An Au–Cu bimetal catalyst for acetylene hydrochlorination with renewable γ-Al2O3 as the support†
Jigang Zhao*a,
Junjian Zenga,
Xiaoguang Chenga,
Lei Wangab,
Henghua Yanga and
Benxian Shena
aState Key Laboratory of Chemical Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: zjg@ecust.edu.cn; Fax: +86 21 64252851; Tel: +86 21 64252916
bTianjinDagu Chemical Co., Ltd., 1 Xinghua Road, Tianjin 300455, P. R. China
First published on 21st January 2015
Abstract
The bimetal catalyst gold(III) chloride–copper(II) chloride (AuCl3–CuCl2) was prepared with several different gamma-aluminium oxide (γ-Al2O3) supports and its catalytic properties towards acetylene hydrochlorination were assessed in a fixed-bed reactor. The comparison indicated that one of the catalysts attained the highest activity with an acetylene conversion of 97%, which was far higher than the others. Catalysts were characterized using detailed X-ray diffraction, nitrogen-Brunauer, Emmett and Teller surface area analysis (N2-BET), ammonia temperature-programmed desorption, Fourier-transform infrared spectroscopy and carbon dioxide temperature-programmed desorption analysis. It is proposed that the base site contributed to its high catalyst activity compared with the other catalysts, instead of the acid site or the textural properties on the support, therefore, the activity and the life of the catalysts can be improved significantly by treating the supports with potassium hydroxide. In addition, the results of N2-BET, thermogravimetric analysis and scanning electron microscopy indicated that the catalysts deactivated rapidly because of carbon deposition, and the actual amount of coke deposition was 18.0% after the reaction. AuCl3–CuCl2/γ-Al2O3 was easily regenerated for reuse as a catalyst by burning off in an air atmosphere for 10 min. The activity of the regenerated catalyst nearly reached the level of the fresh catalyst.
1. Introduction
Vinyl chloride monomer (VCM) is mainly used for the synthesis of poly(vinyl chloride), which is widely used in every aspect of life.1 In China, about 70% of the VCM products are produced from acetylene hydrochlorination, which is based on the catalyst mercury(II) chloride (HgCl2) supported on activated carbon (AC).2,3 However, the toxicity and volatility of HgCl2 can cause serious trouble to the environment and human health, which has driven many researchers to study non-mercuric catalytic systems. Nowadays, gold (Au) is regarded as an efficient catalyst metal.4,5 Conte et al.6,7 studied the AU catalyst in detail and found that Au has high activity in the acetylene hydrochlorination reaction. Its initial activity was higher than that for the HgCl2 catalyst. Alloying Au with other base metals is a promising way to improve the catalytic activity and a bimetallic gold–copper (Au–Cu)/AC catalyst showed promising catalytic activity and an acetylene conversion of 99.5% at 200 h.8 Au-based catalysts have drawn many researchers' attention, however, most of them were focused on AC as the support which has low mechanical strength and poor regeneration capacity.9 In normal practice, the Au dosage was more than 1% of the carbon support, which makes the catalyst too expensive for large scale use. This prompted us to consider whether there was a better support for using acetylene hydrochlorination rather than the AC. According to reports, Au-based catalysts have very low activity when using titanium dioxide or silicon dioxide as the support.10In our previous work, it has been demonstrated that the bimetal Au–Cu catalyst has superior activity and longer lifetimes compared with the industrially preferred catalyst, which is based on carbon supported HgCl2.8 Efforts have also been undertaken to explore aluminium oxide (Al2O3) as a non-mercury catalyst support to reduce the Au content for acetylene hydrochlorination.11 In addition, it was found that for the oxygen-containing groups, especially for the hydroxyl groups, the AC played an important role in the high activity and excellent stability of the Au–Cu/AC catalyst.13 Because of the lack of mesopores and macropores in the carbon, the chloroauric acid (HAuCl4) solution with 40–200 nm gold(III) chloride (AuCl3) particles12 cannot diffuse into the inner part effectively, which will inevitably lead to aggregation of the AuCl3 particles on the surface and also in the outer shell of the AC pellets. The fact that Au3+ was absorbed on the mesopores of the AC shows that this is the main site of activity while the micropores can be ignored in order to improve the usage of Au.12 Meanwhile, the discontinuous distribution and disposable activity were its major shortcomings. All the above facts prompted us to consider whether gamma-aluminium oxide (γ-Al2O3), which has a mesoporous (20–50 nm) structure and contains abundant hydroxyl groups on the surface, could be an efficient support for Au-based catalysts in acetylene hydrochlorination. Recently, γ-Al2O3 was used as the support for preparing Au-based catalysts for carbon monoxide oxidation14,15 and hydrodechlorination of carbon tetrachloride.16 However, as far as we are concerned, there is very little literature11,12 reporting Au-based catalysts supported on γ-Al2O3, and there is still much room for improvement.
In this work, the γ-Al2O3 was employed as the support for the gold(III) chloride–copper(II) chloride (AuCl3–CuCl2) catalyst for acetylene hydrochlorination. Because of its mesoporous structure, AuCl3–CuCl2 supported on γ-Al2O3 can effectively reduce the content of the Au component. It was found that some γ-Al2O3 have some special characteristics, and that they can be efficient supports for the catalyst. The possible mechanisms accounting for the enhanced stability and catalytic efficiency are also discussed. The effects of textural properties and acid/base site of the γ-Al2O3 support on the catalyst activity were also studied in detail. In addition, the reasons for deactivation and the regeneration method were also investigated.
2. Experimental section
2.1 Catalyst preparation
Bimetallic Au–Cu/γ-Al2O3 catalysts were prepared using an incipient wetness impregnation technique.8 γ-Al2O3 (A, B, C, D) from different companies (10 g, Hengxin, Yuanheng, Hong Xing and BaoLai Co. Ltd, China) were initially washed with dilute aqueous hydrochloric acid (HCl; 1 mol l−1) at 25 °C for 1 h to remove the impurities on the surface. Then the catalyst was prepared by impregnating the γ-Al2O3 with 2 ml of HAuCl4·4H2O (Au content assay 49.7%) aqua regia solution (1 g HAuCl4·4H2O/100 ml) and 20 ml of CuCl2·2H2O solution (6.25 g CuCl2·2H2O/500 ml), stirred for 3 h at 353 K, then dried at 423 K for 12 h. The catalysts prepared by different supports were donated as ACAlA, ACAlB, ACAlC, ACAlD. Some of the catalysts were named MACAlB, namely B pretreated with potassium hydroxide (KOH) denoted as MB, and the catalysts were prepared based on the process described elsewhere,8 in order to distinguish the effect of basic sites.2.2 Catalyst characterization
Brunauer, Emmett and Teller (BET) surface area analysis and pore size distribution were performed by obtaining nitrogen (N2) adsorption isotherms at 77 K using a ASAP2020 accelerated surface area and porosimetry analyzer (Micromeritics). Temperature programmed analysis including carbon dioxide temperature-programmed desorption (CO2-TPD) and ammonia temperature-programmed desorption (NH3-TPD) was performed using the ASAP 2920, using 10% CO2 (or NH3) in argon (flow 50 ml min−1, holding for 30 min) as the adsorption gas and a temperature ramp from 50 to 700 °C (ramp rate, 10 °C min−1) when adsorbing. The morphology of the samples was examined using scanning electron microscopy (SEM) with a Nova NanoSEM 450 (FEI, The Netherlands). X-ray diffraction (XRD) data were collected using a D8 advanced X-ray diffractometer (Bruker) with Cu-Kα irradiation at 40 kV and 40 mA in the scanning range from 10° to 80°. Hydrogen temperature-programmed desorption (H2-TPR) was performed using the ASAP 2920, using 10% H2 in argon (flow 50 ml min−1) as the reductive gas and a temperature ramp of 50 to 600 °C (ramp rate, 10 °C min−1) with a thermal conductivity detector recording the signal. Fourier-transform infrared (FT-IR) spectra were obtained using a 6700 FT-IR spectrometer (Thermo Fisher Nicolet). XPS data were collected using an Axis Ultra spectrometer (Kratos Analytical) with a monochromatized Al-Kα X-ray source and a minimum energy resolution of 0.48 eV (Ag 3d5/2).2.3 Catalyst testing
The catalytic performance for acetylene hydrochlorination was evaluated in a fixed-bed microreactor (diameter 10 mm) operating at a pressure of 0.1 MPa and a temperature of 150 °C. The reactor was purged with N2 to remove water in the reaction system before the reaction occurred. Hydrogen chloride passed through the reactor at a flow rate of 50 ml min−1 for 2 h to activate the catalyst. After the reactor was heated to 150 °C, acetylene (20 ml min−1) and hydrogen chloride (22 ml min−1) were fed through the heated reactor, which contained 10 ml of catalyst. The reaction product was analyzed by gas chromatography (GC-920, Al2O3 PLOT column). The catalyst activity was determined by the conversion of acetylene (XC2H2) and selectivity of VCM (SVCM), which are defined as:| XC2H2 = (1 − ΦC2H2) × 100% | (1) |
| SVCM = ΦVCM/(1 − ΦC2H2) × 100% | (2) |
Of which ΦC2H2 is the residual volume fraction of acetylene and ΦVCM is the volume fraction of chloroethylene.
3. Results and discussion
3.1 Catalytic activity
Catalysts with different supports were used in a fixed bed reactor to assess their catalytic performance for acetylene hydrochlorination. The XC2H2 and SVCM with reaction time are illustrated in Fig. 1. It shows that all the catalysts had excellent selectivity toward vinyl chloride (C2H3Cl) (Fig. 1a). However, their activities were quite different (Fig. 1b). The catalysts' activity initially exhibited a growing trend, but then decreased after running for 2 h. ACAlD had the best performance among the samples with the highest conversion of 96% and stabilization of about 90%. For comparison, the three other catalysts had conversions from 40% to 80%, which were significantly lower.The ACAlD had the highest C2H2 conversion, which was contrary to its minimum SBET (Table 1). This could not be explained by the traditional viewpoint that the higher the SBET, the better the catalyst activity when using the AC as the support.14 It can be seen that the volume of porosity per unit volume (Vp) and average pore diameter (Dp) values of sample D were higher than those of the others. However, the corresponding values of sample A were higher than those of sample C, and sample A's catalytic activity was lower than that of sample B. Additionally, samples B and C had almost the same textural parameters, however, they had significantly different catalytic activities. These results demonstrate that the textural parameters of the support were not the main reason for the different catalytic activities. It is the surface chemical properties that play an important role in the catalyst system, and this needs to be studied further, in more detail.
| Sample | SBETa (m2 g−1) | Vpb (cm3 g−1) | Dpc (nm) |
|---|---|---|---|
| a BET specific surface area.b Barret–Joyner–Halenda (BJH) volume of pores.c Average pore diameter. | |||
| A | 244.1 | 0.495 | 7.60 |
| B | 295.0 | 0.400 | 6.25 |
| C | 310.4 | 0.406 | 6.55 |
| D | 188.8 | 0.486 | 9.34 |
| Fresh ACAlA | 245.3 | 0.496 | 7.58 |
| Fresh ACAlB | 294.1 | 0.405 | 6.26 |
| Fresh ACAlC | 311.2 | 0.406 | 6.53 |
| Fresh ACAlD | 186.4 | 0.493 | 9.29 |
| Deactivated ACAlD | 179.4 | 0.479 | 8.89 |
Fig. 1c and d show the influence of the Au3+/Cu2+ content on the structured catalysts supported by D on acetylene conversion and C2H3Cl selectivity. The amounts of Au loaded on each were 0.1 wt%, 0.4 wt%, 0.1 wt%, 0.2 wt%, 0.1 wt% and 0 wt% on six corresponding D supports, which were coated with 1.0 wt%, 2.5 wt%, 2.0 wt%, 2.5 wt%, 3.0 wt% and 0 wt% Cu, respectively. As can be seen from the Fig. 1c, the catalytic activity improved significantly, when added and improved Au components in the catalysts, as compared with single-component Cu catalysts, which confirmed that AuCl3 plays an important role in the catalytic performance. Nevertheless, it was found that when both the Au and Cu were added in certain ratios, as shown, the catalyst could achieve more satisfactory conversion and lower Au dosage compared with single component Au catalysts. This result demonstrates that adding CuCl2 could reduce the amount of precious metal Au required significantly, thus reducing the cost of the catalyst.
3.2 Characterization techniques
Because the AC surface is very dense, the lack of mesoporous structure12 means that the HAuCl4 solution cannot enter the inner part of the dense AC pellets. The high density AuCl3 and CuCl2 crystals accumulated on the surface and also in the outer shell of the AC pellets will inevitably aggregate to form large clusters. By contrast, Al2O3 is rather porous,11 leading to good dispersion of the AuCl3 particles, which enhanced the utilization of Au to a certain extent. This result combined with the previously mentioned C2H2 conversion results can be prove efficiently that using Al2O3 as supports for preparing catalysts can reduce the Au content.
The NH3-TPD patterns are shown in Fig. 2e. All the samples had a weak acidic site and samples A, B, and D had similar weak acids with a desorption peak of about 150 °C, while the tail of the desorption peak of sample D was shifted to higher temperatures (about 450 °C). In addition, according to the size of the area under the NH3-TPD profiles, the amount of acidic sites is in the following order: D > C > A > B. Among them, sample C had an obvious feature of medium acidic sites, and a handful of strong acidic sites in sample A and D could be observed. Although some efforts had been made to correlate the C2H2 conversion with the acid strength and amount of acidic sites, just as in the previous method of investigation of the basic sites, it showed that there was no clear trend between the acidic properties of the support and the overall rate of C2H2 conversion.
The influence of the basic site of the catalysts activity using different γ-Al2O3 as the support has been investigated. The H2-TPR profiles of the fresh ACAlA, ACAlB, ACAlC, and ACAlD are shown in Fig. S3(ESI†) and the transmission electron microscopy images of them are shown in Fig. S4(ESI†). They indicate that the base site on the surface of the γ-Al2O3 support has a positive effect on the dispersion of the active ingredient.
3.3 Initial support modification using potassium hydroxide
Cornelius20 reported that support modification using KOH produced O2− or O−, which can provide a Lewis base site on the surface of Al2O3 during the process of dehydration after calcination. It may be that the different oxygen centers of the O2− or O− on the surface of Al2O3 can affect the nucleation process of Au3+ and Cu2+ species adsorbed on the surface, and this could lead to different particle sizes of active ingredient on different supports (Fig. S5 ESI†), however, the mechanism should be studied further.To confirm that the surface base sites of the support play an important role in the excellent performance of alumina, alumina B, which had the worst catalytic performance, was initially modified by KOH. After being cleaned using HCl, alumina B was impregnated for 10 h in 5 mol l−1 KOH solution and then dried. The sample obtained was designated MB. As the previously mentioned steps were done, the MACAlB catalyst was also prepared with MB as the support. The CO2-TPD profile of MB is shown in Fig. S6(ESI†) and it can be seen that an obvious strong basic site has appeared. Compared with ACAlB, the catalytic performance (Fig. 4) of MACAlB had improved significantly and the highest conversion rate was increased from 51% to 82%. To account for the surface groups of the supports, the FT-IR spectra of different samples (C, B, MB, ACAlB, MACAlB) were analyzed. As can be seen from Fig. 5, the obvious bands were observed at 3497.08 cm−1 and 1640 cm−1, which are the hydroxyl absorption peaks of water. Bands at 729.45 cm−1 and 566.09 cm−1 are regarded as the weak absorption peaks of γ-Al2O3. There were two obvious differences in the patterns between the fresh and modified catalysts: characteristic peaks of hydroxyl radicals from Al–OH on the surface of the γ-Al2O3 at 1161 cm−1 and 1437 cm−1 are characteristic absorption peaks of AlO2−.21 It was found that after loading the active metal, the AlO2− peaks became weaker, which indicates that the process of loading caused the electron transformation. Therefore, a strong effect of the surface chemistry of modified Al2O3 on the Au–Cu/Al2O3 catalytic activity was observed, as shown in Fig. 4.
 | ||
Fig. 4 Curves showing the variation of C2H2 conversion and C2H3Cl selectivity with time for MACAlB. Temperature = 150 °C, pressure = 0.1 MPa, V(HCl)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V(C2H2) = 1.1 V(C2H2) = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, GHSV = 120 h−1. 1, GHSV = 120 h−1. | ||
 | ||
| Fig. 5 FT-IR spectra of pure support C, support B, the fresh catalyst ACAlB, the modified catalyst MACAlB and the modified support MB. | ||
In addition, the life span was also extended, showing that the basic sites on the support play an important role in improving the catalyst activity and its life span.
3.4 Deactivation analysis
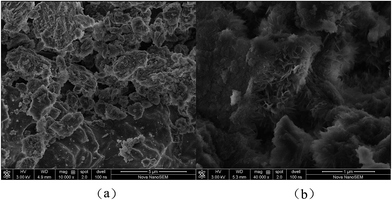
The specific surface area and pore structure parameters of both the fresh ACAlD and the deactivated ACAlD catalyst are shown in Table 1. It can be seen that the parameters of the fresh catalyst are similar to those of the support, which suggests that it did not produce the reunion and bridging phenomenon with small particle size AuCl3 and CuCl2 loaded on the mesoporous pore surface of γ-Al2O3. There was nearly no change in the channel and specific surface area of the fresh catalyst with the support. However, the parameters of the deactivated catalyst were reduced. It seems that there was a phenomenon of channel jam in the catalyst during the reaction.SEM images of the fresh and deactivated catalysts are shown in Fig. 6. It can be clearly seen that channels are relatively abundant in the fresh catalyst (Fig. 6a). However, the surface of the deactivated catalyst was covered with a layer of carbon deposition with a “chip” structure (Fig. 6b). The ‘chip’ structure deposition covered the active sites and was produced quickly and easily and this was one reason for the catalyst's short life span. In addition, this was consistent with the results of the N2 adsorption and desorption analysis (Table 1).
In order to provide direct evidence of coke deposition, thermogravimetric analysis (TGA) was performed under an air atmosphere and the results are shown in Fig. 7. The amount of coke deposition should be equal to the difference in weight loss between the fresh and deactivated catalysts within the temperature range of coke burning. The mass losses of fresh and deactivated ACAlD catalysts in the range of 100–500 °C were 3.9% and 21.9%, respectively, which indicates that the actual amount of coke deposition was 18.0%. This also demonstrates that the coke deposition could be nearly burned off at a temperature of 500 °C in an air atmosphere.
3.5 Regeneration studies
Because of the high price of Au, the possibility of reusing the catalyst was also investigated (Fig. 8). The deactivated catalyst could be easily regenerated by roasting it at 500 °C in an air atmosphere for 10 min. The process was simple and the high mechanical strength of the γ-Al2O3 support guaranteed the intact shape of the catalyst when loading and unloading. It can be seen that the C2H2 conversion rate can reach 96% and the C2H3Cl selectivity was more than 99% for the first regenerated catalyst, which was nearly the same catalytic performance as the fresh catalyst. However, the activity of the twice regenerated catalyst with an C2H2 conversion rate of 92% was reduced. It also showed an easy and efficient regeneration performance.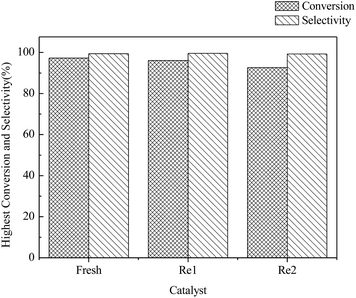 | ||
Fig. 8 The highest C2H2 conversion and C2H3Cl selectivity of the fresh and regenerated catalyst. Temperature = 150 °C, pressure = 0.1 MPa, V(HCl)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V(C2H2) = 1.1 V(C2H2) = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, GHSV = 120 h−1. 1, GHSV = 120 h−1. | ||
Furthermore, it is generally believed that Au3+ is the active component of catalysts for the C2H2 hydrochlorination reaction. The activity recovery of the regenerated catalyst also showed that the reduction and the running off of Au3+ were not the main reasons for the catalyst deactivation.
4. Conclusions
Historically, γ-Al2O3 was used less as an efficient support to prepare catalysts for C2H2 hydrochlorination compared with other carriers. The AuCl3–CuCl2/γ-Al2O3 catalyst can attain a 97% conversion rate for C2H2 conversion, which was close to that using AC as the support. The results show that using γ-Al2O3 as a support gave better mechanical strength and mesoporous (20–50 nm) structure and was thus a potential support for the AuCl3–CuCl2 bimetal catalyst. It was the strong basic sites rather than the textural properties or the acidic sites that determined its unusual activity compared to others. Furthermore, modifying the support with KOH can increase the activity and prolong the life span. The carbon deposition was the main reason for its fast deactivation and the actual amount of coke deposition was 18.0%. Although with a short life span at present, its thermostability and mechanical strength revealed its excellent regeneration ability. Therefore, it is believed that γ-Al2O3 has great potential in view of its increased life span and reduced cost. In the meantime, this should be studied further in future research.Acknowledgements
We are grateful to the Fundamental Research Funds for the Central Universities (no. WA1214003) and the Technology Development Funds for the Tanggu of Binhai New Area, Tianjin, China (no. 2012STHB04-01).References
- X. B. Wei, H. B. Shi, W. Z. Qian, G. H. Luo, Y. Jin and F. Wei, Ind. Eng. Chem. Res., 2009, 48, 128 CrossRef CAS.
- G. J. Hutchings and D. T. Grady, Appl. Catal., 1985, 17, 155 CrossRef CAS.
- N. Pirrone, S. Cinnirella, X. Feng, R. Finkelman, H. R. Friedli, J. Leaner, R. Mason, A. B. Mukherjee, G. B. Stracher, D. G. Streets and K. Telmer, Atmos. Chem. Phys., 2010, 10, 5951 CrossRef CAS.
- H. Z. Gu, X. S. Xu, A. A. Chen, A. Ping and X. H. Yan, Catal. Commun., 2013, 41, 65–69 CrossRef CAS PubMed.
- B. B. Chen, X. B. Zhu, M. Crocker, Y. Wang and C. Shi, Catal. Commun., 2013, 42, 93 CrossRef CAS PubMed.
- M. Conte, A. F. Carley, C. Heirene, D. J. Willock, P. Johnston, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2007, 250, 231 CrossRef CAS PubMed.
- M. Conte, A. F. Carley, G. Attard, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2008, 257, 190 CrossRef CAS PubMed.
- S. J. Wang, B. X. Shen and Q. L. Song, Catal. Lett., 2010, 134, 102 CrossRef CAS.
- X. B. Wei, F. Wei, W. Z. Qian, G. H. Luo, H. B. Shi and Y. Jin, Chin. J. Process Eng., 2008, 8, 1218 CAS.
- H. Y. Zhang, B. Dai, X. G. Wang, L. L. Xu and M. Y. Zhu, J. Ind. Eng. Chem., 2012, 18, 49 CrossRef CAS PubMed.
- J. Zhao, X. Cheng and L. Wang, et al., J. Catal., 2014, 144, 2191–2197 CAS.
- X. Yang, C. Jiang and Z. Yang, J. Mater. Sci. Technol., 2014, 30, 434–440 Search PubMed.
- X. G. Cheng, J. G. Zhao, L. Wang, R. F. Ren, H. H. Yang and B. X. Shen, Chem. Res. Chin. Univ., 2014, 3, 582 Search PubMed.
- H. L. Chih, D. L. Shawn and F. L. Jyh, Catal. Lett., 2003, 89, 235 CrossRef.
- J. C. Yeong and T. Y. Chuin, J. Catal., 2001, 200, 59 CrossRef.
- L. J. Marta, J. Wojciech, B. Magdalena, K. Zbigniew, K. Leszek and K. Zbigniew, Top. Catal., 2009, 52, 1037 CrossRef PubMed.
- E. D. Park and J. S. Lee, J. Catal., 1999, 186, 1–11 CrossRef CAS.
- J. Zhao, J. Xu and J. Xu, J. Chem. Eng., 2014, 262, 1152–1160 CrossRef PubMed.
- J. Okal, W. Tylus and L. Kȩpiński, J. Catal., 2004, 225, 498–509 CrossRef CAS PubMed.
- E. B. Cornelius, T. H. Milliken, G. A. Mills and A. G. Oblad, J. Phys. Chem., 1955, 59, 809 CrossRef CAS.
- S. L. Wang, C. T. Johnston and D. L. Bish, J. Colloid Interface Sci., 2003, 260, 26–35 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13648a |
| This journal is © The Royal Society of Chemistry 2015 |