Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Upcycling of BPA-PC into trimethylene carbonate by solvent assisted organocatalysed depolymerisation†
Ion
Olazabal
 a,
Emelin
Luna
a,
Emelin
Luna
 ab,
Steven
De Meester
b,
Coralie
Jehanno
ab,
Steven
De Meester
b,
Coralie
Jehanno
 *ac and
Haritz
Sardon
*ac and
Haritz
Sardon
 *a
*a
aPOLYMAT, University of the Basque Country UPV/EHU, Avda. Tolosa 72, 20018 Donostia-San Sebastian, Spain. E-mail: haritz.sardon@ehu.es; coralie.jehanno@polykey.eu
bDepartment of Green Chemistry and Technology, Ghent University, Graaf Karel De Goedelaan 5, Kortrijk, 8500, Belgium
cPOLYKEY, Avda. Tolosa 72, 20018 Donostia-San Sebastian, Spain
First published on 28th April 2023
Abstract
In this study, a solvent assisted organocatalysed method for the depolymerisation of bisphenol A-based polycarbonate (BPA-PC) at low temperature is described. It is shown that the selection of a suitable organic catalyst combined with mild conditions allows for the selective formation of trimethylene carbonate (TMC) in high yields (up to 80%) from the depolymerisation of BPA-PC. The key role of 1-methylimidazole as solvent and imidazole as catalyst is demonstrated and the depolymerisation mechanism is elucidated by 1H NMR spectroscopy. It is also demonstrated that this methodology could be extended to other nucleophiles, leading to the synthesis of different cyclic carbonyl-containing intermediates. Such selective depolymerisation processes under mild conditions represent a unique and interesting approach for valorising plastic waste into complex molecules that are otherwise difficult to synthesise.
Introduction
Six-membered cyclic carbonates are important molecules that are commonly used as green solvents, catalysts and as building blocks for polycarbonates, polyurethanes, or poly(ester-carbonate)s synthesis.1–4 They are notably reactive in ring opening polymerisation (ROP), with the resulting polymers finding applications as engineering polymers, electroactive materials for energy storage or isocyanate-free adhesives and coatings.5–8 Unfortunately, the routine synthesis of cyclic carbonates involves the use of highly toxic reagents, such as phosgene surrogates, chlorinated chemicals, or carbon monoxide.9,10 While intensive research has been directed to the coupling reaction of CO2 with diols or oxetanes to form the corresponding cyclic carbonate, this reaction requires not only high-performance catalysts but also high temperatures and/or pressures. As a result the direct coupling approach is less favoured than the insertion of CO2 into epoxides.11–13Recently, the depolymerisation of bisphenol A-based polycarbonate (BPA-PC), a commodity thermoplastic which is the material of choice for a wide range of applications,14,15 has been investigated to recover not only the initial monomer – bisphenol A (BPA) – but also cyclic carbonates. Through the chemical scission of the carbonate linkage, the depolymerisation of BPA-PC represents an alternative source of carbonyl groups to obtain high added-value molecules. In the open literature, articles have reported the deconstruction of BPA-PC into BPA and 5- or 6-member cyclic carbonates with both metallic16,17 and organic18,19 catalysts. However, none of these approaches allowed the production of the simplest but one of the most important cyclic carbonates, trimethylene carbonate (TMC).
TMC is the starting monomer to produce poly(trimethylene carbonate) (PTMC),20–22 an important but costly biocompatible polymer used in various medical applications including as a soft material in scaffolds for soft tissue regeneration and as a hydrophobic segment of amphiphilic block copolymers for drug delivery.23–25 More recently, it has been also investigated as a polymer electrolyte in batteries, demonstrating an excellent stability window and good conductivity values.26,27 However, because of the high reactivity of TMC, care must be taken during its preparation to avoid uncontrolled polymerisation into PTMC. Reports have demonstrated that an optimised catalytic system as well as low temperatures are essential for obtaining high yields of TMC.13 Consequently, the usual harsh conditions required for chemical recycling (high temperatures, pressures, and the use of non-specific catalysts) impede the recovery of the monomer. From this perspective, a selective system able to depolymerise BPA-PC into BPA and TMC while avoiding the side ROP into PTMC is both challenging and of great interest.
Our group has explored the potential of a thermally stable organic catalyst, formed out of an organic base, 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) and an organic acid, methanesulfonic acid (MSA), namely TBD:MSA, to obtain a wide range of cyclic, carbonyl-containing molecules in high yields.29,30 Unfortunately, while employing 1,3-propanediol as nucleophile in bulk at 130 °C, only the corresponding linear carbonate could be synthesised. A previous study based on the use of CO2 as a carbonyl source has demonstrated that cyclisation into TMC was only favoured over the linear PTMC formation (obtaining TMC in yields up to 94%) under mild temperature conditions (i.e. < 60 °C).28 Thus, we hypothesised that the depolymerisation of BPA-PC should similarly be carried out at the lowest possible temperature to obtain reasonable yields of TMC.
Herein, TMC is synthesised from the depolymerisation of BPA-PC using a solvent-assisted procedure. While in the bulk depolymerisation of BPA-PC, high temperatures (100–160 °C) were necessary to complete the reaction,29 it is demonstrated in this study that the use of an appropriate solvent leads to higher polymer solubilisation, allowing for solvent-assisted depolymerisation of BPA-PC at low temperatures (50 °C) (Fig. 1). Combined with an optimised organocatalytic system, this method renders possible the upcycling of BPA-PC waste into highly valuable TMC.
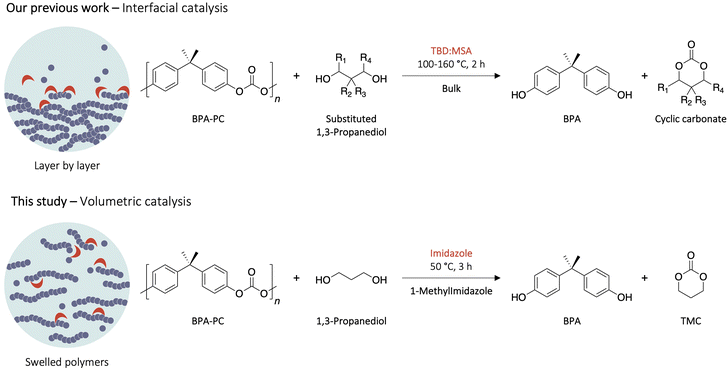 | ||
| Fig. 1 Previous study on the depolymerisation of BPA-PC in bulk (interfacial catalysis) and the reaction presented in this study in appropriate solvent (volumetric catalysis). | ||
Results and discussion
Screening of solvents and catalysts for the depolymerisation of BPA-PC under mild conditions
With the objective of obtaining trimethylene carbonate (TMC) in high yields, the depolymerisation of Bisphenol A-based polycarbonate (BPA-PC) was performed with 1,3-propanediol as nucleophile at a temperature unfavourable for the ring-opening polymerisation (ROP) of the molecule into poly(trimethylene carbonate) (PTMC), i.e. 50 °C, for 3 h (Scheme 1). The effect of the solvent and catalyst were first investigated to assess the optimum reaction conditions. The depolymerisation ratio, corresponding to Bisphenol A (BPA) yield, and the TMC and PTMC yields were determined through 1H NMR spectroscopy (DMSO-d6, 400 MHz, 298 K) (Table 1).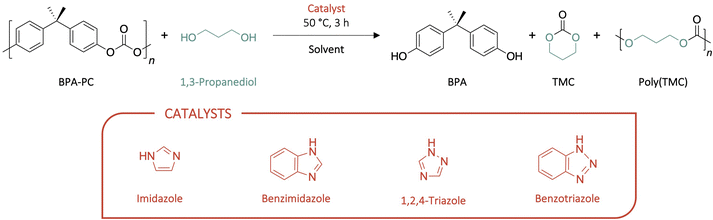 | ||
| Scheme 1 Depolymerisation of BPA-PC with different solvents (10 eq.) and catalysts (1 eq.) for 3 h at 50 °C. | ||
| Entry | Solvent | RED value | Catalyst | Dep. ratioa (%) | TMC yielda (%) | PTMC yielda (%) |
|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy in DMSO-d6 at ambient temperature, (Fig. S1 to S11†). | ||||||
| 1 | 1,3 propanediol | 2.349 | Imidazole | <1 | <1 | <1 |
| 2 | 1-Methylimidazole | 0.928 | Imidazole | >98 | 81 | 18 |
| 3 | THF | 1.015 | Imidazole | 64 | 40 | 22 |
| 4 | Chloroform | 1.295 | Imidazole | 83 | 45 | 37 |
| 5 | Methyl-THF | 1.311 | Imidazole | 30 | 24 | 4 |
| 6 | Toluene | 1.808 | Imidazole | 43 | 10 | 33 |
| 7 | 1-Methylimidazole | 0.928 | Benzimidazole | 82 | 63 | 18 |
| 8 | 1-Methylimidazole | 0.928 | Triazole | 89 | 79 | 9 |
| 9 | 1-Methylimidazole | 0.928 | Benzotriazole | 58 | 52 | 5 |
| 10 | 1-Methylimidazole | 0.928 | TBD | >98 | 26 | 72 |
The selection of solvents was performed based on the affinity of BPA-PC using the Hansen Solubility Parameters (HSP).31 This approach assumes that the interaction of the polymer with a given solvent is described by the sum of three partial solubility parameters corresponding to the nonpolar (dispersion), polar (dipole–dipole), and hydrogen bonding interactions. With the HSP values, the Relative Energy Difference (RED) parameter was calculated (Tables S1 & S2†). The RED value indicates how strong the interaction between the solvent and the polymer is. Good solvents for a given polymer have a RED value inferior to 1, while values superior to 1 indicate low affinity. From this initial screening, tetrahydrofuran (THF), chloroform, 2-methyltetrahydrofuran (methyl-THF), toluene and 1-methylimidazole were selected to evaluate their impact on the depolymerisation of BPA-PC mediated with different organic bases as catalyst.
The different results obtained demonstrate that the reaction is highly solvent-dependent. At 50 °C, the reaction in bulk – 1,3-propanediol used as solvent – did not lead to any depolymerisation, which is consistent with the very poor solubility of BPA-PC in 1,3-propanediol – RED value of 2.329 – (Table 1 – entry 1). On the contrary, all reactions in solvent led to the solvolysis of the polymer, although in very different proportions. Methyl-THF and toluene, for which the RED values are superior to 1, showed low reactivity, with 30 and 43% of depolymerisation after 3 h, respectively (Table 1 – entries 5 & 6). THF, which has a RED value close to 1, provided better performance reaching 64% of depolymerisation. The best result was obtained with 1-methylimidazole which led to the complete depolymerisation of BPA-PC after 3 h (Table 1 – entries 2 & 3). 1-Methylimidazole is the only solvent analysed which has a RED value lower than 1, which confirms its higher solubility compared to common solvents and leads to a system in which the solvent dissolves the surface of the material, thus reducing the mass transfer limitations of heterogeneous systems.32 Nonetheless, one anomaly can be noted; even though chloroform has a RED value of 1.295, it performed rather well, with 83% of depolymerisation after 3 h (Table 1 – entry 4). The extent of the undesired ring opening of TMC was also very different depending on the solvent. While methyl-THF and 1-methylimidazole promoted the formation of TMC over PTMC, the use of toluene favoured the polymerisation into PTMC. Finally, the basicity of 1-methylimidazole may play an additional role in the depolymerisation of BPA-PC as it potentially activates the nucleophilic attack of 1,3-propanediol. In the corresponding 1H NMR spectra, the characteristic signals of 1,3-propanediol methylene groups’ show a slight shift to higher positions – i.e. from δ = 1.56 to 1.61 ppm and from δ = 3.44 to 3.51 ppm – while the signal corresponding to the intensity of the proton of the hydroxyl group is clearly reduced in comparison with the lone compound and the multiplicity of the adjacent methylene group changed from a quadruplet to a triplet (Fig. S3†). These observations suggest the formation of hydrogen bonds between the diol and the moderately basic 1-methylimidazole, thus creating a partial negative charge on the oxygen of 1,3-propanediol which could enhance its reactivity and contribute to the efficiency of this system.
After demonstrating the importance of 1-methylimidazole as a solvent to favour the depolymerisation of BPA-PC, the impact of the catalyst on both the depolymerisation ratio and the TMC/PTMC ratio was investigated. Imidazole and derivatives, including benzimidazole, 1,2,4-triazole and benzotriazole, were explored for the reaction. This azoles were selected for their capacity to catalyse transesterifications when good leaving groups are released and more specifically in the case of phenyl-based esters.33–35 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), which is a strong base which has already shown excellent performance for the depolymerisation of BPA-PC,19 was investigated for comparison (Table 1, entries 7–10). The reaction catalysed by imidazole was the most efficient compared to the depolymerisation mediated by imidazole derivatives. Benzimidazole and 1,2,4-triazole showed good to very good depolymerisation yields, with 83 and 89% conversion, respectively. However, the undesired polymerisation into PTMC seemed to be favoured when using benzimidazole compared to triazole – with 63% and 79% TMC obtained, respectively. Benzotriazole led to only 58% depolymerisation after 3 h, although a higher TMC/PTMC ratio – i.e. 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 – was observed. As expected, the depolymerisation mediated by TBD was much faster, full depolymerisation was attained after only 1 h, but meanwhile the TMC content only reached 26% while PTMC was the major product obtained (72%). This result suggests that strong bases usually employed for such reactions are unselective and promote both the depolymerisation of BPA-PC and the polymerisation of TMC into PTMC.
5 – was observed. As expected, the depolymerisation mediated by TBD was much faster, full depolymerisation was attained after only 1 h, but meanwhile the TMC content only reached 26% while PTMC was the major product obtained (72%). This result suggests that strong bases usually employed for such reactions are unselective and promote both the depolymerisation of BPA-PC and the polymerisation of TMC into PTMC.
Optimisation of the reaction conditions with 1-methylimidazole as solvent and imidazole as catalyst
After demonstrating the importance of 1-methylimidazole as solvent and imidazole as catalyst in the depolymerisation process, the effect of temperature, nucleophile load, solvent-to-polymer ratio as well as the effect of the catalyst concentration were investigated. To evaluate the effect of the temperature on the depolymerisation, the reaction was performed at 40, 50, 60 and 90 °C. The results demonstrate that with increasing temperature, both the depolymerisation ratio and the content of PTMC increases. At 40 °C, the depolymerisation ratio only reached 71% while reactions at 50, 60 and 90 °C led to full conversion after 3 h (Fig. 2a). Continuing the reaction towards 24 h did not further improve the depolymerisation degree of the reaction – i.e. 73% – while PTMC ratio increased up to 9%. As temperature increased, the formation of PTMC also increased; at 50 °C only 18% of PTMC were produced while this value raised up to 84% at 90 °C. These results are in good agreement with previous studies reporting the detrimental effect of temperature (over 55 °C) on the synthesis of TMC from the organocatalysed coupling of oxetane and CO2.28 Considering that increased temperatures promote the ROP of TMC, a minimum temperature is required. For completing the reaction in a reasonable amount of time – less than 3 h – the best balance between the final yield and the TMC/PTMC ratio was obtained for the reaction at 50 °C.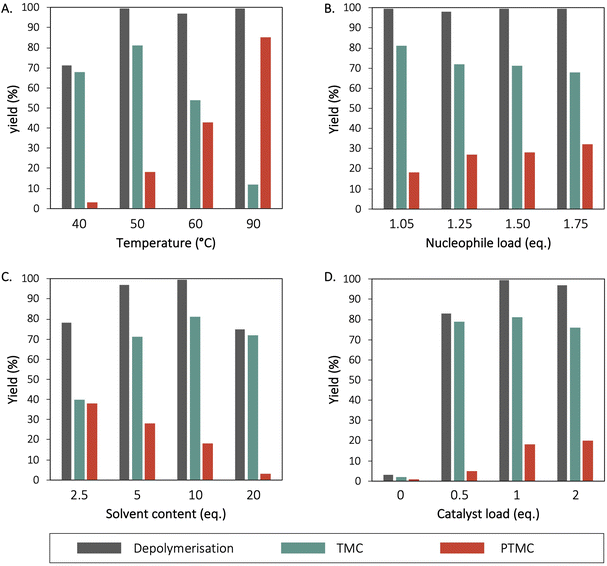 | ||
| Fig. 2 Screening of different parameters for the depolymerisation of BPA-PC with 1,3-propanediol as nucleophile, 1-methylimidazole as solvent and imidazole as catalyst, at different (A) temperatures, (B) nucleophile loads, (C) solvent quantity and (D) catalyst load. Yields were determined by 1H NMR spectroscopy in DMSO-d6 at ambient temperature (Fig. S12 to S23†). | ||
The impact of the quantity of 1,3-propanediol on the formation of TMC and PTMC during the depolymerisation of BPA-PC was also evaluated. The quantity of nucleophile in excess (1.05, 1.25, 1.5 and 1.75 eq.) did not demonstrate any significant impact on the depolymerisation rate, after 3 h, all reactions reached full conversion (Fig. 2b). However, an excess of nucleophile seemed to slightly promote the formation of PTMC. Thus, the TMC/PTMC was 81:18 with 1.05 eq. of 1,3-propanediol vs. 72:32 with 1.75 eq. The 1,3-propanediol may act as an initiator for the polymerisation of TMC, which would explain why an excess of diol leads to higher PTMC content.
Different quantities of solvent were also investigated – i.e. 2.5., 5, 10 and 20 eq. of 1-methylimidazole compared to BPA-PC monomeric unit – to evaluate the effect of the dilution on the depolymerisation (Fig. 2c). The results showed that although a minimum of 1-methylimidazole is needed to promote efficient depolymerisation, increasing the solvent content above 10 eq. does not improve the depolymerisation degree. However, the side polymerisation of TMC seemed disfavoured while 20 eq. of solvent were employed with only 3% of PTMC formed. Higher concentration favours the ROP of TMC, but higher dilution slows down the depolymerisation reaction. A compromise between these two effects can be found with 10 eq. of solvent for which complete depolymerisation is observed while only 18% of PTMC are obtained.
To gain an insight into the efficiency of the process mediated by imidazole, the effect of the catalyst loading was evaluated, with 0.5, 1 or 2 eq. of imidazole used – compared to the BPA-PC monomeric unit. The depolymerisation yield was only 83% with 0.5 eq. of imidazole while the reactions operating with 1 or 2 eq. reached completion after 3 h (Fig. 2d). These results suggest that stoichiometric amounts of imidazole are required to complete the depolymerisation which raises questions on the mechanism.
Mechanism of depolymerisation in the presence of azole catalysts
Mechanisms for imidazole-catalysed reactions have traditionally been described through basic interactions between the nucleophile and the lone pair orbitals of the nitrogen atom with weak activation of the carbonyl group by hydrogen bonding of the amine of imidazole.36 However, this type of mechanism usually exhibits a maximum reaction ratio with 0.1 to 0.2 eq. of catalyst and further increases usually don't lead to improvements in the reaction kinetics or yields. In contrast, with the present system, the maximum catalytic activity occurs at 1 eq. This behaviour suggests that a different mechanism is being followed. To gain an insight into the mechanism, the kinetics of the depolymerisation catalysed by benzimidazole were monitored by 1H NMR spectroscopy in DMSO-d6. Benzimidazole was selected over imidazole because it leads to a more stabilised carboxylate intermediate which facilitates the identification of the characteristic signals of the different steps of the reaction in the 1H NMR spectra.The 1H NMR spectrum for the reaction at t0 only presents the characteristic signals of the benzimidazole (the catalyst) and 1-methylimidazole (the solvent). BPA-PC is insoluble in the solvent which means that no signals of the polymer can be observed in the spectra. Over time a decrease of the benzimidazole signal intensity can be noted while new signals of low intensity arise in the aromatic region (δ = 8.91, 8.05 and 7.84 ppm) that cannot be attributed to the products. Nonetheless, at tf, these signals completely disappeared and the intensity of the characteristic signals of benzimidazole were restored while the signal intensity for the characteristic moieties of TMC and BPA reached a maximum (Fig. S8†). This result suggests the formation of stable intermediates between the catalyst and BPA-PC (Scheme 2). A proposed mechanism consistent with these observations can be described as follows; benzimidazole first performs a nucleophilic attack on the carbonyl of BPA-PC to form an unstable benzimidazole carboxylate chain-end (intermediate 1). The nucleophilic attack of 1,3-propanediol is then favoured by the highly activated carbonyl which leads to the formation of intermediate 2. The catalyst then attacks intermediate 2 to form a carboxylate intermediate composed of 1,3-propanediol and benzimidazole (intermediate 3). The cyclisation of intermediate 3 leads to the formation of TMC while releasing the molecule of benzimidazole.
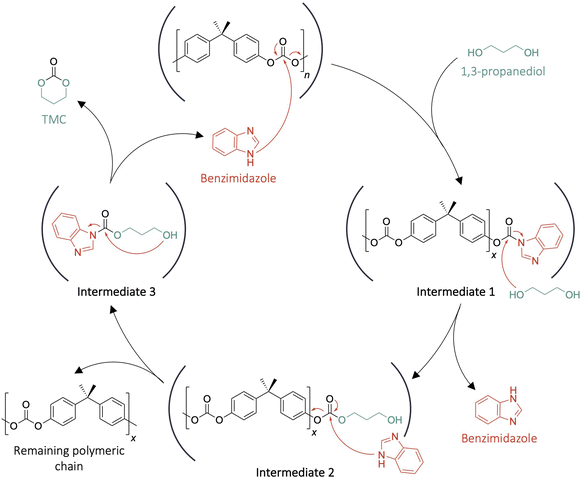 | ||
| Scheme 2 Proposed covalent mechanism for the depolymerisation of BPA-PC with 1,3-propanediol catalysed by benzimidazole. | ||
To examine this hypothesis, the depolymerisation was divided into two model reactions. In the first stage, BPA-PC was mixed with benzimidazole in the absence of 1,3-propanediol under the same conditions as previously described with the objective of characterising the covalent intermediate formed (Intermediate 1) (Fig. 3A). In the second stage, after complete depolymerisation of BPA-PC, 1,3-propanediol was added to the crude reaction to observe the disappearance of the intermediate and the formation of TMC and BPA (Fig. 3B). Reactions were monitored by quantitative 1H NMR spectroscopy and COSY spectra were recorded to complete the characterisation of the intermediates (Fig. S24 & S25†).
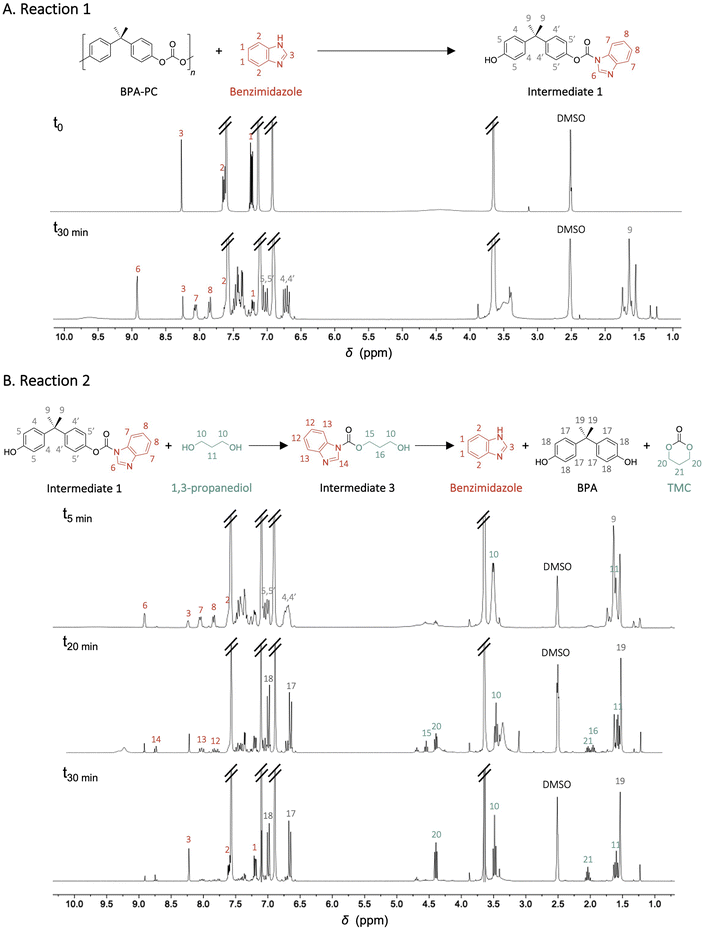 | ||
| Fig. 3 Model reactions for studying the covalent mechanism of the depolymerisation, (A) reaction between BPA-PC and benzimidazole and (B) reaction between the formed intermediate and 1,3-propanediol. | ||
After 30 min of reaction 1, complete disappearance of BPA-PC was observed, and the characteristic signals of intermediate 1 appeared in the 1H NMR spectra. While signals at δ = 8.23, 7.61 and 7.20 ppm corresponding to benzimidazole gradually decreased, new signals appeared in the aromatic regions corresponding to the benzimidazole moiety, – δ = 8.91, 8.05 and 7.84 ppm. Furthermore, multiplets characteristic of the bisphenol moiety at δ = 7.08–6.99 and 6.75–6.67 ppm appeared as well as a signal corresponding to both methyl of the bisphenol synthon at δ = 1.74–1.54 ppm. After the addition of 1,3-propanediol to the crude reaction mixture – reaction 2 – the characteristic signals for intermediate 1 gradually decrease, while signals at δ = 8.02, 7.78 and 4.54 ppm corresponding to intermediate 3 (Fig. S24†) can be identified – mainly at 20 min – before their complete disappearance as the characteristic signals of the final products appeared – δ = 4.39 and 2.04 ppm for TMC and δ = 6.98, 6.67 and 1.54 ppm for BPA. Intermediate 2 was not visible in the 1H NMR spectra, likely because it is rapidly transformed into intermediate 3. However, the presence of intermediate 2 can be reasonably asserted considering previous works which have demonstrated that for such structures, imidazole – or in this case benzimidazole – would be a far better leaving group than a phenol.37 After 30 min of reaction, the concentration of benzimidazole catalyst was completely restored while BPA and TMC were present in similar yields compared to the depolymerisation performed in one step under the same conditions, i.e. 96% and 79% respectively, which confirms the proposed mechanism.
Purification and isolation of TMC
After optimizing the depolymerisation process to obtain the highest depolymerisation ratios and the highest TMC/PTMC ratios, different approaches for the purification and isolation of TMC were investigated. Precipitation in solvent as well as liquid–liquid extraction were both unsuccessful because of the immiscibility of 1-methylimidazole in organic solvents. Following previous work of Hedrick and co-workers, an ionic exchange column was investigated as a separation method by employing an acidic resin – i.e. Amberlyst 15.37 First, the crude product of the depolymerisation reaction containing BPA, TMC, the catalyst and the solvent was dissolved in an excess of dichloromethane before addition to the separation column which retains imidazole and 1-methylimidazole by forming a salt with the attached sulphuric acid of the column. This salt formation being exothermic, to avoid the ring opening of TMC, the column was refrigerated with cold water to preserve a temperature below 45 °C. After concentrating, the solution was precipitated in diisopropyl ether obtaining only moderate yields, up to 30% of TMC.Evaluating the potential of the optimised reaction for the preparation of other cyclic carbonates
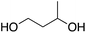
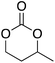
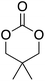
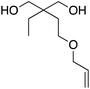
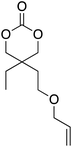
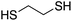
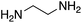
At this point it has been proven that the depolymerisation of BPA-PC through the presented pathway leads to TMC. To demonstrate the versatility of this methodology, other nucleophiles were investigated to obtain different carbonyl-containing cycles using the previously optimised depolymerisation conditions (Scheme 3). More precisely, carbonates, thiocarbonates and ureas were prepared using diols, dithiols, and diamines as nucleophiles, respectively. For diols such as 2,2-dimethyl-1,3-propanediol, butane-1,3-diol, and trimethylolpropane allyl ether, (Table 2 – entries 1 to 3), the corresponding 6-member cyclic carbonates were obtained in high yields – >80% – after 3 h of reaction. The reaction with 1,2-ethanedithiol and 1,2-ethanediamine also gave high yields with 98% and 81% of cyclic compounds, respectively, obtained after only 30 min (Table 2 – entries 14 & 5). These examples support the versatility of the depolymerisation process for different types of nucleophile.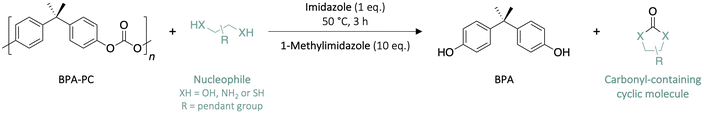 | ||
| Scheme 3 Depolymerisation of BPA-PC with different nucleophiles to obtain carbonyl-containing cyclic molecules. | ||
Conclusion
In this work, a novel procedure for performing the depolymerisation of BPA-PC at low temperature was described. It was demonstrated that the success of this approach lies in the use of a solvent that favours the interaction of the catalyst with BPA-PC and a catalytic system that promotes the cyclisation into TMC over the polymerisation into PTMC. It was found that using 1-methylimidazole as solvent and imidazole as catalyst, complete depolymerisation of BPA-PC is attained with a high yield of TMC – i.e. 81% – at 50 °C in 3 h. To date, sustainable routes for the preparation of TMC are scarce and quite inefficient, mainly because of the high tendency of TMC to ring open. The procedure was extended to other nucleophiles demonstrating the potential of this process to prepare various carbonyl-containing cyclic molecules. Although further work is needed to enhance the isolated yield (currently up to 30%), this study demonstrates the efficiency of solvent-assisted depolymerisation which allows for the preparation of complex molecules from plastic waste through a controlled depolymerisation process under mild conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge grant PDC2021-121461-I00 and TED2021-129852B-C22 funded by MCIN/AEI/10.13039/501100011033 and by the European Union NextGenerationEU/PRTR. I.O. acknowledges the Ministerio de ciencia e innovación under PDC2021-121461-I00 project. C.J. acknowledges the financial support from el Ministerio de ciencia e innovación from the Juan de la Cierva Program (FJC2020045872-I).References
- C. Martín, G. Fiorani and A. W. Kleij, ACS Catal., 2015, 5, 1353–1370 CrossRef.
- Cyclic Carbonate - an overview, ScienceDirect, 2015, vol. Advanced Fluoride-Based Materials for Energy Conversion.
- L. Maisonneuve, O. Lamarzelle, E. Rix, E. Grau and H. Cramail, Chem. Rev., 2015, 115, 12407–12439 CrossRef CAS PubMed.
- X. Zhang, M. Fevre, G. O. Jones and R. M. Waymouth, Chem. Rev., 2018, 118, 839–885 CrossRef CAS PubMed.
- J. Feng, R.-X. Zhuo and X.-Z. Zhang, Prog. Polym. Sci., 2012, 37, 211–236 CrossRef CAS.
- S. Tempelaar, L. Mespouille, O. Coulembier, P. Dubois and A. P. Dove, Chem. Soc. Rev., 2013, 42, 1312–1336 RSC.
- Q. Song, C. Pascouau, J. Zhao, G. Zhang, F. Peruch and S. Carlotti, Prog. Polym. Sci., 2020, 110, 101309 CrossRef CAS.
- W. Yu, E. Maynard, V. Chiaradia, M. C. Arno and A. P. Dove, Chem. Rev., 2021, 121, 10865–10907 CrossRef CAS PubMed.
- H. Babad and A. G. Zeiler, Chem. Rev., 1973, 73, 75–91 CrossRef CAS.
- F. Bigi, R. Maggi and G. Sartori, Green Chem., 2000, 2, 140–148 RSC.
- A. Rehman, F. Saleem, F. Javed, A. Ikhlaq, S. W. Ahmad and A. Harvey, J. Environ. Chem. Eng., 2021, 9, 105113 CrossRef CAS.
- L. Guo, K. J. Lamb and M. North, Green Chem., 2021, 23, 77–118 RSC.
- J. Huang, J. C. Worch, A. P. Dove and O. Coulembier, ChemSusChem, 2020, 13, 469–487 CrossRef CAS PubMed.
- S. Biedermann, P. Tschudin and K. Grob, Anal. Bioanal. Chem., 2010, 398, 571–576 CrossRef CAS PubMed.
- P. Palanza, L. Gioiosa, F. S. vom Saal and S. Parmigiani, Environ. Res., 2008, 108, 150–157 CrossRef CAS PubMed.
- F. Iannone, M. Casiello, A. Monopoli, P. Cotugno, M. C. Sportelli, R. A. Picca, N. Cioffi, M. M. Dell'Anna and A. Nacci, J. Mol. Catal. A: Chem., 2017, 426, 107–116 CrossRef CAS.
- I. E. Nifant'ev, D. A. Pyatakov, A. N. Tavtorkin and P. V. Ivchenko, Polym. Degrad. Stab., 2022, 110210 Search PubMed.
- E. Quaranta, C. C. Minischetti and G. Tartaro, ACS Omega, 2018, 3, 7261–7268 CrossRef CAS PubMed.
- T. Do, E. R. Baral and J. G. Kim, Polymer, 2018, 143, 106–114 CrossRef CAS.
- F. Nederberg, B. G. G. Lohmeijer, F. Leibfarth, R. C. Pratt, J. Choi, A. P. Dove, R. M. Waymouth and J. L. Hedrick, Biomacromolecules, 2007, 8, 153–160 CrossRef CAS PubMed.
- D. J. Darensbourg, W. Choi, O. Karroonnirun and N. Bhuvanesh, Macromolecules, 2008, 41, 3493–3502 CrossRef CAS.
- D. J. Darensbourg, W. Choi and C. P. Richers, Macromolecules, 2007, 40, 3521–3523 CrossRef CAS.
- K. J. Zhu, R. W. Hendren, K. Jensen and C. G. Pitt, Macromolecules, 1991, 24, 1736–1740 CrossRef CAS.
- Z. Zhang, R. Kuijer, S. K. Bulstra, D. W. Grijpma and J. Feijen, Biomaterials, 2006, 27, 1741–1748 CrossRef CAS PubMed.
- K. Fukushima, Biomater. Sci., 2015, 4, 9–24 RSC.
- B. Sun, J. Mindemark, K. Edström and D. Brandell, Solid State Ionics, 2014, 262, 738–742 CrossRef CAS.
- Y. Tominaga, Polym. J., 2017, 49, 291–299 CrossRef CAS.
- J. Huang, C. Jehanno, J. C. Worch, F. Ruipérez, H. Sardon, A. P. Dove and O. Coulembier, ACS Catal., 2020, 10, 5399–5404 CrossRef CAS.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, ACS Macro Lett., 2020, 9, 443–447 CrossRef CAS PubMed.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, Angew. Chem., Int. Ed., 2021, 60, 6710–6717 CrossRef CAS PubMed.
- C. M. Hansen, Hansen Solubility Parameters: A User's Handbook, CRC Press, Boca Raton, 2007 Search PubMed.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Román-Leshkov, N. Wierckx and G. T. Beckham, Nat. Catal., 2021, 4, 539–556 CrossRef CAS.
- T. C. Bruice and G. L. Schmir, J. Am. Chem. Soc., 1957, 79, 1663–1667 CrossRef CAS.
- T. C. Bruice and M. F. Mayahi, J. Am. Chem. Soc., 1960, 82, 3067–3071 CrossRef CAS.
- K. G. Byler, Y. Li, R. A. Houghten and K. Martinez-Mayorga, Org. Biomol. Chem., 2013, 11, 2979–2987 RSC.
- F. Ricciardi, M. M. Joullié, W. A. Romanchick and A. A. Griscavage, J. Polym. Sci., Polym. Lett. Ed., 1982, 20, 127–133 CrossRef CAS.
- E. W. P. Tan, J. L. Hedrick, P. L. Arrechea, T. Erdmann, V. Kiyek, S. Lottier, Y. Y. Yang and N. H. Park, Macromolecules, 2021, 54, 1767–1774 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3py00441d |
| This journal is © The Royal Society of Chemistry 2023 |