Copper and triphenylphosphine-promoted sulfenylation of quinones with arylsulfonyl chlorides†
Xiaoli
Yu
,
Qiujin
Wu
,
Huida
Wan
,
Zhaojun
Xu
,
Xingle
Xu
and
Dawei
Wang
*
The Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, Jiangsu Province, China. E-mail: wangdw@jiangnan.edu.cn
First published on 23rd June 2016
Abstract
The efficient copper and triphenylphosphine-promoted sulfenylation of quinones with arylsulfonyl chlorides has been developed under mild conditions with moderate to good yields. Significantly, this provides an alternative method to synthesise arylthioquinone derivatives. This is the first time that arylthioquinones with arylsulfonyl chlorides as starting material have been prepared, which avoids the use of the awful smelling thioalcohols and thiophenols.
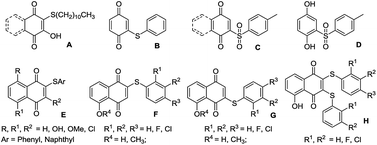
Quinone is a common and unique medical intermediate and building block for biological natural products, such as antibiotics, pharmaceuticals, dyestuff, anticancer drugs, pesticides and pigments owing to its special properties.1 Arylthioquinone is a typical example,2 and Tandon and coworkers reported that 2-methylsulfanyl-[1,4]naphthaquinone showed excellent fungistatic activity, which results in a marked increase in its activity profile.3a Compounds E were found to be antifungal agents as they possess in vitro activity against several fungal cultures capable of causing disease in vertebrates (Scheme 1).3b Ryu et al. reported that 2/3-arylthio- and 2,3-bis(arylthio)-5-hydroxy-/5-methoxy-1,4-naphthoquinones were tested for in vitro antifungal activity against Candida albicans, C. tropicalis and Aspergillus niger.4
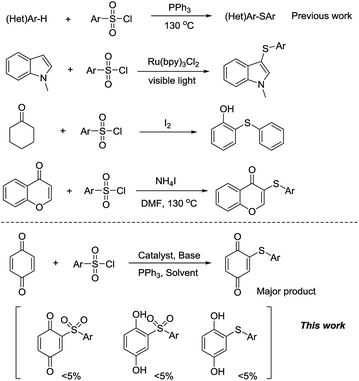
Arylthioquinone derivatives were usually prepared from the quinones and thiophenols.5 Recently, we developed copper-catalyzed cascade reaction of thiophenol hydroxylation and S-arylation to prepare arylthioquinones derivatives through disulfide-directed C–H activation in two steps method.6d However, the awful smell of thioalcohols and thiophenols is unsufferable and sometimes limits their applications. Therefore, searching for alternative reagents for thioalcohols and thiophenols is still an interesting and scientific issue. Chemists have paid great efforts to this area. Several kinds of alternative reagents are proved to be effective to sulfenylation transformations. Sulfonyl hydrazides,7N-(thiophenyl) succinimide,8 sodium sulfinates,9 sulfinic acids10 and arylsulfonyl chlorides are good choice to avoid the upper shortcomings for the cheap or no bad smell nature.11 In 2011, You and co-workers reported an organomediated-sulfenylation of (hetero)arenes to synthesize di(hetero)arylthioethers by directly using inexpensive and easily available arylsulfonyl chlorides as an alternative sulfur source (Scheme 2).12 Later, Zheng et al. described a method to prepare 1-methyl-3-(arylthio)-1H-indoles by the photoredox reactions of N-methylindoles with readily available arylsulfonyl chlorides in moderate yields.13 Adimurthy et al. developed copper-catalyzed regioselective C-3 sulfenylation of imidazo[1,2-a]pyridines using p-tosylchloride as a benign source of sulfenylating agents.14 Wu and Zou reported a copper-catalyzed regioselective sulfenylation of indoles with arylsulfonyl chlorides, which provides a good method to a series of structurally diverse indole thioethers with high regioselectivity.15 Recently, Deng and co-workers developed an iodine-promoted method for the synthesis of 2-arylsulfanylphenols using cyclohexanones as a reliable phenol source with various sulfonyl chlorides and sodium sulfinates as the sulfur source.16 Zhou et al. reported a regioselective and novel ammonium iodide mediated C–S bond formation protocol involving flavones and sulfonyl chlorides.17 From all the aforementioned excellent examples, we found that the commercially available arylsulfonyl chlorides were proved to be promising sulfenylation reagents with the accessibility, substrate compatibility and stability. Therefore, more transformations are needed to explore with arylsulfonyl chlorides as sulfenylation reagents. Based on our previous research on quinone derivatives synthesis,6 herein, we reported the sulfenylation of quinones with arylsulfonyl chlorides under copper and triphenylphosphine conditions in moderate to good yields. Significantly, this provides an alternative method to synthesis of arylthioquinone derivatives. To the best of our knowledge, this is the first time to prepare arylthioquinones with arylsulfonyl chlorides as starting material, which avoids the use of awful smell of thioalcohols and thiophenols.
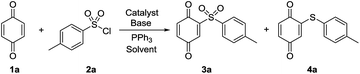
To start the research, benzoquinone and 4-methylbenzenesulfonyl chloride were selected as model substrates to attempt the reaction with palladium as a catalyst and triphenylphosphine as a reducing agent. However, it was found that only a reaction mixture was achieved (Table 1, entry 3). To our pleasure, moderate yield of sulfenylation product was achieved when CuI was added to the reaction mixture instead of palladium catalyst (Table 1, entry 6). For a higher yield, a series of screening reaction conditions were carried on. Firstly, toluene was screened to be more profitable in this reaction than 1,2-dichloroethane. Then several other copper catalysts were explored for their influence on the reaction behavior. However, there were no better catalysts than CuI. Other conditions, like base, were also screened (Table 1, entries 13–16). Several other reductants were tested for this transformation (Table 1, entries 15, 19–21). It was found that P(OMe)3 and Et3SiH could not promote this reaction, while P(nBu)3 produce a slightly lower yield (72%) than PPh3. The best reaction conditions were obtained as follows: CuI as the catalyst, K3PO4 as the base, PPh3 as the reducing agent and toluene as the solvent. It should be noted that the reaction could not happen without phosphorus reagent (Table 1, entry 17). Only low yield was achieved without of copper as a catalyst under triphenylphosphine condition (Table 1, entry 18).
| Entry | Catalyst | Base | Solvent | 3a b , c [%] | 4a b , c [%] |
|---|---|---|---|---|---|
| a Reagents and conditions: 1a (1.0 mmol), 2a (2.5 mmol), catalyst (10 mol%), base (1.5 mmol), PPh3 (2.5 mmol), solvent (5 mL), reflux, 10–24 h. b Yields of isolated product. c 5a and 6a were not checked. d Without PPh3. e 40 °C. f P(OMe)3. g P(nBu)3. h Et3SiH. | |||||
| 1 | Pd(OAc)2 | K2CO3 | DCE | 89 | <5d |
| 2 | [Cp*RhCl2]2/AgSbF6 | K2CO3 | DCE | 85 | <5d |
| 3 | Pd(OAc)2 | K2CO3 | DCE | 26 | 37 |
| 4 | [Cp*RhCl2]2 | K2CO3 | DCE | 33 | 18 |
| 5 | Pd(OAc)2/CuI | K2CO3 | DCE | 12 | 43 |
| 6 | CuI | K2CO3 | DCE | <5 | 61 |
| 7 | CuI | K2CO3 | DCM | <5 | <5 |
| 8 | CuI | K2CO3 | DCE | <5 | <5e |
| 9 | CuI | K2CO3 | Toluene | <5 | 65 |
| 10 | CuI | Cs2CO3 | Toluene | <5 | 73 |
| 11 | CuCl | Cs2CO3 | Toluene | <5 | 59 |
| 12 | Cu(OAc)2 | Cs2CO3 | Toluene | <5 | 47 |
| 13 | CuSO4 | Cs2CO3 | Toluene | <5 | 62 |
| 14 | CuI | tBuOK | Toluene | <5 | 11 |
| 15 | CuI | K3PO4 | Toluene | <5 | 78 |
| 16 | CuI | KOH | Toluene | <5 | <5 |
| 17 | CuI | K3PO4 | Toluene | <5 | —d |
| 18 | — | K3PO4 | Toluene | <5 | 47 |
| 19 | CuI | K3PO4 | Toluene | <5 | <5f |
| 20 | CuI | K3PO4 | Toluene | <5 | 72g |
| 21 | CuI | K3PO4 | Toluene | <5 | <5h |
| 22 | CuI | K3PO4 | MeCN | <5 | 74 |
| 23 | CuI | K3PO4 | Dioxane | <5 | 39 |
| 24 | CuI | K3PO4 | THF | <5 | 37 |
| 25 | CuI | K3PO4 | DMF | <5 | <5 |
| 26 | — | Cs2CO3 | Toluene | 12 | 19 |
| 27 | CuI | — | Toluene | 17 | 26 |
| 28 | — | — | Toluene | <5 | <5 |
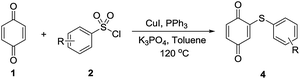
Subsequently, under the optimal conditions, we explored the scope of arylthioquinones synthesis from quinones with arylsulfonyl chlorides and the results are summarized in Table 2. The reactions were carried out at reflux temperature over a period of 10 hours each, with exposure to the air. Generally, nearly all the starting materials were converted to the arylthioquinones with moderate to good yields. As shown in Table 2, methyl-, chloro- and fluoro-substituted arylthioquinones were obtained in 46–83% yields. The substrate with electron-withdrawing substituents gave desired products at almost similar yields relative to that obtained from substrates with electron-donating substituents. The best yield was obtained when the substrate with tBu group in the substrate (Table 2, entry 12). Interestingly, the system could tolerate the methoxy, nitro, trifluoromethyl groups and all these substrates were transformed to the corresponding arylthioquinone derivatives with moderate to good yields (Table 2, entries 13–18).
| Entry | R | Yieldb [%] |
|---|---|---|
| a Reagents and conditions: 1 (1.0 mmol), 2 (2.5 mmol), CuI (10 mol%), K3PO4 (1.5 mmol), PPh3 (2.5 mmol), toluene (5 mL), 10 h, 120 °C. b Yields of isolated product. | ||
| 1 | 4-Me | 78 (4a) |
| 2 | 4-H | 74 (4b) |
| 3 | 4-Et | 71 (4c) |
| 4 | 3-Me | 68 (4d) |
| 5 | 4-F | 79 (4e) |
| 6 | 4-Cl | 81 (4f) |
| 7 | 4-Br | 77 (4g) |
| 8 | 2-Br | 76 (4h) |
| 9 | 3-Cl | 63 (4i) |
| 10 | 2,6-Cl | 52 (4j) |
| 11 | 2-F | 71 (4k) |
| 12 | 4-tBu | 83 (4l) |
| 13 | 4-OMe | 79 (4m) |
| 14 | 3-OMe | 82 (4n) |
| 15 | 2-OMe | 74 (4o) |
| 16 | 3,4-OMe | 83 (4p) |
| 17 | 4-NO2 | 64 (4q) |
| 18 | 4-CF3 | 72 (4r) |
Recently, Deng and co-workers reported the iodine-promoted method for the synthesis of 2-arylsulfanylphenols,16a while only arylthioquinone derivatives were separated with this methodology here after screening of reaction conditions, which maybe attribute to the suitable stability of quinones.
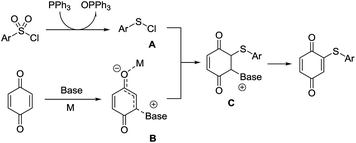
Finally, a possible reaction pathway for this reaction was proposed (Scheme 3). It was reported to produce sulfonyl-quinones under palladium conditions,6a while arylsulfenyl chloride (PhSCl) was formed, when sulfonyl chlorides were dealt with triphenylphosphine as reductive reagents.12 Next, the intermediate B, which might be formed with the help of base through Baylis–Hillman process, reacted with arylsulfenyl chloride to produce arylthioquinone derivatives.
Conclusions
In conclusion, the efficient sulfenylation reaction of quinones with arylsulfonyl chlorides has been developed under copper and triphenylphosphine conditions with moderate to good yields. Significantly, this provides an alternative method to synthesis of arylthioquinone derivatives.Acknowledgements
We gratefully acknowledge financial support of this work by the National Natural Science Foundation of China (21401080, 51503086), the Natural Science Foundation of Jiangsu Province of China (BK20130125), and MOE & SAFEA for the 111 Project (B13025).References
- (a) J. T. Tsay, W. Oh, T. J. Larson, S. Jackowski and C. O. Rock, J. Biol. Chem., 1992, 267, 6807 CAS; (b) X. Qiu, C. A. Janson, A. K. Konstantinidis, S. Nwagwu, C. Silverman, W. W. Smith, S. Khandekar, J. Lonsdale and S. S. Abdel-Meguid, J. Biol. Chem., 1999, 274, 36465 CrossRef CAS PubMed; (c) W. P. Revill, M. J. Bibb, A. K. Scheu, H. J. Kieser and D. A. Hopwood, J. Bacteriol., 2001, 183, 3526 CrossRef CAS; (d) M. Alhamadsheh, N. Waters, S. Sachdeva, P. Lee and K. Reynolds, Bioorg. Med. Chem. Lett., 2008, 18, 6402 CrossRef CAS.
- (a) G. A. Kraus and I. Kim, J. Org. Chem., 2003, 68, 4517 CrossRef CAS; (b) C.-K. Ryu, J.-Y. Shim, M. J. Chae, I. H. Choi, J.-Y. Han, O.-J. Jung, J. Y. Lee and S. H. Jeong, Eur. J. Med. Chem., 2005, 40, 438 CrossRef CAS; (c) B. J. Mulchin, C. G. Newton, J. W. Baty, C. H. Grasso, W. J. Martin, M. C. Walton, E. M. Dangerfield, C. H. Plunkett, M. V. Berridge, J. L. Harper, M. S. M. Timmer and B. L. Stocker, Bioorg. Med. Chem., 2010, 18, 3238 CrossRef CAS.
- (a) V. K. Tandon, R. B. Chhor, R. V. Singh, S. Rai and D. B. Yadav, Bioorg. Med. Chem. Lett., 2004, 14, 1079 CrossRef CAS; (b) V. K. Tandon, R. V. Singh and D. B. Adav, Bioorg. Med. Chem. Lett., 2004, 14, 2091 CrossRef; (c) V. K. Tandon and H. K. Maurya, Tetrahedron Lett., 2009, 50, 5896 CrossRef CAS.
- C.-K. Ryu, J.-Y. Shim, M. J. Chae, I. H. Choi, J.-Y. Han, O.-J. Jung, J. Y. Lee and S. H. Jeong, Eur. J. Med. Chem., 2005, 40, 438 CrossRef CAS.
- (a) M. C. Carreno, G. Ruano, A. Urbano, C. Z. Remor and Y. Arroyo, J. Org. Chem., 2000, 65, 453 CrossRef CAS; (b) C.-K. Ryu, I. H. Choi and R. E. Park, Synth. Commun., 2006, 36, 3319 CrossRef CAS.
- For selected quinone transformations in our group, see: (a) B. Ge, D. Wang, W. Dong, P. Ma, Y. Li and Y. Ding, Tetrahedron Lett., 2014, 55, 5443 CrossRef CAS; (b) D. Wang, B. Ge, L. Du, H. Miao and Y. Ding, Synlett, 2014, 25, 2895 CrossRef CAS; (c) D. Wang, B. Ge, L. Li, J. Shan and Y. Ding, J. Org. Chem., 2014, 79, 8607 CrossRef CAS; (d) D. Wang, X. Yu, W. Yao, W. Hu, C. Ge and X. Shi, Chem.–Eur. J., 2016, 22, 5543 CrossRef CAS.
- (a) F.-L. Yang and S.-K. Tian, Angew. Chem., Int. Ed., 2013, 52, 4929 CrossRef CAS; (b) A. K. Bagdi, S. Mitra, M. Ghosh and A. Hajra, Org. Biomol. Chem., 2015, 13, 3314 RSC; (c) R. Rahaman, N. Devi, K. Sarma and P. Barman, RSC Adv., 2016, 6, 10873 RSC.
- (a) M. Tudge, M. Tamiya, C. Savarin and G. R. Humphrey, Org. Lett., 2006, 8, 565 CrossRef CAS; (b) C. C. Silveira, S. R. Mendes, L. Wolf and G. M. Martins, Tetrahedron Lett., 2010, 51, 2014 CrossRef CAS; (c) T. Hostier, V. Ferey, G. Ricci, D. G. Pardoa and J. Cossy, Chem. Commun., 2015, 51, 13898 RSC.
- (a) Y.-M. Lin, G.-P. Lu, C. Cai and W.-B. Yi, Org. Lett., 2015, 17, 3310 CrossRef CAS; (b) D. Wang, R. Zhang, S. Lin, Z. Yan and S. Guo, RSC Adv., 2015, 5, 108030 RSC; (c) F. Xiao, S. Chen, J. Tian, H. Huang, Y. Liu and G.-J. Deng, Green Chem., 2016, 18, 1538 RSC; (d) Y. Gao, Y. Gao, X. Tang, J. Peng, M. Hu, W. Wu and H. Jiang, Org. Lett., 2016, 18, 1158 CrossRef CAS PubMed; (e) C. Wang, H. Jia, Z. Li, H. Zhang and B. Zhao, RSC Adv., 2016, 6, 21814 RSC.
- (a) C.-R. Liu and L.-H. Ding, Org. Biomol. Chem., 2015, 13, 2251 RSC; (b) R. Rahaman, N. Devi, J. R. Bhagawati and P. Barman, RSC Adv., 2016, 6, 18929 RSC.
- (a) C. Shen, P. Zhang, Q. Sun, S. Bai, T. S. A. Hor and X. Liu, Chem. Soc. Rev., 2015, 44, 291 RSC; (b) G. Kumaraswamy, R. Rajua and V. Narayanaraoa, RSC Adv., 2015, 5, 22718 RSC.
- (a) Q. Wu, D. Zhao, X. Qin, J. Lan and J. You, Chem. Commun., 2011, 47, 9188 RSC; (b) D. Wan, Y. Yang, X. Liu, M. Li, S. Zhao and J. You, Eur. J. Org. Chem., 2016, 55 CrossRef CAS.
- M. Chen, Z.-T. Huang and Q.-Y. Zheng, Chem. Commun., 2012, 48, 11686 RSC.
- C. Ravi, D. C. Mohan and S. Adimurthy, Org. Biomol. Chem., 2016, 14, 2282 RSC.
- Z. Wu, Y.-C. Li, W.-Z. Ding, T. Zhu, S.-Z. Liu and L.-H. Zou, Asian J. Org. Chem., 2016, 5, 625 CrossRef CAS.
- (a) Y. Chen, F. Xiao, H. Chen, S. Liu and G.-J. Deng, RSC Adv., 2014, 4, 44621 RSC; (b) F.-H. Xiao, H. Xie, S.-W. Liu and G.-J. Deng, Adv. Synth. Catal., 2014, 356, 364 CrossRef CAS.
- W. Zhao and A. Zhou, ChemCatChem, 2015, 7, 3464 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, 1H NMR, 13C NMR data for 4a–4r. See DOI: 10.1039/c6ra11301j |
| This journal is © The Royal Society of Chemistry 2016 |