Open Access Article
Open Access ArticleRoadmap of algal autotrophic tissue engineering in the avenue of regenerative wound therapy
Nikhita
Pandian†
af,
Radhika
Chaurasia†
a,
Satyaki
Chatterjee
b,
Bhaskar
Biswas
 c,
Prabir
Patra
de,
Archana
Tiwari
*f and
Monalisa
Mukherjee
c,
Prabir
Patra
de,
Archana
Tiwari
*f and
Monalisa
Mukherjee
 *a
*a
aAmity Institute of Click Chemistry Research and Studies, Amity University, Sector-125, Noida, UP-201313, India. E-mail: mmukherjee@amity.edu; Tel: +91-9873279964
bUniversity of Iceland, Department of Chemistry, Science Institute, Dunhaga 3, 107 Reykjavik, Iceland
cDepartment of Chemistry, University of North Bengal, Darjeeling-734013, India
dDepartment of Biomedical Engineering, College of Engineering and Computer Science, 1 John Marshall Drive, Marshall University, Huntington, WV 25755, USA
eDepartment of Mechanical and Industrial Engineering, College of Engineering and Computer Science, 1 John Marshall Drive, Marshall University, Huntington, WV 25755, USA
fDiatom Research Laboratory, Amity Institute of Biotechnology, Amity University, Noida, Uttar Pradesh 201301, India. E-mail: atiwari9@amity.edu
First published on 18th September 2024
Abstract
In spite of remarkable advancements in tissue engineering and regenerative medicine in recent years, a notable gap remains in the availability of economically feasible and efficient treatments to address the hypoxic conditions within wounds. This perspective delves into cutting-edge strategies leveraging autotrophic tissue engineering for regenerative medicine, and provides new pathways for wound healing and repair. Autotrophic tissue engineering harnesses the innate photosynthetic ability of algae to provide optimal oxygen levels within cell-seeded scaffolds. This innovative approach attempts to fabricate tissue constructs endowed with self-sustainability. It also reduces the dependence on external nutrient sources, and seeks to produce functional scaffolds suitable for 3D bioprinting applications. Similarly, we envision a creative design approach focused on devising a novel methodology to functionalize carbon quantum dots (CQDs) with fucoidan derived from algae through click chemistry.
1. Introduction
Tissue engineering augments the intrinsic regenerative potential of biological tissues, thereby catalysing the restoration of structure and functional processes for tissue repair. The last two decades have seen significant progress in the areas of bone, cartilage, cardiac tissue, pancreatic constructs, and vascular structures.1 Tissue engineering focuses on the development of biocompatible scaffolds, cell-based therapies, and the precise incorporation of growth factors and bioactive molecules to develop engineered constructs contributing to the acceleration and optimization of wound healing.2 The normal wound healing process involves four stages, namely, haemostasis, inflammation, proliferation and remodelling.3 Both local and systemic factors are involved during this process; among these, insufficient supply of oxygen to the wounded area is one of the major bottlenecks for tissue regeneration. Various approaches such as but not limited to the use of recombinant pro-angiogenic growth factors, gene vectors encoding for therapeutic molecules and stem cells have been adopted to enhance vascularization. Each of these approaches holds significant potential in hastening the process of vascularization. Nevertheless, even when all conditions are optimal, the complete restoration of suitable oxygen levels through vascular supply would still require several days to weeks.4 Direct administration of growth factors to augment the regenerative capabilities of engineered tissues faces several limitations, which includes short-term biological half-life of the molecules and the necessity for recurrent administration of large and potentially harmful doses.5Autotrophic tissue engineering represents a novel concept that leverages the capabilities of autotrophic organisms to create or enhance living tissues. Algae, which are autotrophic organisms, have emerged as a promising bioresource for wound healing applications due to their rich repertoire of bioactive compounds. They are capable of consistently supplying oxygen and other essential biomaterials necessary for the process of wound healing. Algae possess potent antioxidant, anti-inflammatory, and antimicrobial properties, and hence can be a potential solution to the global wound care challenge. Through the process of photosynthesis, they convert light into energy-rich organic molecules along with sufficient amount of oxygen. Moreover, alginate, fucoidan, carrageenan, agarose, and ulvan, derived from marine algae, exhibit distinctive physicochemical properties and possess therapeutic benefits. Their unique characteristics, such as gel-forming ability, biocompatibility, and biodegradability, coupled with their potential to modulate cellular behavior and promote tissue repair, position these algal polysaccharides as versatile materials for constructing artificial tissues and facilitating healing processes. Therefore, algae when integrated with cutting-edge tissue engineering play a significant role in tissue regeneration and can be employed in organ transplants, skin substitutes, cartilage, and bone repair, along with other applications.6,7 In this perspective, our objective is to delve into innovative strategies that capitalize on autotrophic tissue engineering. We give insights into the pioneering methodologies employed to augment diverse facets of biomedical engineering and therapy. Additionally, we elaborate on the incorporation of algal derivatives within this framework, evaluating their prospective roles in propelling advancements in the field. Similarly, we envision an inventive design approach aimed at creating a novel strategy to functionalize carbon quantum dots with fucoidan derived from algae using click chemistry, to establish its therapeutic precedence in the near future. Through this comprehensive exploration, our aim is to elucidate the convergence of autotrophic tissue engineering with biomedical applications, elucidating the promising pathways for research and development in this dynamically evolving domain.
2. Tissue engineering components
In vitro human tissue engineering comprises three equivalents, encompassing cell sources, tissue architecture, and niche properties as represented in Fig. 1.8 Cell sources are raw materials for the tissue engineering process, such as primary cells and cell lines, stem cells, organoids and genetically modified cells. Primary cell lines are directly isolated from living tissues or organs and maintain their original physiological characteristics, making them valuable for studying specific tissues or organs,9 whereas stem cells are undifferentiated cells bearing remarkable ability to differentiate into several cell types.10 On the other hand, three-dimensional miniature structures of cells forming organoids resembling organs are generated in the laboratory from stem cells or other precursor cells. Along with these cell sources, genetic engineering tools such as gene editing and gene delivery systems enable the manipulation of genetic material of cells or organisms for research or therapeutic purposes.11 Tissue architecture is the second essential component of tissue engineering, encompassing various structures such as scaffolds, hydrogels, organ-on-chips, and 3D cell printing, all of which serve as supportive mechanical frameworks. The scaffold gives cells a place to adhere, grow, differentiate, and eventually form the extracellular matrix. Additionally, it functions as a delivery system for cells, growth factors, and other biomolecular signals.12 To control tissue development, the scaffold should replicate human tissue composition and characteristics. An ideal scaffold should have a vast network of interconnecting pores, achieve proper mechanical strength, and have channels for delivering nutrients and oxygen to cells. Plant-based biomaterials offer safer alternatives for scaffold construction due to their biocompatibility, cost-effectiveness, and reduced immunogenicity. They also require fewer preparatory treatments for biomedical acceptability compared to animal-derived substances like collagen and gelatin.13 Hydrogels due to their high-water content, soft and elastic nature, and biocompatibility have gained attention as promising materials for various biomedical applications. They allow the diffusion of oxygen, nutrients and drugs, thus mimicking extracellular matrices (ECM).14 They are crucial for creating a bioactive and biocompatible 3D culture system. Hydrogel-based scaffolds tailored for specific cell types have been developed through the utilization of diverse biomaterials and techniques. With the aim to enhance cellular biocompatibility, promote cell adhesion and to insert stable micro or nano dimension structures within scaffolds, a combination of natural and synthetic polymers are utilized. Despite these advancements, the translation of hydrogels into clinical applications remains a formidable challenge. Apligraf®, AlloDerm®, and Juvéderm® represent merely a subset of hydrogels that have obtained approval for clinical utilization.15 Another structural component, organ-on-chip technology, also called microphysiological systems or tissue chips, is an innovative approach in tissue engineering. These small devices are designed to imitate the structure and function of human organs on a miniature scale. They use microfluidic tubes lined with living cells to recreate the natural environment of the organ, offering new possibilities for personalized medicine, toxicity testing, and drug development. For example, a lung-on-chip can replicate lung tissue, while a kidney-on-chip can simulate kidney filtration processes using specific cell types.16 These devices allow for direct visualization and quantitative analysis of biological processes in an intact kidney tubule, potentially aiding research into the molecular causes of kidney function and disease. Similarly, a cardiac muscle membrane was created using polydimethylsiloxane (PDMS) to create cardiac muscle membranes.17 Hence, on a miniature scale, organ-on-chip offers opportunities for personalized treatment, advanced toxicity testing, and enhanced drug development through the replication of human organ functions.18 3D bioprinting or cell printing is a technique that combines tissue engineering principles with 3D printing technology to create complex three-dimensional structures. The bioinks developed for it are made of cells and hydrogels to build complex tissue architectures and patterns. This technique enables the development of 3D tissue scaffolds, creating biomedical components with a high degree of tissue resemblance. The primary types of 3D bioprinting are laser-assisted, inkjet, extrusion, and stereolithography.19 The technique is used in drug testing, regenerative medicine, and tissue engineering to create precise tissues and organs.20 Inkjet bioprinting stands out for its speed, precision, and cost-effectiveness, creating high-resolution constructs with excellent cell viability (over 85%). This technology is ideal for applications requiring intricate details and rapid prototyping.21,22 Extrusion-based bioprinting excels in handling complex biomaterials, including those with high viscosity and cell density (over 95% viability). Its ability to maintain drug release within printed structures makes it a strong contender for tissue engineering applications demanding precise material deposition.23,24 Laser-assisted bioprinting reigns supreme in terms of precision and versatility, allowing for the creation of highly detailed structures with a wide range of biomaterials. This technology is particularly suited for applications requiring microscopic accuracy, such as organ-on-chip models and corneal tissue engineering.25 Growth factors are crucial for tissue repair and maintaining tissue homeostasis.26 These are signalling molecules that control the growth, differentiation, proliferation, and survival of cells. They bind to specific receptors on the cell surface, triggering intracellular signalling pathways that lead to various cellular responses. TGF-β is a growth factor that orchestrates the different events involved in wound healing by regulating ECM synthesis, inducing fibronectin, collagen, and other ECM elements, while inhibiting proteases and promoting protease inhibitors to preserve ECM integrity. Additionally, TGF-β enhances the expression of integrins binding to ECM components, facilitating tissue repair processes.27 Artificial niches in biomaterials aim to mimic the natural cell microenvironment, providing easily moldable, multidimensional structures and minimal cytotoxicity.8 While complex organ tissues like lungs, pancreas, liver, and heart have been successfully recreated, they are still not perfectly reproducible for implantation.28Table 1 summarizes the various products available for tissue engineering in various biomedical applications.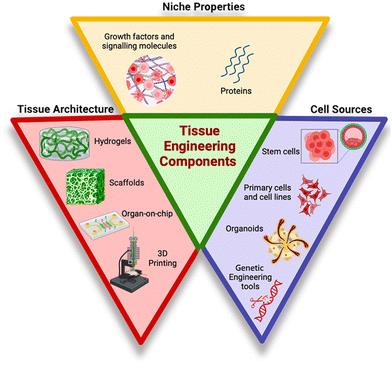 | ||
| Fig. 1 Tissue engineered equivalents.8 | ||
| Product name | Company name | Product type | Composition | Application | Ref. |
|---|---|---|---|---|---|
| 3D Insert™ | 3D Biotek | Scaffold sheet | Polystyrene | 3D cell culture application | 29 |
| HUMIMIC Chip 2 | TissUse | Organ-on-a-chip | Microfluidic circuit | Enables near-lifelike nutrient/oxygen supply for detailed studies on substance distribution, metabolization, and effects prediction | 30 |
| HUMIMIC Chip 3 | |||||
| HUMIMIC Chip 4 | |||||
| HUMIMIC Chip XX/XY | |||||
| OrganoPlate®2-lane 96 | Mimetas | Organ-on-a-chip | Layered tissue without artificial membrane | 31–33 | |
| OrganoPlate®Graft | Automated imaging | ||||
| OrganoFlow® | Robotic liquid handling equipment | ||||
| NERVESIMTM | AxoSim | Organ-on-a-chip | Multi-cellular primary cultures or iPSCs | Nerve conduction velocity for the developed platform is only about 0.13–0.28 m s−1 | 34 |
| BrainSIMTM | Limited automation on existing models | ||||
| AXLung-on-chip system | AlveoliX | Organ-on-a-chip | Siliocon membrane | Recreates the air–blood barrier with an ultra-thin membrane | 35 |
| BioSphincter™ | Cellf BIO | Cell expansion | Smooth muscle cells and neural stem cells | Autologous sphincter grown from patient's own gut cells is implanted, eliminating the rejection risk, and seamlessly integrates into the gastrointestinal tract | 36 |
| SeedEZ | Lena Biosciences | Scaffold | Stem cells | SeedEZ used with all cell types, enables cadherin (cell-to-cell) and integrin (cell-to-ECM) utilization by receptors for biomimetic functions of engineered tissue, suitable for long-term culture and multiple-dose drug testing | 37 |
3. Contemporary methods and hurdles
Tissue engineering faces challenges due to acellularity, thin tissues, low metabolism, and oxygen diffusion. Strategies to reduce hypoxia in vitro are promising, but large volumes of scaffolds in vivo may threaten their effectiveness.38 Research is currently focused on understanding tissue regeneration to exploit major pathways for treating aged and diseased tissues. Bone tissue engineering involves 3D porous scaffolds similar to natural bone, such as hydroxyapatite (HA) scaffolds, which promote new bone production in vivo. However, ceramic materials are not recommended for regeneration of significant bone defects due to their weak mechanical properties.39 Cartilage tissue engineering is centred on collagen, elastin, and proteoglycans, which are the essential components of firm and flexible connective tissues made up of chondrocytes embedded in a highly hydrated extracellular matrix.40 Restoring articular cartilage, which has limited self-regeneration ability, remains a medical challenge. Tissue engineering methods employing suitable scaffolds, mechanical stimulation, and growth factors aim to enhance cartilage healing. However, achieving effective integration with the surrounding environment remains a fundamental challenge in cartilage tissue engineering.41 Inflammation at the transplant site is a critical factor in osteoarthritis development and progression. Type 1 diabetes is a condition that is brought on by the immune system destroying cells.42 The most widely used scaffold for pancreatic tissue engineering for insulin production in diabetic patients has been Matrigel™. It is a solubilized complex basement membrane preparation taken from the Engelbreth–Holm–Swarm mouse sarcoma.43 Matrigel and other materials like collagen and PEG hydrogel have been suggested as 3D scaffolds for pancreatic tissue engineering.44 Islet encapsulation has been suggested as a method to prevent issues with islet transplantation, as it protects the transplanted cells from the host immune system by a biocompatible membrane.45 Today, however, there is still debate over whether immune suppression is necessary when using microencapsulation techniques.Hypoxia can limit cell respiration and growth, but it may also increase angiogenesis through the hypoxia-inducible factor-1 (HIF-1) pathway. Current techniques cannot maintain or deliver adequate oxygen to wounds due to their dependency on gaseous oxygen.46 Biomaterial scaffolds can have intricate designs with large, interconnected pores, mimicking the capillary network, to improve the penetration of the culture medium. However, the extent of blockage by developing tissues remains elusive. Perfusion bioreactors can help overcome this limitation, and oxygen carriers can increase oxygen capacity in the culture medium. Biomaterials have been designed to release angiogenic signals in a controlled manner, and stem cells can be genetically modified to express some angiogenic factors.47 Regenerative therapies encounter immunological barriers, particularly with allogeneic hematopoietic stem cells (HSCs) and transplantation of solid organs. During tolerance-induction treatment, allogeneic immune cells, particularly T cells, may respond negatively when given to an immunosuppressed patient. Many obstacles must be overcome to restore native tissues and organs using regenerative medicine, such as ensuring mechanical integrity of the transplant, vascularization, and innervation.48 Biomaterials are used in tissue engineering to provide surface qualities, shape, and structural traits that support cells and can be loaded with the right growth agents. Natural polymers like gelatin, chitosan, hyaluronic acid, polyhydroxyalkanoates (PHA), and collagen are commonly used due to their compatibility and availability. Silk, a natural polymer, has gained prominence in tissue engineering applications due to its processability, strength, and elasticity.49 The extracellular matrix (ECM) maintains biological cues and aids cell attachment, differentiation, and function. Mammalian tissue-based decellularized matrices have been prepared using various techniques, including chemical, biological, physical, and combined techniques.50 Synthetic polymers like polyglycolide, polylactide, poly(glycerol sebacate), and poly(lactide-co-glycolide) are commonly used in tissue engineering due to their availability, affordability, bioresorbability, and regulated processing.51
4. Algae for advanced tissue engineering
Algae are natural resources that have lately been utilized as revolutionary biological materials. The ability to synthesise complex metabolites with little resource input, together with a greater biomass productivity and rapid growth rate, are the main advantages. Algae are potential candidates for the production of new biochemical probes, biomedical scaffolds or drug carriers due to their distinctive morphological properties and easily functionalized surfaces.52 Microalgae are a renewable source of pharmaceutical compounds which are “Generally Regarded As Safe” (GRAS). The extracts of algae have exhibited a range of pharmacological activities. Because of their GRAS designation, products made from algae are useful not only for pharmaceutical applications but also for the food industry. Their ease of cultivation and production methods such as photoautotrophic, heterotrophic, and mixotrophic processes favour their utility.53 Due to their unique morphology and properties and capabilities algae are highly efficient photosynthetic organisms which are capable of converting light energy into chemical energy.54 They have evolved efficient photosynthetic pathways to capture and utilize light energy, making them ideal candidates for autotrophic tissue engineering. This energy conversion capability can be harnessed to provide a continuous energy source for engineered tissues.55 Algae produce a huge quantity of vital primary metabolites (e.g., carbohydrates, fatty acids, protein and vitamins) and secondary metabolites (e.g., phytosterols, antioxidants, pigments and phycobilin-proteins). Secondary metabolites are non-nutritive molecules that are synthesised by plants for environmental stress protection. With respect to health benefits, these high value secondary metabolites are used in pharmaceutical and nutraceutical industries.56 Interestingly, some algae are considered as a rich source of natural antioxidants such as astaxanthin (ASX), fucoxanthin (FX), zeaxanthin (ZX), canthaxanthin (CTX), violaxanthin (VLX), and neoxanthin (NX).57 Numerous freshwater and marine algae have been reported to contain these carotenoid pigments. The antioxidant and anti-proliferative properties of these carotenoids are well established. The algae Haematococcus pluvialis, C. sorokiniana, C. calcitrans, C. gracilis, S. obliquus, C. vulgaris and C. pyrenoidosa serve as the primary source of astaxanthin which has been licenced for commercial use by the Food and Drug Administration (FDA). Furthermore, by using an algae-based system, we can create novel molecules that are challenging to produce by chemical synthesis. The products of pharmaceutical importance have also been reported to be harvested from algae.58 Spirulina, a type of microalgae, is regarded as a superfood since it has a protein content of 60–70% and is also high in omega-6 fatty acids, vitamin B, vitamin E, phycocyanin and several minerals. It is quite beneficial for diabetes, reducing weight and blood pressure. Spirulina contains a pigment called C-phycocyanin which has antioxidant and anti-inflammatory effects.59 Chlorella, a different microalga, was used as a perfect food during space missions. It helps to stimulate the immune system and has a detoxicant effect. In addition to provitamin A, riboflavin, vitamin E, niacin, vitamin B6, vitamin B12, biotin, pantothenic acid, and folic acid, it contains protein, dietary fibers, fat, carbs, and thiamine B1. It is well known for reducing fibromyalgia, ulcerative colitis, and high blood pressure.59,60Algae are generally non-toxic and have low immunogenicity.61 This characteristic is crucial for biocompatibility in tissue engineering. While immune responses can vary depending on specific strains or individual immune profiles, algae are generally recognized as safe and do not elicit strong immune reactions. This makes them suitable for integration into tissue constructs without provoking significant immune responses or adverse reactions.62 Algae exhibit rapid growth rates and high biomass productivity, allowing for large-scale cultivation.63 There are just a few species of algae that can be consumed by humans such as Aphanizomenon, Chlorella vulgaris, Arthrospira (Spirulina) platensis, Dunaliella, and Nostoc. These algae have a high concentration of physiologically active compounds and a very simple manufacturing procedure, making them an attractive candidate for large-scale growth. Other microalgal species, including Chlorococcum sp., Aphanizomenon, Scenedescmus sp., Nanochloropsis sp., and Tetraselmis chuii, have proven to be a source of useful ingredients in aquaculture, feed, fertilisers, and cosmetics, but they do not yet have the GRAS (Generally Recognised as Safe) status.64 The requirements for growth media can differ amongst microalgal species. However, practically all species have similar fundamental needs, which include basic nutrients, a supply of carbon, either organic or inorganic, as well as nitrogen, phosphorus, and iron. This makes them attractive for tissue engineering applications that require the generation of substantial amounts of autotrophic biomass.
Algae can serve as a source of many different compounds, including antiviral, antibacterial, and antifungal medications, as well as neuroprotective products and therapeutic proteins. The biomass produced from algae is rich in bioactive compounds, which are obtained directly from primary metabolites or created from secondary metabolism.65 Strikingly, these compounds depict antiviral and antifungal activities that can prevent diseases in humans.66 In a study, Pratt et al. extracted a variety of fatty acids from chlorella that had antibacterial effects. It appears that different Gram-positive and Gram-negative bacteria can be killed, or their growth is inhibited by the free fatty acids derived from algae. Biological compounds have demonstrated antibiofilm capabilities in addition to the antibacterial activity of algae, which is important in the treatment of disorders caused by infection.67 Algae, in particular C. reinhardtii, are promising candidates for use as vaccine carriers for viral disease since they are secure and have a single chloroplast that expresses a high concentration of proteins. E2 protein, D2-CTB fusion protein (D2 fibronectin-binding domain of S. aureus containing the cholera toxin B subunit), and E7 oncoprotein are a few examples of these recombinant proteins that are used in vaccines against the classical swine flu virus and the human papillomavirus (HPV).68 Recently, a method for rapid, facile, and environment friendly assimilation of biomass and lipids has been reported in algae with potential utility in the pharmaceutical and nutraceutical sector.69 Algae can therefore be suggested as a sustainable source of high-value bioactive components with therapeutic potential and applications given the reliability and abundance of algae, which may be the reason for their advancing field of scientific literature throughout time.70 Algae encompass a wide range of species with diverse characteristics. This diversity provides options for researchers to select specific algal strains that best suit their tissue engineering goals, such as specific nutrient profiles, growth rates, and compatibility with the target tissue.71 These advantages have been depicted in Fig. 2.
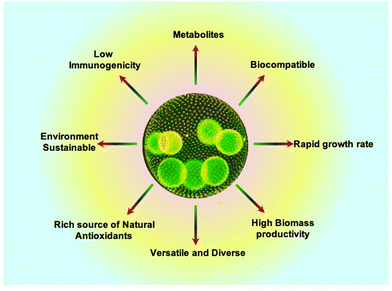 | ||
| Fig. 2 Advantages of algae for tissue engineering.72 | ||
4.1. Autotrophic tissue engineering
Autotrophic tissue engineering aims to develop tissue constructs that can sustain themselves by harnessing energy from light, similar to how plants do through photosynthesis. This approach is particularly relevant when engineering tissues with high metabolic demands or large tissue volumes, as it can provide a continuous energy source to support their growth and function.73 3D bioprinting has become an important tool for biomedical and next-generation biology research because 3D models can bridge the gap between animal models and 2D cell cultures.74 The advancement of autotrophic tissue engineering has a significant bearing on the creation of novel treatments. In addition to the standard problems, tissue engineering calls for extra attention to be paid to the requirements of autotrophic organisms for survival.The delineation of the role of oxygen is also necessary in wounds to retain energy supply (ATP levels) for the growth of new tissues along with deposition of collagen fibres.75 In the wound healing process, oxygen plays a key role in promoting angiogenesis, collagen synthesis, production of reactive oxygen species (ROS), production of growth factors, and ensuring effective fibroblast and leucocyte functioning.76
Schenck et al. (Fig. 3A–F) introduced a new technique called “HULK” (Hyperoxie Unter Licht Konditionierung), aiming to create chimerical tissues using photosynthetic cells to generate local oxygen through photosynthesis. They tested the response of photosynthetic scaffolds in in vivo transplantation, specifically for dermal wound regeneration. The study used a combination of the photosynthetic algae C. reinhardtii and a collagen-based template called “Integra matrix single layer.” In vivo transplantation showed promising results, with chimeric tissues persisting for at least 5 days without significant immune response.73 By creating chimeric animal–plant tissues during skin regeneration in immunocompetent mice, Chávez et al. (Fig. 3G) showed that photosynthetic biomaterials can produce and supply oxygen independently of the circulatory system. The angiogenic recombinant protein vascular endothelial growth factor (VEGF) was expressed by gene-modified algae that was incorporated into biomaterials. This study showcased an algae-based platform for secreting recombinant human growth factors, notably VEGF-165, SDF-1, and PDGF-B, with optimal production achieved using a UV-mutated strain and specific vectors. Genetic manipulation facilitated secretion of human proteins from algae, enhancing therapeutic potential. Additionally, in diabetic chronic wounds, impaired neovascularization due to hypoxia and destabilized HIF-1α exacerbates healing delays, highlighting the clinical need for oxygen supplementation. Algae-based hydrogels offer biocompatibility and versatility, supporting tissue movement and providing a conducive wound environment, addressing a critical gap in diabetic wound care.6,77
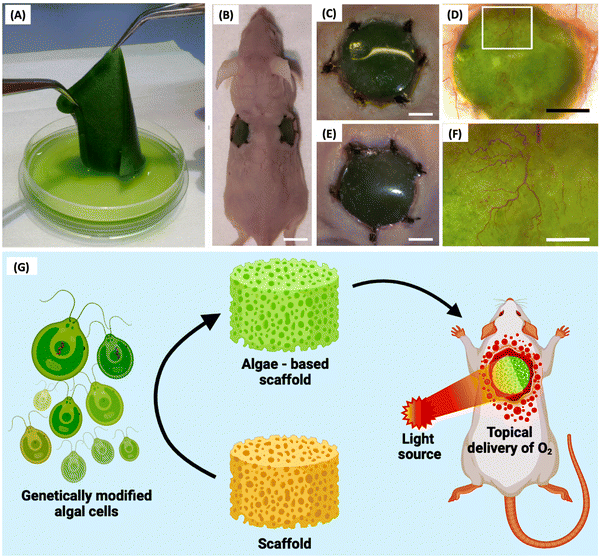 | ||
| Fig. 3 HULK (Hyperoxie Unter Licht Konditionierung) technique. (A) Generation of photosynthetic biomaterials. (B) In vivo scaffold engrafted in a mouse model. (C) and (E) A close-up of the (C) left and (E) right implant show no macroscopic signs of infection or inflammation at the wound area. (D) and (F) Implanted biomaterial showed high vascularization in the presence of microalgae.4 (G) Schematic illustration of genetically modified algal cell incorporated scaffolds engrafted in a wounded mouse and subjected to constant illumination.6 Reproduced with permission © 2015 Acta Biomaterialia. | ||
Bloch et al. studied the photosynthetic oxygen supply to encapsulated islets to mitigate hypoxia in interrupted vascular connections, aiming to evade graft rejection post-transplantation in diabetes mellitus patients. Biohybrid artificial pancreas (BAP), a novel technique, was developed which consisted of pancreatic islets positioned in a compartment that was sealed off from the recipient's immune system by hydrogels or semipermeable membranes. It contained two distinct compartments: one for oxygen-producing algal cells and the other for pancreatic islets that secrete insulin. Co-encapsulating islets and algae within the same compartment could potentially lead to negative consequences for the immobilized cells. These potential issues include disruptions in the physiological function of the encapsulated cells, alterations in algal metabolism from photosynthetic to heterotrophic, excessive algal growth, competition for essential nutrients, and the accumulation of harmful byproducts. To address these concerns, an alternative model was explored as a viable solution. Chlorella sorokiniana, a green, unicellular thermophilic alga, was employed in this experiment as a “photosynthetic oxygen generator” to enable enhanced insulin production from encapsulated pancreatic islets. C. sorokiniana at the ideal body temperature of humans exhibits remarkable potential for adaptation and these algae can be easily grown under laboratory conditions. It was discovered that islet oxygen consumption may be offset by algal-based photosynthetic oxygen production, resulting in optimum insulin secretion from encapsulated islets perfused with oxygen-free media. When the beads were perfused with oxygen-free media, the oxygen transfer from illuminated algae to nearby islets was effective and sustained adequate insulin response to glucose. In the same compartment of alginate beads as the islets, the microalgal cells were also encapsulated. In response to a high level of glucose, the islets boosted insulin secretion in a statistically significant (P = 0.01) manner.78 Maharjan et al. developed a 3D-bioprinted unicellular green algae, Chlamydomonas reinhardtii, to serve as a sustainable oxygen source in engineered tissue constructs. A homogeneous bioink mixture, comprising poly(vinyl alcohol), NaCMC, alginate, and gelatin, was utilized, with optimized proportions to enhance printability and structural stability. Using this bioink, honeycomb-shaped 3D structures as illustrated in Fig. 4 were printed and crosslinked with calcium chloride under constant light irradiation. These structures, containing C. reinhardtii, exhibited increased oxygen production over time, enhancing the viability and functionality of surrounding human cells while reducing hypoxia levels. Bioprinted C. reinhardtii within GelMA-based hydrogel constructs supported liver-derived cells’ viability and function, enabling the creation of vascularized hepatic tissue constructs through enzymatic removal of algae patterns and endothelialization of microchannels with human umbilical vein endothelial cells (HUVECs). O2 patterns formed by algae enhanced the capabilities of nearby human cells. Following the enzymatic breakdown of fugitive algae patterns, channels are endothelialized. The bioprinted C. reinhardtii-laden patterns promoted the survivability and functionality of the HepG2 cells within the surrounding GelMA matrices and acted as a natural photosynthetic O2 generator within hepatic tissue constructions.79
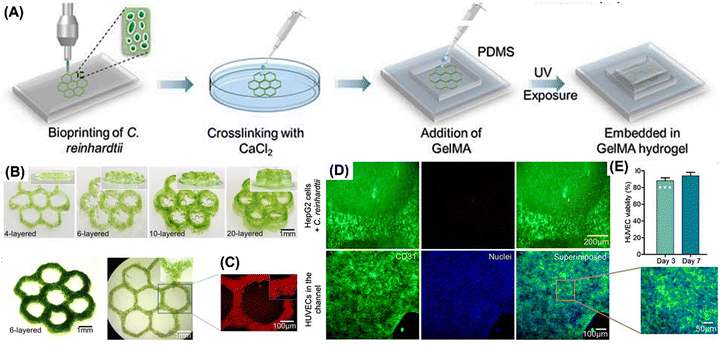 | ||
| Fig. 4 Bioprinting of C. reinhardtii. (A) 3D bioprinting process. (B) 3D bioprinted C. reinhardtii-laden honeycomb patterns with different layers at day 3. (C) Optical and fluorescence micrographs of bioprinted C. reinhardtii-laden honeycomb patterns. (D) HUVEC viability at days 3 and 7 after seeding in microchannels. The results are shown as means ± standard deviations. (E) Fluorescence micrographs of HUVEC immunostaining for CD31 expression (green) on day 7 of seeding in the microchannels.79 Adopted with permission © 2021 Matter. | ||
Miguel et al. introduced the first human trial of a photosynthetic therapy involving implantation of C. reinhardtii algae in scaffolds for complete skin wound regeneration. The algae were embedded in Integra matrix scaffolds with human fibrinogen and thrombin, showing no adverse local or systemic immunological reactions in eight patients during a 90-day follow-up. Macroscopic examination revealed no inflammation, and patients reported minimal discomfort. Laboratory testing indicated no harmful effects, with insignificant immune responses observed. Histological analysis demonstrated integration of the photosynthetic scaffold with the injured area, facilitating tissue regeneration and successful skin grafting. The results demonstrated in Fig. 5 are representative of the first effort to treat patients using photosynthetic cells, and they encourage the introduction of photosynthetic medications into healthcare settings.80
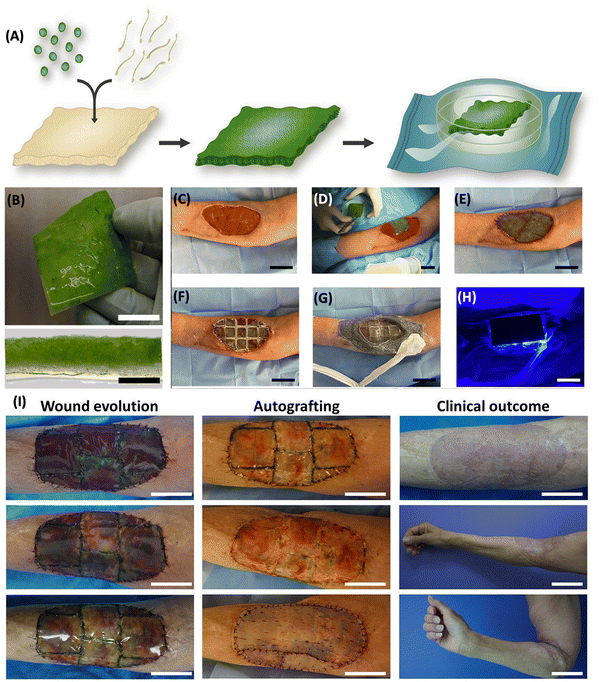 | ||
| Fig. 5 Fabrication of photosynthetic scaffolds and its application. (A) Microalgae and fibrin incorporated into a commercially available collagen scaffold and allowed to develop for four days. (B) The actual picture (top) and cross section (bottom) of a photosynthetic scaffold. Scale bars represent 2 cm (B, top), 2 mm (B, bottom) and 20 μm (C) wound prepared. (D) Photosynthetic scaffold implantation. (E) Scaffold sutured between the wound edges (F) implant covered with a flexible and transparent PDMS membrane (G) implant is secured with adhesive, leaving a window over the scaffold to allow illumination. (H) Light device was then placed on top and illumination intensity was controlled. Scale bars (C–H) represent 5 cm. (I) Wound evolution, autografting procedure, and clinical outcome. Scale bars represent 5 cm except for clinical outcome, middle and bottom, which represent 10 cm. Adapted with permission © 2021 Frontiers.80 | ||
Agarwal et al. combined the dried algal biomass of C. sorokiniana and a bioactive compound loaded hydrogel, which accelerated the wound healing process and also offered antibacterial properties. This algal biomass loaded hydrogel scaffold (AHS) was created using single step synthesis. The AHS comprising varying concentrations of algal biomass was administered to mouse excisional wounds for 14 days. In this investigation, the formulations were applied onto the wound once a day for up to 14 days. When compared to the control, betadine, hydrogel scaffold, and algal biomass groups, the 0.3% AHS group exhibited (Fig. 6) complete wound healing and no scarring. Moreover, the AHS showed strong antibacterial efficacy against the bacterial strains Escherichia coli and Staphylococcus aureus, in addition to having great biocompatibility. Hence the algal hydrogel scaffolds induced migration and proliferation of cells into the wound site rapidly accelerating healing.81
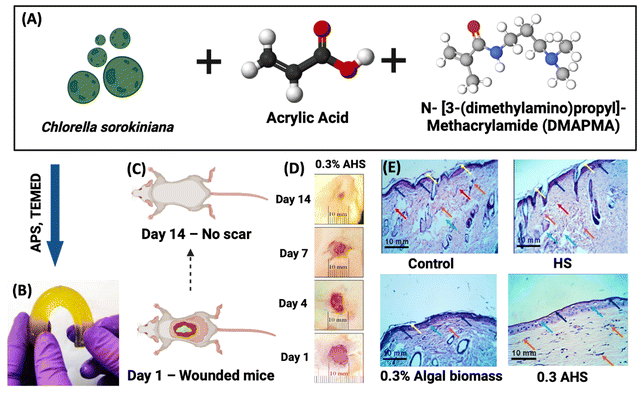 | ||
| Fig. 6 Treating wounded mice with algal biomass-loaded hydrogel scaffolds (AHS). (A) Schematic illustration of Chlorella sorokiniana, acrylic acid and DMAPMA. (B) Actual image of biomass loaded hydrogel scaffolds. (C) Mouse model presentation at day 1 and day 14. (D) 0.3% AHS treated wound healing process from day 1 to day 14. (E) Histological assessment of control, HS, 0.3% AB, and 0.3% AHS treated wounds at day 14 at 40× magnification.81,82 Reproduced with permission © 2022 American Chemical Society. | ||
Cerdas et al. introduced a novel bioactive suture that transcends traditional wound closure functions. By incorporating genetically engineered microalgae into a standard suture matrix, they engineered a construct capable of in situ oxygen generation and controlled release of pivotal growth factors, including VEGF, PDGF-BB, and SDF-1α, crucial for tissue regeneration. The suture exhibited comparable tensile strength to conventional sutures while maintaining stability under diverse conditions.83 Chen et al. introduced a pioneering oxygen-delivery system in the form of a hydrogel patch embedded with living microalgae, specifically targeting chronic wound management, particularly diabetic foot ulcers. In contrast to conventional gaseous oxygen therapies limited by cutaneous penetration, this patch delivers dissolved oxygen, exhibiting a 100-fold enhancement in dermal diffusion. In vitro studies demonstrated the patch's efficacy in stimulating cellular proliferation, migration, and angiogenesis, culminating in accelerated wound closure and improved skin graft integration in diabetic murine models.84 Wanlin et al. developed a novel Spirulina platensis (SP)-based hydrogel for accelerated wound healing, particularly in hypoxic environments. This hydrogel offers a cost-effective alternative to conventional oxygen therapies by generating oxygen in situ. Furthermore, the embedded chlorophyll, when exposed to laser irradiation, produces antimicrobial reactive oxygen species.85 The living microecological hydrogel (LMH) presents a novel therapeutic strategy for wounds characterized by infection and hypoxia. Encapsulating Chlorella and Bacillus subtilis, the LMH provides a symbiotic environment for oxygen generation and antimicrobial activity. Being composed of thermosensitive Pluronic F-127 and adhesive polydopamine, the hydrogel exhibits a liquid-to-solid phase transition upon contact with body temperature, ensuring secure adherence to the wound bed. By mitigating hypoxia and combating bacterial pathogens, the LMH significantly accelerates healing, particularly in infected diabetic wounds.86
4.2. Algal metabolite: fucoidan
Fucoidan is a fructose-rich sulphated polysaccharide that is extracted from a variety of brown algae and brown seaweed species. It has a variety of complex structural elements, but its main constituents are sulphate and L-fucose, with lesser amounts of D-mannose, arabinose, D-galactose, D-rhamnose, D-xylose, D-glucoronic acid and glucose. Due to its strong bio-functional qualities and biocompatibility, it can be used to treat cancer disorders as well as to prevent tumor-induced angiogenesis. Diverse studies have concentrated on fucoidan mixed with various polymeric materials, including alginate, chitosan, and polycaprolactone, for tissue engineering, in particular considering cell support systems because growth factor regulatory roles and drug transport are also significant. These substances are further processed to create films, nanofibers, hydrogels, and scaffolds.87 Fucoidan is extracted from Undaria pinnatifida and brown seaweeds. Fucoidan exhibits beneficial bioactivity and functions as an antioxidant, cancer preventive agent and anticoagulant.88 Sezer et al. created a fucoidan-chitosan structure to accelerate the healing of burn wounds in rabbits. In this research, fucoidan-chitosan hydrogels was studied for treating skin burns. Chitosan was chosen for its ability to form hydrogels, its effectiveness in wound dressing, whereas fucoidan was utilized for its anticoagulant benefits. By swelling the polymers in acidic solution, hydrogels were produced and their swelling, mechanical, and bio-adhesive properties were examined. As the concentration of polymers increased, the formulations became more viscous and were capable of absorbing more water. Fibroplasia and scarring on wounds treated with the fucoidan-chitosan gel and fucoidan solution were cured after 7 days of therapy. However, its best result was shown post 14-day treatment on dermal papillary formation and accelerated wound healing.89Haraguchi et al. studied a thick three-dimensional (3-D) bioengineered cardiac tissue produced using an in vitro co-culture technique that combined mammalian cells and the algae Chlorococcum littorale (Fig. 7). Even in the co-culture setup of algae and mammalian cells, the algae produced large amounts of oxygen at 30 °C, which was consumed actively by rat cardiac cells and C2C12 mouse myoblasts. Mammalian cells consumed O2 and excreted CO2 and metabolites, whereas algae recycled the metabolic waste products (ammonia, CO2) from mammalian cells. This co-culture technique enhanced the culture conditions within thicker multicellular layered tissues. Although anaerobic respiration of the cells was seen in the thicker cardiac cell-layered tissues, the introduction of creative co-cultivation partially converted the metabolism to aerobic respiration. In addition, when algae were co-cultivated, the amount of glucose consumption and ammonia and lactate generation in the culture media all considerably decreased. Histologically, delamination was seen in the heart tissues free of algae, and the release of creatine kinase (Fig. 7D) from the tissues revealed serious cardiac cell injury. On the other hand, it was shown that the layered cell tissues containing algae were in good histological condition, with a drop in creatine kinase release of less than a fifth. 160 μm thick cardiac tissues were formed as a result of co-cultivation with algae, which improved the culture condition of the thicker tissues. Clinical studies have already been successfully carried out in six different fields, including gastrointestinal medicine, cardiovascular medicine, periodontal disease, ophthalmology, otolaryngology and orthopaedic surgery using cell sheet technology to create and regenerate various tissues.82
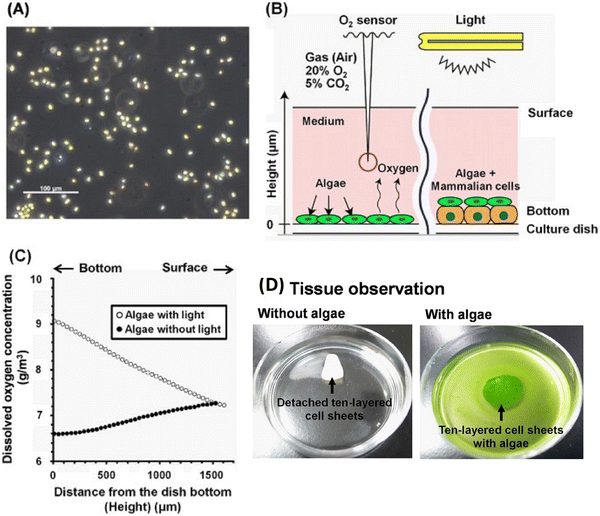 | ||
| Fig. 7 Chlorococcum littorale and oxygen measurement system. (A) Photograph of Chlorococcum littorale. (B) Schematic example of the system for measuring oxygen concentration. (C) Representative oxygen concentration profiles plotted against the height from the bottom of the dish for producing algae in an M199-based culture medium with/without light at 30 °C. (D) Photographs of ten-layered rat cardiac cell sheets without algae (left) and with (right) algae on 60-mm polystyrene culture dishes.82 Adapted with permission © 2017 Nature. | ||
Jin et al. created bone tissue regeneration biocomposites using poly(3-caprolactone) struts coated with alginate and fucoidan. By adjusting alginate levels for controlled fucoidan release, they improved biological activity while maintaining mechanical strength. Tests showed that biocomposites with controlled-release fucoidan promoted better bone regeneration than those without fucoidan or with burst-release.90 Low molecular weight fucoidan was incorporated into a rapid prototyping technique to enhance cell proliferation. This approach aimed to influence osteoconductive properties, including alkaline phosphatase activity, mineral deposition, and collagen type I expression. The ultimate goal was to develop a scaffold with optimal pore structure for bone tissue regeneration. Fucoidan and polycaprolactone were applied using an electrospinning approach to effectively produce micro- and nanofibrous scaffolds for use in bone regeneration.91 Not only alkaline phosphatase gene expression can be increased and bone morphogenic protein (BMP)-2 upregulated, but also bone mineralization can be stimulated and initiated by fucoidan.92 It can be encouraged for usage in regenerating bone tissue because it enhances osteogenic differentiation and osteogenesis in human adipose-derived stem cells and human amniotic fluid stem cells.93 For bone tissue engineering applications, many fucoidan-based composites have been developed as these materials promote osteoblast-like cell growth and osteoblast-mediated mineral deposition.72 Bar-Shai et al. (Fig. 8) investigated two macroalgal species, Ulva sp. and Cladophora sp. (Fig. 8(1)A and D), to assess their suitability as scaffolds based on seaweed cellulose. The scaffolds were created using the decellularization–recellularization technique for in vitro mammalian cell growth. In the decellularization process, pigments and proteins were extracted from fresh algae samples and transformed into acellular scaffolds. For recellularization, NIH3T3-GFP-actin fibroblasts were seeded onto sterilized scaffolds, allowing live cell monitoring through actin-GFP expression. Both scaffolds were non-toxic to fibroblasts. The porous surface of Ulva sp. scaffold promoted fast cell expansion in all directions, attaining saturation by week 3. In contrast, the Cladophora sp. scaffold encouraged the growth of elongated cells along the axis of its fibres, with moderate linear cell division.94 Michele et al. utilized a semi-refined technique to extract kappa-type carrageenan from red seaweed Kappaphycus alvarezii, and compared its chemical and structural characteristics with commercial carrageenan. The hydrogels derived from both the extracted and commercial carrageenan demonstrated significant potential as cell-carrier materials for tissue engineering. They served as scaffolds for in vitro development of multipotent stromal cells obtained from human skin. The study evaluated the effectiveness of encapsulating human SD-MSCs (skin-derived MSCs) in a kappa-type carrageenan hydrogel derived from a non-commercial extraction method to treat skin wounds in a mouse model. The carrageenan hydrogels, acting as scaffolds, exhibited the capability to support the growth and maintenance of human SD-MSCs in vitro, serving as a means to deliver cells to skin injuries. This proved that kappa-carrageenan hydrogels are beneficial for targeted delivery of drugs or cells during specific stages of skin regeneration.95
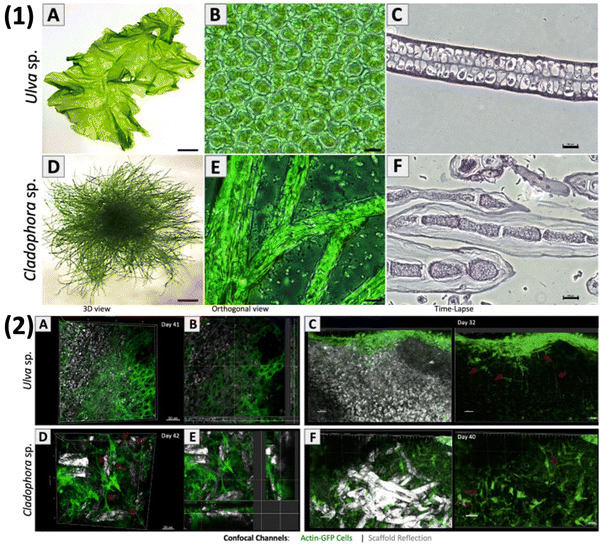 | ||
Fig. 8 Marine green algae: thallus morphology. Macro images of (1) – (A) Ulva sp. and (D) Cladophora sp. Light microscopy (40×) of the middle section shows Ulva sp.'s micro-porous structure and Cladophora sp.'s branching fibrous filamentous structure. Cross-sections reveal tissue sections of (C) Ulva sp.'s porous structure and (F) Cladophora sp.'s fibres. (2) Fluorescence confocal microscopy imaging of living fibroblasts (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × × ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 cells per μL): (A) and (B) Ulva sp.'s porous matrix (day 41) and (D) and (E) Cladophora sp.'s fibrous matrix (day 42). Time-lapse imaging (20×) reveals cell growth and spreading on the cellulose scaffolds: (C) Ulva sp. (day 32) and (F) Cladophora sp. (day 40).94 Adopted with permission © 2021 Nature. 103 cells per μL): (A) and (B) Ulva sp.'s porous matrix (day 41) and (D) and (E) Cladophora sp.'s fibrous matrix (day 42). Time-lapse imaging (20×) reveals cell growth and spreading on the cellulose scaffolds: (C) Ulva sp. (day 32) and (F) Cladophora sp. (day 40).94 Adopted with permission © 2021 Nature. | ||
5. Applications of algae
The “Green Bioprinting” method is anticipated to have advantages over current algae uses in the biotechnological area, including the simplification of harvesting and separation processes as well as the co-immobilization of algae with desired organism's cell. This innovative method creates new opportunities for cutting-edge uses including the incorporation of tissue engineering and algae in the medical industry. Co-cultivating algae with human cells would make it possible for human cells to get oxygen or secondary metabolites within the body system continuously without requiring an external source of oxygen or metabolites. For example, Anja et al. performed 3D printing by using the algae C. reinhardtii encapsulated in 3D alginate-based scaffolds. The algae were able to develop inside the hydrogel matrix after withstanding the plotting procedure. Microscopical examinations and the measurement of the chlorophyll content, which increased 16-fold in just 12 days of cultivation, both showed an increase in the number of cells under illumination. A structured coculture system was created using multichannel system plotting in which human cells and algae are cultured together. This coculture method integrates spatially arranged algae and human cells on a single scaffold. This could promote the creation of novel treatment ideas based on the use of secondary metabolites or oxygen delivered by algae as medicinal agents.96Table 2 summarizes a variety of algae used in tissue engineering.| Algae | Class of algae | Source | Applications | Ref. |
|---|---|---|---|---|
| Chlamydomonas reinhardtii | Green | Temperate soil habitats | Dermal wound regeneration | 97 |
| Chlorococcum littorale | Green | Aquatic and terrestrial environments | Regenerative therapy, 3D tissue model | 82 |
| Spirulina (Arthrospira) | Green | Fresh and marine water | Artificial tissue and for enabling the proliferation of mouse fibroblasts | 98 |
| Ulva armoricana | Green | Brackish water | Bioink, scaffolds for bone tissue engineering | 99 |
| Fucus vesiculosus | Brown | Marine water | Processing films, nanofibers, hydrogels, and scaffolds | 100 |
| Chlorella sp. | Green | Fresh or salt water and in soil | Diabetic wound healing | 101 |
| Porphyridium sp. | Red | Freshwater and terrestrial cells also found in salt marshes and soils of sea cliffs | Cultivation of stem cells, rebuilding of nerves and brain tissue | 102 |
| Nannochloropsis sp. G1-5 | Brown | Marine environments | Skin repair | 103 |
| Haematococcus pluvialis | Green | Freshwater | Tissue regeneration | 104 |
| Gracilaria crassa | Green | Estuaries or bays, usually in shallow subtidal or intertidal zones, less than a meter deep, and either free-floating or attached to rocks | Wound healing, hepatoprotective activities | 105 |
| Turbinaria ornata | Brown | Subtropics and temperate regions | Wound healing, antioxidant, antimicrobial | 105, 106 |
| Laurencia papillosa | Red | Abrasion platforms in the intertidal zone | Antiulcer, hepatoprotective activities | 105 |
| Sargassum illicifolium | Brown | Intertidal coastal regions | Wound healing, antitumor, cytotoxic antioxidant, anthelmintic, anticoagulant, antibacterial, antifungal, hepatoprotective effects | 107 |
| Euglena gracilis | Green | Fresh and brackish water habitats such as ponds rich in organic matter | Cutaneous wound healing, antimicrobial, anti-viral, antitumor, and anti-inflammatory activities | 108, 109 |
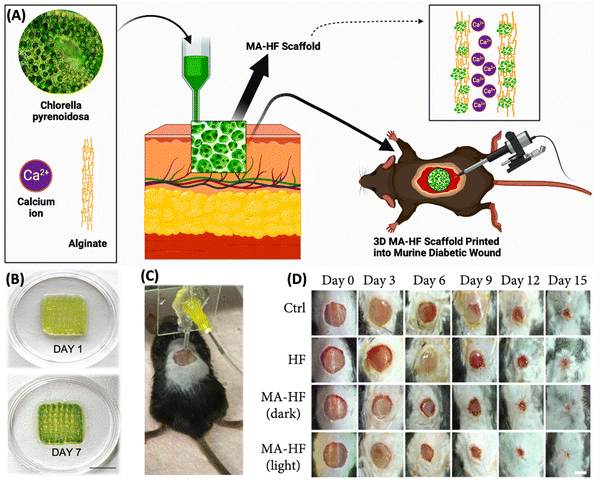
Wang et al. demonstrated (Fig. 9) an in situ microfluidic-assisted bioprinting technique for creating microalgae-filled hollow fibrous (MA-HF) scaffolds with the ability to produce autotrophic oxygen for the adaptation of irregularly shaped wounds and the promotion of their healing. Chlorella pyrenoidosa, a unicellular microalga capable of oxygenic photosynthesis, was added during 3D printing. Due to the quick crosslinking that occurs when Ca ions are present in coaxial microfluidic printing processes using alginate-based progels, direct 3D printing of the microalgae-laden hollow fibrous (MA-HF) scaffolds (Fig. 9A) can be performed in freeform wounds. In low-oxygen culture circumstances, the produced scaffolds enabled cell proliferation, migration, and differentiation by producing sustainable oxygen under light irradiation. Additionally, the living algae-laden scaffold is 3D printed right into diabetic wounds (Fig. 9C), where it would act as a robust autotrophic biosystem to get around the wound's hypoxic environment and hasten wound closure by encouraging angiogenesis and collagen formation. The wound was healed in just 15 days (Fig. 9D), proving that it is possible to in situ bioprint photosynthetic algae-loaded scaffolds for autotrophic wound healing. It provides an intelligent treatment strategy for a range of tissue engineering applications. In order to properly demonstrate the effectiveness in adapting to irregular, curved, or deep wounds in complex biological environments, further optimisation of microfluidic-assisted bioprinting is necessary. The current printing technique predetermined the scaffold shape before printing. Utilizing intraoperative computerised imaging technologies for real-time bioprinting, tissue defects in the future can be mapped using real-time tomography. Microfluidic-assisted bioprinting will also enable the printing of increasingly complex 3D architectures into deep tissue defects and curved tissue defects due to advancements in functional materials and microfluidic chips. The in situ bioprinting system will provide a straightforward and flexible way for swiftly, safely, and automatically correcting numerous faults in order to achieve this.110
Steffens et al. created scaffolds which showed a fibrous and porous structure akin to the natural ECM of the cells and, as a result, displayed properties that made them appropriate for cell culture. They used 7% PDLLA (poly-D,L-lactic acid) which was incorporated with algae Arthrospira. Electrospinning was used to generate nanofibers from the biomaterial. C57/B16N mice were used for this biological experiment. The scaffolds were found to be safe for use due to the low levels of organic solvents and suitable for use in tissues that regenerate rather quickly due to the short degradation times. Additionally, the aggregation characteristic of Spirulina with the application of biocompatible and biodegradable polymers improved stem cell adhesion and vitality. It follows that the scaffolds created in this work have the qualities needed to constitute a novel biomaterial appropriate for use in tissue engineering. The nanofiber matrices must adequately enhance cell adhesion in order for tissue engineering scaffolds to be successfully applied. Adsorption of ECM proteins on the surface of the scaffold creates an interaction between them which is essential for tissue regeneration.111
6. Future prospects and vision
Autotrophic tissue engineering, while promising, faces significant hurdles. Beyond the conventional challenges of tissue engineering, this field requires meticulous consideration of autotrophic organism's survival conditions. Precisely defining oxygen's role in wound healing is crucial, as it directly influences ATP levels and collagen deposition, essential for tissue regeneration. To fully evaluate safety and efficacy, rigorous testing using diverse clinical animal models is imperative. A primary challenge lies in providing adequate oxygenation for dense cellular environments within 3D tissue constructs. While oxygen-releasing dressings and topical oxygen therapy have been explored, inconsistent clinical outcomes hinder their widespread adoption. To advance autotrophic tissue engineering, a synergistic approach is necessary. Genetic engineering can be employed to create scaffolds delivering both oxygen and therapeutic molecules like growth factors, optimizing wound healing. However, current methods of oxygen delivery, such as hyperbaric oxygen and topical gaseous oxygen, suffer from inconsistent efficacy due to limitations in oxygen penetration and delivery. To overcome these challenges, a multi-faceted approach is necessary. This includes optimizing oxygen delivery systems, enhancing algal efficiency, integrating vascular networks, developing multifunctional scaffolds, and conducting robust clinical trials. By addressing these areas, the potential of this promising technology can be fully realized.Autotrophic tissue engineering is an advancing field that holds great potential for the future of medicine and regenerative therapies. As in bone tissue engineering composites, natural hydrogels and HA (hydroxyapatite), including collagen type I, combine the benefits of materials for a product that more closely resembles the structure of bone in vivo. To find the best bone substitute, various combinations of HA with gelatin, chitosan, PLA, alginate, and other polymers which are naturally synthesised from algae have been developed and researched.112,113 Bone is a highly vascularized tissue; the ideal scenario would actually be the injection of a cascade of several growth factors in order to simultaneously promote angiogenesis and osteogenesis and to build a substitute bone tissue that is both functional and vascularized.114
Future developments of cartilage will depend on how the tissue behaves as a unit to disperse uptake load and perform its mechanical function. Tissue engineering is related to the fusion of the developed construct with the native desired host tissue. The scaffold should keep its shape and have strong mechanical properties similar to native cartilage to fit the biological environment if implanted or injected right away. However, in vitro culturing techniques do not need scaffolds with these exact requirements owing to new tissue formation and gradually gains the chondrogenic commitment over the course of the culture phase. Composites are also being created, which combine two or multiple materials into a single scaffold.115 In addition to a hydrogel that was produced either artificially or naturally and added to a synthetic mesh, this group can also include a combination of fibres made from a variety of different natural materials. Selectivity and biocompatibility of phytochemicals obtained from algae are widely utilized in therapies.116,117 Fucoidan are sulfated polysaccharides which are high in fructose and obtained from many kinds of brown algae. Both cancer illnesses and the inhibition of tumor-induced angiogenesis may potentially be treated with it. Through various means, including the trigger of apoptosis, immune system activation and cell cycle arrest, fucoidan mediates its activity. Fucoidan has also been found to induce inflammation through the immune system, cause oxidative stress, and mobilize stem cells. These additional activities of fucoidan have been described and may be connected to the anti-cancer characteristics that have been discovered.118 Numerous fucoidans and their derivatives have been shown to have activity on a variety of tumor cells in vitro and in vivo in animals with transplanted tumors.119
Marine polysaccharides represent a rich reservoir of carbon, serving as a valuable precursor for synthesizing carbon-based nanomaterials such as carbon dots (CQDs), carbon nanosheets, and carbon nanotubes. These polysaccharides offer a distinct advantage due to their diverse heteroatomic composition, encompassing nitrogen (N), sulfur (S), and oxygen (O). The surfaces of nanomaterials like CQDs can undergo natural doping, enhancing their optical and surface characteristics, thereby mitigating the necessity for excessive use of chemical reagents and promoting environmentally friendly methodologies. Since fucoidan exhibits a wide range of applications including antiangiogenic, anti-inflammatory, anticoagulant, and immunostimulant properties, its utilization in tissue engineering would revolutionize the treatment of numerous illnesses and injuries.120–124 Moreover, nanomaterials, characterized by their nanoscopic dimensions and distinctive surface-driven properties, are undergoing extensive investigation within the biomedical domain. CQDs, in particular, have garnered significant attention due to their multifaceted potential, including their efficacy in combating cancer and various microbial agents. For instance, Tang et al. synthesised carbon dots (CDs) derived from fucoidan (FD) through a hydrothermal method for treating Enterococcus faecalis (E. faecalis) biofilm-associated persistent endodontic infections (PEIs).125 Similarly, Das et al. synthesized carbon dots using κ-carrageenan and phenyl boronic acid for biosensing blood glucose and facilitating drug delivery of the antidiabetic medication Metformin.126 Sarkar et al. customized carbon quantum dots (CQDs) utilizing calcium alginate (CA) to produce hydrogel films designed for precise delivery of glycopeptide antibiotic vancomycin within the gastrointestinal tract (GI). Through the integration of CQDs, the drug loading capacity of the CA/CQD film is augmented, while the uptake efficiency is bolstered, particularly near the gastric pH environment.127
Owing to the therapeutic efficacy of CQDs in tissue engineering and regenerative medicine, along with their ease of hydrothermal synthesis, they have gained widespread acceptance.128 Thus, to harness combined use, we propose an analeptic strategy of functionalizing CQDs with algal derived fucoidan via click chemistry. The strategy can be achieved by functionalizing amine-containing CQDs with 2-azido acetic acid via peptide coupling, to have a 2-azido-N-methylacetamide modified CQD surface (4). Subsequently, the pre-modified fucoidan-alkyne will be affixed to the modified CQD (4) through copper-catalysed azide–alkyne cycloaddition (CuAAC) reaction to form fucoidan derived CQD (9) (Scheme 1). Triazole and its derivatives have been proven to possess a wide range of therapeutic properties, including anticancer, antimicrobial, anti-inflammatory, antitubercular, antiviral, analgesic, anticonvulsant, antioxidant, and antidepressant properties. This demonstrates their potential for various applications in scientific fields. The synergistic effect of fucoidan and triazole would also result in enhanced anticancer activity.
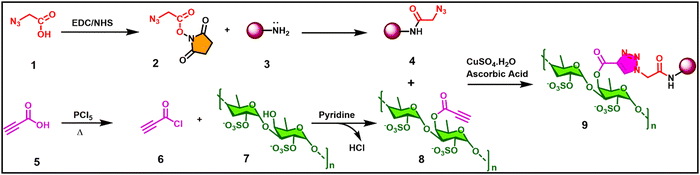 | ||
| Scheme 1 The proposed carbon quantum dots functionalized with a fucoidan derivative via click chemistry. | ||
7. Conclusion
In the quest for optimum oxygen levels within seeded scaffolds, a critical frontier such as autotrophic tissue engineering has emerged in the ever-evolving field of regenerative medicine. Autotrophic tissue engineering provides a potential and beacon of hope, harnessing the power of photosynthesis, a process embraced by algae. This novel approach exploits the intrinsic ability of algae to convert light, water, and carbon dioxide into energy-rich molecules, facilitating tissue constructs to self-sustain through photosynthetic processes and curtailing the demand for external nutritional sources. This cutting-edge perspective opens new avenues in regenerative medicine, where autotrophic tissue engineering unveils its potential to revolutionise wound healing and repair. If fully realized, algal based autotrophic tissue engineering may lead to an effective and sustainable tissue reconstruction, to orchestrate amenable biological processes. Microalgae engineered regenerative medicine has potential to help individuals in need of wound healing and tissue restoration.Abbreviations
| OOAC | Organ-on-a-chip |
| ECM | Extracellular matrix |
| TGF- β | Transforming growth factor beta |
| HA | Hydroxyapatite |
| IL-1b | Interleukin-1b |
| TNF-a | Tumour necrosis factor-a |
| HIF-1 | Hypoxia-inducible factor-1 |
| HSCs | Hematopoietic stem cells |
| HLA | Human leukocyte antigens |
| PHA | Polyhydroxyalkanoates |
| PCL | Polycaprolactone |
| PGA | Polyglycolide |
| PLA | Polylactide |
| PGS | Poly glycerol sebacate |
| PLGA | Polylactide-co-glycolide |
| GRAS | Generally regarded as safe |
| ASX | Astaxanthin |
| FX | Fucoxanthin |
| ZX | Zeaxanthin |
| CTX | Canthaxanthin |
| VLX | Violaxanthin |
| NX | Neoxanthin |
| HPV | Human papillomavirus |
| HULK | Hyperoxie Unter Licht Konditionierung |
| VEGF | Vascular endothelial growth factor |
| SDF-1 | Stromal cell-derived factor 1 |
| PDGH-B | Platelet derived growth factor |
| BAP | Biohybrid artificial pancreas |
| NaCMC | Sodium carboxymethylcellulose |
| HepG2 | Human hepatoma |
| HUVECs | Human umbilical vein endothelial cells |
| TAP | Tris-acetate-phosphate |
| AHS | Algal hydrogel scaffolds |
| BMP | Bone morphogenic protein |
| MA-HF | Microalgae-filled hollow fibrous |
| PDLLA | Poly-D,L-lactic acid |
Author contributions
Nikhita Pandian: investigation, methodology, data curation, writing – original draft preparation, review & editing. Radhika Chaurasia: investigation, methodology, data curation, writing – original draft preparation, writing – review & editing. Satyaki Chatterjee: generation of the fucoidan related click chemistry scheme. Bhaskar Biswas: review and editing. Prabir Patra: review & editing. Archana Tiwari: providing information related to algae, review & editing. Monalisa Mukherjee: conceptualization, methodology, resources, supervision, formal analysis, project administration, funding acquisition, writing – review & editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this perspective.Conflicts of interest
There is no conflict of interest.Acknowledgements
MM thanks the Science and Engineering Research Board, Department of Science and Technology (CRG/2023/001069) and University Grants Commission Department of Atomic Energy Consortium for Scientific Research (UGC-DAE CRS/2021-22/04/642) for funding this work. MM and RC are also thankful to the Department of Science and Technology (DST/WOS-A/CS-106/2021) for their funds. The authors would like to acknowledge the Biorender software for figures. MM, AT, NP and RC would like to acknowledge Amity University Uttar Pradesh, Noida for providing the facilities.References
- F.-M. Chen and X. Liu, Prog. Polym. Sci., 2016, 53, 86–168 CrossRef CAS PubMed.
- F. J. O’Brien, Mater. Today, 2011, 14, 88–95 CrossRef.
- S. Guo and L. A. DiPietro, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed.
- T. L. Schenck, U. Hopfner, M. N. Chávez, H.-G. Machens, I. Somlai-Schweiger, R. E. Giunta, A. V. Bohne, J. Nickelsen, M. L. Allende and J. T. Egaña, Acta Biomater., 2015, 15, 39–47 CrossRef CAS PubMed.
- K. Lee, E. A. Silva and D. J. Mooney, J. R. Soc., Interface, 2011, 8, 153–170 CrossRef CAS PubMed.
- M. N. Chávez, T. L. Schenck, U. Hopfner, C. Centeno-Cerdas, I. Somlai-Schweiger, C. Schwarz, H.-G. Machens, M. Heikenwalder, M. R. Bono, M. L. Allende, J. Nickelsen and J. T. Egaña, Biomaterials, 2016, 75, 25–36 CrossRef.
- G. Chen, F. Wang, X. Zhang, Y. Shang and Y. Zhao, Sci. Adv., 2023, 9(21), eadg3478 CrossRef CAS.
- C.-M. Moysidou, C. Barberio and R. M. Owens, Front. Bioeng. Biotechnol., 2021, 8 DOI:10.3389/fbioe.2020.620962.
- R. Patil, A. Kale, D. Mane and D. Patil, J. Oral Maxillofac. Pathol., 2020, 24, 68 CrossRef PubMed.
- A. Mackay-Sim and P. Silburn, Cell Proliferation, 2008, 41, 85–93 CrossRef PubMed.
- X.-Y. Tang, S. Wu, D. Wang, C. Chu, Y. Hong, M. Tao, H. Hu, M. Xu, X. Guo and Y. Liu, Signal Transduction Targeted Ther., 2022, 7, 168 CrossRef.
- B. P. Chan and K. W. Leong, Eur. Spine J., 2008, 17, 467–479 CrossRef.
- S. Chaudhary and E. Chakraborty, Beni-Suef Univ. J. Basic Appl. Sci., 2022, 11, 3 CrossRef PubMed.
- S. Mantha, S. Pillai, P. Khayambashi, A. Upadhyay, Y. Zhang, O. Tao, H. M. Pham and S. D. Tran, Materials, 2019, 12, 3323 CrossRef CAS PubMed.
- F. Xu, C. Dawson, M. Lamb, E. Mueller, E. Stefanek, M. Akbari and T. Hoare, Front. Bioeng. Biotechnol., 2022, 10 DOI:10.3389/fbioe.2022.849831.
- A. O. Stucki, J. D. Stucki, S. R. R. Hall, M. Felder, Y. Mermoud, R. A. Schmid, T. Geiser and O. T. Guenat, Lab Chip, 2015, 15, 1302–1310 RSC.
- A. Grosberg, A. P. Nesmith, J. A. Goss, M. D. Brigham, M. L. McCain and K. K. Parker, J. Pharmacol. Toxicol. Methods, 2012, 65, 126–135 CrossRef CAS.
- D. E. Ingber, Nat. Rev. Genet., 2022, 23, 467–491 CrossRef CAS PubMed.
- P. Lu, K. Takai, V. M. Weaver and Z. Werb, Cold Spring Harbor Perspect. Biol., 2011, 3, a005058 Search PubMed.
- M. A. Basson, Cold Spring Harbor Perspect. Biol., 2012, 4, a008151–a008151 Search PubMed.
- S. Ostrovidov, S. Salehi, M. Costantini, K. Suthiwanich, M. Ebrahimi, R. B. Sadeghian, T. Fujie, X. Shi, S. Cannata, C. Gargioli, A. Tamayol, M. R. Dokmeci, G. Orive, W. Swieszkowski and A. Khademhosseini, Small, 2019, 15(24) DOI:10.1002/smll.201805530.
- S. Mallakpour, M. Tukhani and C. M. Hussain, Adv. Colloid Interface Sci., 2021, 292, 102415 CrossRef CAS PubMed.
- E. Mancha Sánchez, J. C. Gómez-Blanco, E. López Nieto, J. G. Casado, A. Macías-García, M. A. Díaz Díez, J. P. Carrasco-Amador, D. Torrejón Martín, F. M. Sánchez-Margallo and J. B. Pagador, Front. Bioeng. Biotechnol., 2020, 8 DOI:10.3389/fbioe.2020.00776.
- A. Perez-Valle, C. Del Amo and I. Andia, Int. J. Mol. Sci., 2020, 21, 6679 CrossRef CAS PubMed.
- R. D. Ventura, Med. Lasers, 2021, 10, 76–81 CrossRef.
- R. Morris, K. A. Black and E. J. Stollar, Essays Biochem., 2022, 66, 255–285 CrossRef CAS PubMed.
- G. S. Schultz and A. Wysocki, Wound Repair Regen., 2009, 17, 153–162 CrossRef PubMed.
- G. Pennarossa, S. Arcuri, T. De Iorio, F. Gandolfi and T. A. L. Brevini, Int. J. Mol. Sci., 2021, 22, 830 CrossRef CAS PubMed.
- Z. F. Bielecka, K. Maliszewska-Olejniczak, I. J. Safir, C. Szczylik and A. M. Czarnecka, Biol. Rev., 2017, 92, 1505–1520 CrossRef.
- M. C. Koyilot, P. Natarajan, C. R. Hunt, S. Sivarajkumar, R. Roy, S. Joglekar, S. Pandita, C. W. Tong, S. Marakkar, L. Subramanian, S. S. Yadav, A. V. Cherian, T. K. Pandita, K. Shameer and K. K. Yadav, Cells, 2022, 11, 1828 CrossRef CAS.
- Y. Kato, A. Y. Lim, C. Sakolish, A. Valdiviezo, H. L. Moyer, P. Hewitt, P. Bajaj, G. Han and I. Rusyn, Toxicol. In Vitro, 2022, 85, 105464 CrossRef CAS PubMed.
- F. Bonanini, D. Kurek, S. Previdi, A. Nicolas, D. Hendriks, S. de Ruiter, M. Meyer, M. Clapés Cabrer, R. Dinkelberg, S. B. García, B. Kramer, T. Olivier, H. Hu, C. López-Iglesias, F. Schavemaker, E. Walinga, D. Dutta, K. Queiroz, K. Domansky, B. Ronden, J. Joore, H. L. Lanz, P. J. Peters, S. J. Trietsch, H. Clevers and P. Vulto, Angiogenesis, 2022, 25, 455–470 CrossRef CAS PubMed.
- Bio-Protoc. DOI:10.21769/BioProtoc.4070 .
- M. C. Koyilot, P. Natarajan, C. R. Hunt, S. Sivarajkumar, R. Roy, S. Joglekar, S. Pandita, C. W. Tong, S. Marakkar, L. Subramanian, S. S. Yadav, A. V. Cherian, T. K. Pandita, K. Shameer and K. K. Yadav, Cells, 2022, 11, 1828 CrossRef CAS PubMed.
- A. Sengupta, A. Dorn, M. Jamshidi, M. Schwob, W. Hassan, L. L. De Maddalena, A. Hugi, A. O. Stucki, P. Dorn, T. M. Marti, O. Wisser, J. D. Stucki, T. Krebs, N. Hobi and O. T. Guenat, Front. Pharmacol., 2023, 14 DOI:10.3389/fphar.2023.1114739.
- P. Dadhich and K. N. Bitar, in Current Topics in Faecal Incontinence, IntechOpen, 2020 Search PubMed.
- C. Shay, L. Lang and Y. Teng, Methods Mol. Biol. (N. Y.), 2020, 2138, 167–173 CAS.
- E. Bland, D. Dréau and K. J. L. Burg, J. Tissue Eng. Regener. Med., 2013, 7, 505–514 CrossRef CAS PubMed.
- Z. Xia, X. Yu, X. Jiang, H. D. Brody, D. W. Rowe and M. Wei, Acta Biomater., 2013, 9, 7308–7319 CrossRef CAS PubMed.
- C. Merceron, C. Vinatier, J. Clouet, S. Colliec-Jouault, P. Weiss and J. Guicheux, Jt., Bone, Spine, 2008, 75, 672–674 CrossRef PubMed.
- N. Fahy, M. Alini and M. J. Stoddart, J. Orthop. Res., 2017, 36(1), 52–63 CrossRef PubMed.
- L. Ouziel-Yahalom, M. Zalzman, L. Anker-Kitai, S. Knoller, Y. Bar, M. Glandt, K. Herold and S. Efrat, Biochem. Biophys. Res. Commun., 2006, 341, 291–298 CrossRef CAS PubMed.
- H. P. Shih and M. Sander, Pancreas Development Ex Vivo: Culturing Embryonic Pancreas Explants on Permeable Culture Inserts, with Fibronectin-Coated Glass Microwells, or Embedded in Three- Dimensional Matrigel™, in Stem Cells and Tissue Repair, ed. C. Kioussi, Methods in Molecular Biology, 2014, vol. 1210, pp. 229–237 Search PubMed.
- L. M. Weber, K. N. Hayda and K. S. Anseth, Tissue Eng., Part A, 2008, 14, 1959–1968 CrossRef CAS PubMed.
- D. J. Borg and E. Bonifacio, Curr. Diabetes Rep., 2011, 11, 434–444 CrossRef CAS PubMed.
- J. Malda, T. J. Klein and Z. Upton, Tissue Eng., 2007, 13, 2153–2162 CrossRef CAS PubMed.
- D. J. Polak, J. R. Soc., Interface, 2010, 7(6), S777–S781 Search PubMed.
- N. Amariglio, A. Hirshberg, B. W. Scheithauer, Y. Cohen, R. Loewenthal, L. Trakhtenbrot, N. Paz, M. Koren-Michowitz, D. Waldman, L. Leider-Trejo, A. Toren, S. Constantini and G. Rechavi, PLoS Med., 2009, 6, e1000029 CrossRef PubMed.
- F. J. O’Brien, Mater. Today, 2011, 14, 88–95 CrossRef.
- F. Ge, Y. Lu, Q. Li and X. Zhang, 2020, 15–31.
- N. Ashammakhi, A. GhavamiNejad, R. Tutar, A. Fricker, I. Roy, X. Chatzistavrou, E. Hoque Apu, K.-L. Nguyen, T. Ahsan, I. Pountos and E. J. Caterson, Tissue Eng., Part B, 2022, 28, 633–664 CrossRef PubMed.
- M. Rizwan, G. Mujtaba, S. A. Memon, K. Lee and N. Rashid, Renewable Sustainable Energy Rev., 2018, 92, 394–404 CrossRef.
- Y. Wang, S. Tibbetts and P. McGinn, Foods, 2021, 10, 3002 CrossRef CAS PubMed.
- Y. Qiao, F. Yang, T. Xie, Z. Du, D. Zhong, Y. Qi, Y. Li, W. Li, Z. Lu, J. Rao, Y. Sun and M. Zhou, Sci. Adv., 2020, 6(21) DOI:10.1126/sciadv.aba5996.
- M. I. Khan, J. H. Shin and J. D. Kim, Microb. Cell Fact., 2018, 17, 36 CrossRef PubMed.
- J. Ampofo and Lord Abbey, Foods, 2022, 11, 1744 CrossRef CAS PubMed.
- U. T. Ferdous and Z. N. B. Yusof, Front. Pharmacol., 2021, 12 DOI:10.3389/fphar.2021.593116.
- W. M. Bishop and H. M. Zubeck, J. Nutr. Food Sci., 2012, 2(5) DOI:10.4172/2155-9600.1000147.
- D. Jha, V. Jain, B. Sharma, A. Kant and V. K. Garlapati, ChemBioEng Rev., 2017, 4, 257–272 CrossRef CAS.
- Y. M. A. Naguib, J. Agric. Food Chem., 2000, 48, 1150–1154 CrossRef CAS PubMed.
- H. Cui, Y. Su, W. Wei, F. Xu, J. Gao and W. Zhang, Int. J. Nanomed., 2022, Volume 17, 3101–3122 CrossRef PubMed.
- G. Riccio and C. Lauritano, Mar. Drugs, 2019, 18, 2 CrossRef PubMed.
- W. G. Morais Junior, M. Gorgich, P. S. Corrêa, A. A. Martins, T. M. Mata and N. S. Caetano, Aquaculture, 2020, 528, 735562 CrossRef CAS.
- M. Rizwan, G. Mujtaba, S. A. Memon, K. Lee and N. Rashid, Renewable Sustainable Energy Rev., 2018, 92, 394–404 CrossRef.
- A. Agarwal, S. Jeevanandham, S. Sangam, A. Chakraborty and M. Mukherjee, ACS Omega, 2022, 7, 22061–22072 CrossRef CAS PubMed.
- E. Sanniyasi, G. Venkatasubramanian, M. M. Anbalagan, P. P. Raj and R. K. Gopal, Sci. Rep., 2019, 9, 12185 CrossRef PubMed.
- Y. López and S. M. Soto, Antibiotics, 2019, 9, 9 CrossRef PubMed.
- E. A. Specht and S. P. Mayfield, Front. Microbiol., 2014, 5 DOI:10.3389/fmicb.2014.00060.
- A. Khanra, S. Sangam, A. Shakeel, D. Suhag, S. Mistry, M. P. Rai, S. Chakrabarti and M. Mukherjee, ACS Sustainable Chem. Eng., 2018, 6, 774–780 CrossRef CAS.
- M. Bhattacharjee, Asian J. Pharm. Clin. Res., 2016, 9, 43 CrossRef CAS.
- V. Balasubramaniam, R. D.-N. Gunasegavan, S. Mustar, J. C. Lee and M. F. Mohd Noh, Molecules, 2021, 26, 943 CrossRef CAS PubMed.
- J. Venkatesan, I. Bhatnagar and S.-K. Kim, Mar. Drugs, 2014, 12, 300–316 CrossRef CAS PubMed.
- T. L. Schenck, U. Hopfner, M. N. Chávez, H. G. Machens, I. Somlai-Schweiger, R. E. Giunta, A. V. Bohne, J. Nickelsen, M. L. Allende and J. T. Egaña, Acta Biomater., 2015, 15, 39–47 CrossRef CAS PubMed.
- M. Barreiro Carpio, M. Dabaghi, J. Ungureanu, M. R. Kolb, J. A. Hirota and J. M. Moran-Mirabal, Front. Bioeng. Biotechnol., 2021, 9 DOI:10.3389/fbioe.2021.773511.
- W. L. Yip, Int. Wound J., 2015, 12, 620–624 CrossRef PubMed.
- I. Younis, J. Wound Care, 2020, 29, S4–S10 CrossRef PubMed.
- M. Jarquín-Cordero, M. N. Chávez, C. Centeno-Cerdas, A.-V. Bohne, U. Hopfner, H.-G. Machens, J. T. Egaña and J. Nickelsen, Appl. Microbiol. Biotechnol., 2020, 104, 725–739 CrossRef PubMed.
- K. Bloch, E. Papismedov, K. Yavriyants, M. Vorobeychik, S. Beer and P. Vardi, Artif. Organs, 2006, 30, 715–718 CrossRef CAS PubMed.
- S. Maharjan, J. Alva, C. Cámara, A. G. Rubio, D. Hernández, C. Delavaux, E. Correa, M. D. Romo, D. Bonilla, M. L. Santiago, W. Li, F. Cheng, G. Ying and Y. S. Zhang, Matter, 2021, 4, 217–240 CrossRef CAS PubMed.
- M. L. Obaíd, J. P. Camacho, M. Brenet, R. Corrales-Orovio, F. Carvajal, X. Martorell, C. Werner, V. Simón, J. Varas, W. Calderón, C. D. Guzmán, M. R. Bono, S. San Martín, A. Eblen-Zajjur and J. T. Egaña, Front Med., 2021, 8 DOI:10.3389/fmed.2021.772324.
- A. Agarwal, A. Kumar, P. Garg, A. Chakraborty, R. Verma, M. Sarwat, A. Gupta, P. K. Sasmal, Y. K. Verma, C. Chowdhury and M. Mukherjee, ACS Appl. Polym. Mater., 2022, 4, 5800–5812 CrossRef.
- Y. Haraguchi, Y. Kagawa, K. Sakaguchi, K. Matsuura, T. Shimizu and T. Okano, Sci. Rep., 2017, 7, 41594 CrossRef CAS PubMed.
- C. Centeno-Cerdas, M. Jarquín-Cordero, M. N. Chávez, U. Hopfner, C. Holmes, D. Schmauss, H.-G. Machens, J. Nickelsen and J. T. Egaña, Acta Biomater., 2018, 81, 184–194 CrossRef CAS PubMed.
- H. Chen, Y. Cheng, J. Tian, P. Yang, X. Zhang, Y. Chen, Y. Hu and J. Wu, Sci. Adv., 2020, 6(20) DOI:10.1126/sciadv.aba4311.
- W. Li, S. Wang, D. Zhong, Z. Du and M. Zhou, Adv. Ther., 2021, 4(1) DOI:10.1002/adtp.202000107.
- G. Chen, F. Wang, X. Zhang, Y. Shang and Y. Zhao, Sci. Adv., 2023, 9(21), eadg3478 CrossRef CAS PubMed.
- A. C. Hernández-González, L. Téllez-Jurado and L. M. Rodríguez-Lorenzo, Carbohydr. Polym., 2020, 229, 115514 CrossRef PubMed.
- Y. Zhao, Y. Zheng, J. Wang, S. Ma, Y. Yu, W. White, S. Yang, F. Yang and J. Lu, Mar. Drugs, 2018, 16, 321 CrossRef PubMed.
- A. D. Sezer, E. Cevher, F. Hatıpoğlu, Z. Oğurtan, A. L. Baş and J. Akbuğa, Biol. Pharm. Bull., 2008, 31, 2326–2333 CrossRef CAS PubMed.
- G. Jin and G. Kim, Soft Matter, 2012, 8, 6264–6272 RSC.
- S. Iravani and G. Soufi, Emergent Mater., 2021, 5, 631–652 CrossRef.
- S. I. T. Changotade, G. Korb, J. Bassil, B. Barroukh, C. Willig, S. Colliec-Jouault, P. Durand, G. Godeau and K. Senni, J. Biomed. Mater. Res., Part A, 2008, 87A, 666–675 CrossRef CAS PubMed.
- J. Pereira, S. Portron, B. Dizier, C. Vinatier, M. Masson, S. Sourice, I. Galy-Fauroux, P. Corre, P. Weiss, A.-M. Fischer, J. Guicheux and D. Helley, Tissue Eng., Part A, 2014, 20, 275–284 CrossRef CAS PubMed.
- N. Bar-Shai, O. Sharabani-Yosef, M. Zollmann, A. Lesman and A. Golberg, Sci. Rep., 2021, 11, 11843 CrossRef CAS PubMed.
- M. P. Rode, A. B. Batti Angulski, F. A. Gomes, M. M. da Silva, T. da, S. Jeremias, R. G. de Carvalho, D. G. Iucif Vieira, L. F. C. Oliveira, L. Fernandes Maia, A. G. Trentin, L. Hayashi, K. R. de Miranda, A. K. de Aguiar, R. D. Rosa and G. W. Calloni, J. Biomater. Appl., 2018, 33, 422–434 CrossRef CAS PubMed.
- A. Lode, F. Krujatz, S. Brüggemeier, M. Quade, K. Schütz, S. Knaack, J. Weber, T. Bley and M. Gelinsky, Eng. Life Sci., 2015, 15, 177–183 CrossRef CAS.
- U. Hopfner, T.-L. Schenck, M.-N. Chávez, H.-G. Machens, A.-V. Bohne, J. Nickelsen, R.-E. Giunta and J.-T. Egaña, Acta Biomater., 2014, 10, 2712–2717 CrossRef CAS PubMed.
- M. G. de Morais, B. da, S. Vaz, E. G. de Morais and J. A. V. Costa, BioMed Res. Int., 2014, 2014, 1–9 CrossRef PubMed.
- M. Dash, S. K. Samal, C. Bartoli, A. Morelli, P. F. Smet, P. Dubruel and F. Chiellini, ACS Appl. Mater. Interfaces, 2014, 6, 3211–3218 CrossRef CAS PubMed.
- H. Pajovich and I. Banerjee, J. Funct. Biomater., 2017, 8, 41 CrossRef PubMed.
- H. Wu, P. Yang, A. Li, X. Jin, Z. Zhang and H. X. Lv, Acta Pharm. Sin. B, 2023, 13, 410–424 CrossRef CAS PubMed.
- M. Halperin-Sternfeld, G. Netanel Liberman, R. Kannan, F. Netti, P. X. Ma, S. M. Arad and L. Adler-Abramovich, Biomedicines, 2022, 10, 1388 CrossRef CAS PubMed.
- S. Y. Kim, Y. M. Kwon, K. W. Kim and J. Y. H. Kim, Mar. Drugs, 2021, 19, 690 CrossRef CAS PubMed.
- H.-Y. Chou, D.-L. Ma, C.-H. Leung, C.-C. Chiu, T.-C. Hour and H.-M. D. Wang, Oxid. Med. Cell. Longevity, 2020, 2020, 1–13 CrossRef PubMed.
- K. A. Senthil and A. Murugan, Braz. J. Pharm. Sci., 2013, 49, 669–678 CrossRef.
- K. M. M. Shaibi, B. Leeba, S. Jamuna and R. Babu, Appl. Biochem. Biotechnol., 2022, 194, 395–406 CrossRef CAS PubMed.
- C. Simpi, C. Nagathan, S. Karajgi and N. Kalyane, Pharmacogn. Res., 2013, 5, 146 CrossRef PubMed.
- M. A. Borowitzka, in Microalgae in Health and Disease Prevention, Elsevier, 2018, pp. 23–72 Search PubMed.
- J. Li, Z. Zheng, M. Du, J. Chen, H. Zhu, Z. Hu, Y. Zhu and J. Wang, Front. Bioeng. Biotechnol., 2021, 9 DOI:10.3389/fbioe.2021.713840.
- X. Wang, C. Yang, Y. Yu and Y. Zhao, Research, 2022, 2022, 9794745 CAS.
- D. Steffens, M. Lersch, A. Rosa, C. Scher, T. Crestani, M. G. Morais, J. A. V. Costa and P. Pranke, J. Biomed. Nanotechnol., 2013, 9, 710–718 CrossRef CAS PubMed.
- H.-H. Jin, D.-H. Kim, T.-W. Kim, K.-K. Shin, J. S. Jung, H.-C. Park and S.-Y. Yoon, Int. J. Biol. Macromol., 2012, 51, 1079–1085 CrossRef CAS PubMed.
- D. Alves Cardoso, J. J. J. P. van den Beucken, L. L. H. Both, J. Bender, J. A. Jansen and S. C. G. Leeuwenburgh, J. Biomed. Mater. Res., Part A, 2014, 102, 808–817 CrossRef PubMed.
- J. Filipowska, K. A. Tomaszewski, Ł. Niedźwiedzki, J. A. Walocha and T. Niedźwiedzki, Angiogenesis, 2017, 20, 291–302 CrossRef CAS PubMed.
- A. Selvam, M. Majood, R. Chaurasia, A. Singh, T. Dey, O. Agrawal, Y. K. Verma and M. Mukherjee, J. Mater. Chem. B, 2023, 11, 10761–10777 RSC.
- B.-S. Negreanu-Pirjol, T. Negreanu-Pirjol, D. R. Popoviciu, R.-E. Anton and A.-M. Prelipcean, Pharmaceutics, 2022, 14, 1781 CrossRef CAS PubMed.
- Reetu, M. Mukherjee and M. P. Rai, in Bioinspired and Green Synthesis of Nanostructures, Wiley, 2023, pp. 141–156 Search PubMed.
- F. Atashrazm, R. Lowenthal, G. Woods, A. Holloway and J. Dickinson, Mar. Drugs, 2015, 13, 2327–2346 CrossRef CAS PubMed.
- M. V. Kiselevskiy, N. Yu. Anisimova, N. E. Ustyuzhanina, D. Z. Vinnitskiy, A. I. Tokatly, V. V. Reshetnikova, I. O. Chikileva, I. Zh. Shubina, K. I. Kirgizov and N. E. Nifantiev, Int. J. Mol. Sci., 2022, 23, 11821 CrossRef CAS PubMed.
- M. Majood, P. Garg, R. Chaurasia, A. Agarwal, S. Mohanty and M. Mukherjee, ACS Omega, 2022, 7, 28685–28693 CrossRef CAS PubMed.
- M. Majood, A. Selvam, O. Agrawal, R. Chaurasia, S. Rawat, S. Mohanty and M. Mukherjee, ACS Appl. Mater. Interfaces, 2023, 15, 19997–20011 CrossRef CAS PubMed.
- Y. Wang, J. Chen, J. Tian, G. Wang, W. Luo, Z. Huang, Y. Huang, N. Li, M. Guo and X. Fan, J. Nanobiotechnol., 2022, 20, 78 CrossRef CAS PubMed.
- X. Hao, L. Huang, C. Zhao, S. Chen, W. Lin, Y. Lin, L. Zhang, A. Sun, C. Miao, X. Lin, M. Chen and S. Weng, Mater. Sci. Eng., C, 2021, 123, 111971 CrossRef CAS PubMed.
- S. Chatterjee, A. Chakraborty, J. Banik, S. Mahindru, A. K. Sharma and M. Mukherjee, Drug Discovery Today, 2023, 28, 103601 CrossRef CAS PubMed.
- S. Tang, H. Zhang, L. Mei, K. Dou, Y. Jiang, Z. Sun, S. Wang, M. S. Hasanin, J. Deng and Q. Zhou, J. Nanobiotechnol., 2022, 20, 321 CrossRef CAS PubMed.
- P. Das, P. P. Maity, S. Ganguly, S. Ghosh, J. Baral, M. Bose, S. Choudhary, S. Gangopadhyay, S. Dhara, A. K. Das, S. Banerjee and N. C. Das, Int. J. Biol. Macromol., 2019, 132, 316–329 CrossRef CAS PubMed.
- N. Sarkar, G. Sahoo, R. Das, G. Prusty and S. K. Swain, Eur. J. Pharm. Sci., 2017, 109, 359–371 CrossRef CAS PubMed.
- N. Farshidfar, S. Fooladi, M. H. Nematollahi and S. Iravani, RSC Adv., 2023, 13, 14517–14529 RSC.
Footnote |
| † Equally shared first author. |
| This journal is © The Royal Society of Chemistry 2024 |