In situ synthesis of N-doped carbon nanotubes–BiOCl nanocomposites and their synergistic photocatalytic performance†
LuPing Zhu*ab,
LingLing Wanga,
NaiCi Binga,
Peng Lib,
LiJun Wang*a,
Chao Huangc and
GuiHong Liaod
aSchool of Environmental and Materials Engineering, Shanghai Second Polytechnic University, Shanghai, 201209, China. E-mail: lpzhu@sspu.edu.cn; wang_lijun@yahoo.cn
bEnvironmental Remediation Materials Unit, National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: ZHU.luping@nims.go.jp
cDepartment of Physics and Materials Science, City University of Hong Kong, Hong Kong SAR, China
dTechnical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 23rd December 2015
Abstract
N-Doped carbon nanotube–BiOCl (NCB) nanocomposites were prepared by an in situ growth strategy in liquid ethylene glycol (EG). The prepared samples were characterized by powder X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), UV-vis diffuse reflectance spectroscopy (DRS), and photoluminescence (PL) emission spectroscopy. The photocatalytic investigations showed that the 2-NCB nanocomposites loaded with 2.0 wt% N-doped carbon nanotubes (CNxNTs) possessed the highest rate constant, which is about 2.5, 3.7, and 1.5 times that of pure BiOCl 3D hierarchical structures, BiOCl/MWCNTs composites, and P25, respectively. The enhanced photocatalytic performance could be attributed to the beneficial microstructure, synergistic effects of coupled CNxNTs–BiOCl nanocomposites, and the high migration efficiency of the photogenerated electrons, which may effectively suppress the charge recombination.
1 Introduction
In recent years, photocatalytic decomposition of organic pollutants by using semiconductors, such as TiO2, is of growing interest for the purification of air and water. However, the high recombination of photogenerated electron–hole (e−–h+) pairs in TiO2 leads to low quantum efficiency,1 which greatly limits its application in the field of photocatalysis. So, it is critical to develop novel nontitania-based semiconductor photocatalysts with high photocatalytic performances.2Recently, bismuth oxyhalides (BiOX, X = Cl, Br, and I) have attracted a great deal of attention for their potential application in photocatalysis. BiOX is characterized by a layered structure in which halogen atoms are situated between [Bi2O2] layers.3 The formed internal static electric fields between the [Bi2O2]2+ positive layers and the negative halogen layers are believed to induce an efficient separation of photogenerated electron–hole pairs, thus improving the photocatalytic performance of the catalysts.4 Among them, bismuth oxychloride (BiOCl) is of particular importance because it possesses a relatively high stability and an excellent photocatalytic activity under ultraviolet light irradiation.5,6 However, the recombination of photogenerated charge carriers still exists in the individually BiOCl.7 So, in order to further enhance photocatalytic activity of BiOCl-based semiconductor photocatalysts, it is essential to hamper the recombination of electron–hole species in the semiconductors by molecular electron relay semiconductor structures or efficient electron transport matrices, for instance of carbon materials.7,8 Among the promising carbon materials, CNxNTs have attracted much attention recently due to their unique structure, outstanding properties, and potential applications.9 Nitrogen atoms in the framework of CNxNTs will form chemically active sites which are beneficial to the metal or metal oxide to anchor with the CNxNTs.9a,10,11 Recently, the Pt–Ru,12 Pt,13–15 Ag,16 SnO2,10a,11b CeO2,10a and ZnO11a,17,18 nanoparticles have been immobilized onto the CNxNTs to prepare CNxNTs-based nanomaterials with remarkable catalytic and photocatalytic performance. Moreover, because nitrogen atom has one more electron than carbon atom, the CNxNTs may show the performance of n-type semiconductor for the extra electron acted as the current carrier.10 So, the combination of CNxNTs and BiOCl is expected to obtain the hybrid composite materials with superior photocatalytic performance, owing to the coupling effects between the two components. On one hand, the CNxNTs can serve as an excellent supporting matrix for photocatalyst particles. On the other hand, the excellent electronic conductivity of CNxNTs, which is similar to CNTs and graphene, can also act as an excellent electron-acceptor/transport material to effectively inhibit the recombination of the electron–hole pairs in the NCB composite. Inspired by these facts, we report a facile solvothermal process to prepare NCB nanocomposites by using bismuth chloride, urea, and as-prepared CNxNTs as the starting materials. Their photocatalytic activities were also discussed carefully, which exhibited a higher photocatalytic activity than that of pure BiOCl 3D hierarchical structures, the CNxNTs, and P25, under ultraviolet light irradiation. Furthermore, a mechanism for photocatalytic reaction in the NCB system was proposed.
2 Experimental
2.1 Synthesis of NCB nanocomposites
All the chemicals were of analytical grade from the Sinopharm Chemical Reagent Co., Ltd. and were used without further purification. CNxNTs were obtained by a modified chemical vapor deposition (CVD), is similar to that described in our previous paper.11b,19 The NCB nanocomposites were prepared by using a facile solvothermal process with bismuth chloride, urea, and as-prepared CNxNTs as the precursors. In a typical synthesis, 2 mmol of BiCl3·5H2O was added to 40 mL ethylene glycol (EG) with stirring at room temperature. After BiCl3·5H2O was dissolved fully, CNxNTs of different mass was dispersed in the above solution by ultrasonication for 1 h. Then, an appropriate amount of urea was added to the mixture with magnetic stirring for 30 min. Finally, the formed suspension was transferred into a Teflon-lined stainless-steel autoclave (50 mL). The autoclave was sealed and maintained at 160 °C for 12 h under autogenous pressure. After cooling to room temperature naturally, the resulting products were collected and washed with deionized water and absolute ethanol for several times, and finally dried at 50 °C for 8 h in air. The as-synthesized NCB sample with 1.0, 2.0, 4.0, and 6.0 wt% CNxNTs was labeled as 1-NCB, 2-NCB, 4-NCB, and 6-NCB, respectively. For comparison, the pure BiOCl counterpart was prepared using the same solvothermal process without the addition of as-synthesized CNxNTs.2.2 Characterization
The X-ray diffraction (XRD) patterns of the products were collected on a Bruker D8-Advance X-ray diffractometer with monochromated Cu Kα radiation (λ = 1.5418 Å). The X-ray photoelectron spectroscopy (XPS) analysis were performed on an Axis Ultra DLD instrument from Kratos (UK) using an Al Kα X-ray radiation source at a power of 300 W. Scanning electron microscopy (SEM) images were performed using a Hitachi S-4800 microscope (Japan). Transmission electron microscopy (TEM) images were obtained by using JEM 2100F field-emission transmission electron microscope operated at an accelerating voltage of 200 kV. Diffuse reflectance spectra (DRS) of BiOCl and as-synthesized samples were recorded in the range of 200 to 800 nm by using a Shimadzu 2550 UV-vis spectrophotometer; BaSO4 was used as a reference. The photoluminescence (PL) emission spectra of photocatalysts were detected under excitation at 325 nm using a Shimadzu RF-5301PC spectrofluorophotometer.2.3 Photocatalytic activity measurement
The photocatalytic activities of the as-synthesized samples were evaluated by the photocatalytic degradation of a model pollutant RhB under UV light irradiation at room temperature. A 100 W high-pressure mercury lamp was used as a light source with a main emission wavelength of 365 nm. The experiments were performed as follows: in each run, 20 mg of as-prepared catalyst was added into 200 mL RhB solution (10 mg L−1). Before illumination, the solution was stirred for 30 min in the dark to reach the adsorption–desorption equilibrium between the RhB and the photocatalyst. Then the suspension was stirred and exposed to UV light irradiation. At 10 min intervals, the solution was sampled. The photocatalyst powders and the RhB solution were separated by a centrifugal machine. The concentrations of the RhB were monitored by checking the absorbance at 553 nm during the photodegradation process by using a Shimadzu 2550 UV-vis spectrophotometer.3 Results and discussion
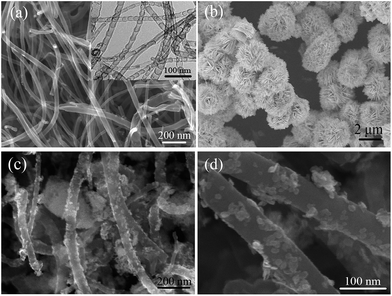
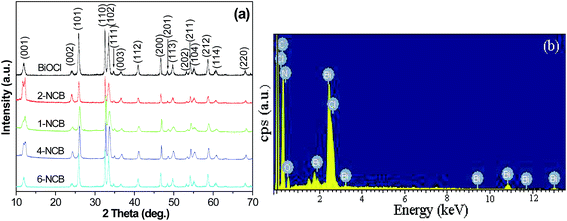
The morphology and structure of the as-synthesized samples were investigated by field-emission SEM and TEM, as shown in Fig. 1. Fig. 1(a) shows the SEM and TEM (inset in Fig. 1(a)) images of the as-prepared pure CNxNTs. It is clearly seen that the CNxNTs with a diameter of about 30–50 nm were very clean, and the bamboo-like structure is the representative feature of CNxNTs which consists of uniform and well-ordered compartments in contrast to the straight CNTs. Fig. 1(b) shows that BiOCl is comprised of many 3D flower-like hierarchical structures with an average diameter of 3 μm. Individual 3D flower-like hierarchical structures are built from several dozen of radially grown nanosheets. The surface of the nanoplates with a thickness of about 30 nm and a width of 0.5 to 1.5 μm was very smooth, probably due to Ostwald ripening.20 However, when the CNxNTs were added, the plate-packed 3D flower-like hierarchical structures were disrupted in NCB nanocomposites. A morphology which significantly differed from that of pure BiOCl was observed. Clearly, a comparatively smaller size of BiOCl plates with an average width of 30 nm were generated and coated on the CNxNTs, the typical SEM images of the NCB nanocomposites are shown in Fig. 1(c) and (d).Fig. 2(a) shows the XRD patterns of the pure BiOCl and NCB nanocomposites. It is found that the main diffraction peaks of NCB nanocomposites are similar to that of pure BiOCl and corresponds to tetragonal phase BiOCl (JCPDS no. 85-0861), which indicates that the presence of CNxNTs does not result in the development of new crystal orientations or changes in preferential orientations of BiOCl. However, it is worth noting that the intensity of the peaks associated with the (001), (002), and (110) diffractions for the sample of NCB nanocomposites are increased significantly. This result indicates that BiOCl grown on CNxNTs adopts a higher crystallinity, which is similar to reports.7 The narrow broadening of the peaks implies a well-crystallized BiOCl powder material. No characteristic diffraction peaks for carbon species were observed in the nanocomposites, probably due to the relatively low diffraction intensity of CNxNTs and overlapping of the main peak of CNxNTs at 26.01 and the (101) peak of tetragonal BiOCl at 25.82.21 Such a phenomenon was also observed in the synthesis of TiO2/graphene composites, graphene/BiOCl, BiOBr–graphene, BiOI–graphene, and BiOCl/MWCNTs.7,22 Additional evidence for the formation of NCB composites came from analysis of the energy-dispersion X-ray spectrum (EDS). Fig. 2(b) shows the EDS of the as-prepared products. As expected, the peaks of C, N, Bi, Cl, and O can be easily found. The atomic ratio of Bi to Cl for BiOCl on the CNxNTs was about 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, according to EDS quantitative microanalysis, which indicates that some bismuth vacancies were present in the NCB nanocomposites.
14, according to EDS quantitative microanalysis, which indicates that some bismuth vacancies were present in the NCB nanocomposites.
To further determine the chemical state and composition of the as-synthesized NCB nanocomposites and compare with that of the as-synthesized BiOCl and CNxNTs sample, X-ray photoelectron spectroscopy (XPS) measurements were carried out in the region of 0–1100 eV. The fully scanned spectra (Fig. 3(a)) demonstrate that Bi, O, Cl, and C elements exist in pure BiOCl sample, while Bi, O, Cl, C, and N exist in 2-NCB nanocomposites, respectively. The C peak in the XPS spectrum of the BiOCl sample can be ascribed to the adventitious carbon-based contaminant which comes from the XPS instrument itself, and the binding energy for the C 1s peak at 284.6 eV is used as the reference for calibration. Fig. 3(b) shows the core-level XPS spectra for the two samples at Bi (4f) binding energy regions. Two strong peaks at 159.8 and 165.1 eV in the high resolution spectra are assigned to Bi 4f7/2 and Bi 4f5/2 spin–orbital splitting photoelectrons, respectively, which is characteristic of Bi3+ in BiOCl. The splitting between the two bands was 5.3 eV, indicating the presence of the normal state of BiOCl in pure BiOCl and 2-NCB. The high resolution XPS spectra of the O 1s region were shown in Fig. 3(c). For 2-NCB and pure BiOCl, the main peak appeared at about 530.9 eV and 530.6 eV, respectively. An about 0.3 eV shift can be observed, which may be attributed to the effect of the interaction between bismuth atom and nitrogen atom. The Cl 2p3/2 and Cl 2p1/2 peaks of 2-NCB and pure BiOCl samples are associated with the binding energy of 198.5 and 200.1 eV, respectively (Fig. 3(d)). In order to further study the interaction between the BiOCl particles and the nitrogen atoms, the N 1s XPS spectra of CNxNTs and NCB nanocomposites were investigated. Fig. 3(e) shows that each XPS spectrum of N 1s scan is composed of two peaks, which are around 399.5 and 400.3 eV, respectively. The peak (P1) at 399.5 eV can be assigned to the pyridine-like N; while the peak (P2) at 400.3 eV can be assigned to the graphite-like N. The binding energy of P1 of NCB nanocomposites is around 400.0 eV, in comparison to the P1 of CNxNTs (399.1 eV), there is 0.9 eV shift of the P1 peak, and for the binding energy of P2, it also exists 0.3 eV shift, which is agreement with the O 1s. As it is known, the electronegativity of nitrogen (2.8–3.4) is stronger than that of bismuth (2.0), if bismuth atom interacts with nitrogen atom, the nitrogen atom may strongly absorb the electron of bismuth atom so that the binding energy of N 1s will reduce. It corresponds to the spectra Fig. 3(e). These shifts demonstrate that there exists strongly interaction between the BiOCl particles and the N atoms (pyridine-like N and graphite-like N) of CNxNTs.
 | ||
| Fig. 3 (a) XPS survey spectra of the samples, (b)–(e) high-resolution XPS spectra of Bi 4f, O 1s, Cl 2p, and N 1s of the samples. | ||
Based on the in situ growth of NCB nanocomposites and the above analysis, a possible formation process of NCB nanocomposites can be schematically illustrated in Fig. 4. Typically, BiOCl grown in the absence of CNxNTs was built from 2D laminar nanoplates, which generated rotation and assembly into 3D hierarchical structures.21 However, when adding the CNxNTs into the EG solution of BiCl3 and urea, NCB nanocomposites was obtained. It is well-known that treating the CNTs with acid could create considerable functional groups such as carboxyl (–COOH) on the outside surface of CNTs which become negatively charged. This will be conducive to the adsorption of the positive ion at the negative electricity activity site. In this work, NCB nanocomposites were prepared by in situ growth strategy in liquid EG without acid treatment of the CNxNTs. Thus the N doping in the CNxNTs may play an important role in the formation of NCB nanocomposites. Due to the electron densities of the N are higher than that of the C atoms in CNxNTs, once the carbon atoms were substituted by nitrogen atoms in the framework of the nanotubes, the extra electrons can be obtained. Then, metal ions could be adsorbed easily on the N of the CNxNTs. The extra electrons of CNxNTs have similar role to the negative electricity group, such as –COOH, on the surface of CNTs after acid treatment. When the CNxNTs was added into the EG solution of BiCl3, the Bi3+ ion might move and adsorb to the negative electricity activity site of the N atoms through electrostatic attraction. The pyridine-like N corresponds to the N atoms occupying the vacancy formed by removing a central C atom among three hexagons and replacing the three surrounding C atoms with three N toms.23 Besides that, the pyridine-like N has two extra nonbonding electrons in its sp2-hybridized orbital, which can act as a nonbonding p-orbital.10a So, the pyridine-like N atoms will form a strongly negative electricity centre, which can catch the metal ions and offer higher stability to form heterojunction between CNxNTs and compounds. In addition, the graphite-like N can also absorb Bi3+ ions and form the Bi–N bond. According to the XPS results, there are the peak shifts of both the pyridine-like N and the graphite-like N structures of the NCB nanocomposites, compared with the pristine CNxNTs. It can be concluded that the Bi3+ ions can be anchored at the sites of the pyridine-like N and the graphite-like N of CNxNTs and form the Bi–N bond. In the following solvothermal process, the Bi3+ reacted with urea and Cl− to form BiOCl primary particles on the surfaces of the CNxNTs. According to our experiments, it is observed that the nanoparticles loading process was not abrupt but usually depended on the sites of N doping in the CNxNTs which act as preferential nucleation sites for the formation of larger crystals. The freshly crystalline nanoparticles are unstable and tend to aggregate and assembly larger flakes at the sites of N doping in the CNxNTs before packing into 3D flower-like hierarchical structures, based on their inner crystallographic orientation. The exact mechanism for the formation of the final samples needs be further investigated.
 | ||
| Fig. 4 Schematic illustration of the growth process of the BiOCl 3D flower-like hierarchitectures and NCB nanocomposites. | ||
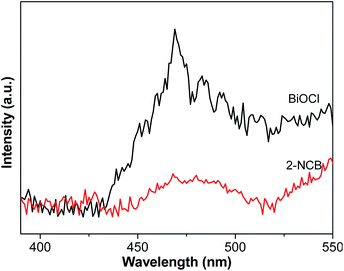
UV-vis spectroscopy was employed to investigate the optical properties of the samples. Fig. 5 shows the diffuse reflectance spectra (DRS) of 2-NCB nanocomposites and pure BiOCl. 2-NCB nanocomposites not only exhibit a slight red-shift at the absorption edge but also an absorption in the both UV and visible range. As a crystalline semiconductor, the optical absorption near the band edge follows the formula:
| (αhν)n = B(hν − Eg) | (1) |
In order to compare the photocatalytic activity of the as-prepared samples over different reaction times, a series of photocatalytic degradation experiments were carried out by using rhodamine B (RhB) as a model pollutant under ultraviolet light irradiation (λ = 365 nm). The characteristic absorption of RhB at λ = 553 nm was used to monitor the photodegradation process. Fig. 6(a) shows the temporal evolution of the spectral changes during the photodegradation of RhB mediated by 2-NCB (0.1 mg mL−1 of catalyst). It was observed that the maximum absorption at λ = 553 nm decreases sharply with the increase of the exposure. The sharp decrease within 10 min indicates that the as-prepared 2-NCB sample exhibited very high photocatalytic activity in the degradation of RhB. In addition, a significant shift of absorbance (at λ = 553 nm) is not observed during the photocatalytic process, as shown in Fig. 6(a). This suggests that the 2-NCB nanocomposites do not change the photocatalytic degradation pathway of RhB in our system, which is in agreement with the reports.21,27 Note that the absorption peak completely disappeared after irradiation for 50 min. No new absorption bands appeared in either the visible or the UV region, indicating that RhB could be completely decolorized by using 2-NCB during that reaction.22c The variations of RhB concentration (C/C0) with irradiation time over different photocatalysts were shown in Fig. 6(b). The blank test showed that the photodegradation efficiency of RhB was extremely low under UV light irradiation in the absence of catalyst, which demonstrated that the direct photolysis of RhB was negligible under UV irradiation. It was observed that the as-synthesized NCB samples, except the 6-NCB, exhibited higher photocatalytic activity than BiOCl. The 2-NCB composite exhibits the best performance on the photodegradation of RhB among the as-prepared samples. Amazingly, nearly 60% of RhB was degraded after being irradiated for 10 min with 2-NCB catalyst, and about 92% was degraded after 30 min, which is higher than the P25 with degradation rate of about 80%. Conversely, the photocatalytic activity of BiOCl was only at 50% under UV light irradiation for 30 min.
To quantitatively analysis the photocatalytic reaction kinetics of the RhB degradation in our experiments, a pseudo-first-order model was used to analyze the photocatalytic degradation processes if the initial concentration of pollutant is low. The rate equation of photocatalytic degradation of RhB can be determined by using the following expression:28
| ln(C/C0) = kt | (2) |
From the viewpoint of practical application, it is crucial that the as-prepared photocatalyst maintains a high activity and stability for long-term use. After the used catalyst was washed with deionized water and absolute ethanol, and finally dried under vacuum at 50 °C for 8 h, it was subjected to repeated reaction at a constant condition. As Fig. S2† illustrated, the N-doped carbon nanotubes–BiOCl nanocomposites loaded with 2.0 wt% N-doped carbon nanotubes possessed a sustainable photocatalytic performance in photodegradation of RhB after 5 cycles. There was insignificant loss of the photodegradation rate of RhB, which indicates that the N-doped carbon nanotubes–BiOCl nanocomposites were highly stable and reusable. This could be attributed to high dispersion of BiOCl nanoplates, and stronger interaction between BiOCl and the N-doped carbon nanotubes.
As we all know, the generation and separation of the photogenerated electron (e−)–hole (h+) pairs are the key factors to affect the photocatalytic reaction. If the photogenerated e−–h+ can be separated effectively, it is conducive to improve the photocatalytic activities. Theoretically, CNxNTs with sp2-hybridized carbon and nitrogen atoms have a high electrical conductivity in storing and shuttling electrons.30,31 When CNxNTs is combined with other materials, electrons would flow from the higher to lower Fermi level at the interface of two materials.31,32 In view of the higher work function of CNxNTs than that of BiOCl, electrons will flow from the conduction band (CB) of light activated BiOCl into CNxNTs to adjust the Fermi energy levels, leading to the formation of a Schottky barrier at the CNxNTs–BiOCl interface, which is same as the report.32 This Schottky barrier can capture electrons from BiOCl to CNxNTs and prevent their back flowing to the BiOCl. Thus, in CNxNTs–BiOCl, CNxNTs served as an acceptor of the photogenerated electrons of BiOCl and effectively suppressed the charge recombination, which can be confirmed by the results of PL (shown in Fig. 7). In the meantime, the photogenerated holes left in valence band (VB) of BiOCl take part in the oxygen production reaction of photodegradation.
PL emission spectrum can be regarded as an effective approach to understanding the charge transportation and separation properties of the photoinduced charge carriers. The higher the PL intensity is, the less efficient carriers participate in the photocatalytic procedure.33 PL emission spectra of BiOCl and NCB under excitation at 325 nm were given in Fig. 7. It is found that NCB exhibited much lower emission intensity than BiOCl, indicating that the presence of CNxNTs can suppress the radiative recombination process, leading to weak recombination of the e−–h+ pairs and high photon efficiency. This is consistent with the photocatalytic activity of the samples.
On the basis of the literatures22d,e and the above results and analysis, a possible mechanism to explain the superior photocatalytic performances of NCB under UV light irradiation can be illustrated, as shown in Fig. 8. Under UV irradiation, electrons (e−) in the valence band (VB) of BiOCl were excited to its conduction band (CB), and at the same time the same amount of holes (h+) were left in VB. Then, the photogenerated electrons on CB of BiOCl transferred to CNxNTs. The photogenerated electrons accumulated on the surface of CNxNTs had good fluidity and could be transferred to surface-absorbed oxygen rapidly to produce activated ˙O2−. The activated ˙O2− further gives rise to hydroxyl radicals (˙OH) via a series of reaction. On the other hand, the holes (h+) left in VB of BiOCl could also react with H2O to produce ˙OH. As a powerful oxidant, the ˙OH could decompose effectively the organic substances such as RhB, MB, and MO.34 In the degradation process, the photogenerated e−/h+ in the nanoheterostructured composites achieved a effective separation.
 | ||
| Fig. 8 Schematic diagram showing band configuration and photocatalytic mechanism of NCB nanocomposites under UV irradiation (CB the bottom of conduction band, VB the top of valence band). | ||
It is well known that the adsorption performance of the targeted contaminant is also very important for the photocatalytic activity of photocatalyst. In order to evaluate the adsorption ability of CNxNTs and 2-NCB, the residue rate of RhB in the dark was determined. As shown in Fig. S3,† after 30 min stirring in the dark, the residue rate of RhB in the solution with CNxNTs as the adsorbent is about 70%, whereas that of 2-NCB as the adsorbent is about 61%. When the stirring time was extended to 40 min, the residue rate of RhB in the solution with CNxNTs and 2-NCB is about 68% and 60%, respectively. This result indicates that the adsorption–desorption equilibrium between the RhB and the photocatalyst can be reached after 30 min stirring in the dark. It is obviously that the adsorption ability of the 2-NCB is higher than that of CNxNTs, which is a prerequisite for good photocatalytic activity.
Therefore, the enhanced photocatalytic activity of the NCB nanocomposites should be attributed to the beneficial microstructure, more effective charge transportations and separations arisen from the effective synergy between the BiOCl and CNxNTs in the NCB nanocomposites.
4 Conclusions
In summary, NCB nanocomposites have been successfully prepared by using a facile solvothermal method. SEM, TEM, XRD and XPS analysis revealed the coating of BiOCl nanoplates on the surface of CNxNTs. The as-synthesized nanocomposites possessed enhanced charge-separation and charge-transportation properties. In comparison with the pure CNxNTs, 3D flower-like hierarchitectures BiOCl, P25 and as-synthesized NCB nanocomposites, the 2-NCB nanocomposites loaded with 2.0 wt% CNxNTs exhibited the best photocatalytic performance for the decomposition of RhB under UV light irradiation, which is about 2.5, 3.7, and 1.5 times that of pure BiOCl 3D hierarchical structures, BiOCl/MWCNTs composites, and P25, respectively. We assumed that the enhanced photocatalytic performance could be attributed to the beneficial microstructure, synergistic effects of coupled CNxNTs–BiOCl nanocomposites, and the high migration efficiency of the photogenerated electrons, which may effectively suppress the charge recombination. This work is expected to provide some insight into the design of new photocatalysts with enhanced photocatalytic activities for environmental purification and other applications.Acknowledgements
This work was supported by the Shanghai Municipal Natural Science Foundation (Grant No. 14ZR1417100, 13ZR1454800), the Innovation Foundation of Shanghai Municipal Education Commission (Grant No. 13YZ134), Shanghai Educational Development Foundation (Grant No. 12CG66), the Shanghai Municipal Lian-Meng Program (Grant No. LM201462), and the key subject of Shanghai Second Polytechnic University (Grant No. 4: Material Science and Engineering, XXKYS1401). LuPing Zhu also gratefully thanks Prof. Jinhua Ye, Environmental Remediation Materials Unit, National Institute for Materials Science (NIMS) for helpful discussion.References
- (a) J. H. Li and J. Z. Zhang, Coord. Chem. Rev., 2009, 253, 3015 CrossRef CAS; (b) Y. Liu, J. Li, M. Wang, Z. Y. Li, H. Liu, P. He, X. R. Yang and J. H. Li, Cryst. Growth Des., 2005, 5, 1643 CrossRef CAS.
- (a) I. Tsuji, H. Kato and A. Kudo, Chem. Mater., 2006, 18, 1969 CrossRef CAS; (b) J. Sato, N. Saito, Y. Yamada, K. Maeda, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi, K. Domen and Y. Inoue, J. Am. Chem. Soc., 2005, 127, 4150 CrossRef CAS PubMed; (c) A. Ishikawa, T. Takata, J. N. Kondo, M. Hara and K. Domen, J. Phys. Chem. B, 2004, 108, 2637 CrossRef CAS.
- K. G. Keramidas, G. P. Voutsas and P. I. Rentzeperis, Z. Kristallogr., 1993, 205, 35 CAS.
- (a) X. Xiao and W. D. Zhang, J. Mater. Chem., 2010, 20, 5866 RSC; (b) Z. H. Ai, W. Ho, S. Lee and L. Z. Zhang, Environ. Sci. Technol., 2009, 43, 4143 CrossRef CAS PubMed; (c) W. Y. Su, J. Wang, Y. X. Huang, W. J. Wang, L. Wu, X. X. Wang and P. Liu, Scr. Mater., 2010, 62, 345 CrossRef CAS; (d) C. H. Wang, C. L. Shao, Y. C. Liu and L. N. Zhang, Scr. Mater., 2008, 59, 332 CrossRef CAS.
- K. L. Zhang, C. M. Liu, F. Q. Huang, C. Zheng and W. D. Wang, Appl. Catal., B, 2006, 68, 125 CrossRef CAS.
- (a) Y. Q. Lei, G. H. Wang, S. Y. Song, W. Q. Fan and H. J. Zhang, CrystEngComm, 2009, 11, 1857 RSC; (b) X. Zhang, Z. Ai, F. Jia and L. Zhang, J. Phys. Chem. C, 2008, 112, 747 CrossRef CAS.
- F. Gao, D. Zeng, Q. Huang, S. Tian and C. Xie, Phys. Chem. Chem. Phys., 2012, 14, 10572 RSC.
- (a) A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed; (b) S. Sharma, A. Ganguly, P. Papakonstantinou, X. P. Miao, M. X. Li, J. L. Hutchison, M. Delichatsios and S. Ukleja, J. Phys. Chem. C, 2010, 114, 19459 CrossRef CAS.
- (a) J. M. Lee, J. S. Park, S. H. Lee, H. Kim, S. Yoo and S. O. Kim, Adv. Mater., 2011, 23, 629 CrossRef CAS PubMed; (b) X. Xu, S. Jiang, Z. Hu and S. Liu, ACS Nano, 2010, 4, 4292 CrossRef CAS PubMed; (c) X. Xu, L. Yang, S. Jiang, Z. Hu and S. Liu, Chem. Commun., 2011, 47, 7137 RSC; (d) Z. Wang, R. Jia, J. Zheng, J. Zhao, L. Li, J. Song and Z. Zhu, ACS Nano, 2011, 5, 1677 CrossRef CAS PubMed; (e) J. Wen, Y. Zhang, N. Tang, X. Wan, Z. Xiong, W. Zhong, Z. Wang, X. Wu and Y. Du, J. Phys. Chem. C, 2011, 115, 12329 CrossRef CAS; (f) L. Y. Feng, Y. Y. Yan, Y. G. Chen and L. J. Wang, Energy Environ. Sci., 2011, 4, 1892 RSC; (g) Y. Li, J. Wang, X. Li, J. Liu, D. Geng, J. Yang, R. Li and X. Sun, Electrochem. Commun., 2011, 13, 668 CrossRef CAS; (h) Y. Wang, Y. Shao, D. W. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790 CrossRef CAS PubMed.
- (a) R. Zhang, L. Li, L. Chen, G. Zhang and K. Shi, J. Alloys Compd., 2011, 509, 8620 CrossRef CAS; (b) L. P. Zhu, L. L. Wang, N. C. Bing, Y. H. Li, Y. H. Jiao, L. J. Wang and G. H. Liao, Mater. Lett., 2014, 121, 85 CrossRef CAS.
- (a) J. S. Park, J. M. Lee, S. K. Hwang, S. H. Lee, H. J. Lee, B. R. Lee, H. I. Park, J. S. Kim, S. Yoo, M. H. Song and S. O. Kim, J. Mater. Chem., 2012, 22, 12695 RSC; (b) L. L. Wang, L. Shen, L. P. Zhu, H. J. Jin, N. C. Bing and L. J. Wang, J. Nanomater., 2012, 2012, 794625 Search PubMed.
- S. Jiang, L. Zhu, Y. Ma, X. Wang, J. Liu, J. Zhu, Y. Fan, Z. Zou and Z. Hu, J. Power Sources, 2010, 195, 7578 CrossRef CAS.
- B. Yue, Y. W. Ma, H. S. Tao, L. S. Yu, G. Q. Jian, X. Z. Wang, Y. N. Lu and Z. Hu, J. Mater. Chem., 2008, 18, 1747 RSC.
- S. J. Jiang, Y. W. Ma, G. Q. Jian, H. S. Tao, X. Z. Wang, Y. N. Lu, Z. Hu and Y. Chen, Adv. Mater., 2009, 21, 4953 CrossRef CAS PubMed.
- X. Lepró, E. Terrés, Y. Vega-Cantú, F. J. Rodríguez-Macías, H. Muramatsu, Y. A. Kim, T. Hayahsi, M. Endo, R. M. Torres and M. Terrones, Chem. Phys. Lett., 2008, 463, 124 CrossRef.
- A. B. Castle, E. Gracia-Espino, C. Nieto-Delgado, H. Terrones, M. Terrones and S. Hussain, ACS Nano, 2011, 5, 2458 CrossRef CAS PubMed.
- K. Ghosh, M. Kumar, H. F. Wang, T. Maruyama and Y. Ando, Langmuir, 2010, 26, 5527 CrossRef CAS PubMed.
- C. Yu, Y. Wang, Y. Liu, C. Guo and Y. Hu, Mater. Lett., 2013, 100, 278 CrossRef CAS.
- L. L. Wang, L. J. Wang, H. Jin and N. Bing, Catal. Commun., 2011, 15, 78 CrossRef CAS.
- J. W. Mullin, Crystallization, Butterworth-Heinemann, London, 3rd edn, 1997, p. 288 Search PubMed.
- L. P. Zhu, G. H. Liao, N. C. Bing, L. L. Wang, Y. Yang and H. Y. Xie, CrystEngComm, 2010, 12, 3791 RSC.
- (a) Y. S. Chen, J. C. Crittenden, S. Hackney, L. Sutter and D. W. Hand, Environ. Sci. Technol., 2005, 39, 1201 CrossRef CAS PubMed; (b) J. Du, X. Lai, N. Yang, J. Zhai, D. Kisailus, F. Su, D. Wang and L. Jiang, ACS Nano, 2011, 5, 590 CrossRef CAS PubMed; (c) X. Tu, S. Luo, G. Chen and J. Li, Chem.–Eur. J., 2012, 18, 14359 CrossRef CAS PubMed; (d) H. Liu, W. R. Cao, Y. Su, Z. Chen and Y. Wang, J. Colloid Interface Sci., 2013, 398, 161 CrossRef CAS; (e) W. Chen, P. Cai, G. Lu, S. Chen, Y. Liu and X. Cao, Environ. Sci. Technol., 2013, 36, 37 CAS.
- (a) S. H. Lim, R. J. Li, W. Ji and J. Y. Lin, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 195406 CrossRef; (b) W. X. Lv, K. Y. Shi, L. Li and S. Z. Shao, Microchim. Acta, 2010, 170, 91 CrossRef CAS.
- M. A. Butler, J. Appl. Phys., 1977, 48, 1914 CrossRef CAS.
- Y. Luo, G. Duan and G. Li, J. Solid State Chem., 2007, 180, 2149 CrossRef CAS.
- Y. H. Zhang, Z. R. Tang, X. Z. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303 CrossRef CAS PubMed.
- (a) W. Zhao, C. Chen, X. Li, J. Zhao, H. Hidaka and N. Serpone, J. Phys. Chem. B, 2002, 106, 5022 CrossRef CAS; (b) L. P. Zhu, N. C. Bing, D. D. Yang, Y. Yang, G. H. Liao and L. J. Wang, CrystEngComm, 2011, 13, 4486 RSC; (c) L. P. Zhu, N. C. Bing, L. L. Wang, H. Y. Jin, G. H. Liao and L. J. Wang, Dalton Trans., 2012, 41, 2959 RSC; (d) L. P. Zhu, L. L. Wang, N. C. Bing, C. Huang, L. J. Wang and G. H. Liao, ACS Appl. Mater. Interfaces, 2013, 5, 12478 CrossRef CAS PubMed.
- J. Yu and L. Qi, J. Hazard. Mater., 2009, 169, 221 CrossRef CAS PubMed.
- R. G. Chen, J. H. Bi, L. Wu, Z. H. Li and X. Z. Fu, Cryst. Growth Des., 2009, 9, 1775 CAS.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282 CrossRef CAS PubMed.
- Z. H. Ai, W. Ho and S. Lee, J. Phys. Chem. C, 2011, 115, 25330 CAS.
- M. Iwamoto, H. Furukawa, K. Matsukami, T. Takenaka and S. Kagawa, J. Am. Chem. Soc., 1983, 105, 3719 CrossRef CAS.
- Y. Zheng, L. Zheng, Y. Zhan, X. Lin, Q. Zheng and K. Wei, Inorg. Chem., 2007, 46, 6980 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: In situ synthesis of N-doped carbon nanotubes–BiOCl nanocomposites and their synergistic photocatalytic performance. See DOI: 10.1039/c5ra24149a |
| This journal is © The Royal Society of Chemistry 2016 |