Synthesis of 4-hydroxyindole fused isocoumarin derivatives and their fluorescence “Turn-off” sensing of Cu(II) and Fe(III) ions†
Sudipta Pathak,
Doyel Das,
Ashis Kundu,
Subhendu Maity,
Nikhil Guchhait and
Animesh Pramanik*
Department of Chemistry, University of Calcutta, 92, A. P. C. Road, Kolkata-700 009, India. E-mail: animesh_in2001@yahoo.co.in
First published on 2nd February 2015
Abstract
A simple and efficient protocol has been developed for the synthesis of 4-hydroxyindole fused isocoumarins from easily available starting materials. Dihydroxy-indenoindoles, the cyclic hemiaminals of the condensation products of ninhydrin and enamines of 1,3-cyclohexanedione, produced indole fused isocoumarins 11-(aryl/alkyl)-8,9,10,11-tetrahydro-6-oxa-11-aza-benzo[a]fluorine-5,7-diones through an acid catalyzed intramolecular rearrangement. The above isocoumarin derivatives furnished novel 4-hydroxyindole fused isocoumarins 11-(aryl or alkyl)-7-hydroxy-11H-6-oxa-11-aza-benzo[a]fluoren-5-ones through dehydrogenation with Pd/C. The synthesized 4-hydroxyindole fused isocoumarins show fluorescence properties with good quantum yields and fluorescence “Turn-off” sensing of Cu2+ and Fe3+ ions. Importantly these molecules are found to be chemosensors only for Cu2+ ions with respect to UV-Vis spectral change and naked eye colour change in the presence and absence of UV radiation.
Introduction
α-Pyranone (2H-pyran-2-one), a six-membered oxygen heterocycle, represents an important class of naturally occurring unsaturated lactones. Its benzo derivatives e.g. isocoumarins are found in many natural products and bioactive synthetic compounds.1,2 This imperative class of naturally occurring lactones has attracted significant attention from chemists due to their varied biological activities.3–8 Tricyclic isocoumarins A (Fig. 1) isolated from Microdochium bolleyi have shown good antifungal, antibacterial and antialgal activities.9 (−)-Cephalosol B is a potent naturally occurring antimicrobial metabolite (Fig. 1).10 Polyheterocycles like isocoumarin C and isoquinolinone D framework also represent some privileged structures for the development of natural product-inspired compounds of potential biological interest (Fig. 1).11,12 So development of new and efficient methodology for the synthesis of isocoumarins and their carbo/hetero annulated analogues have attracted great attention of the synthetic as well as medicinal chemists.13 Various methodologies for the syntheses of isocoumarins have been reported such as the reaction of o-halobenzoic acids and 1,3-diketones via copper-catalyzed tandem sequential cyclization/addition/deacylation process,14 iridium-catalyzed oxidative lactonization or intramolecular cyclization reaction of δ-ketoaldehydes,15 ruthenium-catalyzed aerobic oxidative cyclization of aromatic acids with alkynes,16 FeCl3-promoted regioselective annulation of o-(1-alkynyl)benzoates with disulfides,17 Heck–Matsuda cyclization reaction,18 6-endo-dig cyclization of hetero arylesters to alkynes19 and Pd(II)-mediated cyclization.20In the present paper, we wish to report the synthesis of a new class of 4-hydroxyindole fused isocoumarins. These compounds also show diverse kinds of photophysical properties such as fluorescence “Turn-off” sensing of Cu(II) and Fe(III) ions, chemosensing of Cu2+ ion and differential fluorescence quantum yields and lifetimes depending upon the solvent polarity. Copper is an essential trace element in the human body which plays a critical role in many biological processes and is also required by the human nervous system.21 However, the high levels of copper ion in living cell leads to neurodegenerative diseases.22 Thus, the sensing and recognition of divalent copper ion has attracted considerable attention in recent years23 and there is a need for development of new fluorescent sensors for detection of copper. To the best of our knowledge suitable isocoumarin based molecules have not been discovered to date for this purpose. In continuation of our research interest in the synthesis of various heterocyclic compounds from ninhydrin,24 we wish to report herein a novel class of potentially bioactive and fluorescence active 4-hydroxyindole fused isocoumarin systems.
Results and discussion
Recently a multicomponent reaction of N-heteroannulations involving enaminones, ninhydrin and acid anhydride or aromatic amines has been reported to produce fused pyrazole derivatives.25 In this reaction the acyclic hemiaminals of C-2 alkylated 1,3-indanediones (1) have been proposed to be an important intermediate (Scheme 1). We became interested to examine whether the above reaction would be fruitful starting from the proposed hemiaminals intermediate 1. Therefore initially a series of enamines of 1,3-cyclohexanedione and dimedone were reacted with ninhydrin in chloroform to isolate cyclic hemiaminals 1 according to the literature procedure.26 Subsequently the intermediate 5-benzyl-4b,9b-dihydroxy-4b,5,6,7,8,9b-hexahydroindeno[1,2-b]indole-9,10-dione 1a (R1 = –CH2Ph, R2 = H) was heated in acetic anhydride or various acidic media like p-TSA, acetic acid, lactic acid, formic acid and citric acid under conventional refluxing as well as microwave irradiation. The reaction scarcely proceeded to give any fused pyrazole derivatives25 or any other products in these conditions even after prolong refluxing (Table 1, entries 1–6). But when the same reaction was carried out in 8–11 N aqueous H2SO4 solution at reflux, a white product was precipitated out from the reaction mixture in low yield after 10 h (Scheme 1, Table 1, entries 7–11). The NMR studies confirmed that the product was not the expected multifunctionalized tetracyclic indeno[1,2-b]indole derivatives25 but a new rearranged product. Eventually, the structure elucidation of the product by single crystal X-ray diffraction study established that a novel indole fused isocoumarin 2a (R1 = –CH2Ph, R2 = H) has been formed (Fig. 2). The crystal structure of NaBH4 reduced product of 2a was also determined (Fig. S12, see ESI†). This significant result motivated us to carry out the above reaction at higher acidic condition to achieve better yields. Interestingly, when the acid strength of the reaction medium was increased to about 18 N aqueous H2SO4, the yield of the product 2a was enhanced considerably (Table 1, entries 12 and 13). Further the yield of the reaction was improved significantly when melamine sulfonic acid (Table 1, entry 14) and PEG-OSO3H (Table 1, entry 15) were employed in water under reflux. After performing a series of experiments, we observed that the yield of the product 2a can reach maximum upto 87% without producing any by-product if the reaction was carried out in acetic acid at reflux for 15 min in presence of concentrated H2SO4 (Table 1, entry 16).| Entry | Solvent (10 mL) | Acid | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | Acetic acid | — | 10 | — |
| 2 | H2O | p-TSA | 10 | Trace |
| 3 | H2O | Acetic acid | 10 | — |
| 4 | H2O | Lactic acid | 10 | — |
| 5 | H2O | Formic acid | 10 | — |
| 6 | H2O | Citric acid | 10 | — |
| 7 | H2O | 6.5 (N) H2SO4 | 10 | Trace |
| 8 | H2O | 8.3 (N) H2SO4 | 10 | 12 |
| 9 | H2O | 9.3 (N) H2SO4 | 10 | 17 |
| 10 | H2O | 10.3 (N) H2SO4 | 10 | 23 |
| 11 | H2O | 11.2 (N) H2SO4 | 10 | 24 |
| 12 | H2O | 12.0 (N) H2SO4 | 10 | 26 |
| 13 | H2O | 13.5 (N) H2SO4 | 10 | 26 |
| 14 | H2O | 20 mol% melamine sulfonic acid (MSA) | 5 | 40 |
| 15 | H2O | PEG6000-OSO3H (20 mol%) | 4 | 50 |
| 16 | Acetic acid | H2SO4 (0.5 mL) | 0.25 | 87 |
 | ||
| Fig. 2 ORTEP diagram of X-ray crystal structure of isocoumarin 2a with atom numbering scheme (CCDC number 940456). | ||
Having prepared 2a successfully, we decided to explore the scope and generality of the reaction in the synthesis of other analogues. Accordingly, a variety of cyclic hemiaminals 1 derived from enamines of commercially available primary amines and 1,3-cyclohexanedione or dimidone were reacted under the optimized conditions (Table 1, entry 16). As evident from Table 2, all the hemiaminals 1 reacted well in the reaction affording the desired products 2a–k and 3a–k in good yields. All the structures of the products were determined by detailed study of the spectroscopic data.
| Entry | R1 | R2 | Product | Time (min) | Yielda (%) | Melting point (°C) |
|---|---|---|---|---|---|---|
| a Isolated yield. | ||||||
| 1 |  |
H | 2a | 15 | 87 | 224–226 |
| 2 |  |
H | 2b | 15 | 86 | 295–297 |
| 3 |  |
H | 2c | 20 | 83 | 298–300 |
| 4 | 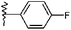 |
H | 2d | 18 | 87 | 294–296 |
| 5 |  |
H | 2e | 35 | 80 | 292–294 |
| 6 |  |
H | 2f | 25 | 85 | 256–258 |
| 7 | 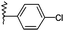 |
H | 2g | 18 | 85 | 255–257 |
| 8 |  |
H | 2h | 25 | 81 | >300 |
| 9 |  |
H | 2i | 35 | 82 | 300–302 |
| 10 | 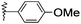 |
H | 2j | 40 | 80 | >300 |
| 11 | 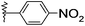 |
H | 2k | 20 | 86 | 216–218 |
| 12 |  |
Me | 3a | 30 | 90 | 252–254 |
| 13 |  |
Me | 3b | 30 | 85 | 294–296 |
| 14 |  |
Me | 3c | 32 | 83 | 280–282 |
| 15 | 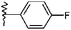 |
Me | 3d | 28 | 86 | >300 |
| 16 |  |
Me | 3e | 45 | 78 | 255–257 |
| 17 |  |
Me | 3f | 34 | 83 | 282–284 |
| 18 |  |
Me | 3g | 36 | 82 | 285–287 |
| 19 |  |
Me | 3h | 40 | 79 | 280–282 |
| 20 |  |
Me | 3i | 40 | 81 | 278–280 |
| 21 |  |
Me | 3j | 45 | 78 | 286–288 |
| 22 | 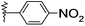 |
Me | 3k | 32 | 85 | 270–272 |
Formation of isocoumarin ring in products 2 or 3 from adducts 1 can be explained on the basis of the proposed mechanism depicted in Scheme 2. In strong acidic condition, protonation of the carbonyl oxygen in dihydroxy-indenopyrrole 1 generates the oxonium ion 5. Then hydroxyl group of 5 attacks the adjacent carbonyl carbon to generate a hydroxy epoxide intermediate 6 which is subsequently converted to intermediate 7 through protonation of the hydroxyl group. The intermediate 7 then loses water molecule to generate cationic intermediate 8 which provokes the ring expansion through the breaking of the central C–C bond affording an eight-membered lactum intermediate 2 with isocoumarin skeleton. It was not possible to isolate any of the intermediates 5–8 under the reaction conditions (Scheme 2).
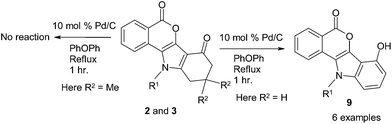
Finally dehydrogenation of 2 with 10% Pd/C (10 mol% of Pd(0)) in the diphenyl ether at reflux produced 4-hydroxyindole fused isocoumarins 11-(aryl/alkyl)-7-hydroxy-11H-6-oxa-11-aza-benzo[a]fluoren-5-one 9a–f in good yields (Scheme 3, Table 3). All the structures of the aromatized products were determined by detailed analysis of the spectroscopic data. Furthermore, the formation of product 9c was unambiguously confirmed through X-ray crystallographic analysis (Fig. 3). Since palladium is electropositive, the high activity of the catalyst also led to dehalogenation reactions.27 In order to stop the dehalogenation, the reaction was carried out for shorter period of time at low temperature, but the reaction did not produce any satisfactory result. A plausible mechanism of dehalogenation is outlined in Scheme 4. When palladium(0) reacted with compound 2, dehydrogenation took place to produce 9 and palladium dihydride (“PdH2”). This palladium dihydride (“PdH2”) species might be responsible for dehalogenation reaction (Scheme 4).28 It should be noted that defluorination did not take place because of high carbon–fluorine (C–F) bond energy. Compounds 3, derived from dimidone, did not aromatize in presence of 10% Pd/C due to the presence of two methyl groups at the same carbon atom of the cyclohexane ring.
 | ||
| Fig. 3 ORTEP diagram of X-ray crystal structure of hydroxylindole fused isocoumarin 9c with atom numbering scheme (CCDC number 940458). | ||
Steady state absorption and emission spectra of all the purified compounds 9a–f have been performed in solvents of different polarity. Probe concentration was maintained at 5 × 10−5 M. UV-Vis absorption spectrum shows structured band with peaks around 380 nm, 339 nm and 311 nm. The emission spectrum shows a structure less band with maximum around 502 nm. The shape and band position of the emission spectra are same regardless of excitation wavelength. Noticeably the molecule displays large Stokes shifted emission of more than ∼100 nm which indicates that the structure of the emitting species and the ground state species are considerably different. The emission maxima for all compounds have been found to shift to longer wavelength with increasing solvent polarity and hydrogen bonding ability (Fig. 4). The most bathochromic emission was found in methanol as solvents. This shifting of emission maxima in protic solvent clearly indicates more stabilization of the emissive species through intermolecular solute solvent intermolecular hydrogen bonding interaction. Concurrently, the measured fluorescence quantum yields (ΦF) are found to decrease with increasing hydrogen bonding ability of the solvent (see ESI, Table S1†). The high ΦF values were observed in aprotic solvent, and the lowest were measured in protic polar methanol. The substantial fluorescence quenching of the compounds 9 in protic solvent methanol compared to that of in CH3CN and DCM might be due to activation of some non-radiative decay channels of molecules 9 through intermolecular hydrogen bonding with the protic solvents.
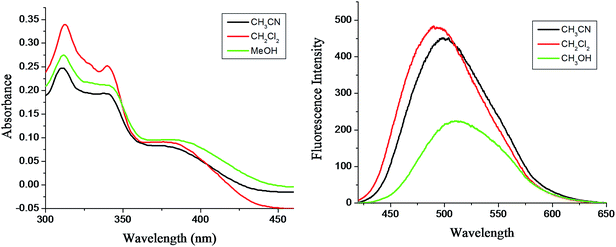 | ||
| Fig. 4 (a) UV-Vis absorption spectra and (b) fluorescence emission spectra (λex = nm) of representative compound 9d in different solvents ([9d] = ∼5 × 10−5 M). | ||
Ground state geometry of 9d has been optimized at B3LYP/6-31+G(d,p) level of theory in methanol solvent. The result shows that the 4-hydroxyindole fused isocoumarin moeity is planar in structure while the phenyl ring at N is perpendicular to the plane of the fused moeity. Further the UV-Vis absorption and emission spectra of 9d have been simulated theoretically in methanol solvent. The calculated excited state energies match well with the experimental values of absorption band positions (Table 4, ESI, Fig. S16†).
| Excitation energy | Oscillator strength |
|---|---|
| 390.74 nm | 0.1734 |
| 339.00 nm | 0.2633 |
| 317.43 nm | 0.1345 |
| 310.70 nm | 0.0149 |
| 306.43 nm | 0.0002 |
Photophysical properties of the ligand 9d, in particular, have been performed in detail in presence of metal ions Ni2+, Co2+, Zn2+, Cu2+, Fe3+, Cd2+, Hg2+ and Ca2+. Complexation of the ligand 9d with Cu2+ and Fe3+ ions was accompanied by the change of the colour of the solution from colourless to pink, which was easily observed by the naked-eye (Fig. 5a). This response was selective to Cu2+ and Fe3+ ions in solution, the colour being more intense in Cu2+ than in Fe3+. The addition of 10 equiv. of the other cations as their perchlorate salts resulted in no appreciable changes in colour under visible as well as UV-light (Fig. 5b). Interestingly, the molecule 9d in presence of Cu2+ ion shows purple colour with UV radiation, but is found to be unchanged with all other metal ion including Fe2+ (Fig. 5b).
 | ||
| Fig. 5 Change in color of solution of 9d in acetonitrile in presence of 10 equiv. of each metal ion (a) under visible light (b) under UV light. | ||
UV-Vis spectra of the ligand 9d solution in acetonitrile show no significant change on increasing concentration of metal perchlorate salts of Ni2+, Co2+, Zn2+, Fe3+, Cd2+, Hg2+ and Ca2+ (ESI, Fig. S11(a–d)†). However in presence of Cu2+ the UV-Vis spectrum of 9d shows appreciable change compared to that in case of free ligand hence indicating ligand–metal interaction. On gradual addition of Cu2+ salt an absorption band develops near 500 nm with simultaneous appearance of two isobestic points at 308 nm and 419 nm (Fig. 6). The emission spectra of ligand 9d in acetonitrile shows no change in fluorescence intensity in presence of metal ions Ni2+, Co2+, Zn2+, Cd2+, Hg2+ and Ca2+.29 On the other hand fluorometric titration in presence of Fe3+ and Cu2+ shows substantial quenching of ligand emission. The extent of fluorescence quenching in Cu2+ is significantly higher compared to that in case of Fe3+ (Fig. 7). Detection limit of Cu2+ by probe 9d has been estimated from fluorescence titration and has been found to be 7.07 × 10−6 M (ESI, Fig. S15†). Selective complexation of an analyte coupled with the fluorescence properties of a bound chromophore resulted in the development of promising molecular fluorescent probes. Fe3+ and Cu2+ orientated probes have been developed widely with the use of highly selective copper and iron-chelating molecules such as naturally occurring fluorescent siderophores or synthetic siderophore analogs connected suitably to a light-emitting group.30 It is well known that d-block ions such as Fe3+ or Cu2+ often open excited state deexcitation pathways via electronic energy transfer (EET) and/or photoinduced electron transfer (PET) involving the metal centre. It is not surprising, therefore, that there are many more examples of “Turn-off” sensors than “Turn-on”.30 In the present case, quenching of fluorescence of the synthetic molecules 9 thus can be treated as fluorescence “Turn-off” sensor selective for Fe3+ or Cu2+ ions.
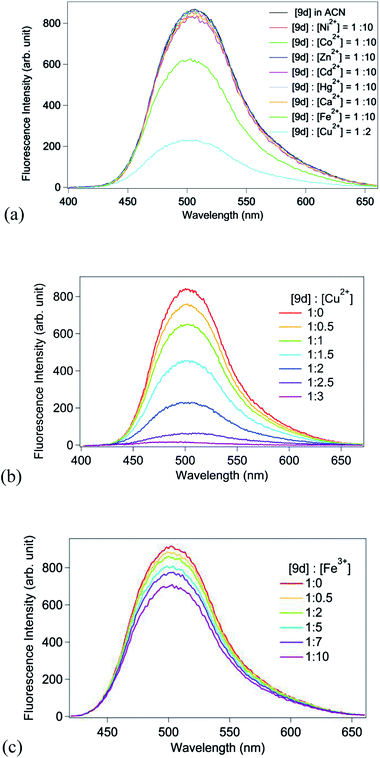 | ||
| Fig. 7 (a) Emission spectra (λex = nm) of 9d in acetonitrile in presence of various metal ions. Fluorescence quenching of 9d on gradual addition of (b) Cu2+ and (c) Fe3+ ions. | ||
Additionally, 1H NMR titration was carried out by gradual addition of perchlorate salt of Cu2+ ions to a DMSO-d6 solution of 9f. The results show that the intensity of the proton signal corresponding to the hydroxyl group (–OH) at 10.25 ppm, which is the most probable binding site for Cu2+ ions, decreases significantly relative to that of other protons upon exposure to Cu2+ (Fig. 8). This effect confirms the formation of weak complex between 9f and Cu2+ ion. Moreover rapid broadening of the proton signal at 10.25 ppm with gradual addition of Cu2+ ions further confirms the fast exchange of –OH protons due to complexation with Cu2+ ion.
 | ||
| Fig. 8 Change in 1H NMR spectra of 9f upon addition of Cu2+ ions, (a) 00 equiv., (b) 0.5 equiv., (c) 1.0 equiv., and (d) 2.0 equiv. in [d6]-DMSO. | ||
Conclusions
In summary, we have successfully developed a synthetic protocol for constructing novel tetracyclic skeleton of 4-hydroxyindole fused isocumarins. This methodology has the advantages of using easily available reactants and inexpensive catalyst and high yields of the product formation. To the best of our knowledge, this is the first report of synthesis of suitable isocoumarin based fluorescence active molecules with high quantum yields and ability to act as fluorescence “Turn-off” sensor for Cu2+ and Fe3+ ions. Most importantly the same molecules are found to be chemosensor for Cu2+ion only with respect to UV-Vis spectral change and naked eye colour change in presence and absence of UV radiation.Experimental section
General procedure for the preparation of 2a–k or 3a–k
Concentrated H2SO4 (0.5 mL) was added to a solution of dihydroxy-indenopyrroles 1 (500 mg) in acetic acid (10 mL). The reaction mixture was heated at reflux for 15–45 min (as mentioned in Table 2). The initial colorless solution was intensified to red. Then it was poured into ice cold water to obtain solid products. The compounds were purified by crystallization technique using acetone and petroleum ether as mixed solvents.General procedure for the preparation of 4a,b
The isocoumarin derivatives 2 (500 mg) were dissolved in 2.5 mL of methanol. NaBH4 (0.3 equiv.) was added in one portion with stirring. A vigorous gas evolution occurs, together with a temperature rise. Stirring was continued for a few minutes (30 min) and then the pH was adjusted to neutrality with addition of dilute aqueous HCl, the mixture was extracted (ethyl acetate) and dried (Na2SO4), and the solvent was evaporated. The crude residue was then purified by column chromatography and crystallized by using petroleum ether and ethyl acetate.General procedure for the preparation of 9a–f
10% Pd/C (97 mg, 0.09 mmol) was added to a stirred solution of 2 (0.9 mmol) in Ph2O (4 mL). The mixture was heated for 2 h at 250 °C under an inert atmosphere of N2. After the mixture was cooled to room temperature, the catalyst was removed by filtration on Celite and the filter cake was rinsed several times with EtOAc. Volatiles were removed under reduced pressure and the resulting mixture was loaded onto a silica gel column. Elution of the column with hexanes (for removal of Ph2O) and then with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 hexane–EtOAc provided yellow solid.
3 hexane–EtOAc provided yellow solid.
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1724 cm−1; anal. calcd for C22H17NO3: C, 76.95; H, 4.99; N, 4.08% found C, 76.82; H, 4.93; N, 4.00%.
1724 cm−1; anal. calcd for C22H17NO3: C, 76.95; H, 4.99; N, 4.08% found C, 76.82; H, 4.93; N, 4.00%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1721 cm−1; anal. calcd for C21H15NO3: C, 76.58; H, 4.59; N, 4.25% found C, 76.44; H, 4.52; N, 4.19%.
1721 cm−1; anal. calcd for C21H15NO3: C, 76.58; H, 4.59; N, 4.25% found C, 76.44; H, 4.52; N, 4.19%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1722 cm−1; anal. calcd for C22H17NO3: C, 76.95; H, 4.99; N, 4.08% found C, 76.80; H, 4.91; N, 4.03%.
1722 cm−1; anal. calcd for C22H17NO3: C, 76.95; H, 4.99; N, 4.08% found C, 76.80; H, 4.91; N, 4.03%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1716 cm−1; anal. calcd for C21H14FNO3: C, 72.62; H, 4.06; N, 4.03% found C, 72.48; H, 3.98; N, 3.97%.
1716 cm−1; anal. calcd for C21H14FNO3: C, 72.62; H, 4.06; N, 4.03% found C, 72.48; H, 3.98; N, 3.97%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1726 cm−1; anal. calcd for C21H14ClNO3: C, 69.33; H, 3.88; N, 3.85% found C, 69.21; H, 3.80; N, 3.78%.
1726 cm−1; anal. calcd for C21H14ClNO3: C, 69.33; H, 3.88; N, 3.85% found C, 69.21; H, 3.80; N, 3.78%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1720 cm−1; anal. calcd for C21H14ClNO3: C, 69.33; H, 3.88; N, 3.85% found C, 69.19; H, 3.81; N, 3.79%.
1720 cm−1; anal. calcd for C21H14ClNO3: C, 69.33; H, 3.88; N, 3.85% found C, 69.19; H, 3.81; N, 3.79%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1718 cm−1; anal. calcd for C21H14ClNO3: C, 69.33; H, 3.88; N, 3.85% found C, 69.46; H, 3.79; N, 3.78%.
1718 cm−1; anal. calcd for C21H14ClNO3: C, 69.33; H, 3.88; N, 3.85% found C, 69.46; H, 3.79; N, 3.78%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1723 cm−1; anal. calcd for C21H14BrNO3: C, 61.78; H, 3.46; N, 3.43% found C, 61.90; H, 3.37; N, 3.36%.
1723 cm−1; anal. calcd for C21H14BrNO3: C, 61.78; H, 3.46; N, 3.43% found C, 61.90; H, 3.37; N, 3.36%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1724 cm−1; anal. calcd for C22H17NO4: C, 73.53; H, 4.77; N, 3.90% found C, 73.40; H, 4.71; N, 3.82%.
1724 cm−1; anal. calcd for C22H17NO4: C, 73.53; H, 4.77; N, 3.90% found C, 73.40; H, 4.71; N, 3.82%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1720 cm−1; anal. calcd for C22H17NO4: C, 73.53; H, 4.77; N, 3.90% found C, 73.43; H, 4.70; N, 3.80%.
1720 cm−1; anal. calcd for C22H17NO4: C, 73.53; H, 4.77; N, 3.90% found C, 73.43; H, 4.70; N, 3.80%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1717 cm−1; anal. calcd for C21H14N2O5: C, 67.38; H, 3.77; N, 7.48% found 67.53; H, 3.69; N, 7.40%.
1717 cm−1; anal. calcd for C21H14N2O5: C, 67.38; H, 3.77; N, 7.48% found 67.53; H, 3.69; N, 7.40%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1715 cm−1; anal. calcd for C24H21NO3: C, 77.61; H, 5.70; N, 3.77% found C, 77.75; H, 5.63; N, 3.71%.
1715 cm−1; anal. calcd for C24H21NO3: C, 77.61; H, 5.70; N, 3.77% found C, 77.75; H, 5.63; N, 3.71%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1722 cm−1; anal. calcd for C23H19NO3: C, 77.29; H, 5.36; N, 3.92% found C, 77.42; H, 5.28; N, 3.83%.
1722 cm−1; anal. calcd for C23H19NO3: C, 77.29; H, 5.36; N, 3.92% found C, 77.42; H, 5.28; N, 3.83%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1725 cm−1; anal. calcd for C24H21NO3: C, 77.61; H, 5.70; N, 3.77% found C, 77.47; H, 5.61; N, 3.69%.
1725 cm−1; anal. calcd for C24H21NO3: C, 77.61; H, 5.70; N, 3.77% found C, 77.47; H, 5.61; N, 3.69%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1724 cm−1; anal. calcd for C23H18FNO3: C, 73.59; H, 4.83; N, 3.73% found C, 73.70; H, 4.75; N, 3.66%.
1724 cm−1; anal. calcd for C23H18FNO3: C, 73.59; H, 4.83; N, 3.73% found C, 73.70; H, 4.75; N, 3.66%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1721 cm−1; anal. calcd for C23H18ClNO3: C, 70.50; H, 4.63; N, 3.57% found C, 70.36; H, 4.56; N, 3.51%.
1721 cm−1; anal. calcd for C23H18ClNO3: C, 70.50; H, 4.63; N, 3.57% found C, 70.36; H, 4.56; N, 3.51%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1719 cm−1; anal. calcd for C23H18ClNO3: C, 70.50; H, 4.63; N, 3.57% found C, 70.39; H, 4.56; N, 3.51%.
1719 cm−1; anal. calcd for C23H18ClNO3: C, 70.50; H, 4.63; N, 3.57% found C, 70.39; H, 4.56; N, 3.51%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1722 cm−1; anal. calcd for C23H18ClNO3: C, 70.50; H, 4.63; N, 3.57% found C, 70.64; H, 4.56; N, 3.51%.
1722 cm−1; anal. calcd for C23H18ClNO3: C, 70.50; H, 4.63; N, 3.57% found C, 70.64; H, 4.56; N, 3.51%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1720 cm−1; anal. calcd for C23H18BrNO3: C, 63.32; H, 4.16; N, 3.21% found C, 63.47; H, 4.07; N, 3.16%.
1720 cm−1; anal. calcd for C23H18BrNO3: C, 63.32; H, 4.16; N, 3.21% found C, 63.47; H, 4.07; N, 3.16%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1721 cm−1; anal. calcd for C24H21NO4: C, 74.40; H, 5.46; N, 3.62% found C, 74.52; H, 5.38; N, 3.55%.
1721 cm−1; anal. calcd for C24H21NO4: C, 74.40; H, 5.46; N, 3.62% found C, 74.52; H, 5.38; N, 3.55%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1726 cm−1; anal. calcd for C24H21NO4: C, 74.40; H, 5.46; N, 3.62% found C, 74.27; H, 5.37; N, 3.55%.
1726 cm−1; anal. calcd for C24H21NO4: C, 74.40; H, 5.46; N, 3.62% found C, 74.27; H, 5.37; N, 3.55%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 1727 cm−1; anal. calcd for C23H18N2O5: C, 68.65; H, 4.51; N, 6.96% found C, 68.76; H, 4.44; N, 6.91%.
1727 cm−1; anal. calcd for C23H18N2O5: C, 68.65; H, 4.51; N, 6.96% found C, 68.76; H, 4.44; N, 6.91%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3491, 1695 cm−1; anal. calcd for C22H19NO3: C, 76.50; H, 5.54; N, 4.06% found C, 76.37; H, 5.49; N, 3.99%.
3491, 1695 cm−1; anal. calcd for C22H19NO3: C, 76.50; H, 5.54; N, 4.06% found C, 76.37; H, 5.49; N, 3.99%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3499, 1700 cm−1; anal. calcd for C22H19NO3: C, 76.50; H, 5.54; N, 4.06% found C, 76.35; H, 5.48; N, 3.99%.
3499, 1700 cm−1; anal. calcd for C22H19NO3: C, 76.50; H, 5.54; N, 4.06% found C, 76.35; H, 5.48; N, 3.99%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3297, 1686 cm−1; anal. calcd for C22H15NO3: C, 77.41; H, 4.43; N, 4.10% found C, 77.54; H, 4.36; N, 4.05%; HRMS (ESI-TOF) m/z calcd: 342.1130 [M + H]; found: 342.1124.
3297, 1686 cm−1; anal. calcd for C22H15NO3: C, 77.41; H, 4.43; N, 4.10% found C, 77.54; H, 4.36; N, 4.05%; HRMS (ESI-TOF) m/z calcd: 342.1130 [M + H]; found: 342.1124.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3285, 1680 cm−1; anal. calcd for C21H13NO3: C, 77.05; H, 4.00; N, 4.28% found C, 77.20; H, 3.93; N, 4.22%; HRMS (ESI-TOF) m/z calcd: 328.0974 [M + H]; found: 328.1272.
3285, 1680 cm−1; anal. calcd for C21H13NO3: C, 77.05; H, 4.00; N, 4.28% found C, 77.20; H, 3.93; N, 4.22%; HRMS (ESI-TOF) m/z calcd: 328.0974 [M + H]; found: 328.1272.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3290, 1682 cm−1; anal. calcd for C22H15NO3: C, 77.41; H, 4.43; N, 4.10% found C, 77.30; H, 4.37; N, 4.05%; HRMS (ESI-TOF) m/z calcd: 342.1130 [M + H]; found: 342.1246.
3290, 1682 cm−1; anal. calcd for C22H15NO3: C, 77.41; H, 4.43; N, 4.10% found C, 77.30; H, 4.37; N, 4.05%; HRMS (ESI-TOF) m/z calcd: 342.1130 [M + H]; found: 342.1246.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3296, 1686 cm−1; anal. calcd for C21H12FNO3: C, 73.04; H, 3.50; N, 4.06% found C, 72.90; H, 3.44; N, 4.00%.
3296, 1686 cm−1; anal. calcd for C21H12FNO3: C, 73.04; H, 3.50; N, 4.06% found C, 72.90; H, 3.44; N, 4.00%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3293, 1687 cm−1; anal. calcd for C22H15NO4: C, 73.94; H, 4.23; N, 3.92% found C, 73.85; H, 4.16; N, 3.86%.
3293, 1687 cm−1; anal. calcd for C22H15NO4: C, 73.94; H, 4.23; N, 3.92% found C, 73.85; H, 4.16; N, 3.86%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) 3289, 1681 cm−1; anal. calcd for C22H15NO4: C, 73.94; H, 4.23; N, 3.92% found C, 73.83; H, 4.17; N, 3.85%; HRMS (ESI-TOF) m/z calcd: 358.1079 [M + H]; found: 358.1073.
3289, 1681 cm−1; anal. calcd for C22H15NO4: C, 73.94; H, 4.23; N, 3.92% found C, 73.83; H, 4.17; N, 3.85%; HRMS (ESI-TOF) m/z calcd: 358.1079 [M + H]; found: 358.1073.Acknowledgements
S.P. and S.M. thank CSIR and A. K. thanks UGC, New Delhi, India, for offering Senior Research Fellowship (SRF). D. D. acknowledges UGC for Dr D. S. Kothari Postdoctoral Fellowship. The financial assistance of CSIR, New Delhi is gratefully acknowledged [Major Research Project, no. 02(0007)/11/EMR-II]. Crystallography was performed at the DST-FIST, India-funded Single Crystal Diffractometer Facility at the Department of Chemistry, University of Calcutta.References
- R. D. Barry, Chem. Rev., 1964, 64, 229 CrossRef CAS.
- E. Napolitano, Org. Prep. Proced. Int., 1997, 29, 631 CrossRef CAS.
- (a) X. Lu, D. Li, N. K. Dalley, S. G. Wood and N. L. Owen, Nat. Prod. Res., 2007, 21, 677 CrossRef CAS PubMed; (b) A. S. Kiselyov, Tetrahedron Lett., 1995, 36, 493 CrossRef CAS; (c) D. A. Goff and R. N. Zuckermann, J. Org. Chem., 1995, 60, 5748 CrossRef CAS; (d) A. Sugimoto, H. Shinba-Tanaka and M. Ishikawa, Synthesis, 1995, 431 CrossRef CAS PubMed; (e) S. Pal, V. Chatare and M. Pal, Curr. Org. Chem., 2011, 15, 782 CrossRef CAS.
- K. F. Devienne, A. F. Calgaro-Helena, D. J. Dorta, I. M. R. Prado, M. S. G. Raddi, W. Vilegas, S. A. Uyemura, A. C. Santos and C. Curti, Phytochemistry, 2007, 68, 1075 CrossRef CAS PubMed.
- J. H. Lee, Y. J. Park, H. S. Kim, Y. S. Hong, K. W. Kim and J. Lee, J. Antibiot., 2001, 54, 463 CrossRef CAS.
- (a) A. Nakhi, R. Adepu, D. Rambabu, R. Kishore, G. R. Vanaja, A. M. Kalle and M. Pal, Bioorg. Med. Chem. Lett., 2012, 22, 4418 CrossRef CAS PubMed; (b) C. Praveen, A. Ayyanar and P. T. Perumal, Bioorg. Med. Chem. Lett., 2011, 21, 4170 CrossRef CAS PubMed.
- (a) D. Engelmeier, F. Hadacek, O. Hofer, G. Lutz-Kutschera, M. Nagl, G. Wurz and H. Greger, J. Nat. Prod., 2004, 67, 19 CrossRef CAS PubMed; (b) K. Nozawa, M. Yamada, Y. Tsuda, K. Kawai and S. Nakajima, Chem. Pharm. Bull., 1981, 29, 3486 CrossRef CAS; (c) P. Yazav and N. V. Purohit, Indian J. Pharm. Sci., 2011, 73, 171 CrossRef PubMed.
- (a) M. Yoshikawa, E. Harada, Y. Naitoh, K. Inoue, H. Matsuda, H. Shimoda, J. Yamahara and N. Murakami, Chem. Pharm. Bull., 1994, 42, 2225 CrossRef CAS; (b) D. K. Ferrazzoli, R. M. S. Gonc-alves, C. R. Gomes and W. Vilegas, Phytomedicine, 2005, 12, 378 CrossRef PubMed.
- W. Zhang, K. Krohn, S. Draeger and B. Schulz, J. Nat. Prod., 2008, 71, 1078 CrossRef CAS PubMed.
- Y. Xie, N. Wang, B. Cheng and H. Zhai, Org. Lett., 2012, 14, 3 CrossRef CAS PubMed.
- B. Chen and S. Ma, Org. Lett., 2013, 15, 3884 CrossRef CAS PubMed.
- P. G. Jagtap, E. Baloglu, G. Southan, W. Williams, A. Roy, A. Nivorozhkin, N. Landrau, K. Desisto, A. L. Salzman and C. Szabo, Org. Lett., 2005, 7, 1753 CrossRef CAS PubMed.
- (a) N. Sharma, M. Asthana, D. Nandini, R. P. Singh and R. M. Singh, Tetrahedron, 2013, 69, 1822 CrossRef CAS PubMed; (b) M. Uchiyama, H. Ozawa, K. Takuma, Y. Matsumoto, M. Yonehara, K. Hiroya and T. Sakamoto, Org. Lett., 2006, 8, 5517 CrossRef CAS PubMed; (c) S. Özcan, E. Şahinc and M. Balcia, Tetrahedron Lett., 2007, 48, 2151 CrossRef PubMed; (d) T. Yao and R. C. Larock, Tetrahedron Lett., 2002, 43, 7401 CrossRef CAS; (e) M. Peuchmaur, V. Lisowski, C. Gandreuil, L. T. Maillard, J. Martinez and J.-F. Hernandez, J. Org. Chem., 2009, 74, 4158 CrossRef CAS PubMed; (f) Y. Nakamura and T. Ukita, Org. Lett., 2002, 4, 2317 CrossRef CAS PubMed; (g) W. E. Bauta, D. P. Lovett, W. R. Cantrell Jr and B. D. Burke, J. Org. Chem., 2003, 68, 5967 CrossRef CAS PubMed; (h) E. Soleimani and M. Zainali, J. Org. Chem., 2011, 76, 10306 CrossRef CAS PubMed; (i) J. L. Bullington and J. H. Dodd, J. Org. Chem., 1993, 58, 4833 CrossRef CAS; (j) M. Chakravarty and K. C. K. Swamy, J. Org. Chem., 2006, 71, 9128 CrossRef CAS PubMed; (k) M. P. Pavan, M. Chakravarty and K. C. Swamy, Eur. J. Org. Chem., 2009, 5927 CrossRef CAS.
- (a) V. Kavala, C.-C. Wang, D. K. Barange, C.-W. Kuo, P.-M. Lei and C.-F. Yao, J. Org. Chem., 2012, 77, 5022 CrossRef CAS PubMed; (b) M. D. Obushak, V. S. Matiychuk and V. V. Turytsya, Tetrahedron Lett., 2009, 50, 6112 CrossRef CAS PubMed; (c) S. Cai, F. Wang and C. Xi, Synthesis, 2012, 44, 1892 CrossRef CAS PubMed; (d) S. Cai, F. Wang and C. Xi, J. Org. Chem., 2012, 77, 2331 CrossRef CAS PubMed; (e) X.-X. Guo, J. Org. Chem., 2013, 78, 1660 CrossRef CAS PubMed.
- T. Suzuki, T. Yamada, K. Watanabe and T. Katoh, Bioorg. Med. Chem. Lett., 2005, 15, 2583 CrossRef CAS PubMed.
- R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2012, 48, 2030 RSC.
- Z. Li, J. Hong, L. Weng and X. Zhou, Tetrahedron, 2012, 68, 1552 CrossRef CAS PubMed.
- E. T. D. Penha, J. A. Forni, A. F. P. Biajoli and C. R. D. Correia, Tetrahedron Lett., 2011, 52, 6342 CrossRef PubMed.
- M. Hellal, J.-J. Bourguignon and F. J.-J. Bihel, Tetrahedron Lett., 2008, 49, 62 CrossRef CAS PubMed.
- (a) P.-Y. Chen, K.-S. Huang, C.-C. Tsai, T.-P. Wang and E.-C. Wang, Org. Lett., 2012, 14, 4930 CrossRef CAS PubMed; (b) A. C. Tadd, M. R. Fielding and M. C. Willis, Chem. Commun., 2009, 6744 RSC; (c) D. Nandi, D. Ghosh, S.-J. Chen, B.-C. Kuo, N. M. Wang and H. M. Lee, J. Org. Chem., 2013, 78, 3445 CrossRef CAS PubMed.
- (a) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS PubMed; (b) S. J. Lippard and J. M. Berg, Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, CA, 1994 Search PubMed.
- (a) E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995 CrossRef CAS PubMed; (b) N. F. Olivieri, N. Engl. J. Med., 1999, 341, 99 CrossRef CAS PubMed.
- (a) R. Sheng, P. Wang, Y. Gao, Y. Wu, W. Liu, J. Ma, H. Li and S. Wu, Org. Lett., 2008, 10, 5015 CrossRef CAS PubMed; (b) P. Li, X. Duan, Z. Chen, Y. Liu, T. Xie, L. Fang, X. Li, M. Yin and B. Tang, Chem. Commun., 2011, 47, 7755 RSC; (c) H. Weizman, O. Ardon, B. Mester, J. Libman, O. Dwir, Y. Hadar, Y. Chen and A. Shanzer, J. Am. Chem. Soc., 1996, 118, 12368 CrossRef CAS.
- (a) S. Das, R. Fröhlich and A. Pramanik, Org. Lett., 2006, 8, 4263 CrossRef CAS PubMed; (b) S. Kundu, A. Pramanik and A. Patra, Synlett, 2002, 823 CrossRef CAS; (c) S. Das, P. Koley and A. Pramanik, Tetrahedron Lett., 2011, 52, 3243 CrossRef CAS PubMed; (d) S. Pathak, A. Kundu and A. Pramanik, Tetrahedron Lett., 2011, 52, 5180 CrossRef CAS PubMed; (e) S. Das, R. Fröhlich and A. Pramanik, Synlett, 2006, 207 CAS; (f) S. Das, A. Pramanik, R. Fröhlich and A. Patra, Tetrahedron, 2004, 60, 10197 CrossRef CAS PubMed; (g) S. Pathak, K. Debnath, S. T. Hossain, S. K. Mukherjee and A. Pramanik, Tetrahedron Lett., 2013, 54, 3137 CrossRef CAS PubMed; (h) S. Pathak and A. Pramanik, Eur. J. Org. Chem., 2013, 4410 CrossRef CAS; (i) S. Pathak and A. Pramanik, RSC Adv., 2014, 4, 23779 RSC.
- B. Jiang, Q.-Y. Li, S.-J. Tu and G. Li, Org. Lett., 2012, 14, 5210 CrossRef CAS PubMed.
- H.-J. Hemmerling and G. Reiss, Synthesis, 2009, 985 CrossRef CAS PubMed.
- (a) M. D. Lebar and B. J. Baker, Aust. J. Chem., 2010, 63, 862 CrossRef CAS; (b) X. Yuan, G. Sun, H. Asakura, T. Tanaka, X. Chen, Y. Yuan, G. Laurenczy, Y. Kou, P. J. Dyson and N. Yan, Chem.–Eur. J., 2013, 19, 1227 CrossRef CAS PubMed; (c) D. Sissouma, L. Maingot, S. Collet and A. Guingant, J. Org. Chem., 2006, 71, 8384 CrossRef CAS PubMed; (d) F. Chang, H. Kim, B. Lee, H. G. Park and J. Park, Bull. Korean Chem. Soc., 2011, 32, 1074 CrossRef CAS.
- (a) J. Cossy and D. Belotti, Org. Lett., 2002, 4, 2557 CrossRef CAS PubMed; (b) S. Mukhopadhyay, G. Rothenberg, D. Gitis and Y. Sasson, Org. Lett., 2000, 2, 211 CrossRef CAS PubMed.
- A. Helal, M. H. O. Rashid, C.-H. Choi and H.-S. Kim, Tetrahedron, 2011, 67, 2794 CrossRef CAS PubMed.
- (a) S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponic and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195 RSC; (b) Y. Peng, Y.-M. Dong, M. Dong and Y.-W. Wang, J. Org. Chem., 2012, 77, 9072 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C data of compounds 2, 3, 4 and 9 and crystallographic data for 9a, 9f. CCDC 940456–940459 and 940478. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01060h |
| This journal is © The Royal Society of Chemistry 2015 |