Open Access Article
Open Access ArticleCommercially available rapid diagnostic tests for the detection of high priority pathogens: status and challenges
Jaime
Castillo-León
 *a,
Ramona
Trebbien
*a,
Ramona
Trebbien
 b,
John J.
Castillo
b,
John J.
Castillo
 c and
Winnie E.
Svendsen
a
c and
Winnie E.
Svendsen
a
aBioengineering Department, Technical University of Denmark, Ørsteds Plads, DK-2800 Kgs. Lyngby, Denmark. E-mail: jaic@dtu.dk
bStatens Serum Institut, 5 Artillerivej, DK-2300 Copenhagen, Denmark
cEscuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia
First published on 1st June 2021
Abstract
The ongoing COVID-19 pandemic has shown the importance of having analytical devices that allow a simple, fast, and robust detection of pathogens which cause epidemics and pandemics. The information these devices can collect is crucial for health authorities to make effective decisions to contain the disease's advance. The World Health Organization published a list of primary pathogens that have raised concern as potential causes of future pandemics. Unfortunately, there are no rapid diagnostic tests commercially available and approved by the regulatory bodies to detect most of the pathogens listed by the WHO. This report describes these pathogens, the available detection methods, and highlights areas where more attention is needed to produce rapid diagnostic tests for future pandemic surveillance.
1. Introduction
Disease outbreaks have occurred since the early days of human history in all regions of the world where humans have migrated. One of the first reported disease outbreaks was the plague of Justinian in 541–542 AD. This pandemic affected the Byzantine Empire, killing as many as 50 million people.1,2 The cause of this pandemic was the bacterium Yersinia pestis, which is the same one that, centuries later, killed almost half of Europe's population during the years 1347–1351.3The main reasons for disease outbreaks during human history are connected to the vast migration of humans throughout the planet and their interaction with new civilizations and animals, resulting in the exchange of pathogens and a speeding up of diseases.4 Colonization expeditions, the foundation and expansion of cities, the opening of new and exotic trade routes, and lately, the phenomenon of globalization have contributed to the development and improvement of human life and increased risks of pandemics occurring.5
Simultaneously, scientific advances, an increase in the understanding of diseases, and improved life and hygienic conditions have been instrumental in preventing, controlling, and mitigating disease outbreaks. The development of new fabrication techniques, such as micro- and nanotechnology, and the improvement of sensing and diagnostic procedures during the last 100 years have been critical tools in the monitoring and preventing disease outbreaks.6 Having the possibility to quickly collect, identify, and analyze an increase in the number of infected people is an essential factor that allows authorities and health organizations to react early and take the necessary measurements to prevent disease spread. However, an unsatisfactory diagnostic capacity compromises outbreak detection and response, which results in the loss of lives.
Despite crucial advances in sensing and diagnostics, the need for more robust, sensitive, and useful devices for the rapid identification of infected individuals is still and will continue to be an urgent need.7 The latest report on the low efficiency of some of the existing rapid tests for detecting COVID-19 has raised questions about the quality of available rapid test devices.8,9
Most of the more effective methods to identify pathogens responsible for disease outbreaks are based on molecular diagnostic assays that require the transportation of the samples to a lab, the use of specialized equipment and trained personnel. Moreover, since the test often takes more than 30 minutes, the more effective methods based on molecular assays are not ideal for analyzing thousands of samples.
The irruption of mobile phones and other devices, such as tablets, has opened a new spectrum of possibilities for combining miniaturized biosensing analytical devices.10 A mobile phone provides an energy source, optical detection, analysis, storage, and data transmission on-site. Even though the combination of miniaturized analytical devices and mobile phones offers the perfect tools to be utilized for quick screening of infected individuals, the reality is that the combination of sensing platforms and mobile phone technology is still only available for research purposes.11
This article highlights some of the deadly diseases that the WHO has identified as potential sources for future epidemics or pandemics.12 Additionally, we present the status of existing commercially available rapid diagnostic tests to quickly identify infected individuals affected by these priority diseases identified by the WHO. Finally, we identify the prevailing challenges in developing more robust and effective rapid diagnostic test devices.
2. Rapid diagnostic test (RDT) – definition and characteristics
The WHO defines a rapid test as an assay that is “designed for use where a preliminary screening test result is required and is especially useful in resource-limited countries”.13 These tests are characterized as being:• High-quality, easy-to-use tests for use in a resource-poor setting
• Quick and easy to perform and requiring little or no additional equipment
• Designed for use with a single or limited number of samples, making them more economical than, e.g., ELISA in low-throughput laboratories
• Possible to store at room temperature for an extended period
• Able to give same-day results, thus providing timely treatment interventions.
The acronym ASSURED, Affordable, Sensitive, Specific, User-friendly, Rapid, and Robust, was outlined by the WHO to summarize the set of criteria that the ideal RDT should have.
A lateral-flow assay works similarly to a chromatographic assay, where the components of a liquid sample are transported by capillary action inside a paper-based membrane. As shown in Fig. 2, an LFA has four main areas. The sample pad, where the sample is dropped; the conjugate pad, an area where the biorecognition elements, antibodies, are combined with labeled tags; test and control lines, where a color change indicates a positive or negative result; and the absorption pad, where excess sample and reagents are collected.
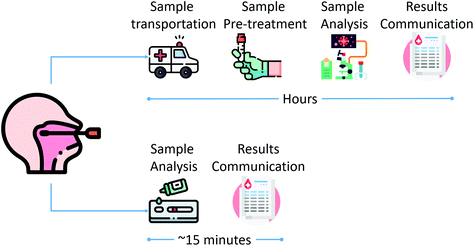 | ||
| Fig. 1 Schematic workflow comparison between laboratory-based (top) and rapid diagnostic (bottom) testing. | ||
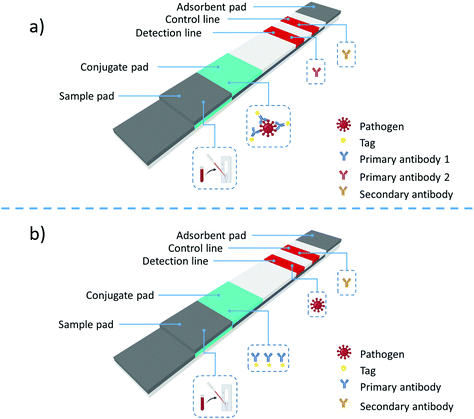 | ||
| Fig. 2 Structure of a lateral-flow assay and the two main types of LFA formats: (a) sandwich format and (b) competitive format. | ||
Two main types of immunoassays can be prepared using LFAs: sandwich and competitive assays. The sandwich assay is used to detect large analytes with multiple antigenic sites. In this format, labeled antibodies are immobilized on the conjugation pad, Fig. 2a. The label can be a metallic nanoparticle or a quantum dot. Once the sample is dropped, it is transported along the LFA membrane by capillary action. When the liquid sample passes through the conjugation pad, it rehydrates the labeled antibodies attached to the antigen epitopes. The labeled antibodies attached to the antigen continue traveling along the LFA membrane and reach the detection and control lines. If the antigen of interest is present, a colored line will appear at the detection lines. The color intensity at the test line is directly proportional to the analyte amount present in the sample. Regardless of the quantity of analyte in the sample, an anti-species antibody at the control line will bind the nanoparticle, yielding a solid control line signal, which demonstrates that the assay is functioning correctly.
When testing lower molecular weight analytes with a single antigenic site, the competitive lateral-flow assay is used, Fig. 2b. In this format, the test line typically contains the analyte antigen, and the conjugate pad contains the detection antibody-labeled conjugate. If the target antigen is present, the antigen will bind to the conjugate and prevent it from binding to the test line's antigen. If the antigen is not present, the conjugates will bind to the analyte at the test line, yielding a signal.
The signal intensity is inversely proportional to the amount of antigen present in the competitive format sample. As in the sandwich assay, the control line will bind the nanoparticle conjugate with or without the antigen, providing confidence that the assay is working correctly. In this case, only two types of antibodies are present in the LFA; a primary antibody coupled with labels and highly specific to the sample antigen, and a second species-specific anti-immunoglubolin antibody located at the control line.
Rapid diagnostic tests are point-of-need devices ideal for testing outside a dedicated laboratory space by personnel with minimal training. They are low-cost, require no or minimum energy, and can provide results within 5–30 minutes. RDTs are vital tools for rapidly detecting disease outbreaks, evaluating vaccine effectiveness, performing surveys of parasite prevalence, and allowing remote diagnosis in organized workforces entering endemic areas.
A key specification of RDTs is the ability to provide a rapid response to an analytical demand exactly where the demand is posed.14
RDTs simplify the analytical test by removing many of the steps required by traditional laboratory-based analysis, while still providing useful and reproducible information, as illustrated in Fig. 1. Additionally, RDTs also decrease the carbon footprint by reducing the amount of energy needed for analysis and skipping the transportation of samples to the lab to complete the analysis.15–17
The technology behind most commercially available rapid diagnostic tests mainly focuses on lateral flow assays (LFA)18 and electrochemical methods.19 LFAs have shown their potential in applications such as pregnancy tests and the detection of illegal drugs. A lateral-flow-based test is ideal for the development of RDTs, mainly because of the advantages offered by using paper as the main component. Paper membranes are cheap to fabricate, easy to store, safe to dispose of, and can be used on-site anywhere and by anyone. Additionally, paper can be readily modified to create hydrophilic or hydrophobic areas to control the reagents and sample flow. Paper-based sensing devices are light weight, compact, and easy to integrate into other technologies. Moreover, paper-based devices have been used in combination with mobile technology, enabling data management.20
The majority of RDTs based on LFAs yield qualitative results, indicating the presence or not of a specific analyte by a change in color, giving a yes/no answer only. Lately, due to the integration of paper-based devices with mobile technology, it is possible to obtain a quantitative answer by relating the color intensity to the sample concentration.10,21,22 Additionally, extra features, such as storing and transmitting data, can be incorporated when combining LFAs with mobile technology.23
Furthermore, LFA tests are disposable with no need for additional chemicals, and using LFAs brings about a massive reduction of the carbon footprint by avoiding transportation of samples to the lab and the use of specialized equipment during analysis.15 A recent study showed that the estimated CO2 emissions of a single full-blood examination test done in a clinical lab were equivalent to driving a standard car for 770 m.16,17
The success of electrochemical RDTs is centered around the amperometric glucose sensor's commercial success, an RDT used by millions of diabetic patients worldwide.24 Electrochemistry has been suggested as a technique that could be used to develop RDTs to detect diseases in outbreaks.19 Electrochemical devices can be miniaturized, functionalized with different biosensing compounds (enzymes, antibodies, nanobodies), and offer the possibility of multiplexing. However, despite all of these advantages, the number of commercially available RDTs for detecting pathogens during disease outbreaks is low compared with lateral flow assays.
Other techniques, such as optical sensing and bioFETS, have only been used in research and have yet to reach the market.25–28
The way the high-priority pathogens can be detected using RDTs can be divided into two groups: direct detection using antigen-detecting and molecular assay RDTs and indirect detection using the body's immune response to the pathogen in the form of antibodies. Lateral-flow immunoassay (LFI) platforms dominate the antigen-detecting RDTs; however, lately, the incorporation of molecular-based techniques has also reached commercialization.
For the second group, antibody-detecting RDTs, LFIs are the primary platform used to develop this kind of RDT.
Table 1 displays a comparison between molecular-based detection methods and antigen- and antibody-detecting assays.
| Test type | Advantages | Challenges |
|---|---|---|
| Molecular diagnostic assays | • High sensitivity and specificity | • Labor intensive |
| • Detects active infection | • Requires specialized laboratory infrastructure and skilled personnel | |
| • Expensive | ||
| • Requires sample transportation | ||
| Antigen-detecting | • Detects active infection | • Lower sensitivity and specificity than molecular diagnostic assays |
| • It can be used as a point of care | • Lower predictive value than for molecular-based tests | |
| • Easy to perform | • A negative result cannot be used to remove an individual from quarantine | |
| • Lower cost than molecular diagnostic assays | ||
| • Rapid results (<30 min) | ||
| Antibody-detecting | • Detects that an individual has been infected | • Provides mainly qualitative results |
| • It can be used as a point of care | • Lower sensitivity and specificity than molecular diagnostic assays | |
| • Easy to perform | • Positive results do not assure the presence of protective immunity | |
| • Lower cost than molecular diagnostic assays | • Interpretation of results depends on the timing of the disease, the epidemiology and prevalence within the setting, and the clinical morbidity of the individual | |
| • Rapid results (<30 min) |
For the RDTs presented in this article, the test sensitivity is calculated as (true positives)/(true positives + false negatives) × 100.
The specificity is calculated as (true negatives)/(true negatives + false positives) × 100.
3. High-priority pathogens identified by the WHO as potential causes of future epidemics
It is a fact that the impact of globalization is accelerating the spread of contagious diseases.5 The increase in air travel and the exchange of goods positively impact the global economy and contribute to the rapid spread of infectious pathogens worldwide. We have experienced the long-transmission potential of global air travel during the last twenty years spreading emerging pathogens.4 The SARS coronavirus emerged in 2002 in China and quickly spread to more than 30 countries, resulting in more than 8000 cases and around 774 deaths worldwide.29 In 2009, the H1N1 influenza pandemic originated in Mexico and spread worldwide, causing 284![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths.30 More recently, Ebola affected African countries and resulted in travel restrictions to and from affected areas. Currently, COVID-19 affects the whole world's population, resulting in an almost complete stop of air travel and paralyzing most goods exchange. When the COVID-19 pandemic hit, it became apparent that testing was the key to containing the spread. Massive and timely testing was implemented in countries like South Korea and New Zealand using a combination of molecular assay-based screening and serological-based rapid tests for the detection of antigenic proteins and antibodies. Mass testing limited the virus's spread by both symptomatic and asymptomatic viral carriers and significantly decreased the death toll in these countries.31
000 deaths.30 More recently, Ebola affected African countries and resulted in travel restrictions to and from affected areas. Currently, COVID-19 affects the whole world's population, resulting in an almost complete stop of air travel and paralyzing most goods exchange. When the COVID-19 pandemic hit, it became apparent that testing was the key to containing the spread. Massive and timely testing was implemented in countries like South Korea and New Zealand using a combination of molecular assay-based screening and serological-based rapid tests for the detection of antigenic proteins and antibodies. Mass testing limited the virus's spread by both symptomatic and asymptomatic viral carriers and significantly decreased the death toll in these countries.31
The rapid transmission of COVID-19 around the world and its significant impact on society and the economy have led to the accelerated development of new tests to detect infected individuals quickly. Recently, thanks to essential economic support from governmental and private institutions, research groups and companies have focused on developing more effective and rapid tests.32,33 Using the information provided by these tests, health authorities could implement measurements like declaring local quarantine or forcing the use of masks instead of a complete lockdown to stop the spread of COVID-19.
However, this is not the case for most of the WHO-distinguished diseases that pose the highest public health risk due to their epidemic potential. These diseases are:
• Crimean-Congo hemorrhagic fever
• Ebola virus disease and Marburg virus disease
• Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome (SARS)
• Nipah and henipaviral diseases
• Rift Valley fever
• Zika
• Coronavirus disease-2019 (COVID-19)
Next, we will describe each of these diseases, their impact areas, and the current rapid test status for their detection.
4. Crimean-Congo hemorrhagic fever (CCHF)
CCHF is a viral hemorrhagic fever with a high case fatality ratio caused by a virus transmitted to humans through a tick bite or direct contact with blood or tissue from infected ticks or viraemic vertebrates, wild animals, and livestock.34 The virus can remain for up to one week in the bloodstream of infected animals, allowing the tick–animal–tick cycle to continue when another tick bites. Infected animals do not show any symptoms, which makes it challenging to identify them. Additionally, it allows the virus to maintain itself in nature.35It is considered the most geographically widespread tick-borne virus, with infection mortality in up to 30% of cases.36 Regions of endemicity include parts of Africa, Asia, the Middle East, Russia, Eastern Europe, and Spain.
CCHF virus is a member of the family Bunyaviridae. It is an enveloped spherical particle of approximately 100 nm in diameter with a tripartite, single-stranded RNA genome of negative polarity. The three genome segments contain one open reading frame flanked by noncoding regions. It also contains four encoded structural proteins: the RNA-dependent RNA polymerase (L protein) expressed by the large (L) segment, the mature glycoproteins GN and GC are encoded by the medium (M) segment, and the nucleoprotein (N) by the small (S) segment.34
Every year, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 15
000 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of CCHF are reported, mainly in endemic countries but also among travelers.
000 cases of CCHF are reported, mainly in endemic countries but also among travelers.
CCHF can be transmitted from human-to-human by direct contact with the body fluids of infected individuals. Human serological studies have revealed a mean seroprevalence of anti-CCHF antibodies of 4.7%. The seroprevalence of anti-CCHF antibodies is 7.5-fold higher among people with high-risk exposures. Increasing trends in CCHFV seroprevalence in both humans and animals, combined with evidence that people with frequent animal contact have more exposure to CCHFV, raise the concern that CCHFV could emerge as a zoonotic pathogen.35
4.1 Symptoms
Patients who develop symptomatic CCHF progress through four stages: an incubation stage, a pre-hemorrhagic stage, a hemorrhagic stage, and a convalescent stage, as depicted in Fig. 3.36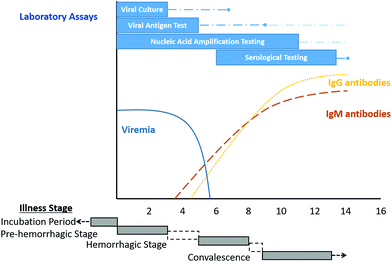 | ||
| Fig. 3 Overview of diagnostic testing for acute illness due to the Crimean-Congo haemorrhagic fever virus in a non-fatal human infection. Reproduced from ref. 36 with permission from the American Society for Microbiology copyright 2020. | ||
CCHF can be misdiagnosed with other viral hemorrhagic fevers, bacterial infections, viral infections, and parasitic infections such as malaria. Due to this, early laboratory confirmation of suspected cases is critical to prepare an adequate response.
CCHF symptoms could include myalgias, chills, fever, dizziness, headache, eye soreness, photophobia, sore throat, nausea, vomiting, and diarrhea.36,37
Mortality occurs in approximately 30% of cases, often in the second week of illness.37
4.2 Diagnostic testing
There are currently two ways to diagnose CCHF in humans: by direct detection of the presence of CCHFV or by measuring the serological response consistent with acute infection by detecting the production of anti-CCHFV IgM or anti-CCHFV IgG antibodies.The direct detection of CCHFV infection using nucleic acid amplification tests, viral antigen detection, and viral culture is most useful during the first week after symptom onset.37,38 Serological tests are most useful after the first week of illness, since IgM antibodies become detectable only after 7 to 9 days of symptom onset, except in some cases where IgM can be detected after four days of illness.39 On the other hand, IgG antibodies become detectable simultaneously with IgM or one or two days later,36cf. Fig. 3.
4.3 Rapid test for the detection of CCHF
Currently, only one rapid test for the detection of CCHFV antibodies is commercially available. A lateral flow assay kit, CCHF Sero K-SeT, developed by CORIS BioConcept (Belgium), is used to detect IgM-specific antibodies in the patient's plasma, cf. Fig. 4. However, a study performed on patients in Iran showed very low sensitivity, 39.7%, and a specificity of 92.9%.40 There is not a commercially available rapid test for the direct detection of the CCHF virus. | ||
| Fig. 4 CCHF Sero K-SeT IgM rapid test showing positive and negative results. (I) Positive result, (II) weakly positive result, and, (III) negative result. Reproduced from ref. 40 with permission from Elsevier copyright 2019. | ||
5. Ebola virus (EV)
The Ebola virus is one of the most virulent pathogens to humans. The virus is endemic in African regions, where the first outbreaks took place in 1976 in a village near the Ebola River in the Democratic Republic of Congo.41 Since then, several outbreaks have been identified in 19 countries in Africa, Europe, Asia, and America.42 The largest recorded outbreak occurred in West African countries between December 2013 and 2016 and had a 40% fatality rate. Encroachment into forest areas due to population growth and direct interaction with wildlife contributed to the spread of the African region's Ebola virus.Ebola virus is a single-stranded RNA virus belonging to the Filoviridae family along with the Marburg virus. Five virus species form the Ebola virus genus: Reston ebolavirus, Tai Forest ebolavirus, Bundibugyo ebolavirus, Zaire ebolavirus, and Sudan ebolavirus. The last two, Zaire ebolavirus and Sudan ebolavirus, are the most pathogenic and cause most outbreaks, with case fatality rates around 90%.41 The genome of the EV includes seven genes: the nucleoprotein, virion protein (VP) 35, VP40, glycoprotein, VP30, VP24, and RNA-dependent RNA polymerase (L)-5′ trailer, cf. Fig. 5.
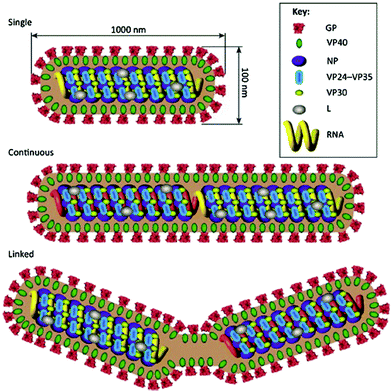 | ||
| Fig. 5 Virion structure of the Ebola virus. Reproduced from ref. 41 with permission from Elsevier copyright 2016. | ||
EV infection spreads by direct contact with bodily fluids from an infected animal or human, after which transmission occurs from human to human. EV has been identified in blood, feces, vomit, urine, semen, saliva, aqueous humor, vaginal fluid, breast milk, tears, and sweat.43 Ingestion of undercooked bushmeat and contact while hunting or preparing a carcass have been identified as transmission methods to humans.41 The fruit bat has been singled out as the natural host and is, in some cases, linked to direct transmission to humans.44,45
5.1 Symptoms
EV commonly manifests with a hemorrhagic fever followed by an incubation period of 2–21 days. The incubation period is characterized by malaise, myalgia, chills, and fever. Other symptoms, like anorexia, nausea, vomiting, diarrhea, chest pain, cough, nasal discharge, prostration, conjunctival injection, headache, confusion, and coma, indicate multisystem involvement.46 Additionally, by days 5–7 of the illness, erythema and desquamation can appear, which are used as differential diagnostic features.5.2 Diagnostic testing
The primary detection assays used during EV outbreaks are ELISA, PCR, immunohistochemistry, virus isolation, and lateral-flow based assays.ELISA and PCR techniques are sensitive methods that can detect EV in blood, serum, and tissue samples. They are lab-based techniques that require special equipment and trained personnel. They target viral nucleic acid, viral antigen, or virus-specific antibodies.47 On the other hand, immunohistochemistry targets viral antigens in tissue samples such as the liver and skin. This is a qualitative imaging method that takes time to perform.
5.3 Rapid test for the detection of EV
Six RDTs for EV are available on the market, Table 2. These devices are based on lateral-flow and used during outbreaks, providing rapid information about the virus's presence in blood samples. OraQuick Ebola is a rapid antigen test approved by the U.S. Food & Drug Administration (the FDA).48 This rapid antigen test is intended for use with whole blood obtained by venipuncture or finger stick. The sensitivity of this test on whole blood is 84%, with a specificity of 98%. Each test costs approximately USD 28.49 After the addition of the sample, results are observed after 30 minutes.| Disease | Test name | Test type | Developer | Sample | Detected | Sensitivity | Specificity | Response time (min) | Approval | Link |
|---|---|---|---|---|---|---|---|---|---|---|
| CCHF | Sero K-Set | LFI | CORIS BioConcept | Plasma | IgM | 98.5% | 98.6% | 15 | — | http://www.corisbio.com |
| EV | Senova Ebola Kit | LFI | Senova | Whole blood, serum, plasma | Antigen | — | — | — | — | http://www.senova.de |
| EV | ReEBOV | LFI | Corgenix | Whole blood, serum, plasma | Antigen | 100% | 92% | 15–25 | — | http://www.corgenix.com |
| EV | SD Ebola Zaire | LFI | SD | Whole blood, serum, plasma | Antigen | 84.5% | 98.9% | — | The WHO EUAL | http://www.sdbiosensor.com |
| EV | OraQuick | LFI | OraSure Technologies | Whole blood | Antigen | 84% | 98% | 30 | The FDA EUA, the WHO EUAL | http://www.orasure.com |
| EV | DPP Ebola Antigen System | LFI | Chembio | EDTA whole blood, plasma | Antigen | 77.1% | 91.7% | 10 | The FDA EUA | https://chembio.com |
| EV | QuickNavi-Ebola | LFI | Denka Seiken | Serum, plasma | Antigen | 85% | 99.8% | — | — | http://www.denka.co.jp |
| LFV | ReLASV Pan-Lassa | LFI | Zalgen Labs | Whole blood, serum, plasma | Antigen | 83.3% | 92.8% | 5–25 | — | http://www.zalgen.com |
| LFV | ReLASV antigen rapid test | LFI | Zalgen Labs | Whole blood, serum, plasma | Antigen | 76.9% | 94.4% | 5–25 | — | http://www.zalgen.com |
| ZV | DPP Zika | LFI | Chembio | Whole blood, serum, plasma | IgG/IgM | 85.8% | 81.4% | 15 | The FDA EUA | http://chembio.com |
| ZV | One Step Zika | LFI | Artron Laboratories Inc | Whole blood | IgG/IgM | 18% | 84% | 10 | — | http://www.artronlab.com |
| ZV | Accu-Tell Zika Virus IgG/IgM AB Cassette | LFI | AccuBioTech | Whole blood, serum, plasma | IgG/IgM | 100% | 100% | 20 | — | https://www.accubiotech.com |
| ZV | Zika IgG/IgM Combo Test Card | LFI | LumiQuick Diagnostics | IgG/IgM | 94.6% | 96.5% | 15–20 | — | https://lumiquick.co | |
| ZV | Zika NS1 Test Card | LFI | LumiQuick Diagnostics | Antigen | — | — | — | — | https://lumiquick.co | |
| ZV | Zika IgG/IgM & NS1 Duo Test | LFI | LumiQuick Diagnostics | IgG/IgM and antigen | — | — | — | — | https://lumiquick.co | |
| ZV | CDIA Zika IgG/IgM Rapid Test Kit | LFI | Creative Diagnostics | Whole blood, serum, plasma | IgG/IgM | — | — | 15 | — | https://www.cd-diatest.com |
| ZV | Zika Rapid Test | LFI | Maternova | Whole blood, serum, plasma | IgG/IgM | — | — | — | — | https://maternova.net |
| ZV | Zika IgG/IgM | LFI | BioPanda | Whole blood, serum, plasma | IgG/IgM | 80% | 94% | 15 | — | https://www.biopanda.co.uk |
| ZV | Zika NS1 | LFI | BioPanda | Whole blood, serum, plasma | Antigen | 99.9% | 98.9% | 15 | — | https://www.biopanda.co.uk |
| ZV | Zika Combo | LFI | BioPanda | Whole blood, serum, plasma | IgG/IgM and antigen | 94.4% | 99.9% | 15 | — | https://www.biopanda.co.uk |
| ZV | Zika v IgG/IgM | LFI | AdvaGen | Whole blood, serum, plasma | IgG/IgM | — | — | — | — | http://www.advagen.com.br |
| ZV | Zika-v NS1 Ag | LFI | AdvaGen | Whole blood, serum, plasma | Antigen | — | — | — | — | http://www.advagen.com.br |
| ZV | Zika IgG/IgM | LFI | Ebram Produtos Laboratoriais | Whole blood, serum, plasma | IgG/IgM | 99.9% | 98.9% | 20 | — | http://ebram.com |
| ZV | ProDetect Zika Rapid Test | LFI | Medical Innovation Ventures | Whole blood, serum, plasma | IgG/IgM | — | — | 10–20 | — | http://mediven.com.my |
| ZV | Rapikit | LFI | Nectar Lifesciences Ltd | Whole blood, serum, plasma | Antigen | 93.5% | 99.4% | — | — | https://rapikit.com |
| SARS-CoV-2 | SARS-COV-2 IgG/IgM Rapid Test Kit | LFI | Abbexa | Whole blood, serum, plasma | IgG/IgM | 96.7% | 96.2% | 10 | — | https://www.abbexa.com |
| SARS-CoV-2 | Panbio SARS-CoV-2 Ag Rapid Test Device | LFI | Abbot | Nasopharyngeal swab | Antigen | 98.1% | 99.8% | 15 | CE European Mark | https://www.globalpointofcare.abbott |
| SARS-CoV-2 | Accu-Tell SARS-CoV-2 IgG/IgM Rapid Test Cassette | LFI | Accu Biotech | Whole blood, serum, plasma | IgG/IgM | 10 | CE European Mark | https://www.accubiotech.com | ||
| SARS-CoV-2 | SARS-CoV-2 IgG/IgM LF | LFI | AdvaGen Biotech | Whole blood, serum, plasma | IgG/IgM | 93.6% | 100% | 15 | — | http://www.advagen.com.br |
| SARS-CoV-2 | RapCov Rapid SARS-CoV-2 Test | LFI | Advaite | Whole blood | IgG/IgM | 90% | 95.2% | 15 | EUA | https://rapcov.com/ |
| SARS-CoV-2 | Assure SARS-CoV-2 IgG/IgM Rapid Test Device | LFI | Assure Tech | Whole blood, serum, plasma | IgG/IgM | 100% | 98.8% | 15 | EUA | http://www.assuretech.com.cn |
| SARS-CoV-2 | SARS-CoV-2 Test Kit | LFI | AccuQuick Tests | Whole blood, serum, plasma | IgG/IgM | 99% | 99% | 10 | CE, EUA | https://www.accuquiktestkits.com |
| SARS-CoV-2 | SARS-COV-2 IgM/IgG Rapid Test Kit | LFI | Aurora | Whole blood, serum, plasma | IgG/IgM | 93.3% | 95% | 15 | CE European Mark | https://www.aurorabiomed.com |
| SARS-CoV-2 | Autobio Anti-SARS-CoV-2 Rapid Test | LFI | Autobio Diagnostics | Serum, plasma | IgG/IgM | 93.3% | 98.8% | 15 | EUA | http://www.autobio.com.cn |
| SARS-CoV-2 | SARS-CoV-2 IgG/IgM Point of Care Rapid Test | LFI | Aytu Biosciences/Orient Gene Biotech | Whole blood, serum, plasma | IgG/IgM | 100% | 100% | 10 | EUA | https://aytubio.com |
| SARS-CoV-2 | BD Veritor System for Rapid Detection of Sars-Cov-2 | LFI | Becton, Dickinson, and Company (BD) | Nasal swabs | Antigen | 84% | 100% | 15 | EUA | https://www.bd.com |
| SARS-CoV-2 | OnSite SARS-CoV-2 IgG/IgM Rapid Test | LFI | Bio Advanced Diagnosticos | Whole blood, serum, plasma | IgG/IgM | 96.7% | 78.1% | 10–15 | — | https://www.bioadvancediag.com.br |
| SARS-CoV-2 | 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold) | LFI | Biolidics | Whole blood, serum, plasma | IgG/IgM | 96.7% | 100% | 10 | CE European Mark | https://www.biolidics.com |
| SARS-CoV-2 | SARS-CoV-2 IgG/IgM Rapid Test | LFI | BioMedomics | Whole blood, serum, plasma | IgG/IgM | 99% | 100% | 10–15 | https://www.biomedomics.com | |
| SARS-CoV-2 | Wantai Sars-CoV-2 Ab Rapid Test | LFI | Beijing Wantai Biological Pharmacy Enterprise Co., Ltd | Whole blood, serum, plasma | IgG/IgM | 81.2% | 99.5% | 15 | EUA | http://www.ystwt.cn |
| SARS-CoV-2 | Sars-CoV-2 IgM/IgG Antibody test Kit | LFI | Biohit Healthcare (Hefei) Co., Ltd | Whole blood, serum, plasma | IgG/IgM | 100% | 99.1% | 15 | EUA | http://www.biohit.cn |
| SARS-CoV-2 | Sars-CoV-2 IgG/IgM Kit | LFI | BioTime | Whole blood, serum, plasma | IgG/IgM | 96.2% | 98.7% | 10 | — | http://www.biotime.cn |
| SARS-CoV-2 | qSars-CoV-2 IgG/IgM Rapid Test | LFI | Cellex, Inc. | Whole blood, serum, plasma | IgG/IgM | 94.6% | 99.5% | 15 | EUA | https://cellexcovid.com |
| SARS-CoV-2 | OnSite SARS-CoV-2 IgG/IgM Rapid Test | LFI | CTK Biotech, Inc. | Whole blood, serum, plasma | IgG/IgM | 97.1% | 97.8% | 15 | https://ctkbiotech.com/ | |
| SARS-CoV-2 | One-Step SARS-CoV-2 Test | LFI | Celer Biotechnologia | Whole blood, serum, plasma | IgG/IgM | — | — | 15 | — | https://celer.ind.br |
| SARS-CoV-2 | SARS-CoV-2 Ag Respi-Strip | LFI (dipstick) | Coris Bioconcept | Nasal mucus swabs | Viral antigen | 91.2% | 99.4% | 30 | — | https://www.corisbio.com/ |
| SARS-CoV-2 | DPP SARS-CoV-2 IgG/IgM System | LFI | Chembio Diagnostics | Whole blood, serum, plasma | IgG/IgM | — | — | 15 | The FDA | http://chembio.com |
| SARS-CoV-2 | Coronavirus Rapid Test | LFI | Diagnostica Industria e Comercio Ltd | Whole blood, serum, plasma | IgG/IgM | 86.4% | 99.6% | 15 | — | https://www.biocondiagnosticos.com.br |
| SARS-CoV-2 | 2019 nCoV IgG/IgM Rapid Test | LFI | Dynamiker | Whole blood, serum, plasma | IgG/IgM | >92% | — | 10 | — | http://en.dynamiker.com |
| SARS-CoV-2 | Coronavirus IgG/IgM (SARS-CoV-2) | LFI | Ebram Produtos Laboratoriais | Whole blood, serum, plasma | IgG/IgM | 93.1% | 95.4% | 15–20 | — | http://ebram.com |
| SARS-CoV-2 | SARS-CoV-2 IgG/IgM ECO test | LFI | Eco Diagnostica Ltd | Whole blood, serum, plasma | IgG/IgM | 100% | 97% | 15 | — | http://ecodiagnostica.com.br |
| SARS-CoV-2 | SARS-CoV-2 Ag ECO Test | LFI | Eco Diagnostica Ltd | Nasopharyngeal or oropharyngeal swab | SARS-CoV-2 antigen | 96.5% | >99.9% | 2–15 | — | http://ecodiagnostica.com.br |
| SARS-CoV-2 | VivaDiag SARS-CoV-2 IgG/IgM Rapid Test | LFI | Everest Links Pte Ltd | Whole blood, serum, plasma | IgG/IgM | 82.9% | 100% | 15 | — | http://everestlinks.com |
| SARS-CoV-2 | FIA SARS-CoV-2 IgM/IgG | LFI | GenBody | Whole blood, serum, plasma | IgG/IgM | 91.7% | 97.5% | 10 | — | http://www.genbody.co.kr |
| SARS-CoV-2 | RightSign SARS-CoV-2 IgG/IgM Rapid Test Cassette | LFI | Hangzhou Biotest Biotech | Whole blood, serum, plasma | IgG/IgM | 93.3% | 100% | 10 | EUA | https://biotestsolutions.com |
| SARS-CoV-2 | LYHER Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Combo Test Kit | LFI | Hangzhou Laihe Biotech | Whole blood, serum, plasma | IgG/IgM | 100% | 98.8% | 15 | EUA | http://www.lyher.com |
| SARS-CoV-2 | SARS-COV-2 IgG/IgM Rapid Test Cassette | LFI | Healgen Scientific | Whole blood, serum, plasma | IgG/IgM | 96.7% | 100% | 10 | EUA | https://www.healgen.com |
| SARS-CoV-2 | 2019-nCoV Ab Test (Colloidal Gold) | LFI | Innovita Biological Technology Co. Ltd | Whole blood, serum, plasma | IgG/IgM | 93.3% | 98.8% | 15 | — | http://www.innovita.com.cn |
| SARS-CoV-2 | Anti SARS-CoV-2 IgG/IgM Rapid Test | LFI | Labtest Diagnostica S.A. | Whole blood, serum, plasma | IgG/IgM | 85% | 98.3% | 15 | — | https://labtest.com.br |
| SARS-CoV-2 | StrongStep® SARS-COV-2 IgG/IgM Combo Test | LFI | Liming Bio | Whole blood, serum, plasma | IgG/IgM | — | — | 15 | — | http://www.limingbio.com |
| SARS-CoV-2 | Rapid Test SARS-CoV-2 | LFI | Livzon Diagnostics | Whole blood, serum, plasma | IgG/IgM | 90.6% | 99.2% | 15 | — | https://livzon-COVID-19test.com/ |
| SARS-CoV-2 | Coronavirus Disease 2019 Antibody (IgMIgG) Combined Test Kit | LFI | Medical Systems Biotechnology | Whole blood, serum, plasma | IgG/IgM | — | — | 5–10 | — | http://en.nbmedicalsystem.com |
| SARS-CoV-2 | Megna SARS-CoV-2 Antibody Test Kit | LFI | Megna Health | Whole blood, serum, plasma | IgG/IgM | 100% | 95% | 15 | EUA | https://www.megnahealth.com |
| SARS-CoV-2 | SARS-COV-2 rapid diagnostic test | LFI | Mologic | Whole blood, serum, plasma | IgA/IgG/IgM | — | — | — | CE European Mark | http://www.mologic.co.uk |
| SARS-CoV-2 | 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold-Based) | LFI | Nanjing Vazyme Medical Technology Co. Ltd | Whole blood, serum, plasma | IgG/IgM | — | — | 10 | — | http://www.vazymebiotech.com |
| SARS-CoV-2 | 2019 COVID Antibody IGG IGM Test Cassette (Colloidal Gold) | LFI | Nantong Diagnose Biotechnology | Whole blood, serum, plasma | IgG/IgM | — | — | — | — | http://www.diagnosbio.com/en/ |
| SARS-CoV-2 | Rapid Test for SARS-COV-2 IgM/IgG Antibody Detection Kit | LFI | Nirmidas Biotech, Inc. | Whole blood, serum, plasma | IgG/IgM | 96.7% | 97.5% | 20 | — | https://www.nirmidas.com |
| SARS-CoV-2 | 2019-nCoV IgG/IgM Rapid Test | LFI | Phamatech | Whole blood, serum, plasma | IgG/IgM | — | — | — | — | https://www.phamatech.com |
| SARS-CoV-2 | SARS-COV-2 IgG/IgM Rapid Test Cassette | LFI | Premier Biotech | Whole blood, serum, plasma | IgG/IgM | 100% | 93% | 10 | EUA | https://premierbiotech.com |
| SARS-CoV-2 | Sofia SARS Antigen FIA | LFI AFI | Quidel | Direct nasal/nasopharyngeal swabs | Antigen | 96.7% | 100% | 15 | EUA | https://www.quidel.com |
| SARS-CoV-2 | SARS-CoV-2 IgM/IgG Ab Rapid Test | LFI | Qingdao Hightop Biotech Co. Ltd | Whole blood, serum, plasma | IgG/IgM | 93% | 97.5% | — | — | http://www.hightopqd.com |
| SARS-CoV-2 | Coronavirus (SARS-COV-2) IgM/IgG Rapid Test Kit | LFI | Ray Biotech | Whole blood, serum, plasma | IgG/IgM | 90.4% | 98.3% | 5–10 | — | https://www.raybiotech.com |
| SARS-CoV-2 | Salocor SARS-CoV-2 (Sars-CoV-2) IgG/IgM Rapid Test Kit | LFI | Salofa Oy | Whole blood, serum, plasma | IgG/IgM | 94.9% | 99.2% | — | EUA | https://www.salofa.com/en/ |
| SARS-CoV-2 | Sars-CoV-2 Antibody Test Strip | LFI (colloidal gold) | Sannuo Biotech (Sinocare) | Whole blood, serum, plasma | IgG/IgM | — | — | — | — | http://www.sinocare.com |
| SARS-CoV-2 | STANDARD Q SARS-COV-2 Ag | LFI | SD Biosensor | Nasopharyngeal swab | Viral antigen | 87.9% | 98.9% | 15–30 | — | http://sdbiosensor.com |
| SARS-CoV-2 | STANDARD Q SARS-COV-2 IgM/IgG Combo | LFI | SD Biosensor | Whole blood, serum, plasma | IgG/IgM | 94.5% | 69.1% | 10–15 | — | http://sdbiosensor.com |
| SARS-CoV-2 | SARS-CoV-2 Rapid IgG/IgM combined Antibody assay Pre-screening test Kit | LFI | Sensing Self | Whole blood, serum, plasma | IgG/IgM | 99.3% | 100% | 10 | — | https://sensingself.me |
| SARS-CoV-2 | Bioeasy 2019-nCoV IgG/IgM Ab GICA Rapid Test (Colloidal Gold) | LFI | Shenzhen Bioeasy Biotechnology Co. Ltd | Whole blood, serum, plasma | IgG/IgM | — | — | 10–15 | — | http://en.bioeasy.com.tr |
| SARS-CoV-2 | Rapid Sars-CoV-2 antigen detection test | LFI | Sona Nanotech | Whole blood, serum, plasma | S1 domain of spike protein | 84.6% | 90% | 15 | — | https://sonanano.com |
| SARS-CoV-2 | SGTI-flex SARS-CoV-2 IgM/IgG | LFI | Sugentech | Whole blood, serum, plasma | IgG/IgM | 90.2% | 100% | 10–15 | — | http://sugentech.com |
| SARS-CoV-2 | Sars-CoV-2 IgM/IgG Antibody Rapid Test | LFI | Sure Biotech | Whole blood, serum, plasma | IgG/IgM | 92.5% | 99.2% | 20 | — | https://www.surebiotech.com |
| SARS-CoV-2 | VivaDiag SARS-CoV-2 IgG/IgM Rapid Test | LFI | VivaChek Biotech | Whole blood, serum, plasma | IgG/IgM | — | — | 15 | — | https://www.vivachek.com |
| SARS-CoV-2 | Sars-CoV-2 IgM/IgG Antibody Test Kit | LFI | Wuhan Easy Diagnosis Biomedicine Co., Ltd | Whole blood, serum, plasma | IgG/IgM | 99.3% | 96.2% | 15 | — | http://www.mdeasydiagnosis.com |
| SARS-CoV-2 | SARS-COV-2 IgG/IgM Rapid Test | LFI | Zhejiang Orient Gene Biotech Co. Ltd | Whole blood, serum, plasma | IgG/IgM | 86.7% | >99% | 15 | — | http://www.orientgene.com |
| SARS-CoV-2 | Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) | LFI | Zhuhai Livzon Diagnostics Inc. | Whole blood, serum, plasma | IgG/IgM | 90.6% | 99.2% | 15 | — | http://www.livzondiagnostics.com |
| SARS-CoV-2 | Lucira COVID-19 All-in-one Test Kit | RT-LAMP | Lucira Health | Nasal swabs | SARS-CoV-2 RNA | 98.4% | 100% | 30 | The FDA EUA | https://www.lucirahealth.com |
The ReEBOV antigen rapid test is a rapid chromatographic immunoassay intended for the qualitative detection of VP40 antigen from Zaire EV, Sudan EV, and Bundibugyo EV. This dipstick test has a sensitivity of 100%, with a specificity of 92%.50 For this rapid test, whole blood from a finger stick is used (1 drop, 30 μL), and results are observed after 15–25 min. The price of the ReEBOV kit is USD 10.51
Brangel and co-workers have developed a serological point-of-care test for the detection of IgG antibodies against EV.23 The novelty of this RDT device is the combination of the lateral-flow technique with mobile technology to obtain a quantitative result and the possibility to store or transmit data and tag the tested individuals geographically. Additionally, the device could simultaneously detect three related EV species: Sudan virus, Bundibugyo virus, and Zaire virus. Assay results are obtained within 15 minutes after the sample is added. The authors of this study reported a sensitivity and specificity of 100% compared with standard ELISA, and this work is an excellent example of the enormous potential of combining RDTs with mobile technology in disease outbreaks. Fig. 6 shows the developed lateral flow device and the smartphone application interface used to detect the intensity of the signal corresponding to the virus's presence and record the patient's details.
 | ||
| Fig. 6 Smartphone lateral-flow point-of-care test for Ebola virus IgG detection. Reproduced from ref. 23 with permission from ACS copyright 2018. | ||
6. Nipah virus (NiV)
Nipah virus is an emerging infectious disease that kills about 75% of those infected. Currently, there is no cure for this disease. NiV is a bat-borne pathogen; Pteropus fruit bats are considered the virus's natural reservoir.52 The bats are endemic to tropical and subtropical regions of Asia, East Africa, the Australian continent, and some oceanic islands.53 There are no FDA-approved drugs to treat or prevent NiV.54,55NiV was discovered in 1998 during the first reported outbreak in the Sungai Nipah village (Malaysia), where locals became infected through contact with pigs, which are the intermediate host of the virus. Since then, recurring NiV outbreaks have been reported in Bangladesh, India, Ghana, Thailand, Madagascar, Cambodia, and Singapore.56
NiV is an enveloped pleomorphic virus belonging to the genus Henipavirus of the family Paramyxoviridae. The virus structure consists of a non-segmented negative-sense single-stranded RNA, approximately 18.2 kb long, encoding six structural proteins: nucleocapsid (N), phosphoprotein (P), matrix protein (M), fusion protein (F), glycoprotein (G), and RNA polymerase (L), cf., Fig. 7. The F and G proteins of NiV are necessary for attachment and entry into the host cell.53,57
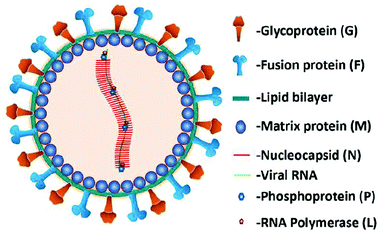 | ||
| Fig. 7 Representation of the Nipah virus structure. Reproduced from ref. 53 with permission from MDPI copyright 2020. | ||
6.1 Symptoms
NiV infection causes febrile encephalitis or pneumonia at the early stage, making it difficult to distinguish from other febrile diseases. Other clinical features of NiV are headache, vomiting, behavioral changes, disorientation, and low levels of consciousness. Incubation periods have been reported to range from 4 to 14 days. Around 20% of encephalitis survivors sustained neurological dysfunction, including persistent seizures, fatigue, and behavioral abnormalities.586.2 Diagnostic testing
The tests for detecting NiV include molecular assays, immunochemistry, histopathology, and serological tests.59 Techniques such as PCR, next-generation sequencing, immunochemistry, and ELISA are among the most used for NiV detection. The type of samples collected for NiV detection in humans include throat and nasal swabs, urine, blood, and cerebrospinal fluid. All testing techniques are laboratory-based; there is no commercially available rapid test for the detection of NiV in humans or animals.606.3 Rapid test for the detection of NiV
In 2014, a lateral-flow assay for the rapid detection of NiV in swine was reported by the Canadian Science Centre for Human and Animal Health.61 The developed rapid test was compared with PCR, and it was concluded that the lateral-flow assay was as sensitive as real-time PCR. Additionally, it was possible to detect viral protein as early as one-day post-inoculation in nasal swabs, and no cross-reaction with Hendra virus and other viruses, e.g., Ebola virus, was observed. This RDT is, however, not commercially available.7. Rift Valley fever (RVF)
Rift Valley fever is a viral zoonosis that affects animals, but can also infect humans. Approximately 1% of humans infected with RVF die from the disease.62 It primarily infects sheep, cattle, and goats and is maintained in nature by transovarial transmission in floodwater Aedes mosquitoes.63 The virus was identified for the first time on a farm in the Rift Valley of Kenya in 1931. Since then, outbreaks have been reported in Niger, Mauritania, Madagascar, Sudan, Kenya, Somalia, Tanzania, Egypt, Yemen, Saudi Arabia, and South Africa.64The trade of animals between endemic areas and Mediterranean European countries actively contributes to viral transmission, thus increasing the risk of contagion in the Mediterranean basin and Europe.65 Moreover, the virus's ability to survive in a range of bioclimatic environments has raised concerns about its potential introduction into Europe and North American areas.66
RVF is a mosquito-borne viral disease from the genera Aedes and Culex. These mosquito species are associated with freshly flooded temporary water bodies and are regarded as maintenance vectors in the Aedes species and as epidemic or amplifying vectors in the case of the Culex species.67 Animals become infected through a bite from an infected mosquito. In the case of humans, they become infected via direct and aerosol exposure to infected animals or animal tissue and when bitten by infected mosquitoes. No human-to-human transmission of RVFV has been documented.
The RVFV genome is composed of three segments of single-stranded RNA: large (L), medium (M), and small (S). The viral RNA-dependent RNA polymerase (L protein) is encoded in the L segment. Four proteins are encoded in the M segment: the structural glycoproteins Gn and Gc, the non-structural protein Nsm, and a large 78 kDa glycoprotein (LGp). The structural glycoproteins mediate binding and entry to the target cell membrane via receptors. The nucleoprotein (N) and the non-structural NS protein, a significant determinant of virulence, are encoded in the S segment.68
7.1 Symptoms
In humans, infection with RVFV is generally asymptomatic in 98% of cases. Only 2% of cases are severe, presenting three different complications: hemorrhagic fever, ocular disease (retinal vasculitis), and meningoencephalitis. Other symptoms corresponding to a mild infection form are flu-like fever, headache, joint pain, sensitivity to light, neck stiffness, vomiting, and appetite loss.69Individuals with clinical symptoms present short febrile illness and no long-term sequelae. During the initial phase of the disease, 1–6 days after infection, antibody levels rise while viremia declines. Thrombosis and other vascular complications can manifest days to weeks after the initial infection.65
7.2 Diagnostic testing
RVFV is detected using molecular assay techniques, namely, reverse transcriptase-polymerase chain reaction (RT-PCR) assay, loop-amplification mediated polymerase chain reaction (LAMP), ELISA, antigen detection tests, and virus isolation by cell culture. RT-PCR and LAMP are the primary assays used for detecting acute infections.70,71 For the detection of antibodies in several species, mainly ELISA platforms are used.72–747.3 Rapid test for the detection of RVFV
Only one device for the rapid detection of RVFV has been reported.75 The first-line lateral flow immunochromatographic strip test was developed to detect nucleoprotein (N) of the RVFV, cf. Fig. 8.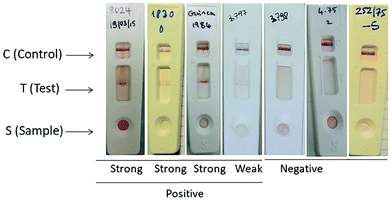 | ||
| Fig. 8 RVF lateral flow test strips for the detection of RVF infection. Reproduced from ref. 75 with permission from PLOS copyright 2019. | ||
The fabricated device showed lower values of specificity and sensitivity compared with molecular-based techniques (PCR), but was, according to the authors of the study, adequate for the specific rapid initial detection of RVF outbreaks. This RDT is obtainable only for research purposes and is not commercially available.
8. Lassa fever virus (LFV)
Lassa fever is a zoonotic disease caused by the Lassa fever virus, a level IV pathogen. The virus’ reservoir is a rodent of the genus Mastomys, known as a multimammate rat.76 It is transmitted to humans by the ingestion of contaminated food or by contact with household items contaminated with rat urine and feces. Human-to-human transmission occurs through direct contact with blood, organs, or other body fluids from infected individuals. Rodent excretion aerosolized during cleaning activities could result in airborne transmission of the virus.77The first case of Lassa fever virus was detected in 1950 in Sierra Leone. Lassa virus belongs to the Arenaviridae family, which is responsible for causing viral hemorrhagic fever in African and South American countries.78 The Lassa virus measures between 70 and 150 nm in diameter, has a spherical shape, and belongs to the Arenaviridae family, cf. Fig. 9. The Lassa virus possesses a glycoprotein envelope with T-shaped spikes measuring 10 nm on its surface. The Lassa virus's genome is a single-stranded, bisegmented RNA consisting of a small (S) and a large (L) RNA fragment of 3.4 and 7 kilobases.77
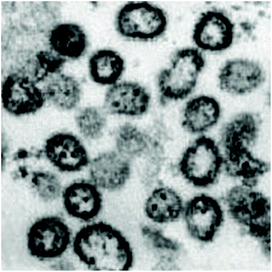 | ||
| Fig. 9 Lassa virus electron micrograph reproduced from ref. 77 with permission from Elsevier copyright 2014. | ||
Lassa fever is endemic in West Africa, where a population of 58 million is at risk. However, imported cases have appeared in Germany, Sweden, the USA, Japan, the Netherlands, and the UK. Every year, approximately 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 300
000 to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of Lassa fever are reported in West Africa, with an estimated death toll of 5000 deaths per year.79
000 cases of Lassa fever are reported in West Africa, with an estimated death toll of 5000 deaths per year.79
8.1 Symptoms
LASV hinders the development of immune cells that are crucial to the immune response and that provide resistance to infection. The severity of LASV is correlated with high viremia levels, with peaks between 4 and 9 days after the onset of the disease. The initial diagnostic of LASV can be challenging, since the symptoms can mimic other endemic diseases, such as typhoid fever and malaria.80Around 30% of individuals infected with Lassa fever present visible bleeding, which could discriminate LASV from other febrile illnesses. Facial swelling, low blood pressure, hepatitis, fluid in the lung cavity, and petechiae or bruising indicate severe infection.81 Deaths due to LASV typically occur within 14 days of onset for 15–20% of severe cases. In the case of non-fatal cases, the patient's condition improves after 1–3 weeks. However, fatigue and renal damage may persist for several more weeks.82
8.2 Diagnostic testing
The current LASV diagnostic test includes laboratory-based serological and nucleic acid amplification tests. However, the majority of commercial assays for PCR and serology are indicated for research use only. Additionally, endemic regions do not always have the financial resources and personnel to utilize these available diagnostics tests.83Immunofluorescent assay tests, western blotting, and ELISA are techniques used for the indirect detection of antibodies and antigens. LASV RNA can be detected using nucleic acid amplification, including techniques such as loop-mediated isothermal amplification (LAMP), strand displacement assays, and PCR. Virus isolation, PCR, and LASV antigen positivity are used to diagnose active infections.84
8.3 Rapid test for the detection of LASV
Apart from the lab-based test, there are two commercially available kits for the rapid detection of LASV on the market: the ReLASV® Pan-Lassa Antigen Rapid Test and the ReLASV® Antigen Rapid Test.85 However, they are labeled for research use only. These rapid detection tests are lateral flow-based dipsticks, cf.—Fig. 10. When applying a drop of whole blood, the nucleoprotein antigen is detected with 91% sensitivity and 86% specificity for LASV lineage IV (Josiah strain). The performance of this test at temperatures between 18 and 30 °C is comparable to molecular tests such as RT-PCR and qPCR.86,87 However, data for the rapid test performance at high temperatures above 30 °C and high humidity are required to better indicate the test's performance in endemic areas. The test cost is around USD 30, with a turnaround time of <30 min.85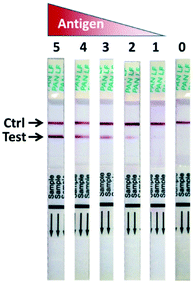 | ||
| Fig. 10 Signal development of the ReLASV Pan-Lassa antigen rapid diagnostic test. Reproduced from ref. 87 with permission from Springer Nature copyright 2018. | ||
9. Zika virus (ZV)
Zika virus is an enveloped, positive-sense, single-stranded RNA virus member of the Flaviviridae family that is transmitted to humans by mosquitoes from the Aedes species. It was discovered in 1947 in the Zika forest (Uganda).88 Since then, several outbreaks of ZV have been reported in various countries in Africa, Asia, and South and Central America. One of the significant ZV outbreaks started in 2015 in Brazil and extended to most countries in the Caribbean, Central, and South American areas. Following this significant outbreak, ZV infection among travelers was reported in European countries, including the UK, Austria, Denmark, France, Finland, Italy, Ireland, Portugal, Germany, Sweden, the Netherlands, Switzerland, and Spain.89Before the large outbreaks in 2014 and 2015, research into ZK pathogenesis, detection, and treatment was neglected due to the mild symptoms and the high percentage of asymptomatic individuals.90
The primary way of ZV transmission is through mosquito bites. However, additional transfer modes have been identified, such as materno-fetal by trans-placental transmission or during delivery when the mother is infected, sexual transmission, or through blood transfusion and organ transplantation,91cf. Fig. 11.
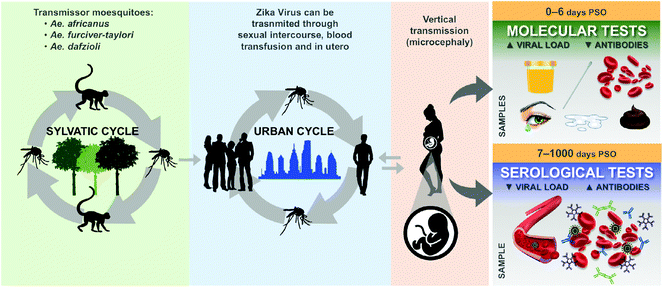 | ||
| Fig. 11 The life cycle of the Zika virus and biological materials used to detect the disease. Reproduced from ref. 98 with permission from John Wiley & Sons copyright 2020. | ||
9.1 Symptoms
Due to serologic cross-reactivity to other flaviviruses, such as the dengue virus, ZV diagnosis has been challenging.88 The incubation period for ZV ranges between 4 and 12 days after infection, followed by a period of up to sixteen days, during which symptoms such as mild fever, skin rash, non-purulent conjunctivitis, headache, arthralgia, and myalgia may appear, cf. Fig. 12.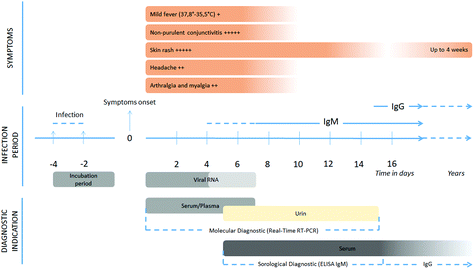 | ||
| Fig. 12 Chronology of viral replication, relationship with clinical and biological symptoms, and the possibility of the detection of Zika virus by laboratory techniques. Reproduced from ref. 98 with permission from John Wiley & Sons copyright 2020. | ||
If a woman is infected during pregnancy, the fetus has a high risk of suffering congenital central nervous malformations and microcephaly. In some cases, fetal losses have been reported during Zika outbreaks.92
9.2 Diagnostic testing
The irruption of ZK in South and North America and Europe has accelerated the development of assays to detect infected individuals.89,93 For instance, after the 2015 outbreak, the FDA granted emergency use authorization to 14 molecular and five serological assays to detect ZV.93One of the primary molecular assays used for ZV detection is real-time reverse transcriptase PCR (rRT-PCR), which targets the ZV E gene in serum, whole blood, urine, cerebrospinal fluid, and amniotic fluid.94 Another molecular assay used for ZV detection is transcription-mediated amplification (TMA). This assay singles out the ZV NS1 and NS4/NS5 genes in serum and urine.95
ELISA has been used for the colorimetric detection of ZV and its differentiation from the dengue virus. This qualitative test detects the presence of ZV IgM antibodies in human sera with a sensitivity of >90%, a specificity of >96%, and a turnaround time of four hours.96
9.3 Rapid test for the detection of ZV
Several RDTs for ZV detection are currently on the market.97,98 They are mainly immunochromatographic assays for the detection of IgG, IgM antibodies, or NS1 protein.99 Chembio Diagnostic Systems developed a lateral flow assay, DPP® Zika IgM System, read by an automated reader,100cf. Fig. 13. This RDT is the only immunochromatographic assay that is FDA Emergency Use Authorization (EUA) approved. According to the manufacturer, the RDT can be used to detect IgM and IgG antibodies to ZV in whole blood, serum, or plasma, and the results are displayed after 15 minutes. The RDT requires only 10 μL of sample to provide a semi-quantitative answer when combined with the reader. Each kit's price is around USD 35/card, while the reader's price is around USD 754.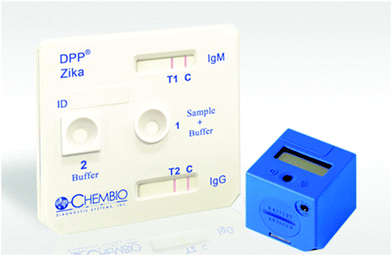 | ||
| Fig. 13 DPP Zika IgM system developed by Chembio for Zika virus detection (image from http://www.chembio.com). | ||
Apart from the DPP Zika RDT, there are other immunochromatographic rapid assays available on the market, some of which are listed below:
• Artron One Step Zika Virus Test Kit, Artron Laboratories Inc (Canada),101
• Accu-Tell Zika Virus IgG/IgM AB Cassette, AccuBioTech (China),102
• Zika NS1 Test Card, Zika IgG/IgM & NS1 Duo Test, LumiQuick Diagnostics Inc. (USA),103
• CDIA Zika IgG/IgM Rapid test Kit, Creative Diagnostics (USA),104
• Zika Rapid Test, Maternova (USA),105
• Zika NS1, Zika IgG/IgM, Zika Combo, Biopanda Reagents (UK),106
• ProDetect Zika IgG/IgM Rapid Test, Medical Innovation Ventures (Malaysia),107
• Zika IgG//IgM Virus Antigen Rapid Test, Nectar Lifesciences Ltd (India).108
Apart from these commercially available RDTs for ZV detection, other devices based on electrochemistry and lateral flow have been reported in the literature, but are only for research purposes.99,109–112
10. Middle East respiratory syndrome coronavirus (MERS-CoV)
MERS-CoV is a lineage C beta coronavirus that was first identified in June 2012 in Saudi Arabia. Since then, several outbreaks have been reported in 27 countries in Asia, Africa, and Europe, with mortality of around 34.4%.113–115 MERS-CoV's genotype is closely related to bat coronaviruses, such as BtCoV-HKU4 and BtCoV-HKU5, but its evolutionary pathway is still not fully understood. MERS-CoV exists as enveloped extracellular particles with protruding spike (S) proteins.116 MERS-CoV targets the cellular receptor using its spike glycoprotein (S), a putative receptor-binding domain.117 The MERS-CoV infection results in profound apoptosis of infected human bronchial epithelial Calu-3 cells within 24 hours.118Several studies link MERS-CoV to dromedary camels.113 Additional studies have indicated potential cross-infection between dromedary camels and humans due to close contact. Compared with other coronaviruses, MERS-CoV has a lower basic reproduction number (R0); this is the average number of infections caused by one infected individual in a fully susceptible population. MERS-CoV R0 is around 1.0 throughout various clusters and outbreaks.119 Transmission between humans occurs through close and unprotected human contact and nosocomial transmission, mainly at healthcare locations and households.71,120–122
10.1 Symptoms
The incubation period for MERS is approximately five days. Individuals infected with MERS-CoV will present chills, fever, shortness of breath, cough, generalized myalgia, nausea, vomiting, and diarrhea. These symptoms are similar to other respiratory diseases, such as SARS. Due to the absence of specific laboratory abnormalities or clinical data to differentiate MERS-CoV pneumonia from pneumonia caused by other viruses, real-time PCR is used to identify the virus from respiratory secretions.117Older age (>60 years), as well as a medical history of chronic lung disease, diabetes, cancer, or renal disease, are considered risk factors that could aggravate the condition of MERS patients, resulting in respiratory failure and the need for mechanical ventilation and support in intensive care units.123
10.2 Diagnostic testing
As mentioned before, the primary method to identify and differentiate MERS-CoV from other respiratory syndromes is real-time PCR using samples from the lower respiratory tract, namely, sputum, tracheal aspirates, or bronchoalveolar lavage.124ELISA, indirect immunofluorescence, and neutralization tests are serological tests used for MERS-CoV detection.125–127 However, these tests require specialized equipment and trained personnel.
10.3 Rapid test for the detection of MERS-CoV
There are no commercially available RDTs for the detection of MERS-CoV in humans. Chen and co-workers developed an immunochromatographic RDT for MERS-CoV detection in human samples for research purposes. Their device showed high specificity, but moderate sensitivity, 81%, with a detection limit that was 25–100 times less sensitive than ELISA.12811. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Coronavirus disease-2019 (COVID-19) is a respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This disease was first reported in December 2019 in Wuhan (China), where it caused an outbreak of atypical pneumonia.129 Four months later, on March 11, 2020, a global pandemic was declared by the WHO.130 As of March 16, 2021, COVID-19 had infected more than 120 million individuals worldwide with a death toll of approximately 2.6 million deaths reported to the WHO.131,132 Additionally, the COVID-19 outbreak has caused a global economic crisis due to the almost complete paralysis of all primary industries, activities, travel, and goods exchange.SARS-CoV-2 is an enveloped positive-sense RNA virus. Its genome comprises around 30 kilobases. It belongs to the same Coronaviridae family as SARS-CoV and the Middle East respiratory syndrome virus (MERS). SARS-Cov2 enters into target cells by using the spike (S) protein of coronaviruses. Entry depends on binding of the surface unit, S1, to a cellular receptor, facilitating viral attachment to target cells,133cf. Fig. 14. After membrane fusion and endocytosis, the viral nucleocapsid releases the genome payload into the infected cell's cytoplasm. Inside the infected cell, the virus forms numerous double-membrane vesicles that protect the viral genome, thus allowing its replication. After this process, the viral genomes are translated into viral polyproteins, which are then cleaved into viral proteins. Immediately after this, viral particles and virions assemble in the endoplasmic reticulum/Golgi compartment and are transported to the cell surface from which they are released via exocytosis and they continue to infect other cells.134
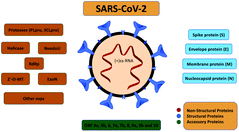 | ||
| Fig. 14 SARS-CoV-2 encoded proteins reproduced from ref. 134 with permission from ACS copyright 2020. | ||
It is unclear which animal was the intermediate host that transmitted the SARS-CoV-2 virus to humans, but bats and pangolin are among the main suspects. SARS-CoV-2 is highly contagious and can be transmitted from human-to-human by contact with saliva, tissue from infected individuals, and contaminated surfaces.
11.1 Symptoms
In most individuals infected with SARS-CoV-2, the disease manifests as a respiratory tract infection, with many different symptoms that are common to other infections, such as influenza. Due to this, a laboratory test is required to distinguish SARS-CoV-2 from other respiratory diseases. Among the reported symptoms are a cough, difficulty breathing, fever, sore throat, nasal congestion, headache, nausea or vomiting, muscle and joint pain, diarrhea, and a loss of taste and smell.135,136 Since SARS-CoV-2 is not fully understood, the list of symptoms is continuously being updated.11The majority of infected patients present mild symptoms and can recover at home without professional medical care. However, people with a medical history of diabetes, heart or lung disease, obesity, and older adults seem to be at higher risk of developing more severe complications. The symptoms may appear 2–14 days after exposure to the virus. The mortality rate for SARS-CoV-2 is around 1%.137
11.2 Diagnostic testing
Due to the current emergency with SARS-CoV-2, pharmaceutical companies and research labs worldwide are focusing on developing quick and effective tests.138,139 Commercially available tests for the detection of SARS-CoV-2 can be classified into two major groups: molecular assays and immunological and serological assays.Molecular assays focus on detecting viral RNA to identify infected individuals during the acute phase of infection. Around 90% of the available molecular assays use PCR or reverse transcription-PCR. The remaining 10% are based on isothermal amplification techniques, hybridization technologies, or CRISPR.140,141 One of the main advantages of these assays is the high sample throughput and, in some cases, an analysis time below 30 minutes.142 However, the tests are based on specialized equipment, skilled personnel, and high complexity labs.
Immunological and serological assays focus on detecting antibodies or antigenic proteins produced by individuals exposed to the virus. Immunological and serological RDTs are usually paper-based lateral-flow assays, providing the advantage of transporting samples and reagents by capillary action, making the device more straightforward, and maintaining a low-cost per device. These tests provide a qualitative answer within 10–20 minutes. Currently, there are more than 200 commercially available rapid lateral flow immunoassays (LFIs) for the detection of SARS-CoV-2, detecting primarily antibody response.143 From these 200 commercially available RDTs, only nine have received EUA by the US FDA.144
Tests based on the detection of the virus antigen are, due to low sensitivity, not recommended by the WHO.145 The sensitivity of antigen-based RDTs for respiratory diseases similar to SARS-CoV-2 varies from 34% to 80%. This variation in sensitivity means that more than half of infected individuals could be overlooked by such tests.146
Additionally, the efficacy of rapid LFIs for SARS-CoV-2 has been questioned in the media during the last months.8,9 The incorrect results are attributed to the lack of specificity and sensitivity of several LFIs on the market due to the use of antibodies that are not specific enough or low-quality materials for their fabrication. These reports highlight the need to develop more robust and efficient RDTs controlled and validated by governmental regulator bodies before becoming available on the market.
Recently, a molecular-based RDT for COVID-19 self-testing at home got EUA approval by the FDA.147 This RT-LAMP test kit developed by Lucira Health has a cost of approximately 50 USD and can detect SARS-CoV-2 RNA in nasal swabs in 30 minutes.148 The user gets a positive or negative answer indicated by illumination of a light in the test unit display shown in Fig. 15.
12. Existing challenges to develop robust rapid diagnostic tests for use during disease outbreaks
The current SARS-CoV-2 pandemic that affects the whole world has exposed the lack of available RDTs to detect high-priority pathogens during disease outbreaks. The number of available RDTs correlates with the geographic extension of disease outbreaks and their impact on developed countries’ populations. Currently, there are no commercially available RDTs for the detection of LFV, RVF, and NV. Outbreaks connected to these viruses have affected areas located in undeveloped countries, with only a few cases reported in Europe or North America. Meanwhile, the number of commercially available RDTs for detecting EV, ZV, and COVID-19 is much higher. These outbreaks have affected not only undeveloped countries, but have also led to a large number of cases in Europe and North America, in the case of EV and ZV, and throughout the whole world, in the case of COVID-19.Looking over the RDTs listed in Table 2, it is clear that most of these devices are based on a lateral-flow immunoassay platform. Lateral-flow immunoassay platforms are simple, low-cost, and provide a rapid response. Two types of immunoassays can be implemented in a lateral-flow platform. The first type is known as a sandwich assay, in which an antigen can be detected by the combination of an antibody pair, where the first antibody labeled with a colloidal gold nanoparticle binds to one antigen epitope, and a second capture antibody, immobilized on the lateral flow strip, binds to a second epitope on the same antigen. In this assay type, the antigen's concentration will be proportional to the signal intensity at the test line.
The second type of test is known as a competitive assay, and it is used for the detection of analytes using an antigen. As opposed to the sandwich assay detection, this test line's intensity is inversely proportional to the sample concentration in the competitive assay.
The fabrication of most LFIs involves combining several materials, e.g., paper membranes, Au nanoparticles, antibodies, and antigens. Despite the overwhelming number of scientific reports and discussion of how the integration of LFIs with novel materials with intrinsic properties, such as carbon nanotubes,149,150 III–V nanostructures,151,152 and graphene,153–155 will disrupt the diagnostic market, only a few materials and techniques have made the transition from the lab bench to commercialization for disease detection during outbreaks. More efficient interactions between researchers in fields like immunology, biochemistry, materials science, diagnostics, and regulating bodies are required to accelerate the development of robust RDTs that fulfill the criteria listed by the WHO for the ideal rapid detection device during disease outbreaks,13Fig. 16.
 | ||
| Fig. 16 Specifications for the ideal RDT for application during disease outbreak, according to criteria listed by the WHO. | ||
One of the challenges when a new pandemic or outbreak occurs is the lack of time to develop new robust assays to detect the new pathogen. Ideally, it should be easy and quick to change RDT technology to accommodate new pathogens, to be used instantly in an occurring outbreak. However, development often takes time, especially when it is an entirely new pathogen where no preexisting data are present.
During an outbreak, health authorities must collect data from testing, patient details, geography, etc. This is to estimate the magnitude of outbreaks, transmission routes, exposure, underlying diseases of importance for the development of the disease, risk behaviors, etc. Epidemiological analyses and data collection are essential for implementing the preventive measures needed during an epidemic outbreak. Results of RDTs should ideally be reported in a way that the health authorities can use data. This could be done by implementing electronic reporting in the RDT technology, e.g., by mobile phone data transfer.
Next, we discuss the areas that need further improvement to achieve more efficient RDTs for disease outbreaks.
12.1 Sensitivity
The majority of available RDTs for virus detection are based on the immobilization of antibodies against the specific virus on a surface acting as a transducer. The efficiency of binding between the antibody and the antigen will determine the assay's ultimate sensitivity. Three factors can influence this binding efficiency: the specificity of the antibody, the antibody's orientation, and the attachment of the antibody to the surface. These parameters influence the performance of RDTs based on LFIs, since they are critically dependent on the concentration of the analyte in a biological fluid. False-negative results from analyte concentrations below the LFI limit of detection are significant concerns.156These are critical factors that need to be considered when developing RDTs. Making available the structure of the virus or pathogen to be detected facilitates the development of antibodies. Recently, nanobodies have appeared as a promising novel class of therapeutic proteins based on single-domain antibody fragments containing the unique structural and functional properties of naturally occurring heavy-chain-only antibodies. These small structures are arousing increasing attention in immunosensing due to their higher binding specificity, greater thermal stability, and a lower tendency for aggregation than conventional antibodies.157,158
Once a specific antibody or nanobody against a particular target is developed, its attachment and correct orientation must ensure efficient binding with the target pathogen. Several methods are available with the aim of obtaining a covalent attachment between the capturing molecule and the surface, acting as a transducer.159,160 The available immobilization methods can be classified as covalent and non-covalent, depending on the coupling chemistry used. Both methods aim to assure the sensing molecule's attachment to the surface without affecting its binding activity and specificity.
Antibodies and nanobodies can adopt different orientations when deposited on a surface. This orientation will affect the binding capacity of these glycoproteins, since their binding sites could entirely or partially face the substrate, thus affecting the immunoassay's performance.161
During the current COVID-19 pandemic, the high number of false negatives when using LFIs for antigen detection has accelerated the development of a rapid molecular diagnostic test with a shorter turnaround time and higher sensitivity.162,163
Current efforts are focus on combining the high sensitivity of molecular diagnostic assays with the simplicity of lateral flow platforms. An example of this is the AmpliVue platform developed by Quidel.164 This platform combines a rapid nucleic acid amplification that does not require a thermal cycler with the advantages of a lateral-flow-based cassette, avoiding the need for an electronic reader. The AmpliVue platforms are used to detect Bordetella pertussis, Clostridium difficile, group B streptococcus, Herpes simplex virus type 1 (HSV-1), and Herpes simplex virus type 2 (HSV-2). The turnaround time of the AmpliVue platform for the detection of HSV-1 is around one hour.
Another attempt to combine lateral flow with genetic analysis was recently developed by Wang and co-workers.165 In their work, the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas system was integrated into a lateral flow assay platform prepared using a nitrocellulose membrane. Using this hybrid platform, it was possible to identify the African swine fever virus in a non-laboratory environment from swine serum samples within 40 minutes.
Using a similar approach, Broughton and co-workers reported developing a rapid (<60 min) CRISPR-Cas12-based lateral flow assay for the detection of SARS-CoV-2 from respiratory swab RNA extracts.166 The platform was validated using contrived reference samples and clinical samples from patients with COVID-19 infection and patients with other viral respiratory infections, obtaining 95% positive predictive agreement and 100% negative predictive agreement. This qualitative assay does not require bulky instrumentation and is currently waiting for EUA approval from the US FDA. Other examples of combining CRISPR with analytically portable and simple sensing platforms have recently been reviewed.167
12.2 Quantitative answer
As shown in Table 2, most commercially available RDTs are based on the immunochromatographic technique using a paper-based membrane to immobilize the sensing chemistry and transport samples and reagents. The response of these devices is colorimetric, i.e., there is a color change and an increase in the color intensity proportional to the sample concentration. Even though a couple of devices provide the option of transforming the change in color into a concentration value, most of the devices offer only a qualitative answer. This “yes” or “no” response might be insufficient or not sufficiently clear if the limit of detection is not clearly stated, i.e., at what concentration the color change will occur. For these situations, a quantitative detection method will provide a more precise and robust answer. A natural combination to provide a simple answer to this challenge could be to use the cameras on mobile phones with lateral-flow assay devices.168,169Additionally, the latest advances in the development of apps and algorithms offer the possibility of incorporating this software in any mobile phone to obtain a concentration value from a picture of the RDT reaction area.170 Moreover, the mobile phone will also add the option to detect and report additional data to health authorities, which is crucial from a surveillance point of view. These additional data are geographic location, temperature conditions, and time of the analysis, just to mention a few. Several publications reporting on the status of the combination of LFA and mobile phones are available,10,171–173 as well as the FDA-approved apps for the analysis of urine and wounds.174 Using these types of apps for the quantitative detection of RDTs during disease outbreaks will provide the health authorities with more complete and faster information to facilitate the implementation of measurements aiming to stop the spread of the disease.
12.3 Alternatives to lateral flow and colorimetric detection
As mentioned previously, paper-based devices offer several advantages in terms of cost and simplicity for the development of RDTs. Additionally, their fabrication is simple and could be integrated with, for instance, conductive materials (gold, graphene) for the manufacture of more advanced electrochemical LF devices.171,175 These systems have been reported in the literature to develop point-of-care biomedical devices.176 Despite the high number of existing articles, the amount of commercially available devices is still meager.Since the irruption of electrochemical biosensors, these devices have been put forward as an ideal option for developing POC systems due to their presenting the possibility of miniaturization, integration with sensing molecules (enzymes, antibodies, aptamers, cells), sensitivity and specificity, and simplicity.177 Still, after more than 20 years of research, only a handful of electrochemical biomedical sensors are commercially available and successful, of which the main one is the glucose biosensor based on an enzymatic reaction.178,179 Despite all the mentioned advantages offered by electrochemical sensors, none of the existing RDTs listed in Table 2 use electrochemistry as their detection technique. One of the reasons for this is that electrochemical sensors utilized in combination with immunochemistry are mainly based on impedance. This method requires the presence of a redox couple in a solution for the quantification of antigen concentration. Typically, a solution of ferro-/ferricyanide is used as the source for the redox couple that will be employed to quantify the amount of sample bound to the antibody.180 This means that, in practice, the user will need to add the sample, wait for the binding between the antigen and the antibody to occur, and then add the redox solution to quantify the presence of the analyte. This extra step makes this method complex and not user-friendly, limiting its use as a point-of-care device.181 Recently, the use of thiols containing a tethered redox molecule was suggested as an alternative to immunoassays based on electrochemical impedance.182,183 The obtained results for the detection of the dengue virus showed that this technique had great potential for facilitating the development of RDTs for biomedical applications.184,185
Recently, a hybrid approach combining electrochemistry detection with the trans-cleavage property of CRISPR, E-CRISPR, was presented by a multidisciplinary research team from Case Western Reserve University.186 Using the E-CRISPR platform, viral nucleic acids, such as papillomavirus 16 (HPV-16) and parvovirus B19 (PB-19), were detected with picomolar sensitivity within one hour. This is another excellent example of nucleic acid assays combined with miniaturized electrochemical platforms and used outside the lab.
Another recent development that could facilitate the use of electrochemical sensors in the development of RDTs is the miniaturization of a potentiostat that can be integrated into smartphones,187cf. Fig. 17a. The smartphone potentiostat allows the evaluation of electrochemical sensors based on amperometric, voltammetric, pulsed techniques, and electrochemical impedance, of which the results can be stored and evaluated using a corresponding app on a mobile phone. PalmSens has developed a miniaturized handheld wireless potentiostat that uses the USB port of a smartphone or tablet to communicate with and send data from an electrochemical sensor,188cf. Fig. 17b. This miniaturized potentiostat enables electrochemical analysis on-site with lower consumption of energy and with data transmission.
 | ||
| Fig. 17 (a) SenSit-smart miniaturized potentiostat connected to a mobile phone, and (b) Sensit-bt miniaturized wireless potentiostat developed by PalmSens (images from http://www.palmsens.com). | ||
13. Conclusions and perspectives
The current SARS-CoV-2 pandemic and previous SARS and MERS pandemics demonstrated the crucial role of rapid testing devices as an essential tool for health authorities to obtain rapid and precise information that could be used to adopt quick decisions aiming to stop the spread of disease. Simultaneously, it exposed the high dependence on lateral-flow devices and colorimetric assays as the primary technique to develop rapid test devices, as defined in the introduction. Even though more precise molecular-based assays are developing rapidly and more user-friendly sensing platforms are emerging, these methods are not yet at the level of simplicity, low-cost and rapid turnaround of lateral-flow-based assay rapid test devices. However, recently a molecular-based RDT device for SARS-COV-2 self-testing at home on nasal swabs received approval from the FDA. This RT-PCR self-testing device can detect SASR-CoV-2 in 30 minutes with 94% positive percent agreement and 98% negative percent agreement.148,189 This molecular-based RDT could be the first step towards developing more precise and straightforward RDTs that could hopefully be applied to detect other pathogens causing pandemics.As shown in Table 2, due to the worldwide extension of the current SARS-CoV-2 pandemic, the development of new RDTs for SARS-CoV-2 received a tremendous boost from government and private organizations. This vast support resulted in the fast development of hundreds of new RDT devices that effectively provided rapid data to decrease the virus's spread, as demonstrated in New Zealand and South Korea. However, the situation is not the same for most of the pathogens identified by the WHO as potential causes of future pandemics.
As discussed, it is necessary to accelerate the integration of more robust techniques with the lateral-flow assay platform's simplicity. Techniques such as electrochemistry, optical, nanoplasmonics, and molecular-based assays have already demonstrated promising results in sensitivity, selectivity, and reproducibility. The irruption of new micro- and nano-fabrication techniques would definitively accelerate integrating these highly sensitive assays with low-cost and simple platforms. It is vital to maintain the simplicity, low-cost, and robustness to facilitate the distribution and use of these new platforms worldwide.
Quick turnaround RDTs should be effectively combined with the advantages of existing mobile technology. Effective communication of data for disease surveillance will be achieved by standardizing the recording and reporting of results. Existing mobile technology provides the possibilities of capturing, recording, and transmitting data using a single device in resource-limited locations. The development of applications that standardize the way data extracted from RDTs are recorded and reported will contribute to a rapid exchange of information between health organizations worldwide. Moreover, the use of the global positioning system integrated into mobile phones will link geographical location data (time, temperature, humidity) to the assay results, allowing the status of disease spread to be precisely mapped and strategies adopted to contain the advance of a particular disease.
Research into RDTs should be supported and conducted to develop these new and smarter technologies. The research should also include the critical perspective that a given technology should have the ability to shift focus if new pathogens occur swiftly. This is essential for immediate implementation and response in an outbreak situation.
Another critical factor that should be highlighted is more effective coordination between government agencies in charge of evaluating and approving new RDTs used in disease surveillance. This collaboration will decrease the release of commercially available RDTs that do not provide complete and reliable information, which delays practical measurements to prevent the disease from spreading.
All the actions suggested in this review should be implemented as soon as possible through coordinated efforts involving research groups, health authorities, and funding bodies. Climate change and closer interaction between humans and animals in previously unexplored environments contribute to the emergence of new diseases, resulting in epidemics and pandemics due to increased human mobility and goods trade.
Author contributions
Jaime Castillo-León: conceptualization, investigation, writing original draft, writing & review. Ramona Trebbien: conceptualization, investigation, writing original draft, writing & review. John J. Castillo: investigation, writing original draft, writing & review. Winnie E. Svendsen: conceptualization, investigation, writing original draft, writing & review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Kaspar Heine Jessen Jürgensen for help with the illustrations.References
- D. M. Wagner, J. Klunk, M. Harbeck, A. Devault, N. Waglechner, J. W. Sahl, J. Enk, D. N. Birdsell, M. Kuch, C. Lumibao, D. Poinar, T. Pearson, M. Fourment, B. Golding, J. M. Riehm, D. J. D. Earn, S. DeWitte, J. M. Rouillard, G. Grupe, I. Wiechmann, J. B. Bliska, P. S. Keim, H. C. Scholz, E. C. Holmes and H. Poinar, Lancet Infect. Dis., 2014, 14, 319–326 CrossRef PubMed
.
- G. Vogel, Science, 2014, 345, 1549–1550 CrossRef CAS PubMed
.
- S. Haensch, R. Bianucci, M. Signoli, M. Rajerison, M. Schultz, S. Kacki, M. Vermunt, D. A. Weston, D. Hurst, M. Achtman, E. Carniel and B. Bramanti, PLoS Pathog., 2010, 6, e1001134 CrossRef PubMed
.
- S. A. Truelove, L. Mier-y-Teran-Romero, P. Gastanaduy, A. Taylor Walker, A. Berro, J. Lessler and M. Johansson, medRxiv, 2020, 10.1101/2020.05.08.20095414.
-
I. of M. (US) F. on M. Threats, S. Knobler, A. Mahmoud, S. Lemon and L. Pray, The Impact of Globalization on Infectious Disease Emergence and Control, National Academies Press, 2006 Search PubMed
.
-
J. Castillo-León, in Lab-on-a-Chip Devices and Micro-Total Analysis Systems, Springer International Publishing, 2015, pp. 1–15 Search PubMed
.
- M. D. Perkins, C. Dye, M. Balasegaram, C. Bréchot, J. V. Mombouli, J. A. Røttingen, M. Tanner and C. C. Boehme, Lancet, 2017, 390, 2211–2214 CrossRef
.
- Are We Failing the Coronavirus-Antibody Test? | The New Yorker, https://www.newyorker.com/news/daily-comment/are-we-failing-the-coronavirus-antibody-test, (accessed 14 July 2020).
- Mayo Clinic doctors find many COVID-19 antibody tests fail their quality standards: ABC News exclusive - ABC News, https://abcnews.go.com/US/mayo-clinic-doctors-find-covid-19-antibodytests/story?id=70803740, (accessed 14 July 2020).
- A. W. Martinez, S. T. Phillips, E. Carrilho, S. W. Thomas, H. Sindi and G. M. Whitesides, Anal. Chem., 2008, 80, 3699–3707 CrossRef CAS PubMed
.
- D. A. Drew, L. H. Nguyen, C. J. Steves, C. Menni, M. Freydin, T. Varsavsky, C. H. Sudre, M. J. Cardoso, S. Ourselin, J. Wolf, T. D. Spector and A. T. Chan, Science, 2020, 368, 1362–1367 CrossRef CAS PubMed
.
- Prioritizing diseases for research and development in emergency contexts, https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts, (accessed 25 August 2020).
- WHO | Simple/Rapid tests, https://www.who.int/diagnostics_laboratory/faq/simple_rapid_tests/en/, (accessed 6 July 2020).
-
L. Anfossi, C. Giovannoli and C. Baggiani, in Rapid Test - Advances in Design, Format and Diagnostic Applications, InTech, 2018 Search PubMed
.
- J. B. Lopez, D. Jackson, A. Gammie and T. Badrick, Clin. Biochem. Rev., 2017, 38, 3–11 Search PubMed
.
- S. McAlister, A. L. Barratt, K. J. Bell and F. McGain, Med. J. Aust., 2020, 212, 377–382 CrossRef PubMed
.
- K. Ni, Y. Hu, X. Ye, H. S. AlZubi, P. Goddard and M. Alkahtani, Energies, 2018, 11, 3105 CrossRef CAS
.
- T. Ozer, C. McMahon and C. S. Henry, Annu. Rev. Anal. Chem., 2020, 13, 85–109 CrossRef PubMed
.
- T. Ozer, B. J. Geiss and C. S. Henry, J. Electrochem. Soc., 2020, 167, 037523 CrossRef CAS PubMed
.
- X. Ding, M. G. Mauk, K. Yin, K. Kadimisetty and C. Liu, Anal. Chem., 2019, 91, 655–672 CrossRef CAS PubMed
.
- K. H. Foysal, S. E. Seo, M. J. Kim, O. S. Kwon and J. W. Chong, Sensors, 2019, 19, 4812 CrossRef CAS PubMed
.
- M. M. Gong and D. Sinton, Chem. Rev., 2017, 117, 8447–8480 CrossRef CAS PubMed
.
- P. Brangel, A. Sobarzo, C. Parolo, B. S. Miller, P. D. Howes, S. Gelkop, J. J. Lutwama, J. M. Dye, R. A. McKendry, L. Lobel and M. M. Stevens, ACS Nano, 2018, 12, 63–73 CrossRef CAS PubMed
.
- J. D. Newman and A. P. F. Turner, Biosens. Bioelectron., 2005, 20, 2435–2453 CrossRef CAS PubMed
.
- Y. Chen, R. Ren, H. Pu, X. Guo, J. Chang, G. Zhou, S. Mao, M. Kron and J. Chen, Sci. Rep., 2017, 7, 10974 CrossRef PubMed
.
- M. Soler, M. C. Estévez, M. Cardenosa-Rubio, A. Astua and L. M. Lechuga, ACS Sens., 2020, 5, 2663–2678 CrossRef CAS PubMed
.
-
W. E. Svendsen, M. Jørgensen, L. Andresen, K. B. Andersen, M. B. B. Larsen, S. Skov and M. Dimaki, in Procedia Engineering, Elsevier, 2011, vol. 25, pp. 288–291 Search PubMed
.
- A. Zulfiqar, F. Patou, A. Pfreundt, C. Papakonstantinopoulos, W. E. Svendsen and M. Dimaki, Sens. Bio-Sensing Res., 2017, 13, 88–95 CrossRef
.
- M. Park, R. S. Thwaites and P. J. M. Openshaw, Eur. J. Immunol., 2020, 50, 308–311 CrossRef CAS
.
- 2009 H1N1 Pandemic (H1N1pdm09 virus) | Pandemic Influenza (Flu) | CDC, https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html, (accessed 25 August 2020).
- M. G. Baker, N. Wilson and A. Anglemyer, N. Engl. J. Med., 2020, e56 CrossRef CAS PubMed
.
- Rapid COVID-19 testing breaks free from the lab - C&EN Digital Magazine, https://cendigitalmagazine.acs.org/2020/08/14/rapid-covid-19-testing-breaks-free-from-the-lab-3/content.html, (accessed 18 August 2020).
-
B. J. Tromberg, T. A. Schwetz, E. J. Pérez-Stable, R. J. Hodes, R. P. Woychik, R. A. Bright, R. L. Fleurence and F. S. Collins, Rapid Scaling Up of Covid-19 Diagnostic Testing in the United States-The NIH RADx Initiative, 2020 Search PubMed
.
-
R. Flick, in Crimean-Congo Hemorrhagic Fever: A Global Perspective, Springer Netherlands, 2007, pp. 35–44 Search PubMed
.
- H. Nasirian, Acta Trop., 2019, 196, 102–120 CrossRef PubMed
.
- V. N. Raabe, J. Clin. Microbiol., 2020, 58, e01580-19 CrossRef PubMed
.
- M. Zivcec, F. E. M. Scholte, C. F. Spiropoulou, J. R. Spengler and É. Bergeron, Viruses, 2016, 8, 106 CrossRef PubMed
.
- L. T. Mazzola and C. Kelly-Cirino, BMJ Glob. Health, 2019, 4, 1114 Search PubMed
.
- W. Wu, S. Zhang, J. Qu, Q. Zhang, C. Li, J. Li, C. Jin, M. Liang and D. Li, Virus Res., 2014, 187, 84–90 CrossRef CAS PubMed
.
- V. Baniasadi, M. H. Pouriayevali, T. Jalali, M. Fazlalipour, K. Azadmanesh and M. Salehi-Vaziri, J. Virol. Methods, 2019, 265, 49–52 CrossRef CAS PubMed
.
-
R. Reece, M. A. Smit and T. P. Flanigan, in Encyclopedia of Immunobiology, Elsevier Inc., 2016, vol. 4, pp. 355–362 Search PubMed
.
- Outbreaks | Ebola (Ebola Virus Disease) | CDC, https://www.cdc.gov/vhf/ebola/outbreaks/index-2018.html, (accessed 6 October 2020).
-
E. Rose, in Life-Threatening Rashes, Springer International Publishing, Cham, 2018, pp. 291–300 Search PubMed
.
- E. M. Leroy, A. Epelboin, V. Mondonge, X. Pourrut, J. P. Gonzalez, J. J. Muyembe-Tamfum and P. Formenty, Vector-Borne Zoonotic Dis., 2009, 9, 723–728 CrossRef PubMed
.
- E. M. Leroy, B. Kumulungui, X. Pourrut, P. Rouquet, A. Hassanin, P. Yaba, A. Délicat, J. T. Paweska, J. P. Gonzalez and R. Swanepoel, Nature, 2005, 438, 575–576 CrossRef CAS PubMed
.
- H. Feldmann and T. W. Geisbert, Lancet, 2011, 377, 849–862 CrossRef
.
- S. Su, G. Wong, X. Qiu, G. Kobinger, Y. Bi and J. Zhou, Lancet Infect. Dis., 2016, 16, 294–295 CrossRef PubMed
.
- First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response | FDA, https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health, (accessed 7 July 2020).
- OraQuick Ebola Rapid Antigen Test, kit/25, https://supply.unicef.org/s0003883.html, (accessed 7 July 2020).
- M. J. Broadhurst, J. D. Kelly, A. Miller, A. Semper, D. Bailey, E. Groppelli, A. Simpson, T. Brooks, S. Hula, W. Nyoni, A. B. Sankoh, S. Kanu, A. Jalloh, Q. Ton, N. Sarchet, P. George, M. D. Perkins, B. Wonderly, M. Murray and N. R. Pollock, Lancet, 2015, 386, 867–874 CrossRef
.
- ReEBOV Antigen Rapid Test Kit/50, https://supply.unicef.org/s0003750.html, (accessed 7 July 2020).
- M. Letko, S. N. Seifert, K. J. Olival, R. K. Plowright and V. J. Munster, Nat. Rev. Microbiol., 2020, 18, 461–471 CrossRef CAS PubMed
.
- V. Soman Pillai, G. Krishna and M. Valiya Veettil, Viruses, 2020, 12, 465 CrossRef PubMed
.
- R. Ojha, A. Pareek, R. K. Pandey, D. Prusty and V. K. Prajapati, ACS Omega, 2019, 4, 13069–13079 CrossRef CAS PubMed
.
- R. K. McLean and S. P. Graham, Front. Vet. Sci., 2019, 6, 16 CrossRef PubMed
.
- S. P. Luby, Antiviral Res., 2013, 100, 38–43 CrossRef CAS PubMed
.
- A. Tamin, B. H. Harcourt, T. G. Ksiazek, P. E. Rollin, W. J. Bellini and P. A. Rota, Virology, 2002, 296, 190–200 CrossRef CAS PubMed
.
- J. J. Sejvar, J. Hossain, S. K. Saha, E. S. Gurley, S. Banu, J. D. Hamadani, M. A. Faiz, F. M. Siddiqui, Q. D. Mohammad, A. H. Mollah, R. Uddin, R. Alam, R. Rahman, C. T. Tan, W. Bellini, P. Rota, R. F. Breiman and S. P. Luby, Ann. Neurol., 2007, 62, 235–242 CrossRef PubMed
.
- L. T. Mazzola and C. Kelly-Cirino, BMJ Glob. Health, 2019, 4, e001118 CrossRef PubMed
.
- Nipah virus, https://www.who.int/news-room/fact-sheets/detail/nipah-virus, (accessed 7 October 2020).
- M. Mingyi and L. Li, A novel pen A novel pen–side strip test side strip test for the detection of for the detection of Nipah Nipah virus in swine virus in swine, http://cahln-rctlsa.com/wp-content/uploads/2014/11/mingyi_li.pdf Search PubMed.
- Rift Valley Fever, https://www.cdc.gov/vhf/rvf/RVF-FactSheet.pdf, (accessed 9 October 2020).
-
Modern Infectious Disease Epidemiology - Concepts, Methods, Mathematical Models, and Public Health, ed. A. Krämer, Springer, 2010 Search PubMed
.
- R. B. Mandell and R. Flick, Hum. Vaccin., 2010, 6, 597–601 CrossRef PubMed
.
- V. Chevalier, Clin. Microbiol. Infect., 2013, 19, 705–708 CrossRef CAS PubMed
.
- B. Bazanow, D. Stygar, E. Romuk, B. Skrzep-Poloczek, J. Pacoń, Ł. Gadzała, M. Welz and J. Pawęska, J. Vector Borne Dis., 2018, 55, 324 CrossRef CAS PubMed
.
- J. Bingham and P. Jansen van Vuren, Microbiol. Aust., 2020, 41, 28 CrossRef
.
- J. Gonzalez-Valdivieso, B. Borrego, A. Girotti, S. Moreno, A. Brun, J. F. Bermejo-Martin and F. J. Arias, Mol. Pharm., 2020, 17, 1608–1620 CrossRef CAS PubMed
.
- K. L. Mansfield, A. C. Banyard, L. McElhinney, N. Johnson, D. L. Horton, L. M. Hernández-Triana and A. R. Fooks, Vaccine, 2015, 33, 5520–5531 CrossRef PubMed
.
- C. A. Le Roux, T. Kubo, A. A. Grobbelaar, P. J. Van Vuren, J. Weyer, L. H. Nel, R. Swanepoel, K. Morita and J. T. Paweska, J. Clin. Microbiol., 2009, 47, 645–651 CrossRef CAS PubMed
.
- C. Drosten, S. Göttig, S. Schilling, M. Asper, M. Panning, H. Schmitz and S. Günther, J. Clin. Microbiol., 2002, 40, 2323–2330 CrossRef CAS PubMed
.
- P. Jansen van Vuren and J. T. Paweska, J. Virol. Methods, 2009, 157, 15–24 CrossRef CAS PubMed
.
- J. T. Paweska, F. J. Burt, F. Anthony, S. J. Smith, A. A. Grobbelaar, J. E. Croft, T. G. Ksiazek and R. Swanepoel, J. Virol. Methods, 2003, 113, 103–112 CrossRef CAS PubMed
.
- B. Faburay, W. C. Wilson, A. Secka, B. Drolet, D. Scott McVey and J. A. Richt, J. Clin. Microbiol., 2019, 57, e01058-19 CrossRef PubMed
.
- C. Cêtre-Sossah, A. Pédarrieu, M. Juremalm, P. Jansen Van Vuren, A. Brun, A. B. Ould EL Mamy, J.-M. Héraud, C. Filippone, J.-P. Ravalohery, H. Chaabihi, E. Albina, L. Dommergues, J. Paweska and E. Cardinale, PLoS Negl. Trop. Dis., 2019, 13, e0007700 CrossRef PubMed
.
- N. E. Yun and D. H. Walker, Viruses, 2012, 4, 2031–2048 CrossRef PubMed
.
-
O. Ogbu, in Encyclopedia of Food Safety, Elsevier, 2014, vol. 2, pp. 208–213 Search PubMed
.
-
D. S. Grant, H. Khan, J. Schieffelin and D. G. Bausch, in Emerging Infectious Diseases: Clinical Case Studies, Elsevier Inc., 2014, pp. 37–59 Search PubMed
.
- E. L. Hamblion, P. Raftery, A. Wendland, E. Dweh, G. S. Williams, R. N. C. George, L. Soro, V. Katawera, P. Clement, A. N. Gasasira, E. Musa and T. K. Nagbe, Int. J. Infect. Dis., 2018, 66, 65–73 CrossRef CAS PubMed
.
- D. M. Emperador, S. A. Yimer, L. T. Mazzola, G. Norheim and C. Kelly-Cirino, BMJ Glob. Health, 2019, 4, e001119 CrossRef PubMed
.
- O. Azeez-Akande, African J. Clin. Exp. Microbiol., 2016, 17, 282 CrossRef
.
- V. Raabe and J. Koehler, J. Clin. Microbiol., 2017, 55, 1629–1637 CrossRef CAS PubMed
.
- A. N. Happi, C. T. Happi and R. J. Schoepp, Curr. Opin. Virol., 2019, 37, 132–138 CrossRef PubMed
.
- L. T. Mazzola and C. Kelly-Cirino, BMJ Glob. Health, 2019, 4, 1116 Search PubMed
.
- Lassa Diagnostic Kits – Zalgen Labs, https://www.zalgen.com/product-category/vhf-diagnostic-kits/lassa-diagnostic-kits/, (accessed 7 October 2020).
- M. L. Boisen, E. Uyigue, J. Aiyepada, K. J. Siddle, L. Oestereich, D. K. S. Nelson, D. J. Bush, M. M. Rowland, M. L. Heinrich, P. Eromon, A. T. Kayode, I. Odia, D. I. Adomeh, E. B. Muoebonam, P. Akhilomen, G. Okonofua, B. Osiemi, O. Omoregie, M. Airende, J. Agbukor, S. Ehikhametalor, C. O. Aire, S. Duraffour, M. Pahlmann, W. Böhm, K. G. Barnes, S. Mehta, M. Momoh, J. D. Sandi, A. Goba, O. A. Folarin, E. Ogbaini-Emovan, D. A. Asogun, E. A. Tobin, G. O. Akpede, S. A. Okogbenin, P. O. Okokhere, D. S. Grant, J. S. Schieffelin, P. C. Sabeti, S. Günther, C. T. Happi, L. M. Branco and R. F. Garry, Sci. Rep., 2020, 10, 1–14 CrossRef PubMed
.
- M. L. Boisen, J. N. Hartnett, J. G. Shaffer, A. Goba, M. Momoh, J. D. Sandi, M. Fullah, D. K. S. Nelson, D. J. Bush, M. M. Rowland, M. L. Heinrich, A. P. Koval, R. W. Cross, K. G. Barnes, A. E. Lachenauer, A. E. Lin, M. Nekoui, D. Kotliar, S. M. Winnicki, K. J. Siddle, M. Gbakie, M. Fonnie, V. J. Koroma, L. Kanneh, P. C. Kulakosky, K. M. Hastie, R. B. Wilson, K. G. Andersen, O. O. Folarin, C. T. Happi, P. C. Sabeti, T. W. Geisbert, E. O. Saphire, S. H. Khan, D. S. Grant, J. S. Schieffelin, L. M. Branco and R. F. Garry, Sci. Rep., 2018, 8, 17 CrossRef PubMed
.
-
J. N. Maslow and C. C. Roberts, in Methods in Molecular Biology, Humana Press Inc., 2020, vol. 2142, pp. 1–8 Search PubMed
.
- J. J. Waggoner and B. A. Pinsky, J. Clin. Microbiol., 2016, 54, 860–867 CrossRef CAS PubMed
.
- H. Song, J. Qi, J. Haywood, Y. Shi and G. F. Gao, DOI:10.1038/nsmb.3213.
- J. M. Mansuy, M. Dutertre, C. Mengelle, C. Fourcade, B. Marchou, P. Delobel, J. Izopet and G. Martin-Blondel, Lancet Infect. Dis., 2016, 16, 405 CrossRef PubMed
.
- M. H. Collins and J. J. Waggoner, ACS Infect. Dis., 2019, 5, 1055–1069 CrossRef CAS PubMed
.
- E. S. Theel and D. Jane Hata, J. Clin. Microbiol., DOI:10.1128/JCM.01972-17.
-
Zika Virus RNA Qualitative Real-Time RT-PCR For use under an Emergency Use Authorization only Rx Only Instructions For Use, 2017, https://testdirectory.questdiagnostics.com/test/test-detail/93870/zika-virus-rna-qualitative-real-time-rt-pcr?cc=MASTER Search PubMed
.
- P. Ren, D. A. Ortiz, A. C. B. Terzian, T. E. Colombo, M. L. Nogueira, N. Vasilakis and M. J. Loeffelholz, J. Clin. Microbiol., 2017, 55, 2198–2203 CrossRef CAS PubMed
.
- ZIKV DetectTM IgM 2.0 Capture ELISA Kit (USA) – InBios International, Inc., https://inbios.com/zikv-detecttm-igm-capture-elisa-kit-usa/, (accessed 7 October 2020).
- A. Goncalves, R. W. Peeling, M. C. Chu, D. J. Gubler, A. M. De Silva, E. Harris, M. Murtagh, A. Chua, W. Rodriguez, C. Kelly, A. Wilder-Smith and A. Wilder-Smith, J. Infect. Dis., 2018, 1060, 1060–1068 CrossRef PubMed
.
- F. A. Jorge, M. V. Thomazella, D. Castro Moreira, L. D. G. Lopes, J. J. V. Teixeira and D. A. Bertolini, Rev. Med. Virol., 2020, 30, e2105 CrossRef PubMed
.
- I. Bosch, H. De Puig, M. Hiley, M. Carré-Camps, F. Perdomo-Celis, C. F. Narváez, D. M. Salgado, D. Senthoor, M. O. Grady, E. Phillips, A. Durbin, D. Fandos, H. Miyazaki, C. W. Yen, M. Gélvez-Ramírez, R. V. Warke, L. S. Ribeiro, M. M. Teixeira, R. P. Almeida, J. E. Muñóz-Medina, J. E. Ludert, M. L. Nogueira, T. E. Colombo, A. C. B. Terzian, P. T. Bozza, A. S. Calheiros, Y. R. Vieira, G. Barbosa-Lima, A. Vizzoni, J. Cerbino-Neto, F. A. Bozza, T. M. L. Souza, M. R. O. Trugilho, A. M. B. De Filippis, P. C. De Sequeira, E. T. A. Marques, T. Magalhaes, F. J. Díaz, B. N. Restrepo, K. Marín, S. Mattar, D. Olson, E. J. Asturias, M. Lucera, M. Singla, G. R. Medigeshi, N. De Bosch, J. Tam, J. Gómez-Márquez, C. Clavet, L. Villar, K. Hamad-Schifferli and L. Gehrke, Sci. Transl. Med., 2017, 9, 29 Search PubMed
.
- Chembio | DPP® Zika IgM System, http://chembio.com/dpp-zika-igm-system/, (accessed 10 July 2020).
- Artron | Zika Test Kit, http://www.artronlab.com/products/new/zika-test.html, (accessed 8 October 2020).
- ZIKA Rapid Test, https://www.accubiotech.com/product-list-zika-rapid-test.html, (accessed 8 October 2020).
- Infectious Diseases - Lumiquick Diagnostics, Inc | Lumiquick Diagnostics, Inc., https://lumiquick.co/infectious-diseases, (accessed 8 October 2020).
- CDIA™ Zika IgG/IgM Rapid Test Kit - Creative Diagnostics, https://www.cd-diatest.com/cdia-sup-tm-sup-zika-igg-igm-rapid-test-kit_p570.html, (accessed 8 October 2020).
- Zika Virus Rapid Test Kit - Maternova Inc., https://maternova.net/products/zika-rapid-test, (accessed 8 October 2020).
- Zika Rapid Test Kits - Biopanda Reagents, https://www.biopanda.co.uk/php/products/rapid/infectious_diseases/zika.php, (accessed 8 October 2020).
- ProDetect® Zika Rapid Test | Mediven - Diagnostics Solutions - COVID-19 Test Kits, http://mediven.com.my/index.php/catalog/prodetect-zika-rapid-test/, (accessed 8 October 2020).
- Zika IgG/IgM virus Antigen Rapid Test - Rapikit, https://rapikit.com/products/zika-iggigm-virus-antigen-rapid-test/, (accessed 8 October 2020).
- A. Priye, S. W. Bird, Y. K. Light, C. S. Ball, O. A. Negrete and R. J. Meagher, DOI:10.1038/srep44778.
- A. Kaushik, A. Yndart, S. Kumar, R. D. Jayant, A. Vashist, A. N. Brown, C. Z. Li and M. Nair, Sci. Rep., 2018, 8, 1–5 Search PubMed
.
- A. Kaushik, S. Tiwari, R. D. Jayant, A. Vashist, R. Nikkhah-Moshaie, N. El-Hage and M. Nair, Trends Biotechnol., 2017, 35, 308–317 CrossRef CAS PubMed
.
- A. Kaushik, S. Tiwari, R. Dev Jayant, A. Marty and M. Nair, Biosens. Bioelectron., 2016, 75, 254–272 CrossRef CAS PubMed
.
- H. A. Mohd, J. A. Al-Tawfiq and Z. A. Memish, Virol. J., 2016, 13, 87 CrossRef PubMed
.
- A. S. Omrani, J. A. Al-Tawfiq and Z. A. Memish, Pathog. Glob. Health, 2015, 109, 354–362 CrossRef PubMed
.
- N. Petrosillo, G. Viceconte, O. Ergonul, G. Ippolito and E. Petersen, Clin. Microbiol. Infect., 2020, 26, 729 CrossRef CAS PubMed
.
-
E. Qing, M. P. Hantak, G. G. Galpalli and T. Gallagher, in Methods in Molecular Biology, Humana Press Inc., 2020, vol. 2099, pp. 9–20 Search PubMed
.
-
J. A. Al-Tawfiq and Z. A. Memish, in Emerging Infectious Diseases: Clinical Case Studies, Elsevier Inc., 2014, pp. 185–190 Search PubMed
.
- X. Tao, T. E. Hill, C. Morimoto, C. J. Peters, T. G. Ksiazek and C.-T. K. Tseng, J. Virol., 2013, 87, 9953–9958 CrossRef CAS PubMed
.
- I. M. Mackay and K. E. Arden, Virol. J., 2015, 12, 222 CrossRef PubMed
.
- A. Assiri, A. McGeer, T. M. Perl, C. S. Price, A. A. Al Rabeeah, D. A. T. Cummings, Z. N. Alabdullatif, M. Assad, A. Almulhim, H. Makhdoom, H. Madani, R. Alhakeem, J. A. Al-Tawfiq, M. Cotten, S. J. Watson, P. Kellam, A. I. Zumla and Z. A. Memish, N. Engl. J. Med., 2013, 369, 407–416 CrossRef CAS PubMed
.
- Z. A. Memish and J. A. Al-Tawfiq, Am. J. Infect. Control, 2014, 42, 1258–1260 CrossRef PubMed
.
- Z. A. Memish, A. M. Assiri and J. A. Al-Tawfiq, Int. J. Infect. Dis., 2014, 29, 307–308 CrossRef PubMed
.
- C. D. Kelly-Cirino, J. Nkengasong, H. Kettler, I. Tongio, F. Gay-Andrieu, C. Escadafal, P. Piot, R. W. Peeling, R. Gadde and C. Boehme, BMJ Glob. Health, 2019, 4, 1179 Search PubMed
.
- S. Al Johani and A. H. Hajeer, J. Infect. Public Health, 2016, 9, 216–219 CrossRef PubMed
.
- Y. Chen, K.-H. Chan, Y. Kang, H. Chen, H. K. Luk, R. W. Poon, J. F. Chan, K.-Y. Yuen, N. Xia, S. K. Lau and P. C. Woo, Emerg. Microbes Infect., 2015, 4, 26 Search PubMed
.
- J. F. W. Chan, S. Sridhar, C. C. Y. Yip, S. K. P. Lau and P. C. Y. Woo, J. Microbiol., 2017, 55, 172–182 CrossRef PubMed
.
- R. Perera, P. Wang, M. Gomaa, R. El-Shesheny, A. Kandeil, O. Bagato, L. Siu, M. Shehata, A. Kayed, Y. Moatasim, M. Li, L. Poon, Y. Guan, R. Webby, M. Ali, J. Peiris and G. Kayali, Eurosurveillance, 2013, 18, 20574 CrossRef PubMed
.
- Y. Chen, K. H. Chan, C. Hong, Y. Kang, S. Ge, H. Chen, E. Y. M. Wong, S. Joseph, N. G. Patteril, U. Wernery, N. Xia, S. K. P. Lau and P. C. Y. Woo, J. Infect., 2016, 73, 82–84 CrossRef PubMed
.
- F. Wu, S. Zhao, B. Yu, Y. M. Chen, W. Wang, Z. G. Song, Y. Hu, Z. W. Tao, J. H. Tian, Y. Y. Pei, M. L. Yuan, Y. L. Zhang, F. H. Dai, Y. Liu, Q. M. Wang, J. J. Zheng, L. Xu, E. C. Holmes and Y. Z. Zhang, Nature, 2020, 579, 265–269 CrossRef CAS PubMed
.
- Archived: WHO Timeline - COVID-19, https://www.who.int/news-room/detail/27-04-2020-who-timeline--covid-19, (accessed 7 October 2020).
- WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/?gclid=CjwKCAjwr7X4BRA4EiwAUXjbt46IjGAwI4MuFY7U11TK1j9x3RoBIXX_x8I7sRflgVp91yqcdtfAZRoC-CAQAvD_BwE, (accessed 7 October 2020).
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html, (accessed 7 October 2020).
- M. Hoffmann, H. Kleine-Weber, S. Schroeder, M. A. Mü, C. Drosten and S. Pö, Cell, 2020, 181, 271–280 CrossRef CAS PubMed
.
- E. E. A. Osman, P. L. Toogood and N. Neamati, ACS Infect. Dis., 2020, 6, 1548–1552 CrossRef CAS PubMed
.
- C. Menni, A. M. Valdes, M. B. Freidin, C. H. Sudre, L. H. Nguyen, D. A. Drew, S. Ganesh, T. Varsavsky, M. J. Cardoso, J. S. El-Sayed Moustafa, A. Visconti, P. Hysi, R. C. E. Bowyer, M. Mangino, M. Falchi, J. Wolf, S. Ourselin, A. T. Chan, C. J. Steves and T. D. Spector, Nat. Med., 2020, 26, 1037–1040 CrossRef CAS PubMed
.
- C. Menni, C. H. Sudre, C. J. Steves, S. Ourselin and T. D. Spector, Lancet, 2020, 395, e107–e108 CrossRef CAS
.
- Y. Roussel, A. Giraud-Gatineau, M.-T. Jimeno, J.-M. Rolain, C. Zandotti, P. Colson and D. Raoult, Int. J. Antimicrob. Agents, 2020, 55, 105947 CrossRef CAS PubMed
.
- B. Udugama, P. Kadhiresan, H. N. Kozlowski, A. Malekjahani, M. Osborne, V. Y. C. Li, H. Chen, S. Mubareka, J. B. Gubbay and W. C. W. Chan, ACS Nano, 2020, 14, 3822–3835 CrossRef CAS PubMed
.
- L. J. Carter, L. V. Garner, J. W. Smoot, Y. Li, Q. Zhou, C. J. Saveson, J. M. Sasso, A. C. Gregg, D. J. Soares, T. R. Beskid, S. R. Jervey and C. Liu, ACS Cent. Sci., 2020, 6, 591–605 CrossRef CAS PubMed
.
- M. Mahmoudi, DOI:10.1021/acs.molpharmaceut.0c00371.
-
D. Administration, FDA Combating COVID-19 With Medical Devices, 2020 Search PubMed
.
- Home - Credo Diagnostics Biomedical Pte. Ltd, https://www.credodxbiomed.com/en/, (accessed 7 October 2020).
- SARS-CoV-2 diagnostic pipeline - FIND, https://www.finddx.org/covid-19/pipeline/?avance=Commercialized&type=Rapid+diagnostic+tests&test_target=all&status=all§ion=show-all&action=default, (accessed 7 October 2020).
- In Vitro Diagnostics EUAs | FDA, https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas#isft1, (accessed 7 October 2020).
- Advice on the use of point-of-care immunodiagnostic tests for COVID-19, https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19, (accessed 7 October 2020).
- A. H. L. Bruning, M. M. G. Leeflang, J. M. B. W. Vos, R. Spijker, M. D. De Jong, K. C. Wolthers and D. Pajkrt, Clin. Infect. Dis., 2017, 1026, 1026–1058 CrossRef PubMed
.
- Coronavirus (COVID-19) Update: FDA Authorizes First COVID-19 Test for Self-Testing at Home | FDA, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-covid-19-test-self-testing-home, (accessed 20 November 2020).
- COVID-19 At-home Test - Lucira Health, https://www.lucirahealth.com/, (accessed 20 November 2020).
- Y. Huang, Y. Wen, K. Baryeh, S. Takalkar, M. Lund, X. Zhang and G. Liu, Microchim. Acta, 2017, 184, 4287–4294 CrossRef CAS PubMed
.
- A. Abera and J. W. Choi, Anal. Methods, 2010, 2, 1819–1822 RSC
.
- C. Gui, K. Wang, C. Li, X. Dai and D. Cui, Nanoscale Res. Lett., 2014, 9, 1–8 CrossRef PubMed
.
- Y. J. Xie, Y. Yang, W. J. Kong, S. H. Yang and M. H. Yang, Chin. J. Anal. Chem., 2015, 43, 618–628 CAS
.
- V. Shirshahi, S. N. Tabatabaei, S. Hatamie and R. Saber, J. Pharm. Biomed. Anal., 2019, 164, 104–111 CrossRef CAS PubMed
.
- E. Morales-Narváez, T. Naghdi, E. Zor and A. Merkoçi, Anal. Chem., 2015, 87, 8573–8577 CrossRef PubMed
.
- R. Álvarez-DIduk, J. Orozco and A. Merkoçi, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed
.
- T. R. Kozel and A. R. Burnham-Marusich, J. Clin. Microbiol., 2017, 55, 2313–2320 CrossRef CAS PubMed
.
- L. Huang, S. Muyldermans and D. Saerens, Expert Rev. Mol. Diagn., 2010, 10, 777–785 CrossRef PubMed
.
- Y. Wang, Z. Fan, L. Shao, X. Kong, X. Hou, D. Tian, Y. Sun, Y. Xiao and L. Yu, Int. J. Nanomed., 2016, 11, 3287–3303 CrossRef CAS PubMed
.
- A. K. Trilling, J. Beekwilder and H. Zuilhof, Analyst, 2013, 138, 1619–1627 RSC
.
- N. G. Welch, J. A. Scoble, B. W. Muir and P. J. Pigram, Biointerphases, 2017, 12, 02D301 CrossRef PubMed
.
- J. Hu, M. Gao, Z. Wang, Y. Chen, Z. Song and H. Xu, Appl. Nanosci., 2020, 1, 3 Search PubMed
.
- J. Van Elslande, E. Houben, M. Depypere, A. Brackenier, S. Desmet, E. Andr, M. Van Ranst, K. Lagrou and P. Vermeersch, Clin. Microbiol. Infect., 2020, 26, 1082 CrossRef CAS PubMed
.
- S. J. C. Pallett, M. Rayment, A. Patel, S. A. M. Fitzgerald-Smith, S. J. Denny, E. Charani, A. L. Mai, K. C. Gilmour, J. Hatcher, C. Scott, P. Randell, N. Mughal, R. Jones, L. S. P. Moore and G. W. Davies, Lancet Respir. Med., 2020, 8, 885–894 CrossRef CAS PubMed
.
- AmpliVue | Quidel, https://www.quidel.com/molecular-diagnostics/amplivue-products, (accessed 23 September 2020).
- X. Wang, E. Xiong, T. Tian, M. Cheng, W. Lin, H. Wang, G. Zhang, J. Sun and X. Zhou, ACS Nano, 2020, 14, 2497–2508 CrossRef CAS PubMed
.
- J. P. Broughton, X. Deng, G. Yu, C. L. Fasching, V. Servellita, J. Singh, X. Miao, J. A. Streithorst, A. Granados, A. Sotomayor-Gonzalez, K. Zorn, A. Gopez, E. Hsu, W. Gu, S. Miller, C. Y. Pan, H. Guevara, D. A. Wadford, J. S. Chen and C. Y. Chiu, Nat. Biotechnol., 2020, 38, 870–874 CrossRef CAS PubMed
.
- J. E. van Dongen, J. T. W. Berendsen, R. D. M. Steenbergen, R. M. F. Wolthuis, J. C. T. Eijkel and L. I. Segerink, Biosens. Bioelectron., 2020, 166, 112445 CrossRef CAS PubMed
.
- A. Carrio, C. Sampedro, J. Sanchez-Lopez, M. Pimienta and P. Campoy, Sensors, 2015, 15, 29569–29593 CrossRef PubMed
.
- C. Ruppert, N. Phogat, S. Laufer, M. Kohl and H. P. Deigner, Microchim. Acta, 2019, 186, 1–9 CrossRef CAS PubMed
.
-
L. Pezzarossa, S. I. Preus, W. E. Svendsen and J. Madsen, A Computer Vision Algorithm for the Digitalization of Colorimetric Lateral Flow Assay Readouts, Institute of Electrical and Electronics Engineers (IEEE), 2020, pp. 1–6 Search PubMed
.
- C. Carrell, A. Kava, M. Nguyen, R. Menger, Z. Munshi, Z. Call, M. Nussbaum and C. Henry, Microelectron. Eng., 2019, 206, 45–54 CrossRef CAS
.
- D. C. Christodouleas, B. Kaur and P. Chorti, ACS Cent. Sci., 2018, 4, 1600–1616 CrossRef CAS PubMed
.
- W. Xiao, C. Huang, F. Xu, J. Yan, H. Bian, Q. Fu, K. Xie, L. Wang and Y. Tang, Sens. Actuators, B, 2018, 266, 63–70 CrossRef CAS PubMed
.
- Healthy.io | Turning the smartphone into a medical device, https://healthy.io/, (accessed 4 August 2020).
- G. Ruiz-Vega, M. Kitsara, M. A. Pellitero, E. Baldrich and F. J. del Campo, ChemElectroChem, 2017, 4, 880–889 CrossRef CAS
.
- P. D. Sinawang, V. Rai, R. E. Ionescu and R. S. Marks, Biosens. Bioelectron., 2016, 77, 400–408 CrossRef CAS PubMed
.
-
J. Wang, in Biosensors and Bioelectronics, Elsevier, 2006, vol. 21, pp. 1887–1892 Search PubMed
.
- Y. Dai and C. C. Liu, Angew. Chem., 2019, 131, 12483–12496 CrossRef
.
- E. T. S. G. da Silva, D. E. P. Souto, J. T. C. Barragan, J. de F. Giarola, A. C. M. de Moraes and L. T. Kubota, ChemElectroChem, 2017, 4, 778–794 CrossRef CAS
.
- X. Chen, Y. Wang, J. Zhou, W. Yan, X. Li and J. J. Zhu, Anal. Chem., 2008, 80, 2133–2140 CrossRef CAS PubMed
.
- T. Bertok, L. Lorencova, E. Chocholova, E. Jane, A. Vikartovska, P. Kasak and J. Tkac, ChemElectroChem, 2019, 6, 989–1003 CrossRef CAS
.
- R. M. B. Oliveira, F. C. B. Fernandes and P. R. Bueno, Electrochim. Acta, 2019, 306, 175–184 CrossRef CAS
.
-
P. R. Bueno, in Nanobiosensors for Personalized and Onsite Biomedical Diagnosis, Institution of Engineering and Technology, 2016, pp. 293–316 Search PubMed
.
- J. Cecchetto, A. Santos, A. Mondini, E. M. Cilli and P. R. Bueno, Biosens. Bioelectron., 2020, 151, 111972 CrossRef CAS PubMed
.
- J. Cecchetto, F. C. B. Fernandes, R. Lopes and P. R. Bueno, Biosens. Bioelectron., 2017, 87, 949–956 CrossRef CAS PubMed
.
- Y. Dai, R. A. Somoza, L. Wang, J. F. Welter, Y. Li, A. I. Caplan and C. C. Liu, Angew. Chem., Int. Ed., 2019, 58, 17399–17405 CrossRef CAS PubMed
.
- Sensit Smart - PalmSens, https://www.palmsens.com/product/sensit-smart/, (accessed 4 August 2020).
- Sensit BT - PalmSens, https://www.palmsens.com/product/sensit-bt/, (accessed 4 August 2020).
- R. Francis, M. Le Bideau, P. Jardot, C. Grimaldier, D. Raoult, J. Yaacoub, B. Khalil and B. La Scola, Clin. Microbiol. Infect., 2021, 27, 128e1–128e7 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |