Nanomolar detection of Hcy, GSH and Cys in aqueous solution, test paper and living cells†
Xingjiang Liu‡
ab,
Wenying Zhang‡a,
Chunxiao Lia,
Wan Zhoua,
Zhanxian Li*a,
Mingming Yu*a and
Liuhe Weia
aCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, China. E-mail: lizx@zzu.edu.cn; yumm@zzu.edu.cn; Fax: +86 371 67781205; Tel: +86 371 67781205
bCollege of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, China
First published on 8th December 2014
Abstract
A novel naphthalimide-based fluorescent probe was designed and synthesized for thiol recognition with high sensitivity and excellent selectivity. The probe can detect thiol quantitatively in a concentration range of 0–6.0 μM and the detection limit could be as low as 1.6 nM. The fluorescence enhancement of this probe upon addition of Cys on test paper and the application of the probe for selective detection of intracellular Hcy, GSH and Cys have been successfully demonstrated.
Introduction
Biological thiols are essential for maintaining the appropriate redox status of proteins, cells, and organisms.1 For example, as an essential amino acid, cysteine (Cys) is involved in protein synthesis, detoxification, and metabolism.2 Cys deficiency is considered to result in many health problems such as slowed growth rate, hair depigmentation, edema, lethargy, liver damage, and so on.3 Similarly, as an important endogenous antioxidant, glutathione (GSH) is considered to be an indicator of oxidative stress.4 Therefore, significant effort has been paid to the development of colorimetric, phosphorescent, and fluorescent probes for these thiol-containing amino acids to achieve high sensitivity, low cost, and ease of detection.5Most of the developed Cys probes are based on chemical reactions such as cyclization reactions with aldehydes,6 Michael additions,7 and cleavage reactions.8 As an especially effective strategy, thiol-induced cleavage of intramolecular fluorescence quenching agents, such as 2,4-dinitro-benzenesulfonamide or 2,4-dinitro-bensensulfonate, has been demonstrated with great success using a number of prototypical fluorescent chromophores such as fluorescein,9 BODIPY,10 resorufin,11 rhodamine,12 benzothiazole13 and other species.14
As prototypical fluorophores, 1,8-naphthalimide-based derivatives have been widely used in fluorescence detection and imaging because they posses many favorable optical properties such as good photostability, high fluorescence quantum yields and large Stokes' shift and also because their absorption and emission spectra are within the visible spectral region. Moreover, their photophysical properties can be easily tuned through judicious structural modifications.15 In fact, based on 1,8-naphthalimide, some biological thiol-fluorescent probes have been reported.16 However, the reported naphthalimide-based thiol fluorescent sensors are very limited and it is necessary to design and synthesize novel naphthalimide derivatives for sensing thiol.
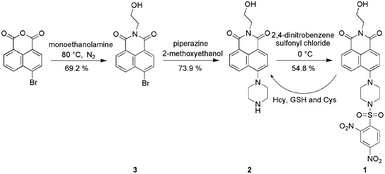
Herein we report a 2,4-dinitrobenzenesulfonate derivative of 1,8-naphthalimide, 1 (Scheme 1) that we designed as a fluorescent probe for biological thiols at a physiological value of pH = 7.40 (Fig. 1). Due to a photoinduced electron transfer (PET) process from the electron donor, fluorophore to the electron accepter 2,4-dinitrobenzenesulfonate moiety, probe 1 is non-fluorescent (Φ = 0.031). However, when treated with biological thiols, probe 1 exhibits a relatively rapid, time-dependent enhancement of its fluorescence signal (Φfl = 0.393). Such finding suggests that thiols selectively cleave the 2,4-dinitrobenzenesulfonate moiety in probe 1, thus eliminating PET-induced fluorescence quenching and concomitantly allowing fluorescence to occur (Scheme 1).
Results and discussions
In this work, synthetic route employed for the preparation of novel 1,8-naphthalimide-based thiol sensitive fluorescent probe 1 is shown in Scheme 1. The reaction of 4-bromo-1,8-naphthalic anhydride and monoethanolamine gives the product 3 in a 69.2% yield. Compound 2 was obtained with a yield of 73.9% by the reaction of compound 3 and piperazine. Compound 2 reacts with 2,4-dinitrobenzenesulfonyl chloride leads to the probe 1 in a 54.8% yield. The chemical structure of 1 was confirmed by HRMS, FT-IR, 1H NMR, and 13C NMR.To investigate the changes in fluorescence emission spectrascope of 1 upon exposure to Cys, fluorescence titrations were conducted with Cys in aqueous solution of 1 (5.0 × 10−6 M, VHEPES buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vethanol = 7
Vethanol = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
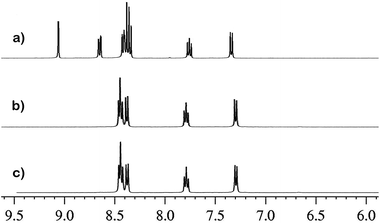
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH = 7.4) (Fig. 1). As is evident from inspection of Fig. 2, probe 1 exhibits negligible fluorescence (Φfl = 0.031) in the buffered solution in the absence of Cys because of the PET process from 1,8-naphthalimide group to 2,4-dintrobenzenesulfonyl unit. However, a significant turn-on fluorescence response with a maximum at 521 nm was observed upon the addition of Cys (82 fold fluorescence enhancement, Φfl = 0.393), which was ascribed to the prohibition of PET upon sensing. Fig. 2 indicates the relationship between the fluorescence peak intensity at 521 nm and the concentration of Cys. In the concentration range of 0 and 3.0 μM, the fluorescence peak intensity of 1 is in good linear relationship with Cys concentration, implying that Cys can be quantitatively detected in a wider concentration range. From this linear calibration graph, the detection limit of probe 1 for Cys is found to be about 1.6 nM based on signal-to-noise ratio (S/N) = 3,17 which was sufficiently low for the detection of thiols under physiological conditions. These results led us to conclude that 1 could be an effective fluorescent ‘turn on’ probe for Cys. The reaction mechanism was studied by 1H NMR and Mass spectra. The reaction product was isolated by column chromatography over silica gel column to confirm that compound 2 was formed through the reaction of Cys with probe 1 (Scheme 1). 1H NMR spectra of the isolated product, the probe 1 itself and the reference compound 2 are shown in Fig. 3. Upon addition of Cys, three protons of probe 1 corresponding to the protons of 2,4-dintrobenzenesulfonyl group around 9.06, 8.65 and 8.39 ppm dramatically disappeared. The reaction product was isolated by column chromatography to confirm that compound 2 was formed through the reaction of Cys with probe 1 (Scheme 1). For pure probe 1, a characteristic peak at m/z = 578.0958 was obtained which corresponds to the species [1 + Na]+, whilst on addition of Cys, the peak at 578.0958 disappeared and a new peak appeared at m/z = 326.1503 corresponding to the species [2 + H] (Fig. S5, S6 and S7 in the ESI†).
3, v/v, pH = 7.4) (Fig. 1). As is evident from inspection of Fig. 2, probe 1 exhibits negligible fluorescence (Φfl = 0.031) in the buffered solution in the absence of Cys because of the PET process from 1,8-naphthalimide group to 2,4-dintrobenzenesulfonyl unit. However, a significant turn-on fluorescence response with a maximum at 521 nm was observed upon the addition of Cys (82 fold fluorescence enhancement, Φfl = 0.393), which was ascribed to the prohibition of PET upon sensing. Fig. 2 indicates the relationship between the fluorescence peak intensity at 521 nm and the concentration of Cys. In the concentration range of 0 and 3.0 μM, the fluorescence peak intensity of 1 is in good linear relationship with Cys concentration, implying that Cys can be quantitatively detected in a wider concentration range. From this linear calibration graph, the detection limit of probe 1 for Cys is found to be about 1.6 nM based on signal-to-noise ratio (S/N) = 3,17 which was sufficiently low for the detection of thiols under physiological conditions. These results led us to conclude that 1 could be an effective fluorescent ‘turn on’ probe for Cys. The reaction mechanism was studied by 1H NMR and Mass spectra. The reaction product was isolated by column chromatography over silica gel column to confirm that compound 2 was formed through the reaction of Cys with probe 1 (Scheme 1). 1H NMR spectra of the isolated product, the probe 1 itself and the reference compound 2 are shown in Fig. 3. Upon addition of Cys, three protons of probe 1 corresponding to the protons of 2,4-dintrobenzenesulfonyl group around 9.06, 8.65 and 8.39 ppm dramatically disappeared. The reaction product was isolated by column chromatography to confirm that compound 2 was formed through the reaction of Cys with probe 1 (Scheme 1). For pure probe 1, a characteristic peak at m/z = 578.0958 was obtained which corresponds to the species [1 + Na]+, whilst on addition of Cys, the peak at 578.0958 disappeared and a new peak appeared at m/z = 326.1503 corresponding to the species [2 + H] (Fig. S5, S6 and S7 in the ESI†).
We also measured the fluorescence changes of compound 1 in the presence of Hcy and GSH (Fig. S1 and S2 in ESI†). Upon addition of Hcy and GSH respectively, similar fluorescence spectra changes of 1 were obtained, indicating the reaction of 1 with Hcy or GSH leads to the same product as that from the reaction of 1 with Cys. The fluorescence peak intensity of 1 is in good linear relationship with Hcy and GSH concentration (the concentration range for Hcy is 0–3.0 μM and 0–6.0 μM is for GSH.) (Fig. S3 and S4 in ESI†), implying that Hcy and GSH can be quantitatively detected in a certain concentration range.
Cys, Hcy and GSH were also done. As shown in Fig. 4, the reaction of 1 and Cys is completely accomplished within 135 min and Cys can be detected within 3 min when the concentration of Cys is more or equal to 5 × 10−5 M. However, the reaction rates of compound 1 with Hcy and GSH are much slower compared to Cys, which may be ascribed to the larger steric hindrance of Hcy and Gsh. Such result implied that compound 1 can detect Cys according to the reaction time.
 | ||
Fig. 4 Kinetics of fluorescence increase rate at 521 nm by the reaction of 1 (5.0 × 10−6 M, VHEPES buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vethanol = 7 Vethanol = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, pH = 7.4) and Cys (dark)/Hcy (red)/GSH (green) with excitation at 380 nm. 3, pH = 7.4) and Cys (dark)/Hcy (red)/GSH (green) with excitation at 380 nm. | ||
The pH value of solution was found to be essential to the cleavage reaction. To investigate the pH effect, the fluorescence intensities of 5.0 μM probe 1 at 521 nm in the absence and presence of 50 μM Hcy, GSH and Cys were examined at pH range from 1.0 to 14.0. As shown in Fig. 5, the probe 1 itself does not show fluorescence emission from pH 1.0 to 14.0. Upon the addition of Cys, Hcy and GSH respectively, there was significant fluorescence increase in pH range of 5.0–10. In the lower pH range from 1.0 to 4, no fluorescence enhancement could be observed, which might be ascribed to the difficulty for thiols to cleave the sulfonyl group under acidic condition. On the contrary, higher pH environment (>10.0) could cause other reactions which made against the formation of compound 2.
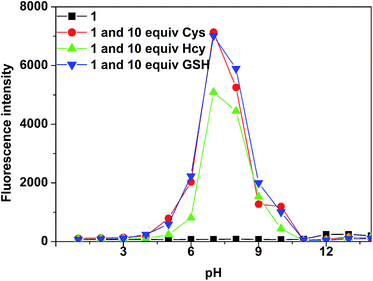 | ||
Fig. 5 Fluorescence intensity change of 1 (5.0 × 10−6 M, VHEPES buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vethanol = 7 Vethanol = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) before and after addition of Cys, Hcy and GSH respectively with different pH. 3) before and after addition of Cys, Hcy and GSH respectively with different pH. | ||
The selectivity of compound 1 was testified in the presence of other physiologically relevant amino acids (e.g., Asp, Ala, Val, Phe, His, Leu, Ser, IIe, Trp, Lys, Arg, Pro, Gly, Met, Tyr, Glu, Thr) at identical conditions. As shown in Fig. 6, 7 and red bars of Fig. 8, Probe 1 uniquely responds to thiol-containing amino acids (Hcy, GSH, Cys) to produce a prominent fluorescence enhancement with a maximum at 521 nm, whereas other amino acids have no influence. To further assess its utility as a thiol-selective probe, its fluorescence response to Cys in the presence of typical thiol-free amino acids (green bars of Fig. 8). The results demonstrated that all of the thioal-free amino acids have no interference in the detection of Cys. Therefore, it can be concluded that the probe 1 showed extremely high selectivity towards thiol at a physiological pH value.
As shown in the above, probe 1 can detect thiols in aqueous solution, it inspired us to further investigate the possibility of detection as solid materials for point-of-care detection application. Test paper was selected and the fluorescence detection properties were studied by a UV lamp with photography as well as solid fluorescence spectroscope. In the detection process, the probe spots were prepared by dropping 1 μL 50 μM solutions of 1 Portable ultraviolet lamp. As shown in Fig. 9, the probe spots emitted bright blue-green fluorescence upon the addition of Cys under UV illumination, while an enhancement of the fluorescence intensity at 493 nm could be recorded. Additionally, the probe spots emitted fluorescence only upon the addition of thiols (Fig. S8 in the ESI†), demonstrating the high selectivity of probe 1 as solid materials toward thiols.
 | ||
| Fig. 9 (a) Images of test papers for the detection of Cys at various concentrations (0, 2.5 × 10−4 M, 1.0 × 10−3 M, 5.0 × 10−3 M, 5.0 × 10−2 M, 0.1 M, from left to right) in PBS solutions (5.0 × 10−5 M, pH = 7.4) under a UV lamp (365 nm). (b) Fluorescence spectra and (c) fluorescence intensity at 493 nm of the probe spots of 1 (5.0 × 10−5 M) on test papers upon addition of various concentrations of Cys (0–9.0 mM). Other conditions are the same as in Fig. 1. | ||
The practical utility of using probe 1 to detect thiols in an image-wise manner within living SH-SY5Y cells was explored (Fig. 10). When SH-SY5Y cells were incubated with 5 μM probe 1 at 37 °C for 30 min, a significant green fluorescence inside the cells was observed with the aid of an inverted fluorescence microscope. Thus, probe 1 is capable of permeating into cells and reacting with thiols to give easily discernable fluorescent images. In a controlled experiment, the SH-SY5Y cells were pre-treated with 1 mM of N-ethylmaleimide (NEM), a thiol-blocking reagent, after subsequent incubation with probe 1, no intracellular fluorescence was observed within the cells. Therefore, the probe 1 is applicable for thiol detection in living cells.
Experimental section
Instruments
Mass spectra were obtained on high resolution mass spectrometer (IonSpec4.7 Tesla FTMS-MALDI/DHB). FT-IR spectra were recorded on a NEXUS-470 spectrometer at frequencies ranging from 400 to 4000 cm−1. Samples were thoroughly mixed with KBr and pressed into pellet form. 1H and 13C NMR spectra were recorded on a Bruker 400 NMR spectrometer. Chemical shifts are reported in parts per million using tetramethylsilane (TMS) as the internal standard.All spectral characterizations were carried out in HPLC-grade solvents at 20 °C within a 10 mm quartz cell. UV-vis absorption spectroscopy was measured with a TU-1901 double-beam UV-vis Spectrophotometer, and fluorescence spectroscopy was determined on a Hitachi F-4500 spectrometer. The fluorescence quantum yields were measured at 20 °C with quinine bisulfate in 1 M H2SO4 (Φfl = 0.546) selected as the ref. 18. Cells imaging was performed with an Olympus IX83 inverted microscope.
Materials and reagents
All commercial grade chemicals and solvents were purchased and were used without further purification. The probe 1 was synthesized according to Scheme 1.19Synthesis of compound 3
28 mL 4-bromo-1,8-naphthalic anhydride (2.76 g, 10.0 mmol) ethanol solution was heated to 80 °C under nitrogen atmosphere and 9 mL monoethanolamine was added to the above solution. After the mixture was refluxed for 4 h, the reaction liquid was cooled to room temperature and filtered. The filter cake was washed for 3 times with methanol and the product 3 was obtained (2.2137 g, 69.2%).Synthesis of compound 2
The mixture of 3 (1.4060 g, 4.39 mmol), piperazine (0.3781 g, 4.39 mmol), and 2-methoxyethanol (35 mL) was refluxed for 5 h. The reaction liquid was filtered and the filter cake was obtained as the crude product. The final product 2 (1.0762 g, 73.9%) was obtained by column chromatography over silica gel column using dichloromethane/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.
Synthesis of compound 1
5 mL dichloromethane solution of 2,4-dinitrobenzenesulfonyl chloride (81.9 mg, 0.31 mmol) was slowly added to the 25 mL dichloromethane solution of compound 2 (100 mg, 0.31 mmol) and the mixture was reacted for 1 h at 0 °C. The final product 1 (0.0943 g, 54.8%) was obtained by column chromatography over silica gel column using dichloromethane/ethanol (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.
Synthesis of the product from the reaction of 1 with Cys
55.5 mg (0.1 mmol) probe 1 was dissolved in 15 mL methanol and 121 mg (1 mmol) Cys was dissolved in water. The mixture of the above two solutions were stirred for 20 h at 20 °C and the final product (compound 2, 19.7 mg, 61.0%) was obtained by column chromatography over silica gel column using dichloromethane/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.
Imaging of SH-SY5Y cells
SH-SY5Y cells (Neuroblastoma cells) were seeded in a 12-well plate in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin. The cells were incubated under an atmosphere of 5% CO2 and 95% air at 37 °C for 24 h. Before the experiments, cells were washed with PBS buffered solution three times. The cells were incubated with probe 1 (5 μM) at 37 °C for 30 min. Cell images were acquired after washing with PBS buffer three times. For control experiment, SH-SY5Y cells were pretreated with 1 mM of N-ethylmaleimide (NEM) for 30 min at 37 °C for 30 min. After washing three times with PBS buffered solution, the pretreated cells were incubated with probe 1 (5 μM) at 37 °C for additional 30 min. Fluorescence imaging was performed after washing the cells three times with PBS buffered solution.Conclusions
In summary, based on selective cleavage of 1 by thiols, a “turn-on” fluorescent probe 1 toward thiols has been developed. In aqueous solution containing 30% ethanol, probe 1 exhibited ultrasensitive fluorescence enhancement for thiols (the LOD can be as low as 1.6 nM) and a good selectivity over other amino acids. The fluorescence turn-on responses of 1 on test paper and for imaging cellular thiols were also applied.Acknowledgements
We are grateful for the financial supports from National Natural Science Foundation of China (50903075 and J1210060), New Teachers' Joint Fund for Doctor Stations from Ministry of Education of the People's Republic of China (20114101120003) and Zhengzhou University.Notes and references
- Z. A. Wood, E. Schröder, J. R. Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32–40 CrossRef CAS
; S. Y. Zhang, C. N. Ong and H. M. Shen, Cancer Lett., 2004, 208, 143–153 CrossRef PubMed
.
- S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostion, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476–483 CrossRef CAS PubMed
; N. M. Giles, A. B. Watts, G. I. Giles, F. H. Fry, J. A. Littlechild and C. Jacob, Chem. Biol., 2003, 10, 677–693 CrossRef
; W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949–15958 CrossRef PubMed
; R. Obeid and W. Hermann, FEBS Lett., 2006, 580, 2994–3005 CrossRef PubMed
.
- N. Fu, D. Su, J. R. Cort, B. Chen, Y. Xiong, W. J. Qian, A. E. Konopka, D. J. Bigelow and T. C. Squier, J. Am. Chem. Soc., 2013, 135, 3567–3575 CrossRef CAS PubMed
; R. G. Upendar, A. Hridesh, T. Nandaraj, G. Suvankar, C. Samit and D. Amitava, Chem. Commun., 2014, 50, 9899–9902 RSC
; M. Isik, R. Guliyev, S. Kolemen, Y. Altay, B. Senturk, T. Tekinay and E. U. Akkaya, Org. Lett., 2014, 16, 3260–3263 CrossRef PubMed
.
- S. Sabelle, P. Y. Renard, K. Pecorella, S. Suzzoni-Dezard, C. Creminon, J. Grassi and C. Mioskowski, J. Am. Chem. Soc., 2002, 124, 4874–4880 CrossRef CAS PubMed
; J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. H. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351–5358 CrossRef PubMed
; M. H. Lee, J. H. Han, P. S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316 CrossRef PubMed
.
- H. S. Jung, X. Q. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019–6031 RSC
.
- X. Zhang, X. Ren, Q. H. Xu, K. P. Loh and Z. K. Chen, Org. Lett., 2009, 11, 1257–1260 CrossRef CAS PubMed
; P. Wang, J. Liu, X. Lv, Y. Liu, Y. Zhao and W. Guo, Org. Lett., 2012, 14, 520–523 CrossRef PubMed
; H. Li, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 39, 5904–5906 RSC
.
- L. Yi, H. Li, L. Sun, L. Liu, C. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2009, 48, 4034–4037 CrossRef CAS PubMed
; G. J. Kim, K. Lee, H. Kwon and H. J. Kim, Org. Lett., 2011, 13, 2799–2801 CrossRef PubMed
; X. Zhou, X. Jin, G. Sun, D. Li and X. Wu, Chem. Commun., 2012, 48, 8793–8795 RSC
; X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690–10693 CrossRef PubMed
; Y. W. Liu, S. Zhang, X. Lv, Y. Q. Sun, J. Liu and W. Guo, Analyst, 2014, 139, 4081–4087 RSC
.
- B. Zhu, X. Zhang, Y. Li, P. Wang, H. Zhang and X. Zhuang, Chem. Commun., 2010, 46, 5710–5712 RSC
; L. Long, W. Lin, B. Chen, W. Gao and L. Yuan, Chem. Commun., 2011, 47, 893–895 RSC
; L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed
.
- H. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki and N. Itoh, Angew. Chem., Int. Ed., 2005, 44, 2922–2925 CrossRef CAS PubMed
.
- J. Shao, H. Guo, S. Ji and J. Zhao, Biosens. Bioelectron., 2011, 26, 3012–3017 CrossRef CAS PubMed
; X. D. Jiang, J. Zhang, X. Shao and W. Zhao, Org. Biomol. Chem., 2012, 10, 1966–1968 Search PubMed
.
- K. Cui, Z. Chen, Z. Wang, G. Zhang and D. Zhang, Analyst, 2011, 136, 191–195 RSC
.
- A. Shibata, K. Furukawa, H. Abe, S. Tsuned and Y. Ito, Bioorg. Med. Chem. Lett., 2008, 18, 2246–2249 CrossRef CAS PubMed
; W. Sun, W. Li, J. Li, J. Zhang, L. Du and M. Li, Tetrahedron Lett., 2012, 53, 2332–2335 CrossRef PubMed
; J. Bouffard, Y. Kim, T. M. Swager, R. Weissleder and S. A. Hilderbrand, Org. Lett., 2008, 10, 37–40 CrossRef PubMed
.
- S. P. Wang, W. J. Deng, D. Sun, M. Yan, H. Zheng and J. G. Xu, Org. Biomol. Chem., 2009, 7, 4017–4020 Search PubMed
; S. Chen, P. Hou, B. Zhou, X. Song, J. Wu, H. Zhang and J. W. Foley, RSC Adv., 2013, 3, 11543–11546 RSC
; J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511–4535 RSC
; Z. Xu, J. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 2563–2565 RSC
; R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Krugerc and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936–3953 RSC
.
- M. Dong, Y. W. Wang and Y. Peng, Org. Lett., 2010, 12, 5310–5313 CrossRef CAS PubMed
.
- S. Chen, P. Hou, J. W. Foley and X. Song, RSC Adv., 2013, 3, 5591–5596 RSC
; L. Duan, Y. Xu, X. Qian, F. Wang, J. Liu and T. Chen, Tetrahedron Lett., 2008, 49, 6624–6627 CrossRef CAS PubMed
; X. Wu, Q. Ma, X. Wei, Y. Hou and X. Zhu, Sens. Actuators, B, 2013, 183, 565–573 CrossRef PubMed
; J. Zhou, H. Lin, B. Jin, X. Liu, H. Fu and D. Shangguan, J. Mater. Chem. C, 2013, 1, 4427–4436 RSC
; D. H. Yu, F. H. Huang, S. S. Ding and G. Q. Feng, Anal. Chem., 2014, 86, 8835–8841 CrossRef PubMed
.
- H. Tian, J. Gan, K. Chen, J. He, Q. L. Song and X. Y. Hou, J. Mater. Chem., 2002, 12, 1262–1267 RSC
.
- Y. Liu, H. Li, M. Pei, G. Zhang, L. Hu and J. Han, Talanta, 2013, 115, 190–194 CrossRef CAS PubMed
; D. Yu, F. Huang, S. Ding and G. Feng, Anal. Chem., 2014, 86, 8835–8841 CrossRef PubMed
.
- Z. X. Li, L. Y. Liao, W. Sun, C. H. Xu, C. Zhang, C. J. Fang and C. H. Yan, J. Phys. Chem. C, 2008, 112, 5190–5196 CAS
.
- L. Peng, Z. Zhou, R. Wei, K. Li, P. Song and A. Tong, Dyes Pigm., 2014, 108, 24–31 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13262a |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2015 |