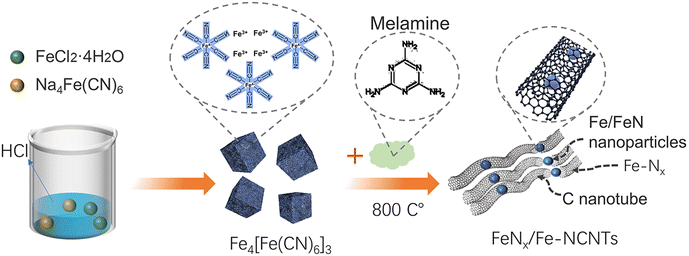
Open Access Article
Open Access ArticlePrussian blue-derived FeNx/Fe-based N-doped carbon nanotube catalysts with high ORR electrochemical performance†
Zhenlu
Zhao‡
 *ab,
Qidi
Lu‡
a and
Xin
Wang
a
*ab,
Qidi
Lu‡
a and
Xin
Wang
a
aSchool of Material Science and Engineering, University of Jinan, Jinan 250022, Shandong, China
bState Key Lab of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, China. E-mail: mse_zhaozl@ujn.edu.cn
First published on 12th February 2024
Abstract
The oxygen reduction reaction (ORR) kinetics are slow, limiting the overall reaction rate. It is still challenging to construct transition metal-based electrocatalysts with high activity and stability. In this study, FeNx/Fe-based skewer-like N-doped carbon nanotubes (FeNx/Fe-NCNTs) are successfully prepared by a simple one-step pyrolysis strategy. The Fe-based nanoparticles are evenly dispersed at the tip of the carbon nanotubes and the junction of the bamboo segments, indicating that the FeNx/Fe-NCNTs catalyst has abundant ORR active sites. When applied to the ORR, FeNx/Fe-NCNTs exhibit initial and half-wave potentials of 0.96 and 0.86 V (vs. RHE) in 0.1 M KOH, comparable to those of commercial 20% Pt/C catalysts. Moreover, the FeNx/Fe-NCNTs showed a low Tafel slope value of 81.2 mV dec−1 and the electron transfer number (n) is between 3.81 and 3.93, indicating a fast four-electron transfer path. The results show that FeNx/Fe-NCNTs have better electron transfer efficiency and stability, making them an excellent ORR catalyst.
Introduction
The demand for clean economic energy as an alternative to fossil fuels is currently growing in response to environmental concerns and energy consumption.1–3 Fuel cells have been recognized as major alternative devices.4,5 However, the sluggishness of the oxygen reduction reaction (ORR) in fuel cells is a major challenge. The high cost and lack of resources of highly active platinum (Pt)-based catalysts in the ORR have prevented their large-scale commercial application.6–10 Transition metal and carbon-based materials have become the subject of lively discussion among researchers.11–15As for carbon materials, how to improve the activity has also become a major focus of research. In recent years, nitrogen-doped carbon materials have shown excellent catalytic properties. The disparity in electronegativity between nitrogen and carbon atoms results in an imbalance in their electron spin density, and charge polarisation is an important reason for improving the catalytic performance of N-doped carbon materials. Additionally, the encapsulation of abundant transition metals (eg. Cu, Ni, Co, Fe) in N-doped carbon materials has positive implications for enhancing the electrocatalytic performance, because the abundant 3d electrons in transition metal elements are easily lost or captured, allowing for the controlled synthesis of multi-component, multi-structures as catalysts with multiple catalytic active centers, especially for Fe-based N-doped carbon materials.16–19 However, developing transition metal oxygen reduction catalysts with high activity and excellent stability is still a problem in industry, since nanoparticles based on transition metals loaded on the outer surface of carbon materials are susceptible to Ostwald curing during the catalytic process, resulting in poor long-term stability.20–24
Prussian blue, a type of transition metal-based material, is widely utilized in synthesizing catalysts for electro-chemical power devices. Its widespread application results from its low cost, stable 3D framework, non-toxic and non-hazardous properties, and abundant natural resources.25,26 Prussian blue can be used as a sacrificial template for conversion to inorganic nanoparticles and carbon composites can be created through a simple carbonization process, resulting in the uniform dispersion of metal or metal-based nanoparticles within the carbon matrix.27
In this regard, we explored a simple preparation method of Fe-based carbon nanotube materials for highly active ORR electrocatalysts with Fe-based nanoparticles as the main active site of the catalyst. Na4Fe(CN)6 and FeCl2·4H2O were homogeneously mixed, and a certain amount of HCl was added to prepare a Prussian blue precursor material, which was aged in air for 24 h. An appropriate amount of melamine was added and then homogeneously sintered in a tube furnace at 800 °C to form FeNx/Fe-based N-doped carbon materials. The material (FeNx/Fe-NCNTs) exhibits high ORR electrocatalytic activity in alkaline media, where the half-wave potential reaches 0.86 V and the onset potential is as high as 0.96 V (vs. RHE). The FeNx/Fe-NCNTs show higher ORR activity with a peroxide yield of less than 8%, an electron transfer number close to 4, and improved stability compared to commercial 20% Pt/C.
Experimental
Preparation of Prussian blue
Synthesis of the Prussian blue (Fe4[Fe(CN)6]3) precursor: 5 ml of an aqueous solution containing 300 mM FeCl2·4H2O and 600 mM Na4Fe(CN)6 was prepared, then 45 ml of concentrated hydrochloric acid (37%) was added, and the resultant solution was placed in a fume cupboard and aged for 24 h in air. The solution was centrifuged at 9000 rpm to obtain a precipitate and then it was washed three times with deionized water and ethanol. The samples were dried for 24 h at 70 °C.Preparation of FeNx/Fe-NCNTs
0.2 g of Prussian blue (Fe4[Fe(CN)6]3) synthesized above was ground with 3 g of melamine and mixed well in a mortar and pestle, and then the ground sample was transferred to a porcelain boat. The porcelain boat was placed into a two-zone temperature-controlled tube furnace, and the sample was heated up to 800 °C under a N2 atmosphere at a ramping rate of 3 °C min−1. The FeNx/Fe-NCNTs were obtained by holding at 800 °C for 2 h.Results and discussion
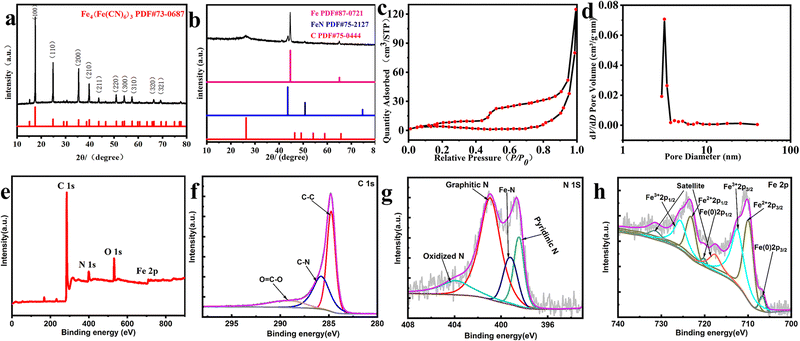
As depicted in Fig. 1, FeNx/Fe-NCNTs were prepared by adding hydrochloric acid to control the synthesis of synthetic Prussian blue precursors. The composition and structure of the FeNx/Fe-NCNT catalyst were investigated by X-ray diffractometry (XRD). The XRD patterns (Fig. 2a) show crystal diffraction peaks of Prussian blue at 17.374° (100), 24.710° (110), 35.164° (200), 39.491° (210), 43.692° (211), 50.673° (220), 53.886° (300), 57.166° (310), 60.457° (311), 63.202° (222), 66.227° (320), 68.997° (321), 74.677° (400) and 76.807° (410). The diffraction peaks above correspond well with those of Prussian blue, which also proves the successful preparation of Prussian blue precursor. The XRD pattern of the FeNx/Fe-NCNTs (Fig. 2b) shows that there are crystallization diffraction peaks at 26.381°, 43.915°, 44.673° and 65.021°. The diffraction peaks at 44.673° and 65.021° correspond to the (110) and (200) crystal planes of Fe. The 43.915° diffraction peak corresponds to the (111) crystal plane of FeN, and the 26.381° diffraction peak corresponds to the (002) crystal plane of C. Fig. 2c shows the N2 adsorption–desorption curve of the FeNx/Fe-NCNTs catalyst, which shows a typical Type IV isotherm indicating that the material has a large number of mesopores and has a clear hysteresis line. The FeNx/Fe-NCNTs have a high surface area of 153.379 m2 g−1. Fig. 2d displays the pore size distribution of the material, which is predominantly distributed within the 2–6 nm range. The high quantity of pores facilitates electron transfer, which improves the catalytic efficiency of the catalysts.28
Fig. 2e–h show the X-ray photoelectron spectroscopy (XPS) images of the samples to further analyze the material composition of the FeNx/Fe-NCNTs. Fig. 2e shows the survey spectrum of the FeNx/Fe-NCNTs. The survey spectrum indicates the presence of C, N, O and Fe. In the high-resolution XPS spectra of C 1s in Fig. 2f, the characteristic peaks at 284.8, 286.1 and 289.1 eV can be observed, belonging to C–C, C–N and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O bonds, respectively.29Fig. 2g shows the high-resolution XPS spectrum of N 1s with peaks at 398.4 eV attributed to pyridine nitrogen, 399.3 eV to Fe–N, 401.0 eV to graphitic nitrogen, and 403.9 eV to nitrogen oxide.30–32 The material's ORR activity benefits from the presence of pyridine nitrogen and graphitic nitrogen.31 The high-resolution XPS spectrum of Fe 2p is shown in Fig. 2h. The peak at 706.7 eV belongs to zero-valent Fe 2p3/2, the peak at 709.9 eV belongs to Fe2+ 2p3/2, and the peak at 712.4 eV is Fe3+ 2p3/2.30,31 In summary, the iron present in FeNx/Fe-NCNTs is distributed throughout the N doped carbon matrix in the form of Fe, FeN, and Fe–Nx.33
C–O bonds, respectively.29Fig. 2g shows the high-resolution XPS spectrum of N 1s with peaks at 398.4 eV attributed to pyridine nitrogen, 399.3 eV to Fe–N, 401.0 eV to graphitic nitrogen, and 403.9 eV to nitrogen oxide.30–32 The material's ORR activity benefits from the presence of pyridine nitrogen and graphitic nitrogen.31 The high-resolution XPS spectrum of Fe 2p is shown in Fig. 2h. The peak at 706.7 eV belongs to zero-valent Fe 2p3/2, the peak at 709.9 eV belongs to Fe2+ 2p3/2, and the peak at 712.4 eV is Fe3+ 2p3/2.30,31 In summary, the iron present in FeNx/Fe-NCNTs is distributed throughout the N doped carbon matrix in the form of Fe, FeN, and Fe–Nx.33
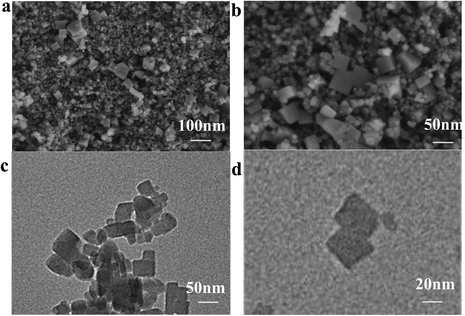
Fig. 3a and b shows images obtained by field emission scanning electron microscopy (FESEM) of Prussian blue. The images show that the size of the prepared cubes ranged from 20 to 100 nm. The results indicate the successful preparation of the cubes.26 The transmission electron microscopy (TEM) images of Prussian blue in Fig. 3c and d further demonstrate the cubic structured Prussian blue material. The morphology and structure of the samples containing FeNx/Fe-NCNTs were further characterized through the use of FESEM and TEM. As shown in the FESEM images of Fig. 4a and b, the FeNx/Fe-NCNT samples have narrow and dense carbon nanotube structures, which have an average diameter of about 500 nm, with uniform thickness and length. The TEM images of Fig. 4c and d show the presence of Fe-based nanoparticles, especially at the tips and bamboo-like junctions of the FeNx/Fe-NCNTs. These nanoparticles significantly increase the material's surface area, number of defects, and electrical conductivity while simultaneously supporting an increase in active sites. The overall effect is an improvement in the ORR performance of the material.34Fig. 4e–h displays the energy dispersive X-ray spectral (EDS) mapping of the material, revealing that the Fe-based nanoparticles are predominantly concentrated at the carbon nanotube tips while also existing within the interior and on the surface of the carbon nanotubes. Nitrogen is found to be uniformly distributed throughout the structure, indicating that pyrolysis can uniformly dope a large amount of N heteroatoms into the carbon material.35 EDS further confirms that the iron within FeNx/Fe-NCNTs is distributed throughout the N-doped carbon matrix as Fe, FeN, and Fe–Nx.
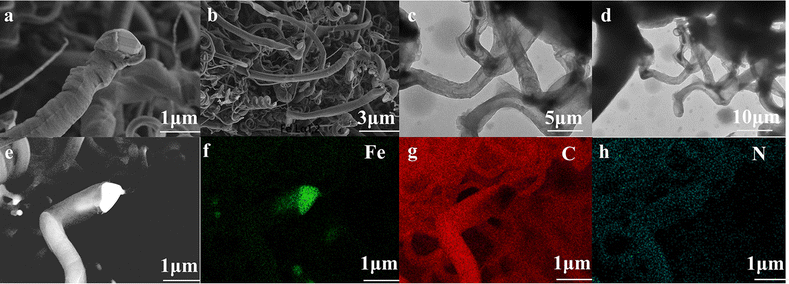 | ||
| Fig. 4 (a) and (b) FESEM images of FeNx/Fe-NCNTs; (c) and (d) TEM images of FeNx/Fe-NCNTs; (e) EDS surface scan composition distribution of FeNx/Fe-NCNTs: (f) Fe; (g) C; (h) N. | ||
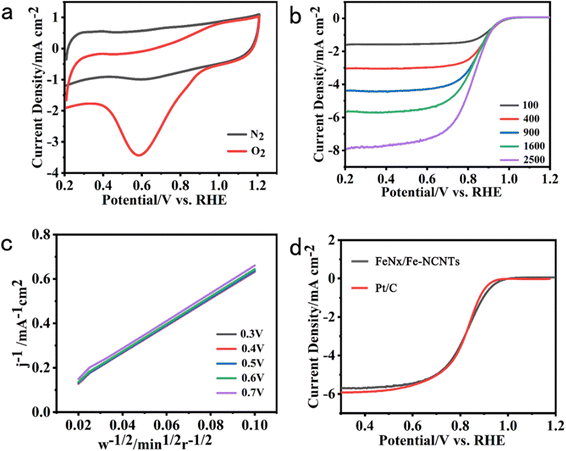
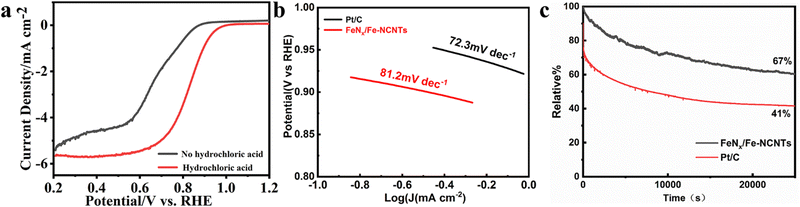
The electrochemical ORR properties of FeNx/Fe-NCNTs are next investigated, and the electrocatalytic activity of the FeNx/Fe-NCNTs is tested by cyclic voltammetry (CV). As shown in Fig. 5a, there is a clear redox peak in the O2-saturated 0.1 M KOH solution compared to the N2-saturated 0.1 M KOH solution, and there is a peak at about 0.61 V. Furthermore, a peak at approximately 0.61 V indicates the exceptional ORR activity of the catalyst. Linear scanning voltammetry (LSV) curves measured at different velocities further show that FeNx/Fe-NCNTs have a high ORR activity (Fig. 5b) with an onset potential of 0.96 V and a half-wave potential (E1/2) of 0.86 V (vs. RHE), which are almost comparable to that of the commercial 20% Pt/C (0.87 V), or even have a better onset potential (Fig. 5d). The K–L curve, as computed from the LSV (Fig. 5c), yields 3.81–3.93 for the electron transfer number (n) within the potential range of 0.3 to 0.7 V. This indicates that the material's ORR in the limit current region involves nearly 4 transferred electrons. The ORR activity of the FeNx/Fe-NCNTs and other reported transition metal-based carbon electrocatalysts were compared (Table S1, ESI†). The FeNx/Fe-NCNTs exhibit excellent ORR activity. The ORR activity of the FeNx/Fe-NCNTs is improved by the synergistic enhancement effect of the N-doped carbon nanotubes and FeNx/Fe-based nanoparticles, which facilitates mass and electron transport and improves the activity of the active sites.
From Fig. 6a, it can be seen that the FeNx/Fe-NCNTs catalyst with 45 ml of concentrated hydrochloric acid has a better ORR activity with an onset potential of 0.96 V and a half-wave potential (E1/2) of 0.86 V (vs. RHE). The sample without hydrochloric acid treatment has a worse ORR activity with an onset potential of only 0.85 V and a half-wave potential of only 0.67 V (vs. RHE). Concentrated hydrochloric acid plays a vital role in enhancing the catalyst activity under similar pyrolysis conditions. This effect may relate to the synthesis of the Prussian blue precursor. In the preparation of this experiment, 45 ml of concentrated hydrochloric acid (37% HCl) was added as an inhibitor for the synthesis of Prussian blue. The initial Gibbs free energy difference (ΔG) between the reactants and products of the synthesized Prussian blue is less than zero and the reaction reacts spontaneously. Protons can react with [Fe(CN)6]4− to form H4[Fe(CN)6], which makes the Gibbs free energy of the synthesis of Prussian blue reaction decrease and the trend and rate of the reaction decrease. Volatile hydrochloric acid was used as a source of protons, and with the volatilization of the hydrochloric acid, the concentration of protons in the reaction system decreased, and the synthetic Prussian blue reaction proceeded gradually and spontaneously to form small-sized Prussian blue. The reaction system cannot be hermetically sealed. If it is hermetically sealed, then the internal inhibitor concentration will become so great that the reaction to synthesize Prussian blue will not be able to continue spontaneously.36 The Tafel slope curve of our prepared FeNx/Fe-NCNTs is shown in Fig. 6b above, from which it can be seen that the Tafel slope value of FeNx/Fe-NCNTs is 81.2 mV dec−1, which is close to that of the commercial 20% Pt/C catalyst (72.3 mV dec−1). The results show that the FeNx/Fe-NCNTs catalysts have superior electron transfer efficiency and chemical reaction kinetics. Fig. 6c shows the chronoamperometry of FeNx/Fe-NCNTs at 0.6 V (vs. RHE). It maintains 66.7% performance after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s of chronoamperometry, while commercial 20% Pt/C has higher current loss and only maintains about 53.6% performance, indicating that our synthesized FeNx/Fe-NCNTs have better long-term stability, which makes them an excellent electrochemical catalyst for the ORR.
000 s of chronoamperometry, while commercial 20% Pt/C has higher current loss and only maintains about 53.6% performance, indicating that our synthesized FeNx/Fe-NCNTs have better long-term stability, which makes them an excellent electrochemical catalyst for the ORR.
Conclusion
In summary, a bamboo nanotube-like structural material with uniform diameter was successfully prepared by a simple one-step pyrolysis strategy, and Fe-based nanoparticles were homogeneously dispersed at the tips of the carbon nanotubes and the junctions of the bamboo joints. The FeNx/Fe-NCNTs catalysts are rich in active sites. The ORR test of the FeNx/Fe-NCNTs material shows that the onset potential and half-wave potential in 0.1 M KOH are 0.96 and 0.86 V (vs. RHE), respectively, which are almost comparable to the commercial 20% Pt/C catalysts with even more excellent onset potential. This indicates that our prepared materials have excellent ORR electrocatalytic activity. In addition, FeNx/Fe-NCNTs have better stability, which makes them an excellent ORR electrochemical catalyst. The FeNx/Fe-NCNTs were prepared in a simple method with convenient operation steps and possessed excellent ORR electrocatalytic activity.Author contributions
Zhenlu Zhao conceived and designed the project. Qidi Lu prepared the nanomaterials, conducted the structural characterizations, and performed electrical measurements. Zhenlu Zhao, Qidi Lu and Xin Wang analyzed and discussed the data. Zhenlu Zhao and Qidi Lu wrote the manuscript. All authors have given approval to the final version of the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the Natural Science Foundation of Shandong Province (No. ZR2021MB017), Foundation of State Key Laboratory of Electro-analytical Chemistry (SKLEAC201907), and Study Abroad Fund.References
- B. You and Y. Sun, Acc. Chem. Res., 2018, 51, 1571–1580 CrossRef CAS PubMed.
- Z. Dimitrova and W. B. Nader, Energy, 2022, 239, 121933–121944 CrossRef.
- V. Mazumder, M. Chi, K. L. More and S. Sun, J. Am. Chem. Soc., 2010, 132, 7848–7849 CrossRef CAS PubMed.
- L. Wu, Q. Li, C. H. Wu, H. Zhu, A. Mendoza-Garcia, B. Shen, J. Guo and S. Sun, J. Am. Chem. Soc., 2015, 137, 7071–7074 CrossRef CAS PubMed.
- H. Liu, Y. Zheng, G. Wang and S. Z. Qiao, Adv. Energy Mater., 2015, 5, 1401186–1401192 CrossRef.
- S.-Y. Lin, Y.-P. Chen, Y. Cao, L. Zhang, J.-J. Feng and A.-J. Wang, J. Power Sources, 2022, 521, 230926–230934 CrossRef CAS.
- Y. Feng, K. Song, W. Zhang, X. Zhou, S. J. Yoo, J.-G. Kim, S. Qiao, Y. Qi, X. Zou, Z. Chen, T. Qin, N. Yue, Z. Wang, D. Li and W. Zheng, J. Energy Chem., 2022, 70, 211–218 CrossRef CAS.
- X. Liu, L. Wang, P. Yu, C. Tian, F. Sun, J. Ma, W. Li and H. Fu, Angew. Chem., Int. Ed., 2018, 57, 16166–16170 CrossRef CAS PubMed.
- X. Wang, H. Zhu, C. Yang, J. Lu, L. Zheng and H.-P. Liang, Carbon, 2022, 191, 393–402 CrossRef CAS.
- H. Karimi-Maleh, C. Karaman, O. Karaman, F. Karimi, Y. Vasseghian, L. Fu, M. Baghayeri, J. Rouhi, P. Senthil Kumar, P.-L. Show, S. Rajendran, A. L. Sanati and A. Mirabi, J. Nanostruct. Chem., 2022, 12, 429–439 CrossRef CAS.
- J. Liang, Y. Zheng, J. Chen, J. Liu, D. Hulicova-Jurcakova, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 3892–3896 CrossRef CAS PubMed.
- S. Sarkar, N. Kamboj, M. Das, T. Purkait, A. Biswas and R. S. Dey, Inorg. Chem., 2020, 59, 1332–1339 CrossRef CAS PubMed.
- H.-S. Zhai, L. Cao and X.-H. Xia, Chin. Chem. Lett., 2013, 24, 103–106 CrossRef CAS.
- J. Hu, P. Zhang, W. An, L. Liu, Y. Liang and W. Cui, Appl. Catal., B, 2019, 245, 130–142 CrossRef CAS.
- Q. Liu, T. Chen, Y. Guo, Z. Zhang and X. Fang, Appl. Catal., B, 2017, 205, 173–181 CrossRef CAS.
- S. Sarkar, S. S. Sumukh, K. Roy, N. Kamboj, T. Purkait, M. Das and R. S. Dey, J. Colloid Interface Sci., 2020, 558, 182–189 CrossRef PubMed.
- R. Li, J. Huang, M. Cai, J. Huang, Z. Xie, Q. Zhang, Y. Liu, H. Liu, W. Lv and G. Liu, J. Hazard. Mater., 2020, 384, 121435–121445 CrossRef CAS PubMed.
- P. Teppor, R. Jäger, E. Härk, I. Tallo, U. Joost, M. Kook, P. Paiste, K. Šmits, K. Kirsimäe and E. Lust, J. Electrochem. Soc., 2018, 165, F1217–F1223 CrossRef CAS.
- O. Pariiska, D. Mazur, Y. Kurys, R. Socha, V. Koshechko and V. Pokhodenko, J. Solid State Electrochem., 2021, 25, 2309–2319 CrossRef CAS.
- J. Zhang, S. Guo, J. Wei, Q. Xu, W. Yan, J. Fu, S. Wang, M. Cao and Z. Chen, Chem. – Eur. J., 2013, 19, 16087–16092 CrossRef CAS PubMed.
- D. Deng, L. Yu, X. Chen, G. Wang, L. Jin, X. Pan, J. Deng, G. Sun and X. Bao, Angew. Chem., Int. Ed., 2013, 52, 371–375 CrossRef CAS PubMed.
- Q. Li, H. Pan, D. Higgins, R. Cao, G. Zhang, H. Lv, K. Wu, J. Cho and G. Wu, Small, 2015, 11, 1443–1452 CrossRef CAS PubMed.
- Y.-G. Guo, J.-S. Hu and L.-J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS.
- Y. Ren, R. Chang, X. Hu, J. Guo, G. Hao and A. Lu, Chin. Chem. Lett., 2023, 34, 108634 CrossRef CAS.
- S. Cheng, C. Shen, H. Zheng, F. Liu and A. Li, Appl. Catal., B, 2020, 269, 118785–118794 CrossRef CAS.
- J. Sanetuntikul and S. Shanmugam, Electrochim. Acta, 2014, 119, 92–98 CrossRef CAS.
- J. Huai, K. Ma, Y. Lu, T. Chen and Z. Zhao, ChemCatChem, 2019, 11, 4818–4821 CrossRef CAS.
- X. Xie, L. Peng, H. Yang, G. I. N. Waterhouse, L. Shang and T. Zhang, Adv. Mater., 2021, 33, 2101038–2101045 CrossRef CAS PubMed.
- H. Niu, Y. Zhang, Y. Liu, B. Luo, N. Xin and W. Shi, J. Mater. Chem. A, 2019, 7, 8503–8509 RSC.
- X. Wan, Q. Liu, J. Liu, S. Liu, X. Liu, L. Zheng, J. Shang, R. Yu and J. Shui, Nat. Commun., 2022, 13, 2963–2973 CrossRef CAS PubMed.
- F. Fan, H. Zhou, R. Yan, C. Yang, H. Zhu, Y. Gao, L. Ma, S. Cao, C. Cheng and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 41609–41618 CrossRef CAS PubMed.
- S.-Y. Tang, Y.-S. Wang, Y.-F. Yuan, Y.-Q. Ba, L.-Q. Wang, G.-P. Hao and A.-H. Lu, New Carbon Mater., 2022, 37, 237–244 CrossRef CAS.
- J. Han, H. Bao, J.-Q. Wang, L. Zheng, S. Sun, Z. L. Wang and C. Sun, Appl. Catal., B, 2021, 280, 119411–119420 CrossRef CAS.
- X. Liu, W. Yang, L. Chen, Z. Liu, L. Long, S. Wang, C. Liu, S. Dong and J. Jia, ACS Appl. Mater. Interfaces, 2020, 12, 4463–4472 CrossRef CAS PubMed.
- Z. Zhao, C. Gao, K. Ma and Y. Lu, Appl. Surf. Sci., 2020, 504, 144380–144385 CrossRef CAS.
- W. Zhang, Y. Li, C. Shi, R. Qi and M. Hu, J. Am. Chem. Soc., 2021, 143, 6447–6459 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ya00615h |
| ‡ Zhenlu Zhao and Qidi Lu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |