr value for vibration-coupled deactivation has been estimated as 4 × 103 s−1, sufficiently low for a minor impact of vibration-coupled decay on the net NR decay. Accordingly, the authors concluded that NR decay rates can be decreased and PLQY can be improved by increasing the energy separation between the emissive and non-emissive states. In addition, on the basis of a DFT calculation for 10, they propose a change from the 6-coordinated lowest triplet state to the 3MC state with a 5-coordinated trigonal bipyramid structure accompanied by rupturing of one Ir–N bond, as shown in Fig. 7. Later, structural change in the excited state is supported by theoretical calculations, which showed a lengthened Ir–N bond up to 2.70 Å in the 3MC state due to the strong σ-antibonding interactions between the metal and the N atom in the ppy ligand.85
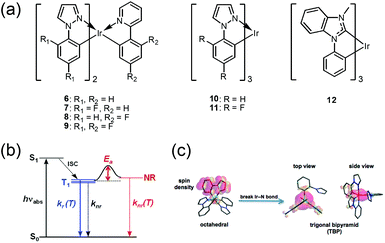 | ||
| Fig. 7 (a) Chemical structures of 6–12. (b) Three types of decay process from T1 state to the ground state: temperature-dependent radiative process, kr(T), or one of two NR decay processes. (c) Changes in chemical structures and spin density surfaces calculated for triplet states of six- and five-coordinated forms of 10. Adapted with permission from ref. 84. Copyright 2009, American Chemical Society. | ||
Haga et al. reported a similar phenomenon in Ir(III) complexes with tridentate pyrazolyl ligands, 13 and 14.86 The intense absorptions at 320–400 nm and weak bands at >410 nm were attributed to the 1MLCT transition and 3LC mixed with 3MLCT transitions, respectively. In those complexes, the emission spectra at 77 K are highly structured, with the 0–0 vibration band being highest; this is characteristic of emission from a 3LC-dominated state with a minor 3MLCT contribution. The emission behaviour is highly temperature-dependent, as revealed by the shift from considerably short lifetimes (0.18–140 ns) at 298 K to much longer lifetimes (3.9–13.1 μs) at 77 K. The temperature dependence of τp for 13 at 90–300 K reveal biphasic behaviour, which was analysed based on the following bi-exponential eqn (2):
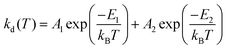 | (2) |
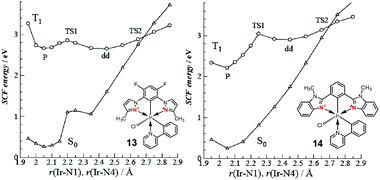 | ||
| Fig. 8 Potential–energy curves involving thermal deactivation of phosphorescent state (P) calculated as function of bond length Ir–N(1) (Ir–N(4)) for 13 and 14. Adapted with permission from ref. 86. Copyright 2008, American Chemical Society. | ||
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ em (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
| a Measured at 77 K. | ||||||||||
| 1 | 510 | 1.9 | 0.40 | 2.1 × 105 | 3.2 × 105 | 0.31 | — | — | — | 9 |
| — | — | — | — | — | — | 518 | (0.30, 0.63) | 14.3 | 160 | |
| 514 | — | — | — | — | — | 510 | (0.28, 0.63) | 5.7 | 88 | |
| 2 | 471, 495 | 1.74 | 0.94 | 5.5 × 105 | 0.3 × 105 | — | — | (0.15, 0.36) | 5.1 | 174 |
| 3 | 449, 479 | 1.08 | 0.66 | 6.1 × 105 | 3.1 × 105 | 0.28 | — | — | — | 80 and 96 |
| 4 | 428, 456 | 1.25 | 0.27 | 2.2 × 105 | 5.8 × 105 | 0.50 | — | — | — | 80 and 96 |
| 5 | 425, 450 | 0.15 | 0.03 | 2.0 × 105 | 6.5 × 106 | 0.72 | — | — | — | 80 and 96 |
| 6 | 500 | 1.7 | 0.95 | 5.6 × 105 | 0.3 × 105 | — | — | — | — | 84 |
| 7 | 475 | 2.6 | 0.93 | 3.6 × 105 | 0.3 × 105 | — | — | — | — | 84 |
| 8 | 500 | 1.2 | 0.55 | 4.6 × 105 | 3.8 × 105 | — | — | — | — | 84 |
| 9 | 457 | 1.3 | 0.60 | 4.6 × 105 | 3.1 × 105 | — | — | — | — | 84 |
| 10 | 412a | 0.002 | <0.01 | — | — | — | — | — | — | 84 |
| 11 | 388a | 0.007 | <0.01 | — | — | — | — | — | — | 84 |
| 12 | 382 | 1.1 | 0.37 | 1.1 × 105 | 3.4 × 105 | — | — | — | — | 84 |
| 13 | 456a | 0.0002 | <0.001 | — | — | 0.75 | — | — | — | 86 |
| 14 | 555 | 1.78 | 0.78 | 4.4 × 105 | 1.2 × 105 | 0.42 | — | — | — | 86 |
3. Design of blue-emissive Ir(III) complex
3.1. Introducing F atom(s) to cyclometalating ligand
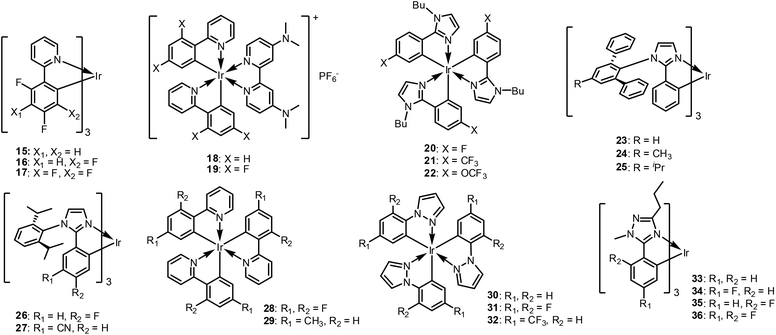
In 2006, De Cola et al. explored the number of F atoms in the same molecular architecture.89 When one more F atom is substituted at the 3-position of the phenyl ring, (16, Fig. 9), the emission shows a hypsochromic shift to 459 nm. Interestingly, however, the emission of the tetra-fluorinated complex (17, Fig. 9), shifts to lower energy at 468 nm. De Cola et al. suggested that the lower emission energy of 17 is due to the more positive reduction potentials caused by the presence of more F atoms. Note also that these two complexes have different emission quantum yields (0.30 and 0.53 for 16 and 17, respectively) and τem at RT (1600 and 2300 ns for 16 and 17, respectively). The highest EQE value of 5.5% was observed for an electroluminescent device containing 17. Those authors also observed different stabilities for devices fabricated with the fac- and mer-isomers. That is, the latter exhibited fast spectral changes in emission from the blue to green region of the spectrum.
Further theoretical studies, performed by Tian et al. in 2011 revealed that the differences between 16 and 17 are due to the different transition dipole moments.90 The same effects were also observed for cationic Ir(III) complexes. De Angelis et al. reported a combined experimental and theoretical study comparing 18 (a dfppy-based complex) and 19 (a ppy-based complex, see Fig. 9).91 Those authors controlled the phosphorescent emission wavelength and improved the quantum yields by modulating the electronic structures of the cyclometalated Ir(III) complexes through F functionalisation. The F-functionalised 19 showed blue-shifted emission at 463 nm with a higher PLQY (0.85) than 18 (λmax = 491 nm, PLQY = 0.80) in acetonitrile solution. This was related to a dramatic decrease in the NR deactivation rate constant, in agreement with the ‘Energy-Gap Law’. The τem values were also found to increase from 18 (2.4 μs) to 19 (4.1 μs), yielding an overall reduction in the kr; this suggests increasing π–π* character in the emitting excited states. DFT and TD-DFT calculations with solvent effects were conducted to characterise the lowest triplet excited states and revealed that the extensive mixing of the 3MLCT and π–π* contributions agrees with the τem increment for 19 compared to 18.
Other modifications were made by replacing phenyl pyridine ligands with phenyl heterocyclic ring systems, such as imidazole, pyrazole, and triazole. Note that imidazole-based homoleptic Ir(III) complexes have been used as blue dopants because the imidazole group can heighten the LUMO energy level, thereby enlarging the energy gap and increasing the T1 energy level. Kitamura et al. reported homoleptic and heteroleptic Ir(III) tirs(phenylimidazolinate) complexes.92 Upon replacement of the pyridyl ring with the imidazolyl ring, the LUMO mainly populates the phenyl ring; this is quite different from the LUMOs of ppy-based Ir(III) complexes. Accordingly, substitution of an F atom into the phenyl ring (20, Fig. 9) yields the most blue-shifted emission of 453 nm at RT (PLQY = 0.60), with a value of 446 nm at 77 K. In this case, π-electron-donating substituents can induce a blue-shift of the emission spectra. They fabricated an OLED device using 22, which exhibited efficient luminescence compared with a 1-based device. The two devices showed similar emission colours, but the emission luminance of the former was smaller (Lmax = 889 cd m−2 at 15 V compared to Lmax = 3490 cd m−2 at 13 V), because of inefficient carrier injection into the emitting layer.
Kang et al. also studied imidazole-based Ir(III) complexes, but with a bulky terphenyl unit on the N atom of the imidazoyl ring, (23–25, Fig. 9).93 Those researchers concluded that a terphenyl ligand without alkyl chains (23) is advantageous in terms of lifetime, whereas the terphenyl with alkyl chains (24, 25) is efficient in terms of PLQY. When employed in OLED devices, the EQEs of 24 and 25 were 21.1% and 21.3%, respectively, which were higher than that of 23 (19.2%). The authors explained that the high PLQYs of 24 and 25 assisted the triplet emission of the blue devices by effectively harvesting triplet excitons.
In 2018, Lee et al. reported diisopropylphenyl-functionalised phenylimidazole-based Ir(III) complexes (rather than a terphenyl group) (26 and 27, Fig. 9), which showed more blue-shifted emission at 454 nm.94 Even though the diisopropylphenyl group has lesser bulkiness than the terphenyl ring, this group still efficiently limits intermolecular aggregation and prevents the different self-quenching processes. Substitution of CN at position-5 (27), was found to significantly affect the device lifetime; a longer device lifetime exceeding 550 h at 200 cd m−2 was obtained, with EQEmax = 17.6% and CIE (0.15, 0.28).
Pyrazole-attached Ir(III) complexes were also investigated. For example, Thompson et al. reported that the MLCT transitions of the pyrazolyl-based complexes, 30–32 (Fig. 9) are hypsochromically shifted relative to pyridyl-based complexes (1, 28, and 29), due to the higher triplet energy of phenylpyrazole (3.28 eV) compared to ppy (2.88 eV).9 F substitution effectively shifts the emission further to the blue region, up to 21 nm. Notably, phosphorescence of 31 was observed at 390 nm, which has very rarely been reported to date. However, these homoleptic pyrazolyl-based complexes are not emissive at the RT. Therefore, pryrazolyl-based ligands tend to be used in heteroleptic rather than homoleptic systems (vide infra).
Samuel et al. previously reported a series of phenyl triazoletype Ir(III) complexes.80 As triazole has a higher LUMO energy than pyridine,95 replacement of the pyridyl moiety with a triazolyl ring was expected to shift the emission further to the blue region than that of the corresponding ppy-based Ir(III) complex. As expected, 6 has λmax at 449 nm, which is significantly blue shifted compared to that of 1 (λmax = 510 nm). Ir(III) complexes with F atom(s) 6–8 show hypsochromically shifted emissions relative to non-F-based complexes, by up to 425 nm. However, the PLQYs decrease with increased F atoms. This is because of the increased ligand triplet energy in the emissive energy state, which decreases the material radiative decay rate through vibronic-coupled NR decay, as explained in the previous section.
Powell et al. theoretically studied the effects of fluorination on the role of metal–ligand bond fission in the NR decay of excited states in cylcometalated Ir(III) complexes.26,96 The calculated activation barrier to the 3MC state shows a clear correlation with the experimentally obtained NR decay rate for a series of Ir(III) complexes. As 3MC state formation requires breaking of an Ir–N bond, Powell et al. compared Ir–N bond distances in the 3MC states of cylcometalated Ir(III) complexes. For 33 and 34 (Fig. 9), the Ir–N bond length changes in the 3MC state relative to the ground-state structure are much smaller than those of 35 and 36 (Fig. 9). Accordingly, the activation barrier to the 3MC state is lower for 35 and 36, which yields low PLQY.
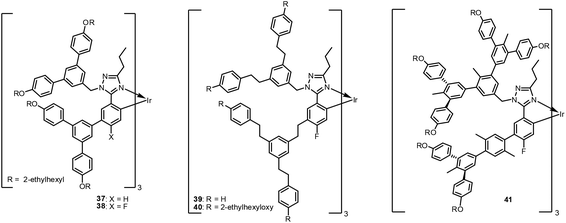
Samuel et al. extended their work to dendronised triazole-based Ir(III) complexes, to attain a solution-processable material.97–99 F-Attached dendrimers 38–40 (Fig. 10) show phosphorescence emissions at approximately 441 nm; however, these materials are unsuitable for device applications because of their low triplet energy and vibrational quenching, which yield luminescence quenching or low thermal properties.97,98 To solve this problem, dendrons were later modified to have a twisted geometry and successfully utilised in a blue PH-OLED device.99 Dendrimer 41 shown in Fig. 10 is highly emissive, with PLQY = 0.94 and 0.6, and τem of 3.6 and 2.8 μs, in solution and neat film, respectively. Its high emissivity is attributed to the dendron rigidifying effect, which reduces the geometry change in the excited state. Using 41, Samuel et al. fabricated a preliminary OLED device and showed EQEmax of 3.9% with CIE coordinates of (0.16, 0.17). Dendritic approaches are still being investigated, especially for solution-processed phosphorescent OLEDs.100–106 Available photophysical- and electrochemical data of 15–41 with their device performances are summarised in Table 2.
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ em (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
| a Measured at 77 K. | ||||||||||
| 15 | 468 | 1.6 | 0.43 | 2.7 × 105 | 3.6 × 105 | 0.78 | — | — | — | 9 |
| 495 | — | 0.06 | — | — | — | — | (0.27, 0.38) | 0.6 | 88 | |
| 16 | 459, 486 | 0.1 | 0.03 | 1.9 × 105 | 4.4 × 105 | — | — | — | — | 89 |
| 17 | 468, 497 | 0.1 | 0.03 | 2.3 × 105 | 2.0 × 105 | — | 478, 511 | — | 3.2 | 89 |
| 18 | 491, 520 | 2.4 | 0.80 | 3.3 × 105 | 0.8 × 105 | 0.72 | — | — | — | 91 |
| 19 | 463, 493 | 4.1 | 0.85 | 2.1 × 105 | 0.4 × 105 | 1.00 | — | — | — | 91 |
| 20 | 453, 482 | 3.4 | 0.60 | 1.7 × 105 | 1.2 × 105 | — | — | — | — | 92 |
| 21 | 486, 518 | 2.8 | 0.40 | 1.4 × 105 | 2.1 × 105 | — | — | — | — | 92 |
| 22 | 461, 492 | 3.3 | 0.53 | 1.6 × 105 | 1.4 × 105 | — | 464, 494 | (0.23, 0.35) | — | 92 |
| 23 | 463, 487 | — | 0.38 | — | — | — | — | (0.17, 0.30) | 19.2 | 93 |
| 24 | 459, 487 | — | 0.45 | — | — | — | — | (0.17, 0.28) | 21.1 | 93 |
| 25 | 462, 487 | — | 0.50 | — | — | — | — | (0.17, 0.29) | 21.3 | 93 |
| 26 | 454, 484 | 1.1 | 0.87 | 7.9 × 105 | 1.2 × 105 | — | 455, 484 | (0.17, 0.30) | 18.9 | 94 |
| 27 | 462, 494 | 1.8 | 0.99 | 5.5 × 105 | 0.5 × 104 | — | 462, 494 | (0.15, 0.28) | 22.5 | 94 |
| 28 | 468 | 1.6 | 0.05 | 2.7 × 105 | 3.6 × 105 | 0.78 | — | — | — | 9 |
| 29 | 510 | 2.0 | 0.50 | 2.5 × 105 | 2.5 × 105 | 0.30 | — | — | — | 9 |
| 30 | 414a | 14a | — | — | — | 0.39 | — | — | — | 9 |
| 31 | 390a | 27a | — | — | — | 0.80 | — | — | — | 9 |
| 32 | 428 | 0.05 | — | — | — | 0.73 | — | — | — | 9 |
| 33 | 449 | 1.08 | 0.66 | 6.1 × 105 | 3.1 × 105 | 0.28 | — | — | — | 96 |
| 34 | 443 | 0.15 | 0.06 | 4.0 × 105 | 6.3 × 105 | 0.50 | — | — | — | 96 |
| 35 | 428 | 1.25 | 0.27 | 2.2 × 105 | 5.8 × 106 | 0.50 | — | — | — | 96 |
| 36 | 425 | 0.15 | 0.03 | 2.0 × 105 | 6.5 × 106 | 0.72 | — | — | — | 96 |
| 37 | 468, 495 | 2.8 | 0.76 | 2.7 × 105 | 0.9 × 105 | 0.31 | — | (0.18, 0.35) | 7.9 | 98 |
| 38 | 441, 468 | 22.0 | 0.59 | 0.3 × 105 | 0.2 × 105 | 0.53 | — | — | — | 97 and 99 |
| 39 | 441, 468 | 1.7 | 0.46 | — | — | 0.47 | — | — | — | 98 and 99 |
| 40 | 441, 470 | 1.7 | 0.45 | 2.7 × 105 | 3.3 × 105 | 0.45 | — | — | — | 98 and 99 |
| 41 | 435, 465 | 3.6 | 0.94 | 2.6 × 105 | 1.7 × 104 | 0.61 | 438, 466 | (0.16, 0.16) | 3.9 | 99 |
Thompson et al. reported photophysical and electrochemical properties of nineteen cyclometalated Ir(III) complexes with various ancillary ligands and three types of main ligand (dfppy, ppy, and terpyridine (tpy)).106 The ancillary ligands were chosen to be ‘non-chromophoric’, so as to drive the excited-state properties dominated by the main ligands and Ir. For this approach, the 3LC state energy was expected to be relatively constant for all complexes, whereas the 1MLCT state energies could be altered by varying the ancillary ligand. As expected, the F-substituted main ligand (dfppy) showed a blue-shifted emission relative to the tpy-based Ir(III) complex. The effects of the ancillary ligands on the excited-state properties of the cyclometalated Ir(III) complexes were independent of the choice of main ligand. That is, the cyclometalated Ir(III) complexes with the same ancillary ligands but different main ligands exhibited different kr values. Thompson et al. explained that the degree of 1MLCT character mixed into the T1 state decreases when the Ir(III) complex has a deeper HOMO energy level. This yields an increased energy gap between the singlet and triplet energies and consequently, lowers the kr values.
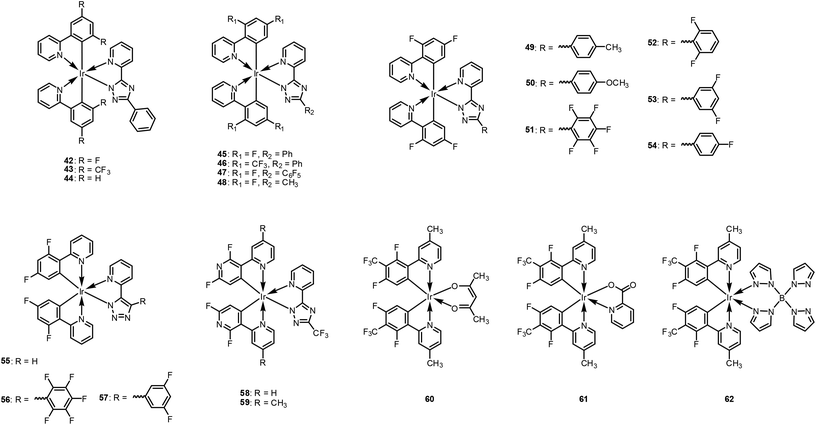
De Cola et al. studied triazole-based ancillary ligand systems, 42–48, as described in Fig. 11.107 Those researchers designed ppy main ligands while varying the phenyl-ring substitution positions at 4/6 or 5 with different substituents, i.e., H, F, and CF3. The ancillary ligands were based on phenyltriazole with substituent variations at the triazole ring positions. They performed a detailed theoretical analysis of the effects of the substituents in the main ligand to study the emissive state properties. Calculation results showed that the substituents not only affect the emission energy but, also, change the ordering of the lowest excited triplet states. The same group reported a series of heteroleptic Ir(III) complexes, 45, 48, and 49–54 (Fig. 11), in which the 1,2,4-triazole ancillary ligands are varied with different substituents while the main ligand is fixed as dfppy.108 By increasing the electron-withdrawing ability, the HOMO energy level is lowered, and consequently, the HOMO–LUMO gap is widened. This yields blue-shifted emission with narrower full width at half maximum. In this series, complexes 51 and 52 exhibit lower PLQY, which may be due to the torsional angle between the phenyl and triazole rings arising from F atoms in the ortho position. In these cases, the lowest MLCT states are shifted from the pyridyl-triazole to the ppy. Two preliminary devices were constructed using 49 and 51, with both exhibiting EQEs exceeding 7% together with a blue colour (CIEx,y = 0.17, 0.27). De Cola et al.'s work was extended to 1,2,3-triazole-based Ir(III) complexes, 55–57 (Fig. 11).109 The emission spectra in the solution for 1,2,3-triazole-based complexes are slightly blue-shifted with respect to their analogous complexes with 1,2,4-triazole moieties, 45, 48, and 49–54, having enhanced colour purity to blue.
Bryce et al. introduced F-substituted 2,3′-bipyridine derivatives rather than ppy derivatives as the main ligand, to lower the HOMO level and develop efficient deep-blue phosphors, i.e., 58 and 59 (Fig. 11).110 Both of these complexes show intense blue phosphorescence emission at 437 and 435 nm, respectively, with high PLQYs exceeding 0.65. When implemented in OLED devices, the 59-based device exhibited 13% EQE with a deep blue colour (CIEx,y = 0.16, 0.17).
Recently, our group investigated the role of the ancillary ligand on the emission behaviours.111 A series of heteroleptic Ir(III) complexes, 60–62, were prepared, comprised of 2-(2,4-difluoro-3-(trifluoromethyl)phenyl)-4-methylpyridine (dfCF3) as the main ligand and the following different ancillary ligands: acetylacetonate (60), picolinate (61), and tetrakis-pyrazolyl borate (62). The heteroleptic Ir(III) complexes of 60–62 exhibit emission peaks at 470, 455, and 450 nm, respectively, in dichloromethane solution. For 60–62, the Huang–Rhys factors are estimated to be 0.76, 0.87, and 0.97, respectively, as shown in Fig. 12a. Interestingly, however, the NR rate constants are in the order 60 (4.89 × 105 s−1) > 61 (1.17 × 105 s−1) > 62 (0.28 × 105 s−1). To explain this phenomenon, we measured the temperature-dependent τem to estimate the activation barrier from the radiative triplet state to NR state, 3MC. The activation barriers were calculated as 46, 61, and >100 meV for 60–62, respectively. Theoretical quantum chemical calculations were also conducted to support the experimental data. According to these calculations, the activation barrier for 62 (7.9 kcal mol−1) is significantly higher than those of 60 (1.7 kcal mol−1) and 61 (2.9 kcal mol−1), as depicted in Fig. 12b. We suggested that reorganisation to a trigonal bipyramidal geometry may be difficult because of the steric demands of the borate ligand. Further, the bulkiness of the borate ligand may restrict the free rotation of the pyrazolyl group. Thus, the activation barrier to 3MC for 62 is much higher than those of 60 and 61. Accordingly, a 62-based blue phosphorescent OLED device exhibited the best performance among the series, with high current and power efficiencies of 32.9 cd A−1 and 25.4 lm W−1, respectively. Available photophysical- and electrochemical data of 49–62 with their device performances are summarised in Table 3.
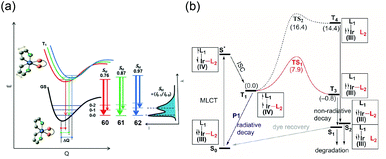 | ||
| Fig. 12 (a) Energy potential curves with Huang–Rhys factors for 60–62 and (b) reaction profiles for Ir–N bond cleavage to form NR states for 62. Adapted with permission from ref. 111. Copyright 2009, The Royal Society of Chemistry. | ||
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ em (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
| 49 | 463, 492 | 0.1 | 0.03 | 2.0 × 105 | 2.8 × 105 | 0.91 | — | — | — | 108 |
| 50 | 464, 492 | 0.1 | 0.01 | 1.2 × 105 | 0.8 × 105 | 0.91 | — | — | — | 108 |
| 51 | 458, 487 | 0.2 | 0.04 | 3.0 × 105 | 1.6 × 106 | 1.02 | 461, 490 | (0.17, 0.27) | 7.4 | 108 |
| 52 | 460, 490 | 0.1 | 0.04 | 2.7 × 105 | 1.4 × 106 | 0.99 | — | — | — | 108 |
| 53 | 459, 488 | 0.2 | 0.04 | 2.9 × 105 | 7.1 × 105 | 0.99 | — | — | — | 108 |
| 54 | 459, 489 | 0.1 | 0.03 | 2.1 × 105 | 4.2 × 105 | 0.97 | — | — | — | 108 |
| 55 | 460, 489 | 1.2 | 0.32 | 2.6 × 105 | 5.6 × 105 | 0.98 | 495 | (0.18, 0.40) | 0.4 | 109 |
| 56 | 457, 487 | 1.3 | 0.32 | 2.3 × 105 | 5.3 × 105 | 1.02 | — | — | — | 109 |
| 57 | 458, 487 | 1.1 | 0.27 | 2.5 × 105 | 6.8 × 105 | 0.99 | 495 | (0.17, 0.40) | 0.48 | 109 |
| 58 | 437, 466 | 3.0 | 0.65 | 2.2 × 105 | 1.2 × 105 | — | 430–440 | (0.15, 0.13) | 11.2 | 110 |
| 59 | 435, 464 | 3.0 | 0.70 | 2.3 × 105 | 1.0 × 105 | — | 460–470 | (0.14, 0.11) | 13.0 | 110 |
| 60 | 470, 494 | 0.9 | 0.56 | 6.2 × 105 | 4.9 × 105 | 0.88 | — | (0.14, 0.26) | 19.9 | 111 |
| 61 | 455, 484 | 1.8 | 0.79 | 4.4 × 105 | 1.2 × 105 | 1.12 | — | (0.14, 0.18) | 15.5 | 111 |
| 62 | 450, 478 | 4.6 | 0.87 | 1.9 × 105 | 0.3 × 105 | 1.18 | — | (0.14, 0.20) | 22.6 | 111 |
3.2. Strategies for retarding the NR decay process
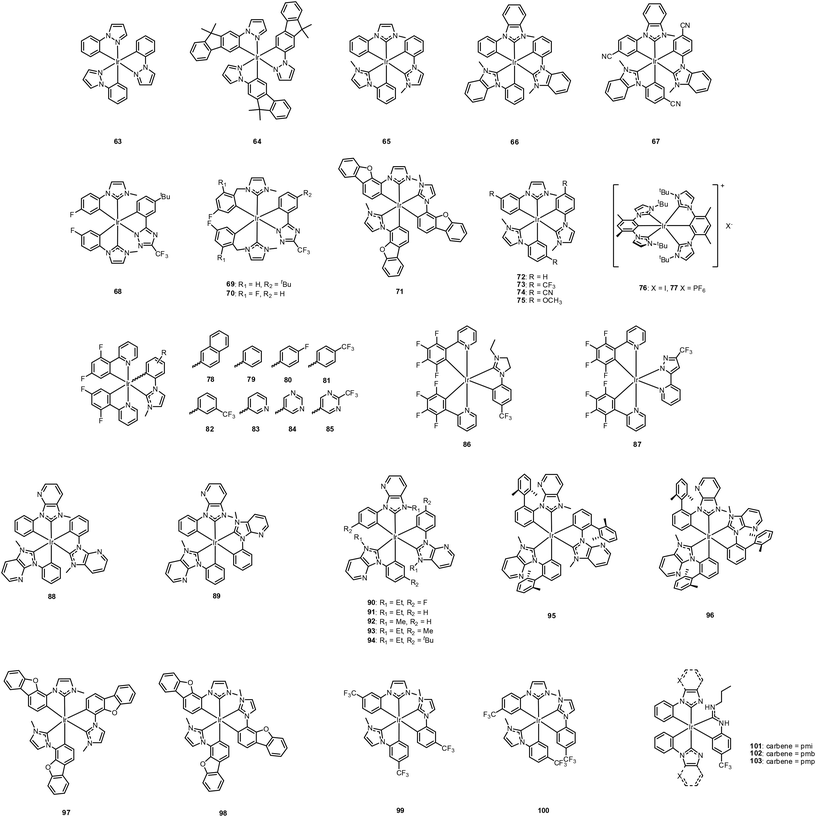
As deep-blue-emitting compounds have high-energy T1 states, the 3MC state, which is a NR state, can be generated using relatively small thermal energies, as described above. Generation of this NR state not only decreases the PLQY, but also promotes electrons into metal–ligand σ* orbitals. The latter induces deformation of the Ir–N bond that limits photostability and causes device efficiency degradation.127 Therefore, strategies to retarding 3MC state generation for deep-blue emissive cyclometalated Ir(III) complexes are necessary. Note that the modification must only affect the 3MC state and not the frontier orbitals, which are involved in the luminescent T1 state. The following are some strategies developed for this purpose.Later, Da Como et al. prepared CN-substituted pmb-based Rh, Pt, and Ir carbene complexes to investigate the role of the SOC and ΔEST in controlling the radiative phosphorescence rate.132 The CN-substituted Ir(III) complex, 67 (Fig. 14), exhibits enhanced PLQY up to 0.78, much higher than 66 (note that the PLQY of 66 was later corrected from 0.04 to 0.37).57 This idea was extended to heteroleptic Ir(III) complexes with F-coordinated benzyl carbene main ligands and a 2-pyridyl triazolate ancillary ligand by Wu et al., who reported complexes 68–70 (Fig. 14).133 Those researchers observed significant blue-shifted emission through F-atom attachment, along with higher PLQY with insertion of a saturated methylene spacer in the main ligand. The enhanced PLQY was rationalised by estimating different NR decay rate constants for the reported complexes. Among the considered complexes, 70 was applied as a dopant in a blue OLED device. CIE coordinates of (0.158, 0.128) with an EQE of up to 6.0% were obtained. However, this value dropped to 2.7% at a practical brightness of 100 cd m−2. The results of a further theoretical investigation revealed remarkably destabilised HOMO energies through introduction of a methylene spacer in 69 and 70; thus, the HOMO–LUMO energy gaps in these complexes were increased.134
Kido et al. reported another carbene complex, 71, that exhibited blue emission with λmax at 445 nm.135 When 71 was doped with 3,6-bis(diphenylphosphoryl)-9-phenylcarbazole at 10 wt% (PO9, host) in film, high ηPL values of 0.70 were obtained with a τp of 19.6 μs at RT. This behaviour was compared with 1 and it was concluded that the strong ligand field effect in the carbene complex induces significant shifts in the d-orbital energies, which then facilitates high PLQY and longer lifetime. Additionally, 71 was combined with other red and green phosphors to fabricate a white OLED; the device exhibited ηp,max and ηp,1000 values of 59.9 and 43.3 lm W−1, respectively. Karatsu et al. systematically studied substitution effects on Ir(pmb)3 (66) moieties, 72–75 (Fig. 14), using electron-donating (–OCH3) or withdrawing (–CF3 and –CN) groups on the phenyl ring.136 The luminescent properties were found to be greatly affected by the functional group on the phenyl moiety, whereas the geometries and electrochemical properties remained within a similar range.
In 2013, De Cola et al. first reported efficient deep-blue-emissive cationic bi-pincer Ir(III) carbene complexes, 76 and 77 (Fig. 14), which were obtained using a pincer-type ligand, (4,6-dimethyl-1,3-phenylene-κC2)bis(1-butylimidazol-2-ylidene), with I− and PF6− as counter anions.137 Both complexes exhibit vibronic progression from the 3LC/3MLCT excited states with two main emission maxima at 394 and 406 nm. The PLQYs of 76 and 77 in solution are higher than those of the other reported bis-tridentate Ir(III) complexes at 0.41 and 0.38, respectively, because of the presence of strong-field ligands and a more rigid tridentate system. Interesting features are found in the solid state. The crystals of 76 exhibit a large, dominant redshifted emission at 500 nm, whereas those of 77 exhibit dual emission with the original high-energy emission and an additional low-energy emission at approximately 500 nm. The effect of the counter anion is remarkably apparent in the amorphous film. At a 50% doping ratio, significant low-energy emission appears for 76; however, 77 exhibits only a minor band in this region. Although the fabricated preliminary device exhibited very low efficiency (<1%), the EL spectrum showed saturated blue emission with maxima at 386 and 406 nm.
A series of N-heterocyclic carbene Ir(III) complexes, 78–85 (Fig. 14), having dfppy as the main ligand and NHCs as ancillary ligands, were systematically studied by Zuo et al. in 2015.138 By modifying the phenyl moiety in the NHCs with electron-withdrawing substituents or replacing the phenyl ring with N-heteroaromatic rings, the HOMO–LUMO gaps were increased and the emissions were blue-shifted accordingly. Among this series of carbene-based Ir(III) complexes, the 82-based blue phosphorescent OLED device exhibits good performance, with a maximum ηc of 37.83 cd A−1, an EQE of 10.3%, and an Lmax of 8709 cd m−2. More recently, Wong et al., reported heteroleptic iridium(III) complexes with NHC and acidic pyrazolyl-pyridine (fppz) moieties as ancillary ligands, 86 and 87, respectively.139 These ancillary ligands successfully maintained the relatively high LUMO energy levels, because the LUMOs of these Ir(III) complexes are almost completely located on the antibonding π* orbital of the pyridyl ring of the main ligand, F4ppy. Thus, sky-blue emissions at a wavelength of around 465 nm with PLQYs of over 0.60. Accordingly, OLED devices were fabricated which afford high luminance values of over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 at a driving voltage of 10 V with peak current efficiencies of 47.6 and 45.5 cd A−1, corresponding to EQEs of 20.6% and 19.6% for 86-based and 87-based devices, respectively.
000 cd m−2 at a driving voltage of 10 V with peak current efficiencies of 47.6 and 45.5 cd A−1, corresponding to EQEs of 20.6% and 19.6% for 86-based and 87-based devices, respectively.
In 2016, impressive device efficiency enhancement using carbene-based Ir(III) complexes was reported by the Forrest group.140N-Phenyl, N-methylpyridoimidazole was used as a cyclometalating ligand and both fac- (88) and mer- (89) isomers of Ir(pmp)3 were prepared (Fig. 14). Although their absorption and emission a slightly red-shifted compared to 66, from 380 to 418 nm for 88 and to 465 nm for 89, both complexes exhibit significantly higher PLQYs (≈0.77) compared to 66 (0.37).57 This enhancement is due to the N atom in the pyridoimidazol-2-yl moiety, which modulates the LUMO energy level. As a result, the emissive triplet state is stabilised, and a concomitant increase is obtained in the energy gap between the emissive T1 state and the non-emissive 3MC state. Notably, both complexes show enhanced device performance. That is, the 88-based device achieves a remarkable reduction in efficiency roll-off at high current density with high luminance (EQE = 10.1%), along with a deep-blue colour coordinate of (0.16, 0.09). The 89-based device shows even higher efficiency with a high EQE of 14.4% and high luminance of >22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. In particular, 88 can serve as the HTL and EBL material simultaneously. Available photophysical- and electrochemical data of 63–103 with their device performances are summarised in Table 4.
000 cd m−2. In particular, 88 can serve as the HTL and EBL material simultaneously. Available photophysical- and electrochemical data of 63–103 with their device performances are summarised in Table 4.
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ em (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
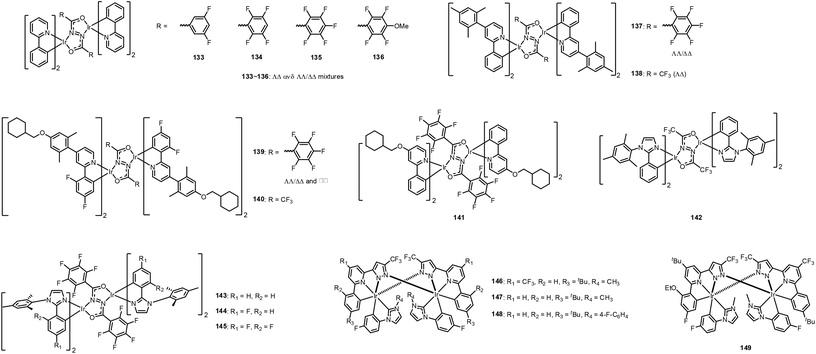
| a Measured at 77 K. b Doped film. | ||||||||||
| 63 | 414a | 14.0 | — | — | — | 0.41 | — | — | — | 131 |
| 64 | 480 | 37.0 | 0.38 | 0.1 × 105 | 0.2 × 105 | 0.31 | — | — | — | 131 |
| 65 | 380a | 0.4 | 0.02 | 0.5 × 105 | 2.0 × 106 | 0.22 | — | — | — | 131 |
| 66 | 389 | 0.22 | 0.04 | 1.8 × 105 | 4.3 × 106 | — | — | — | — | 131 |
| 67 | 380 | — | 0.78 | 4.9 × 104 | 1.3 × 104 | — | — | — | — | 132 |
| 68 | 392, 461 | 0.001 | <0.001 | 6.0 × 105 | 1.2 × 109 | — | — | — | — | 133 |
| 69 | 460 | 0.22 | 0.22 | 1.0 × 106 | 3.5 × 106 | — | — | — | — | 133 |
| 70 | 458 | 0.38 | 0.73 | 1.9 × 106 | 7.0 × 105 | — | 434, 460 | (0.16, 0.13) | 6.0 | 133 |
| 71 | 445 | 19.6b | 0.70b | 0.4 × 106 | 0.2 × 105 | — | — | — | — | 135 |
| 72 | 390, 407 | 1.3 | 0.44 | 3.4 × 10−5 | 4.3 × 10−5 | 0.45 | — | — | — | 136 |
| 73 | 396, 416 | 6.1 | 0.84 | 1.4 × 10−5 | 0.3 × 10−5 | 0.74 | — | — | — | 136 |
| 74 | 521, 445 | 14.0 | 0.71 | 0.5 × 10−5 | 0.2 × 10−5 | 0.84 | — | — | — | 136 |
| 75 | 403, 415 | 5.0 | 0.76 | 1.5 × 10−5 | 0.5 × 10−5 | 0037 | — | — | — | 136 |
| 76 | 384, 406 | 8.9 | 0.41 | 4.6 × 10−5 | 6.6 × 10−5 | 0.68 | — | — | — | 137 |
| 77 | 384, 406 | 9.4 | 0.38 | 4.1 × 10−5 | 6.5 × 10−5 | 0.65 | 386, 406 | — | — | 137 |
| 78 | 483 | 2.1 | 0.14 | 0.6 × 105 | 3.7 × 105 | — | — | — | — | 138 |
| 79 | 483 | 1.8 | 0.65 | 3.6 × 105 | 1.9 × 105 | — | — | — | — | 138 |
| 80 | 473 | 1.8 | 0.73 | 4.1 × 105 | 1.5 × 105 | — | — | — | — | 138 |
| 81 | 469 | 1.9 | 0.57 | 3.0 × 105 | 0.2 × 105 | — | — | — | — | 138 |
| 82 | 469 | 1.8 | 0.69 | 3.8 × 105 | 1.7 × 105 | — | 470 | — | 10.3 | 138 |
| 83 | 473, 498 | 1.8 | 0.61 | 3.4 × 105 | 2.2 × 105 | — | — | — | — | 138 |
| 84 | 471, 497 | 1.7 | 0.33 | 1.9 × 105 | 3.9 × 105 | — | — | — | — | 138 |
| 85 | 455, 479 | 1.9 | 0.32 | 1.7 × 105 | 3.6 × 105 | — | — | — | — | 138 |
| 86 | 469, 462 | 17.6 | 0.60 | 0.3 × 105 | 0.2 × 105 | 465 | (0.19, 0.39) | 20.6 | 139 | |
| 87 | 462, 459 | 16.6 | 0.68 | 0.4 × 105 | 0.2 × 105 | — | (0.19, 0.37) | 19.6 | 139 | |
| 88 | 418 | 1.2 | 0.76 | 6.4 × 105 | 2.0 × 105 | 0.23 | — | — | — | 140 |
| 89 | 465 | 0.8 | 0.78 | 1.0 × 106 | 2.7 × 105 | — | — | — | — | 140 |
| 90 | 430 | 3.8 | 0.98 | 2.6 × 105 | 0.5 × 104 | — | — | — | — | 142 |
| 91 | 454 | 4.5 | 0.85 | 1.9 × 105 | 0.3 × 105 | — | — | (0.15, 0.19) | 7.6 | 142 |
| 92 | 468 | 4.3 | 0.99 | 2.3 × 105 | 0.2 × 104 | — | — | (0.15, 0.19) | 10.8 | 142 |
| 93 | 469 | 4.7 | 0.45 | 1.0 × 105 | 1.2 × 105 | — | 450 | (0.15, 0.19) | 15.2 | 142 |
| 94 | 422 | 5.3 | 0.33 | 0.6 × 105 | 1.2 × 105 | — | — | — | — | 142 |
| 95 | 515 | 0.17 | 0.03 | 1.8 × 105 | 5.9 × 106 | 0.43 | — | — | — | 143 |
| 96 | 555 | 0.0037 | 0.001 | 2.7 × 105 | 2.7 × 108 | 0.29 | — | — | — | 143 |
| 97 | 444, 472 | 11.2 | 0.68 | 6.1 × 104 | 2.9 × 104 | (0.14, 0.11) | 18.5 | 144 | ||
| 98 | 450, 477 | 11.0 | 0.53 | 4.8 × 104 | 4.1 × 104 | (0.14, 0.14) | 18.2 | 144 | ||
| 99 | 414, 424 | 0.3, 1.8 | 0.25 | — | — | 0.88 | — | — | — | 121 |
| 100 | 412, 427 | 0.7, 1.8 | 0.72 | — | — | 0.80 | 431 | (0.15, 0.08) | 7.2 | 121 |
| 101 | 418b | 6.1b | 0.13b | 0.2 × 10−5 | 1.4 × 10−5 | 0.16 | — | — | — | 145 |
| 102 | 418b | 1.8b | 0.31b | 1.7 × 10−5 | 3.8 × 10−5 | 0.21 | — | — | — | 145 |
| 103 | 459b | 0.9b | 0.48b | 5.6 × 10−5 | 6.1 × 10−5 | 0.25 | — | — | — | 145 |
Zhou and Powell later calculated the possible reaction pathways of 88 and 66 to the NR 3MC states, the correlation between the lowest activation barrier to the 3MC states, and the experimental NR rate constants.141 According to their calculations, the Ir–C bond of the NHC ligand is mostly elongated in the excited state for both isomers. In addition, those authors calculated the key parameters of the T1 states: (1) the Franck–Condon state in the ground-state, (2) the optimised 3MLCT state, (3) an intermediate 3MC state, and (4) the optimised 3MC state. When a benzannulated component of the NHC ligands is replaced with a fused pyridyl ring, transitioning from 66 to 88, the energy barrier to the NR state is increased. Zhou and Powell explained that the presence of the N atom with greater electronegativity may strengthen the Ir–C (NHC) bond and generate a higher energy barrier in the 3MC state, yielding a slower knr for 88. More importantly, they found that the metal–ligand bond in the 3MC state does not break away and is reversible. The PLQY enhancement of 88 may be due to this unique property.
In 2017, Wong et al. prepared a series of N-heterocyclic carbene Ir(III) complexes, 90–94, to study the effect of electron-withdrawing/donating nature of the substituent on the phenyl ring by modifying 89.142 The device fabricated using 94 (Fig. 14) exhibited the maximum EQE, at 19.0% with restricted efficiency roll-off. Recently, Kang et al. modified the phenyl ring of 88 with a bulkier substituent, a xylyl ring at the ortho positions of pmb, to investigate the NR decay pathway according to the ligand bulkiness.143 The detailed photo-dynamics in the excited states were studied using transient absorption and time-resolved emission techniques in a series of Ir(III) complexes. When the bulkier ligand was attached, the PLQYs of both the fac- (95, Fig. 14) and mer- (96) isomers were anomalously quenched in the solution at 300 K. They found new, broad TA bands for 95 and 96 at approximately 720 nm with increasing delay time; this band was associated with structural changes in the excited triplet state which yielded fast localisation migration via inter-ligand charge transfer and influenced the deactivation pathway to quench the emission intensity.
N-Dibenzofuranyl-N-methylimidazole-based Ir(III) carbene complexes with fac- (97) and mer- (98, Fig. 14) isomers was also reported by our group.144 For the previously reported Ir(III) carbene complexes, the mer-isomer is equally or more efficiently luminescent than the fac-isomer in solution and in the solid state;131,140 however, the fac-isomer labelled 97 exhibits better luminescence properties in solution and device application than the mer-isomer labelled 98. We rationalised this difference using DFT calculations, which revealed that the energy barrier from T1 to 3MC of 98 is lower than that of 97. Among the two OLED devices, the 97-based device showed a higher EQE value (18.5%) than the 98-based device (18.2%). Furthermore, the CIEx,y for the 97-based device showed a deeper blue coordination of (0.14, 0.11) compared to the 98-based device with (0.14, 0.14).
Zysman-Colman et al. reported two kinds of –CF3 functionalised mer-Ir(pmi)3 complexes, 99 and 100 (Fig. 14), in which the substitution position of –CF3 on the phenyl ring was varied to obtain a high PLQY through sufficient stabilisation of the HOMO energy level.121 Both complexes show structured deep-blue emission (λmax ≈ 425 nm) in degassed dichloromethane at RT. However, the PLQYs are significantly affected depending on the substitution position. For 99, the PLQY is relatively low at 0.25, whereas that of 100 is considerably higher at 0.72, being comparable to 89. An optimised device with 100 as a blue dopant and 99 as an efficient exciton/electron blocker exhibited deep-blue CIE coordinates of (0.154, 0.052), with an EQEmax of 13.4% and an EQE of 12.5% at 100 cd m−2.
Most recently, Teets et al. reported a new type of Ir(III) carbene complex with an acyclic diaminocarbene (ADC) as an ancillary ligand, 101–103 (Fig. 14), referred to as a ‘mixed-carbene complex’.145 These mixed-complexes were prepared through a cascade reaction with nucleophilic addition reaction followed by base-assisted cyclometalation reaction of the ADC intermediate. Compared to homoleptic Ir(III) carbene complexes, the mixed-carbene complexes show the same or even better photoluminescence characteristics; for example, higher PLQY. As ADC is an even stronger σ-donor ligand than NHCs, because of its greater 2p in its s orbital, the molecular design strategy for saturated blue emissive material may be extended following this approach.
3.3. Rigid structure for restricted intramolecular motion
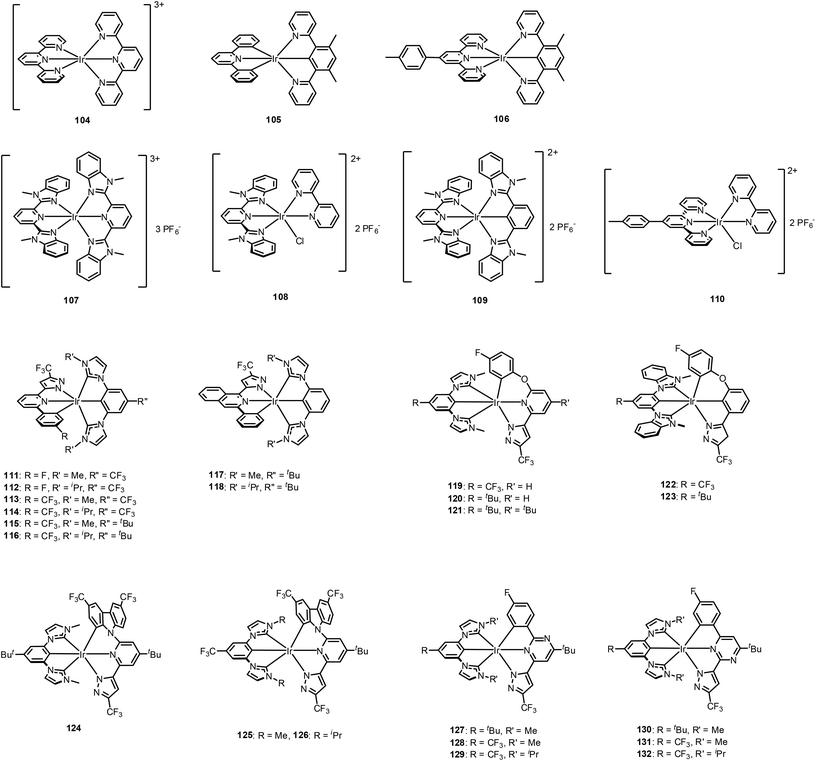
Increments of PLQY are often observed for cyclometalated Ir(III) complexes in solid states, such as frozen solutions and doped polymer films. For example, Chou et al. found that the solid-state PLQY can be increased by more than one order compared to that in solution.146 This result suggests that an cyclometalated Ir(III) complex with rigid conformation that can suppress motional relaxations may yield increased PLQY. (The structural origin of these behaviours can be understood by considering the temperature-independent decay process (vibronic-coupled NR decay process) described above.) Therefore, cyclometalated Ir(III) complexes with rigid structures have been extensively studied for restriction of intramolecular motions to minimise the NR decay process.Following the above works, extensive studies were subsequently performed to develop a blue-emissive bis-tridentate Ir(III) complex. In 2016, the Chi group reported cooperation between bis(imidazolylidene) benzenes and functionalised 2-pyrazolyl-6-phenyl pyridine (or isoquinoline) chelates ligands to form bis-tridentated Ir(III) complexes, 111–118 (Fig. 15).155 Although the most blue-shifted emission among this series is at 467 nm for solution 111, the methyl-substituted complexes exhibit almost unitary PLQY whereas the isopropyl-substituted complexes exhibit lower PLQY. This difference was rationalised by considering the reduction in the torsional vibration degrees of freedom for the methyl groups. The researchers also noted that increases in the rigidity and multidentate coordination mode are key factors yielding the excellent PL and EL efficiency obtained for these complexes. Depending on the π-conjugation and/or electronic characteristics of the chelates, the emission colour can be finely tuned. Accordingly, the authors successfully fabricated efficient, independent red, green, and blue OLED devices. They also obtained a white OLED device by combining these dopants in the emitting layer. For example, 95% doping in a 9-(3-(9H-carbazol-9-yl)phenyl)-9H-carbazole-3-carbonitrile (mCPCN) host yielded blue-emitting OLEDs with an EQE of 27% and CIEx,y of (0.18, 0.40). To enlarge the HOMO–LUMO energy band gap of this sky-blue dopant, the same group designed new Ir(III) complexes in which the 6-pyrazolyl-2-ppy was replaced with 6-pyrazolyl-2-phenoxylpyridine to break the π-conjugation between the pyridyl and fluorophenyl units.156 As expected, all emission spectra of these 6-pyrazolyl-2-phenoxylpyridine based complexes, 119–121(Fig. 15), are clearly blue-shifted versus the emission spectra for the 6-pyrazolyl-2-ppy-based Ir(III) complexes, 111–116 (Fig. 15). Note that deep-blue EL with an EQE of >20% and CIEx,y = (0.15, 0.17) were achieved using 121 as a dopant material. Available photophysical- and electrochemical data of 104–132 with their device performances are summarised in Table 5.
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ em (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
| 104 | 506 | 9.5 | 0.11 | 0.1 × 105 | 1.0 × 105 | >1.7 | — | — | — | 153 |
| 105 | 585 | 3.9 | 0.21 | 3.4 × 105 | 1.2 × 106 | — | — | — | — | 152 |
| 106 | 560 | — | — | — | — | — | — | — | — | 152 |
| 107 | 550, 592 | 1.0 | 0.04 | 0.4 × 105 | 8.9 × 106 | 1.65 | — | — | — | 153 |
| 108 | 547, 582 | 5.7 | 0.19 | 0.3 × 105 | 1.4 × 106 | 1.64 | — | — | — | 153 |
| 109 | 593, 623 | 1.6 | 0.10 | 0.6 × 105 | 5.7 × 105 | 1.18 | — | — | — | 153 |
| 110 | 509, 541 | 1.2 | 0.04 | 0.4 × 105 | 9.1 × 105 | >1.7 | — | — | — | 153 |
| 111 | 467, 501 | 5.4 | 0.99 | 1.8 × 105 | 0.1 × 104 | — | — | (0.18, 0.40) | 27.0 | 155 |
| 112 | 468, 503 | 6.7 | 0.86 | 1.3 × 105 | 0.2 × 105 | — | — | (0.19, 0.39) | 20.8 | 155 |
| 113 | 490, 526 | 4.7 | 0.92 | 2.0 × 105 | 0.2 × 105 | — | — | (0.29, 0.57) | 30.0 | 155 |
| 114 | 492, 529 | 4.4 | 0.80 | 1.8 × 105 | 0.5 × 105 | — | — | (0.28, 0.57) | 28.3 | 155 |
| 115 | 501, 535 | 2.8 | 1.00 | 3.6 × 105 | — | — | — | (0.34, 0.60) | 31.4 | 155 |
| 116 | 503, 537 | 3.0 | 0.98 | 3.3 × 105 | 0.7 × 104 | — | — | (0.34, 0.60) | 30.0 | 155 |
| 117 | 593, 643 | 8.2 | 1.00 | 1.2 × 105 | — | — | — | (0.63, 0.38) | 27.4 | 155 |
| 118 | 595, 647 | 6.9 | 0.98 | 1.4 × 105 | 0.3 × 104 | — | — | (0.63, 0.38) | 20.0 | 155 |
| 119 | 471 | 25.1 | 0.81 | 0.3 × 105 | 0.7 × 104 | 0.67 | — | — | — | 156 |
| 120 | 478 | 4.42 | 0.82 | 1.9 × 105 | 0.4 × 105 | 0.53 | — | (0.15, 0.24) | 19.7 | 156 |
| 121 | 472 | 8.66 | 0.72 | 0.8 × 105 | 0.3 × 105 | 0.52 | — | (0.15, 0.17) | 20.7 | 156 |
| 122 | 473 | 61.2 | 0.68 | 0.1 × 105 | 0.5 × 104 | 0.80 | — | — | — | 156 |
| 123 | 473 | 18.6 | 0.79 | 0.4 × 105 | 0.1 × 105 | 0.62 | — | — | — | 156 |
| 124 | 486 | 2.8 | 1.00 | 3.6 × 105 | — | 0.55 | 484 | (0.19, 0.34) | 19.6 | 157 |
| 125 | 473 | 3.2 | 0.84 | 2.6 × 105 | 0.5 × 105 | 0.69 | 468 | (0.17, 0.25) | 21.6 | 157 |
| 126 | 476 | 2.7 | 0.83 | 3.1 × 105 | 0.6 × 105 | 0.68 | 472 | (0.17, 0.26) | 19.6 | 157 |
| 127 | 506 | 2.59 | 0.92 | 7.7 × 10−5 | — | 0.48 | — | — | — | 158 |
| 128 | 489 | 4.43 | 0.94 | 8.2 × 10−5 | — | 0.75 | — | — | — | 158 |
| 129 | 491 | 3.89 | 1.00 | 9.2 × 10−5 | — | 0.59 | — | — | — | 158 |
| 130 | 515 | 0.93 | 0.96 | 2.8 × 10−5 | — | 0.37 | — | — | — | 158 |
| 131 | 473 | 1.52 | 0.97 | 3.3 × 10−5 | — | 0.67 | — | — | — | 158 |
| 132 | 477 | 1.53 | 1.00 | 3.7 × 10−5 | — | 0.62 | — | — | — | 158 |
Next, the Chi group employed a carbazolyl unit to replace the phenoxy group.157 The resultant carbazole-based Ir(III) complexes, 124–126 (Fig. 15), exhibit structureless emission bands indicating the dominant charge transfer contribution in the emissive process, with maxima at 486, 473, and 476 nm, respectively. The PLQY is almost unitary for 124, and slightly lower, at approximately 83%, for both 125 and 126 in solution at RT. Surprisingly, the radiative lifetimes (τrad) of 124–126 are significantly shorter (2.77–3.80 μs) than those of the previously reported Ir(III) complexes, 111 and 121, (τrad = 5.41 and 12.0 μs). This may be advantageous for application of these materials in device structures, because it may reduce the population density of the long-lived triplet excitons. Importantly, the Chi group also conducted photo-degradation experiments on the complexes in deaerated toluene under irradiation. The carbazole-based bis-tridentate Ir(III) complexes exhibits superior photostability to the tri-bisdentate deep-blue Ir(III) carbene complexes, 88 and 89. Furthermore, the rate of photodegradation of the carbazole-based Ir(III) complexes is lower than those of 111 and 121, indicating that the six-membered N-containing metallacycle in these complexes is more stable than the corresponding five-membered metallacycle system as a result of the robust chelating framework. More recently, improved photo-stabilities with shortened τem were obtained by replacing the central pyridine unit with a pyrimidine unit, 127–132 (Fig. 15).158 Various methodologies for fine-tuning the electronic transitions of bis-tridentate Ir(III) complexes for high performance and durable phosphorescent OLEDs are being developed at present.159–162
Recently, Bryce et al., reported a series of diarylhydrazide-bridged diiridium complexes functionalised with ppy-based cyclometalating ligands, 133–141 (Fig. 16).171 Among them, 141 exhibits sky-blue emission in doped film with λmax = 460 nm; this value indicates 10 nm hypsochromic shifting compared to 1. This complex also exhibits high PLQY (0.69), high kr (4.26 × 105 s−1), and relatively short τp (2.24 μs) in doped film. A combination of X-ray molecular structure analyses and NMR studies have revealed that the intramolecular π–π interactions between the bridging and cyclometalating ligands rigidify the complexes and play an important role in the observed, excellent photophysical properties. Bryce et al. continued their work using hydrazide-bridged diiridium complexes, 142–145 (Fig. 16).172 Complexes 143–145 are strongly emissive (PLQYs = 0.47–0.55) when doped into PMMA, whereas complex 142 exhibits a relatively small PLQY of 0.11. Those researchers interpreted these findings as indicating that the intramolecular π–π interactions are promoted by introduction of F substituents to the phenyl rings of the bridging ligand. In 2018, Chi et al. reported sublimable diiridium complexes bearing both functional 2-pyrazolyl-6-phenyl pyridine chelate and bidentate phenyl imidazolylidene chelate, 146–149 (Fig. 16).173 Among them, 149 exhibits blue-shifted emission peaks at 468, 500, and 536 nm due to the combined inductive and mesomeric effects. Importantly, very high PLQYs of >0.90 were observed for all diiridium complexes in both solution and film states at RT. Accordingly, the resulting OLED device using 149 showed impressive blue-emitting characteristics with an EQE of 14.2%. It should be noted OLED devices with 146 and 147 showed much higher EQEs of 18.3% (yellowish green colour) and 27.6% (green colour), respectively. These investigations of diiridium complexes offer a new method for future development of deeper-blue emitting Ir(III) complexes. Available photophysical- and electrochemical data of 133–149 with their device performances are summarised in Table 6.
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ emmax (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
| a Doped film. | ||||||||||
| 133 | 516a | 1.8a | 0.61a | 3.4 × 105 | 2.2 × 105 | 0.42, 0.67 | — | — | — | 171 |
| 134 | 503a | 2.0a | 0.59a | 3.0 × 105 | 2.1 × 105 | 0.52, 0.77 | — | — | — | 171 |
| 135 | 503a | 2.1a | 0.71a | 3.4 × 105 | 1.4 × 105 | 0.56, 0.81 | — | — | — | 171 |
| 136 | 507a | 2.0a | 0.66a | 3.3 × 105 | 1.7 × 105 | 0.50, 0.76 | — | — | — | 171 |
| 137 | 507a | 11.4a | 0.72a | 5.2 × 105 | 2.0 × 105 | 0.58, 0.90 | — | — | — | 171 |
| 138 | 504a | 1.1a | 0.66a | 5.8 × 105 | 3.0 × 106 | 0.62, 0.78 | — | — | — | 171 |
| 139 | 470 | 1.2 | 0.65a | 5.5 × 105 | 3.0 × 105 | 0.93, 1.28 | — | — | — | 171 |
| 472a | 1.2a | 0.60a | 5.5 × 105 | 3.4 × 105 | 0.97, 1.33 | |||||
| 140 | 471a | 1.1a | 0.46a | 4.1 × 105 | 4.8 × 105 | 0.95, 1.12 | — | — | — | 171 |
| 141 | 460a | 1.6a | 0.69a | 4.3 × 105 | 2.0 × 105 | 0.81, 1.18 | — | — | — | 171 |
| 142 | 500a | 1.8a | 0.11a | 0.6 × 105 | 4.9 × 105 | 0.34, 0.60 | — | — | — | 172 |
| 143 | 501a | 2.8a | 0.55a | 2.0 × 105 | 1.6 × 105 | 0.30, 0.67 | — | — | — | 172 |
| 144 | 486a | 4.2a | 0.47a | 1.1 × 105 | 1.3 × 105 | 0.49, 0.89 | — | — | — | 172 |
| 145 | 480a | 4.6a | 0.52a | 1.1 × 105 | 1.1 × 105 | 0.68, 1.12 | — | — | — | 172 |
| 146 | 531 | 3.0 | 0.98 | 3.3 × 105 | 0.1 × 105 | 0.64, 0.94 | 530 | 0.36, 0.61 | 18.3 | 173 |
| 147 | 487, 521, 559 | 4.6 | 0.95 | 2.1 × 105 | 0.1 × 105 | 0.50, 0.78 | 489 | 0.19, 0.53 | 27.6 | 173 |
| 148 | 485, 519, 554 | 7.0 | 0.64 | 0.9 × 105 | 0.5 × 105 | 0.58, 0.89 | 173 | |||
| 149 | 531 | 2.9 | 0.91 | 3.1 × 105 | 0.3 × 105 | 0.61, 0.92 | — | — | — | 173 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C H bond involving phenolic OH and a non-bonded carboxylate O atom was confirmed. Attachment of the hydroxyl group does not affect the steady state photophysical properties. Note that 150 shows similar emission behaviour to 2, with emission maxima at 468 and 494 nm and a PLQY of 0.94. However, the rigid structure of 150 induced by the intramolecular hydrogen bond significantly affects the excited-state kinetics. The τem of 150 is substantially longer than those of either 151 or 2 at both 300 and 77 K. The role of the intramolecular hydrogen bond in the excited state of 150 was confirmed by comparing the lifetimes in dichloromethane (DCM) solution and DMSO solution, where the latter can prevent the intramolecular hydrogen bond but induce the intermolecular hydrogen bond. The τem of 150 in DMSO solution (2.13 μs) is significantly shorter than that measured in DCM solution (3.19 μs). These results clearly indicate that the intramolecular hydrogen bond in the ancillary ligand maintains the constrained geometry in the excited state and affords stable blue phosphorescence, facilitating fast radiative emission and retarding NR emission processes. The effect of an intramolecular hydrogen bond in real applications was evaluated for two OLED devices fabricated using 150 and 2 as dopant materials. The 150- and 2-based devices exhibited similar efficiencies, with EQEs of 18.1 and 19.0%, respectively. However, the device operation lifetime was significantly improved for the 150-based device compared to 2-based device. Zhang et al. theoretically investigated this strategy using heteroleptic Ir(III) complexes with 3-(2,6-difluoropyridin-3-yl)pyridazine as a main ligand and picolinic acid 154 or 3-hydroxypicolinic acid 155 as an ancillary ligand (Fig. 17).175 They performed independent calculations for this complex with and without an intramolecular hydrogen bond, to confirm the influence of the hydrogen bond on the PLQY. According to their calculations, 155 has a larger quantum yield than the other two complexes, which is mainly attributed to it having the smallest temperature-dependent knr(T). The potential energy profiles of the deactivation pathway from the 3MLCT/π–π* state to the S0 state via the 3MCd–d state for 154 and 155 suggest that 3MCd–d state formation is most difficult for 155, because of the rigid environment induced by the intramolecular hydrogen bond. Both studies clearly indicate that formation of an intramolecular hydrogen bond constitutes a new method for the design of new phosphors with the desired properties. Available photophysical- and electrochemical data of 150–155 with their device performances are summarised in Table 7.
C H bond involving phenolic OH and a non-bonded carboxylate O atom was confirmed. Attachment of the hydroxyl group does not affect the steady state photophysical properties. Note that 150 shows similar emission behaviour to 2, with emission maxima at 468 and 494 nm and a PLQY of 0.94. However, the rigid structure of 150 induced by the intramolecular hydrogen bond significantly affects the excited-state kinetics. The τem of 150 is substantially longer than those of either 151 or 2 at both 300 and 77 K. The role of the intramolecular hydrogen bond in the excited state of 150 was confirmed by comparing the lifetimes in dichloromethane (DCM) solution and DMSO solution, where the latter can prevent the intramolecular hydrogen bond but induce the intermolecular hydrogen bond. The τem of 150 in DMSO solution (2.13 μs) is significantly shorter than that measured in DCM solution (3.19 μs). These results clearly indicate that the intramolecular hydrogen bond in the ancillary ligand maintains the constrained geometry in the excited state and affords stable blue phosphorescence, facilitating fast radiative emission and retarding NR emission processes. The effect of an intramolecular hydrogen bond in real applications was evaluated for two OLED devices fabricated using 150 and 2 as dopant materials. The 150- and 2-based devices exhibited similar efficiencies, with EQEs of 18.1 and 19.0%, respectively. However, the device operation lifetime was significantly improved for the 150-based device compared to 2-based device. Zhang et al. theoretically investigated this strategy using heteroleptic Ir(III) complexes with 3-(2,6-difluoropyridin-3-yl)pyridazine as a main ligand and picolinic acid 154 or 3-hydroxypicolinic acid 155 as an ancillary ligand (Fig. 17).175 They performed independent calculations for this complex with and without an intramolecular hydrogen bond, to confirm the influence of the hydrogen bond on the PLQY. According to their calculations, 155 has a larger quantum yield than the other two complexes, which is mainly attributed to it having the smallest temperature-dependent knr(T). The potential energy profiles of the deactivation pathway from the 3MLCT/π–π* state to the S0 state via the 3MCd–d state for 154 and 155 suggest that 3MCd–d state formation is most difficult for 155, because of the rigid environment induced by the intramolecular hydrogen bond. Both studies clearly indicate that formation of an intramolecular hydrogen bond constitutes a new method for the design of new phosphors with the desired properties. Available photophysical- and electrochemical data of 150–155 with their device performances are summarised in Table 7.
| Photophysical properties | Oxidation (V) | Device performances | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ em (nm) | τ em (μs) | PLQY | k r (s−1) | k nr (s−1) | EL (λmax, nm) | CIE (x, y) | EQEmax (%) | |||
| 150 | 468, 494 | 3.2 | 0.94 | 3.0 × 10−5 | 0.3 × 10−5 | — | — | (0.15, 0.35) | 18.1 | 174 |
| 151 | 501, 526 | 0.2 | 0.20 | 9.1 × 10−5 | 3.6 × 10−4 | — | — | — | — | 174 |
| 152 | 469, 495 | 0.9 | 0.72 | 7.7 × 10−5 | 3.0 × 10−5 | — | — | — | — | 174 |
| 153 | 506, 529 | 0.2 | 0.15 | 9.4 × 105 | 5.3 × 10−4 | — | — | — | — | 174 |
| 154 | 472 | 14.9 | 0.70 | 4.7 × 104 | 2.0 × 104 | — | — | — | 175 | |
| 155 | 533 | 18.3 | 0.77 | 4.2 × 104 | 1.3 × 104 | — | — | — | 175 | |
4. Conclusions
Phosphorescent cyclometalated Ir(III) complexes are currently being used as key materials in highly efficient OLEDS, because of the high quantum efficiency and colour tunability, which are attributed to the various synthetic protocols and which afford functionalisation diversity. However, exploration of efficient blue phosphorescent Ir(III) complexes remains a challenge because of their relatively inadequate CIE coordinates and low PLQY efficiency. In this review, we summarised the fundamental photophysics and design strategies of blue phosphorescent Ir(III) complexes, including recent progress. Experimental and theoretical studies have shown that the HOMO and LUMO energy levels can be controlled by substituting electron-withdrawing groups into cyclometalate ligands and replacing phenyl rings with N-heteroaromatic rings, respectively. To suppress the NR decay processes in the excited states of cyclometalate Ir(III) complexes, two mechanisms should be considered: the vibronic-coupled NR decay process and crossing from the emissive state to an upper non-emissive 3MC excited state. This review surveyed several strategies towards development of efficient blue phosphorescent Ir(III) complexes, including the effects of a rigid structure providing restricted intramolecular motion and utilisation of ligands with strong σ donation ability to destabilise the 3MC state. Based on the strategies described herein, further improvements regarding the colour purity and phosphorescent efficiency of Ir(III) complexes can be expected, through design of smart ligands that can control the molecular geometry and electronic perturbation. We hope that this review will provide helpful guidance for future researchers towards the development of highly efficient blue phosphorescent Ir(III) complexes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (2019R1F1A1058578).References
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Highly efficient phosphorescent emission from organic electroluminescent devices, Nature, 1998, 395, 151–154 CrossRef CAS
.
- M. A. Baldo, M. E. Thompson and S. R. Forrest, High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer, Nature, 2000, 403, 750–753 CrossRef CAS
.
- Y. You and W. Nam, Photofunctional triplet excited states of cyclometalated Ir(III) complexes: beyond electroluminescence, Chem. Soc. Rev., 2012, 41, 7061–7084 RSC
.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Very high-efficiency green organic light-emitting devices based on electrophosphorescence, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS
.
- C. Adachi, M. A. Baldo, S. R. Forrest and M. E. Thompson, High-efficiency organic electrophosphorescent devices with tris(2-phenylpyridine)iridium doped into electron-transporting materials, Appl. Phys. Lett., 2000, 77, 904–906 CrossRef CAS
.
- B. W. D'Andrade, M. A. Baldo, C. Adachi, J. Brooks, M. E. Thompson and S. R. Forrest, High-efficiency yellow double-doped organic light-emitting devices based on phosphor-sensitized fluorescence, Appl. Phys. Lett., 2001, 79, 1045–1047 CrossRef
.
- C. Adachi, M. A. Baldo, S. R. Forrest, S. Lamansky, M. E. Thompson and R. C. Kwong, High-efficiency red electrophosphorescence devices, Appl. Phys. Lett., 2001, 78, 1622–1624 CrossRef CAS
.
- S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, R. Kwong, I. Tsyba, M. Bortz, B. Mui, R. Bau and M. E. Thompson, Synthesis and characterization of phosphorescent cyclometalated iridium complexes, Inorg. Chem., 2001, 40, 1704–1711 CrossRef CAS PubMed
.
- A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, Synthesis and characterization of facial and meridional tris-cyclometalated iridium(III) complexes, J. Am. Chem. Soc., 2003, 125, 7377–7387 CrossRef CAS PubMed
.
- R. J. Holmes, S. R. Forrest, Y.-J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Blue organic electrophosphorescence using exothermic host–guest energy transfer, Appl. Phys. Lett., 2003, 82, 2422–2424 CrossRef CAS
.
- C. Wu, H.-F. Chen, K.-T. Wong and M. E. Thompson, Study of ion-paired iridium complexes (soft salts) and their application in organic light emitting diodes, J. Am. Chem. Soc., 2010, 132, 3133–3139 CrossRef CAS PubMed
.
- B. Liu, F. Dang, Z. Feng, Z. Tian, J. Zhao, Y. Wu, X. Yang, G. Zhou, Z. Wu and W.-Y. Wong, Novel iridium(III) complexes bearing dimesitylboron groups with nearly 100% phosphorescent quantum yields for highly efficient organic light-emitting diodes, J. Mater. Chem. C, 2017, 5, 7871–7883 RSC
.
- X. Yang, G. Zhou and W.-Y. Wong, Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices, Chem. Soc. Rev., 2015, 44, 8484–8575 RSC
.
- Y. Im, S. Y. Byun, J. H. Kim, D. R. Lee, C. S. Oh, K. S. Yook and J. Y. Lee, Recent progress in high-efficiency blue-light-emitting materials for organic light-emitting diodes, Adv. Funct. Mater., 2017, 27, 1603007 CrossRef
.
- Z. Wu, N. Sun, L. Zhu, H. Sun, J. Wang, D. Yang, X. Qiao, J. Chen, S. M. Alshehri, T. Ahamad and D. Ma, Achieving extreme utilization of excitons by an efficient sandwich-type emissive layer architecture for reduced efficiency roll-off and improved operational stability in organic light-emitting diodes, ACS Appl. Mater. Interfaces, 2016, 8, 3150–3159 CrossRef CAS PubMed
.
- J.-H. Lee, H. Shin, J.-M. Kim, K.-H. Kim and J.-J. Kim, Exciplex-forming co-host-based red phosphorescent organic light-emitting diodes with long operational stability and high efficiency, ACS Appl. Mater. Interfaces, 2017, 9, 3277–3281 CrossRef CAS PubMed
.
- K.-H. Kim, E. S. Ahn, J.-S. Huh, Y.-H. Kim and J.-J. Kim, Design of heteroleptic Ir complexes with horizontal emitting dipoles for highly efficient organic light-emitting diodes with an external quantum efficiency of 38%, Chem. Mater., 2016, 28, 7505–7510 CrossRef CAS
.
- S.-Y. Kim, W.-I. Jeong, C. Mayr, Y.-S. Park, K.-H. Kim, J.-H. Lee, C.-K. Moon, W. Brütting and J.-J. Kim, Organic light-emitting diodes with 30% external quantum efficiency based on a horizontally oriented emitter, Adv. Funct. Mater., 2013, 23, 3896–3900 CrossRef CAS
.
- S. Seo, S. Shitagaki, N. Ohsawa, H. Inoue, K. Suzuki, H. Nowatari and S. Yamazaki, Exciplex-triplet energy transfer: A new method to achieve extremely efficient organic light-emitting diode with external quantum efficiency over 30% and drive voltage below 3 V, Jpn. J. Appl. Phys., 2014, 53, 042102 CrossRef
.
- K.-H. Kim, S. Lee, C.-K. Moon, S.-Y. Kim, Y.-S. Park, J.-H. Lee and J.-J. Kim, Phosphorescent dye-based supramolecules for high-efficiency organic light-emitting diodes, Nat. Commun., 2014, 5, 4769 CrossRef CAS PubMed
.
- X. Li, J. Zhang, Z. Zhao, L. Wang, H. Yang, Q. Chang and Z. Lu, Deep blue phosphorescent organic light-emitting diodes with CIEy value of 0.11 and external quantum efficiency up to 22.5%, Adv. Mater., 2018, 30, 1705005 CrossRef PubMed
.
- J.-H. Lee, S.-H. Cheng, S.-J. Yoo, H. Shin, J.-H. Chang, C.-I. Wu, K.-T. Wong and J.-J. Kim, An exciplex forming host for highly efficient blue organic light emitting diodes with low driving voltage, Adv. Funct. Mater., 2015, 25, 361–366 CrossRef CAS
.
- H. Shin, J.-H. Lee, C.-K. Moon, J.-S. Huh, B. Sim and J.-J. Kim, Sky-blue phosphorescent OLEDs with 34.1% external quantum efficiency using a low refractive index electron transporting layer, Adv. Mater., 2016, 28, 4920–4925 CrossRef CAS PubMed
.
- R. Zaen, K.-M. Park, K. H. Lee, J. Y. Lee and Y. Kang, Blue phosphorescent Ir(III) complexes achieved with over 30% external quantum efficiency, Adv. Opt. Mater., 2019, 7, 1901387 CrossRef CAS
.
- Y. Zhang, J. Lee and S. R. Forrest, Tenfold increase in the lifetime of blue phosphorescent organic light-emitting diodes, Nat. Commun., 2014, 5, 5008 CrossRef CAS PubMed
.
- X. Zhou, P. L. Burn and B. J. Powell, Bond fission and non-radiative decay in iridium(III) complexes, Inorg. Chem., 2016, 55, 5266–5273 CrossRef CAS PubMed
.
- J. Lee, C. Jeong, T. Batagoda, C. Coburn, M. E. Thompson and S. R. Forrest, Hot excited state management for long-lived blue phosphorescent organic light-emitting diodes, Nat. Commun., 2017, 8, 15566 CrossRef CAS PubMed
.
- D. Jacquemin and D. Escudero, The short device lifetimes of blue PhOLEDs: insights into the photostability of blue Ir(III) complexes, Chem. Sci., 2017, 8, 7844–7850 RSC
.
- S. Reineke, K. Walzer and K. Leo, Triplet-exciton quenching in organic phosphorescent light-emitting diodes with Ir-based emitters, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125328 CrossRef
.
- Y. Luo and H. Aziz, Correlation between triplet–triplet annihilation and electroluminescence efficiency in doped fluorescent organic light-emitting devices, Adv. Funct. Mater., 2010, 20, 1285–1293 CrossRef CAS
.
- C. Murawski, K. Leo and M. C. Gather, Efficiency roll-off in organic light-emitting diodes, Adv. Mater., 2013, 25, 6801–6827 CrossRef CAS PubMed
.
- S. Kim, H. J. Bae, S. Park, W. Kim, J. Kim, J. S. Kim, Y. Jung, S. Sul, S.-G. Ihn, C. Noh, S. Kim and Y. You, Degradation of blue-phosphorescent organic light-emitting devices involves exciton-induced generation of polaron pair within emitting layers, Nat. Commun., 2018, 9, 1211 CrossRef PubMed
.
- Y. You and S. Y. Park, Phosphorescent iridium(III) complexes: toward high phosphorescencequantum efficiency through ligand control, Dalton Trans., 2009, 1267–1282 RSC
.
- W.-Y. Wong and C.-L. Ho, Functional metallophosphors for effective charge carrier injection/transport: new robust OLED materials with emerging applications, J. Mater. Chem., 2009, 19, 4457–4482 RSC
.
- Y. Chi and P.-T. Chou, Transition-metal phosphors with cyclometalating ligands: fundamentals and applications, Chem. Soc. Rev., 2010, 39, 638–655 RSC
.
- H. Fu, Y.-M. Cheng, P.-T. Chou and Y. Chi, Feeling blue? Blue phosphors for OLEDs, Mater. Today, 2011, 14(10), 472–479 CrossRef CAS
.
- K. S. Yook and J. Y. Lee, Organic materials for deep blue phosphorescent organic light-emitting diodes, Adv. Mater., 2012, 24, 3169–3190 CrossRef CAS PubMed
.
- C.-L. Ho and W.-Y. Wong, Small-molecular blue phosphorescent dyes for organic light-emitting devices, New J. Chem., 2013, 37, 1665–1683 RSC
.
- R. Visbal and M. C. Gimeno,
N-heterocyclic carbene metal complexes: photoluminescence and applications, Chem. Soc. Rev., 2014, 43, 3551–3574 RSC
.
- X. Yang, X. Xu and G. Zhou, Recent advances of the emitters for high performance deep-blue organic light-emitting diodes, J. Mater. Chem. C, 2015, 3, 913–944 RSC
.
- I. Omae, Application of the five-membered ring blue light-emitting iridium products of cyclometalation reactions as OLEDs, Coord. Chem. Rev., 2016, 310, 154–169 CrossRef CAS
.
- D. Ma, T. Tsuboi, Y. Qiu and L. Duan, Recent progress in ionic iridium(III) complexes for organic electronic devices, Adv. Mater., 2017, 29, 1603253 CrossRef PubMed
.
- C. E. Housecroft and E. C. Constable, Over the LEC rainbow: Colour and stability tuning of cyclometallated iridium(III) complexes in light-emitting electrochemical cells, Coord. Chem. Rev., 2017, 350, 155–177 CrossRef CAS
.
- Y. Chi, T.-K. Chang, P. Ganesan and P. Rajakannu, Emissive bis-tridentate Ir(III) metal complexes: Tactics, photophysics and applications, Coord. Chem. Rev., 2017, 346, 91–100 CrossRef CAS
.
- W. Song and J. Y. Lee, Degradation mechanism and lifetime improvement strategy for blue phosphorescent organic light-emitting diodes, Adv. Opt. Mater., 2017, 1600901 CrossRef
.
- T.-Y. Li, J. Wu, Z.-G. Wu, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Rational design of phosphorescent iridium(III) complexes for emission color tunability and their applications in OLEDs, Coord. Chem. Rev., 2018, 374, 55–92 CrossRef CAS
.
- I. N. Mills, J. A. Porras and S. Bernhard, Judicious design of cationic, cyclometalated Ir(III) complexes for photochemical energy conversion and optoelectronics, Acc. Chem. Res., 2018, 51(2), 352–364 CrossRef CAS PubMed
.
- H.-T. Maoa, G.-F. Li, G.-G. Shan, X.-L. Wang and Z.-M. Su, Recent progress in phosphorescent Ir(III) complexes for nondoped organic light-emitting diodes, Coord. Chem. Rev., 2020, 413, 213283 CrossRef
.
- R. Bai, X. Meng, X. Wang and L. He, Blue-emitting iridium(III) complexes for light-emitting dlectrochemical cells: Advances, challenges, and future prospects, Adv. Funct. Mater., 2020, 1907169 CrossRef
.
- C. Zhang, R. Liu, D. Zhang and L. Duan, Progress on light-emitting electrochemical cells toward blue emission, high efficiency, and long lifetime, Adv. Funct. Mater., 2020, 1907156 CrossRef
.
- A. Jablonski, Efficiency of anti-Stokes fluorescence in dyes, Nature, 1933, 131, 839–840 CrossRef CAS
.
- T. Hofbeck and H. Yersin, The triplet state of fac-Ir(ppy)3, Inorg. Chem., 2010, 49, 9290–9299 CrossRef CAS PubMed
.
- P. J. Hay, Theoretical studies of the ground and excited electronic states in cyclometalated phenylpyridine Ir(III) complexes using density functional theory, J. Phys. Chem. A, 2002, 106, 1634–1641 CrossRef CAS
.
- A. V. Kukhta, I. N. Kukhta, S. A. Bagnich, S. M. Kazakov, V. A. Andreev, O. L. Neyra and E. Meza, Interactions of low-energy electrons with Ir(ppy)3 in the gas phase, Chem. Phys. Lett., 2007, 434, 11–14 CrossRef CAS
.
- A. R. G. Smith, P. L. Burn and B. J. Powell, Exact exchange and the density functional theory of metal-to-ligand charge-transfer in fac-Ir(ppy)3, Org. Electron., 2016, 33, 110–115 CrossRef CAS
.
- E. Jansson, B. Minaev, S. Schrader and H. Ågren, Time-dependent density functional calculations of phosphorescence parameters for fac-tris(2-phenylpyridine) iridium, Chem. Phys., 2007, 333, 157–167 CrossRef CAS
.
- B. Minaev, H. Ågren and F. De Angelis, Theoretical design of phosphorescence parameters for organic electro-luminescence devices based on iridium complexes, Chem. Phys., 2009, 358, 245–257 CrossRef CAS
.
- T. Matsushita, T. Asada and S. Koseki, Relativistic study on emission mechanism in tris (2-phenylpyridine) iridium, J. Phys. Chem. C, 2007, 111, 6897–6903 CrossRef CAS
.
- S. I. Bokarev, O. S. Bokareva and O. Kühn, Electronic excitation spectrum of the photosensitizer [Ir(ppy)2(bpy)]+, J. Chem. Phys., 2012, 136, 214305 CrossRef PubMed
.
- S. I. Bokarev, O. S. Bokareva and O. Kühn, A theoretical perspective on charge transfer in photocatalysis. The example of Ir-based systems, Coord. Chem. Rev., 2015, 304–305, 133–145 CrossRef CAS
.
- S. Obara, M. Itabashi, F. Okuda, S. Tamaki, Y. Tanabe, Y. Ishii, K. Nozaki and M. Haga, Highly phosphorescent iridium complexes containing both tridentate bis (benzimidazolyl)-benzene or-pyridine and bidentate phenylpyridine: synthesis, photophysical properties, and theoretical study of Ir-bis(benzimidazolyl)benzene complex, Inorg. Chem., 2006, 45, 8907–8921 CrossRef CAS PubMed
.
- K.-C. Tang, K. L. Liu and I.-C. Chen, Rapid intersystem crossing in highly phosphorescent iridium complexes, Chem. Phys. Lett., 2004, 386, 437–441 CrossRef CAS
.
- H.-S. Duan, P.-T. Chou, C.-C. Hsu, J.-Y. Hung and Y. Chi, Photophysics of heteroleptic iridium(III) complexes of current interest; a closer look on relaxation dynamics, Inorg. Chem., 2009, 48, 6501–6508 CrossRef CAS PubMed
.
- F. Spaenig, J.-H. Olivier, V. Prusakova, P. Retailleau, R. Ziessel and F. N. Castellano, Excited-state properties of heteroleptic iridium(III) complexes bearing aromatic hydrocarbons with extended cores, Inorg. Chem., 2011, 50(21), 10859–10871 CrossRef CAS PubMed
.
- J. H. Klein, T. L. Sunderland, C. Kaufmann, M. Holzapfel, A. Schmiedel and C. Lambert, Stepwise versus pseudo-concerted two-electron-transfer in a triarylamine–iridium dipyrrin–naphthalene diimide triad, Phys. Chem. Chem. Phys., 2013, 15, 16024–16030 RSC
.
- W. J. Finkenzeller and H. Yersin, Emission of Ir(ppy)3. Temperature dependence, decay dynamics, and magnetic field properties, Chem. Phys. Lett., 2003, 377, 299–305 CrossRef CAS
.
- G. J. Hedley, A. Ruseckas and I. D. W. Samuel, Vibrational Energy Flow Controls Internal Conversion in a Transition Metal Complex, J. Phys. Chem. A, 2011, 114, 8961–8968 CrossRef PubMed
.
- R. W. Harrigan and G. A. Crosby, Symmetry assignments of the lowest CT excited states of ruthenium(II) complexes via a proposed electronic coupling model, J. Chem. Phys., 1973, 59, 3468 CrossRef CAS
.
- D. C. Baker and G. A. Crosby, Spectroscopic and magnetic evidence for multiple-state emission from tris(2,2′-bipyridine) ruthenium(II) sulfate, Chem. Phys., 1974, 4, 428–433 CrossRef CAS
.
- H. Yersin and D. Donges, Low-lying electronic states and photophysical properties of organometallic Pd(II) and Pt(II) compounds.
Modern research trends presented in detailed case studies, Top. Curr. Chem., 2001, 214, 81–186 CrossRef CAS
.
- H. Wiedenhofer, S. Schuetzenmeier, A. v. Zelewsky and H. Yersin, Characterization of triplet sublevels by highly resolved vibrational satellite structures. Application to Pt(2-thpy)2, J. Phys. Chem., 1995, 99(36), 13385–13391 CrossRef CAS
.
- Y. Koide, S. Takahashi and M. Vacha, Simultaneous two-photon excited fluorescence and one-photon excited phosphorescence from single molecules of an organometallic complex Ir(ppy)3, J. Am. Chem. Soc., 2006, 128(34), 10990–10991 CrossRef CAS PubMed
.
- E. Baranoff and B. F. E. Curchod, FIrpic: archetypal blue phosphorescent emitter for electroluminescence, Dalton Trans., 2015, 44, 8318–8329 RSC
.
- K. K.-W. Lo, C.-K. Chung, T. K.-M. Lee, K. H.-K. Tsang and N. Zhu, New luminescent cyclometalated iridium(III) diimine complexes as biological labeling reagents, Inorg. Chem., 2003, 42, 6886–6897 CrossRef CAS PubMed
.
- H. Guo, M. L. Muro-Small, S. Ji, J. Zhao and F. N. Castellano, Naphthalimide Phosphorescence Finally Exposed in a Platinum(II) Diimine Complex, Inorg. Chem., 2010, 49, 6802–6804 CrossRef CAS PubMed
.
- D. V. Kozlov, D. S. Tyson, C. Goze, R. Ziessel and F. N. Castellano, Room temperature phosphorescence from ruthenium(II) complexes bearing conjugated pyrenylethynylene subunits, Inorg. Chem., 2004, 43, 6083–6092 CrossRef CAS PubMed
.
- A. F. Rausch, M. E. Thompson and H. Yersin, Blue light emitting Ir(III) compounds for OLEDs-new insights into ancillary ligand effects on the emitting triplet state, J. Phys. Chem. A, 2009, 113, 5927–5932 CrossRef CAS PubMed
.
- T. Tsuboi, H. Murayama, S.-J. Yeh, M.-F. Wu and C.-T. Chen, Photoluminescence characteristics of blue phosphorescent Ir3+-compounds FIrpic and FIrN4 doped in mCP and SimCP, Opt. Mater., 2008, 31, 366–371 CrossRef CAS
.
- A. Endo, K. Suzuki, T. Yoshihara, S. Tobita, M. Yahiro and C. Adachi, Measurement of photoluminescence efficiency of Ir(III) phenylpyridine derivatives in solution and solid-state films, Chem. Phys. Lett., 2008, 460, 155–157 CrossRef CAS
.
- S.-C. Lo, C. P. Shipley, R. N. Bera, R. E. Harding, A. R. Cowley, P. L. Burn and I. D. W. Samuel, Blue phosphorescence from iridium(III) complexes at room temperature, Chem. Mater., 2006, 18, 5119–5129 CrossRef CAS
.
- R. E. Harding, S.-C. Lo, P. L. Burn and I. D. W. Samuel, Non-radiative decay mechanisms in blue phosphorescent iridium(III) complexes, Org. Electron., 2008, 9, 377–384 CrossRef CAS
.
- K. Huang and A. Rhys, Theory of light absorption and non-radiative transitions in F-centres, Proc. R. Soc. London, Ser. A, 1950, 204, 406 CAS
.
- P. K. Mallick, G. D. Danzer, D. P. Strommen and J. R. Kincaid, Vibrational spectra and normal-coordinate analysis of tris(bipyridine)ruthenium(II), J. Phys. Chem., 1988, 92, 5628–5634 CrossRef CAS
.
- T. Sajoto, P. I. Djurovich, A. B. Tamayo, J. Oxgaard, W. A. Goddard and M. E. Thompson, Temperature dependence of blue phosphorescent cyclometalated Ir(III) complexes, J. Am. Chem. Soc., 2009, 131, 9813–9822 CrossRef CAS PubMed
.
- R. D. Costa, F. Monti, G. Accorsi, A. Barbieri, H. J. Bolink, E. Ortí and N. Armaroli, Photophysical properties of charged cyclometalated Ir(III) complexes: A joint theoretical and experimental study, Inorg. Chem., 2011, 50, 7229–7238 CrossRef CAS PubMed
.
- L. Yang, F. Okuda, K. Kobayashi, K. Nozaki, Y. Tanabe, Y. Ishii and M. Haga, Syntheses and phosphorescent properties of blue emissive iridium complexes with tridentate pyrazolyl ligands, Inorg. Chem., 2008, 47, 7154–7165 CrossRef CAS PubMed
.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Ru(II) polypyridine complexes: photophysics, photochemistry, eletrochemistry, and chemiluminescence, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS
.
- J. H. Seo, Y. K. Kim and Y. Ha, Efficient blue-green organic light-emitting diodes based on heteroleptic tris-cyclometalated iridium(III) complexes, Thin Solid Films, 2009, 517, 1807–1810 CrossRef CAS
.
- R. Ragni, E. A. Plummer, K. Brunner, J. W. Hofstraat, F. Babudri, G. M. Farinola, F. Naso and L. De Cola, Blue emitting iridium complexes: synthesis, photophysics and phosphorescent devices, J. Mater. Chem., 2006, 16, 1161–1170 RSC
.
- X. Li, B. Minaev, H. Ågren and H. Tian, Theoretical study of phosphorescence of iridium complexes with fluorine-substituted phenylpyridine ligands, Eur. J. Inorg. Chem., 2011, 2517–2524 CrossRef CAS
.
- F. De Angelis, S. Fantacci, N. Evans, C. Klein, S. M. Zakeeruddin, J.-E. Moser, K. Kalyanasundaram, H. J. Bolink, M. Grätzel and M. K. Nazeeruddin, Controlling phosphorescence color and quantum yields in cationic iridium complexes: a combined experimental and theoretical study, Inorg. Chem., 2007, 46, 5989–6001 CrossRef CAS PubMed
.
- T. Karatsu, M. Takahashi, S. Yagai and A. Kitamura, Photophysical properties of substituted homoleptic and heteroleptic phenylimidazolinato Ir(III) complexes as a blue phosphorescent material, Inorg. Chem., 2013, 52, 12338–12350 CrossRef CAS PubMed
.
- H. Cho, J. Lee, J.-I. Lee, N. S. Cho, J. H. Park, J. Y. Lee and Y. Kang, Phenylimidazole-based homoleptic iridium(III) compounds for blue phosphorescent organic light-emitting diodes with high efficiency and long lifetime, Org. Electron., 2016, 34, 91–96 CrossRef CAS
.
- Y. Kwon, S. H. Han, S. Yu, J. Y. Lee and K. M. Lee, Functionalized phenylimidazole-based facial-homoleptic iridium(III) complexes and their excellent performance in blue phosphorescent organic light-emitting diodes, J. Mater. Chem. C, 2018, 6, 4565–4572 RSC
.
- K. Tamao, M. Uchida, T. Izumizawa, K. Furukawa and S. Yamaguchi, Silole derivatives as efficient electron transporting materials, J. Am. Chem. Soc., 1996, 118, 11974–11975 CrossRef CAS
.
- A. R. G. Smith, M. J. Riley, P. L. Burn, I. R. Gentle, S.-C. Lo and B. J. Powell, Effects of fluorination on iridium(III) complex phosphorescence: magnetic circular dichroism and relativistic time-dependent density functional theory, Inorg. Chem., 2012, 51, 2821–2831 CrossRef CAS PubMed
.
- S.-C. Lo, R. N. Bera, R. E. Harding, P. L. Burn and I. D. W. Samuel, Solution-processible phosphorescent blue dendrimers based on biphenyl-dendrons and fac-tris(phenyltriazolyl)iridium(III) cores, Adv. Funct. Mater., 2008, 18, 3080–3090 CrossRef CAS
.
- S.-C. Lo, R. E. Harding, E. Brightman, P. L. Burn and I. D. W. Samuel, The development of phenylethylene dendrons for blue phosphorescent emitters, J. Mater. Chem., 2009, 19, 3213–3227 RSC
.
- S.-C. Lo, R. E. Harding, C. P. Shipley, S. G. Stevenson, P. L. Burn and I. D. W. Samuel, High-triplet-energy dendrons: enhancing the luminescence of deep blue phosphorescent iridium(III) complexes, J. Am. Chem. Soc., 2009, 131, 16681–16688 CrossRef CAS PubMed
.
- Y. Wang, Y. Lu, B. Gao, S. Wang, J. Ding, L. Wang, X. Jing and F. Wang, Self-host blue-emitting iridium dendrimer containing bipolar dendrons for nondoped electrophosphorescent devices with superior high-brightness performance, ACS Appl. Mater. Interfaces, 2016, 8, 29600–29607 CrossRef CAS PubMed
.
- Y. Wang, Y. Lu, B. Gao, S. Wang, J. Ding, L. Wang, X. Jing and F. Wang, Single molecular tuning of the charge balance in blue-emitting iridium dendrimers for efficient nondoped solution-processed phosphorescent OLEDs, Chem. Commun., 2016, 52, 11508–11511 RSC
.
- Y. Wang, S. Wang, J. Ding, L. Wang, X. Jing and F. Wang, Dendron engineering in self-host blue iridium dendrimers towards low-voltage-driving and power-efficient nondoped electrophosphorescent devices, Chem. Commun., 2017, 53, 180–183 RSC
.
- J. Wang, J. Peng, C. Yao, R. Liu, W. Yao, C. Zhang, M. He and C. Jiang, Self-host blue-emitting iridium dendrimer for solution-processed non-doped phosphorescent organic light-emitting diodes with flat efficiency roll-off and less phase segregation, Org. Electron., 2017, 45, 49–56 CrossRef CAS
.
- G. Zhang, F. Hermerschmidt, A. Pramanik, D. Schollmeyer, M. Baumgarten, P. Sarkar, E. J. W. List-Kratochvil and K. Müllen, Bulky, dendronized iridium complexes and their photoluminescence, J. Mater. Chem. C, 2019, 7, 15252–15258 RSC
.
- C. Adachi, R. C. Kwong, P. Djurovich, V. Adamovich, M. A. Baldo, M. E. Thompson and S. R. Forrest, Endothermic energy transfer: A mechanism for generating very efficient high-energy phosphorescent emission in organic materials, Appl. Phys. Lett., 2001, 79, 2082–2084 CrossRef CAS
.
- J. Li, P. I. Djurovich, B. D. Alleyne, M. Yousufuddin, N. N. Ho, J. C. Thomas, J. C. Peters, R. Bau and M. E. Thompson, Synthetic control of excited-state properties in cyclometalated Ir(III) complexes using ancillary ligands, Inorg. Chem., 2005, 44, 1713–1727 CrossRef CAS PubMed
.
- I. Avilov, P. Minoofar, J. Cornil and L. De Cola, Influence of substituents on the energy and nature of the lowest excited states of heteroleptic phosphorescent Ir(III) complexes: A joint theoretical and experimental study, J. Am. Chem. Soc., 2007, 129, 8247–8258 CrossRef CAS PubMed
.
- E. Orselli, G. S. Kottas, A. E. Konradsson, P. Coppo, R. Fröhlich, L. De Cola, A. van Dijken, M. Büchel and H. Börner, Blue-emitting iridium complexes with substituted 1,2,4-triazole ligands: Synthesis, photophysics, and devices, Inorg. Chem., 2007, 46, 11082–11093 CrossRef CAS PubMed
.
- E. Orselli, R. Q. Albuquerque, P. M. Fransen, R. Fröhlich, H. M. Janssenc and L. De Cola, 1,2,3-Triazolyl-pyridine derivatives as chelating ligands for blue iridium(III) complexes. Photophysics and electroluminescent devices, J. Mater. Chem., 2008, 18, 4579–4590 RSC
.
- Y. Feng, X. Zhuang, D. Zhu, Y. Liu and M. R. Bryce, Rational design and characterization of heteroleptic phosphorescent iridium(III) complexes for highly efficient deep-blue OLEDs, J. Mater. Chem. C, 2016, 4, 10246–10252 RSC
.
- Y.-J. Cho, S.-Y. Kim, J.-H. Kim, D. W. Crandell, M.-H. Baik, J. Lee, C. H. Kim, H.-J. Son, W.-S. Han and S. O. Kang, Important role of ancillary ligand in the emission behaviours of blue-emitting heteroleptic Ir(III) complexes, J. Mater. Chem. C, 2017, 5, 4480–4487 RSC
.
- V. Sivasubramaniam, F. Brodkorb, S. Hanning, H. P. Loebl, V. van Elsbergen, H. Boerner, U. Scherf and M. Kreyenschmidt, Fluorine cleavage of the light blue heteroleptic triplet emitter FIrpic, J. Fluor. Chem., 2009, 130, 640–649 CrossRef CAS
.
- B. Liu, M. A. Jabed, J. Guo, W. Xu, S. L. Brown, A. Ugrinov, E. K. Hobbie, S. Kilina, A. Qin and W. Sun, Neutral cyclometalated iridium(III) complexes bearing substituted N-heterocyclic carbene (NHC) ligands for high-performance yellow OLED application, Inorg. Chem., 2019, 58, 14377–14388 CrossRef CAS PubMed
.
- J.-H. Zhao, Y.-X. Hu, H.-Y. Lu, Y.-L. Lü and X. Li, Progress on benzimidazole-based iridium(III) complexes for application in phosphorescent OLEDs, Org. Electron., 2017, 41, 56–72 CrossRef CAS
.
- G. Sarada, B. Sim, W. Cho, J. Yoon, Y.-S. Gal, J.-J. Kim and S.-H. Jin, New sky-blue and bluish–green emitting Ir(III) complexes containing an azoline ancillary ligand for highly efficient PhOLEDs, Dyes Pigm., 2016, 131, 60–68 CrossRef CAS
.
- N. M. Shavaleev, R. Scopelliti, M. Grätzel, M. K. Nazeeruddin, A. Pertegás, C. Roldán-Carmona, D. Tordera and H. J. Bolink, Pulsed-current versus constant-voltage light-emitting electrochemical cells with trifluoromethyl-substituted cationic iridium(III) complexes, J. Mater. Chem. C, 2013, 1, 2241–2248 RSC
.
- D. Cortés-Arriagada, L. Sanhueza, I. González, P. Dreyse and A. Toro-Labbé, About the electronic and photophysical properties of iridium(III)-pyrazino [2,3-f,][1,10]-phenanthroline based complexes for use in electroluminescent devices, Phys. Chem. Chem. Phys., 2016, 18, 726–734 RSC
.
- P. Tao, Y. Zhang, J. Wang, L. Wei, H. Li, X. Li, Q. Zhao, X. Zhang, S. Liu, H. Wang and W. Huang, Highly efficient blue phosphorescent iridium(III) complexes with various ancillary ligands for partially solution-processed organic light-emitting diodes, J. Mater. Chem. C, 2017, 5, 9306–9314 RSC
.
- Y. Miao, P. Tao, L. Gao, X. Li, L. Wei, S. Liu, H. Wang, B. Xu and Q. Zhao, Highly efficient chlorine functionalized blue iridium(III) phosphors for blue and white phosphorescent organic light-emitting diodes with the external quantum efficiency exceeding 20%, J. Mater. Chem. C, 2018, 6, 6656–6665 RSC
.
- J.-P. Zhang, Y. Wang, J.-B. Ma, L. Jin, F.-T. Liu and F.-Q. Bai, Density functional theory investigation on iridium(III) complexes for efficient blue electrophosphorescence, RSC Adv., 2018, 8, 19437–19448 RSC
.
- A. K. Pal, S. Krotkus, M. Fontani, C. F. R. Mackenzie, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, High-efficiency deep-blue-emitting organic light-emitting diodes based on iridium(III) carbene complexes, Adv. Mater., 2018, 30, 1804231 CrossRef PubMed
.
- A. F. Henwood and E. Zysman-Colman, Lessons learned in tuning the optoelectronic properties of phosphorescent iridium(III) complexes, Chem. Commun., 2017, 53, 807–826 RSC
.
- A. K. Pal, A. F. Henwood, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Blue-to-green emitting neutral Ir(III) complexes bearing pentafluorosulfanyl groups: a combined experimental and theoretical study, Inorg. Chem., 2017, 56, 7533–7544 CrossRef CAS PubMed
.
- C. D. Ertl, J. Cerdá, J. M. Junquera-Hernández, A. Pertegás, H. J. Bolink, E. C. Constable, M. Neuburger, E. Orti and C. E. Housecroft, Colour tuning by the ring roundabout: [Ir(C^N)2(N^N)]+ emitters with sulfonyl-substituted cyclometallating ligands, RSC Adv., 2015, 5, 42815–42827 RSC
.
- J.-H. Kim, S.-Y. Kim, S. Jang, S. Yi, D. W. Cho, H.-J. Son and S. O. Kang, Blue phosphorescence with high quantum efficiency engaging the trifluoromethylsulfonyl group to iridium phenylpyridine complexes, Inorg. Chem., 2019, 58, 16112–16125 CrossRef CAS PubMed
.
- H. Benjamin, M. A. Fox, A. S. Batsanov, H. A. Al-Attar, C. Li, Z. Ren, A. P. Monkman and M. R. Bryce, Pyridylpyrazole N^N ligands combined with sulfonyl-functionalised cyclometalating ligands for blue-emitting iridium(III) complexes and solution-processable PhOLEDs, Dalton Trans., 2017, 46, 10996–11007 RSC
.
- R. J. Holmes and S. R. Forrest, Saturated deep blue organic electrophosphorescence using a fluorine-free emitter, Appl. Phys. Lett., 2005, 87, 243507 CrossRef
.
- S. Díez-González, N. Marion and S. P. Nolan,
N-heterocyclic carbenes in late transition metal catalysis, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed
.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, An overview of N-heterocyclic carbenes, Nature, 2014, 510, 485–496 CrossRef CAS PubMed
.
- C.-H. Chien, S. Fujita, S. Yamato, T. Hara, T. Yamagata, M. Watanabe and K. Mashima, Stepwise and one-pot syntheses of Ir(III) complexes with imidazolium-based carbene ligands, Dalton Trans., 2008, 916–923 RSC
.
- T. Sajoto, P. I. Djurovich, A. Tamayo, M. Yousufuddin, R. Bau and M. E. Thompson, Blue and near-UV phosphorescence from iridium complexes with cyclometalated pyrazolyl or N-heterocyclic carbene ligands, Inorg. Chem., 2005, 44, 7992–8003 CrossRef CAS PubMed
.
- S. Haneder, E. Da Como, J. Feldmann, J. M. Lupton, C. Lennartz, P. Erk, E. Fuchs, O. Molt, I. Münster, C. Schildknecht and G. Wagenblast, Controlling the radiative rate of deep-blue electrophosphorescent organometallic complexes by singlet-triplet gap engineering, Adv. Mater., 2008, 20, 3325–3330 CrossRef CAS
.
- C.-F. Chang, Y.-M. Cheng, Y. Chi, Y.-C. Chiu, C.-C. Lin, G.-H. Lee, P.-T. Chou, C.-C. Chen, C.-H. Chang and C.-C. Wu, Highly efficient blue-emitting iridium(III) carbene complexes and phosphorescent OLEDs, Angew. Chem., Int. Ed., 2008, 47, 4542–4545 CrossRef CAS PubMed
.
- Y. Liu, X. Sun, G. Gahungu, X. Qu, Y. Wang and Z. Wu, DFT/TDDFT investigation on the electronic structures and photophysical properties of phosphorescent Ir(III) complexes with conjugated/non-conjugated carbene ligands, J. Mater. Chem. C, 2013, 1, 3700–3709 RSC
.
- H. Sasabe, J. Takamatsu, T. Motoyama, S. Watanabe, G. Wagenblast, N. Langer, O. Molt, E. Fuchs, C. Lennartz and J. Kido, High-efficiency blue and white organic light-emitting devices incorporating a blue iridium carbene complex, Adv. Mater., 2010, 22, 5003–5007 CrossRef CAS PubMed
.
- K. Tsuchiya, S. Yagai, A. Kitamura, T. Karatsu, K. Endo, J. Mizukami, S. Akiyama and M. Yabe, Synthesis and photophysical properties of substituted tris(phenylbenzimidazolinato) IrIII carbene complexes as a blue phosphorescent material, Eur. J. Inorg. Chem., 2010, 926–933 CrossRef CAS
.
- N. Darmawan, C.-H. Yang, M. Mauro, M. Raynal, S. Heun, J. Pan, H. Buchholz, P. Braunstein and L. De Cola, Efficient near-UV emitters based on cationic bis-pincer iridium(III) carbene complexes, Inorg. Chem., 2013, 52, 10756–10765 CrossRef CAS PubMed
.
- T.-Y. Li, X. Liang, L. Zhou, C. Wu, S. Zhang, X. Liu, G.-Z. Lu, L.-S. Xue, Y.-X. Zheng and J.-L. Zuo,
N-Heterocyclic carbenes: Versatile second cyclometalated ligands for neutral iridium(III) heteroleptic complexes, Inorg. Chem., 2015, 54, 161–173 CrossRef CAS PubMed
.
- Z. Chen, S. Suramitr, N. Zhu, C.-L. Ho, S. Hannongbua, S. Chen and W.-Y. Wong, Tetrafluorinated phenylpyridine based heteroleptic iridium(III) complexes for efficient sky blue phosphorescent organic light-emitting diodes, J. Mater. Chem. C, 2020, 8, 2551–2557 RSC
.
- J. Lee, H.-F. Chen, T. Batagoda, C. Coburn, P. I. Djurovich, M. E. Thompson and S. R. Forrest, Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency, Nat. Mater., 2015, 15, 92–99 CrossRef PubMed
.
- X. Zhou and B. J. Powell, Nonradiative decay and stability of N-heterocyclic carbene iridium(III) complexes, Inorg. Chem., 2018, 57, 8881–8889 CrossRef CAS PubMed
.
- Z. Chen, L. Wang, S. Su, X. Zheng, N. Zhu, C.-L. Ho, S. Chen and W.-Y. Wong, Cyclometalated iridium(III) carbene phosphors for highly efficient blue organic light-emitting diodes, ACS Appl. Mater. Interfaces, 2017, 9, 40497–40502 CrossRef CAS PubMed
.
- B.-S. Yun, J.-H. Kim, S.-Y. Kim, H.-J. Son, D. W. Cho and S. O. Kang, Photophysical properties of structural isomers of homoleptic Ir-complexes derived from xylenyl-substituted N-heterocyclic carbene ligands, Phys. Chem. Chem. Phys., 2019, 21, 7155–7164 RSC
.
- Y.-J. Cho, S.-Y. Kim, J.-H. Kim, J. Lee, D. W. Cho, S. Yi, H.-J. Son, W.-S. Han and S. O. Kang, Probing photophysical properties of isomeric N-heterocyclic carbene Ir(III) complexes and their applications to deep-blue phosphorescent organic light-emitting diodes, J. Mater. Chem. C, 2017, 5, 1651–1659 RSC
.
- H. Na, L. M. Canada, Z. Wen, J. I. Wu and T. S. Teets, Mixed-carbene cyclometalated iridium complexes with saturated blue luminescence, Chem. Sci., 2019, 10, 6254–6260 RSC
.
- Y.-C. Chiu, J.-Y. Hung, Y. Chi, C.-C. Chen, C.-H. Chang, C.-C. Wu, Y.-M. Cheng, Y.-C. Yu, G.-H. Lee and P.-T. Chou, En Route to High External Quantum Efficiency (∼12%), Organic True-Blue-Light-Emitting Diodes Employing Novel Design of Iridium(III) Phosphors, Adv. Mater., 2009, 21, 2221–2225 CrossRef CAS
.
- N. P. Ayala, C. M. Flynn Jr., L. Sacksteder, J. N. Demas and B. A. DeGraff, Synthesis, luminescence, and excited-state complexes of the tris(1,10-phenanthroline)- and bis(terpyridine)iridium(III) cations, J. Am. Chem. Soc., 1990, 112, 3837–3844 CrossRef CAS
.
- J.-P. Collin, I. M. Dixon, J.-P. Sauvage, J. A. G. Williams, F. Barigelletti and L. Flamigni, Synthesis and photophysical properties of iridium(III) bisterpyridine and its homologues: a family of complexes with a long-lived excited state, J. Am. Chem. Soc., 1999, 121, 5009–5016 CrossRef CAS
.
- A. J. Wilkinson, H. Puschmann, J. A. K. Howard, C. E. Foster and J. A. G. Williams, Luminescent complexes of iridium(III) containing N∧C∧N-coordinating terdentate ligands, Inorg. Chem., 2006, 45, 8685–8699 CrossRef CAS PubMed
.
- V. L. Whittle and J. A. G. Williams, A new class of iridium complexes suitable for stepwise incorporation into linear assemblies: Synthesis, electrochemistry, and luminescence, Inorg. Chem., 2008, 47, 6596–6607 CrossRef CAS PubMed
.
- V. L. Whittle and J. A. G. Williams, Cyclometallated, bis-terdentate iridium complexes as linearly expandable cores for the construction of multimetallic assemblies, Dalton Trans., 2009, 3929–3940 RSC
.
- A. J. Wilkinson, A. E. Goeta, C. E. Foster and J. A. G. Williams, Synthesis and luminescence of a charge-neutral, cyclometalated iridium(III) complex containing N∧C∧N- and C∧N∧C- coordinating terdentate ligands, Inorg. Chem., 2004, 43, 6513–6515 CrossRef CAS PubMed
.
- T. Yutaka, S. Obara, S. Ogawa, K. Nozaki, N. Ikeda, T. Ohno, Y. Ishii, K. Sakai and M. Haga, Syntheses and properties of emissive iridium(III) complexes with tridentate benzimidazole derivatives, Inorg. Chem., 2005, 44, 4737–4746 CrossRef CAS PubMed
.
- J. A. G. Williams, A. J. Wilkinson and V. L. Whittle, Light-emitting iridium complexes with tridentate ligands, Dalton Trans., 2008, 2081–2099 RSC
.
- C.-Y. Kuei, W.-L. Tsai, B. Tong, M. Jiao, W.-K. Lee, Y. Chi, C.-C. Wu, S.-H. Liu, G.-H. Lee and P.-T. Chou, Bis-tridentate Ir(III) complexes
with nearly unitary RGB phosphorescence and organic light-emitting diodes with external quantum efficiency exceeding 31%, Adv. Mater., 2016, 28, 2795–2800 CrossRef CAS PubMed
.
- H.-H. Kuo, Y.-T. Chen, L. R. Devereux, C.-C. Wu, M. A. Fox, C.-Y. Kuei, Y. Chi and G.-H. Lee, Bis-tridentate Ir(III) metal phosphors for efficient deep-blue organic light-emitting diodes, Adv. Mater., 2017, 29, 1702464 CrossRef PubMed
.
- H.-H. Kuo, Z. Zhu, C.-S. Lee, Y.-K. Chen, S.-H. Liu, P.-T. Chou, A. K.-Y. Jen and Y. Chi, Bis-tridentate iridium(III) phosphors with very high photostability and fabrication of blue-emitting OLEDs, Adv. Sci., 2018, 5, 1800846 CrossRef PubMed
.
- Y.-K. Chen, H.-H. Kuo, D. Luo, Y.-N. Lai, W.-C. Li, C.-H. Chang, D. Escudero, A. K.-Y. Jen, L.-Y. Hsu and Y. Chi, Phenyl-and pyrazolyl-functionalized pyrimidine: versatile chromophore of bis-tridentate Ir(III) phosphors for organic light-emitting diodes, Chem. Mater., 2019, 31, 6453–6464 CrossRef CAS
.
- H.-H. Kuo, L.-Y. Hsu, J.-Y. Tso, W.-Y. Hung, S.-H. Liu, P.-T. Chou, K.-T. Wong, Z.-L. Zhu, C.-S. Lee, A. K.-Y. Jen and Y. Chi, Blue-emitting bis-tridentate Ir(III) phosphors: OLED performances vs. substituent effects, J. Mater. Chem. C, 2018, 6, 10486–10496 RSC
.
- V. Adamovich, P.-L. Boudeault, M. A. Esteruelas, D. Gómez-Bautista, A. M. López, E. Oñate and J.-Y. Tasi, Preparation via a NHC dimer complex, photophysical properties, and device performance of heteroleptic bis (tridentate) iridium(III) emitters, Organometallics, 2019, 38, 2738–2747 CrossRef CAS
.
- J.-L. Liao, P. Rajakannu, S.-H. Liu, G.-H. Lee, P.-T. Chou, A. K.-Y. Jen and Y. Chi, Iridium(III) complexes bearing tridentate chromophoric chelate: phosphorescence fine-tuned by phosphine and hydride ancillary, Inorg. Chem., 2018, 57, 8287–8298 CrossRef CAS PubMed
.
- L.-Y. Hsu, Q. Liang, Z. Wang, H.-H. Kuo, W.-S. Tai, S.-J. Su, X. Zhou, Y. Yuan and Y. Chi, Bis-tridentate IrIII Phosphors bearing two fused five-six-membered metallacycles: A strategy to improved photostability of blue Emitters, Chem. – Eur. J., 2019, 25, 15375–15386 CrossRef CAS PubMed
.
- V. L. Whittle and J. A. G. Williams, A New Class of Iridium Complexes Suitable for Stepwise Incorporation into Linear Assemblies: Synthesis, Electrochemistry, and Luminescence, Inorg. Chem., 2008, 47, 6596–6607 CrossRef CAS PubMed
.
- A. Tsuboyama, T. Takiguchi, S. Okada, M. Osawa, M. Hoshino and K. Ueno, A novel dinuclear cyclometalated iridium complex bridged with 1,4-bis[pyridine-2-yl]benzene: its structure and photophysical properties, Dalton Trans., 2004, 1115–1116 RSC
.
- F. Neve, A. Crispini, S. Serroni, F. Loiseau and S. Campagna, Novel dinuclear luminescent compounds based on iridium(III) cyclometalated chromophores and containing bridging ligands with ester-linked chelating sites, Inorg. Chem., 2001, 40, 1093–1101 CrossRef CAS PubMed
.
- L. Donato, C. E. McCusker, F. N. Castellano and E. Zysman-Colman, Mono- and dinuclear cationic iridium(III) complexes bearing a 2,5-dipyridylpyrazine (2,5-dpp) ligand, Inorg. Chem., 2013, 52, 8495–8504 CrossRef CAS PubMed
.
- J. Fernández-Cestau, N. Giménez, E. Lalinde, P. Montaño, M. T. Moreno and S. Sánchez, Synthesis, characterization, and properties of doubly alkynyl bridging dinuclear cyclometalated iridium(III) complexes, Organometallics, 2015, 34, 1766–1778 CrossRef
.
- D. G. Congrave, Y.-T. Hsu, A. S. Batsanov, A. Beeby and M. R. Bryce, Synthesis, Diastereomer separation, and optoelectronic and structural properties of dinuclear cyclometalated iridium(III) complexes with bridging diarylhydrazide ligands, Organometallics, 2017, 36, 981–993 CrossRef CAS
.
- G. Li, D. G. Congrave, D. Zhu, Z. Su and M. R. Bryce, Recent advances in luminescent dinuclear iridium(III) complexes and their application in organic electroluminescent devices, Polyhedron, 2018, 140, 146–157 CrossRef CAS
.
- G. Li, D. Zhu, X. Wang, Z. Su and M. R. Bryce, Dinuclear metal complexes: multifunctional properties and applications, Chem. Soc. Rev., 2020, 49, 765–838 RSC
.
- D. G. Congrave, Y.-T. Hsu, A. S. Batsanov, A. Beeby and M. R. Bryce, Sky-blue emitting bridged diiridium complexes: beneficial effects of intramolecular π–π stacking, Dalton Trans., 2018, 47, 2086–2098 RSC
.
- D. G. Congrave, A. S. Batsanov and M. R. Bryce, Highly luminescent 2-phenylpyridine-free diiridium complexes with bulky 1,2-diarylimidazole cyclometalating ligands, Dalton Trans., 2018, 47, 16524–16533 RSC
.
- J.-L. Liao, P. Rajakannu, P. Gnanasekaran, S.-R. Tasi, C.-H. Lin, S.-H. Liu, C.-H. Chang, G.-H. Lee, P.-T. Chou, Z.-N. Chen and Y. Chi, Luminescent diiridium complexes with bridging pyrazolates: Characterization and fabrication of OLEDs using vacuum thermal deposition, Adv. Opt. Mater., 2018, 6, 1800083 CrossRef
.
- S. Yi, J.-H. Kim, Y.-J. Cho, J. Lee, T.-S. Choi, D. W. Cho, C. Pac, W.-S. Han, H.-J. Son and S. O. Kang, Stable blue phosphorescence iridium(III) cyclometalated complexes prompted by intramolecular hydrogen bond in ancillary ligand, Inorg. Chem., 2016, 55, 3324–3331 CrossRef CAS PubMed
.
- X. Wang, H. Yang, Y. Wen, L. Wang, J. Li and J. Zhang, Comprehension of the effect of a hydroxyl group in ancillary ligand on phosphorescent property for heteroleptic Ir(III) complexes: a computational study using quantitative prediction, Inorg. Chem., 2017, 56, 8986–8995 CrossRef CAS PubMed
.
| This journal is © the Partner Organisations 2020 |