Oxidative desulfurization of dibenzothiophene based on air and cobalt phthalocyanine in an ionic liquid
Juan Zhang*,
Junpan Li,
Tengjie Ren,
Yanhui Hu,
Jingjing Ge and
Dishun Zhao
College of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang, 050018, P.R. China. E-mail: mytomorrow@126.com
First published on 28th October 2013
Abstract
Cobalt phthalocyanines (CoPc(Cl)n) were synthesized by a microwave method and characterized by UV-Vis spectroscopy, FTIR, elemental analysis and ICP. Dibenzothiophene (DBT) was oxidized with air as the oxidant and cobalt phthalocyanines (CoPc(Cl)n) as catalysts in an ionic liquid at room temperature and atmospheric conditions. The removal ratio of DBT reached 90.0% at room temperature after 2 h, with 0.3 wt% of CoPc(Cl)16 in an ionic liquid, and the ratio of the ionic liquid to the model oil was 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature. The effect of the substituents on the cobalt phthalocyanines on their catalytic activities was investigated. The results show that the catalytic activity of cobalt phthalocyanines with different substituents increases in the order CoPc(Cl)4 < CoPc(Cl)8 < CoPc(Cl)12 < CoPc(Cl)16, indicating that electron-withdrawing group substituents have a positive effect on the catalytic properties. The activity of CoPc(Cl)16 was kept unchanged after 5 runs of oxidation. The oxidation product detected by FTIR and mass spectrometry was single DBTO2. The desulfurization of different sulfur compounds and real gasoline was also investigated in this system. The sulfur removal ratio of five different sulfur compounds was over 80%. The sulfur content in real gasoline (1000 ppm) was decreased to 30 ppm after desulfurization.
1 at room temperature. The effect of the substituents on the cobalt phthalocyanines on their catalytic activities was investigated. The results show that the catalytic activity of cobalt phthalocyanines with different substituents increases in the order CoPc(Cl)4 < CoPc(Cl)8 < CoPc(Cl)12 < CoPc(Cl)16, indicating that electron-withdrawing group substituents have a positive effect on the catalytic properties. The activity of CoPc(Cl)16 was kept unchanged after 5 runs of oxidation. The oxidation product detected by FTIR and mass spectrometry was single DBTO2. The desulfurization of different sulfur compounds and real gasoline was also investigated in this system. The sulfur removal ratio of five different sulfur compounds was over 80%. The sulfur content in real gasoline (1000 ppm) was decreased to 30 ppm after desulfurization.
Introduction
Developed countries are setting new standards for the amount of sulfur allowed in diesel: in the United States, sulfur levels in diesel fuel must be below 15 ppm by 2014.1 Conventional hydrodesulfurization (HDS) is facing a major operational and economic challenge to meet the new stricter regulations built on the content of sulfur. To meet these specifications, a new desulfurization approach is needed. Among these new methods, oxidative desulfurization (ODS) is considered to be one of the promising new methods for super deep desulfurization of fuel oil.2–6 Lo et al. first combined chemical oxidation and solvent extraction to achieve the one-pot desulfurization of light oils.7 However, the efficiency of sulfur removal with ionic liquids (ILs) is relatively low due to the similar polarity between the sulfur-containing molecules and the remaining diesel fuels. More recently, a new and effective approach combining chemical oxidation with IL extraction (ECODS) has been explored in order to improve the efficiency of desulfurization.8–14 However, H2O2 is used as the oxidant in the above-mentioned IL systems and the by-product is H2O, which is necessary to remove by distillation in order to recover the ionic liquid and the product. In addition, H2O2 can become expensive and makes these catalysts expensive on the large scale. Molecular oxygen (O2) is cheap, available and environment friendly.Molecular oxygen would be an ideal oxidant for ODS if it could oxidize DBTs under acceptable conditions. Murata et al.15 used O2 as the oxidant in the presence of cobalt catalysts to oxidize dibenzothiophene. However, aldehydes must be used as co-oxidants. Sundararaman et al.16 tested unsupported CuO as a catalyst and air as the oxidant to oxidize sulfur compounds. However, the reaction temperature was higher than 100 °C. Metallophthalocyanines have attracted considerable interest because of their structural similarity to the active center of naturally occurring haemproteins.17 Metallophthalocyanines (MPcs) have been used as efficient biomimetic catalysts for oxidation, reduction and other reactions of organic compounds.18–23 Zhou et al.24 reported that the direct oxidation of dibenzothiophene (DBT) for deep desulfurization based on molecular oxygen and iron tetranitrophthalocyanine (FePc(NO2)4) catalyst was performed in a hydrocarbon solvent under water-free conditions. Conversion of DBT in decalin reached 98.7 (wt)% at 100 °C and 0.3 MPa of initial pressure with 1 (wt)% of FePc(NO2)4 over the whole solution for 2 h. However, conversion of DBT was only about 30% at room temperature in the above mentioned system, pure oxygen was used as the oxidant and poisonous acetonitrile was used as the solvent. Cobalt phthalocyanines are highly active catalysts with air or molecular oxygen as the oxidant at room temperature.25 V. B. Sharma et al. reported that secondary alcohols have been efficiently oxidized to their corresponding ketones in excellent yields with molecular oxygen using cobalt phthalocyanine as the catalyst in the presence of powder potassium hydroxide at room temperature.26 S. L. Jain et al. reported that a variety of hydroxyketones were efficiently oxidized to diketones in near quantitative yields with molecular oxygen, under alkaline conditions, using cobalt phthalocyaninetetrasulphonamide as the catalyst at room temperature.27 However, catalytic oxidation of dibenzothiophene has not been reported yet with a cobalt phthalocyanine as the catalyst.
In this study, a series of cobalt phthalocyanines attached with different strong electron-withdrawing groups, tetrachlorin cobalt phthalocyanine (CoPc(Cl)n), was employed as the catalyst for the direct oxidation of DBT using O2 from air in an ionic liquid solvent as a green and stable solvent at room temperature, under water-free conditions, at room temperature and atmosphere. The influence of the conditions on the performance of the oxidation as well as the effect of the substituents on the cobalt phthalocyanines on the catalytic activity and stability were investigated. The oxidative desulfurization combined with extraction was performed with a simulated fuel (DBT dissolved in n-octane).
Results and discussion
Oxidation of DBT
The oxidation of DBT under different conditions is shown in Fig. 1. When [PBy]BF4 and the model fuel are mixed simply without stirring, the removal ratio of DBT is very low due to the slow speed of mass transfer. When [PBy]BF4 and the model fuel are mixed with stirring, the removal ratio of DBT increases obviously, because stirring increases the chances of contact between the oil phase and the ionic liquid, and accelerates the mass transfer. However, extraction exists in a phase equilibrium and the removal ratio of DBT is only 26% when extracted with ionic liquids. Oxidation of DBT in n-octane using O2 in the absence of catalyst was also examined at room temperature after 2 h, and no oxidation of DBT was observed. Comparatively, in the presence of 0.3 (wt)% (catalyst/model oil) of CoPc(Cl)16 and under the same conditions, the removal ratio of DBT is up to 90% after 2 h. These results indicate that the catalyst is necessary for the oxidation and plays an important role in the reaction.Effect of the ratio of ionic liquid to model oil on the desulfurization reaction
The effect of the ratio of ionic liquid to model oil on the removal ratio of DBT is shown in Fig. 2. Ionic liquids as extracting agents can affect the mass transfer speed of DBT from the oil phase to the ionic liquid phase. First, ionic liquids extract DBT from the oil phase to the ionic liquid phase, and DBT is oxidized in the ionic liquid phase where the oxidation product remains. The ratio of ionic liquid to model oil on the removal ratio of DBT is a key factor for the oxidation reaction of DBT. From Fig. 2, we can see that the removal ratio of DBT increases gradually as the ratio increases. The extraction capacity of the ionic liquid increases with the amount of ionic liquid, but the extraction capacity of the ionic liquid is limited as the two phases exist in equilibrium. When the ratio is over 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the removal ratio of DBT no longer increases. Higher ratios can not accelerate the reaction speed and thus, the best ratio was found to be 1.8
1, the removal ratio of DBT no longer increases. Higher ratios can not accelerate the reaction speed and thus, the best ratio was found to be 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Effect of cobalt phthalocyanines with different substituents on the desulfurization reaction
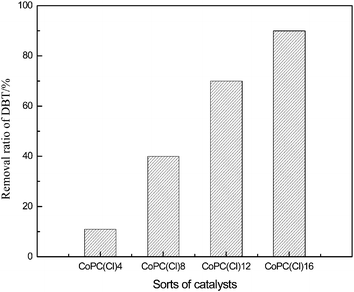
A series of oxidation reactions were carried out to investigate the effect of the substituents on the removal ratio of DBT with 0.3 wt% of CoPc(Cl)n after 2 h. As shown in Fig. 3, the compounds with n from 4 to 8 lead to a remarkable increase in the removal ratio of DBT. With n = 16, the conversion of DBT was up to 90.4%, compared to 18% obtained with n = 4. The chloride ion is an electron-withdrawing substituent by inductive and resonant effects; so the electron density of the Pc ring increases in the order of CoPc(Cl)4 < CoPc(Cl)8 < CoPc(Cl)12 < CoPc(Cl)16. More chloride ions can enhance the conjugating strength of phthalocyanine, accelerate the electron transmission and increase the catalytic activity. Buck et al.28 found that substituents on the cobalt phthalocyanine influenced the catalytic activity. Electron-withdrawing substituents enhance the catalytic activity while electron-donating substituents decrease it. This study is in accordance with the conclusion obtained by Buck. In subsequent experiments, CoPc(Cl)16 was always used.Effect of the air flow rate on the desulfurization reaction
Molecular oxygen has been widely used as an oxidant in the ODS process.29 However, the use of air as the oxidant30 in the ODS system is seldom. Because there is only a 21% (volume ratio) of molecular oxygen in the air, its oxidation susceptibility is week, as considered by some researchers. In our previous study,31 we found that air can be a good oxidant if used with a highly active catalyst. In addition, it is cheap, non-polluting and commercially available. Fig. 4 shows that air is an effective oxidant in this ODS system The curve presented in Fig. 4 shows that the conversion of DBT increases significantly with the increasing air flow rate from 0 to 100 mL min−1, and then decreases slowly from 100 to 300 mL min−1. It may demonstrate that oxidation of DBT can be facilitated by the dissolved oxygen in the liquid phase. Because air passes through the ionic liquid from the bottom of the reactor, when the air flow is increased, most of the oxygen (O2) flows away before the reaction can occur. In other words, the time the oxygen (O2) remains in solution is shortened and thus, the oxidation efficiency of the system decreases. An air flow rate between 50 and 100 mL min−1 is the most appropriate .Stability of cobalt phthalocyanine catalysts in the oxidation reaction
To investigate the reusability of the cobalt phthalocyanine catalyst, oxidation of DBT was performed in 5 runs in the presence of CoPc(Cl)16, where the cobalt phthalocyanine catalyst was reused. The catalyst was separated after each run, and dried at 90 °C under vacuum. The results presented in Table 1 show that the activity of CoPc(Cl)16 is kept unchanged after 5 cycles.| Times | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Removal ratio of DBT/% | 90.4 | 90.5 | 90.3 | 90 | 90.2 |
The ionic liquid is the transparent pale yellow liquid before the reaction, becomes dark green after adding CoPc(Cl)16 during the reaction, and is still transparent pale yellow after CoPc(Cl)16 is removed by centrifugation at the end of the reaction. The color of CoPc(Cl)16 after the reaction did not change. This suggests that CoPc(Cl)16 did not decompose during the oxidation. It is commonly accepted that phthalocyanines modified with electron-withdrawing substituents have a better stability,32 because the electron deficiency of the Pc ring offers resistance to any possible oxidant electrophilic attack that would result in the decomposition of phthalocyanine.
By this procedure, DBTO2 is accumulated in the IL phase in successive runs. After the fifth cycle, the IL phase was re-extracted with tetrachloromethane at room temperature. Then, tetrachloromethane was separated from the IL and distilled under vacuum at 60 °C until a white crystal solid was produced (Fig. 5 photo (d)). The white crystalline solid was characterized by IR spectroscopy (Fig. 6). The two absorption bands at 1164 and 1289 cm−1 are attributed to the sulfone groups.33 The mass spectrum of DBTO2 is shown in Fig. 6, The fragmentation peaks at m/z 216 (239-23 (element Na) = 216) are distinctly detected.
Desulfurization of different sulfur compounds by CoPc(Cl)16
The removal of different sulfur-containing compounds, including thiophene (Th), methyl thiophene (MT), benzothiophene (BT), dibenzothiophene (DBT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT) by catalyst CoPc(Cl)16 was studied. As shown in Table 2, the sulfur removal ratio decreases in the following order Th (100.0%) > DBT (90.4%) > BT(89.1) > MT(81.9) > 4,6-DMDBT (80%) at room temperature after 2 h. The different desulfurization efficiency of the different sulfur compounds may be influenced mainly by steric hindrance and the electron density on the sulfur atom. However the sulfur removal ratio in all cases is higher than 80%, which indicates that CoPc(Cl)16 is a good catalyst for different sulfur-containing compounds.| Sulfur compound | Th | MT | BT | DBT | 4,6-DMDBT |
|---|---|---|---|---|---|
| Sulfur removal ratio/% | 100 | 81.9 | 89.1 | 90.4 | 80 |
Desulfurization of gasoline by CoPc(Cl)16
Real gasoline (1000 ppm) was oxidized under the above mentioned optimal reaction conditions. Fig. 7 shows the gas chromatograms of gasoline before and after the oxidation desulfurization reaction. We can see that most of the sulfur-containing compounds have been removed. The sulfur content of gasoline decreased to 30 ppm after oxidation. In addition, the oxidation products found in the gasoline phase were transferred to the ionic liquid phase.Conclusions
In this paper, four cobalt phthalocyanines (CoPc(Cl)n, n = 4, 8, 12, 16) were synthesized successfully. The cobalt phthalocyanines were dissolved in an IL ([PBy]BF4) to oxidize DBT for deep desulfurization with air at room temperature. The removal ratio of DBT reached 90.0% at room temperature after 2 h under optimal reaction conditions (ratio of ionic liquid to model oil 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 0.3 wt% CoPc(Cl)16 per 10 mL model oil; 100 mL min−1 air flow rate, room temperature), which is the remarkable advantage of this process over desulfurization by mere solvent extraction with ILs or catalytic oxidation without ILs. Moreover, the activity of cobalt phthalocyanines with different substituents increases in the order CoPc(Cl)4 < CoPc(Cl)8 < CoPc(Cl)12 < CoPc(Cl)16. The catalytic oxidation system containing cobalt phthalocyanines and [PBy]BF4 could be recycled five times without a significant decrease in activity. The oxidized product of DBT is DBTO2 as observed by infrared spectroscopy and mass spectrometry. The reaction conditions of this oxidation system are mild (room temperature and atmospheric conditions), the use of air as the oxidant is cheap and safe, and the catalyst and ionic liquid are reproducible. The sulfur removal ratio of five sulfur compounds was found to be over 80%. The sulfur content of real gasoline (1000 ppm) was decreased to 30 ppm after desulfurization. This oxidation system is a promising desulfurization technology.
1; 0.3 wt% CoPc(Cl)16 per 10 mL model oil; 100 mL min−1 air flow rate, room temperature), which is the remarkable advantage of this process over desulfurization by mere solvent extraction with ILs or catalytic oxidation without ILs. Moreover, the activity of cobalt phthalocyanines with different substituents increases in the order CoPc(Cl)4 < CoPc(Cl)8 < CoPc(Cl)12 < CoPc(Cl)16. The catalytic oxidation system containing cobalt phthalocyanines and [PBy]BF4 could be recycled five times without a significant decrease in activity. The oxidized product of DBT is DBTO2 as observed by infrared spectroscopy and mass spectrometry. The reaction conditions of this oxidation system are mild (room temperature and atmospheric conditions), the use of air as the oxidant is cheap and safe, and the catalyst and ionic liquid are reproducible. The sulfur removal ratio of five sulfur compounds was found to be over 80%. The sulfur content of real gasoline (1000 ppm) was decreased to 30 ppm after desulfurization. This oxidation system is a promising desulfurization technology.
Experimental
Preparation of catalyst
CoPc(Cl)n (n = 4,8,12,16) was prepared following the microwave method published by Shaabani et al.34 A mixture of a given phthalic anhydride, hexamethyl disilazane, (NH4)2SO4, CoCl2 and DMF was irradiated in a glass sealed tube in a domestic microwave oven. As the reaction proceeded, a blackish green solid gradually appeared. After completion of the reaction, the solid was washed with methanol and dissolved in concentrated sulfuric acid, and then the solution was poured into water to give a blue precipitate. The precipitate was filtered and washed successively with distilled water and dried in an oven. The blackish green solid CoPc(Cl)n was obtained in 76% yield. UV-Vis: λmax (nm): 325, 498 and 681. IR (KBr, ν cm−1): 741 w and 763 m (characteristic peaks of the Pc ring), 1090 m (C–H of Pc), 1146 m (Co–N of Pc), 1579 w (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of Pc) and 1603 w (
C of Pc) and 1603 w (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of Pc). Elemental Analysis found (CoPc(Cl)16): C, 34.52%, N, 10.85%; calculated: C, 34.25%, N, 9.99%. ICP found: Co, 5.36%; calculated: Co, 5.23%.
N of Pc). Elemental Analysis found (CoPc(Cl)16): C, 34.52%, N, 10.85%; calculated: C, 34.25%, N, 9.99%. ICP found: Co, 5.36%; calculated: Co, 5.23%.
Analysis of the catalysts
The catalysts were characterized by Infrared Spectroscopy (IR, Nicolet-380), Elemental Analysis (EA, Vario-EL-CUBE) and Inductively Coupled Plasma-Atom Emission Spectrometry (ICP, icpe-900).Oxidative desulfurization procedure
In a typical DBT oxidation, 10 mL of model fuel (DBT in n-octane, S (wt)% = 1000 μg g−1), 10 mL of ionic liquid and a given catalyst were added to a quartz sand core funnel at room temperature. Air was fed from the bottom of the funnel and 0.57 (wt)% DBT in n-octane was used for the investigation of the bulk material. Liquid samples were taken at intervals of 20 min to determine the concentration variation of the sulfur-containing compounds with time. Both feed and products were analyzed on a Tianmei-789011 gas chromatograph equipped with a FPD. The oxidation products were identified by means of LC-MS (HP1100LC/MSD). The conversion of the sulfur-containing compounds in the feed was used as a measure of the catalytic performance (Scheme 1).Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (no. 21106032), and Hebei University of Science and Technology doctor funding (no. 000172).References
- T. V. Rao, B. Sain, S. Kafola, B. R. Nautiyal, Y. K. Sharma, S. M. Nanoti and M. O. Garg, Energy Fuels, 2007, 21, 3420 CrossRef CAS.
- K. Castillo, J. G. Parsons, D. Chavez and R. R. Chianelli, J. Catal., 2009, 268(2), 329 CrossRef CAS PubMed.
- J. Zhang, A.-J. Wang, X. Li and X.-H. Ma, J. Catal., 2011, 279, 269 CrossRef CAS PubMed.
- J. L. García-Gutiérrez, G. A. Fuentes, M. E. Hernández-Terán, P. García, F. Murrieta-Guevara and F. Jiménez-Cruz, Appl. Catal., A, 2008, 334(1–2), 366 CrossRef PubMed.
- J. Shen, H. P. Li and H. Zhao, Pet. Sci. Technol., 2008, 26, 2182 CrossRef CAS.
- N. Y. Chan, T.-Y. Lin and T. F. Yen, Energy Fuels, 2008, 22, 3326 CrossRef CAS.
- W. H. Lo, H. Y. Yang and G. T. Wei, Green Chem., 2003, 5(5), 639 RSC.
- P. S. Kulkarni and C. A. M. Afonso, Green Chem., 2010, 12, 1139 RSC.
- H. Y. Lü, J. B. Gao, Z. X. Jiang, F. Jing, Y. X. Yang, G. Wang and C. Li, J. Catal., 2006, 239(2), 369 CrossRef PubMed.
- C. Li, Z. Jiang, J. Gao, Y. Yang, S. Wang, F. Tian, F. Sun, X. Sun, P. Ying and C. Han, Chem.–Eur. J., 2004, 10, 2277 CrossRef CAS PubMed.
- H. Li, W. Zhu, Y. Wang, J. Zhang, J. Lu and Y. Yan, Green Chem., 2009, 11, 810 RSC.
- D. S. Zhao, J. L. Wang and E. P. Zhou, Green Chem., 2007, 9, 1219 RSC.
- Y. S. Chi, C. P. Li, Q. Z. Jiao, Q. S. Liu, P. F. Yan, X. M. Liu and U. Welz-Biermann, Green Chem., 2011, 13, 1224 RSC.
- W. S. Zhu, H. M. Li, X. Jiang, Y. S. Yan, J. D. Lu, L. N. He and J. X. Xia, Green Chem., 2008, 10, 641 RSC.
- S. Murata, K. Murata, K. Kidena and M. Nomura, Energy Fuels, 2004, 18(1), 116 CrossRef CAS.
- R. Sundararaman, X. Ma and C. Song, Ind. Eng. Chem. Res., 2010, 49, 5561 CrossRef CAS.
- V. I. Iliev, A. I. Ileva and L. D. Dimitrov, Appl. Catal., A, 1995, 126, 333 CrossRef CAS.
- A. B. Sorokin and A. Tuel, Catal. Today, 2000, 57, 45 CrossRef CAS.
- V. B. Sharma, S. L. Jain and B. Sain, Tetrahedron Lett., 2003, 44(2), 383 CrossRef CAS.
- R. Naik, P. Joshi and R. K. Deshpande, J. Mol. Catal. A: Chem., 2005, 238(1–2), 46 CrossRef CAS PubMed.
- M. Alvaro, E. Carbonell, M. Espla and H. Carcia, Appl. Catal., B, 2005, 57(1), 37 CrossRef CAS PubMed.
- S. L. Jain and B. Sain, J. Mol. Catal. A: Chem., 2001, 176(1–2), 101 CrossRef CAS.
- N. Grootboom and T. Nyokong, J. Mol. Catal. A: Chem., 2002, 179(1–2), 113 CrossRef CAS.
- X. R. Zhou, J. Li, X. N. Wang, K. Jin and W. Ma, Fuel Process. Technol., 2009, 90(2), 317 CrossRef CAS PubMed.
- S. M. S. Chauhan, A. Kumar and K. A. Srinivas, Chem. Commun., 2003, 2348 RSC.
- V. B. Sharma, S. L. Jain and B. Sain, Tetrahedron Lett., 2003, 44, 383 CrossRef CAS.
- S. L. Jain and B. Sain, J. Mol. Catal. A: Chem., 2001, 176, 101 CrossRef CAS.
- T. Buck, H. Bohlen and D. Wǒhrle, J. Mol. Catal., 1993, 80, 253–267 CrossRef CAS.
- S. K. Murata, K. Kidena and M. Nomura, Energy Fuels, 2004, 18, 116 CrossRef CAS.
- W. Guo, C. Y. Wang, P. Lin and X. P. Lu, Appl. Energy, 2011, 88, 175 CrossRef CAS PubMed.
- J. Zhang, D. S. Zhao, Z. Ma and Y. N. Wang, Catal. Lett., 2010, 138(1–2), 111 CrossRef CAS.
- S. Nthapo and N. Tebello, J. Mol. Catal. A: Chem., 2004, 209, 51 CrossRef PubMed.
- Y. Shiraishi, K. Tachibana, T. Hirai and I. Komasawa, Ind. Eng. Chem. Res., 2002, 41, 4362 CrossRef CAS.
- A. Shaabani, R. Maleki-Moghaddam, A. Maleki and A. H. Rezayan, Dyes Pigm., 2007, 74, 279 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |