Luminescent properties of Ag(I)/Cu(I) coordination polymers: crystal structures and high intensity luminescence of a PMMA-doped hybrid material based on a quinoline-2,3-dicarboxylic acid ligand†
Yang Songa,
Rui-Qing Fan*a,
Hui-Jie Zhanga,
Zhi-Wei Liua,
Xue-Tao Wanga,
Cai-Tu Tana,
Yu-Lin Yang*a and
Yu-Lei Wangb
aDepartment of Chemistry, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: fanruiqing@hit.edu.cn; ylyang@hit.edu.cn; Fax: +86-0451-86413710
bNational Key Laboratory of Science and Technology on Tunable Laser, Harbin Institute of Technology, Harbin 150080, P. R. China
First published on 3rd February 2015
Abstract
Three one-dimensional (1D) Ag(I)/Cu(I) coordination polymers, formulated as [Ag(2,3-Hqldc)]n (Ag1), [Ag(3-qlc)]2n (Ag2) and [CuI(3-Hqlc)]n (Cu1) based on the ligand quinoline-2,3-dicarboxylic acid (H2qldc), were synthesized through hydrothermal (solvothermal) method and structurally characterized by single-crystal X-ray diffraction, IR spectroscopy and elemental analysis. Molecular structural analysis reveals that Ag1 was a 1D + 1D → 1D infinite chain synthesized at a relatively low temperature 80 °C, which further forms a three-dimensional (3D) structure by π–π stacking interactions. Ag2 forms a 1D dimer chain structure and via π⋯π packing interactions shows a two-dimensional (2D) supramolecular network. Both Ag1 and Ag2 display stable blue luminescent in the solid state and in organic solvents (DMSO, CH3CN and CH3OH) at 298 K and 77 K. However, Cu1 possess a 1D ladder chain structure, which further forms a 2D structure by hydrogen bonding interactions. Cu1 shows tunable luminescence at 298 K and 77 K in the solid state with a large red-shift of 70 nm and the CIE color shifts from bright yellow (0.51, 0.48) to red (0.67, 0.30), indicating thermochromic luminescence for Cu1. After doping with poly(methylmethacrylate) (PMMA), not only are the luminescence intensity and lifetimes enhanced, but the thermal stability is also increased in comparison with Cu1. After Cu1 was doped with PMMA (Cu1@PMMA), the lifetime of the polymer film material Cu1@PMMA increases and reaches a maximum at 1.0% (τ = 95.57 μs), which is more than eight times longer than that of Cu1 (τ = 13.78 μs). Cu1@PMMA is confirmed as a bright yellow luminescent polymer film material.
Introduction
Silver(I) and copper(I) coordination polymers have interesting luminescent properties1 that have been utilized in areas such as light emitting diodes, luminescence probes2 and emitting layers in polymer light-emitting diodes.3 Although the coordination polymers have better luminescence and higher thermal stability, polymer-doped systems have clear technical advantages over glass ones, such as flexibility and prominent processing ability, which are important for optical fibers and fiber amplifiers.4 When Isamu Akasaki, Hiroshi Amano and Shuji Nakamura produced bright blue light beams from their semi-conductors in the early 1990s, they triggered a fundamental transformation in lighting technology. Red and green diodes had been around for a long time but without blue light, white lamps could not be created. Despite considerable efforts, both in the scientific community and in industry, the blue LED had remained a challenge for three decades.5 As luminescent materials, silver(I) coordination polymers can be used as stable blue luminescent complexes6 in optical devices. However, until now, only a few cases have been reported about blue emitting silver(I) coordination polymers, and the realization of high luminescence efficiency remains a big challenge.7In addition, monovalent copper complexes show a variety of structure and rich photophysical properties. Nevertheless, Cu(I) is easy to oxidize into Cu(II) in the process of synthesis, thus create monovalence copper complexes as luminescent material is difficult but very meaningful. A popular polymer matrix used as a host for luminescent transition coordination polymers is poly(methyl methacrylate) (PMMA), which is a low-cost, simply prepared polymer with excellent optical quality.8 Until now most of the reports are rare earth doped with PMMA.9 There is no doubt that rare earth element shows excellent luminescent properties, but luminous color of rare earth element has defects which is not easy to control. Compared with noble metal Pt, Au, Ag even rare earth, Cu(I) with the advantage of cheap and non-poison, which also can modify ligands and control the emitting. Hence, copper-based systems with favorable luminescence are beginning to receive more attention.10 When copper(I) coordination polymers incorporate with PMMA forming a new complex-containing polymer/film,11 it will lead to significant flexibility, versatility, thermal and photostability.12
In this work, we choose quinoline-2,3-dicarboxylic acid (H2qldc) as the ligand. H2qldc as a derivative of 2,3-H2pydc which has a relatively large π-conjugated system in the quinoline ring, which might not only contribute to the desirable fluorescence properties resulting from the interaction between 2,3-qldc2− anions and metal ions, but also easily assemble into high dimensionality, supramolecular networks via π⋯π packing interactions between two adjacent aromatic rings as well as hydrogen bonding interactions. To the best of knowledge, cases of metal–organic complexes linked by H2qldc have been presented. Y. P. Cai,13 J. H. Lin,14 G. B. Che15 and their co-workers use 2,3-H2qldc ligand to prepare a series of coordination polymers from zero- to two-dimension with Co(II), Zn(II), Cd(II), Mn(II) and Ln(III) ions, which are summarized in Table S1.†
Three Ag(I)/Cu(I) coordination polymers constructed from H2qldc, namely, [Ag(2,3-Hqldc)]n (Ag1), [Ag(3-qlc)]2n (Ag2) and [CuI(3-Hqlc)]n (Cu1) have been synthesized under hydrothermal conditions. During the solvothermal synthetic process, unexpected situ decarboxylation of H2qldc was observed. Through controlling the temperature (from 80 °C to 120 °C) makes the ligand 2,3-H2qldc decarboxylation and results in the formation of 3-Hqlc ligand. After decarboxylation, the dihedral angle between the planes of carboxyl and quinoline in one bridging organic ligand is 3.70° in the Ag2, which is much smaller than those of 61.98° and 30.49° in the Ag1. The nice coplanar feature of the structure in Ag2 can enhance the luminescent efficiency. The structure of Ag2 has been reported by C. B. Liu, et al.16 but they directly use 3-Hqlc as ligand rather than decarboxylation of H2qldc. In this work, we mainly talked about the decarboxylation process of H2qldc and compared with coordination polymer Ag1. What's more, we also discussed the luminescent properties of Ag1 and Ag2 in details. Both Ag1 and Ag2 display stable blue luminescent in the solid state and in different solvents (DMSO, CH3CN and CH3OH) at 298 K and 77 K. However, Cu1 shows the interesting property of thermochromic luminescence, which is rarely reported in literatures, and most of cases only talked about the luminescent properties in the solid state rather than the effect of solvent and temperature, more comparisons are summarized in Table S2.†
Experimental section
General characterization
All reagents were analytical grade (99.7%) from commercial sources and were used directly without any further purification. Elemental analyses (C, H, and N) were performed on a Perkin-Elmer 240c elemental analyzer. IR spectra were recorded by Nicolet Impact 410 FTIR spectrometer (range in 4000–400 cm−1). 13C NMR and 1H NMR spectra were recorded on a Bruker ACF 400 MHz at room temperature. X-ray powder diffraction (XRPD) patterns were analyzed with monochromated Cu Kα radiation of 40 mA, 40 kV. UV-vis spectra were recorded on a Perkin-Elmer Lambda 20 spectrometer. The solid-state and solution photoluminescence analyses were carried out on an Edinburgh FLS920 fluorescence spectrometer in the range of 200–800 nm at 298 K and 77 K.Synthesis of [Ag(2,3-Hqldc)]n (Ag1)
A mixture of AgNO3 (0.034 g, 0.20 mmol), 2,3-H2qldc (0.021 g, 0.10 mmol) with a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was dissolved in 8 mL H2O and stirred for 20 min. The final mixture was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 80 °C for 5 days. After slowly cooling to the room temperature, the mixture was washed with distilled water and colorless block-shaped crystals were filtered off and dried at room temperature (yield ca. 65%, based on silver metal). Elemental analysis (%): calc. for C11H6AgNO4 (Mr: 324.04): C, 40.77; N, 4.32; H, 1.87%. Found: C, 40.72; N, 4.38; H, 1.89%. IR (cm−1): 3515 (br, s), 3404 (br, s), 3142 (w), 2235 (m), 1591 (vs), 1372 (vs), 1285 (s), 1132 (vs), 1001 (m), 750 (vs), 673 (s), 553 (w), 476 (w). 1H NMR (400 MHz, DMSO-d6): δ = 8.98 (s, 1H, H2qldc–H1), 8.24 (s, 1H, H2qldc–H5), 8.14 (d, 1H, H2qldc–H4′), 7.95 (d, 1H, H2qldc–H2), 7.77 (d, 1H, H2qldc–H3) ppm. 1H NMR (400 MHz, CD3CN): δ = 9.13 (s, 1H, H2qldc–H1), 8.24 (s, 1H, H2qldc–H5), 8.18 (d, 1H, H2qldc–H4), 8.06 (d, 1H, H2qldc–H2), 7.84 (d, 1H, H2qldc–H3) ppm. 1H NMR (400 MHz, CD3OD): δ = 8.94 (s, 1H, H2qldc–H1), 8.13 (d, 2H, H2qldc–H4,5), 7.95 (d, 1H, H2qldc–H2), 7.76 (d, 1H, H2qldc–H3) ppm.
1 was dissolved in 8 mL H2O and stirred for 20 min. The final mixture was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 80 °C for 5 days. After slowly cooling to the room temperature, the mixture was washed with distilled water and colorless block-shaped crystals were filtered off and dried at room temperature (yield ca. 65%, based on silver metal). Elemental analysis (%): calc. for C11H6AgNO4 (Mr: 324.04): C, 40.77; N, 4.32; H, 1.87%. Found: C, 40.72; N, 4.38; H, 1.89%. IR (cm−1): 3515 (br, s), 3404 (br, s), 3142 (w), 2235 (m), 1591 (vs), 1372 (vs), 1285 (s), 1132 (vs), 1001 (m), 750 (vs), 673 (s), 553 (w), 476 (w). 1H NMR (400 MHz, DMSO-d6): δ = 8.98 (s, 1H, H2qldc–H1), 8.24 (s, 1H, H2qldc–H5), 8.14 (d, 1H, H2qldc–H4′), 7.95 (d, 1H, H2qldc–H2), 7.77 (d, 1H, H2qldc–H3) ppm. 1H NMR (400 MHz, CD3CN): δ = 9.13 (s, 1H, H2qldc–H1), 8.24 (s, 1H, H2qldc–H5), 8.18 (d, 1H, H2qldc–H4), 8.06 (d, 1H, H2qldc–H2), 7.84 (d, 1H, H2qldc–H3) ppm. 1H NMR (400 MHz, CD3OD): δ = 8.94 (s, 1H, H2qldc–H1), 8.13 (d, 2H, H2qldc–H4,5), 7.95 (d, 1H, H2qldc–H2), 7.76 (d, 1H, H2qldc–H3) ppm.
Synthesis of [Ag(3-qlc)]2n (Ag2)
A mixture of AgNO3 (0.034 g, 0.20 mmol), 2,3-H2qldc (0.021 g, 0.10 mmol), with a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was dissolved in 8 mL H2O and stirred for 20 min. The final mixture was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 120 °C for 5 days. After slowly cooling to the room temperature, the mixture was washed with distilled water and colorless block-shaped crystals were filtered off and dried at room temperature (yield ca. 52%, based on silver metal). Elemental analysis (%): calc. for C20H12Ag2N2O4 (Mr: 560.06): C, 42.89; N, 5.00; H, 2.16%. Found: C, 42.85; N, 5.03; H, 2.19%. IR (cm−1): 3437 (br, s), 3044 (w), 1597 (vs), 1558 (vs), 1394 (vs), 1317 (s), 793 (vs), 761 (m), 586 (m), 476 (w). 1H NMR (400 MHz, DMSO-d6): δ = 9.32 (s, 1H, 3-qlc–H1), 9.01 (s, 1H, 3-qlc–H2), 8.20 (d, 1H, 3-qlc–H6), 8.12 (d, 1H, 3-qlc–H3), 7.89 (d, 1H, 3-qlc–H5), 7.67 (d, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3CN): δ = 9.39 (s, 1H, 3-qlc–H1), 9.84 (s, 1H, 3-qlc–H2), 8.13 (d, 2H, 3-qlc–H3,5), 7.89 (d, 1H, 3-qlc–H6), 7.71 (d, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3OD): δ = 9.39 (s, 1H, 3-qlc–H1), 9.05 (s, 1H, 3-qlc–H2), 8.28 (s, 1H, 3-qlc–H6), 8.13 (d, 1H, 3-qlc–H3), 7.93 (d, 1H, 3-qlc–H5), 7.74 (d, 1H, 3-qlc–H4) ppm.
1 was dissolved in 8 mL H2O and stirred for 20 min. The final mixture was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 120 °C for 5 days. After slowly cooling to the room temperature, the mixture was washed with distilled water and colorless block-shaped crystals were filtered off and dried at room temperature (yield ca. 52%, based on silver metal). Elemental analysis (%): calc. for C20H12Ag2N2O4 (Mr: 560.06): C, 42.89; N, 5.00; H, 2.16%. Found: C, 42.85; N, 5.03; H, 2.19%. IR (cm−1): 3437 (br, s), 3044 (w), 1597 (vs), 1558 (vs), 1394 (vs), 1317 (s), 793 (vs), 761 (m), 586 (m), 476 (w). 1H NMR (400 MHz, DMSO-d6): δ = 9.32 (s, 1H, 3-qlc–H1), 9.01 (s, 1H, 3-qlc–H2), 8.20 (d, 1H, 3-qlc–H6), 8.12 (d, 1H, 3-qlc–H3), 7.89 (d, 1H, 3-qlc–H5), 7.67 (d, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3CN): δ = 9.39 (s, 1H, 3-qlc–H1), 9.84 (s, 1H, 3-qlc–H2), 8.13 (d, 2H, 3-qlc–H3,5), 7.89 (d, 1H, 3-qlc–H6), 7.71 (d, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3OD): δ = 9.39 (s, 1H, 3-qlc–H1), 9.05 (s, 1H, 3-qlc–H2), 8.28 (s, 1H, 3-qlc–H6), 8.13 (d, 1H, 3-qlc–H3), 7.93 (d, 1H, 3-qlc–H5), 7.74 (d, 1H, 3-qlc–H4) ppm.
Synthesis of [CuI(3-Hqlc)]n (Cu1)
A mixture of CuI (0.019 g, 0.10 mmol), 2,3-H2qldc (0.011 g, 0.05 mmol) with a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was dissolved in 6 mL CH3CN and stirred for 20 min. The final mixture was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 120 °C for 5 days. After cooling to the room temperature, the mixture was washed with distilled water and yellow block-shaped crystals were filtered off and dried at room temperature (yield ca. 72%, based on copper metal). Elemental analysis (%): calc. for C10H7CuINO2 (Mr: 363.61): C, 33.00; N, 3.85; H, 1.93%. Found: C, 33.59; N, 3.88; H, 1.96%. IR (cm−1): 3437 (br, s), 3076 (br, m), 1700 (vs), 1613 (vs), 1427 (m), 1285 (vs), 925 (w), 782 (vs), 465 (w). 1H NMR (400 MHz, DMSO-d6): δ = 9.40 (s, 1H, 3-qlc–H1), 9.16 (s, 1H, 3-qlc–H2), 8.32 (d, 2H, 3-qlc–H3,5), 7.93 (d, 1H, 3-qlc–H6), 7.78 (d, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3CN): δ = 9.40 (s, 1H, 3-qlc–H1), 8.89 (s, 1H, 3-qlc–H2), 8.13 (d, 2H, 3-qlc–H3,5), 7.89 (s, 1H, 3-qlc–H6), 7.67 (s, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3OD): δ = 9.93 (s, 1H, 3-qlc–H1), 9.59 (s, 1H, 3-qlc–H2), 8.86 (d, 1H, 3-qlc–H6), 8.70 (d, 1H, 3-qlc–H3), 8.51 (d, 1H, 3-qlc–H5), 8.28 (d, 1H, 3-qlc–H4) ppm.
1 was dissolved in 6 mL CH3CN and stirred for 20 min. The final mixture was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 120 °C for 5 days. After cooling to the room temperature, the mixture was washed with distilled water and yellow block-shaped crystals were filtered off and dried at room temperature (yield ca. 72%, based on copper metal). Elemental analysis (%): calc. for C10H7CuINO2 (Mr: 363.61): C, 33.00; N, 3.85; H, 1.93%. Found: C, 33.59; N, 3.88; H, 1.96%. IR (cm−1): 3437 (br, s), 3076 (br, m), 1700 (vs), 1613 (vs), 1427 (m), 1285 (vs), 925 (w), 782 (vs), 465 (w). 1H NMR (400 MHz, DMSO-d6): δ = 9.40 (s, 1H, 3-qlc–H1), 9.16 (s, 1H, 3-qlc–H2), 8.32 (d, 2H, 3-qlc–H3,5), 7.93 (d, 1H, 3-qlc–H6), 7.78 (d, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3CN): δ = 9.40 (s, 1H, 3-qlc–H1), 8.89 (s, 1H, 3-qlc–H2), 8.13 (d, 2H, 3-qlc–H3,5), 7.89 (s, 1H, 3-qlc–H6), 7.67 (s, 1H, 3-qlc–H4) ppm. 1H NMR (400 MHz, CD3OD): δ = 9.93 (s, 1H, 3-qlc–H1), 9.59 (s, 1H, 3-qlc–H2), 8.86 (d, 1H, 3-qlc–H6), 8.70 (d, 1H, 3-qlc–H3), 8.51 (d, 1H, 3-qlc–H5), 8.28 (d, 1H, 3-qlc–H4) ppm.
Synthesis of Cu+ complex-doped PMMA polymer films
The PMMA polymer was doped with the Cu(I) coordination polymer Cu1 in the proportions 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4% (w/w). The PMMA powder was dissolved in 6 mL N,N′-dimethylformamide (DMF), followed by addition of the required amount of coordination polymer Cu1 in DMF solution, and the resulting mixture was heated at 40 °C for 60 min. The polymer film was obtained after evaporation of excess solvent at 60 °C.Syntheses
Hydrothermal methods are widely used in the syntheses of inorganic–organic polymers, especially when organic carboxylate ligands are applied. Because under such reaction conditions, the problems of ligand solubility can be minimized.17 The structures of Ag1 and Ag2 were accomplished by introducing the N and O containing ligands into the reaction system, which may act as deprotonation and decarboxylation reagents. Coordination polymers Ag1 and Ag2 reported here were synthesized under hydrothermal reaction conditions, while reaction temperature showed a remarkable influence on the final structure. Ag1 was synthesized at a relatively low temperature 80 °C to get the 1D + 1D → 1D infinite chain, which is forms by the H2qldc with μ2–η1:η1:η0:η0 bridging mode and Ag1 further forms three-dimensional (3D) structure by π–π stacking interaction. Ag2 was synthesized at a higher temperature 120 °C, forming 1D dimer chain structure and via π⋯π packing interactions show two-dimensional (2D) supramolecular network. Meanwhile, after replacing AgNO3 with CuI, and changing water to organic solvent CH3CN, Cu1 was synthesized at a higher temperature 120 °C, which formed a 1D ladder chain.Interestingly, the decarboxylation of H2qldc was observed in the solvothermal synthetic process of Ag2 and Cu1. In order to study the decarboxylation process of H2qldc, a series of control experiments were conducted. We did the experiment under the condition of without adding metal salts, only ligand H2qldc in 8 mL H2O was placed in a Teflon-lined stainless steel vessel (20 mL) under autogenous pressure and heated at 120 °C for 5 days. We found that the decarboxylation of free H2qldc was performed under solvothermal conditions at 120 °C (without any other substance). The 13C NMR spectra of the reaction product as well as H2qldc are shown in Fig. S1 and S2 in the ESI,† in which the characteristic peaks of –COOH appeared at 167.99 and 166.22 ppm, but for the reaction product only one characteristic peak of –COOH appeared at 166.30 ppm. As aforementioned reaction fact, coordination polymer Ag1 can be obtained at 80 °C. Based on these facts, we can deduce that, the coordination between the ligand and the metal center may be occurred before the decarboxylation of H2qldc in Ag1. Meanwhile, when Ag1 assembling, the decarboxylation is too difficult to perform. As shown in TG curve, until 452.2 °C, Ag1 begins to lose the two carboxylates. Compared with Ag1, coordination polymer Ag2 was not obtained at 80 °C (other conditions remain unchanged except for the reaction temperature). The phenomenon can be explained as: in Ag2, the coordination reaction may be occurred after the decarboxylation process. The aryl group in H2qldc ligand as electron attracting group is beneficial to the cleavage of C–C bond. The reaction mechanism of Ag1 and Ag2 are shown in Scheme 1.
X-ray crystal structure determination
The X-ray diffraction data taken at room temperature for coordination polymers Ag1 and Cu1 are collected on a Rigaku R-AXIS RAPID IP diffractometer equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The structures of Ag1 and Cu1 are solved by direct methods and refined on F2 by the full-matrix least squares using the SHELXTL-97 crystallographic software.18 Anisotropic thermal parameters are refined to all of the non-hydrogen atoms. The hydrogen atoms are held in calculated positions on carbon atoms and nitrogen atoms and that are directly included in the molecular formula on water molecules. ESI.† Crystal structure data and details of the data collection and the structure refinement are listed as Table 1. Selected bond lengths and bond angles for complexes are summarized in Table 2.| Identification code | Ag1 | Cu1 |
|---|---|---|
| a R1 = ∑||Fo| − |Fc||/∑|Fo.b wR2 = [∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]]1/2. | ||
| Empirical formula | C11H6AgNO4 | C10H7CuINO2 |
| Formula mass | 324.04 | 363.61 |
| Crystal system | Triclinic | Monoclinic |
| Space group | p![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P2(1)/n |
| a (Å) | 6.6702(13) | 4.2490(8) |
| b (Å) | 8.7729(18) | 15.962(3) |
| c (Å) | 9.1647(18) | 15.297(3) |
| α (°) | 78.49(3) | 90.00 |
| β (°) | 72.67(3) | 90.03(3) |
| γ (°) | 68.92(3) | 90.00 |
| V (Å3) | 475.13(2) | 1037.5(4) |
| Z | 2 | 4 |
| Dc/(g cm−3) | 2.265 | 2.328 |
| μ (Mo Kα)/mm−1 | 2.121 | 5.056 |
| F(000) | 316 | 688 |
| Crystal size | 0.20 × 0.18 × 0.15 mm | 0.14 × 0.14 × 0.12 mm |
| 2θ range (°) | 3.24–27.48 | 3.69–27.46 |
| Limiting indices | −8 ≤ h ≤ 8 | −5 ≤ h ≤ 5 |
| −11 ≤ k ≤ 11 | −20 ≤ k ≤ 20 | |
| −11 ≤ l ≤ 11 | −19 ≤ l ≤ 19 | |
| Data/restraints/parameters | 2168/0/155 | 2368/0/136 |
| GOF on F2 | 0.983 | 1.267 |
| Final R indices [I > 2σ(I)] | ||
| R1a | 0.0324 | 0.0814 |
| wR2b | 0.0937 | 0.2183 |
| R indices (all data) | ||
| R1 | 0.0404 | 0.0842 |
| wR2 | 0.0995 | 0.2192 |
| CCDC | 1036463 | 1036466 |
| a Symmetry transformations used to generate equivalent atoms: #1: −x, −y + 2, −z; #2: −x, −y + 1, −z + 1. | |||
|---|---|---|---|
| Ag1 | |||
| Ag(1)–N(1) | 2.261(3) | N(1)–Ag(1)–O(1)#2 | 128.8(1) |
| Ag(1)–O(4)#3 | 2.390(2) | O(1)#2–Ag(1)–O(4)#3 | 105.4(1) |
| Ag(1)–O(1)#2 | 2.295(3) | N(1)–Ag(1)–O(4)#3 | 120.8(0) |
| Cu1 | |||
| I(1)–Cu(1) | 2.645(2) | Cu(1)–I(1)–Cu(1)#1 | 80.4(1) |
| I(1)–Cu(1)#1 | 2.688(2) | Cu(1)–I(1)–Cu(1)#2 | 61.3(3) |
| I(1)–Cu(1)#2 | 2.694(3) | Cu(1)#1–I(1)–Cu(1)#2 | 104.3(1) |
| Cu(1)–N(1) | 2.051(1) | N(1)–Cu(1)–I(1) | 122.3(4) |
| Cu(1)–I(1)#1 | 2.688(2) | N(1)–Cu(1)–I(1)#1 | 109.8(4) |
| Cu(1)–I(1)#2 | 2.694(3) | I(1)–Cu(1)–I(1)#1 | 99.6(1) |
| Cu(1)–Cu(1)#2 | 2.723(4) | N(1)–Cu(1)–I(1)#2 | 100.9(4) |
| I(1)–Cu(1)–I(1)#2 | 118.7(2) | ||
| I(1)#1–Cu(1)–I(1)#2 | 104.3(1) | ||
| N(1)–Cu(1)–Cu(1)#2 | 134.9(4) | ||
| I(1)–Cu(1)–Cu(1)#2 | 60.2(3) | ||
| I(1)#1–Cu(1)–Cu(1)#2 | 114.0(4) | ||
| I(1)#2–Cu(1)–Cu(1)#2 | 58.5(5) | ||
Results and discussion
IR spectroscopy
The IR spectra of coordination polymers Ag1, Ag2, Cu1 and the free ligand 2,3-H2qldc are shown in Fig. S3.† The asymmetric stretching vibrations of the carboxyl group in Ag1 and Ag2 are observed at ca. 1590 cm−1 and the band of symmetric stretching vibrations are observed at ca. 1394 cm−1. The frequent separation between the asymmetric and symmetric stretching of carboxyl group can be mode to distinction between these binding states. The deviation Δ(vas(COO−) − vs(COO−)) was approximately 200 cm−1, in the range of 100 cm−1 to 200 cm−1, which indicates that the coordination interaction exists between the metal ion and the oxygen atoms of carboxyl group. Therefore, the stretching vibrations of the carboxyl group shift toward lower frequencies and the intensity is greatly reduced compared to that of free ligand (1700 cm−1). The absence of strong bands ranging from 1579 to 1590 cm−1 for coordination polymers Ag1 and Ag2 indicate that the ligand in these coordination polymers are deprotonated, compared with those of free ligands in these complexes. Coordination polymer Cu1 possesses identical ligand 2,3-H2qldc and their IR spectra are similar. The asymmetric stretching vibration of the carboxyl group is observed at ca. 1700 cm−1, and the band of symmetric stretching vibration is observed at ca. 1285 cm−1, as the same as ligand 2,3-H2qldc, which indicates that the oxygen atoms of carboxyl group does not coordinate to the metal ion. Weak bands in the region of 476–597 cm−1 are observed in the spectra of coordination polymers, which are assigned to ν(M–N) and ν(M–O) stretching vibrations.19 These are in agreement with the results of single-crystal X-ray diffraction analysis.Description of crystal structure
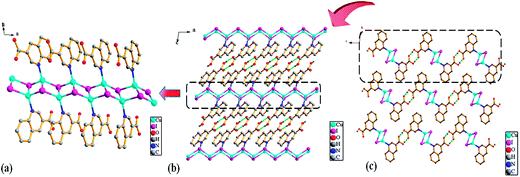
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The asymmetric unit of Ag1 and Ag2 are shown in Fig. 1. The asymmetric unit of Ag1 contains one Ag(I) cation, one 2,3-Hqldc− anion. Ag(I) center is three-coordinated by one nitrogen atom (N1) from one 2,3-Hqldc− anion and two oxygen atoms (O1A and O4A) from two other 2,3-Hqldc− anions, forming a plane triangle. Ag–N bond length is 2.261(3) Å, Ag–O bond lengths are 2.295(3) and 2.390(2) Å respectively. All Ag–N and Ag–O bond lengths as well as the bond angles around Ag(I) ion are in the range expected for such coordination polymers.1b Two adjacent silver ions are connected through N1 and O4 atoms deriving from one 2,3-Hqldc− anion, giving rise to an infinite 1D zigzag chain. What's more, two adjacent 1D zigzag chains are linked by bridging O atom, forming a new 1D + 1D → 1D chain with a Ag⋯Ag separation of 4.168 Å (Fig. 2). It is noteworthy that, in the Ag1 crystal structure, it exits weak intermolecular forces including aromatic rings. Further analysis of the crystal packing reveals that adjacent 1D chains are further connected by face-to-face π⋯π interactions between quinoline ring with a centroid to centroid distance of 3.697 Å (Fig. 3a) and 3.785 Å (Fig. 3b). They play an important role in stabilizing the network structure, and further resulting in a 3D supramolecular architecture.
. The asymmetric unit of Ag1 and Ag2 are shown in Fig. 1. The asymmetric unit of Ag1 contains one Ag(I) cation, one 2,3-Hqldc− anion. Ag(I) center is three-coordinated by one nitrogen atom (N1) from one 2,3-Hqldc− anion and two oxygen atoms (O1A and O4A) from two other 2,3-Hqldc− anions, forming a plane triangle. Ag–N bond length is 2.261(3) Å, Ag–O bond lengths are 2.295(3) and 2.390(2) Å respectively. All Ag–N and Ag–O bond lengths as well as the bond angles around Ag(I) ion are in the range expected for such coordination polymers.1b Two adjacent silver ions are connected through N1 and O4 atoms deriving from one 2,3-Hqldc− anion, giving rise to an infinite 1D zigzag chain. What's more, two adjacent 1D zigzag chains are linked by bridging O atom, forming a new 1D + 1D → 1D chain with a Ag⋯Ag separation of 4.168 Å (Fig. 2). It is noteworthy that, in the Ag1 crystal structure, it exits weak intermolecular forces including aromatic rings. Further analysis of the crystal packing reveals that adjacent 1D chains are further connected by face-to-face π⋯π interactions between quinoline ring with a centroid to centroid distance of 3.697 Å (Fig. 3a) and 3.785 Å (Fig. 3b). They play an important role in stabilizing the network structure, and further resulting in a 3D supramolecular architecture.
 | ||
| Fig. 1 The structural unit of Ag1 (a) and Ag2 (b) with labeling scheme and 50% thermal ellipsoids (hydrogen atoms are omitted for clarity). | ||
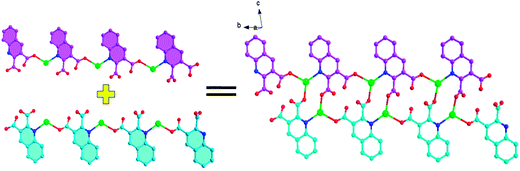 | ||
| Fig. 2 Ball-and-stick representation of the 1D chain structure and the 1D + 1D → 1D chain structure in Ag1. | ||
Notably, though the chemical environments of the central Ag cations are the same in Ag1 and Ag2, the major difference is the dihedral angle between the carboxyl and quinoline in one bridging organic ligand. The dihedral angle between the two planes in one bridging ligand is 3.70° in the Ag2, which is much smaller than those of 61.98° and 30.49° in Ag1. Hence, Ag2 overcomes the shortcoming of the coplanarity, which is constructed by carboxyl and quinoline ring and shows fantastic coplanar structure.
Photoluminescent properties and lifetimes of coordination polymers
The emission spectra of Ag1, Ag2 and Cu1 are recorded in different solvents and in the solid state at 298 K and 77 K. The photoluminescent data for emission are gathered in Table 3. To investigate the details of surrounding environment effects, the emission spectra of Ag1, Ag2 and Cu1 in solutions (DMSO, CH3CN and CH3OH) with the same concentration (1.0 × 10−5 mol L−1) were examined by luminescence spectrophotometry at 298 K and 77 K. The 1H NMR data indicates that Ag1, Ag2 and Cu1 still keep polymeric structure in solutions and don't decompose (Fig. S6–S8†). As shown in Fig. 6, there are high energy (HE) emission and low energy (LE) emission in Ag1 and Ag2. The corresponding maximum emissions are observed at 427 and 631 nm in DMSO (416 and 624 nm in CH3CN, 404 and 611 nm in CH3OH) for Ag1, and 446 and 609 nm in DMSO (431 and 618 nm in CH3CN, 412 and 628 nm in CH3OH) for Ag2. The luminescence spectra of Ag1 and Ag2 show that the HE peaks probably result from intraligand transitions in H2qldc, and the Ag atoms may play some role in these transitions.22 What's more, the LE emissions should be, without doubt, phosphorescence because of their long wavelengths and long lifetime in microsecond scale.23 In addition, the maximum emission band for coordination polymer Cu1 is located at 602 nm in DMSO (590 nm in CH3CN, 578 nm in CH3OH), upon excitation at 360 nm at 298 K, the red-shifted emission compared with ligand H2qldc can be assigned to metal-to-ligand charge transfer (MLCT), a combination of iodide-to-copper charge transfer (XMCT) and d–s transitions in Cu1 (ref. 24) (Scheme 2). The change of temperature from 298 K to 77 K (ref. 25) caused the red shift of the emission peaks substantially in all the solutions of Ag1, Ag2 and Cu1, the emission peaks show rich structural features at 77 K. Such fascinating phenomenon may be attributed to the different state of solution at the low temperature. At 77 K, the viscosity of the solvent increases, and the interaction between fluorescent substance and solvent, the forces of attraction and hydrogen bonds may be stronger in contrast with those at 298 K. In this case, excited state is more stable, and the energy level difference between ground state and excited state decreases. Accordingly, the luminescent emission peaks of Ag1, Ag2 and Cu1 in solution display red shifts significantly from 298 K to 77 K.| Coordination polymers/ligands | Absorption (nm) (ε/dm3 cm−1 mol−1) | Excitation (λ, nm) | Emission (λmax, nm) | CIE (x, y) | Quantum yields (Φ)b | Lifetimes (μs) | Conditionsa | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| τ1 (μs) | A1% | τ2 (μs) | A2% | 〈τ〉 (μs) | |||||||
| a Concentration in DMSO, CH3CN and CH3OH solutions: (M) = 1 × 10−5 M.b Determined using quinine sulfate in 0.1 M sulfuric acid (Φ = 0.546) for Ag1, Ag2 and rhodamine B chloride in ethanol (Φ = 0.690) for Cu1 as a standard. | |||||||||||
| Ag1 | 267 (79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 236) 236) |
300 | 427, 631 | 0.17, 0.12 | 0.185 | 1.72 | 46.39 | 13.71 | 53.61 | 12.54 | DMSO, 298 K |
227 (139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893) 893) |
300 | 416, 624 | 0.16, 0.05 | 0.143 | 1.77 | 45.68 | 13.49 | 54.32 | 12.33 | CH3CN, 298 K | |
222 (84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282) 282) |
300 | 404, 611 | 0.15, 0.06 | 0.092 | 0.91 | 6.37 | 7.29 | 55.40 | 7.20 | CH3OH, 298 K | |
| — | 300 | 455 | 0.23, 0.29 | — | 0.89 | 54.51 | 7.08 | 45.49 | 6.27 | Solid, 298 K | |
| — | 300 | 453, 639 | 0.18, 0.22 | — | 1.37 | 51.73 | 18.03 | 48.27 | 16.78 | DMSO, 77 K | |
| — | 300 | 434, 626 | 0.16, 0.16 | — | 2.04 | 43.31 | 15.55 | 56.69 | 14.32 | CH3CN, 77 K | |
| — | 300 | 425, 614 | 0.15, 0.15 | — | 1.12 | 40.59 | 13.02 | 59.41 | 12.36 | CH3OH, 77 K | |
| — | 300 | 476 | 0.23, 0.30 | — | 1.81 | 54.10 | 18.10 | 45.90 | 16.38 | Solid, 77 K | |
| Ag2 | 276 (63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
300 | 446, 609 | 0.23, 0.14 | 0.254 | 1.16 | 49.43 | 11.29 | 50.57 | 10.37 | DMSO, 298 K |
222 (54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294) 294) |
300 | 431, 618 | 0.17, 0.06 | 0.212 | 1.44 | 64.73 | 10.99 | 35.27 | 9.14 | CH3CN, 298 K | |
215 (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294) 294) |
300 | 412, 628 | 0.18, 0.17 | 0.126 | 1.12 | 56.03 | 10.03 | 43.97 | 8.92 | CH3OH, 298 K | |
| — | 300 | 446 | 0.23, 0.27 | — | 1.20 | 50.40 | 8.33 | 49.60 | 7.42 | Solid, 298 K | |
| — | 300 | 471, 632 | 0.18, 0.26 | — | 1.29 | 48.60 | 13.03 | 51.40 | 12.03 | DMSO, 77 K | |
| — | 300 | 452, 628 | 0.15, 0.21 | — | 1.09 | 57.14 | 12.37 | 42.86 | 11.18 | CH3CN, 77 K | |
| — | 300 | 445, 631 | 0.17, 0.21 | — | 1.25 | 56.00 | 10.89 | 44.00 | 9.66 | CH3OH, 77 K | |
| — | 300 | 479 | 0.24, 0.33 | — | 1.74 | 43.02 | 19.02 | 56.98 | 17.90 | Solid, 77 K | |
| Cu1 | 297 (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669) 669) |
360 | 602 | 0.61, 0.39 | 0.283 | 1.40 | 50.24 | 16.07 | 49.76 | 14.88 | DMSO, 298 K |
276 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 888) 888) |
360 | 590 | 0.58, 0.41 | 0.224 | 1.71 | 47.03 | 13.17 | 52.97 | 11.99 | CH3CN, 298 K | |
274 (76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 551) 551) |
360 | 578 | 0.50, 0.49 | 0.151 | 1.09 | 44.33 | 11.47 | 55.67 | 10.74 | CH3OH, 298 K | |
| — | 360 | 576 | 0.51, 0.48 | — | 1.98 | 40.38 | 14.85 | 59.62 | 13.78 | Solid, 298 K | |
| — | 360 | 627 | 0.65, 0.34 | — | 1.84 | 52.33 | 28.11 | 47.67 | 26.35 | DMSO, 77 K | |
| — | 360 | 612 | 0.57, 0.43 | — | 1.22 | 46.15 | 17.14 | 53.85 | 16.22 | CH3CN, 77 K | |
| — | 360 | 606 | 0.57, 0.43 | — | 1.12 | 51.29 | 13.00 | 48.71 | 12.01 | CH3OH, 77 K | |
| — | 360 | 646 | 0.67, 0.30 | — | 2.58 | 46.03 | 22.50 | 53.97 | 1.847 | Solid, 77 K | |
| H2qldc | 274 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939) 939) |
300 | 434 | 0.20, 0.20 | 0.016 | — | — | — | — | — | DMSO, 298 K |
237 (71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
300 | 419 | 0.16, 0.06 | 0.012 | — | — | — | — | — | CH3CN, 298 K | |
235 (101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444) 444) |
300 | 406 | 0.18, 0.12 | 0.008 | — | — | — | — | — | CH3OH, 298 K | |
| — | 300 | 398 | 0.20, 0.19 | — | — | — | — | — | — | Solid, 298 K | |
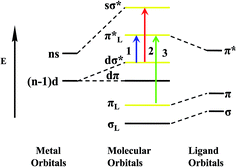 | ||
| Scheme 2 Schematic energy level diagram of coordination polymers Ag1, Ag2 and Cu1 molecular orbitals ((1) MLCT; (2) d–s transitions, (3) intraligand transitions). | ||
The luminescence quantum yields of coordination polymers Ag1, Ag2, Cu1 and H2qldc are determined in DMSO, CH3CN, and CH3OH. The value of the quantum yields for H2qldc in DMSO, CH3CN and CH3OH are 0.016, 0.012 and 0.008, respectively, while those of Ag1 are 0.185, 0.143 and 0.092 (0.254, 0.212 and 0.126 for Ag2, 0.283, 0.224 and 0.151 for Cu1). The quantum yields of Ag1, Ag2 and Cu1 are much higher than that of the free ligand, which can be easily explained by aggregation of the pure ligands in the solvents, which would also be in accordance with their low quantum yield. Complexation with the silver or copper might make aggregation and π-stacking more difficult than for the pure ligands and when coordination polymers assemble, the enhancement after the connection of the ligand to metal center increases the conformational rigidity of the ligands, and the loss of energy via thermal vibration decay may be reduced. Compared with coordination polymers Ag1, Ag2 and Cu1, the luminescence quantum yields of Ag2 and Cu1 is significantly higher than Ag1, which is attributed to the decarboxylation effect. After decarboxylation making the dihedral angle of carboxyl and quinoline decreases and Ag1 has two dihedral angles are 61.98° and 30.49° respectively. However, dihedral angle of Ag2 is 3.70° and Cu1 is 5.47°. The nice coplanar feature of the structure in Ag2 and Cu1 can enhance the mobility of π electrons in organic aromatic rings, which is in favor of luminescent emission.
In the solid state, the maximum emission band for coordination polymer Ag1 is located at 455 nm (446 nm for Ag2), upon excitation at 300 nm, exhibiting blue emissions at 298 K. The solid-state fluorescence spectra of Ag1 and Ag2 exhibit strong red-shifted photoluminescence emission in comparison with that of free H2qldc ligand (λem = 398 nm). It is noted that there is no emission band for H2qldc ligand can be observed in the region, the photoluminescence origin of the emission bands for the coordination polymers Ag1 and Ag2 should be attributed to MLCT22b,26 (Scheme 2). At 77 K, the emission band for coordination polymer Ag1 is located at 476 nm and Ag2 is located at 479 nm (λex = 300 nm), exhibiting blue emissions in the solid state (Fig. 7), which shows that Ag(I) coordination polymers can be used as stable blue materials. The change of temperature from 298 K to 77 K causes a bathochromic shift of emission peaks in the solid state of Ag1 and Ag2. Obviously, the red shift of coordination polymer Ag2 is longer than Ag1, which due to the existence of Ag–Ag interaction in Ag2. Meanwhile, one of carboxyl removed, making the nice coplanar feature of the structure in Ag2 and improve the mobility of π electrons in organic aromatic rings, so that it shows the excellent luminescence. Ag2 shows stable luminescence at 298 K and 77 K in the solid state with the CIE color shifts from deep-blue (0.23, 0.27) to light blue (0.24, 0.33). Both Ag1 and Ag2 display stable blue luminescent in the solid state at 298 K and 77 K. The luminescent lifetimes for Ag1 and Ag2 in the solid state are determined. The luminescent lifetimes for Ag1 at 298 K and 77 K are 6.27 μs and 16.38 μs, respectively, while those of Ag2 are 7.42 μs and 17.90 μs. The luminescent lifetimes of Ag1 and Ag2 at 77 K are more than twice as those at 298 K in the solid state. A general trend is that the lifetime of emission of low-temperature (77 K) is longer than that in room temperature (298 K), which is attributed to the decrease of thermal vibration and nonradiative transition at low temperature.27
 | ||
| Fig. 7 Normalized emission spectra of coordination polymers Ag1, Ag2 and Cu1 in the solid state at 298 K and 77 K and the corresponding color coordinate diagram of emission. | ||
Coordination polymer Cu1 is different from Ag1 and Ag2 in the solid state, under UV irradiation, at room temperature, Cu1 emits an intense yellow light. The fascinating and visually impressive phenomenon of thermochromic luminescence of Cu1 is revealed by immersing the samples into liquid nitrogen (77 K). When exposed to UV light, the crystalline solid shows a color changing process from bright yellow luminescence at room temperature to red luminescence at low temperature of liquid nitrogen. Once the samples gradually warmed up to room temperature, the yellow emission is recovered, indicating a reversible thermochromic luminescence for Cu1. The variable temperature luminescence spectrum is shown in Fig. 7. Cu1 shows tunable luminescence at 298 K and 77 K in the solid state. The emission maximum shifts from 576 nm to 646 nm in the solid state luminescence spectrum when the temperature is decreased from 298 to 77 K. The red shift of 70 nm for Cu1 is large enough to make the color change visible by the naked eye and the CIE color shifts from bright yellow (0.51, 0.48) to red (0.67, 0.30). Their emission bands could be attributed to triplet cluster-centered (3CC) excited states, a combination of XMCT and d–s transitions21 (Scheme 2). The solid state lifetimes at room temperature is on the scale of microseconds (13.78 and 20.73 μs at 298 K and 77 K respectively), suggestive of their phosphorescent character. The long decay lifetime displayed by the Cu(I) coordination polymer, characteristic of triplet state emission.
Thermal analysis and XRPD patterns of Ag1, Ag2 and Cu1
Thermogravimetric experiments were conducted to study the thermal stability of coordination polymers Ag1, Ag2 and Cu1 (Fig. S9†). The experiments were performed on samples consisting of numerous single crystals in the 25–700 °C range. The TG curve shows that Ag1 has two weight-loss stages. The loss of 53.49% occurring at the first step is attributed to decomposition of 3-Hqlc in the range of 238.1–251.8 °C (calculated 53.44%). The second weight-loss of 13.22% at 452.2–556 °C can be ascribe to loss the 2-position carboxyl (calculated 13.58%). The TG curve of Ag2 shows the coordination polymer is very steady and there is no weight loss until 245.8 °C, the first weight-loss of 8.01% which corresponding to loss the 3-position carboxyl (calculated 7.86%) and the following weight-loss of 53.57% during 338.2–367.2 °C, which is attributed to the decomposition of the 3-qlc ligands (calculated 53.62%). The remaining weight of 33.29% for Ag1 and 38.42% for Ag2, corresponds to the percentage (calculated 32.98% and 38.52%) of the Ag components, indicating that the final product is metal Ag. Through analyzing the TG curves of Ag1 and Ag2, we deduce that once 2-position carboxyl coordinates to Ag atom, the sample is very steady and it begins to lose the weight until 452.2 °C in Ag1. In the TG curve of Cu1, there are two weight-loss steps. The loss 45.74% occurring at the first step is attributed to the sublimation of I2 and lose the weight of 3-position carboxyl in the range of 237.8–320.4 °C (calculated 45.29%). The second weight-loss of 31.63% at 320.4–402.9 °C corresponds to the decomposition of quinoline (calculated 33.76%). The remaining weight of 22.63% for Cu1, the observed weight loss of 22.63% is in good agreement with the calculated value (20.95%), indicating that the final product is CuO.The XRPD patterns for coordination polymers Ag1, Ag2 and Cu1 are shown in Fig. S10.† The diffraction peaks of both simulated and experimental patterns match well in key positions, indicating thus the phase purities of coordination polymers Ag1, Ag2 and Cu1.
Properties of PMMA polymer doped with coordination polymer Cu1
TG analysis of Cu1@PMMA film exhibits no weight-loss in the temperature range of 25–285 °C in contrast to that for coordination polymer Cu1 (Fig. 8). Furthermore, TG analysis of Cu1@PMMA film shows a slight increase of 48 °C in comparison with the pure PMMA, which suggests that the thermal stability of the Cu1@PMMA film is essentially improved by doping coordination polymer Cu1. The IR spectra of Cu1, PMMA and Cu1@PMMA are shown in Fig. S11.†Based on the excellent luminescence of Cu(I) coordination polymers, making it incorporated into polymer matrixes represents a new class of materials. The materials can serve as ideal candidates in the pursuit of application in farm plastic-film with optical transfer function.28 As an extension of this work, we describe the incorporation of the newly designed, and intensity luminescent Cu1 into PMMA, a low-cost and easily prepared polymer with excellent optical quality. The excitation spectrum of the PMMA polymer films doped with Cu1 at different concentrations [0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4% (w/w)] is shown in Table 4. The emission spectrum of PMMA doped with coordination polymer Cu1 is at 578 nm (λex = 360 nm). The lifetimes for coordination polymer Cu1 and the PMMA matrixes doped with coordination polymer Cu1 are listed in Table 4. Noticeably, with the increase of the content of coordination polymer Cu1, the lifetime of the Cu1@PMMA films increases and reaches a maximum at 1.0% and then decreases with further increasing of the content of coordination polymer Cu1 (Fig. 9). It can be attributed to the fact that with a low concentration of coordination polymer Cu1 in the PMMA polymer, the coordination polymer Cu1 can disperse uniformly in the PMMA matrix and the PMMA effectively sensitizes the luminescence of the coordination polymer Cu1. Upon further increasing of the content of coordination polymer Cu1 to more than 1.0%, some aggregates formed in the film and the excition migration between the Cu1 resulted in the luminescence quenching of coordination polymer Cu1.29 Noticeably, with the increasing of the content of Cu1, the lifetime of Cu1@PMMA films reaches a maximum at 1.0% (τ = 95.57 μs), which is more than eight times longer than that of Cu1 (τ = 13.78 μs). All τ values for the doped polymer systems are higher than coordination polymer Cu1, indicating that radiative processes are operative in all the doped polymer films. Cu1@PMMA is confirmed as a yellow luminescence polymer film material.
| Excitation (λ, nm) | Emission (λmax, nm) | Integrated area | Lifetimes (μs) | |||||
|---|---|---|---|---|---|---|---|---|
| τ1 (μs) | A1% | τ2 (μs) | A2% | 〈τ〉 (μs) | ||||
| Cu1@0.2% PMMA | 360 | 578 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862.07 862.07 |
1.17 | 8.66 | 35.86 | 91.34 | 37.75 |
| Cu1@0.4% PMMA | 360 | 578 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384.10 384.10 |
1.41 | 8.75 | 46.05 | 91.25 | 45.92 |
| Cu1@0.6% PMMA | 360 | 578 | 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 371.01 371.01 |
1.18 | 6.44 | 50.26 | 93.56 | 50.18 |
| Cu1@0.8% PMMA | 360 | 578 | 229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 682.46 682.46 |
1.01 | 4.25 | 83.43 | 95.75 | 83.39 |
| Cu1@1.0% PMMA | 360 | 578 | 334![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 392.39 392.39 |
2.08 | 2.93 | 95.63 | 97.07 | 95.57 |
| Cu1@1.2% PMMA | 360 | 578 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 764.47 764.47 |
1.13 | 9.42 | 47.14 | 90.58 | 47.03 |
| Cu1@1.4% PMMA | 360 | 578 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401.74 401.74 |
1.03 | 8.57 | 43.69 | 91.43 | 43.60 |
 | ||
| Fig. 9 The emission spectra of PMMA polymer doped with coordination polymer Cu1 in 0.2–1.4% at 298 K under excitation at 360 nm. | ||
Conclusion
In summary, three 1D coordination polymers [Ag(2,3-Hqldc)]n (Ag1), [Ag(3-qlc)]2n (Ag2) and [CuI(3-Hqlc)]n (Cu1) were successfully synthesized under hydro(solvo) thermal methods based on quinoline-2,3-dicarboxylic acid (H2qldc). Through controlling the temperature makes the ligand H2qldc decarboxylation and results in the formation of 3-Hqlc ligand. After decarboxylation, the nice coplanar feature of the structure in Ag2 can enhance the luminescent efficiency. Both Ag1 and Ag2 display stable blue luminescent in the solid state and in solvents (DMSO, CH3CN and CH3OH) at 298 K and 77 K. The luminescent lifetimes of Ag1 and Ag2 at 77 K are longer than at 298 K both in the solid state and in solvents. The quantum yields of Ag1 and Ag2 are much higher than those of free ligand. The superior photoluminescent properties make coordination polymers Ag1 and Ag2 promising materials for the development of optical devices. While Cu1 shows tunable luminescence by changing the temperature from 298 K to 77 K in the solid state, the luminescence variation from bright yellow to red indicating thermochromic luminescence for Cu1. In extending work, Cu1 is doped with PMMA matrix to obtain PMMA-supported doped polymer film materials, which displays excellent yellow luminescent properties with enhanced luminescent intensities, long lifetimes and thermal stability, thus it can be used in the pursuit of application in farm plastic-film with optical transfer function.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 21371040 and 21171044), the National key Basic Research Program of China (973 Program, no. 2013CB632900), and the Fundamental Research Funds for the Central Universities (Grant no. HIT. IBRSEM. A. 201409), also Program for Innovation Research of Science in Harbin Institute of Technology (PIRS of HIT no. A201416 and B201414).References
- (a) Y. Y. Tang, C. X. Ding, S. W. Ng and Y. S. Xie, RSC Adv., 2013, 3, 18134 RSC; (b) B. Li, R. W. Huang, H. C. Yao, S. Q. Zang and T. C. W. Mak, CrystEngComm, 2014, 16, 723 RSC.
- I. Takashima, A. Kanegae, M. Sugimoto and A. Ojida, Inorg. Chem., 2014, 53, 7080 CrossRef CAS PubMed.
- S. Y. Liu, X. L. Qi, R. B. Lin, X. N. Cheng, P. Q. Liao, J. P. Zhang and X. M. Chen, Adv. Funct. Mater., 2014, 24, 5866 CrossRef CAS.
- K. Kuriki and Y. Koike, Chem. Rev., 2002, 102, 2347 CrossRef CAS PubMed.
- L. Shi, Z. Liu, G. Dong, L. Duan, Y. Qiu, J. Jia, W. Guo, D. Zhao, D. Cui and X. Tao, Chem.–Eur. J., 2012, 18, 8092 CrossRef CAS PubMed.
- Q. Zhu, C. Shen, C. Tan, T. Sheng, S. Hu and X. Wu, Chem. Commun., 2012, 48, 531 RSC.
- (a) S. Q. Liu, H. Konaka, Y. Suenaga, M. Maekawa, T. Mizutani, G. L. Ning and M. Munakata, Inorg. Chem., 2005, 44, 1031 CrossRef CAS; (b) Y. B. Dong, J. P. Ma and R. Q. Huang, Inorg. Chem., 2006, 45, 3325 CrossRef CAS PubMed.
- W. Q. Fan, J. Feng, S. Y. Song, Y. Q. Lei, G. L. Zheng and H. J. Zhang, Chem.–Eur. J., 2010, 16, 1903 CrossRef CAS PubMed.
- (a) W. Li, P. Yan, G. Hou, H. Li and G. Li, Dalton Trans., 2013, 42, 11537 RSC; (b) H. G. Liu, S. Park, K. Jang, X. S. Feng, C. Kim, H. J. Seo and Y. I. Lee, J. Lumin., 2004, 106, 47 CrossRef CAS.
- D. G. Cuttell, P. E. Fanwick, D. R. McMillin and R. A. Walton, J. Am. Chem. Soc., 2002, 124, 6 CrossRef CAS PubMed.
- W. L. Jia, Y. Tao, J. P. Lu and S. N. Wang, Inorg. Chem., 2005, 44, 5706 CrossRef CAS PubMed.
- (a) Z. M. Hudson, C. Sun, M. G. Helander, Y. L. Chang, Z. H. Lu and S. Wang, J. Am. Chem. Soc., 2012, 134, 13930 CrossRef CAS PubMed; (b) P. Myllymaki, M. Roeckerath, J. M. Lopes, J. Schubert, K. Mizohata, M. Putkonen and L. Niinisto, J. Mater. Chem., 2010, 20, 4207 RSC.
- (a) M. F. Wang, X. J. Hong, Q. G. Zhan, H. G. Jin, Y. T. Liu, Z. P. Zheng, S. H. Xu and Y. P. Cai, Dalton Trans., 2012, 41, 11898 RSC; (b) X. J. Hong, M. F. Wang, H. Y. Jia, W. X. Li, J. Li, Y. T. Liu, H. G. Jin and Y. P. Cai, New J. Chem., 2013, 37, 933 RSC.
- Y. Gong, M. M. Zhang, J. B. Qin, J. Li, J. P. Meng and J. H. Lin, Dalton Trans., 2014, 43, 8454 RSC.
- C. B. Liu, Q. Li, X. Wang, G. B. Che and X. J. Zhang, Inorg. Chem. Commun., 2014, 39, 56 CrossRef CAS PubMed.
- C. B. Liu, Y. Cong and H. Y. Sun, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, m1177 CAS.
- L. Li, J. Luo, S. Wang, Z. Sun, T. Chen and M. Hong, Cryst. Growth Des., 2011, 11, 3744 CAS.
- (a) G. M. Sheldrick, SHELXL 97 Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, SHELXL 97 Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- G. C. Xu, L. Zhang, Y. H. Zhang, J. X. Guo, M. Q. Shi and D. Z. Jia, CrystEngComm, 2013, 15, 2873 RSC.
- B. Liu, S. F. Pan and W. Z. Chen, Inorg. Chem., 2014, 53, 10485 CrossRef CAS PubMed.
- D. Sun, S. Yuan, H. Wang, H. F. Lu, S. Y. Feng and D. F. Sun, Chem. Commun., 2013, 49, 6152 RSC.
- (a) J. Zheng, Y. D. Yu, F. F. Liu, B. Y. Liu, G. Wei and X. C. Huang, Chem. Commun., 2014, 50, 9000 RSC; (b) K. K. Bisht, A. C. Kathalikkattil and E. Suresh, RSC Adv., 2012, 2, 8421 RSC.
- J. P. Zhang, X. C. Huang and X. M. Chen, J. Am. Chem. Soc., 2005, 127, 5495 CrossRef CAS PubMed.
- (a) D. Braga, L. Maini, P. P. Mazzeo and B. Ventura, Chem.–Eur. J., 2010, 16, 1553 CrossRef CAS PubMed; (b) J. Song, Y. Hou, L. Zhang and Y. Fu, CrystEngComm, 2011, 13, 3750 RSC; (c) Z. L. Fang, X. Y. Wu, R. M. Yu and C. Z. Lu, CrystEngComm, 2014, 16, 8769 RSC.
- (a) E. C. Constable, M. Neuburger, P. Rosel, G. E. Schneider, J. A. Zampese, C. E. Housecroft, F. Monti, N. Armaroli, R. D. Costa and E. Orti, Inorg. Chem., 2013, 52, 885 CrossRef CAS PubMed; (b) P. Ivanov, S. Stanimirov, S. Kaloyanova and I. Petkov, J. Fluoresc., 2012, 22, 1501 CrossRef CAS PubMed.
- Y. Y. Liu, Y. Y. Xu, Y. Y. Wang, J. Z. Huo, B. Ding, Y. Wang and X. G. Wang, Z. Anorg. Allg. Chem., 2014, 640, 2463 CrossRef CAS.
- A. V. Dijken, D. Vanmaekelbergh and A. Meijerink, J. Phys. Chem. B, 2000, 104, 1715 CrossRef.
- D. B. Raj, B. Francis, M. L. Reddy, R. R. Butorac, V. M. Lynch and A. H. Cowley, Inorg. Chem., 2010, 49, 9055 CrossRef CAS PubMed.
- W. Li, P. Yan, G. Hou, H. Li and G. Li, RSC Adv., 2013, 3, 18173 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1036463 and 1036466. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra16863a |
| This journal is © The Royal Society of Chemistry 2015 |