Enhanced cycling performance of the Li4Ti5O12 anode in an ethers electrolyte induced by a solid–electrolyte interphase film†
Yuxuan Zhu‡
a,
Jingxia Qiu‡bc,
Yueqing Huanga,
Po Wang*a and
Chao Lai*a
aSchool of Chemistry and Chemical Engineering, Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, Jiangsu Normal University, Xuzhou, Jiangsu 221116, P. R. China. E-mail: laichao@jsnu.edu.cn
bInstitute for Energy Research, Jiangsu University, Zhenjiang, Jiangsu 21203, P. R. China
cSchool of Chemistry and Molecular Biosciences, Faculty of Science, The University of Queensland, Brisbane, QLD 4072, Australia
First published on 18th June 2015
Abstract
The generation of a uniform solid–electrolyte interphase film on the surface of the Li4Ti5O12 anode at potentials above 1.0 V in an ethers electrolyte with lithium bis(trifluoromethanesulfonyl)imide as the lithium salt is reported. A significant enhancement in the cycling life can be obtained in the ethers electrolyte compared to in the conventional carbonates electrolyte. At a rate of 2 C, the discharge capacity can be retained at a stable 155.4 mA h g−1 after 300 cycles with a high capacity retention of 97.5%, which is the best result reported for a Li4Ti5O12 anode with a particle size above 300 nm.
Introduction
Rechargeable Li-ion batteries have been receiving extensive attention and are considered as one of the most attractive technologies for applications in electric vehicles and hybrid electric vehicles because of their high energy density, light weight, no memory effects and environmental benignity.1–3 Currently, graphite is the predominant anode material for commercial lithium-ion batteries. However, the poor rate performance has hindered its application, and the existence of lithium dendrite on the electrode at the end of the charge, where the potential is quite close to that of the lithium electrode, may cause internal short-circuits and a high safety risk in the batteries.1–3 As an alternative to graphite, Li4Ti5O12 has been investigated as an appealing material for lithium-ion batteries.3–6 It retains the advantages with low cost, low toxicity and high safety. Most importantly, lithium insertion/extraction occurs with negligible expansion of the unit cell, which makes Li4Ti5O12 a promising anode.As a safe anode material, Li4Ti5O12 still encounters some serious problems, such as the poor conductivity and releasing gas after many cycles.3–16 Over the past few decades, extensive research has focused on improving the rate performance of Li4Ti5O12, and various nanostructures and conductive composites of Li4Ti5O12 anode materials have been developed.3,6–12 However, there are hardly any reports about Li4Ti5O12-based batteries releasing gas, which is regarded as the main obstacle for the commercial application of Li4Ti5O12.13–16 For Li4Ti5O12 anodes, a carbonate solvent will decompose on the surface of TiO2, which is produced from Li4Ti5O12 after a long cycling life, and a large amount of gas, such as CO2 and CO, will be generated, especially at high temperatures.15,16 Such decomposition not only generates a serious releasing gas phenomenon, but also destroys the stable cycling performance of Li4Ti5O12. It has been suggested that surface coatings will be an effective method to prohibit the contact between the carbonate and the surface of the active material, which would then restrict the generation of gas in the batteries.13–16 However, it is technically difficult to produce a uniform and adequate coating layer, and a complex synthesis process is always needed, limiting their practical applications. Thus, the key to addressing the problem of released gas is how to build a uniform and adequate surface coating layer via a simple strategy.
Solid–electrolyte interphases (SEIs) have been suggested as the crucial key to the stability and durability of Li-ion batteries.16–19 During the charging process, a SEI layer can be generated due to the reductive decomposition of the electrolyte on the surface of the electrode. The SEI film is a Li+ conductor but an insulator of electron transport, and thus it can prevent further electrolyte decomposition to improve the cycling life performance of the Li-ion battery.16–19 For Li4Ti5O12 anodes, the SEI film is also an ideal coating layer to separate the electrode from the electrolyte, and thus it can effectively restrict the generation of gas in Li4Ti5O12-based batteries. Although this will be a big challenge since the formation of a SEI film happens at a low potential, the strategy of producing a SEI layer on the surface of Li4Ti5O12 is still appealing. In lithium–sulfur batteries, an ethers electrolyte (1,3-dioxolane and dimethoxyethane) is used with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as the lithium salt. The 1,3-dioxolane in the electrolyte can easily decyclize in the presence of acids, such as H+ and Lewis acids (metal ions). Thus, the ring-opening reaction of 1,3-dioxolane is possible after the generation of Ti3+ during the discharge process for the Li4Ti5O12 anode, and then it can produce a surface layer. Following on from such a design, in this work, we first investigate the electrochemical performance of Li4Ti5O12 in the ethers electrolyte with LiTFSI as the lithium salt. Surprisingly, a uniform surface coating layer is generated during the initial cycle, and an obviously enhanced cycling performance is obtained.
Experimental
2.1 Materials characterization
The commercial Li4Ti5O12 was characterized by an X-ray diffractometer (XRD, Model LabX-6000, Shimadzu) with CuKα radiation. The morphology and structure of the Li4Ti5O12 were examined by scanning electron microscopy (SEM, JOEL, JSM-7001F), and high-resolution transmission electron microscopy (HR-TEM) (FEI Model Tecnai 20) with an acceleration voltage of 200 kV.2.2 Electrochemical measurements
The anode slurry was prepared by mixing 70 wt% Li4Ti5O12, 20 wt% acetylene black and 10 wt% binder (polytetrafluoroethylene, PTFE) in ethanol. Then, the slurry was ground, punched into round disks, and dried at 60 °C for 12 h in a vacuum oven. The average mass of the electrode was ca. 4 mg cm−2.The half cells were assembled with the Li4Ti5O12 electrode as the working electrode, and lithium metal sheets as the reference electrode and counter electrode. Copper was used as the current collector and a porous polypropylene film acted as a separator. Two different kinds of electrolytes were used in the system. One was a mixed solution composed of 1.0 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ethylene carbonate (EC)/dimethyl carbonate (DMC), designated as LiPF6/carbonates. The other was 1.0 M LiTFSI dissolved in dimethoxyethane (DME) and dioxolane (DOL) (1
1 (w/w) ethylene carbonate (EC)/dimethyl carbonate (DMC), designated as LiPF6/carbonates. The other was 1.0 M LiTFSI dissolved in dimethoxyethane (DME) and dioxolane (DOL) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume), designated as LiTFSI/ethers. The charge–discharge processes of the Li4Ti5O12 electrode in different electrolyte systems were investigated at different C rates in the voltage range of 1.0–2.5 V vs. Li/Li+ using a Land-CT2001A battery tester (Wuhan, PRC). Electrochemical impedance spectra (EIS) were collected using a Solartron 1287 electrochemical workstation. A perturbation of 5 mV was applied and data were collected under PC control (custom software) from 100 kHz to 10 mHz.
1, volume), designated as LiTFSI/ethers. The charge–discharge processes of the Li4Ti5O12 electrode in different electrolyte systems were investigated at different C rates in the voltage range of 1.0–2.5 V vs. Li/Li+ using a Land-CT2001A battery tester (Wuhan, PRC). Electrochemical impedance spectra (EIS) were collected using a Solartron 1287 electrochemical workstation. A perturbation of 5 mV was applied and data were collected under PC control (custom software) from 100 kHz to 10 mHz.
Results and discussion
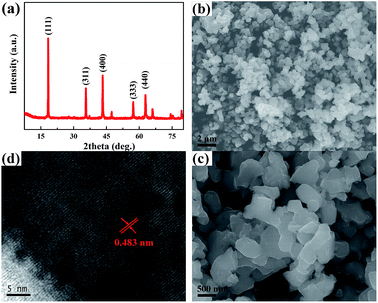
The morphology and structure of commercial Li4Ti5O12 was characterized using the XRD pattern, and SEM and TEM images, as displayed in Fig. 1. From Fig. 1a, it can be seen that the powder XRD pattern agrees well with the JCPDS (card no. 49-0207) of Li4Ti5O12, revealing that the sample has a single-phase cubic structure in the Fd3m space group.4–13 As shown in the low- and high-magnification SEM images in Fig. 1b and c, the Li4Ti5O12 sample has a uniform size distribution with diameters in the range of 300–500 nm. The HRTEM image (Fig. 1d) indicates that the entire particle of Li4Ti5O12 is highly crystallized with a lattice spacing of 0.483 nm, which is consistent with the (111) plane of Li4Ti5O12.Lithium-ion battery cells were assembled to test the electrochemical performances of Li4Ti5O12 in the LiTFSI/ethers and LiPF6/carbonates electrolyte systems. The typical voltage profiles of the electrodes for the 1st cycle, 2nd cycle, 30th cycle and 60th cycle in the LiPF6/carbonates and LiTFSI/ethers electrolytes are presented at a current density of 0.1 C (1 C = 175 mA g−1) in Fig. 2a and b. In the conventional carbonates electrolyte, distinct discharge and charge potential plateaus around 1.51 and 1.57 V can be observed, which suggest a good kinetic process. This is a typical feature of a two-phase lithium insertion and extraction process, corresponding to the transformation of Li4Ti5O12 and Li7Ti5O12.4–13,20,21 Nevertheless, in the ethers electrolyte, similar discharge–charge curves can be observed except that an additional long flat plateau appeared at a potential around 1.2 V in the initial discharging process, similar to the reductive decomposition of an electrolyte on the surface of the graphite anode below 1 V.22,23 For titanium-based anode materials, especially on the nanoscale, the continuous decomposition of the electrolyte and formation of an unstable SEI film are common phenomena, leading to the serious gas issues.24,25 Thus, the long distinctive discharge plateau may correspond to the formation process of a stable SEI film on the Li4Ti5O12.
In addition, it was observed that the sample in the LiTFSI/ethers electrolyte performed much better than that in LiPF6/carbonates. The initial discharge capacity in the ethers electrolyte was 431.9 mA h g−1, and a high capacity of 169.5 mA h g−1 was preserved after the 60th cycle at 0.1 C. However, in the carbonates electrolyte system, the sample demonstrated an initial discharge capacity of 184 mA h g−1, which gradually decreased to 158.0 mA h g−1 after the 60th cycle.
To illustrate the discharge mechanism of Li4Ti5O12 in the ethers electrolyte, EIS tests were conducted with an open-circuit potential and a discharge state of 2.5 V, and the results are given in Fig. 2c and d. For the Li4Ti5O12 anode in the carbonates electrolyte, it can be seen that the Nyquist plot consists of one depressed semicircle at high frequencies and a straight line at low frequencies before discharge. The diameter of the high frequencies semicircle refers to the charge transfer resistance, related to the electrochemical reaction at the electrode–electrolyte, while the straight line is attributed to the Warburg element, related to the Li-ion diffusion in the Li4Ti5O12.18,22,26–28 For the Li4Ti5O12 in the ethers electrolyte, an additional semicircle can be observed, and this suggests the formation of a solid electrolyte interphase (SEI) on the surface of the Li4Ti5O12 electrode, which may result from the decomposition or polymerization of the ether solvents, as illustrated below.27,28 The fitting results of the EIS tests are given in Table S1,† and it was found that the charge transfer resistance of the cell using the ethers electrolyte was much smaller than that using the carbonates electrolyte (Rct). This is mainly due to the high conductivity of the LiTFSI/ethers electrolyte (Rs), thus leading to a better electrochemical performance.29 The change in the Li-ion diffusion coefficient, DLi, in the different electrolytes can be calculated from the low frequency linear Warburg regions, according to the following equation:30,31
| DLi = 1/2[(Vm/FAδw)dE/dx]2 |
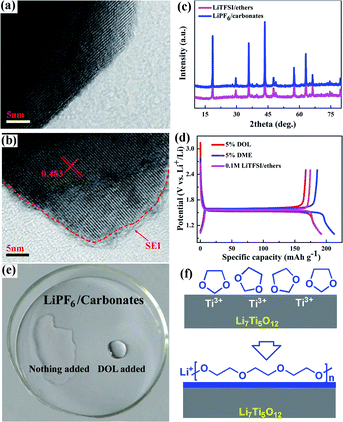
Evidence for the formation of a SEI film on the surface of Li4Ti5O12 can be easily obtained from the HRTEM images in Fig. 3a and b. As shown, after the first cycle, a homogeneous thin layer of a stable SEI was produced outside the crystal lattice on the electrode in the ethers electrolyte, while no surface layer can be observed for the electrode in the carbonates electrolyte. The d-spacing of the Li4Ti5O12 after the formation of the SEI film is ca. 0.48 nm, suggesting that there is no phase change in the ethers electrolyte. The structural stability of Li4Ti5O12 in the ethers electrolyte can be further confirmed by the XRD patterns after various cycles. Fig. 3c presents the XRD patterns of the charged electrodes in the LiTFSI/ethers and LiPF6/carbonates electrolytes directly after an acetone wash after 300 cycles at a rate of 2 C. All the diffraction peaks can be indexed to a single-phase cubic spinel structure in the Fd3m space group, suggesting that the crystal structure of Li4Ti5O12 has not changed after many cycles.4–13 By considering all the results above, it can be concluded that a uniform SEI film can be generated on the surface of Li4Ti5O12 in the ethers electrolyte, and such a coating layer is favorable for producing high cycling stability.
A comprehensive comparison of the electrochemical behaviors of Li4Ti5O12 in various electrolytes is given in Fig. 3d to explain how the formation of the SEI on the surface of the electrode facilitates the performance of the electrode. As presented, with DOL, DME and LiTFSI salt added into the LiPF6/carbonates electrolyte, the Li4Ti5O12 electrode shows similar discharge–charge curves compared to that in the LiPF6/carbonates electrolyte. There is no additional potential plateau as there is in the pure LiTFSI/ethers electrolyte, suggesting that the formation of the SEI film may be related to the amount of the solvents in the electrolyte. To further illustrate the formation mechanism of the SEI film, the DOL and LiPF6/carbonates electrolytes were mixed with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As shown in Fig. 3e, the mixture became extremely viscous after 6 h as a result of the polymerization of DOL,32,33 while no change occurred by adding DME. One possible reaction mechanism is proposed in Fig. 3f. As demonstrated, after discharging to 1.5 V, Ti3+ will generate, acting as an initiator for the polymerization of DOL, and producing a uniform coating layer on the surface of the Li4Ti5O12 electrode.32–34 Such a hypothesis is mainly based on the instability of DOL during the discharge process,34 especially in the presence of Ti3+. More experiments are still needed to clearly clarify the formation mechanism of the SEI in the ethers electrolyte.
1. As shown in Fig. 3e, the mixture became extremely viscous after 6 h as a result of the polymerization of DOL,32,33 while no change occurred by adding DME. One possible reaction mechanism is proposed in Fig. 3f. As demonstrated, after discharging to 1.5 V, Ti3+ will generate, acting as an initiator for the polymerization of DOL, and producing a uniform coating layer on the surface of the Li4Ti5O12 electrode.32–34 Such a hypothesis is mainly based on the instability of DOL during the discharge process,34 especially in the presence of Ti3+. More experiments are still needed to clearly clarify the formation mechanism of the SEI in the ethers electrolyte.
As discussed above, Li4Ti5O12 in the ethers electrolyte shows a much lower interface resistance, and the formation of the SEI film is favorable to obtain a stable cycling performance.18,35 Accordingly, the rate performance (2 C) of the Li4Ti5O12 in the ethers electrolyte was also investigated. Fig. 4a shows the 2nd discharge–charge curves of the Li4Ti5O12 electrode in the ethers and carbonates electrolytes. As shown, there is an obvious plateau at around 1.05 V using the ethers electrolyte, suggesting the formation of the SEI film. Different from its discharge–charge curves at a rate of 0.1 C, there is a noticeable overcharge phenomenon. This may be because it does not form a fully covering and stable SEI film on the surface of the electrode during the fast discharge–charge process, and there are still exposed catalytically active regions. During the charge process, the decomposition of the electrolyte and dissociation of the SEI film may happen together, leading to the overcharge phenomenon. Such a hypothesis can be confirmed by the EIS results after different cycles at a rate of 2 C. As presented in Fig. S2,† after the initial two cycles, the straight line turns into an arc-like profile in the low-frequency region, which represents a finite Nernst diffusion process in a thin layer,36 suggesting the gradual formation of a surface film on the electrode. After the initial few cycles, the overcharge phenomenon disappears, as shown in Fig. 4b. Furthermore, the cell using the ethers electrolyte demonstrates a much lower polarization potential compared to that using the conventional carbonates electrolyte, indicating a better kinetic process. The cycling performance profiles of Li4Ti5O12 in the LiTFSI/ethers and LiPF6/carbonates electrolytes at a constant current density of 2 C are given in Fig. 4c. As presented, in the initial 100 cycles, both electrolytes can support a superior cycling performance. However, after that, the discharge capacity decreases rapidly in the carbonates electrolyte, while there is virtually no capacity decay in the ethers electrolyte. The stable reversible capacity of the electrode in LiTFSI/ethers is 159.5 mA h g−1 (6th), and the capacity can be retained at 155.4 mA h g−1 after 300 cycles with a retention of 97.5%. On the other hand, the capacity is only 118 mA h g−1 in the LiPF6/carbonates electrolyte after 300 cycles. Therefore, the electrode in the LiTFSI/ethers electrolyte exhibits a significantly enhanced cycling performance due to the formation of the SEI film.17–19
 | ||
| Fig. 4 The second (a) and 50th (b) discharge–charge curves of the Li4Ti5O12 anode in different electrolytes; cycle curves of the Li4Ti5O12 anode in different electrolytes (c). | ||
Conclusions
In summary, we have demonstrated a novel and simple strategy, using an ethers electrolyte with lithium bis(trifluoromethanesulfonyl)imide as the lithium salt, to produce a uniform SEI layer on the surface of the Li4Ti5O12 electrode at high potential. The formation of the SEI film can be confirmed by the discharge–charge curves, EIS and HRTEM images. The electrode using the ethers electrolyte exhibits much better cycling and rate performances than that using the conventional carbonates electrolyte. The excellent cycling stability can be ascribed to the formation of a uniform SEI layer, which can successfully separate the electrode from the electrolyte to maintain the structure stability of the electrode. Furthermore, it may prohibit the gas generation phenomenon in the Li4Ti5O12-based batteries. At a rate of 0.1 C, the capacity can be retained at a stable 169.5 mA h g−1 in the ethers electrolyte, while in the carbonates electrolyte it is only 158.0 mA h g−1. When the rate increases to 2 C, a high discharge capacity of 155.4 mA h g−1 can still be obtained after 300 cycles in the ethers electrolyte, and the capacity retention can reach to 91.7% compared to that at a rate of 0.1 C. However, in the conventional carbonates electrolyte, the cell shows a poor cycling life, and the capacity is ca. 118 mA h g−1 after 300 cycles.Acknowledgements
This work was supported by the Chinese National Science Funds (no. 51202094); the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- W. Xu, J. L. Wang, F. Ding, X. L. Chen, E. Nasybutin, Y. H. Zhang and J. G. Zhang, Energy Environ. Sci., 2014, 7, 513 CAS.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- G. N. Zhu, Y. G. Wang and Y. Y. Xia, Energy Environ. Sci., 2012, 5, 6652 CAS.
- Y. Q. Wang, L. Gu, Y. G. Guo, H. Li, X. Q. He, S. Tsukimoto, Y. Ikuhara and L. J. Wan, J. Am. Chem. Soc., 2012, 134, 7874 CrossRef CAS PubMed.
- L. Yu, H. B. Wu and X. W. Lou, Adv. Mater., 2013, 25, 2296 CrossRef CAS PubMed.
- X. Hao and B. M. Bartlett, Adv. Energy Mater., 2013, 3, 753 CrossRef CAS PubMed.
- X. L. Jia, Y. F. Kan, G. Q. Ning, Y. F. Lu and F. Wei, Nano Energy, 2014, 10, 344 CrossRef CAS PubMed.
- Y. Oh, S. Nam, S. Wi, J. Kang, T. Hwang, S. Lee, H. H. Park, J. Cabana, C. Kim and B. Park, J. Mater. Chem. A, 2014, 2, 2023 CAS.
- J. Liu, K. Song, P. A. Aken, J. Maier and Y. Yu, Nano Lett., 2014, 14, 2597 CrossRef CAS PubMed.
- H. H. Xu, X. L. Hu, Y. M. Sun, W. Luo, C. J. Chen and Y. H. Huang, Nano Energy, 2014, 10, 163 CrossRef CAS PubMed.
- C. F. Lin, X. Y. Fan, Y. L. Xin, F. Q. Cheng, M. O. Lai, H. H. Zhou and L. Lu, J. Mater. Chem. A, 2014, 2, 9982 CAS.
- C. Lai, Y. Y. Dou, X. Li and X. P. Gao, J. Power Sources, 2010, 195, 3676 CrossRef CAS PubMed.
- W. Li, X. Li, M. Z. Chen, Z. W. Xie, J. X. Zhang, S. Q. Dong and M. Z. Qu, Electrochim. Acta, 2014, 139, 104 CrossRef CAS PubMed.
- L. Wei, Z. Y. Wu, H. Z. Luo, R. S. Song and F. Li, J. Electrochem. Soc., 2015, 162, A3038 Search PubMed.
- R. Bernhard, S. Meini and H. A. Gasteiger, J. Electrochem. Soc., 2014, 161, A497 CrossRef CAS PubMed.
- Y. B. He, B. Li, M. Liu, C. Zhang, W. Lv, C. Yang, J. Li, H. D. Du, B. Zhang, Q. H. Yang, J. K. Kang and F. Kang, Sci. Rep., 2012, 2, 913 Search PubMed.
- S. Bhattacharya, A. R. Ahmet and A. T. Alpas, Carbon, 2014, 77, 99 CrossRef CAS PubMed.
- Y. B. He, M. Liu, Z. D. Huang, B. Zhang, Y. Yu, B. H. Li, F. Y. Kang and J. K. Kim, J. Power Sources, 2013, 239, 269 CrossRef CAS PubMed.
- M. D. Bhatt and C. O’Dwyer, J. Electrochem. Soc., 2014, 161, A1415 CrossRef CAS PubMed.
- K. S. Park, A. Benayad, D. J. Kang and S. G. Doo, J. Am. Chem. Soc., 2008, 130, 14930 CrossRef CAS PubMed.
- M. Kitta, T. Akita, S. Tanaka and M. Kohyama, J. Power Sources, 2014, 257, 120 CrossRef CAS PubMed.
- C. Liao, K. S. Han, L. Baggetto, D. A. Hillesheim, R. Custelcean, E. S. Lee, B. K. Guo, Z. H. Bi, D. E. Jiang, G. M. Veith, E. W. Hagaman, G. M. Brown, C. Bridges, A. Manthiram, S. Dai and X. G. Sun, Adv. Energy Mater., 2014, 4, 1301368 Search PubMed.
- P. Murmann, P. Niehoff, R. Schmitz, S. Nowak, H. Gores, N. Ignatiev, P. Sartori, M. Winter and R. Schmitz, Electrochim. Acta, 2013, 114, 658 CrossRef CAS PubMed.
- C. P. Han, Y. B. He, H. F. Li, B. H. Li, H. D. Du, Y. Qin and F. Y. Kang, Electrochim. Acta, 2015, 157, 266 CrossRef CAS PubMed.
- S. Brutti, V. Gentili, H. Menard, B. Scrosati and P. G. Bruce, Adv. Energy Mater., 2012, 2, 322 CrossRef CAS PubMed.
- C. Lai, X. L. Cao, X. C. Yuan, Y. L. Wang and S. H. Ye, Solid State Ionics, 2013, 249–250, 151 CrossRef CAS PubMed.
- C. L. Wei, W. He, X. D. Zhang, S. J. Liu, C. Jin, S. K. Liu and Z. Huang, RSC Adv., 2015, 5, 28662 RSC.
- M. Umeda, K. Dokko, Y. Fujita, M. Mohamedi, I. Uchida and J. R. Selman, Electrochim. Acta, 2001, 47, 885 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303 CrossRef CAS.
- Y. G. Wang, H. M. Liu, K. X. Wang, H. Eiji, Y. R. Wang and H. S. Zhou, J. Mater. Chem., 2009, 19, 6789 RSC.
- J.-Y. Shin, J. H. Joo, D. Samuelis and J. Maier, Chem. Mater., 2012, 24, 543 CrossRef CAS.
- N. Nemoto, Y. Ito and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 699 CrossRef CAS PubMed.
- N. Djelali, D. Aliouche, L. D. Oubeka and G. Pierre, Asian J. Chem., 2006, 18, 1839 CAS.
- L. Kong, H. Zhan, Y. J. Li and Y. H. Zhou, Electrochem. Commun., 2007, 9, 2557 CrossRef CAS PubMed.
- J. J. Xu, Y. Y. Hu, T. Liu and X. D. Wu, Nano Energy, 2014, 5, 67 CrossRef CAS PubMed.
- Y. Liu, X. Huang, Q. Q. Qiao, Y. L. Wang, S. H. Ye and X. P. Gao, Electrochim. Acta, 2014, 147, 696 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06566f |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |