Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemoselective derivatisation and ultrahigh resolution mass spectrometry for the determination of hydroxyl functional groups within complex bio-oils†
Diana Catalina Palacio Lozano *,
Hugh E. Jones
*,
Hugh E. Jones ,
Mark P. Barrow
,
Mark P. Barrow and
Martin Wills
and
Martin Wills
Department of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: diana.palacio-lozano@warwick.ac.uk
First published on 12th June 2023
Abstract
Bio-oils are a renewable alternative resource for the production of fine chemicals and fuels. Bio-oils are characterised by a high content of oxygenated compounds with a diverse array of different chemical functionalities. Here, we performed a chemical reaction to transform the hydroxyl group of the various components in a bio-oil prior to characterisation with ultrahigh resolution mass spectrometry (UHRMS). The derivatisations were first evaluated using twenty lignin-representative standards with different structural features. Our results indicate a highly chemoselective transformation of the hydroxyl group despite the presence of other functional groups. Mono- and di-acetate products were observed in acetone–acetic anhydride (acetone–Ac2O) mixtures for non-sterically hindered phenols, catechols and benzene diols. Dimethyl sulfoxide–Ac2O (DMSO–Ac2O) reactions favoured the oxidation of primary and secondary alcohols and the formation of methylthiomethyl (MTM) products of phenols. The derivatisations were then performed in a complex bio-oil sample to gain insights into the hydroxyl group profile of the bio-oil. Our results indicate that the bio-oil before derivatisation is composed of 4500 elemental compositions containing 1–12 oxygen atoms. After the derivatisation in DMSO–Ac2O mixtures, the total number of compositions increased approximately five-fold. The reaction was indicative of the variety of hydroxyl group profiles within the sample in particular the presence of phenols that were ortho and para substituted, non-hindered phenols (about 34%), aromatic alcohols (including benzylic and other non-phenolic alcohols) (25%), and aliphatic alcohols (6.3%) could be inferred. Phenolic compositions are known as coke precursors in catalytic pyrolysis and upgrading processes. Thus, the combination of chemoselective derivatisations in conjunction with UHRMS can be a valuable resource to outline the hydroxyl group profile in elemental chemical compositions in complex mixtures.
Introduction
Chemical products such as automotive and industry oil and grease, equipment lubricants, hydraulic oils, food additives, additives for food contact materials, cosmetic and hair-care product ingredients, and additives for pharmaceutical formulations, are mainly produced from highly refined fossil-fuels.1 Non-fossil feedstocks, such as those produced by biomass pyrolysis or catalytic depolymerisation of plastic waste, are not only a renewable alternative resource for the decarbonisation of some transport sectors, they are also considered the only readily available source of carbon material to produce chemicals and polymers traditionally produced from refined fossil fuels.2–4 Alternative renewable resources can be a key contributor in addressing the challenges of socio-economic growth and climate change mitigation and adaptation.5 Bottlenecks in both fundamental knowledge and technological aspects, limit the advances in the chemical processes required for a successful energy and chemistry transition to produce fine chemicals from renewable biological materials.6Biomass is composed of cellulose, hemicellulose, and lignin units.7,8 A popular route for the conversion of biomass into liquids (bio-oils) is pyrolysis.9 The thermal decomposition occurring in a biomass during a pyrolysis process leads to the formation of thousands of different, mostly oxygen-containing, compositions with a wide variety of monofunctional or multifunctional groups e.g. a combination of acids, alcohols, aldehydes, ethers, ethers, furans, phenols, and ketones, among others. In general, bio-oils need further processing to reduce their oxygen content in an effort to improve a bio-oil's quality and miscibility with petroleum fuels.10 Previous works have determined that the chemical functionality of model compounds clearly impact the formation of monocyclic and polycyclic hydrocarbons. In general, phenols and cyclic ketones have been shown to convert to a much lower extent under catalytic reactions and to produce mostly coke or other oxygenated compounds.11–13 Kim et al.,14 showed that phenols with saturated side chains (methyl, ethyl and propyl) produced a higher yield of naphthalenes as a consequence of side chain cleavage over a zeolite catalyst and their subsequent oligomerisation, cyclisation, and aromatisation. Due to the negative effect of phenolic compositions, pre-treatments such as methylation of the phenolic hydroxyl groups has been proposed to improve the hydrocarbon production from lignin material.12 The structural characterisation of the compositions present in the bio-oil can then facilitate or assist the prediction of some co-processing products e.g., coke and light gases.
Bio-oils are complex mixtures not solely because of the large number of poly-oxygenated compositions, but because each molecule can exist in several isomeric forms, and contain different functional groups,15 and therefore exhibit different properties that affect their behaviour under storage, processing, and upgrading. The structural characterisation of such a complex mixture remains a key goal for energy and environmental research. The hydroxyl group is the most abundant functional group in bio-oils16 and natural product derivatives17 and can be found in carbohydrates, alcohols, carboxylic acids, and phenols. Spectroscopic techniques such as 31P NMR (nuclear magnetic resonance) of derivatives can provide valuable quantitative information about the aliphatic phenolic, carboxylic hydroxyl groups, and water content, of bio-oils.18,19 The quantitative characterisation by 31P NMR is based on the derivatisation with a phosphitylation agent (P-agent) that allows moieties with a hydroxyl group (aliphatic, phenols and carboxylic OH) to react with the P-agent forming an active moiety for 31P NMR analysis. Nevertheless, spectroscopic techniques are unsuitable for individual compound analysis within a complex mixture.16
A molecular level characterisation of complex mixtures can be achieved with ultrahigh resolution mass spectrometry (UHRMS) techniques. UHRMS techniques are well-known for their ability to resolve thousands of individual molecular compositions with the highest mass accuracy in a single analysis, allowing the assignment of unique elemental compositions.20,21 Due to its ultrahigh resolving power and mass accuracy, Fourier transform ion cyclotron resonance mass spectrometers (FTICR MS) are commonly used for the analysis of complex mixtures, allowing access to high-molecular weight compositions in bio-oils and crude oils.21,22 The structural identification of the individual compositions in bio-oils is the key goal for a comprehensive chemical composition analysis. Derivatizations as an additional step in sample preparation before UHRMS detection have recently been applied for semi-targeted analysis of functional groups in complex mixtures. Examples include the characterization of thiols in fossil fuels by Michael addition derivatisation,23 Ag+ complexation for the characterisation of olefin mixtures,24 the derivatization of ketone/aldehyde functional groups in weathered petroleum,25 the carbonyl group derivatization in an oak pyrolysis bio-oil by the use of 3-chloroaniline,26 and the use of esterification reactions to gain insights in reactivity of bio-oil compositions.15 Changes in the total abundance of heteroatomic class distributions after derivatisations are a clear indication of the presence of the targeted functional groups in complex mixtures.
Here, UHRMS in combination with derivatisation using acetic anhydride (Ac2O) in the presence of different solvents was used to pinpoint those compositions containing a hydroxyl group in a complex bio-oil obtained from lignocellulosic material. We have also included a comprehensive evaluation of the reactions using lignin-derivative standard compounds to gain a greater understanding of the functional group profile changes when the reactions are applied in a complex mixture. The detection of more than 1900 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 unique compositions observed after reactions in acetone–Ac2O and dimethyl sulfoxide (DMSO)–Ac2O mixtures, respectively, are a clear indication of the wide variety of structural arrangements of the hydroxyl group within the bio-oil. We have also been able to infer and semi-quantify the presence of primary and secondary alcohols, non-hindered phenols, and aromatic alcohols in bio-oil's elemental compositions.
000 unique compositions observed after reactions in acetone–Ac2O and dimethyl sulfoxide (DMSO)–Ac2O mixtures, respectively, are a clear indication of the wide variety of structural arrangements of the hydroxyl group within the bio-oil. We have also been able to infer and semi-quantify the presence of primary and secondary alcohols, non-hindered phenols, and aromatic alcohols in bio-oil's elemental compositions.
Materials and methods
Chemicals and bio-oil
Twenty lignin-derived model compounds containing a range of hydroxyl groups typically represented in bio-oils were used to evaluate the acetylation reactions.27 The standards and their respective identifiers are listed in Table 1. The identifier of each model compound is based on their structural features, e.g. Ph for phenols, Ct for catechols and benzene diols, Ox for phenols with oxygenated functional groups, Ol for hydroxyls in primary or secondary positions, and CA for those standards containing a carboxylic acid group. The details of vendors and purities can be found in Table S1.† The acetylation reactions were also performed in a pyrolysis bio-oil obtained from lignocellulosic bio-oil. The bio-oil was purchased from BTG BioLiquids (Enschede, The Netherlands). Briefly, the bio-oil was produced from lignocellulosic biomass in a fast pyrolysis process operating with a short vapor resident time at 450–600 °C, at near atmospheric pressure in the absence of oxygen.| Name | Exact mass | Molecular formula | Identifier |
|---|---|---|---|
| Small phenols | |||
| 2-Methoxyphenol | 124.0524 | C7H8O2 | Ph-1 |
| 2,4-Dimethylphenol | 122.0732 | C8H10O | Ph-2 |
| o-Cresol | 108.0575 | C7H8O | Ph-3 |
| 4-Ethylphenol | 122.0732 | C8H10O | Ph-4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Catechols and benzene diols | |||
| Hydroquinone | 110.0368 | C6H6O2 | Ct-5 |
| 1-(2,5-Dihydroxyphenyl)propan-1-one | 166.0630 | C9H10O3 | Ct-6 |
| 5-Methylbenzene-1,3-diol | 124.0524 | C7H8O2 | Ct-7 |
| Pyrocatechol | 110.0368 | C6H6O2 | Ct-8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Phenols with other oxygenated functional groups | |||
| Ethyl 2-hydroxybenzoate | 166.0630 | C9H10O3 | Ox-9 |
| 1-(3-Hydroxy-4-methoxyphenyl)ethan-1-one | 166.0630 | C9H10O3 | Ox-10 |
| 3-Ethoxy-4-hydroxybenzaldehyde | 166.0630 | C9H10O3 | Ox-11 |
| 4-Hydroxy-3-methoxybenzaldehyde | 152.0473 | C8H8O3 | Ox-12 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Primary and secondary alcohols | |||
| 3-(Hydroxymethyl)phenol | 124.0524 | C7H8O2 | Ol-13 |
| Chroman-4-ol | 150.0681 | C9H10O2 | Ol-14 |
| 1-Phenylethan-1-ol | 122.0732 | C8H10O | Ol-15 |
| 2-Phenylethan-1-ol | 122.0732 | C8H10O | Ol-16 |
| 3-Cyclohexylpropan-1-ol | 142.1358 | C8H10O | Ol-17 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Carboxylic acids | |||
| 2-Hydroxy-3-phenylpropanoic acid | 166.0630 | C9H10O3 | CA-18 |
| 2-(4-Hydroxyphenyl)propanoic acid | 166.0630 | C9H10O3 | CA-19 |
| 4-Hydroxy-3,5-dimethylbenzoic acid | 166.0630 | C9H10O3 | CA-20 |
Acetylation reactions
Each standard was first prepared as a 0.1 mmol in 1 mL (0.1 M) solution in HPLC grade methanol (Sigma-Aldrich, Gillingham, United Kingdom). This sample set was used as a blank to determine the GC retention time of each of the non-derivatised standards. Additionally, a further three sets of each standard were prepared at a concentration of 0.1 mmol in 1 mL (0.1 M) in three different HPLC grade solvents: methanol, dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Gillingham, United Kingdom), and acetone (Avantor, Lutterworth, United Kingdom). Each vial of these three sets was then spiked with 36 μL (39 mg, 0.38 mmol) of acetic anhydride (Ac2O 99%, Fischer Scientific, Loughborough, UK). The standards were left to react at room temperature for approximately seven days, after which they were stored at −23 °C until analysis.Similarly, 79 mg of the bio-oil were weighed into each of 4 scintillation vials, for two sets of reactions. One set of two vials were diluted by the addition of 1 mL of HPLC grade acetone and the other set were diluted by the addition of 1 mL of HPLC grade DMSO. One vial of each set was then spiked with 360 μL (390 mg, 3.8 mmol) of acetic anhydride and were left to react at room temperature. The other pair of vials to which acetic anhydride was not added act as blanks for each reaction. The samples without acetic anhydride (non-derivatised blanks) are named hereafter bio-oil acetone and bio-oil DMSO whereas the derivatised samples are named bio-oil acetone–Ac2O and bio-oil DMSO–Ac2O.
Sample preparation
The raw standards and the derivatised standards (a total of 80 samples) were dissolved to a final concentration of 0.1 mg mL−1 in methanol before the GC-APCI-TOF MS (gas chromatography-atmospheric pressure chemical ionisation-time of flight mass spectrometry) analysis. The bio-oils before and after derivatisation were prepared at a final concentration of 0.1 mg mL−1 in HPLC grade methanol for direct infusion APCI FTICR MS experiments.Analytical techniques
High or ultrahigh resolution mass spectrometry techniques were used for the analysis of the samples. High resolution MS coupled to gas chromatography was used for the analysis of standard molecular compositions before and after derivatisation reactions. Direct infusion ultrahigh resolution mass spectrometry was used for the analysis of the more complex bio-oil samples. The experimental parameters are given below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at m/z 200). The ions were detected in a mass range of 40–1000 Da with a transfer time of 90.0 μs, a collision energy offset of 5.0 eV, and an acquisition rate of 1 spec per sec. The GC TOF MS data obtained for each standard is about 440 MB in size. The chromatographic method was optimised using a standard mixture of even carbon number fatty acid methyl esters (C4–C24, Sigma Aldrich, Gillingham, United Kingdom). 1 μL of each sample was injected into a 30 m Stabilwax-DA polar phase column (0.25 mmID, 0.25 μm, Thames Restek, UK) with helium (99.9995% purity) as the carrier gas. The GC column is connected to a Rxi guard column (0.25 mmID) using a SilTite μ-union Connector (Thames Restek, UK). The inlet was set at a temperature of 260 °C. The oven temperature was set at an initial temperature of 40 °C for 10 minutes and the temperature was increased at a rate of 5 °C min−1 until 255 °C. The oven was then maintained at 255 °C for 10 min. The interface of the GC to the mass spectrometer consists of a heated transfer line that was kept at a temperature of 270 °C. Methanol blanks were run in between experiments to ensure there was no carryover between samples. The standards were ionised in positive-ion mode by a corona discharge needle operating at 2400 nA. Other ionisation parameters include dry gas flow 5.0 L min−1, drying gas temperature 220 °C, and nebuliser gas flow rate of 2.0 bar.
000 at m/z 200). The ions were detected in a mass range of 40–1000 Da with a transfer time of 90.0 μs, a collision energy offset of 5.0 eV, and an acquisition rate of 1 spec per sec. The GC TOF MS data obtained for each standard is about 440 MB in size. The chromatographic method was optimised using a standard mixture of even carbon number fatty acid methyl esters (C4–C24, Sigma Aldrich, Gillingham, United Kingdom). 1 μL of each sample was injected into a 30 m Stabilwax-DA polar phase column (0.25 mmID, 0.25 μm, Thames Restek, UK) with helium (99.9995% purity) as the carrier gas. The GC column is connected to a Rxi guard column (0.25 mmID) using a SilTite μ-union Connector (Thames Restek, UK). The inlet was set at a temperature of 260 °C. The oven temperature was set at an initial temperature of 40 °C for 10 minutes and the temperature was increased at a rate of 5 °C min−1 until 255 °C. The oven was then maintained at 255 °C for 10 min. The interface of the GC to the mass spectrometer consists of a heated transfer line that was kept at a temperature of 270 °C. Methanol blanks were run in between experiments to ensure there was no carryover between samples. The standards were ionised in positive-ion mode by a corona discharge needle operating at 2400 nA. Other ionisation parameters include dry gas flow 5.0 L min−1, drying gas temperature 220 °C, and nebuliser gas flow rate of 2.0 bar.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, while the data acquired at 4 MW had a corresponding resolving power of 690
000, while the data acquired at 4 MW had a corresponding resolving power of 690![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The bio-oils were ionised with a corona discharge operating at 1200 nA to reduce in-source fragmentation. Drying gas flows, drying gas temperature, and nebuliser gas flow rate were set at 5.0 L min−1, 220 °C, and 2.0 bar, respectively.
000. The bio-oils were ionised with a corona discharge operating at 1200 nA to reduce in-source fragmentation. Drying gas flows, drying gas temperature, and nebuliser gas flow rate were set at 5.0 L min−1, 220 °C, and 2.0 bar, respectively.Data processing
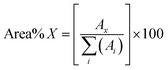 | (1) |
Data analysis
| DBE = c − h/2 + n/2 + 1 | (2) |
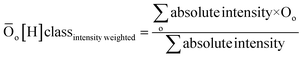 | (3) |
Results and discussion
GC-TOF MS of lignin-representative compounds
Bio-oils are distinguished from fossil-fuels by their characteristic content of highly oxygenated chemical compounds. The chemical profile of bio-oils can include aromatic and aliphatic hydrocarbons, phenolic derivatives, carboxylic acids, esters, ethers, and chemicals containing an array of different functional groups. Identifying the specificity of the conversion of the hydroxyl moiety amidst an array of more reactive functional groups then needs to be evaluated. In this section, acetic anhydride in the presence of acetone, methanol and DMSO was evaluated to transform the hydroxyl group in lignin-representative model compounds.Standards before and after reactions in acetic anhydride were characterised by GC-TOF MS. GC-TOF MS allows the identification of the raw standards and reaction products based on their retention time, and additionally generates smaller data set size files in comparison with GC-FTICR MS.28 The chromatograms and relevant extracted ion chromatograms can be found in Fig. S1–S20 in the ESI.† The retention time of the raw standards was used to identify the respective reactant and products of the reactions with acetic anhydride, and the yields were then calculated using eqn (1). A list of the retention times and their relative abundances can be found in the ESI† (“Retention time data.xlsx”). A summary of the products of the reactions are shown in Fig. 1 and an extended version of the reaction products can be found in Fig. S21 and S22.†
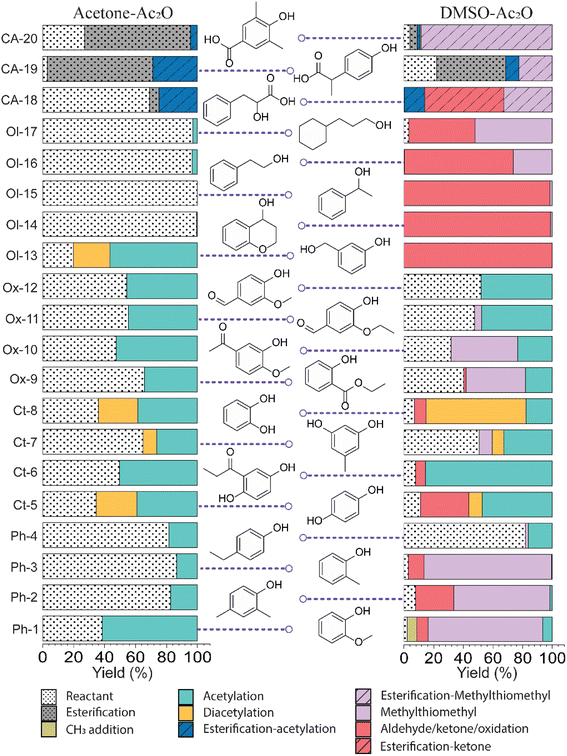 | ||
| Fig. 1 Reaction yields observed on lignin-representative standard molecules in mixtures of acetone–Ac2O and DMSO–Ac2O. | ||
The standards analysed in this work correspond to several types of phenols, aromatic/cyclohexane alcohols, phenols with other functional groups (ester, ethers, ketone, aldehydes and carboxylic acids), and compounds that contain up to two hydroxyl groups. As can be seen in Fig. S1–S20,† the raw standards were shown to be ionisable by APCI. Most of the standards were detected as [M + H]+ ions. It is noticeable however, that standards with a hydroxyl group in a primary or secondary position, e.g., Ol-13, Ol-14, Ol-15, Ol-16, and Ol-17, were detected as either an odd-electron ion [M]+˙ or a pseudo-molecular [M − H]+ ion.
Derivatization of lignin-representative model compounds
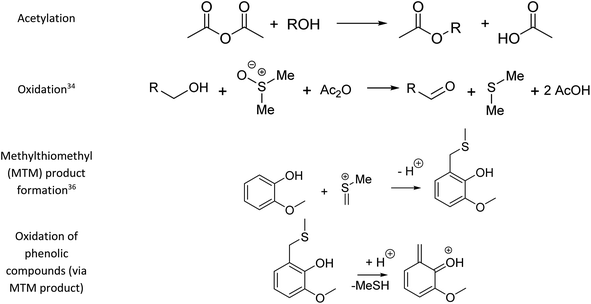
Fig. 1 summarises the results of the derivatisation studies in acetone and in DMSO; full details including proposed reaction mechanisms can be found in Section 1.3 of the ESI.†Ol-14, Ol-15, Ol-16, and Ol-17, that were shown to be non-reactive with Ac2O in acetone and MeOH mixtures, gave products in excellent yield in DMSO–Ac2O mixtures (yields > 97%). The hydroxyl groups in Ol-14, Ol-15 and to a lesser extent CA-18 (the former, a composition with secondary alcohol and a carboxylic acid functional group) were oxidised to form a ketone under the experimental conditions, whereas Ol-13 and Ol-16 presented a combined reaction of acetylation and oxidation as major product. In Ol-13 for instance, an acetate ester of the phenol plus aldehyde formation from the primary alcohol were observed with a yield of 84%. Phenols such as Ph-2, Ph-3, and CA-20 were also shown to be very reactive in DMSO–Ac2O (yield > 97%). In contrast to alcohols, small phenols such as Ph-3 were mono- and di-methylthiomethylated by DMSO in the presence of Ac2O. According to Hayashi and Oda,36 phenols produced a higher yield of ortho-methylthiomethylated (MTM) products whereas phenols without an unsubstituted ortho position e.g. 2,6-dimethylphenol, gave para-alkylated products. Our results showed that varied yields of MTM products were observed in almost all phenols with an available ortho position in the benzene ring. The GC data presented in Fig. S1–S20,† suggest that isomeric mono-MTM and di-MTM products can be produced. For instance, Ox-9, a composition with ortho, meta and para positions, produced a mono-MTM product detected at retention times of 36.999, 37.362 and 37.639 minutes. Following results in the literature, we have proposed an ortho-MTM (33%), para-MTM (4.2%) and a meta-MTM (2.7%) for the compositions eluting at 36.999, 37.362, and 37.639 min, respectively. Thus, compositions with multiple available positions in the benzene ring (non-hindered) produced MTM products with higher yields. Exceptions were observed in vanillin and ethyl-vanillin (Ox-12 and Ox-11, respectively), where the aldehydes seem to hinder the MTM-attachment, leading to a preferable monoacetate product.
MTM by-products of the standards categorised as alcohols, catechols, and benzene diols were produced in negligible yields (<8%). Is interesting to note that an orange-coloured solution was observed for Ct-7 in DMSO–Ac2O whereas a dark brown solution was observed in the reaction of Ct-5, Ct-6, and Ct-8. Our results indicate that the former standards give similar reaction yields (89–92%) with products including monoacetate, diacetate and those of oxidation. Oxidation products were also observed in those samples producing orange and light-yellow solutions after reactions in DMSO–Ac2O (e.g. Ct-23, Ph-1, among others). 1,2-Phenylene diacetate was the major product of catechol in DMSO–Ac2O while a monoacetate (4-hydroxyphenyl acetate) and not the diacetate was the major acetate product observed in the reaction of hydroquinone. The difference in colour changes observed in catechols and benzene diols is believed to be related to the formation of the oxidised product (see for instance the mass spectrum obtained for Ct-5 DMSO–Ac2O at Rt 43.539 min in Fig. S5†). Hydroquinones and catechols play important role in chemical industries, biological processes, and environmental science. Our results indicate that a very distinctive and interesting reaction, likely corresponding to the oxidation of hydroquinone to the corresponding quinone, is possible in DMSO–Ac2O. Theoretical studies of the scale of oxidation potential of hydroquinones and catechols in DMSO indicates that the oxidation potential of hydroquinone is much smaller than that of catechol,38 however a more dedicated study of the reactions of catechols and hydroquinones in DMSO–Ac2O is necessary to clarify the reaction mechanisms involved. Such a study is out of the scope of this paper. In summary, alcohols and sterically hindered phenols presented the higher reaction yields in DMSO–Ac2O. While oxidation of alcohols was the main product observed for the standards categorised here as Ol, sterically hindered phenols were preferably methylthiomethylated in DMSO–Ac2O. Methylthiomethyl by-products were not observed in the reaction of catechols and vanillin. Finally, a negligible yield of the acetate products was observed in the reaction of both the standards classified as CA (containing carboxylic acids) and secondary alcohols.
| Group | Acetone–Ac2O | DMSO–Ac2O | |||
|---|---|---|---|---|---|
| Main product | Yield (%) | Main products | Yield (%) | ||
| a Excluding yields of Ct-25: catechol with a methyl group.b A yield of 25% was observed for CA-20. | |||||
| Ol (primary) |  |
— | 0.6 | Aldehyde | ∼97 |
| Ol (secondary) | 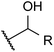 |
— | 3.5b | Ketone | ∼99.8 |
| Ph |  |
Monoacetate | 62–35 | Monoacetate MTM | 69–49 |
| Ph (hindered) |  |
Monoacetate | ∼18 | MTM | ∼97 |
| Catechols and benzene diols | 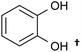 |
Monoacetate diacetate | ∼65a | Monoacetate diacetate oxidation | 92–89 |
Hydroxyl group of complex mixtures: bio-oil from lignocellulosic biomass
Direct infusion mass spectrometry measurements allow the simultaneous detection of all ionisable compounds in a single experiment. Mass spectrometers with a high resolving power can unambiguously discriminate individual elemental molecular compositions, however, additional chromatographic separation is needed to be able to discriminate between isomeric compounds.39 Therefore, the signal intensity of each detected m/z-value, may correspond to a combined signal contribution of ionised compounds with the same m/z-value but with a different functional group profile. Here, the derivatisation of the bio-oils in mixtures of acetone–Ac2O and DMSO–Ac2O is used to determine compositions containing a hydroxyl group. The results are summarised in Table 3 and the mass spectra are shown in Fig. S23–S24.†| Bio-oil | Acetone | Acetone–Ac2O | DMSO | DMSO–Ac2O | |
|---|---|---|---|---|---|
| A | B | C | D | ||
a Percentage contribution to total signal calculated with direct infusion FTICR data. The intensity of each MS was normalised to 100%.b 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, 2852 and 639 corresponding to OoSs[H], Oo[H] and O heteroatomic classes respectively. 800, 2852 and 639 corresponding to OoSs[H], Oo[H] and O heteroatomic classes respectively. |
|||||
| Assignments | Total | 4055 | 5471 | 4599 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 089 089 |
| Mass range | (Da) | 120–830 | 120–850 | 120–926 | 120–1200 |
| Heteroatomic class total number (%) | Oo[H] | 4032 (>99) | 5393 (>98) | 4338 (94.3) | 6260 (28.3) |
| O | — | — | — | 639 (2.9) | |
| OoSs[H] | — | — | — | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 (68.1) 060 (68.1) |
|
| Other | 23 | 78 | 261 | 130 (0.6) | |
| Elemental contribution (%)a | Carbon | 70 | 65 | 70 | 63 |
| Hydrogen | 6.5 | 6 | 6.1 | 5.9 | |
| Oxygen | 24 | 29 | 24 | 24 | |
| Sulfur | — | — | — | 7.6 | |
| Nitrogen | <0.2 | <0.1 | <0.1 | <0.2 | |
| Lignin (%) | 0.7 < H/C < 1.5 | 92 | 93 | 89 | 87 |
| 0.1 < O/C < 0.67 | |||||
| Commonality | Before/after reaction | Acetone mixtures | DMSO mixtures | ||
| Common assignments | Common in A and B = 3569 | Common in C and D = 3798 | |||
| Unique assignments | 486 | 1902 | 801 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291b 291b |
|
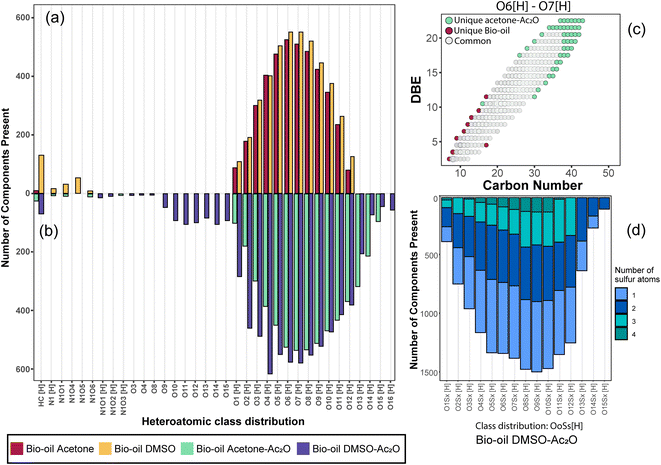
The elemental composition profile of the bio-oil after derivatisation in DMSO–Ac2O mixtures is very distinctive from that of the bio-oil blank, while some differences were also observed when the reaction was performed in acetone–Ac2O (see Fig. 2(b)). A shift towards higher oxygen-containing species was evident when the bio-oil was subjected to derivatisations in acetone–Ac2O, which is expected if acetate esters of phenols are formed by the addition of acetyl groups to the organic mixture. Unfortunately, the products from this reaction can overlap with a molecular composition already detected in the bio-oil blank (see Fig. 2(c)), henceforth a common molecular formula between the bio-oil before and after the derivatisation reaction cannot be discriminated with direct infusion experiments. A transformation of the hydroxyl group to a new chemical class is more advisable for direct infusion experiments, e.g. the addition of sulfur as described below. Despite this disadvantage, it was possible to detect 1835 unique molecular compositions in the bio-oil acetone–Ac2O. The former molecules correspond to acetate products of the reaction mainly assigned to O8[H]–O15[H] oxygen-containing heteroatomic classes. Considering the results observed in standard compositions, these molecular compositions are attributed to acetate products of non-sterically hindered phenols. The bio-oil elemental chemical compositions increased by five-fold after derivatisation in DMSO–Ac2O mixture. This is clear evidence of the diverse hydroxyl group profile present in the bio-oil. As can be seen in Fig. 2(b) the compositions detected in the bio-oil DMSO–Ac2O sample correspond to even- and odd-oxygen-containing molecular classes ([M + H]+ and M˙+, respectively), O1–13S1–4[H] (see Fig. 2(d)), and some other heteroatomic compositions in lower abundance. OoSs[H] heteroatomic classes (68.1% of the total signal contribution) correspond to methylthiomethyl by-products likely produced from the reaction of phenols with ortho, para, and meta positions (non-sterically hindered, see Fig. 2(d)) and guaiacol-like molecules (see products in Ph-1, Ph-2, and Ph-3). The synthesis of methylthiomethyl esters of carboxylic acids in DMSO have been previously reported by Yu et al.41 However, such a reaction was not observed in the CA-standards with our reaction conditions.
It is important to mention that the addition of sulfur after the reaction in DMSO–Ac2O introduces additional molecular compositions with a mass difference of 3.37 mDa corresponding to C3 vs. SH4 (e.g., C20H16O4[H] vs. C17H20O4S1[H], see Fig. S24†). A resolving power of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 is needed to separate C20H16O4[H] from C17H20O4S1[H], thus ultrahigh resolution mass spectrometers such as FTICR MS are uniquely suited to characterise this sample. According to Table 3, the reactions in DMSO–Ac2O allowed the detection of 3491 oxygen-containing molecules that were not detected in the corresponding blank. Interesting, about 640 molecules were detected as odd-electron ions (M˙+) which were not detected in the blank. As shown in our previous section, DMSO–Ac2O changes the structure of the analyte to form products that were not observed in acetone–Ac2O. These molecules can be associated with products of oxidation of primary or secondary alcohols and the production of O-acetates.
000 is needed to separate C20H16O4[H] from C17H20O4S1[H], thus ultrahigh resolution mass spectrometers such as FTICR MS are uniquely suited to characterise this sample. According to Table 3, the reactions in DMSO–Ac2O allowed the detection of 3491 oxygen-containing molecules that were not detected in the corresponding blank. Interesting, about 640 molecules were detected as odd-electron ions (M˙+) which were not detected in the blank. As shown in our previous section, DMSO–Ac2O changes the structure of the analyte to form products that were not observed in acetone–Ac2O. These molecules can be associated with products of oxidation of primary or secondary alcohols and the production of O-acetates.
A comparison of the unique elemental molecular compositions of the reactions between acetone–Ac2O and DMSO–Ac2O is shown in Fig. 3 (see also Fig. S28†). In Fig. 3, common compositions between each reaction mixture and its corresponding blank have been excluded e.g. 3569 and 3798 common compositions found in the sample sets acetone vs. acetone–Ac2O, and DMSO vs. DMSO–Ac2O, respectively (see Table 3). As can be seen in Fig. 3a and b, a clear shift towards higher oxygen content species was observed in acetone–Ac2O mixtures. As previously discussed, this is a consequence of o-acetate formation of non-hindered phenols. In contrast, a higher dispersity of oxygenated compositions was detected in the reactions in DMSO–Ac2O. Odd-electron ions are uniquely detected in DMSO–Ac2O and correspond mainly to oxygenated species containing 10–15 oxygen atoms with a double bond equivalent between 14–22 (DBE plots can be found in Fig. S29†). The even-electron ions ([H]-class) detected in DMSO–Ac2O mixtures presented a double distribution, one with a distribution with O1[H] to O4[H] oxygenated compositions and another distribution corresponding to O9[H]–O13[H]. More than 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ions corresponding to OoSs[H] heteroatomic class give us an indication of a high number of molecules containing phenols with, more likely, ortho and para positions.
000 ions corresponding to OoSs[H] heteroatomic class give us an indication of a high number of molecules containing phenols with, more likely, ortho and para positions.
The mean- H/C and O/C values per each heteroatomic class are shown in Fig. 3c. As can be seen in this figure, the O/Cmean of each class is similar between the blank samples (bio-oil acetone and bio-oil DMSO). In contrast, both bio-oils reacting in acetone–Ac2O and DMSO–Ac2O show an increased O/Cmean as the oxygen content in the molecule increases. This is a clear indication of the formation of acetate products after the reaction. The H/Cmean values of the sample bio-oil acetone–Ac2O remains very close to that of the blank bio-oil acetone for all the oxygenated classes. In contrast, most of the Oo[H] classes in the DMSO samples presented a decreased H/Cmean value. A decrease in hydrogen-to-carbon value is an indication of oxidation of alcohols, a reaction that has been previously reported.34 This reaction was also observed in alcohols, some catechols, and benzene diol molecular standards as shown in Table 2 and Fig. 1. The presence of p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, for instance, can lead to oxidation products of bio-oils reacting in DMSO–Ac2O mixtures.
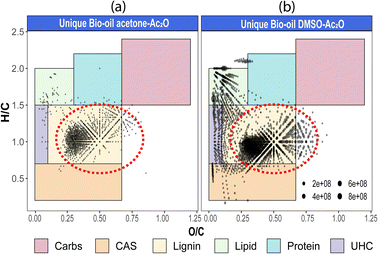
Van Krevelen diagrams of the unique species detected before and after derivatisation in each solvent are shown in Fig. 4 (see also van Krevelen diagrams per heteroatomic class in Fig. S29–S31†). As shown in Fig. 4, the compositions were classified by regions in which structures share common H/C and O/C elemental ratios.40 Comparison of the elemental compositions in both mixtures reveals that most of the unique compositions in acetone–Ac2O mixtures have elemental compositions similar to lignin, whereas lignin-type alongside with several unsaturated hydrocarbons (UHC), lipid-like, and condensed aromatic compositions were detected in the unique compositions after reactions in DMSO–Ac2O. As highlighted with a red circle in Fig. 3(b), odd-electron ion species and OO ≥ 5[H] are more likely o-acetate products of lignin-derivates (see also Fig. S31–32†), while several species assigned to O1[H]–O4[H] occupied the compositional space of UHC and lipid-like elemental compositions in DMSO–Ac2O mixtures. Huba et al.,42 have shown that aliphatic alcohols and aliphatic aldehydes, such as tetracosanol (C24H50O) and 1-octadecanal, respectively, are not efficiently ionised by APCI whereas aliphatic ketones presented a higher ionisation efficiency. Consequently, the lipid-like molecular compositions, with an H/C = 1.5–2, are more likely ketone products of the reactions in DMSO–Ac2O (Fig. 3). Lipophilic extractives such β-sitosterol (H/C = 2, O/C = 1/29) have been previously reported in wood-based fast pyrolysis.43 This indicates that the ketone products detected at a lipid-like compositional space in DMSO–Ac2O mixtures likely correspond to the oxidation of the secondary alcohol in sterol molecules. About 7% of the molecules detected uniquely in DMSO–Ac2O are in the compositional space associated with unsaturated hydrocarbons are probably oxidation products of moieties containing cyclic alcohols (cyclic ketones products).
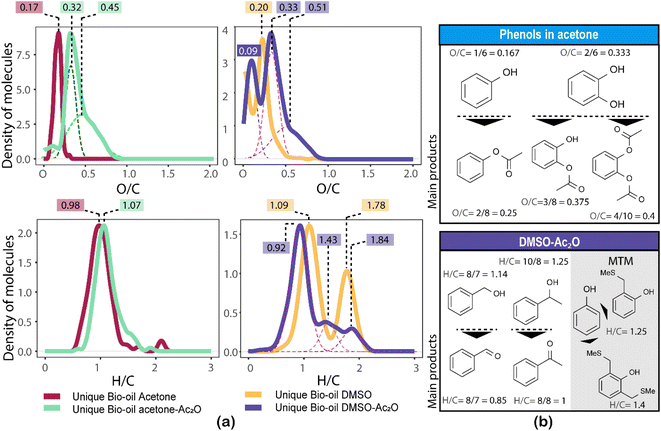
The formation of an acetate product after the reaction will produce a molecule with a mass incremented by 42.010565 Da, which corresponds to the addition of C2H2O, this addition will in turn increase the O/C-value of the molecule. Similarly, the addition of multiple acetate products to a single molecule will also increase the O/C-value. For instance, the monoacetate and diacetate product of C6H6O2 (O/C = 2/6 = 0.333) will have an O/C-value of 0.375 (O/C = 3/8) and 0.4 (O/C = 4/10), respectively.
Similarly, the oxidation of primary and secondary alcohols will reduce the H/C value of the molecules (see related examples in Fig. 5). Consequently, the density of molecules along the H/C and O/C axes will allow a quick visual comparison of the oxygen and hydrogen profile of the samples after derivatisation (see Fig. 5(a)). Two highly populated areas are observed at similar mean O/C-values for the bio-oil in both acetone–Ac2O and DMSO–Ac2O mixtures. The first distribution has a mean O/C-value of 0.32 and might correspond to mainly mono-acetate products while di-acetate products might be contributing to the density of molecules with a O/C-value of 0.45 and 0.51 in acetone–Ac2O and DMSO–Ac2O, respectively. The higher reaction yields observed in DMSO–Ac2O explains the relatively higher mean O/C-value observed for the reaction of bio-oil in this mixture. An additional high density of molecules located at a mean O/C-value of 0.09 was observed in DMSO–Ac2O, these compositions correspond to the contribution of species at the lowest O/C-values in the van Krevelen diagram shown in Fig. 4 (lipid-like and UHC-like molecular compositions).
The marginal increase in the H/C-value after the reactions in acetone–Ac2O can be a consequence of esterification side reactions analogous to the ones observed in CA-standards. In contrast, the density of unique molecules detected in DMSO–Ac2O are observed at a reduced H/C-value. Common monomers within lignin chemistry include vanillin alcohol (H/C = 1.25), homovanillyl alcohol (H/C = 1.33), veratryl alcohol (H/C = 1.33) and dihydroconiferyl alcohol (H/C = 1.4) are prone to oxidise in DMSO–Ac2O to form molecules with an H/C value between 1–1.2 (peak with a mean H/C = 0.92). The oxidation of lipid-like compositions and unsaturated hydrocarbons, which comprise a mixture of aliphatic and aromatic moieties, are more likely contributing to the density of molecules at a mean H/C = 1.84 and 1.43 respectively. Methylthiomethyl products are mostly located within the lignin-type compositional space in van Krevelen diagrams (see Fig. S32–S35†). This indicates the presence of molecules containing phenols such as the ones shown in Fig. 5(b).
Semi-quantitative speciation of the hydroxyl group
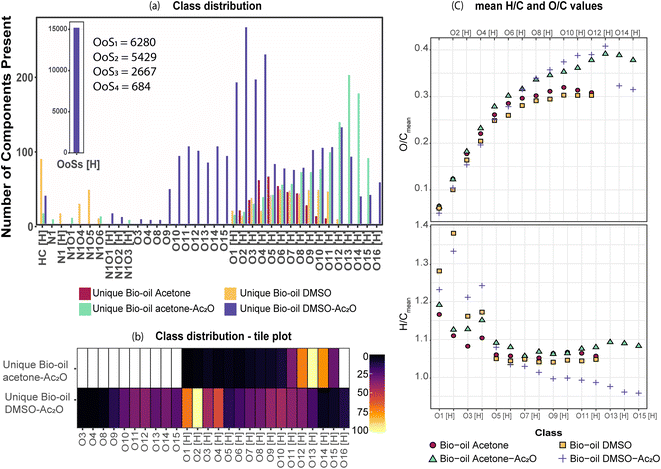
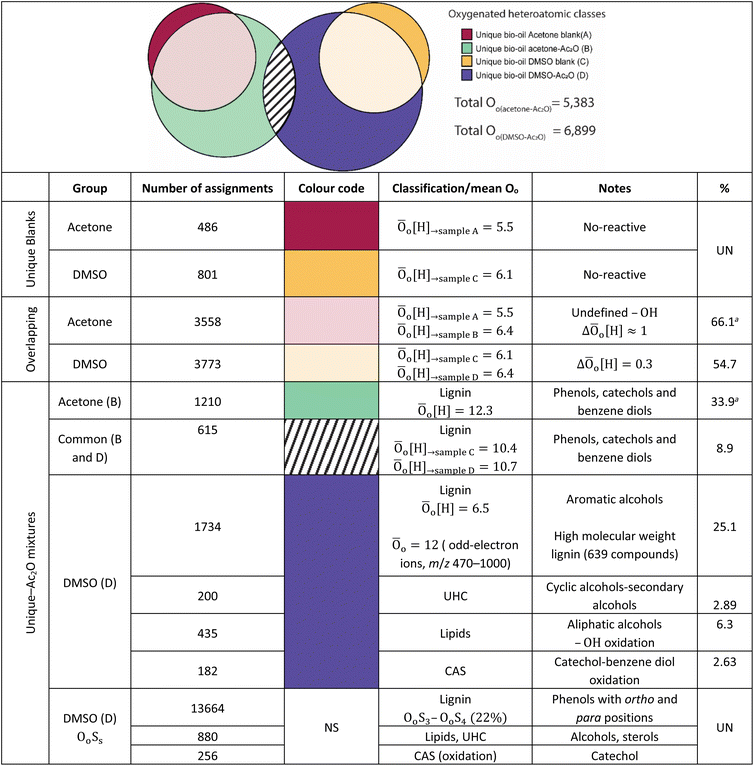
FTICR MS capabilities allows the simultaneous detection and unique assignment of thousands of individual elemental compounds in a single mass spectrum. Several factors limit the quantification by MS: ionisation yields differ between the different compounds, matrix effects, unknown chemical structures, selective solubility, alongside an undefined number of isomeric contributions per individual elemental compound.44,45 Thus, considering these factors only semi-quantification can be achieved. It is important to mention, however, that oxygenated compositions as the ones presented in Table 1 have been shown to be ionizable by positive ion-mode APCI.The elemental compositions of each sample are compared to deliver a semi-quantitative speciation of the hydroxyl group profile within the sample. A Venn diagram contained in Table 4, is used to illustrate such comparison. The area of the circles has been re-sized to correspond to the total number of oxygenated compositions detected in each sample i.e., 4032 (acetone blank), 5383 (acetone–Ac2O), 4338 (DMSO blank) and 6899 (DMSO–Ac2O).
As can be seen in Table 4, a significant number of compositions overlapped with a composition already detected within the blank. The percentage of non-reactive and overlapping material is then undefined with the current experimental data. It is interesting to note that the mean oxygen content is increased by about one oxygen atom when the derivatisation is performed in acetone–Ac2O, which indicates that mono-acetate products are contributing to the total intensity within the overlapping compositions. In comparison, the overlapping compositions in DMSO did not present a significant increase of the oxygen content after derivatisation.
The separation of the overlapping products and reactants will require the use of a separation technique before ion detection that are currently out of the scope of this study. Our data indicates that the hydroxyl group is mostly present in phenolic-like containing molecules (∼34%), followed by aromatic alcohols (benzyl alcohol-type, ∼25%). Fewer unique compositions corresponding to aliphatic alcohols (6.3%) and primary secondary alcohols, including cyclic alcohols (2.89) were also detected in DMSO–Ac2O mixtures. The percentage of phenol-like compositions falls close to the range typically reported for phenol oligomers in pyrolysis bio-oils, 26–33%,46,47 similarly 2–5% of alcohols have been previously reported in literature. Our method then also provides unique insights on the distribution of aromatic alcohols, aliphatic and cyclic alcohols in complex mixtures, information that is currently scarce in literature.
Our data also present clear evidence of the diversity of ortho, para, and meta positions in phenolic compositions. A semi-quantitation of these compositions is, however, more difficult. Firstly, an attachment of up to two methylthiomethyl chains to the ortho, para, or meta positions was observed within the reaction products of the standards, hence, the quantitation of MTM chain attachment is difficult. Secondly, the fragmentation of the MTM chain to produce a OoS1 elemental composition was observed for the standard compositions (see for instance Fig. S3†), although the corona current was reduced for the acquisition of the UHRMS data of the bio-oils (see Method section), the degree of fragmentation of OoSs compositions is difficult to estimate. Thirdly, competition between formation of MTM and ketones can be observed (e.g., Ol-16 and Ol-27). Finally, bio-oil compositions were characterised by a high-oxygen content in a single elemental molecule, therefore a combination of reactions cannot be discriminated. Thus, the presence of OoSs compositions is used as a qualitative metric of the distribution of ortho, para and meta positions in phenols. According to Fig. 3, S1 to S4 oxygenated molecular compositions (OoS1–4[H]) were detected. Compositions with at least two ortho, para or meta positions will yield a OoS1 and OoS2 elemental composition (78%, calculated data shown in Fig. 3), this indicates the high number of moieties containing phenols similar to Ph-1, Ph-2 and Ph-3 (hindered phenols). Notice that catechols, benzene diols, and phenols containing an aldehyde (such as vanillin) are more likely transformed to acetate products and, therefore, a lower signal contribution from these compositions to the OoSs heteroatomic classes is expected. Alkylated phenols have been previously reported by Garcia-Perez et al., in 2007.48 Examples include but are not limited to 2-methylphenol, 3,4-dimethylphenol, 3-methyl-1,2-benzenediol, and 3,4-diehtylphenol. These phenols can be transformed to MTM products in DMSO–Ac2O. About 22% of sulfur-oxygenated containing classes contain three to four sulfur atoms. The attachment of more than two MTM chains was not observed within the products observed in the reaction of the standards, is then likely that the bio-oil is composed of an arrangement of monomers such as p-coumaryl alcohol and coniferyl alcohol. Oligomers containing p-coumaryl end groups (two ortho positions) are likely precursors of the OoS3–4[H] molecular compositions whereas coniferyl alcohol end groups are more likely precursors of compositions containing one to two sulfur–oxygen containing species (78%). This result is in agreement with previous literature.49
According to the data discussed in previous sections, acetone–acetic anhydride reactions and dimethyl sulfoxide–acetic reactions can be used to provide unique and distinctive insights into the hydroxyl functional group profile in complex mixtures. In summary, alcohols are non-reactive in acetone–Ac2O mixtures, phenols with ortho or para positions are mostly transformed to an MTM-product in DMSO–Ac2O, non-hindered phenols reacted in both mixtures, and alcohols oxidised when reacting in DMSO–Ac2O mixtures. The quantification of the hydroxyl group in individual moieties of a complex mixture will require the separation of each reactive chemical within the bio-oil, calibration curves, authentic standards covering the mass range of detection (4000–5000, elemental compositions were detected), and measuring ionisation response against concentration. Individual isomers are, however, not discriminated by direct infusion mass spectrometry and thus hyphenated techniques such as gas chromatography, GC × GC, and liquid chromatography, or alternatively, ion mobility50–52 can be used to deliver a more detailed insight of the hydroxyl group profile. Future work will focus on the use of chemical derivatisations combined with hyphenated mass spectrometry to allow the separation of isomeric compositions and overlapping reactant/products elemental molecular compositions.
Conclusions
In the first part of this research, derivatisations using acetic anhydride in different solvents were employed to evaluate the reactivity of the hydroxyl groups in lignin-representative standards. The observed reactions are summarised as follows:•Reactions in acetone–Ac2O: (1) mono and di-acetate products of phenols without saturated side chains with acceptable yields (65–35%) were observed, (2) primary and secondary alcohols were essentially non-reactive (yield < 3.5%), and (3) low reaction yields (<18%) were observed in sterically hindered phenols.
•Reactions in DMSO–Ac2O: (1) primary and secondary alcohols formed aldehyde or ketone products, respectively, with a high yield (97–99.8%) (oxidation reactions), (2) hindered phenol compounds such as o-cresol and guaiacol formed primarily a methylthiomethyl product (–CH2SCH3) with about 92% yield, and (3) phenol, catechol and benzene diol molecules formed primarily mono and di-acetate products.
•The standards were essentially non-reactive in MeOH–Ac2O mixtures.
Thus, the reactions in both acetone and DMSO, presented a high chemo-selective transformation of the hydroxyl group and DMSO–Ac2O reactions are advisable when higher yield reactions of the hydroxyl group are required.
The derivatisations combined with direct infusion ultrahigh resolution mass spectrometry were used to pinpoint elemental compositions containing a hydroxyl group in a bio-oil. Our results show that about 2000 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 new elemental compositions were detected in the bio-oil after derivatisations in acetone–Ac2O and DMSO–Ac2O mixtures, respectively. Those compositions correspond to reaction products from chemical moieties containing at least one hydroxyl group. The bio-oil acetone–Ac2O presented unique compositions in the compositional space corresponding to lignin-like structures, which indicates the formation of acetate products of non-hindered phenols. The extraordinary increased number of compositions of the bio-oil DMSO–Ac2O sample is clear evidence of the diverse hydroxyl group profile present in the bio-oil. DMSO–Ac2O reactions have the unique advantage of transforming the hydroxyl group to a new chemical class, e.g., oxygen-containing species, Oo, to heteroatomic classes corresponding to OoSs, species that can be easily separated by FTICR MS. Our results indicate that about 15
400 new elemental compositions were detected in the bio-oil after derivatisations in acetone–Ac2O and DMSO–Ac2O mixtures, respectively. Those compositions correspond to reaction products from chemical moieties containing at least one hydroxyl group. The bio-oil acetone–Ac2O presented unique compositions in the compositional space corresponding to lignin-like structures, which indicates the formation of acetate products of non-hindered phenols. The extraordinary increased number of compositions of the bio-oil DMSO–Ac2O sample is clear evidence of the diverse hydroxyl group profile present in the bio-oil. DMSO–Ac2O reactions have the unique advantage of transforming the hydroxyl group to a new chemical class, e.g., oxygen-containing species, Oo, to heteroatomic classes corresponding to OoSs, species that can be easily separated by FTICR MS. Our results indicate that about 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 elemental compositions correspond to the methylthiomethyl product (OoSs-heteroatomic classes) likely corresponding to phenolic moieties with ortho and para positions. Additionally, unique compositions occupying the compositional space of lipid-like and UHC-like structures might correspond to oxidised reactions that uniquely occur in DMSO–Ac2O mixtures, confirming the presence of primary and secondary alcohols, including cyclic alcohols, within the chemical moieties found in a bio-oil. A semi-quantitative analysis indicates that about 34% and 25% of the new elemental compositions detected after reaction with Ac2O correspond to the transformation of phenolic-like and aromatic-alcohols, respectively.
000 elemental compositions correspond to the methylthiomethyl product (OoSs-heteroatomic classes) likely corresponding to phenolic moieties with ortho and para positions. Additionally, unique compositions occupying the compositional space of lipid-like and UHC-like structures might correspond to oxidised reactions that uniquely occur in DMSO–Ac2O mixtures, confirming the presence of primary and secondary alcohols, including cyclic alcohols, within the chemical moieties found in a bio-oil. A semi-quantitative analysis indicates that about 34% and 25% of the new elemental compositions detected after reaction with Ac2O correspond to the transformation of phenolic-like and aromatic-alcohols, respectively.
The combination of the chemoselective transformation of a functional group in conjunction with ultrahigh resolution mass spectrometry can be used as a qualitative metric of the functional group profile of complex mixtures such as bio-oils. The production of fine chemicals and hydrocarbons from renewable sources such as bio-oils, relies on more efficient deoxygenation routes. Knowledge about the hydroxyl group profile of bio-oils, in particular the possible content of hindered and non-hindered phenols can be beneficial to predict the contribution of specific products if bio-oils are co-processed or upgraded via acidic catalysts.
Data availability
The research data (and/or materials) supporting this publication can be accessed at http://wrap.warwick.ac.uk/Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would also like to thank David Stranz (Sierra Analytics, Modesto, CA, USA) for access to Composer 1.5.6. DCPL thanks the Leverhulme Trust for an Early Career Fellowship (ECF-2020-393) and funding. MPB thanks the Engineering and Physical Sciences Research Council (EPSRC) for funding the 15 T solariX XR and timsTOF Pro instrumentation (grant EP/V007718/1) and HEJ's PhD studentship via the EPSRC Centre for Doctoral Training in Molecular Analytical Science (grant number EP/L015307/1). MPB also thanks Warwick Innovations (formerly Warwick Ventures) for funding towards development of KairosMS.References
- R. Pirow, A. Blume, N. Hellwig, M. Herzler, B. Huhse, C. Hutzler, K. Pfaff, H. J. Thierse, T. Tralau, B. Vieth and A. Luch, Crit. Rev. Toxicol., 2019, 49, 742–789 CrossRef CAS PubMed.
- T. Rajabloo, W. De Ceuninck, L. Van Wortswinkel, M. Rezakazemi and T. Aminabhavi, J. Environ. Manage., 2022, 302, 114055 CrossRef CAS PubMed.
- C. Zhao, C. Hong, J. Hu, Y. Xing, W. Ling, B. Zhang, Y. Wang and L. Feng, Fuel, 2023, 333, 126388 CrossRef CAS.
- N. Gómez-Marín and A. V. Bridgwater, Renewable Sustainable Energy Rev., 2021, 137, 110496–110510 CrossRef.
- N. Bauer, K. Calvin, J. Emmerling, O. Fricko, S. Fujimori, J. Hilaire, J. Eom, V. Krey, E. Kriegler, I. Mouratiadou, H. Sytze de Boer, M. van den Berg, S. Carrara, V. Daioglou, L. Drouet, J. E. Edmonds, D. Gernaat, P. Havlik, N. Johnson, D. Klein, P. Kyle, G. Marangoni, T. Masui, R. C. Pietzcker, M. Strubegger, M. Wise, K. Riahi and D. P. van Vuuren, Glob. Environ. Change, 2017, 42, 316–330 CrossRef.
- G. Centi, G. Iaquaniello and S. Perathoner, BMC Chem. Eng., 2019, 1, 1–16 CrossRef.
- Supergen Bioenergy Hub, Carbon Recycling Network, Biomass Biorefinery Network & High Value Biorenewables Network, 2021 Search PubMed.
- R. Rowell, R. Pettersen and M. Tshabalala, Handb. Wood Chem. Wood Compos. 2nd edn, 2012, pp. 33–72 Search PubMed.
- M. R. Barr, M. Volpe, A. Messineo and R. Volpe, Fuel, 2020, 276, 1–6 CrossRef.
- S. D. Stefanidis, K. G. Kalogiannis, E. F. Iliopoulou, A. A. Lappas and P. A. Pilavachi, Bioresour. Technol., 2011, 102, 8261–8267 CrossRef CAS PubMed.
- M. Bertero and U. Sedran, Catal. Today, 2013, 212, 10–15 CrossRef CAS.
- J.-Y. Kim, S. Heo and J. W. Choi, Fuel, 2018, 232, 81–89 CrossRef CAS.
- R. T. J. Gerards, A. Fernandes, I. Graça and M. F. Ribeiro, Fuel, 2020, 260, 116372 CrossRef CAS.
- J. Kim, J. Moon, J. H. Lee, X. Jin and J. W. Choi, Fuel, 2020, 279, 118484 CrossRef CAS.
- D. C. Palacio Lozano, H. E. Jones, R. Gavard, M. J. Thomas, C. X. Ramírez, C. A. Wootton, J. A. Sarmiento Chaparro, P. B. O'Connor, S. E. F. Spencer, D. Rossell, E. Mejia-Ospino, M. Witt and M. P. Barrow, Anal. Chem., 2022, 94, 7536–7544 CrossRef CAS PubMed.
- M. Staš, M. Auersvald, L. Kejla, D. Vrtiška, J. Kroufek and D. Kubička, TrAC, Trends Anal. Chem., 2020, 126, 115857 CrossRef.
- J. Cramer, C. P. Sager and B. Ernst, J. Med. Chem., 2019, 62, 8915–8930 CrossRef CAS PubMed.
- Y. Wang, Y. Han, W. Hu, D. Fu and G. Wang, J. Sep. Sci., 2020, 43, 360–371 CrossRef CAS PubMed.
- N. Hao, H. Ben, C. G. Yoo, S. Adhikari and A. J. Ragauskas, Energy Fuels, 2016, 30, 6863–6880 CrossRef CAS.
- D. C. Palacio Lozano, M. J. Thomas, H. E. Jones and M. P. Barrow, Annu. Rev. Anal. Chem., 2020, 13, 405–430 CrossRef PubMed.
- A. Abou-Dib, F. Aubriet, J. Hertzog, L. Vernex-Loset, S. Schramm and V. Carré, Molecules, 2022, 27(24), 8889–8920 CrossRef CAS PubMed.
- D. C. Palacio Lozano, R. Gavard, J. P. Arenas-Diaz, M. J. Thomas, D. D. Stranz, E. Mejía-Ospino, A. Guzman, S. E. F. Spencer, D. Rossell and M. P. Barrow, Chem. Sci., 2019, 10, 6966–6978 RSC.
- M. Wang, S. Zhao, X. Liu and Q. Shi, Anal. Chem., 2016, 88, 9837–9842 CrossRef CAS PubMed.
- Y. Zhang, C. Huang, F. Kong, Y. Wang, Q. Shi and L. Zhang, Fuel, 2022, 319, 123760 CrossRef CAS.
- S. F. Niles, M. L. Chacón-Patiño, H. Chen, A. M. McKenna, G. T. Blakney, R. P. Rodgers and A. G. Marshall, Environ. Sci. Technol., 2019, 53, 6887–6894 CrossRef CAS PubMed.
- J. Hertzog, V. Carré, A. Dufour and F. Aubriet, J. Am. Soc. Mass Spectrom., 2018, 29, 543–557 CrossRef CAS PubMed.
- I. Z. Awan, N. Tanchoux, F. Quignard, S. Albonetti, F. Cavani and F. Di Renzo, Stud. Surf. Sci. Catal., 2019, 178, 257–275 CrossRef CAS.
- R. Gavard, H. E. Jones, D. C. Palacio Lozano, M. J. Thomas, D. Rossell, S. E. F. Spencer and M. P. Barrow, Anal. Chem., 2020, 92, 3775–3786 CrossRef CAS PubMed.
- D. C. Palacio Lozano, H. E. Jones, T. Ramirez Reina, R. Volpe and M. Barrow, Green Chem., 2021, 23, 8949–8963 RSC.
- H. E. Jones, D. C. Palacio Lozano, C. Huener, M. J. Thomas, D. J. Aaserud, J. C. Demuth, M. P. Robin and M. P. Barrow, Energy Fuels, 2021, 35, 11896–11908 CrossRef CAS.
- D. J. Trader and E. E. Carlson, Mol. Biosyst., 2012, 8, 2484 RSC.
- I. W. Ashworth, E. Bush, L. C. Chan, J. Cherryman, B. G. Cox, J. Muir, S. R. Korupoju and J. Keshwan, Org. Process Res. Dev., 2012, 16, 1646–1651 CrossRef CAS.
- S. Kim, J. Yun, J. Cho, H. Choi, Y. S. Shin, H. Jeong and J. C. Jung, Mol. Catal., 2022, 532, 112721 CrossRef CAS.
- J. D. Albright and L. Goldman, J. Am. Chem. Soc., 1967, 89, 2416–2423 CrossRef CAS.
- S. Zavgorodny, E. Besidsky, A. Sanin, M. Pokrovskaya and G. Gurskaya, Tetrahedron Lett., 1991, 32, 7593–7596 CrossRef.
- Y. Hayashi and R. Oda, J. Org. Chem., 1967, 32, 457–458 CrossRef CAS.
- R. Oda and Y. Hayashi, Bull. Inst. Chem. Res., Kyoto Univ., 1969, 47, 1969 Search PubMed.
- X. Zhu, C. Wang and H. Liang, J. Org. Chem., 2010, 75, 7240–7257 CrossRef CAS PubMed.
- D. C. Palacio Lozano, H. E. Jones, T. Ramirez Reina, R. Volpe and M. P. Barrow, Green Chem., 2021, 23, 8949–8963 RSC.
- W. C. Hockaday, J. M. Purcell, A. G. Marshall, J. A. Baldock and P. G. Hatcher, Limnol. Oceanogr.: Methods, 2009, 7, 81–95 CrossRef.
- S. Yu, K. C. Nam and S. Lee, Bull. Korean Chem. Soc., 2018, 39, 906–908 CrossRef CAS.
- A. K. Huba, K. Huba and P. R. Gardinali, Sci. Total Environ., 2016, 568, 1018–1025 CrossRef CAS PubMed.
- T. Ohra-aho, M. Ghalibaf, R. Alén, C. Lindfors and A. Oasmaa, Energy Fuels, 2022, 36, 5797–5804 CrossRef CAS PubMed.
- E. A. Smith and Y. J. Lee, Energy Fuels, 2010, 5190–5198 CrossRef CAS.
- F. Stankovikj, A. G. McDonald, G. L. Helms and M. Garcia-Perez, Energy Fuels, 2016, 30, 6505–6524 CrossRef.
- D. Wang, D. Li, Y. Liu, D. Lv, Y. Ye, S. Zhu and B. Zhang, Sep. Purif. Technol., 2014, 134, 132–138 CrossRef CAS.
- M. Staš, D. Kubička, J. Chudoba and M. Pospíšil, Energy Fuels, 2014, 28, 385–402 CrossRef.
- M. Garcia-Perez, A. Chaala, H. Pakdel, D. Kretschmer and C. Roy, Biomass Bioenergy, 2007, 31, 222–242 CrossRef CAS.
- E. Terrell and M. Garcia-Perez, Energy Fuels, 2020, 34, 8466–8481 CrossRef CAS.
- C. A. Olanrewaju, C. E. Ramirez and F. Fernandez-Lima, Energy Fuels, 2021, 35, 13722–13730 CrossRef CAS.
- M. Staš, M. Auersvald and P. Vozka, Energy Fuels, 2021, 35, 8541–8557 CrossRef.
- J. Hertzog, C. Mase, M. Hubert-Roux, C. Afonso, P. Giusti and C. Barrère-Mangote, Energy Fuels, 2021, 35, 17979–18007 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02779a |
| This journal is © The Royal Society of Chemistry 2023 |