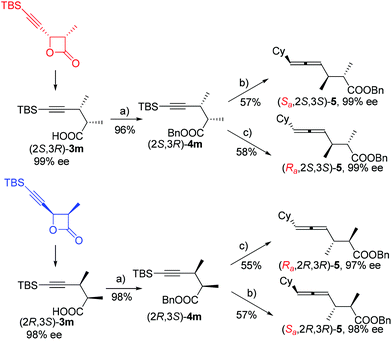
Highly selective 4-alkynoic acids synthesis via iron-mediated complete inversion of stereogenic carbon centers†
Xiaobing
Zhang
,
Youai
Qiu
,
Chunling
Fu
and
Shengming
Ma
*
Laboratory of Molecular Recognition and Synthesis, Department of Chemistry, Zhejiang University, 310027 Hangzhou, Zhejiang, P. R. China. E-mail: masm@sioc.ac.cn; Fax: (+86) 21-62609305
First published on 19th February 2014
Abstract
An Fe-catalyzed highly regio- and diastereoselective reaction of 4-ethynyloxetan-2-ones with different Grignard reagents affording alkynes 3 in good yields with absolute configuration of the β-carbon atom completely inverted has been developed. The optically active acids may be easily converted to esters, which may easily undergo desilylation and highly diastereoselective allenylation to afford optically active 4,5-allenoates with two chiral centers and one axial allene chirality.
Introduction
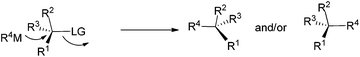
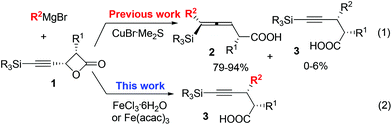
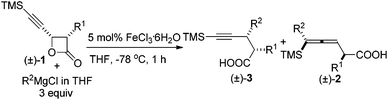
As is well known, the stereoselectivity in the reaction of a chiral carbon center bearing a leaving group such as halide, tosylate, or tosylamide with a hard carbon nucleophile, such as Grignard reagents, lithium reagents, or lithium cuprates, is a basic topic of current interest with either high stereoselectivity for inversion or partial racemization.1–9 Although iron compounds or complexes have been recently reported to be very efficient catalysts for many organic transformations,10 the FeCl3-catalyzed reactions of ArMgX with optically active alkyl bromides proceed with complete racemization.11 Surprisingly, such reactions with the more readily available yet stable acetates are quite limited (Scheme 1).12On the other hand, in 2000, Nelson et al. reported the Cu(I)-catalyzed SN2′ type coupling reaction of 4-(trimethylsilylethynyl) oxetan-2-one 1 with alkyl Grignard reagents for the synthesis of β-allenoic acid derivatives 2 together with the formation of γ-propargylic acids 3 as the minor products (eqn (1));13–15 Fürstner et al. reported the Fe(acac)3-catalyzed reaction of Grignard reagents with 1-alkynyl epoxides affording 2,3-allenols16 and the [Fe(MgX)2]n-catalyzed coupling reaction of primary propargylic bromides with Grignard reagents affording alkynes.17–19 Herein, we wish to report the highly regio- and stereo-selective Fe-catalyzed reaction of 4-(trimethylsilylethynyl)oxetan-2-one 1 with Grignard reagents affording 4-alkynoic acids 3 with absolute configuration of the chiral center being completely inverted.
Results and discussion
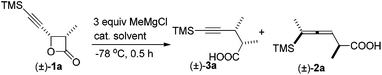
During the study of the reaction of cis-3-methyl-4-(trimethylsilylethynyl)oxetan-2-one 1a with 3 equiv. of MeMgCl, it was observed that the reaction catalyzed by 10 mol% of Fe(acac)3 at −78 °C afforded 2,3-dimethyl-5-(trimethylsilyl)pent-4-ynoic acid 3a as the major product with a selectivity of 3a/2a = 97/3 and dr value of 3a being about 100/0 as judged by the 1H NMR analysis of the crude reaction mixture (entry 1, Table 1). Other solvents such as Et2O and toluene led to a lower yield and selectivity (entries 2 and 3, Table 1). FeCl3·6H2O demonstrated a similar results and the loading of catalyst could be reduced to 5 mol% (entries 6 and 7, Table 1). Anhydrous FeCl3 catalyzed this transformation affording 3a in 61% yield together with 2a in 3% yield (entry 8, Table 1). Iron salt is vital to the coupling reaction: neither 3a nor 2a was provided from the blank test and the control experiment with CuCl as catalyst (entries 9 and 10, Table 1).| Entry | Cat. (mol%) | Solvent | Yield of 3ab (%) | 3a/2a |
|---|---|---|---|---|
| a The reaction was conducted using 0.2 mmol of 1a, 3 equiv. of MeMgCl, and the indicated amount of the corresponding catalyst in 3 mL of solvent. b NMR yield. c The reaction time was 1.0 h. d 1 mmol of 1a was applied and the reaction time was 1 h. e Recovery: 80%. f Recovery: 77%. | ||||
| 1 | Fe(acac)3 (10) | THF | 86 | 97/3 |
| 2 | Fe(acac)3 (10) | Et2O | 79 | 95/5 |
| 3 | Fe(acac)3 (10) | Toluene | 77 | 95/5 |
| 4 | Fe(acac)2 (10) | THF | 87 | 97/3 |
| 5c | FeCl2 (10) | THF | 87 | 98/2 |
| 6 | FeCl3·6H2O (10) | THF | 83 | 96/4 |
| 7d | FeCl3·6H2O (5) | THF | 88 | 98/2 |
| 8 | FeCl3 (10) | THF | 61 | 95/5 |
| 9e | CuCl (10) | THF | 0 | — |
| 10f | — | THF | 0 | — |
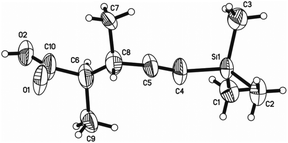
The structure, as well as the relative configurations of the two chiral centers of 3a, was unambiguously established by its single crystal X-ray diffraction analysis (Fig. 1),20 which clearly indicated that the in situ generated metallic reagent undergoes an SN2 substitution reaction at the β-position of 4-(trimethylsilylethynyl)oxetan-2-one affording 4-alkynoic acid 3 with the configuration of this carbon center inverted.
Under this set of optimized reaction conditions, the scope and the limitations of this reaction were explored. The reaction of 1b with MeMgCl afforded 2,3-dimethyl-5-(trimethylsilyl)pent-4-ynoic acid 3b with a yield of 84% and a selectivity of 97/3 (entry 1, Table 2). Aryl Grignard reagents could also be used in the reaction providing the corresponding products 3 in 73–89% yield with a regioselectivity of 93/7–97/3 (entries 2 and 5–8, Table 2). Different alkyl or allyl substituents could be introduced into the 2-position of the products in yields of 78%–87% with excellent ratios of 97/3–99/1 of 3/2 (entries 3 and 9–11, Table 2).
| Entry | R1 | R2 | Yield of 3 (%) | 3/2 |
|---|---|---|---|---|
| a The reaction was conducted using 1 mmol of 1, 3 equiv. of Grignard reagents, and 5 mol% of FeCl3·6H2O in 5 mL of THF. b 0.4 mmol of 1a was applied. c The reaction time was 1.5 h. d 0.5 mmol of 1d was applied. e TES was used instead of TMS. | ||||
| 1 | H (1b) | Me | 84 (3b) | 97/3 |
| 2 | H (1b) | Ph | 80 (3c) | 94/6 |
| 3 | Me (1a) | Me | 84 (3a) | 98/2 |
| 4b | Me (1a) | Ph | 88 (3d) | 96/4 |
| 5 | Me (1a) | p-MeC6H4 | 78 (3e) | 95/5 |
| 6 | Me (1a) | m-MeC6H4 | 79 (3f) | 93/7 |
| 7 | Me (1a) | p-MeOC6H4 | 73 (3g) | 95/5 |
| 8 | Me (1a) | 2-Thienyl | 89 (3h) | 97/3 |
| 9c | CH2CH2Cl (1c) | Me | 78 (3i) | 97/3 |
| 10d | Allyl (1d) | Me | 87 (3j) | 99/1 |
| 11c,e | n-C3H7 (1e) | Me | 77 (3k) | 98/2 |
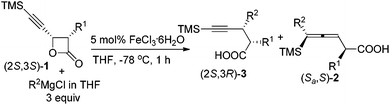
Based on these observations, we were anxious to see whether various optically active substrates 1 may be applied to this Fe-catalyzed coupling reaction. Interestingly, when optically active 4-(trimethylsilylethynyl)oxetan-2-ones were treated with different Grignard reagents, the corresponding optically active 4-alkynoic acids 3 were formed without any racemization (Table 3). Furthermore, we may easily conduct the reaction of 0.8976 g of (2R,3R)-1g and 0.8934 g of (2S,3S)-1g affording 0.9015 g of (2R,3S)-3m and 0.8967 g of (2S,3R)-3m in excellent yield and selectivity (entries 7 and 8, Table 3).
| Entry | 1 | R2 | Yield of 3 (%) (ee%) | 3/2 |
|---|---|---|---|---|
| R1 (ee%) | ||||
| a The reaction was conducted using 1 mmol of 1, 3 equiv. of Grignard reagents, and 5 mol% of FeCl3·6H2O in 5 mL of THF. b The reaction time was 2 h. c TBS was used instead of TMS and 4 mmol of the 1g were applied. | ||||
| 1 | (S,S)-1a Me (98) | Me | 81 (99) (S,R)-3a | 98/2 |
| 2 | (S,S)-1a Me (98) | Ph | 77 (98) (S,R)-3d | 95/5 |
| 3b | (S,S)-1a Me (98) | PMP | 72 (97) (S,R)-3g | 95/5 |
| 4 | (R,R)-1d allyl(99) | Me | 81 (98) (R,S)-3j | 99/1 |
| 5 | (S,S)-1fnPr (97) | Me | 74 (97) (S,R)-3l | 98/2 |
| 6 | (R,R)-1fnPr (>99) | Me | 73 (>99) (R,S)-3l | 97/3 |
| 7c | (S,S)-1g Me(>99) | Me | 94 (99) (S,R)-3m | 98/2 |
| 8c | (R,R)-1g Me(>99) | Me | 94 (98) (R,S)-3m | 99/1 |
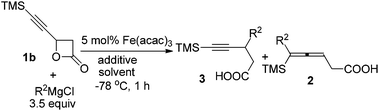
However, the reaction of FeCl3·6H2O-catalyzed coupling of 1b bearing just one chiral center with n-C4H9MgCl afforded a complex mixture (entry 1, Table 4) while a ratio of 75/25 of 3/2 was observed when Fe(acac)3 was used instead of FeCl3·6H2O (entry 2, Table 4). Et2O appeared better than THF (entry 3, Table 4) and the addition of 1 equiv. of NaI, added as ligand, also helped (entry 4, Table 4). By comparing the results with these shown in Table 3, we reasoned that water from FeCl3·6H2O may have a unique role. In fact, the addition of 0.3 equiv. of H2O did further improve the regioselectivity of this coupling reaction using Fe(acac)3 as the catalyst (entries 4 and 5, Table 4). The reactions of 1b with n-C4H9MgCl and n-C5H11MgCl afforded the corresponding products 3n and 3o in 71% and 69% yields with an excellent ratio of 94/6 of 3/2 (entries 5 and 6, Table 4).
| Entry | R2MgCl | Solvent | Additive | Yield of 3b (%) | 3/2 |
|---|---|---|---|---|---|
| a The reaction was conducted using 0.2 mmol of 1b, 3.5 equiv. of the Grignard reagent, 5 mol% of Fe(acac)3, 1 equiv. of NaI (if required), 0.3 equiv. H2O (if required), 2 mL of solvent. b Isolated yield of 3. c FeCl3·6H2O was applied. d 1 mmol of 1b was applied. | |||||
| 1c | n-C4H9 | THF | — | Complex mixture | |
| 2 | n-C4H9 | THF | — | 60 (3n) | 75/25 |
| 3 | n-C4H9 | Et2O | — | 70 (3n) | 89/11 |
| 4 | n-C4H9 | Et2O | NaI | 64 (3n) | 91/9 |
| 5d | n-C4H9 | Et2O | NaI–H2O | 71 (3n) | 94/6 |
| 6d | n-C5H11 | Et2O | NaI–H2O | 69 (3o) | 94/6 |
Furthermore, we conducted the SN2 substitution reaction of (R)-1b with a single chiral center with n-C4H9MgCl affording (S)-3n without any racemization in 68% yield with a ratio of 94/6 of (S)-3n/(Ra)-2n (eqn (3)).
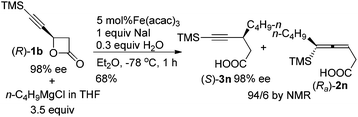 | (3) |
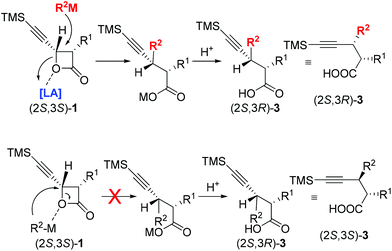
These “inverted” observations led to the conclusion that the mechanism is different from the reported one under Fe-catalysis with the coordination-directed direct delivery of the R group16 from the in situ generated highly reduced iron–magnesium cluster reagent.17 We believed that the attack of the R2 from in situ generated highly reduced iron–magnesium cluster reagent R2–M at the 4-position of 4-(trimethylsilyl)ethynyloxetan-2-one (2S,3S)-1 may be mediated by the Lewis acidic species in the solution coordinating with the oxygen atom to afford (2S,3R)-3. The steric effect of the trimethylsilylacetylenyl group is beneficial to this transformation. The configuration of the chiral center in the products (2S,3R)-3 followed the Walden inversion which shows that the coupling reaction proceeds via an SN2 mechanism with complete inversion of the carbon center (Scheme 2).21
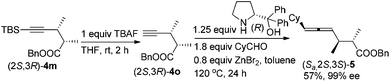
It is well established that the extra C–C triple bond in the products is synthetically very useful in organic synthesis, undergoing many reactions22 such as highly stereoselective ionic additions, semi-hydrogenation, complete hydrogenation, and manipulation based on the terminal acidic C–H bond. In addition to these known protocols, here, (2S,3R)-5-(t-butyldimethylsilyl)-2,3-dimethylpent-4-ynoic acid (2S,3R)-3m was converted to the benzyl ester easily, which was followed by desilylation by treating with TBAF. This terminal alkynoic acid ester may undergo an enantioselective allenylation of terminal alkyne reaction (EATA) with (R)- or (S)-diphenylprolinol and CyCHO23 providing (Sa,2S,3S)-benzyl-6-cyclohexyl-2,3-dimethylhexa-4,5-dienoate (Sa,2S,3S)-5 or (Ra,2S,3S)-benzyl-6-cyclohexyl-2,3-dimethylhexa-4,5-dienoate (Ra,2S,3S)-5 in excellent de and ee (Scheme 3). The absolute configuration of the allene unit has been assigned based on our previous report.23 Likewise, its enantiomers (Sa,2R,3R)-5 and (Ra,2R,3R)-5 could be prepared similarly by using (2R,3S)-5-(t-butyldimethylsilyl)-2,3-dimethylpent-4-ynoic acid (2R,3S)-3m as the starting material.
In addition, (2S,3R)-4m may undergo a desilylation reaction followed by a Sonogashira coupling24 with PhI to afford (2S,3R)-benzyl 2,3-dimethyl-5-phenylpent-4-ynoate (2S,3R)-6, which further extends the potential of this reaction by introducing an extra substituent at this location (eqn (4)).
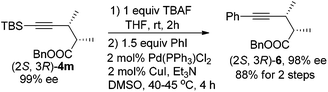 | (4) |
In summary, we have achieved an Fe-catalyzed highly regio- and stereoselective coupling reaction of 4-ethynyl-oxetan-2-ones with different Grignard reagents affording 4-alkynoic acids in good yields with the absolute configuration of the β-carbon atom completely inverted. The optically active acids may be easily esterified, which may be followed by desilylation to unveil the attractive terminal C–C triple bond for arylation or enantioselective allenylation (EATA) to afford optically active 4,5-allenoates with defined advanced steric nature. Although the mechanism, especially the real nature of the in situ generated metallic species and the role of NaI as well as water, is far from clear, due to the easy availability of the Fe-catalyst and optically active starting materials,25 high regio- and stereo-selectivity, and potential of the products, this reaction will be useful in organic synthesis involving multiple chiralities. Further studies including the effect of water in this area are ongoing in our laboratory.
Experimental
1. Fe-catalyzed SN2 coupling reaction of Grignard reagent with 1. Synthesis of 2,3-dimethyl-5-(trimethylsilyl)pent-4-ynoic acid 3a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to afford 3a (167.3 mg, 84%): Solid: m.p. 67.3–68.4 °C (hexane–ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 10.54 (brs, 1 H, COOH), 2.83 (pentet, J = 7.2 Hz, 1 H, CH), 2.42 (pentet, J = 7.2 Hz, 1 H, CH), 1.32 (d, J = 6.9 Hz, 3 H, CH3), 1.22 (d, J = 6.9 Hz, 3 H, CH3), 0.14 (s, 9 H, 3 × CH3Si); 13C NMR (CDCl3, 75 MHz) δ 181.6, 108.0, 86.6, 45.1, 30.0, 19.4, 14.8, 0.1; IR (neat, cm−1) 2977, 2938, 2899, 2169, 1712, 1460, 1427, 1373, 1268, 1245, 1212, 1088; MS (EI) m/z (%) 198 (M+, 0.23), 183 ((M − CH3)+, 20.67), 75 (100); Elemental analysis calcd for C10H18O2Si: C, 60.56, H, 9.15, found: C, 60.40, H, 8.95%.
1 to afford 3a (167.3 mg, 84%): Solid: m.p. 67.3–68.4 °C (hexane–ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 10.54 (brs, 1 H, COOH), 2.83 (pentet, J = 7.2 Hz, 1 H, CH), 2.42 (pentet, J = 7.2 Hz, 1 H, CH), 1.32 (d, J = 6.9 Hz, 3 H, CH3), 1.22 (d, J = 6.9 Hz, 3 H, CH3), 0.14 (s, 9 H, 3 × CH3Si); 13C NMR (CDCl3, 75 MHz) δ 181.6, 108.0, 86.6, 45.1, 30.0, 19.4, 14.8, 0.1; IR (neat, cm−1) 2977, 2938, 2899, 2169, 1712, 1460, 1427, 1373, 1268, 1245, 1212, 1088; MS (EI) m/z (%) 198 (M+, 0.23), 183 ((M − CH3)+, 20.67), 75 (100); Elemental analysis calcd for C10H18O2Si: C, 60.56, H, 9.15, found: C, 60.40, H, 8.95%.
2. Desilylation and enantioselective allenylation of 4m. Synthesis of (Sa,2S,3S)-benzyl 6-cyclohexyl-2,3-dimethylhexa-4,5-dienoate (Sa,2S,3S)-5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford (2S,3R)-benzyl 2,3-dimethylpent-4-ynoate (2S,3R)-4o, which was used directly in the next step.
1) to afford (2S,3R)-benzyl 2,3-dimethylpent-4-ynoate (2S,3R)-4o, which was used directly in the next step.
To a reaction tube was added ZnBr2 (90.3 g, 0.40 mmol). This reaction tube was then dried under vacuum with a heating gun. (R)-Diphenylprolinol (156.4 mg, 0.62 mmol), CyCHO (101.2 mg, 0.90 mmol)/toluene (1 mL), and (2S,3R)-benzyl 2,3-dimethylpent-4-ynoate (2S,3R)-4o/toluene (2 mL) were then added sequentially under a N2 atmosphere. The reaction tube was then placed in a pre-heated oil bath at 120 °C. After the reaction was complete as monitored by TLC, the reaction mixture was cooled to rt and the crude reaction mixture was filtered through a short pad of silica gel (2 cm) eluted with ether. After evaporation, the residue was purified by flash chromatography on silica gel (petroleum ether–ethyl ether = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford (Sa,2S,3S)-5 (88.5 mg, 57%, 99% ee: HPLC conditions: OJ-H column, rate = 0.7 mL min−1, eluent: hexane–i-PrOH = 400
1) to afford (Sa,2S,3S)-5 (88.5 mg, 57%, 99% ee: HPLC conditions: OJ-H column, rate = 0.7 mL min−1, eluent: hexane–i-PrOH = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, λ = 214 nm, tR 14.8 min (minor), 15.8 min (major)): Liquid; [α]20D = +11.6 (c = 1.82, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.40–7.30 (m, 5 H, ArH), 5.19–5.00 (m, 4 H, CH2 + 2 × CH
1, λ = 214 nm, tR 14.8 min (minor), 15.8 min (major)): Liquid; [α]20D = +11.6 (c = 1.82, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.40–7.30 (m, 5 H, ArH), 5.19–5.00 (m, 4 H, CH2 + 2 × CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.58–2.35 (m, 2 H, 2 × CH), 2.01–1.85 (m, 1 H, CH), 1.80–1.56 (m, 5 H, 5 protons of Cy), 1.35–0.95 (m, 11 H, 5 protons of Cy + 2 × CH3); 13C NMR (CDCl3, 75 MHz) δ 202.4, 175.6, 136.0, 128.5, 128.1, 98.2, 94.2, 66.0, 45.5, 37.1, 36.6, 33.04, 33.00, 26.1, 26.0, 18.4, 14.1; IR (neat, cm−1) 2966, 2925, 2851, 1959, 1735, 1498, 1449, 1379, 1345, 1254, 1219, 1158; MS (EI) m/z (%) 312 (M+, 1.10), 165 (100), 91 (100), 69 (100); HRMS calcd for C21H28O2 (M+): 312.2089, found: 312.2092.
), 2.58–2.35 (m, 2 H, 2 × CH), 2.01–1.85 (m, 1 H, CH), 1.80–1.56 (m, 5 H, 5 protons of Cy), 1.35–0.95 (m, 11 H, 5 protons of Cy + 2 × CH3); 13C NMR (CDCl3, 75 MHz) δ 202.4, 175.6, 136.0, 128.5, 128.1, 98.2, 94.2, 66.0, 45.5, 37.1, 36.6, 33.04, 33.00, 26.1, 26.0, 18.4, 14.1; IR (neat, cm−1) 2966, 2925, 2851, 1959, 1735, 1498, 1449, 1379, 1345, 1254, 1219, 1158; MS (EI) m/z (%) 312 (M+, 1.10), 165 (100), 91 (100), 69 (100); HRMS calcd for C21H28O2 (M+): 312.2089, found: 312.2092.
Acknowledgements
Financial support from the National Basic Research Program of China (2011CB808700) and National Natural Science Foundation of China (21232006) is greatly appreciated. S. Ma is a Qiu Shi Adjunct Professor at Zhejiang University. We thank Miss Qiong Yu in this group for reproducing the preparation of 3d, (2S,3R)-3l, and 3o.Notes and references
- For such reports on the reaction of R2CuLi with halides with racemization, see: (a) B. H. Lipshutz and R. S. Wilhelm, J. Am. Chem. Soc., 1982, 104, 4696 CrossRef CAS; (b) G. M. Whitesides, W. F. Fischer, J. S. Filippo, R. W. Bashe and O. H. House, J. Am. Chem. Soc., 1969, 91, 4871 CrossRef CAS.
- For such reports on the reaction of Ph2CuLi with tosylates, see: (a) G. R. Johnson and G. A. Dutra, J. Am. Chem. Soc., 1973, 95, 7783 CrossRef; (b) S. Hanessian, B. Thavonekham and B. DeHoff, J. Org. Chem., 1989, 54, 5831 CrossRef CAS.
- For such a report on the reactions of alkyl magnesium halides with tosylates, see: C.-T. Yang, Z.-Q. Zhang, J. Liang, J.-H. Liu, X.-Y. Lu, H.-H. Chen and L. Liu, J. Am. Chem. Soc., 2012, 134, 11124 CrossRef CAS PubMed.
- For such a report on the reactions of diphenylmethyl tosylate with lithium reagents, see: Y. Bolshan, C. Chen, J. R. Chilenski, F. Gosselin, D. J. Mathre, P. D. O'Shea, A. Roy and R. D. Tillyer, Org. Lett., 2004, 6, 111 CrossRef CAS PubMed.
- For such a report on the reaction of alkylmagnesium halides with alkyl tosylamides, see: M.-B. Li, X.-L. Tang and S.-K. Tiana, Adv. Synth. Catal., 2011, 353, 1980 CrossRef CAS.
- For such a report on the Cu-catalyzed reactions of i-PrZnCl·MgCl2 with α-OTf-substituted esters, see: C. Studte and B. Breit, Angew. Chem., Int. Ed., 2008, 47, 5451 CrossRef CAS PubMed.
- For such a report on the ZnCl2-catalyzed reactions of RMgX with α-chloro-substituted ketones, see: C. F. Malosh and J. M. Ready, J. Am. Chem. Soc., 2004, 126, 10240 CrossRef CAS PubMed.
- For such a report on the palladium-catalyzed reactions of ArMgX with optically active α-bromo-substituted ethylbenzene, see: A. López-Pėrez, J. Adrio and J. C. Carretero, Org. Lett., 2009, 11, 5514 CrossRef PubMed.
- For such a report on the reactions of organozincs with α-chloro aldimines, see: G. R. Stanton, P. O. Norrby, P. J. Carroll and P. J. Walsh, J. Am. Chem. Soc., 2012, 134, 17599 CrossRef CAS PubMed.
- For some recent reviews, see: (a) A. Correa, O. G. Mancheño and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (b) R. H. Morris, Chem. Soc. Rev., 2009, 38, 2282 RSC; (c) A. A. O. Sarhan and C. Bolm, Chem. Soc. Rev., 2009, 38, 2730 RSC; (d) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (e) K. Gopalaiah, Chem. Rev., 2013, 113, 3248 CrossRef CAS PubMed.
- M. Nakamura, K. Matsuo, S. Ito and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS PubMed.
- For such reports on the Cu-catalyzed reactions of RMgX with 3-phenyl or 3,3-diphenyl-substituted allylic acetates, see: (a) J. Norinder and J.-E. Bäckvall, Chem.–Eur. J., 2007, 13, 4094 CrossRef CAS PubMed; (b) J. Norinder, K. Bogár, L. Kanupp and J.-E. Bäckvall, Org. Lett., 2007, 9, 5095 CrossRef CAS PubMed; (c) L. K. Thalen, A. Sumic, K. Bogár, J. Norinder, A. K. A. Persson and J.-E. Bäckvall, J. Org. Chem., 2010, 75, 6842 CrossRef CAS PubMed; (d) M. C. Warner, A. Nagendiran, K. Bogár and J.-E. Bäckvall, Org. Lett., 2012, 14, 5094 CrossRef CAS PubMed; (e) Y. Takashima and Y. Kobayashi, J. Org. Chem., 2009, 74, 5920 CrossRef CAS PubMed; (f) Q. Wang and Y. Kobayashi, Tetrahedron Lett., 2010, 51, 5592 CrossRef CAS; (g) A. M. Lauer, F. Mahmud and J. Wu, J. Am. Chem. Soc., 2011, 133, 9119 CrossRef CAS PubMed.
- Z. Wan and S. G. Nelson, J. Am. Chem. Soc., 2000, 122, 10470 CrossRef CAS.
- X. Jiang, C. Fu and S. Ma, Eur. J. Org. Chem., 2010, 687 CrossRef CAS.
- X. Zhang, C. Fu, Y. Yu and S. Ma, Chem.–Eur. J., 2012, 18, 13501 CrossRef CAS PubMed.
- For Fe(acac)3-catalyzed reaction of Grignard reagents with 1-alkynyl epoxides affording 2,3-allenols, see: A. Fürstner and M. Méndez, Angew. Chem., Int. Ed., 2003, 42, 5355 CrossRef PubMed.
- For [Fe(MgX)2]n-catalyzed coupling reaction of propargylic bromides with Grignard reagents affording alkynes, see: R. Martin and A. Fürstner, Angew. Chem., Int. Ed., 2004, 43, 3955 CrossRef CAS PubMed.
- For palladium-catalyzed coupling reaction of propargylic carbonates or mesylates with organozincs affording alkynes, see: (a) S. Ma and A. Zhang, J. Org. Chem., 2002, 67, 2287 CrossRef CAS PubMed; (b) S. Ma and G. Wang, Angew. Chem., Int. Ed., 2003, 42, 4215 CrossRef CAS PubMed.
- For nickel-catalyzed coupling reactions of propargylic halides or carbonates with arylzinc reagents affording alkynes, see: (a) S. W. Smith and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 12645–12647 CrossRef CAS PubMed; (b) A. J. Oelke, J. Sun and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 2966–2969 CrossRef CAS PubMed.
- Crystal data for 3a: C10H18O2Si: MW = 198.33, monoclinic, space group P21/c, final R indices R1 = 0.1016, wR2 = 0.2706, R indices (all data) R1 = 0.1246, wR2 = 0.2869, a = 6.4270 (7) Å, b = 24.105 (2) Å, c = 8.3807 (8) Å, α = 90°, β = 98.770 (8)°, γ = 90°, V = 1283.2 (2) Å3, T = 293 K, Z = 4, reflections collected/unique: 5110/2342 (Rint = 0.0227), number of observations [>2σ(I)] 1704, parameters: 145. CCDC 940135.
- M. B. Smith and J. March, Advanced Organic Chemistry, Wiley, 5th edn, 2001, p. 389 Search PubMed.
- For selected examples, see: (a) W. Zhang, S. Zhang and Z. Xi, Acc. Chem. Res., 2011, 44, 541 CrossRef CAS PubMed; (b) K. Cao, P. Lee, T. Fujita and N. Yoshikai, J. Am. Chem. Soc., 2010, 132, 12249 CrossRef PubMed; (c) T. Sugiishi and H. Nakamura, J. Am. Chem. Soc., 2012, 134, 2504 CrossRef CAS PubMed; (d) E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326 CrossRef PubMed; (e) A. König, M. Bette, C. Wagner, R. Lindner and D. Steinbon, Organometallics, 2011, 30, 5919 CrossRef.
- J. Ye, W. Fan and S. Ma, Chem.–Eur. J., 2013, 19, 716 CrossRef CAS PubMed.
- S. Ma, H. Ren and Q. Wei, J. Am. Chem. Soc., 2003, 125, 4817 CrossRef CAS PubMed.
- S. Nelson, T. Peelen and Z. Wan, J. Am. Chem. Soc., 1999, 121, 9742 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and detailed characterization data for all new compounds. CCDC 940135. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qo00066d |
| This journal is © the Partner Organisations 2014 |