Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Benign solvents for recycling and re-use of a multi-layer battery pouch†
Jean E.
Marshall
 *a,
Bethany
Middleton
a,
Dominika
Gastol
*a,
Bethany
Middleton
a,
Dominika
Gastol
 b,
Roberto
Sommerville
b,
Roberto
Sommerville
 b,
Con R.
McElroy
b,
Con R.
McElroy
 c,
Emma
Kendrick
c,
Emma
Kendrick
 b and
Vannessa
Goodship
a
b and
Vannessa
Goodship
a
aWMG, University of Warwick, Coventry, CV4 7AL, UK. E-mail: Jean.Marshall@warwick.ac.uk
bSchool of Metallurgy and Materials, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
cDepartment of Chemistry, University of York, Heslington, York YO10 5DD, UK
First published on 4th May 2022
Abstract
This article describes a process for the repair and re-use of an aluminium-containing pouch used as an outer casing for a Lithium-ion battery cell. As Lithium-ion batteries become more widespread, particularly with their increasing use in the automotive industry and in consumer electronics, recycling them is becoming an important challenge. Current recycling approaches for Li-ion batteries focus on reclamation of the high-value metals found in the electrodes. However, in order to minimise the environmental impact of the battery it would be optimal to be able to reclaim and re-use other components. Since many battery cells consist of an electrode stack held inside an outer pouch, we herein describe the structure of such a pouch and suggest methods for selectively stripping and repairing the inner layer, allowing the material to be re-used. We investigated the use of three different solvents (xylene, limonene, and 2,2,5,5-tetramethyloxolane) for the selective stripping of the polypropylene (PP) layer on the inner side of a pouch material. Each solvent was tested on both ‘pristine’ pouch material (as obtained from the manufacturer) and pouch material that had previously been part of a battery. For ‘used’ pouch material, the overall thickness of the film decreased from 150–160 μm to 70–80 μm in under 1 h for all three solvents (corresponding to the removal of the PP layer), whereas for the ‘pristine’ pouch higher temperatures and longer times were required; this result is suggestive of some polymer degradation during the life of the pouch. Following the removal of the PP layer, virgin PP was subsequently added to renew the multi-layer structure.
1 Introduction
A recent report by the International Energy Agency (IEA) demonstrates the extent to which electric vehicles are increasingly attractive to consumers,1 stating that “There were 10 million electric cars on the worlds roads at the end of 2020, following a decade of rapid growth. Electric car registrations increased by 41% in 2020, despite the pandemic-related worldwide downturn in car sales in which global car sales dropped 16%”. Battery Electric Vehicles (BEVs) differ from fossil fuel-powered cars in that they contain electric motors instead of internal combustion engines, and are powered by battery packs. Electric vehicles (EVs) have numerous environmental advantages over traditional cars – they do not emit exhaust fumes from a tailpipe, and their batteries can be recharged using energy generated from renewable sources. However, there is still a large environmental cost associated with the manufacture of batteries for EVs,2–5 and therefore as the number of EVs on the road increases it is becoming a matter of pressing concern to recycle battery packs as they come to their end-of-life.6Recycling Li-ion batteries poses some difficult challenges – there are several different chemical components present in each battery, and separating each of these out to make pure waste streams can be a complex process.7,8 The majority of automotive battery packs are based on lithium-ion chemistry,9–11 with a lithium-containing metal oxide at the cathode and graphite at the anode. Furthermore, the design of an electrical cell can take different formats. For electric vehicles, there are 3 main formats in use-pouch cells, cylindrical cells and prismatic cells.12 According to a recent estimate,13 approximately 40% of cells produced in passenger vehicles are based on the pouch format. Any recycling process for these electrical cells will need to be tailored to specific cell chemistry and format. Furthermore, the toxicity and flammability of some of these chemical components can impose strict requirements on the facilities needed to safely carry out a recycling procedure. Despite these issues, it is vital that effective recycling processes and infrastructure are developed in this area because spent battery packs will pose hazards both to human health and to the environment if they end up in landfill.14,15
Current processes for recycling Li-ion batteries tend to focus on reclaiming the materials that are of the highest commercial value in the battery. These are the metals contained in the cathode material (cobalt, nickel, etc.) and the copper current collector used in the negative electrode. In commercial recycling processes, Li-ion cells are mechanically shredded, then some combination of pyrometallurgy and hydrometallurgy is employed in order to extract the desired reclaimed materials.16,17 Pyrometallurgy involves simply burning off the undesired materials present in order to extract valuable metals,18 while hydrometallurgy involves dissolution of the active material in acid followed by pH adjustment to precipitate the desired material.19 The focus upon high value metals means that the less valuable materials such as Lithium, Graphite and Phosphorus are not considered (despite being on the critical materials list for Europe), and the even lower value components such as the organic separator, laminated pouch or electrolyte are often just used as energy sources. Clearly, while these techniques allow some high-value materials to be reclaimed, they lead to mixed waste streams and a large part of the ‘mass’ of the battery is not recovered. Other approaches that have been proposed to improve the recycling procedure include mechanochemical techniques,20 selective leaching,21,22 froth flotation23 and shear de-agglomeration.24
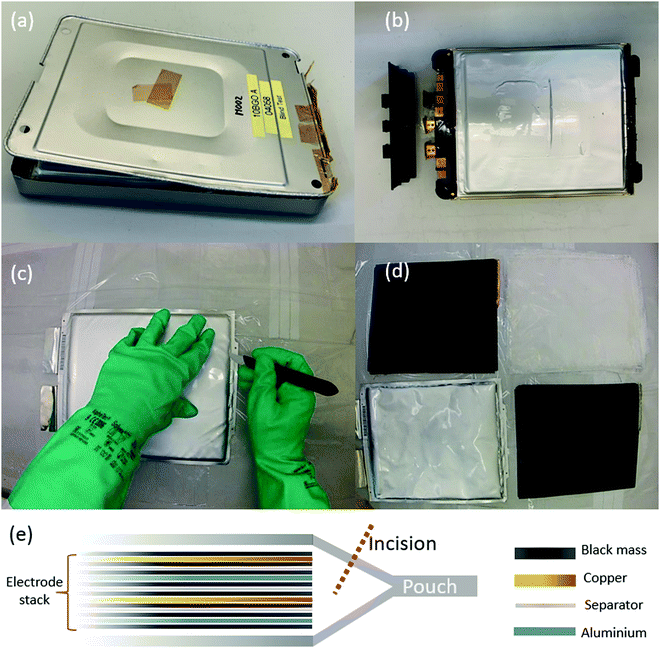
We recently showed that if Li-ion pouch cells are manually disassembled (rather than shredded), it is possible to separate the different chemical components of the cell into rather purer waste streams than is possible using a shredding approach.25 In that study, one of these waste streams was termed ‘plastics’, consisting of the outer pouch of the cell as well as the (polypropylene) separator. For a cell with a total mass of approximately 790 g, 25 g of the waste is in the outer pouch. Although this was grouped with the plastics, the pouch actually consists of an aluminium layer with a polymer coating on each side; we herein present a characterisation of the materials in a battery pouch, with a discussion of how used materials can be repaired or recycled. Fig. 1 shows photographs of a cell taken out of a module, followed by a diagram depicting the contents of the electrode stack inside the cell. The electrode stack contains layers of aluminium foil, copper foil, cathode/anode material (‘black mass’) and a separator; all of these materials are infused with an electrolyte which contains a lithium salt, and care must be taken during disassembly due to the flammable nature of this electrolyte.
A patent for a commercial pouch material26 describes a process in which an aluminium foil is coated in adhesive then polymer is attached to each side of the foil. This gives rise to a layered structure, as shown in Fig. 2(a). A cross-section of our pouch material (D-EL408PH(3), manufactured by Dai Nippon Printing Co.) was examined by SEM, and shown to also have a layer structure, as shown in Fig. 2(b), and therefore we suggest that our pouch was manufactured by a similar process.
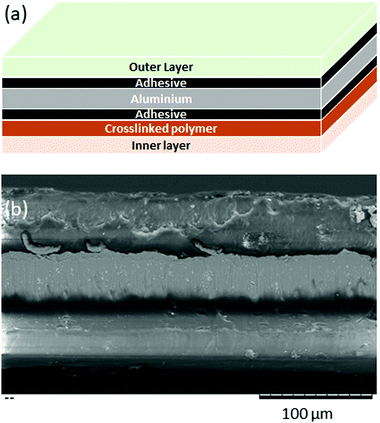 | ||
| Fig. 2 (a) Schematic diagram of a layered pouch film (b) SEM image of a cross section through a pouch film, showing the layered structure. | ||
At the time of writing, the cost of the pouch material is 77 SGD (42 GBP) per m2, which we estimate to be 190 GBP per kg, while both aluminium and polypropylene cost less than 1 GBP per kg.27,28 Therefore, it is clear that a substantial part of the value of the pouch is in its design and manufacture; being able to re-use the pouch material is potentially more cost-effective than being able to reclaim the raw materials from it. Metal-containing pouches are used in many industries other than batteries; for example, in food/pharmaceutical industries where effective barrier materials are required to protect the contents of the pouch from ambient conditions. Batteries contain flammable and toxic materials, so reclaimed battery pouches are unlikely to be re-used in food contact or medical applications; however, lessons learned from attempts to reclaim battery pouches could be applied to reclaiming used pouch material from these other sectors.
An optimal reclamation route for the pouch material would involve being able to clean and repair it so that it can be re-used. Any contaminants associated with the other components of the battery (such as the electrolyte) will principally be found on the ‘inner’ side of the pouch (adjacent to the electrode stack), therefore it would be advantageous to be able to selectively strip the inner polymer layer (while leaving the outer layer intact) and re-coat the inside of the pouch. In this manner, the economic value of the pouch material can be retained while it is made ready for re-use. We herein present a characterisation study of a commercial pouch material, followed by solvent extraction to selectively strip the inner layer from the pouch, followed by the addition of a new polymer layer to the inner side of the pouch. Previous studies by other authors have indicated that solvent stripping methods can be favourable, in terms of energy cost, compared to other recycling approaches.29,30 As part of our study, we investigate two relatively ‘benign’ solvents, limonene and TMO, and show that these solvents can selectively strip the inner layer allowing the pouch to be repaired.
2 Results and discussion
As mentioned above, this study was carried out using pouch material D-EL408PH(3), manufactured by Dai Nippon Printing Co. The Material Safety Data sheet for this pouch material gives the approximate composition of the material, as shown in Table 1.| Material | % | Melting point (°C) |
|---|---|---|
| Polyethylene terephthalate (PET) | 8 ± 0.8 | 260 |
| Nylon | 8 ± 0.8 | 220 |
| Adhesive | 3 ± 0.3 | |
| Aluminium | 49 ± 4.9 | 650–660 |
| Acid-modified polypropylene | 16 ± 1.6 | 160 |
| Polypropylene (PP) | 16 ± 1.6 | 140 |
This composition is compatible with an aluminium foil layer coated on both sides with polymer. We investigated ‘pristine’ pouch material (as received from the manufacturer) and compared this to ‘End of Life (EoL)’ pouch material (from a battery pack at the end of life).
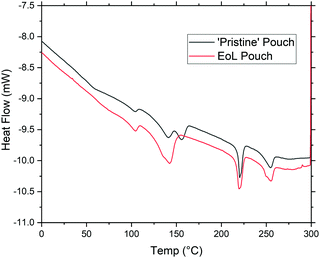
Fig. 3 shows dynamic scanning calorimetry (DSC) data to compare a ‘pristine’ pouch to a pouch at its EoL. For the ‘pristine’ (as-received from the manufacturer) pouch, signals are clearly seen at 140, 160, 220, and 260 °C; these correspond to polypropylene, acid-modified polypropylene, nylon, and polyethylene terephthalate, respectively. Interestingly, however, for the EoL pouch the signal at 160 °C has disappeared completely, while the signal at 140 °C has increased in intensity. Since polypropylene does not bond well to many substrates, we assume that the acid-modified polypropylene is included to ensure good adhesion between polypropylene and aluminium; if this polymer degrades during the life of the battery, this has interesting implications for being able to selectively strip the polypropylene layer. While the manufacturer does not provide details of the acid-modified layer here, we assume it is of a similar type to that described in a 2011 patent for acid-modified polypropylene.31 This type of modified polypropylene will contain more reactive chemical functionality than PP homopolymer, and can then be more susceptible to applied voltages and the presence of electrolyte.
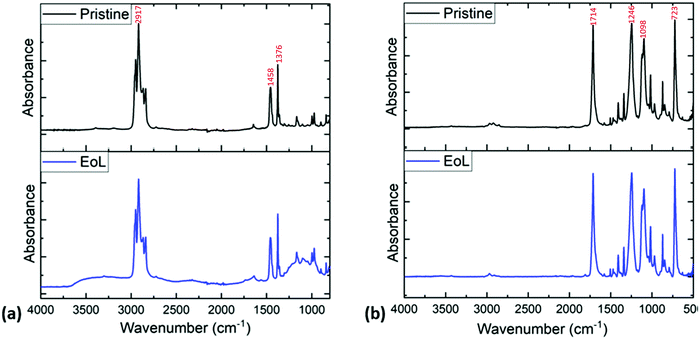
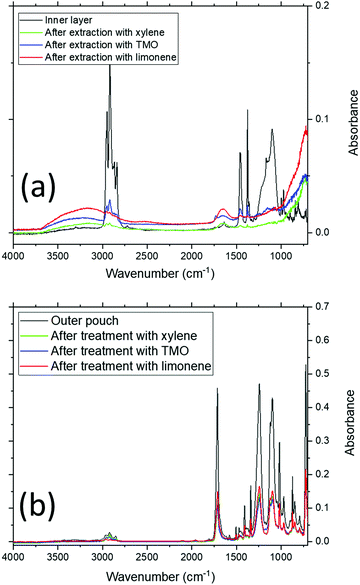
Fig. 4 shows sample FT-IR analysis of the pouch surfaces. Fig. 4(a) shows the FT-IR data collected on the inner surfaces of pristine and EoL pouches, while Fig. 4(b) shows FT-IR data collected on the outer surfaces. The FT-IR data for the inner layer of the pristine pouch is compatible with a polypropylene layer, with C–H bending modes at 1458 and 1376 cm−1; this is consistent with previous literature data for the FTIR spectrum of polypropylene.32,33 It is clear from these spectra that the inner layer of the EoL pouches is similar to that of the pristine pouch, but not exactly the same; there is increased absorption in the 3000–3500 cm−1 and 900–1200 cm−1 regions. This is consistent with the DSC data in Fig. 3, which suggested a chemical change in the inner layer of the EoL pouch compared to the pristine pouch. Meanwhile, as shown in Fig. 4(b), the FTIR spectra of the outer layer appears identical for pristine and EoL pouches, and has a carbonyl stretching absorption at 1714 cm−1 consistent with PET.34
It is clear, then, that in order to ‘repair’ some of the pouch material we would like to strip the inner polypropylene (PP) layer while leaving the outer polymer layer intact. The key challenge here is PP's relative lack of solubility in many common solvents. It is a hydrocarbon polymer and hydrophobic, but is known to have some solubility in a few non-polar solvents such as xylene.35 Xylene is an aromatic solvent, produced by catalytic reforming36; however, it is substantially toxic and can produce depression of the nervous system at ambient concentrations above 100 ppm.37 For a recycling process, we would ideally select the most ‘benign’ solvents possible, in terms of both environmental impact and hazards to human health. Therefore, in addition to xylene, we also investigated two other non-polar solvents, limonene and 2,2,5,5-tetramethyloxolane (TMO). Limonene is a naturally-occurring compound that has low toxicity compared to xylene,38 while TMO is a cyclic ether that can be produced by a demonstrated ‘green’ synthetic route.39–41 Hazards associated with these three solvents are given in Table 2.42–44
| Solvent | Boiling point (°C) | Hazards | |
|---|---|---|---|
| Xylene (mix of isomers) | 136–140 | H226 | Flammable liquid and vapor |
| H304 | May be fatal if swallowed and enters airways | ||
| H312 + H332 | Harmful in contact with skin or if inhaled | ||
| H315 | Causes skin irritation | ||
| H319 | Causes serious eye irritation | ||
| H335 | May cause respiratory irritation | ||
| H373 | May cause damage to organs (hearing organs) through prolonged or repeated exposure | ||
| H373 | May cause damage to organs (central nervous system, liver, kidney) through prolonged or repeated exposure if inhaled | ||
| H412 | Harmful to aquatic life with long lasting effects | ||
| Limonene (mix of isomers) | 170–180 | H226 | Flammable liquid and vapor |
| H315 | Causes skin irritation | ||
| H317 | May cause an allergic skin reaction | ||
| H410 | Very toxic to aquatic life with long lasting effects | ||
| TMO | 112 | H225 | Highly flammable liquid and vapor |
| H302 | Harmful if swallowed |
To rationalise the choice of solvents, xylene, limonene and TMO were examined using Hansen Solubility Parameters (HSP) and the associated software HSP (see ESI for further information).† All three solvents sit in a similar area of HSP space with high dispersivity and low polarisability and hydrogen bonding. This suggests all three solvents should perform in a similar manner. Expanding the list to include established solvents described as being incompatible with PP shows a range of solvents with low hydrogen bonding ability, low to moderate polarisability, moderate to high dispersivity. The three proposed solvents lie within this grouping, again suggesting good perfomance. Finally including solvents to which PP shows excellent resistance gave a broad covering of HSP space although with moderate to high hydrogen bonding ability being almost ubiquitous.
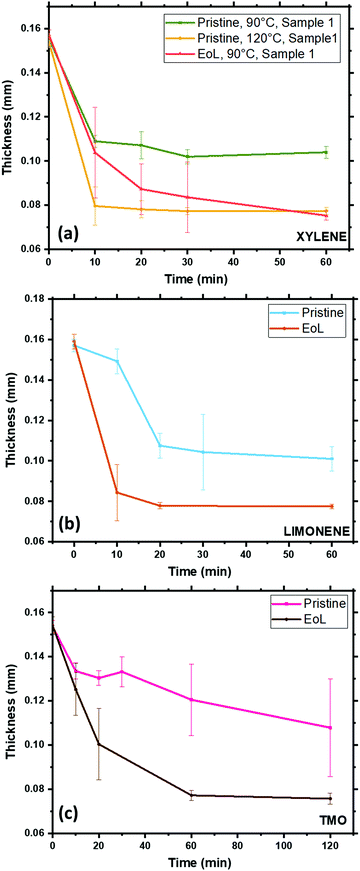
In order to test the response of the pouch material to the solvents chosen, pieces of pouch material were immersed in these 3 solvents at different temperatures and times, before being removed from the solvent bath, rinsed and dried. The thickness of the film was measured for each sample after drying, and these film thicknesses are collated in Fig. 5. There are clear trends in these data. When xylene is the solvent, at 90 °C, EoL pouches decrease in thickness to an eventual value of 70–80 μm within 1 h, and after this the thickness stabilises, presumably as no further PP is stripped from the surface. In the case of the ‘pristine’ pouch, however, this process still occurs rapidly at 120 °C but at 90 °C the process occurs more slowly and it appears that not all of the PP layer is stripped after 1 h. The fact that PP is stripped more readily from EoL pouches than for the pristine pouch is strongly suggestive of a chemical change occurring in the inner pouch layer during the battery's use; this finding is consistent with the DSC and FTIR data. Similar trends are found for TMO and limonene, with the EoL pouch being stripped more quickly than the pristine pouch. From these data, limonene is a particularly suitable replacement for xylene, stripping PP from the EoL pouch in 10–20 min at 90 °C. Fig. 6 shows the FTIR data obtained on the inner and outer surfaces after extraction in the 3 solvents; as expected, the polymer peaks on the inner surface are gone or dramatically reduced after solvent treatment, while the polymer peaks on the outer surface remain.
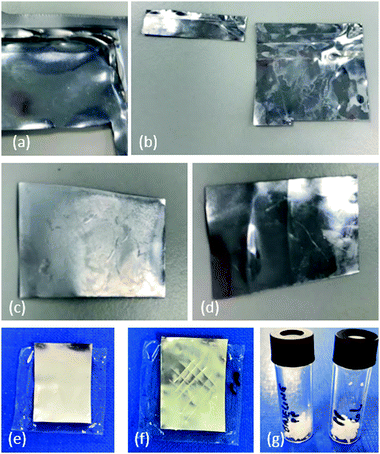
During manufacture of this type of pouch, the edges are bonded together by being compressed under heating, to form the pouch structure. As may be appreciated from the schematic diagram in Fig. 1(e), when the pouch is opened (to remove the electrode stack for recycling) by an incision made with a blade, the edges will remain bonded together. For the purpose of recycling the pouch, it would be advantageous to be able to re-use the edge pieces along with the rest of the pouch material. We therefore also immersed an edge piece of the material in xylene in order to see whether the edges could be separated straightforwardly. As shown in Fig. 7(a and b), separation occurs readily during this soaking process. During a disassembly of the battery, using an approach of the type described in our previous work,25 an incision will be made into the pouch in order to disassemble it, and therefore recycled pieces will be smaller and will need to be repurposed for smaller pouch cells. However, if the incisions required are kept to the minimum necessary to extract the cell contents, any bonded edges can be soaked in solvent for material recovery.
Fig. 7(c) shows a piece of inner pouch that has been only partially stripped with solvent; the inner surface appears dull and inhomogeneous. After being fully stripped, however, the surface becomes shiny again as shown in Fig. 7(d).
In order to further investigate the reusability of these pouch materials, an aluminium surface that had been stripped with solvent was covered with a new PP film, then compressed and heated to 160 °C so that the PP film softened and adhered to the Al surface, as shown in Fig. 7(e). Adhesion was tested by a simple cross-hatch test; the PP film was scored in a cross-hatch pattern as shown in Fig. 7(f) before being covered with duct tape. When the tape was pulled off the surface, none of the PP layer was removed. The adhesion of the ‘new’ PP film to the aluminium surface is further investigated in the SEM studies below.
As well as the aluminium layer, it is possible to recover the PP that has been stripped from the surface during solvent treatment. A vessel containing limonene, that had been used to strip the PP layer from of pouch material, was cooled to room temperature; acetone (a non-solvent for PP) was added and the PP readily precipitated out as a white solid. As shown in Fig. 7(g), this white solid has an identical appearance when recovered from pristine and EoL pouches.
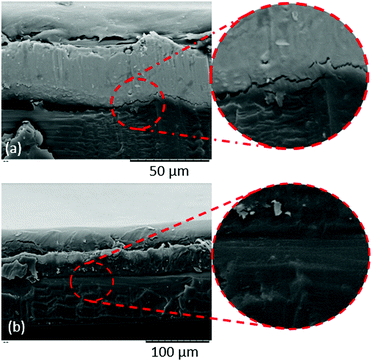
We also carried out SEM-EDS analysis in order to further confirm the findings of this study. Fig. 8(a) shows elemental maps of a cross-section through a ‘pristine’ pouch. A thin Al film is clearly shown in the centre of the cross section, with carbon layers on either side due to the polymer layers; one of those carbon layers is more oxygen-rich than the other, presumably due to the polyester structure of PET in the outer layer (compared with the inner layer, which is PP and therefore expected to contain largely carbon and hydrogen only). The same analysis was carried out on ‘pristine’ pouch that had been immersed in xylene at 120 °C for 2 h, and the results are shown in Fig. 8(b); the Al layer is intact as expected while the ‘carbon’ layer has been stripped from one side of the pouch. The remaining carbon layer is the ‘oxygen-rich’ layer, i.e. the PET-containing outer layer. These images corroborate our finding that xylene can selectively strip the PP layer. The stripping process is quantified by SEM-EDS data; sample data is shown in Table 3. This table compares SEM-EDS data taken from the inner surface of an EoL pouch, compared to an EoL pouch after 120 minutes of being soaked in xylene at 120 °C. While the inner surface before solvent treatment consists of 96% carbon (consistent with a PP layer), after solvent stripping the Al surface is clearly exposed.
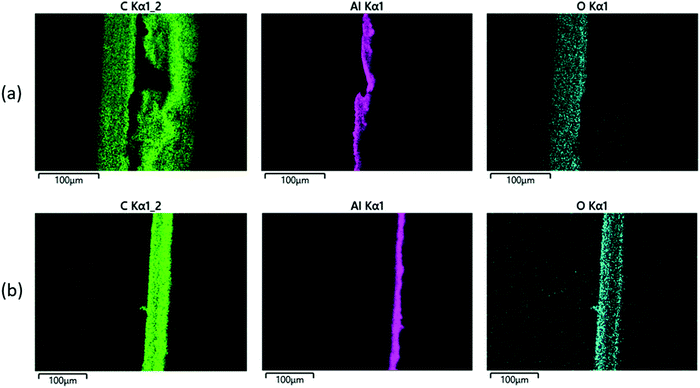 | ||
| Fig. 8 SEM-EDS elemental maps of a cross-section through a pouch, for (a) a pristine pouch, and (b) a pristine pouch after treatment with xylene. | ||
| Element | Inner pouch surface | Inner pouch surface after stripping with xylene |
|---|---|---|
| C | 96.4 | 19.57 |
| O | 3.35 | 1.67 |
| Al | 0.03 | 75.29 |
| P | 0.14 | |
| Si | 0.07 | |
| F | 2.61 | |
| Fe | 0.73 | |
| Ca | 0.05 | |
| Cr | 0.08 |
As shown in Fig. 7(e), we also attempted to ‘repair’ some of the selectively stripped pouch material by covering it with a new PP film. A simple crosshatch adhesion test did indicate that there was some bonding between the aluminium surface and the PP film. To investigate this further, a new PP film was applied to (a) a pouch film where the PP layer had been fully stripped (using limonene at 90 °C for 60 min on an EoL pouch film) and (b) a pouch film where the PP layer had only been partially stripped (limonene at 90 °C for 10 min on an EoL pouch film). A cross section was cut through each of the ‘repaired’ materials and SEM images were taken of the two cross sections. Images of the cross sections are shown in Fig. 9. Neither sample showed perfect bonding between the Al film and the PP (and this is to be expected, since in the manufacturing process there is an adhesion layer containing acid-modified PP to bind the PP to the aluminium. For an industrial recycling process, this binding layer would require further optimisation). In each case, some cracking and voids between the Al layer and the polymer film were observed. Qualitatively, the amount of voids and cracks observed were fewer in the case of the PP attached to the material that had only partially been stripped of the original PP layer. This finding may have important implications for future recycling processes in this area, since the ability to not only selectively strip certain layers from the multi-layer structure, but to only partially strip certain layers, may actually make the repair/recycle procedure more effective.
3 Experimental
Xylene and Limonene were obtained from Sigma Aldrich and used as received, TMO was synthesised adapting a published route, carrying out reactive distillation at 112 °C using 1 kg of 2,5-dimethylhexane-2,5-diol (Sigma Aldrich) and 1 g of HCZB 30 (Clariant, calcined at 500 °C for 1 hour).39Pouch cells were opened manually under a fume hood, using a ceramic scalpel/scissors. Electrodes and separator materials were removed and separated using a bespoke suction device. All cell components (including the pouch material) were soaked, separately, in an IPA bath in ambient conditions for 24 h. Components were then dried in a vacuum oven at 50/75 °C at 100 mbar for 24 h.
DSC was carried out using a Mettler Toledo TGA/DSC 3+ STARe system, at a heating rate of 10 °C per min.
FT-IR spectra in Fig. 4 were obtained using a Bruker Tensor 27 IR and OPUS software. FT-IR spectra in Fig. 6 were obtained using a JASCO FT/IR 4200 system with ATR attachment.
SEM imaging was carried out using a Hitachi Tabletop Microscope TM3030Plus (Hitachi High-Tech Corporation, Japan). For analysis of the pouch materials, each sample was coated in AuPd, sputtered at 80 mA for 30 s under vacuum (0.01 mbar).
Thickness variation tests were carried out by placing some of each solvent in a vessel, which was then brought to the desired temperature. Several small (approx 2.5 × 2 cm) pieces of pouch material would then be placed in the vessel with the solvent. Before being placed in the vessel, a corner of each piece was marked asymmetrically with a scalpel so that on removal from the solvent it would still be obvious which side was the inner pouch and which the outer, even after removal of a polymer layer. At various time intervals, a piece of pouch would be removed from the vessel, rinsed with acetone and dried, before its thickness was measured with a digital micrometer. For each combination of solvent/temperature, this process was repeated twice (‘sample 1’ and ‘sample 2’ on each graph).
For the ‘repair’ stage, pieces of pouch that had been stripped of PP were placed in contact with a film of pristine PP. This was then placed on a hotplate under a weight so as to keep conformal contact of the PP with the pouch. The hotplate was heated to 160 °C for 20 minutes, after which time the polymer had softened. The material was removed from the heat source and allowed to cool to room temperature before further tests were carried out.
4 Conclusions
This study demonstrates that it is possible to selectively remove some polymer layers from a multilayer material consisting of an aluminium sheet coated on one side in polypropylene and on the other side by a combination of polymers including PET. All three of the solvents tested (xylene, limonene and TMO) showed a propensity to strip the inner PP layer from the pouch while leaving the central aluminium foil and the outer polymer layer intact. Since both limonene and TMO carry fewer hazard warnings than does xylene, these data have implications for all future reprocessing procedures involving polypropylene, and so the usefulness of this study is not limited to the field of batteries. In particular, this study has implications for the recycling of multilayer structures, where it is economically more viable to repair part of the multilayer than to recycle them all.It is interesting that the inner layer was more readily stripped from pouches that had previously been used in a battery than from the ‘pristine’ pouch film as received from the manufacturer; DSC and FT-IR analysis corroborates our assertion that this finding means that some parts of the polymer degrade during use. Our findings may also have further implications for ‘repair’ procedures using this material, since it may be more effective to bond a new PP layer to a partially stripped PP surface than to an aluminium surface.
Author contributions
Jean E. Marshall: conceptualization, methodology, data curation, writing – original draft. Bethany Middleton: methodology, investigation, writing – review and editing. Dominika Gastol: methodology, investigation. Roberto Sommerville: methodology, investigation. Con R. McElroy: investigation, methodology, resources. Emma Kendrick: funding acquisition, project administration, writing – review and editing. Vannessa Goodship: funding acquisition, project administration, writing – review and editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We gratefully acknowledge Adrian Lopera-Valle for DSC measurements of the pouch. We acknowledge funding from UKRI project “Reclamation and Re-manufacture of lithium ion batteries,” reference number 104425, and Faraday Institution ReLIB FIRG005 and FIRG006.References
- IEA, Global EV Outlook 2021, IEA, Paris, 2021, https://www.iea.org/reports/global-ev-outlook-2021.
- S. Wang and J. Yu, Waste Manage. Res., 2021, 39, 156–164 CrossRef CAS PubMed.
- Q. Dai, J. C. Kelly, L. Gaines and M. Wang, Batteries, 2019, 5, 48 CrossRef CAS.
- S. Zhao and F. You, ACS Sustainable Chem. Eng., 2019, 7, 5082–5094 CrossRef CAS.
- L. A.-W. Ellingsen, G. Majeau-Bettez, B. Singh, A. K. Srivastava, L. O. Valøen and A. H. Strømman, J. Ind. Ecol., 2014, 18, 113–124 CrossRef CAS.
- J. Scoyer, Recycling of Li-Ion Batteries and New Generation Batteries, World Scientific, 2017, ch. 3, pp. 105–159 Search PubMed.
- P. Marques, R. Garcia, L. Kulay and F. Freire, J. Cleaner Prod., 2019, 229, 787–794 CrossRef CAS.
- R. Sommerville, J. Shaw-Stewart, V. Goodship, N. Rowson and E. Kendrick, Sustainable Mater. Technol., 2020, 25, e00197 CrossRef CAS.
- M. V. Reddy, A. Mauger, C. M. Julien, A. Paolella and K. Zaghib, Materials, 2020, 13, 1884 CrossRef CAS PubMed.
- B. Scrosati, History of lithium batteries, 2011, https://link.springer.com/article/10.1007/s10008-011-1386-8 Search PubMed.
- K. Brandt, Solid State Ionics, 1994, 69, 173–183 CrossRef CAS.
- H. Löbberding, S. Wessel, C. Offermanns, M. Kehrer, J. Rother, H. Heimes and A. Kampker, World Electr. Veh. J., 2020, 11, 77 CrossRef.
- G. Fisher, Pouch, Cylindrical or Prismatic: Which Battery Format Will Rule the Market?, http://www.addionics.com/post/pouch-cylindrical-or-prismatic-which-battery-format-will-rule-the-market, 2021, Online, accessed 28-04-2022 Search PubMed.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- J. Dunn, M. Slattery, A. Kendall, H. Ambrose and S. Shen, Environ. Sci. Technol., 2021, 55, 5189–5198 CrossRef CAS PubMed.
- L. Brückner, J. Frank and T. Elwert, Metals, 2020, 10, 1107 CrossRef.
- H. Bae and Y. Kim, Mater. Adv., 2021, 2, 3234–3250 RSC.
- M. A. Rajaeifar, M. Raugei, B. Steubing, A. Hartwell, P. A. Anderson and O. Heidrich, J. Ind. Ecol., 2021, 1–12 Search PubMed.
- Y. Yao, M. Zhu, Z. Zhao, B. Tong, Y. Fan and Z. Hua, ACS Sustainable Chem. Eng., 2018, 6, 13611–13627 CrossRef CAS.
- M. M. Wang, C. C. Zhang and F. S. Zhang, Waste Manage., 2017, 67, 232–239 CrossRef CAS PubMed.
- J. Li, P. Shi, Z. Wang, Y. Chen and C. C. Chang, Chemosphere, 2009, 77, 1132–1136 CrossRef CAS PubMed.
- Y. Xin, X. Guo, S. Chen, J. Wang, F. Wu and B. Xin, J. Cleaner Prod., 2016, 116, 249–258 CrossRef CAS.
- R. Zhan, Z. Oldenburg and L. Pan, Sustainable Mater. Technol., 2018, 17, e00062 CrossRef CAS.
- R. Zhan, T. Payne, T. Leftwich, K. Perrine and L. Pan, Waste Manage., 2020, 105, 39–48 CrossRef CAS PubMed.
- J. Marshall, D. Gastol, R. Sommerville, B. Middleton, V. Goodship and E. Kendrick, Metals, 2020, 10, 773 CrossRef CAS.
- M.-Y. Sung, J.-H. Shim, J.-G. Kim and S.-M. Kim, Aluminum pouch film for secondary battery, packaging material including same, secondary battery including same, and method for manufacturing aluminum pouch film for secondary battery, US20140377636A1, 2013 Search PubMed.
- Scrap Metal Prices UK, https://www.scrapmetalpricesuk.co.uk/, Accessed: 2021-09-27.
- Recycling today, https://www.recyclingtoday.com/article/plastic-scrap-prices-trend-upward/, Accessed: 2021-09-27.
- C. Samorì, D. Cespi, P. Blair, P. Galletti, D. Malferrari, F. Passarini, I. Vassura and E. Tagliavini, Green Chem., 2017, 19, 1714–1720 RSC.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C.-V. Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef PubMed.
- K. Morikawa and K. Ohno, Acid-modified polypropylene resin, method for producing same, and resin composition using same, US7989083B2, 2011 Search PubMed.
- R. Kumar, S. Ali, A. Naqvi, H. Virk, U. De, D. Avasthi and R. Prasad, Indian J. Phys., 2009, 83, 969–976 CrossRef CAS.
- M. Mazhar, M. Abdouss, Z. Shariatinia and M. Zargaran, Chem. Ind. Chem. Eng. Q., 2014, 20, 87–96 CrossRef CAS.
- A. P. Pereira, M. Silva, E. Jr, A. Paula and F. Tommasini, Mater. Res., 2017, 20, 1–10 Search PubMed.
- J. Poulakis and C. Papaspyrides, Resour., Conserv. Recycl., 1997, 20, 31–41 CrossRef.
- K. Ziegler-Skylakakis, J. Fabri, U. Graeser and T. A. Simo, in Xylenes, American Cancer Society, 2019, pp. 1–20 Search PubMed.
- R. Kandyala, S. P. Raghavendra and S. Rajasekharan, J. Oral Maxillofac. Pathol., 2010, 14, 1 CrossRef PubMed.
- Y. W. Kim, M. J. Kim, B. Y. Chung, D. Y. Bang, S. K. Lim, S. M. Choi, D. S. Lim, M. C. Cho, K. Yoon, H. S. Kim, K. B. Kim, Y. S. Kim, S. J. Kwack and B. M. Lee, J. Toxicol. Environ. Health, Part B, 2013, 16, 17–38 CAS.
- F. Byrne, B. Forier, G. Bossaert, C. Hoebers, T. J. Farmer, J. H. Clark and A. J. Hunt, Green Chem., 2017, 19, 3671–3678 RSC.
- A. Pellis, F. P. Byrne, J. Sherwood, M. Vastano, J. W. Comerford and T. J. Farmer, Green Chem., 2019, 21, 1686–1694 RSC.
- F. P. Byrne, W. M. Hodds, S. Shimizu, T. J. Farmer and A. J. Hunt, J. Cleaner Prod., 2019, 240, 118175 CrossRef CAS.
- Sigma-Aldrich, Xylenes, https://www.sigmaaldrich.com/GB/en/product/aldrich/214736, 2021, Accessed: 2021-09-27.
- Sigma-Aldrich, Limonene, https://www.sigmaaldrich.com/GB/en/product/aldrich/334111, 2021, Accessed: 2021-09-27.
- Sigma-Aldrich, 2,2,5,5-Tetramethyltetrahydrofuran, https://www.sigmaaldrich.com/GB/en/product/aldrich/223700, 2021, Accessed: 2021-09-27.
Footnote |
| † Electronic supplementary information (ESI) available: Hansen solubility simulations for PP in solvents. See DOI: https://doi.org/10.1039/d2ma00239f |
| This journal is © The Royal Society of Chemistry 2022 |