High-yield fabrication of suspended two-dimensional materials for atomic resolution imaging†
Jaehyun Hanab,
Jun-Young Leeab,
Jeongun Choeab and
Jong-Souk Yeo*ab
aSchool of Integrated Technology, Yonsei University, Songdo-dong, Yeonsu-gu, Incheon 406-840, Korea. E-mail: jongsoukyeo@yonsei.ac.kr
bYonsei Institute of Convergence Technology, Yonsei University, Songdo-dong, Yeonsu-gu, Incheon 406-840, Korea
First published on 8th August 2016
Abstract
Two-dimensional (2D) atomic crystals are very interesting materials due to their unique properties, which are significantly different than those observed in conventional three-dimensional (3D) materials. The atomic crystal structure of 2D materials is very important in determining the properties of these materials. In this comparative study, the 2D atomic structures of graphene and h-BN, synthesized using low pressure chemical vapor deposition (LPCVD), were analyzed using a spherical aberration-corrected high resolution transmission electron microscope (HRTEM) for the in situ observation of the atomic scale phenomena. In order to prepare the suspended membrane of 2D materials on a TEM grid with high yield, a two-step approach for soft removal of PMMA is employed for atomic resolution imaging. Using our new method of preparing TEM samples, we obtained fully covered and well suspended graphene and h-BN membranes on TEM mesh grids with clean surfaces and few defects. Honeycomb lattice structures of monolayer graphene and h-BN were clearly observed, and their atomic defects were analyzed as growing into circular and triangular holes, respectively, upon irradiation using an electron beam.
Introduction
Since the discovery of graphene, tremendous research related to 2-dimensional (2D) materials such as graphene, hexagonal boron nitride (h-BN), and 2D-chalcogenides has been conducted due to their outstanding properties. The unique properties of these 2D materials make them ideal candidates for use in transparent conductors, extremely thin insulators, and various optoelectronic devices.1–4 Generally, mechanical exfoliation,5,6 solution based exfoliation,7,8 and chemical vapor deposition (CVD) are used for the synthesis of 2D materials.9 Recently, an enhanced CVD method has enabled the growth of high quality and large area 2D materials, and these are suitable for industrial applications.10–12 However, it is still challenging to use 2D materials grown by the CVD method in optoelectronic applications because the inherent atomic scale defects in the materials degrade their properties and influence structural uniformity.13,14 Defects such as vacancies, grain boundaries, and dislocations critically influence the mechanical and chemical stability, and they can also negatively affect the optical and electrical properties.15,16 Therefore, many studies have focused on growing defect-free and wafer-scale single-crystal 2D layers.17–19 In contrast, controlled formation of defects has recently shown the potential for use in DNA sequencing, photo-detection, gas sensors, water purification, and various other applications.20–23Due to the importance of these materials, analyzing various phenomena of 2D crystals at the atomic scale, including the structural characteristics and the dynamics of defect formation, is a fundamental and important task. High resolution transmission electron microscopy (HRTEM) is a powerful and standard tool for analyzing and observing nanoscale materials. Therefore, in this paper, we investigated the atomic scale phenomena of 2D crystals such as graphene and h-BN grown by low pressure CVD. Using a novel sample preparation method for atomically thin membranes of graphene and h-BN, atomic resolution images of honeycomb lattice structures were clearly observed using spherical aberration-corrected HRTEM operated using a low energy accelerated electron beam at about 80 kV. The defect formation dynamics, including circular holes in graphene and triangular holes in h-BN upon electron beam irradiation, were investigated and compared.
For atomics scale analysis using HRTEM, sample preparation is the most important issue for obtaining high resolution images. Polymer (i.e., polymethyl methacrylate or PMMA) based wet transfer onto a TEM grid has been widely used to make TEM samples of 2D materials grown by the CVD method. In this process, the spin coated PMMA layer acts as a rigid support for 2D layers during an etching step of a metal catalyst. Finally, the rigid PMMA layer must be removed by using a chemical solution (acetone) to leave the suspended 2D membrane on the TEM grid without tearing, folding, and breaking. Unfortunately, 2D layers are not robust, and may experience collapse as the PMMA dissolves. However, there are no practical alternatives for sample transfer unless we handle with extreme care or utilize additional equipment such as a critical point dryer to increase the yield of suspended 2D membranes on the TEM grid.24,25 Here, we have employed a novel two-step approach for soft removal of PMMA in addition to a conventional TEM sample preparation process; using this method, we obtained large area and suspended 2D materials that remain on the grid without collapsing. Especially, 2D materials well maintained their suspended membrane on the grid using a highly concentrated PMMA solution than a lower one.
Experimental section
Growth of graphene and h-BN
Graphene and h-BN were synthesized using low pressure CVD. Chemical–mechanical polished Cu foil (25 μm thick, Alfa Aesar) was used as a metal catalyst and was placed at the center of a 4 inch quartz tube as shown in Fig. 1. Then, the furnace temperature was increased up to 1050 °C in the presence of 300 sccm of H2 at 1 Torr for 1 h to anneal the Cu foil and remove the native CuO layer. For graphene growth, 10 sccm of CH4 was additionally inserted into the quartz tube for a growth time of about 10 min.9 In the case of h-BN growth, we used ammonia borane, which was inserted into the sub-heater of Fig. 1b.26 Then, the sub-heater was heated up to 130 °C, and ammonia borane was decomposed into hydrogen, monomeric aminoborane, and borazine, the precursor of h-BN. In this process, removing the monomeric aminoborane is an important step for high quality h-BN growth. Here, we employed a filter paper to prevent the introduction of monomeric aminoborane to furnace.19 We have grown the h-BN monolayer slowly by taking longer time compared to a graphene growth in order to limit the formation of thick h-BN layer contaminated with nano particles (aminoborane). Therefore, h-BN was grown for 1 h with 300 sccm of H2 at 1 Torr on a Cu foil. | ||
| Fig. 1 Schematic images of LPCVD system used for graphene growth (a) and h-BN growth with a sub-heater for the decomposition of ammonia borane (b). | ||
Two-step method for soft dissolving PMMA
PMMA (950 PMMA A Resists, MicroChem) supported graphenes or h-BN layers were transferred onto a mesh grid. Conventional process usually removes the rigid PMMA layer directly by acetone for the fabrication of suspended 2D membrane. Here, we have employed a novel two-step method for soft dissolving PMMA in addition to a conventional sample preparation process. Coated PMMA layer on the graphene and h-BN was mildly dissolved using a diluted PMMA (2, 4, and 9% PMMA in anisole) solution for 10 min as a first step. Then, PMMA-residue was removed using acetone for 10 min. These processes are further elaborated in the section of Results and discussion.Characterization
Raman spectroscopy (Horiba, Lab Ram ARAMIS) and X-ray photoelectron spectroscopy (XPS: Thermo Scientific, K-Alpha) were used to analyze the vibration modes and binding energies, respectively, of graphene and h-BN. Surface morphology was observed using field emission scanning electron microscopy (FE-SEM: JEOL, JSM-7100F). Atomic scale images of graphene and h-BN were observed using spherical aberration-corrected TEM (JEOL, JEM-ARM 200F). Formation and the opening dynamics of defects were investigated to identify circular and triangular defects from graphene and h-BN, respectively.Results and discussion
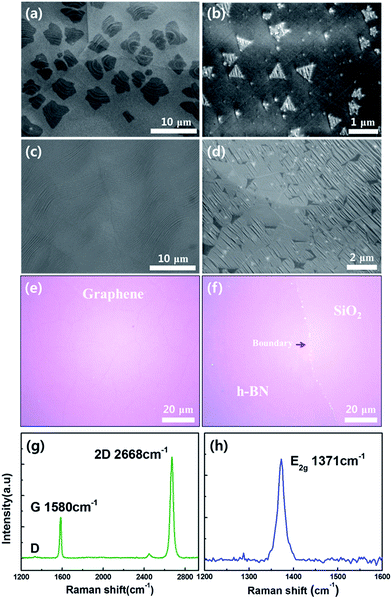
Fig. 2a and b show SEM images of graphene and h-BN; star and triangular shaped domains were observed for samples grown for 1 min and 10 min, respectively.27,28 Compared to the shape of graphene domains, h-BN generally grows in triangular shapes because the nitrogen terminated edges are more stable than the boron terminated edge.26,29 The samples of graphene and h-BN were grown for 10 min and 1 h, respectively, to fully cover the surface of the Cu foil (Fig. 2c and d). Graphene and h-BN layer were transferred onto the Si/SiO2 (300 nm) substrate using a PMMA supported wet transfer method to characterize the properties (Fig. 2e and f).30 Even with an atomically thin layer, the graphene layers on the SiO2 can easily be observed with the naked eye because the optical absorption is about 2.3% per layer in the visible range.31 On the contrary, the h-BN layer is hard to differentiate from the substrate because it has a high optical transmittance.32 The vibration modes were measured using Raman spectroscopy with a 532 nm laser as shown in Fig. 2g and h. For graphene, the intensity ratio of the 2D to G peak was higher than 2, and the sample showed no D peak, indicating that a high quality monolayer graphene was grown.9 It is well known that the Raman spectra of the E2g phonon mode of h-BN shows a blue shift from about 1366 to 1371 cm−1 as the number of layers decreases.33 The measured Raman peak of Fig. 2h was located at 1371 cm−1 and had high symmetry, meaning that we fabricated a high quality monolayer of h-BN.34Graphene and h-BN were transferred onto the quartz tube for analysis of optical properties using UV-visible spectrometry (Fig. S1a and b†). Fig. S1a† shows the transmittance of the monolayer graphene, which is 97.5% at a wavelength of 550 nm. Fig. S1b† shows the absorption spectrum of h-BN, which had almost zero absorbance in the visible light range. This spectrum was used to estimate the optical band gap of h-BN, which was about 6.05 eV from the Tauc plot shown in Fig. S1c.† These results were very close to the conventional values of graphene and h-BN grown by CVD.19,26,31 XPS survey scan spectra were employed to verify the atomic binding energies and stoichiometry. High resolution spectra were obtained to determine the locations of C, B, and N energy peaks. The binding energies of C 1s, B 1s, and N 1s peaks are shown in Fig. S1d–f,† respectively. The measured C 1s peak at 284.5 eV (Fig. S1d†) matched well with the sp2 C–C bonding of graphene.35 Fig. S1e and f† show the energy positions of B 1s and N 1s peaks at 190.2 eV and 397.2 eV, respectively, which were typical values for h-BN. The atomic ratio of B/N was confirmed to be 1.02 based on the integrated peak intensity and correction, indicating the ideal stoichiometry of h-BN was obtained.36,37 These various analyses strongly suggest that high quality monolayer graphene and h-BN were grown by using a LPCVD method on the Cu foil.
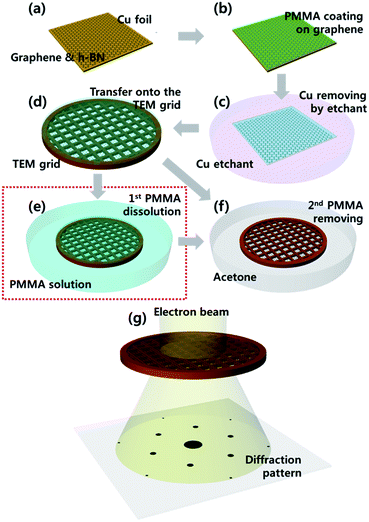
To fabricate the suspended 2D membranes for using TEM analysis, graphene and h-BN layers were transferred onto the carbon mesh grid. This process was conducted using the novel two-step method for soft dissolving PMMA illustrated in the schematic images of Fig. 3. The synthesized graphene and h-BN layers on the Cu foil (Fig. 3a) were coated by PMMA, which was used as a supporting layer. Then, the coated layers were baked at 180 °C for 2 min (Fig. 3b). The graphene grown on the back side of the Cu foil was etched using an O2 plasma treatment. Then, the Cu foil was etched and rinsed by Cu etchant and DI water (Fig. 3c). The PMMA-coated graphene layer was transferred onto the carbon mesh grid, and was dried for one day (Fig. 3d). Conventionally, the PMMA layer on the graphene is directly removed by acetone (Fig. 3f). However, we employed a two-step approach of dissolving PMMA in soft manner. Coated PMMA as a rigid supporting layer was mildly dissolved and removed using a diluted PMMA solution (9% in anisole) for 10 min as a first step (Fig. 3e); this step was added to the conventional process. Then, the solution based PMMA-residue was removed using acetone for 10 min as a second step shown in Fig. 3f. Finally, we prepared a TEM grid with the suspended 2D membrane, and we analyzed these as shown in Fig. 3g.
Fig. 4a and b are low magnification TEM images of suspended graphene membranes on the carbon mesh grid prepared by conventional and our proposed methods, respectively. As shown in Fig. 4a, the whole area of graphene was torn and entangled with the conventional method so it is difficult to find a region of unfolded graphene on the mesh of the TEM grid. With the conventional removal of PMMA from the sample of PMMA supported graphene, either dissolving layer of the rigid PMMA can cause tear in the graphene or rapid evaporation of acetone can lead to the entanglement due to the liquid capillary force during the process step illustrated in Fig. 3f. In the case of the TEM sample prepared by our new method proposed in Fig. 4b, almost all the area of the mesh grid was covered with graphene membrane uniformly. It was hard to find a folded membrane or an empty hole as shown in the red circles with the magnified inset image in Fig. 4b. In our proposed method, the rigid PMMA layer dissolves slowly and uniformly in the liquid PMMA solution so the underlying graphene layer is mechanically relaxed, resulting in better contact with the TEM grid. Also, the PMMA soft dissolution process leads to a decrease in the aforementioned liquid capillary force.38
Additionally, we analyzed the effect of PMMA-concentration in anisole for soft dissolving rigid PMMA layer. Graphene layers supported by PMMA were transferred onto the Cu grids (2000 mesh) including larger size hole than carbon mesh grid shown in Fig. 4. Size of holes is about 7–8 μm. SEM images of Fig. 5a–c show the Cu grid with suspended graphene membrane treated by the novel method using the various PMMA-concentrations 2, 4, and 9% in anisole, respectively. The squared holes are Cu mesh and the translucent gray membranes are suspended graphene layers after removing rigid PMMA. Here, it is hard to find the fully suspended graphene membrane on the Cu mesh in the Fig. 5a treated by 2% PMMA in anisole. In contrast, we are able to confirm that graphene membranes treated by 4 and 9% PMMA in anisole are well suspended fully covering the holes in the mesh grid. For further analysis, most of the holes with suspended graphene in the grid were observed and then categorized into three groups, depending upon the coverage ratio of the holes with the area suspended by graphene such as 0–30% (partially suspended), 30–70% (half suspended), and 70–100% (fully suspended). Statistical histogram of Fig. 5d clearly shows that suspended graphene easily collapses at lower PMMA-concentration, and this tendency reverses with the higher concentration. Especially, half of the holes are observed to have fully suspended graphene when treated by 9% PMMA in anisole. Consequently, our proposed method has increased the yield dramatically for the suspended graphene membrane on the mesh grid. This process was also applied to the TEM sample preparation for h-BN. The prepared TEM grids with uniformly suspended graphene layer or h-BN layer were analyzed by spherical aberration-corrected HRTEM to observe the atomic scale phenomena.
Fig. 6 shows atomic scale high resolution images of monolayer graphene. The spherical aberration-corrected HRTEM was operated at 80 kV. Fig. 6a and its inset image clearly show a typical hexagonal lattice structure of monolayer graphene and the atomic sites of carbon, respectively. Monolayer graphene was continuously exposed to and damaged by an accelerated electron beam, leading to the circular hole shown in Fig. 6b. Generally, the knock-on threshold voltage of graphene is 86 kV which is the energy required for breaking atomic bonds; irradiation using an electron beam at 80 kV is therefore not sufficient to eject the carbon atoms from the graphene lattice.39 However, intrinsic atomic defects existed in the graphene, and some contaminants were on the surface of graphene or the TEM chamber. These induce the lower knock-on threshold voltage,40 thus allowing the formation of defects in graphene membrane by electron beam irradiation. Graphene consists of sp2 bonded carbon atoms, and all the elements occupy the same energy state. Therefore, there is no preferred direction for sputtering of atoms. Accordingly, defects are formed in graphene as circular holes starting from a monovacancy.41,42 As exposure time is increased, pre-existing holes grow to larger size, and new small holes are formed as shown in Fig. 6c. Also, using the in situ TEM analysis, we were able to observe clearly the dynamics of two holes merging into one as shown in Fig. 6d.
Atomic scale phenomena on the h-BN membrane were also characterized with a similar process used for analyzing the graphene membrane. Since h-BN is an atomically thin insulating material where the charged electrons accumulate on the h-BN membrane, obtaining atomic resolution of the monolayer h-BN is not easy due to the drift of an electron beam during TEM imaging. We were able to observe the honeycomb lattice structure of h-BN and the atomic sites of boron and nitrogen by using spherical aberration-corrected HRTEM at reduced acceleration voltage of 80 kV as shown in Fig. 7a, S2† and its inset image. Interestingly, these show notable things that are clearly different from graphene, which are triangular holes. These holes start from the monovacancies. Typically, the monovacancy consists of the site where a boron atom is ejected. This is because the knock-on threshold voltage of boron (74 kV) is lower than that of nitrogen (84 kV), which means that boron is preferentially sputtered by electron beam irradiation.43,44 These boron monovacancies induce the instability of neighboring atoms, resulting in the growth of vacancies to larger sized defects. In this process during the electron beam irradiation, the relatively stable nitrogen atoms remain along the edge of the defect with a zigzag structure, thus the defects are shaped into triangular holes in the h-BN membrane as shown in the schematic image of Fig. 7b.45 This nature of h-BN can be equally applied to merging process of two different holes, thus a larger hole is formed turning into triangular shape as shown in Fig. S2† (as labeled with a blue dashed line).
Fig. 7c and d are the atomic images taken at the same region of a few layered h-BN initially and after 21 seconds of electron irradiation. From the images, we can observe various things explained above such as triangular holes with different size, monovacancies like red arrow in Fig. 7c, and merging process of holes (blue dashed line). Also, the images show the growth process of triangular holes. Fig. 7e and f were obtained by magnifying the same regions from Fig. 7c and d (red dashed line) after the prolonged irradiation with an electron beam. These images agree with the previously mentioned schematic images explaining the opening dynamics of the triangular holes. Initially, a few boron and nitrogen atoms, placed in edges of defect, are accidently sputtered away as shown in Fig. 7e (as labeled by the blue circle on its schematic image). This allows for subsequent removal of atoms along the edge of the triangular defects following the blue arrow in the schematic image of Fig. 7f. As a result, the triangular hole grows larger by forming a new nitrogen terminated zigzag edge. Also, this sputtering process is observed in Fig. S3.†
Defects of h-BN show dominantly triangular shape as mentioned above but the shape of multilayered h-BN is affected by their stacking structures.45 For example, hexagonally shaped hole or star-shaped defect was observed for the AA′ stacked bilayer h-BN as shown in Fig. 8a. Atomic models in Fig. 8b show the projected views of bilayer h-BN with AA′ stacking structure along the [001] and [110] directions, respectively. In the model, each layer alternates B and N atoms along the out-of-plane direction, resulting in the triangles rotated oppositely in 180 degrees for the h-BN defects with nitrogen terminated edge as shown in Fig. 8c.46 Overlapping of the opposite triangles at the same location forms hexagonal hole or star-shaped defect shown in the atomic resolution image in Fig. 8a.
Conclusions
In summary, the atomic structure and dynamics of defect formation in graphene and h-BN were clearly analyzed using spherical aberration-corrected HRTEM. In order to analyze these structures with atomic resolution imaging, we proposed a two-step method for the soft removal of PMMA in preparing suspended membrane of 2D materials for TEM samples. Using this new process, graphene and h-BN membranes were uniformly suspended on the TEM grid without tearing and folding of the membrane for atomic resolution HRTEM imaging. The high-yield fabrication of suspended 2D membrane can help our understanding on the atomic scale dynamics for the defects in 2D materials, thus enabling wider applications in optical and electrical devices such as optical detectors, gas and bio sensors.Acknowledgements
This research was supported by the MSIP (Ministry of Science, ICT and Future Planning), Korea, under the “IT Consilience Creative Program” (IITP-2015-R0346-15-1008) supervised by the IITP (Institute for Information & Communications Technology Promotion).References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- K. S. Novoselov, V. Fal, L. Colombo, P. Gellert, M. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- C. Dean, A. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim and K. Shepard, Nat. Nanotechnol., 2010, 5, 722–726 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- K. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos and A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS PubMed.
- D. Pacile, J. Meyer, C. O. Girit and A. Zettl, Appl. Phys. Lett., 2008, 92, 133107 CrossRef.
- R. J. Smith, P. J. King, M. Lotya, C. Wirtz, U. Khan, S. De, A. O'Neill, G. S. Duesberg, J. C. Grunlan and G. Moriarty, Adv. Mater., 2011, 23, 3944–3948 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung and E. Tutuc, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim and Y. I. Song, Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS PubMed.
- J. Han, J. Y. Lee and J. S. Yeo, Carbon, 2016, 105, 205–213 CrossRef CAS.
- Y. Zhan, Z. Liu, S. Najmaei, P. M. Ajayan and J. Lou, Small, 2012, 8, 966–971 CrossRef CAS PubMed.
- F. Banhart, J. Kotakoski and A. V. Krasheninnikov, ACS Nano, 2010, 5, 26–41 CrossRef PubMed.
- C. Attaccalite, M. Bockstedte, A. Marini, A. Rubio and L. Wirtz, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 144115 CrossRef.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., 2009, 48, 7752–7777 CrossRef CAS PubMed.
- A. L. Gibb, N. Alem, J.-H. Chen, K. J. Erickson, J. Ciston, A. Gautam, M. Linck and A. Zettl, J. Am. Chem. Soc., 2013, 135, 6758–6761 CrossRef CAS PubMed.
- J.-H. Lee, E. K. Lee, W.-J. Joo, Y. Jang, B.-S. Kim, J. Y. Lim, S.-H. Choi, S. J. Ahn, J. R. Ahn and M.-H. Park, Science, 2014, 344, 286–289 CrossRef CAS PubMed.
- S. Behura, P. Nguyen, S. Che, R. Debbarma and V. Berry, J. Am. Chem. Soc., 2015, 137, 13060–13065 CrossRef CAS PubMed.
- J. Han, J.-Y. Lee, H. Kwon and J.-S. Yeo, Nanotechnology, 2014, 25, 145604 CrossRef PubMed.
- C. A. Merchant, K. Healy, M. Wanunu, V. Ray, N. Peterman, J. Bartel, M. D. Fischbein, K. Venta, Z. Luo and A. C. Johnson, Nano Lett., 2010, 10, 2915–2921 CrossRef CAS PubMed.
- Y. Han, Z. Xu and C. Gao, Adv. Funct. Mater., 2013, 23, 3693–3700 CrossRef CAS.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. D. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- S. Liu, B. Lu, Q. Zhao, J. Li, T. Gao, Y. Chen, Y. Zhang, Z. Liu, Z. Fan and F. Yang, Adv. Mater., 2013, 25, 4549–4554 CrossRef CAS PubMed.
- W. Regan, N. Alem, B. Alemán, B. Geng, Ç. Girit, L. Maserati, F. Wang, M. Crommie and A. Zettl, Appl. Phys. Lett., 2010, 96, 113102 CrossRef.
- Y. D. Kim, H. Kim, Y. Cho, J. H. Ryoo, C.-H. Park, P. Kim, Y. S. Kim, S. Lee, Y. Li and S.-N. Park, Nat. Nanotechnol., 2015, 10, 676–681 CrossRef CAS PubMed.
- K. K. Kim, A. Hsu, X. Jia, S. M. Kim, Y. Shi, M. Hofmann, D. Nezich, J. F. Rodriguez-Nieva, M. Dresselhaus and T. Palacios, Nano Lett., 2011, 12, 161–166 CrossRef PubMed.
- X. Li, C. W. Magnuson, A. Venugopal, R. M. Tromp, J. B. Hannon, E. M. Vogel, L. Colombo and R. S. Ruoff, J. Am. Chem. Soc., 2011, 133, 2816–2819 CrossRef CAS PubMed.
- S. Sharma, G. Kalita, R. Vishwakarma, Z. Zulkifli and M. Tanemura, Sci. Rep., 2015, 5, 10426 CrossRef PubMed.
- Y. Liu, S. Bhowmick and B. I. Yakobson, Nano Lett., 2011, 11, 3113–3116 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed.
- R. Nair, P. Blake, A. Grigorenko, K. Novoselov, T. Booth, T. Stauber, N. Peres and A. Geim, Science, 2008, 320, 1308 CrossRef CAS PubMed.
- K. Watanabe, T. Taniguchi and H. Kanda, Nat. Mater., 2004, 3, 404–409 CrossRef CAS PubMed.
- R. V. Gorbachev, I. Riaz, R. R. Nair, R. Jalil, L. Britnell, B. D. Belle, E. W. Hill, K. S. Novoselov, K. Watanabe and T. Taniguchi, Small, 2011, 7, 465–468 CrossRef CAS PubMed.
- L. Ci, L. Song, C. Jin, D. Jariwala, D. Wu, Y. Li, A. Srivastava, Z. Wang, K. Storr and L. Balicas, Nat. Mater., 2010, 9, 430–435 CrossRef CAS PubMed.
- E. Moreau, F. Ferrer, D. Vignaud, S. Godey and X. Wallart, Phys. Status Solidi A, 2010, 207, 300–303 CrossRef CAS.
- N. Guo, J. Wei, L. Fan, Y. Jia, D. Liang, H. Zhu, K. Wang and D. Wu, Nanotechnology, 2012, 23, 415605 CrossRef PubMed.
- K. Park, D. Lee, K. Kim and D. Moon, Appl. Phys. Lett., 1997, 70, 315–317 CrossRef CAS.
- X. Li, Y. Zhu, W. Cai, M. Borysiak, B. Han, D. Chen, R. D. Piner, L. Colombo and R. S. Ruoff, Nano Lett., 2009, 9, 4359–4363 CrossRef CAS PubMed.
- B. W. Smith and D. E. Luzzi, J. Appl. Phys., 2001, 90, 3509–3515 CrossRef CAS.
- Q. M. Ramasse, R. Zan, U. Bangert, D. W. Boukhvalov, Y.-W. Son and K. S. Novoselov, ACS Nano, 2012, 6, 4063–4071 CrossRef CAS PubMed.
- A. Hashimoto, K. Suenaga, A. Gloter, K. Urita and S. Iijima, Nature, 2004, 430, 870–873 CrossRef CAS PubMed.
- J. Kotakoski, A. Krasheninnikov, U. Kaiser and J. Meyer, Phys. Rev. Lett., 2011, 106, 105505 CrossRef CAS PubMed.
- J. C. Meyer, A. Chuvilin, G. Algara-Siller, J. Biskupek and U. Kaiser, Nano Lett., 2009, 9, 2683–2689 CrossRef CAS PubMed.
- A. Zobelli, A. Gloter, C. Ewels, G. Seifert and C. Colliex, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 245402 CrossRef.
- G. H. Ryu, H. J. Park, J. Ryou, J. Park, J. Lee, G. Kim, H. S. Shin, C. W. Bielawski, R. S. Ruoff and S. Hong, Nanoscale, 2015, 7(24), 10600–10605 RSC.
- A. Shmeliov, J. S. Kim, K. B. Borisenko, P. Wang, E. Okunishi, M. Shannon, A. I. Kirkland, P. D. Nellist and V. Nicolosi, Nanoscale, 2013, 5, 2290–2294 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13344d |
| This journal is © The Royal Society of Chemistry 2016 |