Hybrid polymer dot-magnetic nanoparticle based immunoassay for dual-mode multiplexed detection of two mycotoxins†
Yi-Chen
Chen‡
a,
Yu-Han
Syu‡
a,
Jhen-Yan
Huang
a,
Chun-Yi
Lin
a and
Yang-Hsiang
Chan
 *abc
*abc
aDepartment of Applied Chemistry, National Yang Ming Chiao Tung University, Hsinchu, 30010, Taiwan. E-mail: yhchan@nycu.edu.tw
bCenter for Emergent Functional Matter Science, National Yang Ming Chiao Tung University, Hsinchu, 30010, Taiwan
cDepartment of Medicinal and Applied Chemistry, Kaohsiung Medical University, Kaohsiung, 80708, Taiwan
First published on 20th July 2023
Abstract
We designed polymer dot-magnetic nanoparticle nanohybrids for signal enhancement in a test strip platform. Besides, the multicolor emissions of the Pdots embed multiplexing ability for this test strip. Two mycotoxins, aflatoxin B1 and zearalenone, were tested with the determined limits of detection of 2.15 ng mL−1 and 4.87 ng mL−1, respectively.
Since the global pandemic of COVID-19 in recent years, there is no doubt that immunochromatography test strips (ICTS), the most widely used assay of point-of-care (POC) diagnostics, have become a prerequisite for daily testing to slow down disease transmission and thus reduce the number of hospitalizations. The incentive for the widespread use of ICTS lies in many aspects, including prompt result interpretation, large-scale testing, low cost, easy use, etc.1–5 Therefore, ICTS have been adopted in detecting diverse targets.6–14
Though powerful, traditionally commercial ICTS possess several challenges, such as lower sensitivity and poorer specificity than standard laboratory facilities.4,15 Aiming at the improvement of the sensitivity and specificity of ICTS, recently, assay optimization and signal amplification strategies have been applied to ameliorate the assay kinetics and enhance the probe signals.16,17 Unfortunately, these techniques often lead to the issues of lengthening the assay test time18,19 and/or reducing detection specificity.20,21 To address this dilemma, a platform which can integrate assay optimization and signal amplification to achieve both higher sensitivity and specificity is highly desired. Herein, we integrated highly fluorescent polymer dots (Pdots) with Fe3O4 magnetic nanoparticles (MNPs) as the reporter to achieve a dual-mode immunoassay. The ultrahigh fluorescence of the Pdots22–40 can greatly enhance the detection sensitivity of ICTS.21,41–43 However, we found that the high sensitivity due to signal amplification was also accompanied by an unwanted increase in false positives. This drive inspired us to create test strip platforms that are both sensitive and selective, while retaining the multiplexing capability of Pdots. Here, we endowed Pdots with magnetic properties by encapsulating MNPs inside the Pdots to form Pdot-MNP nanohybrids. It is important to note that numerous MNP-based test strip platforms have been developed, primarily employing MNPs for sample preconcentration purposes,4 but it is relatively uncommon to directly utilize the magnetic signal as a reporter for quantitative measurements of the targeted analytes.
In addition, the multi-color property of Pdots provides the chance to develop multiplexed ICTS for simultaneously detecting multiple analytes. Here, we selected two mycotoxins, aflatoxin B1 (AFB1) and zearalenone (ZEN), as the proof-of-concept targets. Mycotoxins are secondary metabolites with low molecular weight produced by filamentous fungi, including species such as Aspergillus, Penicillium, and Fusarium. AFB1 is the predominant mycotoxin of Aspergillus and classified by the International Agency for Research on Cancer as a group I carcinogen.44 ZEN, an estrogenic metabolite mainly produced by Fusarium, has a significant reproductive toxicity and teratogenic effects on humans.45 Both AFB1 and ZEN are common contaminants in cereal grains, nuts, and complete feeds such as rice, maize, wheat, corn, and peanuts. Therefore, the maximum levels and tolerance limits for AFB1 and ZEN in food have been strictly regulated by many countries.46,47 For example, in the European Union, the maximum AFB1 tolerance levels are 5 ppb and 2 ppb for nuts and cereals, respectively.46 In Taiwan, the maximum tolerance levels are 12 ppb and 400 ppb for AFB1 and ZEN, respectively.48 The performance of the detection for AFB1 and ZEN using Pdot-MNP-based ICTS is elaborated as follows.
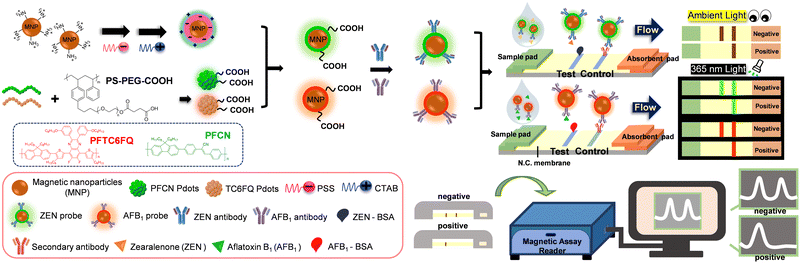
To endow fluorescent Pdots with magnetic properties, we integrated Pdots with MNPs by electrostatic interactions (Scheme 1, see ESI† for detailed discussions). Fig. S1A (ESI†) showcases the optical properties of different materials, including bare MNPs (dashed brown line), MNP@PFCN (dashed/solid green line), and MNP@PFTC6FQ (dashed/solid red lines). Before coating with Pdots, the MNPs showed strong absorption below 400 nm and no fluorescence in the visible region. After coating with Pdots, representative absorption and emission peaks of Pdots clearly appeared. The rationale behind the selection of PFCN Pdots with green emission and PFTC6FQ Pdots with orange-red fluorescence was based on the noticeable difference in color between the two under 365 nm excitation. When the two fluorescence colors are mixed, it leads to a conspicuous color transition from the original colors, which is advantageous for facilitating multiplexing detection. In Fig. S1B (ESI†), the TEM image shows the MNPs before the Pdot coating process. The MNPs exhibit an average hydrodynamic diameter of 10 nm, as determined through dynamic light scattering (DLS) measurements. The TEM images of MNPs subsequent to the encapsulation of PFCN and PFTC6FQ Pdots are depicted in Fig. S1C and D (ESI†), respectively. Furthermore, the hydrodynamic sizes of the MNP@PFCN and MNP@PFTC6FQ nanohybrids increased to 48 nm and 54 nm, respectively. The above results, which encompass zeta potential measurements, optical characterization, TEM imaging, and DLS data, confirm the successful surface modification of MNPs with Pdots.
 | ||
| Scheme 1 Schematic showing the approach used by a MNP@Pdot-based lateral flow test strip for detection of mycotoxins. | ||
The as-prepared MNP@PFCN nanoparticles were then conjugated with ZEN antibodies, while MNP@PFTC6FQ nanorods were decorated with AFB1 antibodies. Following conjugation, the MNP@Pdot nanohybrids exhibit dual capabilities of magnetic/fluorescent readout and targeted recognition. Scheme 1 shows the production of a nitrocellulose membrane comprising a control line and a test line. As there were no commercially available paired ZEN/AFB1 antibodies, a competitive immunoassay was utilized. Consequently, the control line was equipped with IgG secondary antibodies, whereas the test line was customized to include ZEN-BSA, AFB1-BSA, or a combination of both for multiplexed detection. It is important to note that the direct use of bare ZEN or AFB1 antigens is not viable due to their low affinity with the nitrocellulose membrane. A plastic case was used to assemble and accommodate each set of test strips, which consisted of a sample pad, a nitrocellulose membrane, and an absorbent pad. After the analytes and MNP@Pdot probes were added to the sample well of the test strip, the running buffer was introduced. The capillary action facilitated the movement of analytes and probes across the test strip, transferring them from the sample pad to the absorbent pad. The running buffer acted as a carrier for the analytes and probes, facilitating their movement through the test strip. The MNP@Pdot probes interacted with the analytes during their journey, allowing for the detection and quantification of the target analytes. Overall, this process enabled the efficient and effective detection of the analytes of interest. If ZEN is absent in the sample, the MNP@PFCN hybrids will attach to the ZEN antigens on the test line, resulting in the immediate visualization of green fluorescence from PFCN under UV irradiation. On the contrary, If ZEN is present in the sample, no green fluorescence from PFCN will be visible on the test line. This method is ideal for fast qualitative identification. The same mechanism also applies to MNP@PFTC6FQ in detecting AFB1. The magnetic intensity ratios of the test line to the control line (T/C) can be utilized for quantitative measurements in both cases, owing to the minimal magnetic signal background interference in food. We expect that this bimodal signal readout ICTS, which combines fluorescent and magnetic properties, will be an effective tool for diagnosing mycotoxins in food.
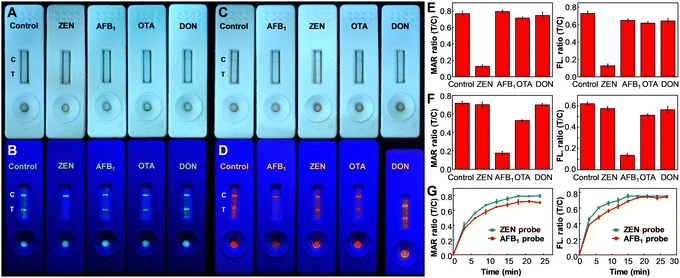
To begin the assessment, the specificity of MNP@PFCN and MNP@PFTC6FQ nanoprobes for ZEN and AFB1, respectively, was examined. According to Fig. 1A and B, the MNP@PFCN probes exhibited a notable specificity towards ZEN, with negligible non-specific binding observed for other antigens such as AFB1, ochratoxin A (OTA), and deoxynivalenol (DON). The ability of fluorescence to distinguish signals was found to be even better than the color depth from MNPs in Fig. 1A, as evidenced by the highly distinguishable fluorescence signals of Pdots in Fig. 1B. This highlights the excellent qualitative performance of fluorescence. In a similar fashion, MNP@PFTC6FQ nanohybrids demonstrated high specificity towards AFB1, as shown in Fig. 1C and D. It is worth noting that we observed a magnetic intensity ratio of T/C that was more than five times higher for MNP@PFCN towards ZEN than towards other reagents (as shown in the left panel of Fig. 1E). In contrast, the fluorescence intensity ratio of T/C for MNP@PFCN towards ZEN was about four times higher than towards other reagents (as depicted in the right panel of Fig. 1E). This indicates that the detection sensitivity based on magnetic signals is higher than that based on fluorescence signals. The same trend is observed in the case of MNP@PFTC6FQ probes, as depicted in Fig. 1F. The superior sensitivity of magnetic signals compared to fluorescence signals can be attributed to the minimal magnetic background from food and nitrocellulose membranes. This leads to higher signal-to-noise ratios and enables more accurate and reliable quantitative detection. The above results suggest that the fluorescence intensities are ideal for qualitative screening purposes, while magnetic signals are more appropriate for precise quantitative measurements. According to the aforementioned findings, the bimodal readout ICTS platform exhibits exceptional specificity for detecting ZEN or AFB1, with minimal occurrences of false negatives and false positives. Fig. 1G illustrates the monitoring of magnetic and fluorescence signals from the test and control lines at varying reaction times, which is a crucial aspect for a competent ICTS platform to rapidly acquire test results. It can be inferred that a reaction time of 20 min is adequate for both probes to decipher the testing results. In addition to specificity, the sensitivity of detection is equally important to assess the performance of these new probes (see ESI† and Fig. S4). Using magnetic signals, the detection linearity of ZEN with MNP@PFCN ranged from 0 to 100 ng mL−1, which is suitable for the maximum tolerance level of ZEN (400 ng mL−1) regulated in Taiwan, taking into account the sample pretreatment process that requires a dilution of 4–10 times. MNP@PFTC6FQ probes also exhibited a good dynamic range from 0 to 25 ng mL−1 for AFB1 (Fig. S4E and F, ESI†). The limit of detection for ZEN and AFB1 was determined to be 4.87 ng mL−1 and 2.15 ng mL−1, respectively.49–52 The practicability of this MNP@Pdot-based ICTS platform in real samples was also evaluated in this work (see ESI,† Fig. S5).
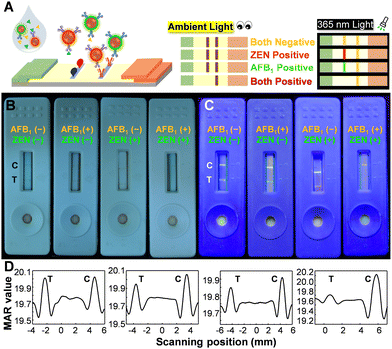
The multiplexing detection ability can be imparted to Pdots through their ability to emit multiple colors and absorb a wide range of spectra. Fig. 2A illustrates the construction of the test strip, in which a blend of antigens of ZEN and AFB1 was utilized to modify the test line. Conceptually, magnetic signals can be utilized to quantify the overall quantity of ZEN and AFB1, whereas fluorescence color is well-suited for discriminating between ZEN and AFB1. The performance of the multiplexed sensing capability was depicted in Fig. 2B and C. The negative control sample (lacking ZEN and AFB1) displayed a visible test line to the naked eye, emitting a distinct white-yellow emission light, manifesting the occurrence of minimal false-positive effects on this platform. When only AFB1 was present, a faintly discernible test line was observed by the naked eye, accompanied by green fluorescence. In the presence of only ZEN, a barely noticeable test line was observed by the naked eye, accompanied by orange fluorescence. In the case of the presence of both ZEN and AFB1, a test line cannot be detected visually and emissions are barely noticeable. It is noteworthy that the control line displayed a yellow-white fluorescence color, which was produced by the merging of green (PFCN) and red (PFTC6FQ) fluorescence. The magnetic quantitation data presented in Fig. 2D support the findings mentioned above; however, it is not possible to differentiate the presence of either ZEN or AFB1 alone. The findings indicate that the bimodal MNP@Pdot-based ICTS platform is highly dependable and has low cross-interference, making it suitable for the simultaneous detection of ZEN and AFB1 in mixed mycotoxin samples.
We have devised a bimodal ICTS platform based on MNP@Pdot for the qualitative and quantitative diagnosis of ZEN and AFB1 in food. The test can be performed on-site and interpreted within 20 minutes. This ICTS has the potential for multiplexed detection of both ZEN and AFB1 at the same time, ensuring food safety by detecting their levels. Furthermore, the detection limit of this ICTS system is low enough to conform to the maximum tolerance levels of ZEN (400 ng mL−1) and AFB1 (12 ng mL−1) regulated in Taiwan. We expect that this user-friendly yet powerful ICTS will have widespread utility in quickly screening for ZEN and AFB1 in food.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Budd, et al. , Nat. Rev. Bioeng., 2023, 1, 13–31 CrossRef.
- X. Gong, et al. , J. Mater. Chem. B, 2017, 5, 5079–5091 RSC.
- A. Sena-Torralba, R. Álvarez-Diduk, C. Parolo, A. Piper and A. Merkoçi, Chem. Rev., 2022, 122, 14881–14910 CrossRef CAS PubMed.
- Y. Liu, L. Zhan, Z. Qin, J. Sackrison and J. C. Bischof, ACS Nano, 2021, 15, 3593–3611 CrossRef CAS PubMed.
- S. Nayak, N. R. Blumenfeld, T. Laksanasopin and S. K. Sia, Anal. Chem., 2017, 89, 102–123 CrossRef CAS PubMed.
- Y.-T. Tsao, et al. , Trends Mol. Med., 2020, 26, 1118–1132 CrossRef CAS PubMed.
- Y. Wu, et al. , Biosens. Bioelectron., 2020, 157, 112168 CrossRef CAS PubMed.
- L. Liu, D. Yang and G. Liu, Biosens. Bioelectron., 2019, 136, 60–75 CrossRef CAS PubMed.
- T. Mahmoudi, M. D. L. Guardia and B. Baradaran, Trends Anal. Chem., 2020, 125, 115842 CrossRef CAS.
- A. Jones, L. Dhanapala, R. N. T. Kankanamage, C. V. Kumar and J. F. Rusling, Anal. Chem., 2020, 92, 345–362 CrossRef CAS PubMed.
- T. Mahmoudi, M. D. L. Guardia, B. Shirdel, A. Mokhtarzadeh and B. Baradaran, Trends Anal. Chem., 2019, 116, 13–30 CrossRef CAS.
- V.-T. Nguyen, S. Song, S. Park and C. Joo, Biosens. Bioelectron., 2020, 152, 112015 CrossRef CAS PubMed.
- J. Wang, et al. , Angew. Chem., Int. Ed., 2021, 60, 13042–13049 CrossRef CAS PubMed.
- Y.-H. Chen, et al. , Anal. Chem., 2021, 93, 5556–5561 CrossRef CAS PubMed.
- B. Udugama, et al. , ACS Nano, 2020, 14, 3822–3835 CrossRef CAS PubMed.
- T. Liang, et al. , Anal. Chem., 2016, 88, 2311–2320 CrossRef CAS PubMed.
- X. He, et al. , ACS Sens., 2019, 4, 1691–1700 CrossRef CAS PubMed.
- J. D. Bishop, H. V. Hsieh, D. J. Gasperino and B. H. Weigl, Lab Chip, 2019, 19, 2486–2499 RSC.
- T.-T. Tsai, et al. , Sci. Rep., 2018, 8, 17319 CrossRef PubMed.
- L. Zhan, et al. , Microsyst. Nanoeng., 2020, 6, 54 CrossRef CAS PubMed.
- Y.-Q. Yang, Y.-C. Yang, M.-H. Liu and Y.-H. Chan, Anal. Chem., 2020, 92, 1493–1501 CrossRef CAS PubMed.
- K. Pu, et al. , Nat. Nanotechnol., 2014, 9, 233–239 CrossRef CAS PubMed.
- K. Pu, N. Chattopadhyay and J. Rao, J. Controlled Release, 2016, 240, 312 CrossRef CAS PubMed.
- X. Lim, Nature, 2016, 531, 26–28 CrossRef CAS PubMed.
- C. Wu, B. Bull, C. Szymanski, K. Christensen and J. McNeill, ACS Nano, 2008, 2, 2415–2423 CrossRef CAS PubMed.
- C. Wu, et al. , Angew. Chem., Int. Ed., 2011, 50, 3430–3434 CrossRef CAS PubMed.
- Y.-H. Chan and P.-J. Wu, Part. Part. Syst. Charact., 2015, 32, 11–28 CrossRef CAS.
- S. Li, et al. , Chem. Mater., 2016, 28, 8669–8675 CrossRef CAS.
- Y. Lyu, C. Xie, S. A. Chechetka, E. Miyako and K. Pu, J. Am. Chem. Soc., 2016, 138, 9049–9052 CrossRef CAS PubMed.
- H.-Y. Liu, et al. , J. Am. Chem. Soc., 2015, 137, 10420–10429 CrossRef CAS PubMed.
- C.-S. Ke, et al. , ACS Nano, 2017, 11, 3166–3177 CrossRef CAS PubMed.
- L. Feng, et al. , Chem. Soc. Rev., 2013, 42, 6620–6633 RSC.
- Y. Lyu, X. Zhen, Y. Miao and K. Pu, ACS Nano, 2017, 11, 358–367 CrossRef CAS PubMed.
- Y. Lyu, et al. , ACS Nano, 2016, 10, 4472 CrossRef CAS PubMed.
- K. Sun, et al. , ACS Nano, 2016, 10, 6769–6781 CrossRef CAS PubMed.
- X. Chen, et al. , Adv. Mater., 2017, 29, 1604859 Search PubMed.
- W. K. Tsai and Y. H. Chan, J. Chin. Chem. Soc., 2019, 66, 9–20 CrossRef CAS.
- Y. Jiang and K. Pu, Acc. Chem. Res., 2018, 51, 1840–1849 CrossRef CAS PubMed.
- X. Zhen, C. Xie and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 3938–3942 CrossRef CAS PubMed.
- Y. Jiang and J. McNeill, Chem. Rev., 2017, 117, 838–859 CrossRef CAS PubMed.
- C.-C. Fang, et al. , Anal. Chem., 2018, 90, 2134–2140 CrossRef CAS PubMed.
- P.-Y. You, F.-C. Li, M.-H. Liu and Y.-H. Chan, ACS Appl. Mater. Interfaces, 2019, 11, 9841–9849 CrossRef CAS PubMed.
- Y.-C. Yang, M.-H. Liu, S.-M. Yang and Y.-H. Chan, ACS Sens., 2021, 6, 4255–4264 CrossRef CAS PubMed.
- World Health Organization, International agency for research on cancer. https://monographs.iarc.who.int/agents-classified-by-the-iarc/(accessed May 27, 2023).
- J. W. Bennett and M. Klich, Clin. Microbiol. Rev., 2003, 16, 497–516 CrossRef CAS PubMed.
- L. Wu, et al. , J. Anim. Sci. Biotechnol., 2016, 7, 63 CrossRef PubMed.
- Economic Research Service, United States Department of Agriculture. https://www.ers.usda.gov/webdocs/publications/41603/15640_aer828h_1_.pdf?v=0 (accessed May 27, 2023).
- Taiwan Food and Drug Administration. https://consumer.fda.gov.tw/Law/Detail.aspx?nodeID=518&lawid=741 (accessed May 27, 2023).
- D. Zhu, L. Xue, G. Li, Y. Che and H. Jiang, Org. Chem. Front., 2014, 1, 501–505 RSC.
- P. Alam, et al. , Chem. Eur. J., 2017, 23, 14911–14917 CrossRef CAS PubMed.
- F. M. d R. Lima, et al. , Microchim. Acta, 2018, 185, 521 CrossRef PubMed.
- X. Pei, et al. , Anal. Chem., 2014, 86, 4893–4900 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Video of testing the real samples, synthesis of the polymers, and Fig. S1–S7. See DOI: https://doi.org/10.1039/d3cc02586a |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |