Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Assembly of a calix[4]arene-supported MnIIIMnII cluster mediated by halogen interactions†
Stephanie M.
Taylor
a,
Jamie M.
Frost
a,
Ross
McLellan
b,
Ruaraidh D.
McIntosh
b,
Euan K.
Brechin
*a and
Scott J.
Dalgarno
*b
aEaStCHEM School of Chemistry, University of Edinburgh, West Mains Road, Edinburgh, EH9 3JJ, Scotland, UK. E-mail: ebrechin@staffmail.ed.ac.uk; Fax: +44 (0)131 650 6453; Tel: +44 (0)131 650 7545
bInstitute of Chemical Sciences, Heriot – Watt University, Riccarton, Edinburgh, EH14 4AS, Scotland, UK. E-mail: S.J.Dalgarno@hw.ac.uk; Fax: +44 (0)131 451 3180; Tel: +44 (0)131 451 8025
First published on 26th June 2014
Abstract
A new calix[4]arene-supported MnIIIMnII cluster, formed by the introduction of 3,5-dichlorobenzoate to a system known to afford MnIII2MnII2 Single-Molecule Magnets (SMMs), assembles in a layered manner through halogen interactions; structural and magnetic properties of this new cluster are presented.
Calix[4]arenes (C[4]s) have been used extensively in the formation of supramolecular structures, as well as in various aspects of coordination chemistry due to their polyphenolic nature.1p-tBu-calix[4]arene (TBC[4], Fig. 1A) is readily accessible2a and is a typical starting point for the alteration of the general C[4] framework.2b The C[4] polyphenolic pocket (at what is termed the lower-rim) is an attractive feature for metal complexation,3 and in this regard we (amongst others) have used TBC4 and related C[4]s for the construction of polynuclear transition metal (TM), lanthanide metal (LnM) and 3d–4f clusters that possess interesting magnetic properties.4 In our studies we have discovered a range of structural/cluster motifs, and those most frequently encountered include a) [MnIII2MnII2(TBC[4])2] Single-Molecule Magnets (SMMs),4b,c b) [MnIII4LnIII4(C[4])4] clusters that are magnetic refrigerants or SMMs depending on the lanthanide employed4f,g and [CuII9(TBC[4])3] clusters4d that are versatile anion binding materials.
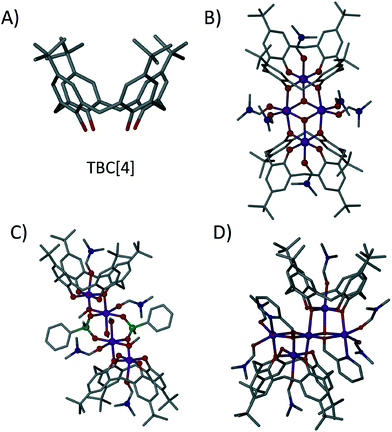 | ||
| Fig. 1 A) p-tBu-calix[4]arene, TBC[4]. B) TBC[4]-supported [MnIII2MnII2] SMM motif.4b,c C) Phenylphosphinate-bridged dimer of TBC[4]-supported [MnIIIMnII] dimers.4e D) TBC[4]-supported MnIII3MnII2 cluster formed with hmp as an ancillary ligand.4j Colour code: C – grey, O – red, N – royal blue, Mn – purple, P – green. H atoms are omitted for clarity. Figures not to scale. | ||
The synthesis of the TBC[4]-supported MnIII2MnII2 cluster shown in Fig. 1B is high yielding and we recently began to investigate the role of ancillary ligands in hybrid TBC[4]-supported cluster formation with a view to disrupting formation of this favourable structural motif. Addition of either sodium phenylphosphinate or 2-(hydroxymethyl)pyridine (hmpH) to the reaction used to form the [MnIII2MnII2(TBC[4])2] SMM affords very different results.4e,j The former results in a modulated TBC[4]-supported MnIII2MnII2 cluster in which two MnIIIMnII dimers are linked by two bridging phenylphosphinates (Fig. 1C).4e The latter results in a MnIII3MnII2 cluster in which both ligands display characteristic metal complexation properties (Fig. 1D).4j In both examples the TBC[4]s house MnIII ions within the cavity formed by the four lower-rim oxygen atoms, which also bridge to MnII ions located at the centre of the cage; behaviour entirely consistent with other systems involving additional metal ions such as lanthanides.4f,g
Thia-, sulfinyl- and sulfonyl-bridged C[4]s have also been used in polynuclear metal cluster formation,5,6 but the resulting materials differ markedly to those formed with methylene-bridged C[4]s due to the influence of the bridging atoms on the resulting coordination chemistry. The Liao6a–c and Hong6d,e groups recently used these alternative thia-C[4]-supported TM4 sub-units as building blocks in a series of studies, where they showed that polybenzoates can been used as ancillary ligands to sequentially direct the formation/assembly of novel metal–organic nanocapsules. Given this, as well as our successful use of ancillary ligands in hybrid TBC[4]-supported cluster formation, we began to investigate the effect of aryl monocarboxylates on the structure of the [MnIII2MnII2(TBC[4])2] SMM motif, prior to investigating topologically directing analogues. Here we report the first result of these studies, obtained by the introduction of sodium 3,5-dichlorobenzoate (Nadcb) to the reaction used to form the [MnIII2MnII2(TBC[4])2] SMM. The benzoate was found to have a marked effect on cluster formation, affording a new [MnIIIMnII(TBC[4])(dcb)(μ-dmso)(dmso)3(H2O)] species (1)7 that assembles though halogen⋯halogen interactions into bi-layers that are reminiscent of the packing observed in TBC4 solvates.
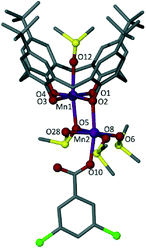
Reaction of MnCl2·4H2O with TBC[4] and Nadcb in a basic dmso solution afforded single crystals of 1 that were suitable for X-ray diffraction studies.‡ The crystals were found to be in a monoclinic cell and structure solution was performed in the space group C2/c. The structure of 1 (Fig. 2) is best described as a mixed valence MnIIIMnII dimer in which Mn1 is in the third oxidation state and bonded centrally within the plane of all four phenoxide oxygens of a tetraanionic TBC[4] (Mn–O range 1.910(7)–1.958(6) Å). The formation of this [MnIII–TBC[4]]− moiety is expected, and is an extraordinarily common motif observed in our coordination chemistry experiments performed with the C[4] framework. The Jahn–Teller axis deviates from linearity (∠O5–Mn1–O12 = 172.19°) and along this vector Mn1 is bonded to a bridging dmso (Mn1–O5 2.282(6) Å) as well as the oxygen of a disordered ligated solvent (Mn1–O12 2.312(6) Å).7 Distorted octahedral Mn2 is in the second oxidation state and is connected to Mn1 by a μ-phenoxide and a μ-dmso (Mn2–O2 2.200(7) Å and Mn2–O5 2.266(6) Å respectively). One aqua ligand (Mn2–O28 2.204(8) Å), two ligated dmso molecules (one of which is disordered over two positions, Mn–O range of 2.12(1)–2.24(1) Å) and a monodentate dcb ligand (Mn2–O10 2.169(8) Å) complete the coordination sphere of Mn2, as shown in Fig. 2. A hydrogen-bonding interaction is observed between the aqua ligand and the non-bonded C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the appended dcb, occurring with an O28⋯O11 distance of 2.650 Å.
O of the appended dcb, occurring with an O28⋯O11 distance of 2.650 Å.
By comparing Figures 1B and 2 it is clear that 1 resembles one half of the MnIII2MnII2 butterfly. Thus, incorporation of 3,5-dichlorobenzoate has a profound impact on cluster formation. We recently reported cases of ancillary ligands “interrupting” the assembly process of the [MnIII2MnII2(TBC[4])2] SMM; for example addition of sodium phenylphosphinate resulted in an ‘elongated’ or ‘expanded’ form of the archetypal [MnIII2MnII2(TBC[4])2] SMM.4e This dimer of dimers (Fig. 1C) is important in the context of the present study as each (ferromagnetically coupled) MnIIIMnII subunit resembles the metallic skeleton in 1. The μ-O atom of dmso in 1 is replaced with a μ-O atom from a μ3-phosphinate that in turn connects to the symmetry equivalent (s.e.) dimer. The use of 2-(hydroxymethyl)pyridine (hmpH) afforded a ferromagnetic MnIII3MnII2 cluster (Fig. 1D) in which the present motif is also found, with hmp occupying the equivalent positions of coordinated water molecule and benzoate anion in 1.4j These closely related structures all show the versatility of TBC[4] towards cluster formation with ancillary ligands, and demonstrate the retention of common coordination motifs for individual ligand types.
Analysis of the extended structure of 1 reveals that molecules assemble in a head-to-head bilayer that is akin to the packing found in TBC[4] solvates (Fig. 3A and B).4b,8 Each appended benzoate moiety interdigitates between the cavities of TBC[4] moieties of s.e. molecules of 1 within each layer, thus forming alternating up–down chain type assemblies along the c axis (Fig. 3B). Closer inspection reveals that individual layers are bridged by halogen⋯halogen interactions between s.e. molecules of 1, with two crystallographically unique Cl⋯Cl distances of 3.278(6) and 4.224(7) Å (Fig. 3C); these s.e. molecules are related by a twofold axis such that x, y, z becomes 1 − x, y, 1/2 − z. The halogen bonding is type I and a survey of the literature shows that these distances are similar to those previously reported for aryl chlorides.9 The individual layers in 1 are well separated and accordingly display a closest interlayer Mn⋯Mn contact of 14.636 Å. Within the layers adjacent clusters are less well isolated and display closest Mn⋯Mn contacts of 7.669 Å.
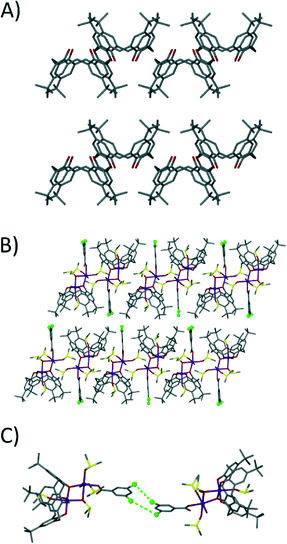 | ||
| Fig. 3 A) Common anti-parallel bi-layer arrangement found for TBC[4] in the solid state.4b,8 B) Extended structure in 1 showing modulated anti-parallel bilayer packing and intercalation of appended dcb. C) Type I halogen interactions9 between symmetry equivalents of 1 across the bi-layer arrangement. | ||
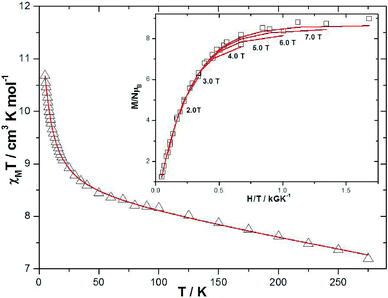
Dc magnetic susceptibility studies on powdered microcrystalline samples of 1 were performed in the 275–5 K temperature range in an applied field of 0.1 T, and are plotted as the χMT product versus T in Fig. 4. The room temperature value of 7.195 cm3 K mol−1 is close to the expected 7.375 cm3 K mol−1 for non-interacting MnIII and MnII ions with g = 2.00. As the temperature is decreased the χMT product increases, reaching a maximum value of ~10.7 cm3 K mol−1 at 5 K. This behaviour is suggestive of weak ferromagnetic exchange between the metal ions. For the interpretation of the magnetic properties of 1 we employed the following spin-Hamiltonian:
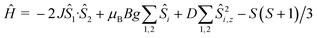 | (1) |
Conclusions
We have synthesised and characterised a [MnIIIMnII] cluster that results from addition of sodium 3,5-dichlorobenzoate to the reaction used to form C[4]-supported [MnIII2MnII2] SMMs. The appended benzoate has disrupted formation of the SMM motif and the resulting cluster can be regarded as a half of this common structural type. The new [MnIIIMnII] cluster reported here, despite having an unusual shape, packs so as to mimic the solid state behaviour of both TBC[4] and the [MnIII2MnII2] SMMs, with assembly occurring through halogen bonding interactions which bridge bi-layers. The magnetic behaviour of the dimer is also analogous to the parent butterfly structures, the exchange between the MnIII and MnII ions being weakly ferromagnetic. This study has used ancillary ligands containing one carboxylate on the aromatic ring with a view to establishing structure/cluster altering viability of this ligand type. We will now focus on using polytopic benzoates in order to form a new family of molecules with tailored geometries and magnetic properties. Given the importance of halogen bonding in driving assembly11 we will also investigate the structurally directing role of halogens placed at the C[4] upper-rim; this will be undertaken with a view to controlling the arrangement of [MnIII2MnII2] SMMs in the solid state relative to TBC[4]-supported analogues. The results of these new avenues of investigation will be reported in due course.Acknowledgements
We thank EPSRC for financial support of this work (EP/I03255X/1 & EP/I031421/1).Notes and references
- For examples of self-assembled and coordination driven calixarene assemblies see: L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS PubMed; T. Gerkensmeier, W. Iwanek, C. Agena, R. Froehlich, S. Kotila, C. Nather and J. Mattay, Eur. J. Org. Chem., 1999, 2257 CrossRef; J. L. Atwood, L. J. Barbour, M. J. Hardie and C. L. Raston, Coord. Chem. Rev., 2001, 222, 3 CrossRef; E. S. Barrett, T. J. Dale and J. Rebek Jr., J. Am. Chem. Soc., 2007, 129, 3818 CrossRef PubMed; S. J. Dalgarno, S. A. Tucker, D. B. Bassil and J. L. Atwood, Science, 2005, 309, 2037 CrossRef PubMed; O. Ugono and K. T. Holman, Chem. Commun., 2006, 2144 RSC.
- (a) C. D. Gutsche, Acc. Chem. Res., 1983, 16, 161 CrossRef CAS , and references therein; (b) C. D. Gutsche, Calixarenes 2001, Kluwer Academic Publishers, 2001, ch. 1 and references therein Search PubMed.
- A significant amount of work has been reported in which various groups have used the C[n] lower-rim as a platform to synthesise novel transition, lanthanide and alkali metal complexes (and in some cases clusters) using air sensitive rather than ambient techniques/conditions. For representative papers please see: A. J. Petrella and C. L. Raston, J. Organomet. Chem., 2004, 689, 4125 CrossRef CAS PubMed; D. M. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086 CrossRef PubMed; E. D. Gueneau, K. M. Fromm and H. Goesmann, Chem. Eur. – J., 2003, 9, 509 CrossRef PubMed; G. Guillemot, B. Castellano, T. Prangé, E. Solari and C. Floriani, Inorg. Chem., 2007, 46, 5152 CrossRef PubMed; G. Guillemot, E. Solari, C. Rizzoli and C. Floriani, Chem. – Eur. J., 2002, 8, 2072 CrossRef; U. Radius and J. Attner, Inorg. Chem., 2004, 43, 8587 CrossRef PubMed , and references therein.
- (a) C. Aronica, G. Chastanet, E. Zueva, S. A. Borshch, J. M. Clemente-Juan and D. Luneau, J. Am. Chem. Soc., 2008, 130, 2365 CrossRef CAS PubMed; (b) G. Karotsis, S. J. Teat, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 8285 CrossRef CAS PubMed; (c) S. M. Taylor, G. Karotsis, R. D. McIntosh, S. Kennedy, S. J. Teat, C. M. Beavers, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. – Eur. J., 2011, 17, 7521 CrossRef CAS PubMed; (d) G. Karotsis, S. Kennedy, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2010, 46, 3884 RSC; (e) S. M. Taylor, R. D. McIntosh, C. M. Beavers, S. J. Teat, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2011, 47, 1440 RSC; (f) G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928 CrossRef CAS PubMed; (g) G. Karotsis, S. Kenndy, S. J. Teat, C. M. Beavers, D. A. Fowler, J. J. Morales, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, J. Am. Chem. Soc., 2010, 132, 12983 CrossRef CAS PubMed; (h) S. Sanz, K. Ferreira, R. D. McIntosh, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2011, 47, 9042 RSC; (i) S. Sanz, R. D. McIntosh, C. M. Beavers, S. J. Teat, M. Evangelisti, E. K. Brechin and S. J. Dalgarno, Chem. Commun., 2012, 48, 1449 RSC; (j) S. M. Taylor, R. D. McIntosh, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2012, 48, 11190 RSC.
- For clusters containing thia- and sulfonyl-C[4]-supported TM4 moieties in the absence of ancillary ligands see: C. Desroches, G. Pilet, S. A. Borshch, S. Parola and D. Luneau, Inorg. Chem., 2005, 44, 9112 CrossRef CAS PubMed; C. Desroches, G. Pilet, P. A. Szilagyi, G. Molnar, S. A. Borshch, A. Bousseksou, S. Parola and D. Luneau, Eur. J. Inorg. Chem., 2006, 2, 357 CrossRef; M. Lamouchi, E. Jeanneau, A. Pillonnet, A. Brioude, M. Martini, O. Stephan, F. Meganem, G. Novitchi, D. Luneau and C. Desroches, Dalton Trans., 2012, 41, 2707 RSC; T. Kajiwara, N. Iki and M. Yamashita, Coord. Chem. Rev., 2007, 251, 1734 CrossRef PubMed; Y. Bi, X.-T. Wang, W. Liao, X. Wang, X. Wang, H. Zhang and S. Gao, J. Am. Chem. Soc., 2009, 131, 11650 CrossRef PubMed.
- For cages constructed with ancillary poly-benzoate ligands see; (a) M. Liu, W. Liao, C. Hu, S. Du and H. Zhang, Angew. Chem., Int. Ed., 2012, 51, 1585 CrossRef CAS PubMed; (b) M. Liu and W. Liao, CrystEngComm, 2012, 14, 5727 RSC; (c) H. Tian, S. Du, Y. Bi and W. Liao, Chem. Commun., 2013, 49, 8211 RSC; (d) K. Xiong, F. Jiang, Y. Gai, D. Yuan, L. Chen, M. Wu, K. Su and M. Hong, Chem. Sci., 2012, 3, 2321 RSC; (e) K. Su, F. Jiang, J. Qian, M. Wu, Y. Gai, J. Pan, D. Yuan and M. Hong, Inorg. Chem., 2014, 53, 18 CrossRef CAS PubMed.
- There is disorder present within the TBC[4] cavity with respect to the solvent coordinated to Mn1. In the interests of brevity, discussion has been limited to one species..
- See the following review for details of TBC[4] assembly in anti-parallel bi-layers: S. J. Dalgarno, P. K. Thallapally, L. J. Barbour and J. L. Atwood, Chem. Soc. Rev., 2007, 36, 236 RSC.
- For example see: V. R. Pedireddi, D. S. Reddy, B. S. Goud, D. C. Craig, A. D. Rae and G. R. Desiraju, J. Chem. Soc., Perkin Trans. 2, 1994, 2353 RSC; V. R. Hathwar, S. M. Roopan, R. Subashini, F. N. Khan and R. N. Guru Row, J. Chem. Sci., 2010, 122, 677 CrossRef CAS PubMed.
- W. H. Press, S. A. Teukolsky, W. T. Vetterling and B. P. Flannery, Numerical Recipes in C: The Art of Scientific Computing, Cambridge University Press, Cambridge, 2nd edn, 1992 Search PubMed.
- A. Priimagi, G. Cavallo, P. Metrangolo and G. Resnati, Acc. Chem. Res., 2013, 46, 2686 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 995697. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ce00729h |
‡ TBC[4] was synthesised according to literature procedure,2a while 3,5-dichlorobenzoic acid was purchased from Sigma-Aldrich. Synthesis of 1: MnCl2·4H2O (0.15 g, 0.75 mmol), TBC[4] (0.1 g, 0.15 mmol), Nadcb (0.213 g, 1.0 mmol) and NEt3 (0.1 g, 1.0 mmol) were dissolved in a mixture of DMSO (10 ml) and EtOH (10 ml). After 2 hours of stirring the solution was filtered an allowed to stand; crystals grew upon evaporation of the mother liquor over several days (23 mg, 12%). Elemental analysis (%) calculated for 1, C60H84.6Cl2O12.3S3.3Mn2: C, 55.89%; H, 6.61%. Found: C, 55.97%; H, 6.52%. General crystallographic details: data were collected on a Bruker X8 Apex II CCD Diffractometer operating at 100(2) K with Mo-Kα radiation (λ = 0.71073 Å). Crystal data (CCDC 995697): C60H84.60Cl2O12.30S3.30Mn2, M = 1289.25, black block, 0.20 × 0.15 × 0.15 mm3, monoclinic, space group C2/c, a = 38.315(3), b = 12.5251(10), c = 29.193(2) Å, β = 109.401(2)°, V = 13214.2(18) Å3, Z = 8, 2θmax = 41.74°, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 reflections collected, 6899 unique (Rint = 0.0679). Final GooF = 1.575, R1 = 0.0930, wR2 = 0.2546, R indices based on 4417 reflections with I > 2sigma(I) (refinement on F2). Unit cells of several single crystals and further analysis by powder X-ray diffraction (Fig. S2†) confirmed the bulk composition to be 1. 773 reflections collected, 6899 unique (Rint = 0.0679). Final GooF = 1.575, R1 = 0.0930, wR2 = 0.2546, R indices based on 4417 reflections with I > 2sigma(I) (refinement on F2). Unit cells of several single crystals and further analysis by powder X-ray diffraction (Fig. S2†) confirmed the bulk composition to be 1. |
| This journal is © The Royal Society of Chemistry 2014 |