Synthesis, reaction, and optical properties of cyclic oligomers bearing 9,10-diphenylanthracene based on an aromatic tertiary amide unit†
Ryohei Yamakadoa,
Shin-ichi Matsuokaa,
Masato Suzukia,
Daisuke Takeuchib,
Hyuma Masuc,
Isao Azumayad and
Koji Takagi*a
aDepartment of Materials Science and Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan
bChemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan
cCenter for Analytical Instrumentation, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba, Chiba 263-8522, Japan
dFaculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan
First published on 6th January 2014
Abstract
Novel cyclic aromatic amide oligomers containing highly fluorescent 9,10-diphenylanthracene units were prepared by condensation of a monomer with amino and ester functional groups using lithium bis(trimethylsilyl)amide. The cyclic trimer (C3A, 40%) and tetramer (C4A, 8%) were isolated using preparative gel permeation chromatography. Some of the aromatic proton signals derived from anthracene were observed in the upfield region (6.96–6.87 ppm) in the 1H nuclear magnetic resonance spectrum of C3A. X-ray crystallographic analysis indicated that three anthracene moieties are inclined with respect to the cyclic skeleton and partly overlap each other. The fluorescence peak maximum of C3A (438 nm) showed a small red-shift compared with that of an acyclic model compound (425 nm). Reduction of the amide carbonyl group in C3A gave the cyclic trimer HC3A, which has a tertiary amine unit. The fluorescence peak maximum of HC3A (489 nm) was largely red-shifted from that of C3A and exhibited strong solvent dependence. A linear correlation was observed between the Stokes shift (Δν), ranging from 2981 to 6646 cm−1, and the Reichardt's solvent polarity parameter [ET(30)].
Introduction
The well-defined three-dimensional spatial arrangement of π-conjugated chromophores has been attracting much attention with regard to the fundamental understanding of the relationship between this spatial arrangement and the optoelectronic properties of a compound.1,2 This is because such organic molecules are mostly used not in an isolated state but in a condensed state (film or crystal) in optoelectronic devices such as light-emitting diodes,3 field-effect transistors,4 and photovoltaic cells.5 In fluorescence resonance energy transfer (FRET) between energy-donor and energy-acceptor chromophores, the FRET efficiency can be described by the Förster equation.6 The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between the two chromophores and the relative orientation of their dipole moments. Accordingly, both the chemical structure and the three-dimensional arrangement have to be taken into account when developing new materials with optimal properties for optoelectronic applications. A number of cyclic oligomers, including [2,2]paracyclophane,7–12 calix[4]arene,13–15 bicyclo[4.4.1]undecane,16–18 and pillar[5]arene,19,20 have been used as scaffolds for the three-dimensional arrangement of π-conjugated systems. For example, Bazan et al.7–9 and Morisaki et al.10–12 independently reported that the [2,2]paracyclophane unit shows transannular through-space π–π interactions and is an excellent building block for the elongation of π-conjugated systems. It has also been demonstrated that an enantiopure ortho-difunctionalized [2,2]paracyclophane chiral skeleton can align π-conjugated oligomers to give optically active properties.21 We previously investigated the alignment of pyrene using cyclic aromatic amide trimer, meta-calix[3]amide, as the scaffold.22 As a result of steric effects, the pyrenyl groups attached to the benzene ring were aligned in a screw-like fashion; this was confirmed by X-ray crystallographic analysis. However, the distance and orientation of π-conjugated chromophores on the benzene ring are difficult to control, especially in the solution state, because meta-calix[3]amide has a conformational flexibility derived from syn and anti isomers.23 Recently, we achieved three-dimensional arrangement of bithiophenes with dynamic triple-stranded helicity using the planar chirality of meta-calix[3]amide.24 Although the interconversion between syn and anti isomers was nicely suppressed, the synthetic procedure consisted of multi-step reactions to be concerned with the low efficiency. We then envisaged that the install of π-conjugated chromophores directly in the cyclic structure would be better strategy to furnish the precision arrangement of π-conjugated chromophores and the aromatic tertiary amide unit must be an excellent linker to construct the molecule.9,10-Diphenylanthracene (DPA) and its derivatives are highly fluorescent, and they are useful soft materials for the light-emitting application.25,26 Accordingly, it is important to investigate the relationship between the three-dimensional arrangement and the optical properties. Yoshizawa et al. reported a tubular macrocycle containing four anthracene units connected by meta-phenylene linkers.27 Despite the close arrangement of the chromophores, the compound showed neither excimer emission nor fluorescence quenching, as a result of the enforced orthogonal conformation. Toyota et al.28 and Zhao et al.29 synthesized aryleneethynylene macrocycles containing the 9,10-anthrylene unit, and demonstrated self-assembly behavior in the solution and liquid-crystalline phases. However, currently known macrocycles mostly have hydrocarbon frameworks.30–32 The introduction of heteroatoms and push–pull interactions is expected to impart charge-transfer properties to cyclic oligomers bearing π-conjugated chromophores.33
In this paper, we will describe the synthesis of new cyclic aromatic amide trimer (C3A) and tetramer (C4A) containing the DPA unit. The conformations in the solution and solid states were studied using nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography, and ultraviolet (UV) absorption/fluorescence spectroscopy were measured to discuss their optical properties. Reduction of the amide carbonyl group was also performed to investigate the influence of the linker on the optical properties.
Results and discussion
Synthesis
Monomer 1, containing amino and ester functional groups, was synthesized as shown in Scheme 1. 4-Iodoacetanilide was alkylated with NaH and 1-bromooctane to obtain 3 in 85% yield. The Suzuki coupling reaction of 3 with 10-bromoanthracene-9-boronic acid was performed using Pd(PPh3)4, giving 4 in 68% yield. The acetyl group in 4 was removed using concentrated HCl, and the obtained product 5 was subjected to a second Suzuki coupling reaction with 4-methoxycarbonylphenylboronic acid pinacol ester, giving 1 as a yellow powder in 82% yield. The cyclic oligomerization of 1 was performed using a 1 M tetrahydrofuran (THF) solution of lithium bis(trimethylsilyl)amide (LiHMDS) (Scheme 2). According to the method previously reported by Yokozawa et al.,34 a THF solution of 1 was slowly added to LiHMDS (5 equiv. relative to 1) at room temperature and 0 °C (Method A). Although 1 was almost completely consumed irrespective of the reaction temperature (Fig. S20,† broken line), a proton signal assignable to the methyl ester was clearly detected in the 1H NMR spectrum, implying the production of linear oligomers instead of cyclic oligomers. Higher-molecular-weight oligomers were obtained by carrying out the reaction at room temperature. In contrast, when the cyclic oligomerization was performed by dropwise addition of LiHMDS to a THF solution of 1 (Method B), no proton signal derived from the methyl ester was observed in the 1H NMR spectrum. In the matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrum of the crude products, cyclic oligomers (3-mer–6-mer) were specifically detected. No significant temperature dependence was observed in the gel permeation chromatography (GPC) profiles between room temperature and 0 °C (Fig. S20,† solid line). Cyclic trimer (C3A) and tetramer (C4A) were isolated, using preparative GPC, in 40% and 8% yields, respectively. Cyclic trimer C3A was preferentially obtained, as in the cyclic oligomerization of other aminobenzoic acid derivatives.35–37 The carbonyl stretching vibrations of the amide bond were confirmed by infrared (IR) spectroscopy (C3A: 1649 cm−1, C4A: 1645 cm−1), and the cyclic architectures were verified by the MALDI-TOF mass spectra (Fig. S21 and S22†). Subsequently, reduction of the amide carbonyl group in C3A was carried out using LiAlH4 and AlCl3 in THF–Et2O, giving the cyclic trimer HC3A, which has a methylene group between the benzene ring and the nitrogen atom, in 71% yield (Scheme 2). The peak derived from the amide carbonyl group completely disappeared in the IR spectrum, and the structure of HC3A was identified from the 1H NMR spectrum (Fig. S15†) and MALDI-TOF mass spectrum (Fig. S23†). Acyclic model compound 2 was also synthesized as shown in ESI.† | ||
| Scheme 2 Synthetic route to cyclic aromatic oligomers C3A, C4A, and HC3A. The chemical structure of acyclic model compounds 2 and DPAA. | ||
Characterization
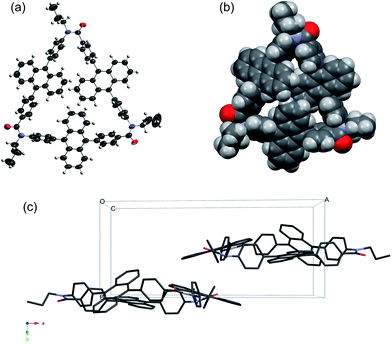
The 1H NMR spectrum of C3A showed seven proton signals in the aromatic region, which were fully assigned using H–H correlation spectroscopy (COSY) (Fig. S11†) and rotating Overhauser enhancement and exchange spectroscopy (ROESY) (Fig. S12†). It should be noted that two broad multiplet signals with a combined integral ratio of 12 appeared at 6.96–6.87 ppm at room temperature; these were assigned to the f and g protons of the anthracene ring (Fig. 1). Since this upfield shift is not observed in acyclic model compound 2, the shielding effect by the other anthracene rings located in close proximity is the most probable cause. Variable-temperature NMR studies showed that the corresponding proton signals broadened with decreasing temperature, and a signal with an integral ratio of 6 was observed at 6.4 ppm at 223 K (Fig. S17†). It can be deduced that the rotational motion of anthracene rings is suppressed at low temperatures, resulting in separate detection of intra-annular (f and g) and extra-annular (f′ and g′) protons. The signals from the b and c protons also broadened with decreasing temperature, but the proton signals derived from the benzene ring did not show obvious changes within the examined temperature range. Unfortunately, the calculation of the rotational energy barrier was difficult because the paired proton signal at the down field region was overlapped with other proton signals. In contrast, in the 1H NMR spectrum of C4A, the corresponding proton signals of the anthracene ring were observed in the normal region (7.27–7.09 ppm) (Fig. S13†). The difference between the chemical shifts in the spectra of C3A and C4A is probably originated from the conformations and ring sizes of the cyclic oligomers; this was further investigated using X-ray crystallography.The relative positions of the three DPA groups were determined by X-ray crystallography, using cyclic aromatic amide oligomers with n-propyl substituents on the amide nitrogen (C3A′ and C4A′) instead of C3A and C4A. Single crystals of C3A′ and C4A′ were obtained by recrystallization from CHCl3 and hexane. Fig. 2 shows the crystal structure of C3A′; three anthracene moieties are inclined with respect to the cyclic skeleton and partly overlap each other (Fig. 2b). The phenyl and anthracene planes have a twisted conformation, with (N)Ph–Anth(Ph) and (Ph)Anth–Ph(CO) torsion angles of 78.2° and 87.2°, respectively, on average. It is of particular interest that C3A′ forms a chiral crystal (space group: P21) as a result of fixing of the axis chirality, although C3A′ is achiral in the solution state. In contrast, four anthracene moieties lie horizontally and a plane of mirror symmetry is observed in the case of C4A′ (space group: P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) (Fig. 3). The crystals stacked along the a-axis and some of the neighboring column molecules filled the space between the molecules (Fig. 3c).
) (Fig. 3). The crystals stacked along the a-axis and some of the neighboring column molecules filled the space between the molecules (Fig. 3c).
The UV and fluorescence spectra of C3A, C4A and 2 in dichloromethane (DCM) were recorded (Fig. 4). The UV spectrum of C3A and C4A are similar to that of 2. In contrast, the fluorescence peak maximum of C3A (λmax = 438 nm) and C4A (λmax = 439 nm) were slightly red-shifted compared with that of 2 (λmax = 425 nm). The relative fluorescence quantum yields of C3A (15%) and C4A (6%) were lower than that of DPA (87%). The fluorescence spectra were independent of the solution concentration between 10−4 and 10−6 M. Consequently, the red-shifted fluorescence emission of C3A and C4A can be explained by intramolecular non-bonding interactions among three anthracene units (vide supra). The difference of the fluorescence spectra between C3A and C4A was not detected because the overlap of the π-cloud is not significant in C3A. We also investigated the photophysical experiments in the solid state (Fig. S25†). The emission maximum of C3A in solid state is red-shifted as compared to that in solution due to the intermolecular π–π interaction. Furthermore C4A showed a larger red-shift than that of C3A. As shown in Fig. 2 and 3, C4A has a higher planarity than C3A, therefore C4A make the intermolecular π–π interaction efficiently in the solid state.
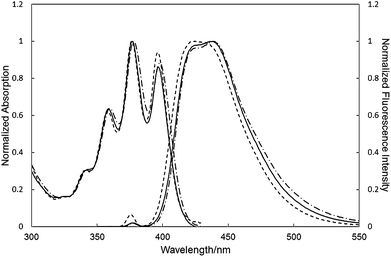 | ||
| Fig. 4 UV and fluorescence spectra of C3A (solid line), C4A (broken line), and 2 (dotted line) in DCM (r.t., 10−5 M). See Table S1† for maximum wavelengths. | ||
The absorption and emission spectra of C3A and HC3A were then investigated to clarify the influence of linker on the optical properties of the molecules. In DCM, the UV spectrum of HC3A became broader and the fluorescence band (λmax = 489 nm) was shifted to longer wavelength by 50 nm compared with that of C3A (438 nm) (Fig. 5a). This red-shift could be related to twisted intramolecular charge transfer (TICT) from the electron-donating aniline moiety to the electron-accepting anthracene unit. To further support this suggestion, the UV and fluorescence spectra were measured in various solvents. Both the UV and fluorescence spectra demonstrated a small solvent dependence in the case of C3A (Fig. S24†), indicating that the ground-state and excited-state structures of C3A were not affected by the solvent character. In sharp contrast, the fluorescence spectrum of HC3A became broader and gradually red-shifted with increasing solvent polarity (Fig. 5b). As indicated in Fig. 5c, the Stokes shift (Δν) showed a linear correlation with the Reichardt's solvent polarity parameter [ET(30)].38 According to the previous reports,39–42 these phenomena are consistent with TICT from the aniline moiety to the anthracene unit in HC3A.
Fig. 6 shows the UV and fluorescence spectra of HC3A and a reference compound, N,N-dipropyl-4-(10-phenyl-9-anthracenyl)aniline (DPAA). The absorption peak maxima of HC3A and DPAA were similar in both cyclohexane and dimethyl sulfoxide (DMSO), but the tailings to the longer wavelength were detected in HC3A. In cyclohexane, which is less polar than DMSO, the fluorescence band of HC3A (λmax = 449 nm) was shifted by 5 nm to a longer wavelength than that of DPAA (λmax = 444 nm), as a result of intramolecular interactions among three anthracene moieties similarly to C3A. In contrast, in more polar DMSO, the fluorescence maximum of HC3A (λmax = 543 nm) was blue-shifted by 16 nm compared with that of DPAA (λmax = 559 nm). Accordingly, HC3A was less sensitive to solvent polarity than DPAA was. We suggest that TICT in the excited state of HC3A is relatively blocked compared with DPAA as a result of restricted bond rotation because of the cyclic architecture. On protonation of the aniline moiety in HC3A with trifluoroacetic acid, a significant blue-shift of the fluorescence spectrum and an increase in the emission intensity were observed (Fig. S27†). It can be deduced that the electron density of the aniline moiety is decreased by protonation and, as a result, TICT from the aniline moiety to the anthracene unit is inhibited. Moreover, the original UV and fluorescence spectra were recovered by addition of excess triethylamine to the proton adduct of HC3A. The complete recovery of spectra indicates that the deprotonation was selectively occurred and no electronic interaction between triethylamine and anthracene43 can be ignored.
 | ||
| Fig. 6 UV and fluorescence spectra of HC3A (solid line) and DPAA (broken line) (r.t., 10−5 M) in (a) CH and (b) DMSO. | ||
Conclusion
In conclusion, we prepared a novel cyclic aromatic amide trimer (C3A) and tetramer (C4A) containing the DPA unit. The spectroscopic and X-ray crystallographic data for C3A show that three anthracene moieties are arranged in a triangular manner and partly stacked intramolecularly, in both the solution and solid states. Reduction of the carbonyl group in C3A gave the cyclic trimer HC3A, constructed from electron-donating aniline and electron-accepting anthracene units. The fluorescence spectrum of C3A showed a small red-shift from that of acyclic model compound 2. The fluorescence spectrum of HC3A was largely red-shifted from that of C3A and the collection of spectra in various solvents suggested the occurrence of TICT from the aniline moiety to the anthracene unit.Experimental section
Materials and instruments
All materials were obtained from commercial suppliers and used without purification. 4-Iodoacetanilide44 and N,N-dipropyl-4-(10-phenyl-9-anthracenyl)aniline (DPAA)45 were synthesized following to the previous reports. 1H and 13C NMR spectra were investigated on Bruker (Billerica, MA, USA) Avance 200, 400, 500, and 600 FT-NMR spectrometers using tetramethylsilane (1H NMR, δ 0.00) and solvent residual peaks as the internal standards (1H NMR and 13C NMR). IR spectra were recorded on a JASCO (Tokyo, Japan) FT-IR 460Plus spectrophotometer in the attenuated total reflectance (ATR) method. Melting points (M.p.) were determined on a Yanaco (Kyoto, Japan) micro melting point apparatus MP-500D. MALDI-TOF MS analyses were performed on a JEOL (Tokyo, Japan) JMS-S3000 in the spiral mode using dithranol as a matrix. Purifications with preparative GPC were carried out on a Japan analytical industry (Tokyo, Japan) LC-9210 system using tandem JAIGEL 1H, 2H, and 2.5H columns (CHCl3 as an eluent, flow rate = 3.5 mL min−1) equipped with an ultraviolet (UV) detector monitored at 254 nm. UV and fluorescence spectra were recorded on a Shimadzu (Kyoto, Japan) UV-1650PC spectrophotometer and a Shimadzu RF-5300PC spectrofluorometer, respectively, using a 10 mm quartz cell. Fluorescence quantum yields (QY) in solution were determined relative to quinine sulfate in 0.05 M H2SO4 having a QY of 0.55.Monomer synthesis
M.p. 135–136 °C. 1H NMR (δ, 200 MHz, ppm, CDCl3) 8.64 (d, J = 9.7 Hz, 2H), 7.68–7.55 (4H), 7.51–7.34 (6H), 3.83 (t, J = 8.0 Hz, 2H), 2.03 (s, 3H), 1.73–1.60 (2H), 1.36–1.17 (10H), 0.88 (t, J = 6.6 Hz, 3H). 13C NMR (δ, 50 MHz, ppm, CDCl3) 170.2, 142.8, 138.0, 136.3, 133.9, 131.0, 130.2, 129.4, 128.2, 127.2, 126.7, 124.3, 123.3, 93.3, 49.0, 32.0, 29.3, 27.8, 22.8, 13.5. IR (cm−1) 2958, 2920, 2853, 1638, 1592, 1511, 1402, 1296, 1026, 936, 879, 765, 751, 620.
M.p. 85–86 °C. 1H NMR (δ, 200 MHz, ppm, CDCl3) 8.57 (d, J = 8.8 Hz, 2H), 7.80 (d, J = 8.8 Hz, 2H), 7.55 (t, J = 7.9 Hz, 2H), 7.34 (d, J = 7.9 Hz, 2H), 7.12 (d, J = 8.3 Hz, 2H), 6.77 (d, J = 8.8 Hz, 2H), 3.79 (bs, 1H), 3.19 (t, J = 6.7 Hz, 2H), 1.69 (m, 2H), 1.36–1.17 (10H), 0.90 (t, J = 6.3 Hz, 3H). 13C NMR (δ, 50 MHz, ppm, CDCl3) 147.9, 138.5, 133.1, 131.5, 130.2, 129.0, 128.2, 126.4, 125.4, 123.7, 122.0, 113.4, 44.1, 31.9, 29.6, 29.5, 29.4, 27.8, 22.8, 13.5. IR (cm−1) 3943, 3443, 2946, 2916, 2847, 1604, 1518, 1464, 1437, 1341, 1289, 1258, 1179, 1029, 933, 874, 813, 756, 722, 654, 611.
M.p. 192–193 °C. 1H NMR (δ, 600 MHz, ppm, CDCl3) 8.28 (d, J = 8.4 Hz, 2H), 7.86 (m, 2H), 7.61–7.55 (4H), 7.32, (m, 4H), 7.26 (d, J = 8.7 Hz, 2H), 6.82 (d, J = 8.7 Hz, 2H), 3.82 (bs, 1H), 3.24 (t, J = 6.9 Hz, 2H), 1.72 (m, J = 6.9 Hz, 2H) 1.48 (m, J = 6.9 Hz, 2H), 1.42–1.27 (8H), 0.90 (t, J = 6.8 Hz, 3H). 13C NMR (δ, 150 MHz, ppm, CDCl3) 167.1, 147.9, 144.6, 138.5, 135.1, 132.1, 131.6, 130.3, 129.7, 129.6, 129.3, 127.5, 126.9, 126.3, 125.3, 124.8, 112.4, 52.2, 44.2, 31.9, 29.7, 29.5, 29.2, 27.2, 22.7, 14.2. IR (cm−1) 3400, 2923, 2853, 1707, 1606, 1521, 1478, 1433, 1391, 1319, 1271, 1176, 1111, 1020, 942, 818, 765, 709, 670, 609. Anal. calcd for C36H37NO2: C, 83.85; H, 7.23; N, 2.72. Found: C, 83.65; H, 7.30; N, 2.65%.
General procedure of cyclic oligomerization
Reduction of amide carbonyl group
C3A (29 mg/0.02 mmol) in THF (0.24 mL) was added dropwise to ether (0.48 mL) solution of LiAlH4 (9.0 mg/0.24 mmol) and AlCl3 (32 mg/0.24 mmol) at 0 °C, and the system was stirred for 2 h at room temperature. The reaction mixture was quenched with cold H2O (2 mL), cold 2 M aq. NaOH (2 mL), and H2O (6 mL). After an aqueous phase was extracted with DCM, the combined organic phase was washed with brine. After drying over MgSO4, solvents were removed by the rotary evaporator. The crude product was purified by preparative GPC (CHCl3 as an eluent) to obtain HC3A (26 mg, 93%). M.p. 127–128 °C. 1H NMR (δ, 600 MHz, ppm, CDCl3) 7.69 (m, 6H), 7.54 (m, 6H), 7.50 (d, J = 7.5 Hz, 6H), 7.41 (d, J = 7.5 Hz, 6H), 7.25 (d, J = 7.9 Hz, 6H), 7.97 (12H), 6.90, (d, J = 7.43 Hz, 6H), 4.90 (s, 6H), 3.78 (t, J = 7.4 Hz, 6H), 1.97 (m, 6H), 1.50 (m, 6H), 1.46–1.31 (24H), 0.95 (t, J = 6.1 Hz, 9H). 13C NMR (δ, 150 MHz, ppm, CDCl3) Not available due to low solubility. IR (cm−1) 3063, 2928, 2853, 1606, 1518, 1395, 1183, 1027, 767, 672. MALDI-TOF MS Calcd for C105H105N3˙+ (M˙+): 1407.83. Found: 1407.83.X-ray crystallographic analysis
Crystallographic data were collected on a CCD diffractometer with Cu Kα (λ = 1.54178 Å) radiation. Data collections were carried out at low temperature using liquid nitrogen. All of the crystal structures were solved by direct methods with SHELXS-97 and refined with full-matrix least-squares SHELXL-97.46 All non-hydrogen atoms were refined anisotropically and hydrogen atoms were included at their calculated positions.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 648 reflections measured, 12
648 reflections measured, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985 unique (Rint = 0.0334), μ = 4.570 mm−1. The final R1 and wR2 were 0.0856 and 0.2403 (I > 2σ(I)), 0.1008 and 0.2640 (all data).† Some atoms of propyl groups and chloroform molecules are disordered to two positions respectively. The occupancies of the disordered atoms were refined.
985 unique (Rint = 0.0334), μ = 4.570 mm−1. The final R1 and wR2 were 0.0856 and 0.2403 (I > 2σ(I)), 0.1008 and 0.2640 (all data).† Some atoms of propyl groups and chloroform molecules are disordered to two positions respectively. The occupancies of the disordered atoms were refined.![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.5211(2), b = 13.4415(3), c = 21.8375(4) Å, α = 92.4470(10), β = 96.5220(10), γ = 111.4330(10)°, V = 2844.21(10) Å3, Z = 1, Dc = 1.384 Mg m−3, 2θmax = 136.06°, T = 120 K, 35
, a = 10.5211(2), b = 13.4415(3), c = 21.8375(4) Å, α = 92.4470(10), β = 96.5220(10), γ = 111.4330(10)°, V = 2844.21(10) Å3, Z = 1, Dc = 1.384 Mg m−3, 2θmax = 136.06°, T = 120 K, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078 reflections measured, 9867 unique (Rint = 0.0300), μ = 4.417 mm−1. The final R1 and wR2 were 0.0905 and 0.2627 (I > 2σ(I)), 0.1174 and 0.2948 (all data).† Some atoms of propyl groups and chloroform molecules are disordered to two positions respectively. The occupancies of the disordered atoms were refined.
078 reflections measured, 9867 unique (Rint = 0.0300), μ = 4.417 mm−1. The final R1 and wR2 were 0.0905 and 0.2627 (I > 2σ(I)), 0.1174 and 0.2948 (all data).† Some atoms of propyl groups and chloroform molecules are disordered to two positions respectively. The occupancies of the disordered atoms were refined.Acknowledgements
This work was performed under the Cooperative Research Program of the “Network Joint Research Center for Materials and Devices”. We thank Prof. S. Higashibayashi and Prof. A. Nagai (IMS) for kindly donating the instruments.Notes and references
- J.-L. Brédas, J. Cornil, D. Beljonne, D. A. dos Santos and Z. Shuai, Acc. Chem. Res., 1999, 32, 267 CrossRef
.
- J.-L. Brédas, D. Beljonne, V. Coropceanu and J. Cornil, Chem. Rev., 2004, 104, 4971 CrossRef PubMed
.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS
.
- H. Sirringhaus, N. Tessler and R. H. Friend, Science, 1998, 280, 1741 CrossRef CAS
.
- N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474 CAS
.
- T. Förster, Ann. Phys., 1948, 2, 55 CrossRef
.
- G. C. Bazan, W. J. Oldham, Jr, R. J. Lachicotte, S. Tretiak, V. Chernyak and S. Mukamel, J. Am. Chem. Soc., 1998, 120, 9188 CrossRef CAS
.
- J. W. Hong, B. S. Gaylord and G. C. Bazan, J. Am. Chem. Soc., 2002, 124, 11868 CrossRef CAS PubMed
.
- J. W. Hong, H. Y. Woo, B. Liu and G. C. Bazan, J. Am. Chem. Soc., 2005, 127, 7435 CrossRef CAS PubMed
.
- Y. Morisaki and Y. Chujo, Macromolecules, 2002, 35, 587 CrossRef CAS
.
- Y. Morisaki and Y. Chujo, Macromolecules, 2003, 36, 9319 CrossRef CAS
.
- Y. Morisaki, F. Fujimura and Y. Chujo, Organometallics, 2003, 22, 3553 CrossRef CAS
.
- X. H. Sun, C. S. Chan, M. S. Wong and W. Y. Wong, Tetrahedron, 2006, 62, 7846 CrossRef CAS PubMed
.
- C. Song and T. M. Swager, Org. Lett., 2008, 10, 3575 CrossRef CAS PubMed
.
- A. Molad, I. Goldberg and A. Vigalok, J. Am. Chem. Soc., 2012, 134, 7290 CrossRef CAS PubMed
.
- S. Mataka, K. Takahashi, T. Mimura, T. Hirota, K. Takuma, H. Kobayashi and M. Tashiro, J. Org. Chem., 1987, 52, 2653 CrossRef CAS
.
- K. M. Knoblock, C. J. Silvestri and D. M. Collard, J. Am. Chem. Soc., 2006, 128, 13680 CrossRef CAS PubMed
.
- V. J. Chebny, R. Shukla, S. V. Lindeman and R. Rathore, Org. Lett., 2009, 11, 1939 CrossRef CAS PubMed
.
- T. Ogoshi, K. Umeda, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2009, 4874 RSC
.
- T. Ogoshi, R. Shiga, M. Hashizume and T. Yamagishi, Chem. Commun., 2011, 47, 6927 RSC
.
- Y. Morisaki, R. Hifumi, L. Lin, K. Inoshita and Y. Chujo, Polym. Chem., 2012, 3, 2727 RSC
.
- R. Yamakado, S. Matsuoka, M. Suzuki, K. Takagi, K. Katagiri and I. Azumaya, Tetrahedron, 2013, 69, 1516 CrossRef CAS PubMed
.
- K. Takagi, S. Sugimoto, R. Yamakado and K. Nobuke, J. Org. Chem., 2011, 76, 2471 CrossRef CAS PubMed
.
- R. Yamakado, K. Mikami, K. Takagi, I. Azumaya, S. Sugimoto, S. Matsuoka, M. Suzuki, K. Katagiri, M. Uchiyama and A. Muranaka, Chem. – Eur. J., 2013, 19, 11853 CrossRef CAS PubMed
.
- S. S. Babu, M. J. Hollamby, J. Aimi, H. Ozawa, A. Saeki, S. Seki, K. Kobayashi, K. Hagiwara, M. Yoshizawa, H. Möhwald and T. Nakanishi, Nat. Commun., 2013, 4, 1969 CrossRef PubMed
.
- Y. Fujiwara, R. Ozawa, D. Onuma, K. Suzuki, K. Yoza and K. Kobayashi, J. Org. Chem., 2013, 78, 2206 CrossRef CAS PubMed
.
- K. Hagiwara, Y. Sei, M. Akita and M. Yoshizawa, Chem. Commun., 2012, 48, 7678 RSC
.
- K. Miyamoto, T. Iwanaga and S. Toyota, Chem. Lett., 2010, 39, 288 CrossRef CAS
.
- S. Chen, Q. Yan, T. Li and D. Zhao, Org. Lett., 2010, 12, 4784 CrossRef CAS PubMed
.
- G. Venkataramana, P. Dongare, L. N. Dawe, D. X. Thompson, Y. Zhao and G. Bodwell, Org. Lett., 2011, 13, 2240 CrossRef CAS PubMed
.
- V. H. Gessner and T. D. Tilley, Org. Lett., 2011, 13, 1154 CrossRef CAS PubMed
.
- T. Li, K. Yue, Q. Yan, H. Huang, H. Wu, N. Zhu and D. Zhao, Soft Matter, 2012, 8, 2405 RSC
.
- W. C. W. Leu, A. E. Fritz, M. Diganantonio and C. S. Hartley, J. Org. Chem., 2012, 77, 2285 CrossRef CAS PubMed
.
- A. Yokoyama, T. Maruyama, K. Tagami, H. Masu, K. Katagiri, I. Azumaya and T. Yokozawa, Org. Lett., 2008, 10, 3207 CrossRef CAS PubMed
.
- I. Azumaya, H. Kagechika, K. Yamaguchi and K. Shudo, Tetrahedron Lett., 1996, 37, 5003 CrossRef CAS
.
- H. Kakuta, I. Azumaya, H. Masu, M. Matsumura, K. Yamaguchi, H. Kagechika and A. Tanatani, Tetrahedron, 2010, 66, 8254 CrossRef CAS PubMed
.
- N. Fujimoto, M. Matsumura, I. Azumaya, S. Nishiyama, H. Masu, H. Kagechika and A. Tanatani, Chem. Commun., 2012, 48, 4809 CAS
.
- C. Reichardt, Chem. Rev., 1994, 94, 2319 CrossRef CAS
.
- A. Simiarzuk, Z. R. Grabowski, A. Krówczyñski, M. Asher and M. Ottolenghi, Chem. Phys. Lett., 1977, 51, 315 CrossRef
.
- J. Herbich, A. Kapturkiewicz and W. Rettig, Chem. Phys., 1991, 158, 143 CrossRef CAS
.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899 CrossRef PubMed
.
- Z. Li, H. Ishizuka, Y. Sei, M. Akita and M. Yoshizawa, Chem. – Asian J., 2012, 7, 1789 CrossRef CAS PubMed
.
- T. Nakayama, T. Hamana, P. Jane, S. Akimoto, I. Yamazaki and K. Hamanoue, J. Phys. Chem., 1996, 100, 18431 CrossRef CAS
.
- P. Hrobárik, V. Hrobáriková, I. Sigmundová, P. Zahradnik, M. Fakis, I. Polyzos and P. Persephonis, J. Org. Chem., 2011, 76, 8726 CrossRef PubMed
.
- M. Kawai, K. Itaya and S. Toshima, J. Phys. Chem., 1980, 84, 2368 CrossRef CAS
.
- A short history of SHELX, G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data, GPC profiles, MALDI-TOF mass spectra, UV/fluorescence spectra, and X-ray crystal structures of materials. CCDC 961419 and 961420. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra46652c |
| This journal is © The Royal Society of Chemistry 2014 |