A highly Li+-conductive HfNb24O62 anode material for superior Li+ storage†
Qingfeng
Fu
ab,
Haijie
Cao
a,
Guisheng
Liang
b,
Lijie
Luo
b,
Yongjun
Chen
 b,
Vignesh
Murugadoss
b,
Vignesh
Murugadoss
 cde,
Shide
Wu
cf,
Tao
Ding
g,
Chunfu
Lin
cde,
Shide
Wu
cf,
Tao
Ding
g,
Chunfu
Lin
 *ab and
Zhanhu
Guo
*ab and
Zhanhu
Guo
 *c
*c
aInstitute of Materials for Energy and Environment, School of Materials Science and Engineering, Qingdao University, Qingdao 266071, China. E-mail: linchunfu@qdu.edu.cn
bSchool of Materials Science and Engineering, Hainan University, Haikou 570228, China
cIntegrated Composites Laboratory (ICL), Department of Chemical and Biomolecular Engineering, University of Tennessee, Knoxville, TN 37996, USA. E-mail: zguo10@utk.edu
dKey Laboratory of Materials Processing and Mold (Zhengzhou University), Ministry of Education, National Engineering Research Center for Advanced Polymer Processing Technology, Zhengzhou University, Zhengzhou 450002, China
eCollege of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, China
fHenan Provincial Key Laboratory of Surface and Interface Science, Zhengzhou University of Light Industry, Zhengzhou 450001, China
gCollege of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, China
First published on 7th December 2019
Abstract
Highly Li+-conductive HfNb24O62 is explored as a new intercalation-type niobium-based oxide anode material for superior Li+ storage. HfNb24O62 owns a Wadsley–Roth shear structure with a large unit-cell volume, leading to a large Li+ diffusion coefficient. HfNb24O62 shows a large capacity, safe operating potential, high rate performance and good cyclability.
Safe lithium-ion batteries (LIBs) with high power and energy density for electric vehicles (EVs) and hybrid electric vehicles (HEVs) have attracted intense attention.1 However, the traditional graphite anode material is limited in such applications due to its low kinetics and unsafe lithiation potential.2 As a promising substitute for graphite, Li4Ti5O12 is appealing due to its safe operating potential (∼1.57 V) and high rate performance after proper modifications.3 However, the small theoretical capacity of Li4Ti5O12 (175 mA h g−1) has limited its large-scale applications. Therefore, new anode materials for safe, high-power and high-energy LIBs have been pursued.
In recent years, intercalation-type niobium-based oxides, such as Ti2Nb2xO4+5x (x = 2, 5 and 24),4–11 MNb11O29 (M = Al, Cr, Fe and Ga),12–15 and W5Nb16O55,16 have been developed as potential candidates to replace Li4Ti5O12 due to their comparably safe operating potentials and much larger theoretical/practical capacities. Niobium is an appealing redox center in electrode materials because of its safe potential window in the range of 1.0–2.0 V for the redox couples of Nb4+/Nb5+ and Nb3+/Nb4+, which can avoid the formation of dangerous lithium dendrites.4 In light of multivalent properties, niobium-based oxides can deliver large theoretical capacities of 374–416 mA h g−1, which are close to that of graphite and significantly surpass that of Li4Ti5O12. In addition, with reported open Wadsley–Roth shear crystal structures consisting of 96–100% MO6 octahedra and 0–4% MO4 tetrahedra,4 niobium-based oxides have been identified as typical intercalation-pseudocapacitive materials, which are beneficial for the capacity and fast-charging property. Despite such attractive features, niobium-based oxide anodes still show poor rate performance due to their intrinsically low Li+ conductivities. Therefore, developing new niobium-based oxide anode materials with high Li+ conductivities, Li+-storage capacities, rate performance, and long-term stability is essential.
In this study, HfNb24O62 was synthesized by a facile and cost-effective solid-state reaction, and applied as a novel niobium-based oxide anode material with a high Li+ conductivity to boost the Li+ storage. In octahedral coordination, the Hf4+ ionic radius (0.710 Å) is larger than those of Ti4+ (0.605 Å), Al3+ (0.535 Å), Cr3+ (0.615 Å), Fe3+ (0.550 Å), Ga3+ (0.620 Å) and W6+ (0.600),17 leading to increased lattice constants and unit-cell volume in HfNb24O62. A larger unit-cell volume usually corresponds to faster Li+ diffusivity, benefiting the Li+ transport kinetics.18 Moreover, HfNb24O62 exhibits a monoclinic Wadsley–Roth shear crystal structure (space group of C2) consisting of 96% edge-sharing (Hf,Nb)O6 octahedra linked by 4% (Hf,Nb)O4 tetrahedra, in which Hf4+ and Nb5+ are disorder located. This three-dimensional skeleton bestows fast Li+ diffusivity and high structural stability, guaranteeing fast lithiation/delithiation and excellent cyclability. Both the HfNb24O62/Li half-cell and LiNi0.5Mn1.5O4//HfNb24O62 full-cell exhibit superior electrochemical properties for fast-charging, large-capacity, safe and durable Li+ storage.
The detailed crystal structure of the as-prepared HfNb24O62 material was studied by XRD and a Rietveld refinement of the XRD pattern (Fig. 1a).19 The characteristic peaks of HfNb24O62 are consistent with those of TiNb24O62 (space group of C2).6 HfNb24O62 owns a robust crystal structure, with the structural unit having a 3 × 4 (Hf,Nb)O6 octahedron-block and a half (Hf,Nb)O4 tetrahedron at the block corner (Fig. 1b). The lattice parameters were Rietveld-refined to be a = 29.92508(125) Å, b = 3.82525(14) Å, c = 21.21133(87) Å, β = 95.068(5)°, and V = 2418.588(167) Å3. Detailed lattice parameters are given in Table S1 (ESI†). Due to the larger Hf4+ (0.710 Å) ionic radius than Ti4+ (0.605 Å), the unit-cell volume of HfNb24O62 is larger than that of TiNb24O62,6 which implies wider Li+ transport pathways in the HfNb24O62 lattice. The survey XPS spectrum of HfNb24O62 reveals the presence of Hf, Nb, O and C (reference) elements (Fig. S1a, ESI†). The detailed Hf-4f (Fig. S1b, ESI†) and Nb-3d (Fig. S1c, ESI†) spectra indicate that the Hf and Nb valence states are +4 and +5,4,20 respectively.
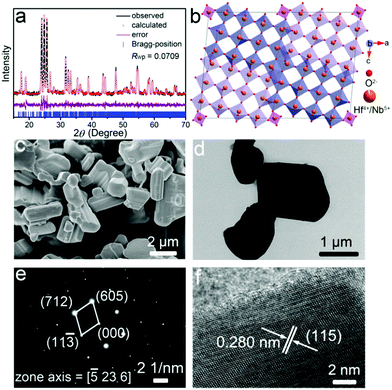 | ||
| Fig. 1 (a) XRD pattern, (b) crystal structure, (c) FESEM image, (d) TEM image, (e) SAED pattern, and (f) HRTEM image of HfNb24O62. Rwp: weighted profile residual. | ||
The FESEM (Fig. 1c) and TEM (Fig. 1d) images reveal that the HfNb24O62 material is composed of irregular and dense particles with particle sizes varying from ∼2 to ∼10 μm, giving a low specific surface area of 0.38 m2 g−1 (Fig. S2, ESI†). The corresponding SAED pattern in Fig. 1e confirms high crystallinity and reveals a single-crystalline feature of the HfNb24O62 primary particles. An interplanar spacing of 0.280 nm measured from the HRTEM image (Fig. 1f) corresponds to the (115) crystallographic plane of HfNb24O62. Besides, the EDX mappings (Fig. S3, ESI†) indicate that the Nb, O and Hf elements are well-dispersed in the whole microparticle, verifying the formation of pure HfNb24O62.
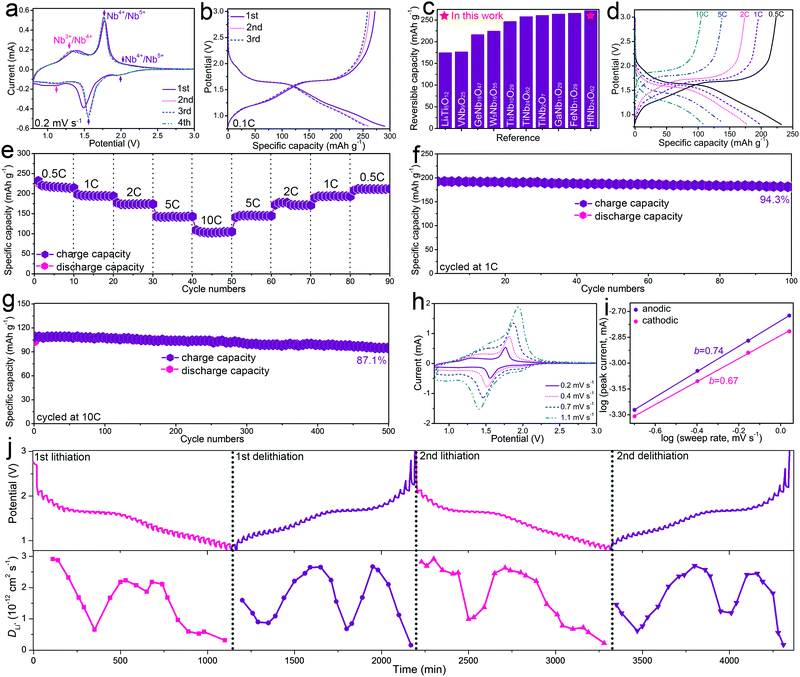
The electrochemical properties of HfNb24O62 were mainly studied using half-cells. Fig. 2a shows the CV curves of the HfNb24O62/Li cell for the initial four cycles at a sweep rate of 0.2 mV s−1. The difference in the discharge portion of the first CV curve from the subsequent cycles is likely due to the incomplete Li+ extraction from the HfNb24O62 lattice in the first cycle.21 After the first cycle, the peak positions and corresponding currents are very stable, suggesting good electrochemical reversibility and cyclability of HfNb24O62. In the subsequent sweeps, two highly overlapping CV peak pairs located at ∼2.0/∼2.0 and 1.77/1.55 V were assigned to the reversible transformation for the redox couple of Nb4+/Nb5+. The shoulder peaks located at 1.34/1.14 V correspond to the redox couple of Nb3+/Nb4+.12 Consequently, the estimated HfNb24O62 mean operating potential (∼1.66 V) based on the intermediate potential of two intensive peaks at 1.77/1.55 V is close to those of Li4Ti5O12 (∼1.57 V),3 TiNb2O7 (∼1.64 V),5 TiNb24O62 (∼1.66 V)6 and Ti2Nb10O29 (∼1.70 V).11 Such a reasonably high operating potential of HfNb24O62 indicates its high safety performance.
Fig. 2b displays the initial three-cycle discharging/charging curves of the HfNb24O62/Li cell recorded at 0.1C. The observed typical potential plateau at ∼1.66 V in the discharging/charging curves resulted from a double-phase transformation reaction. The sloping regions at 3.0–1.67 and 1.65–0.8 V were assigned to two different solid–solution reactions.8 HfNb24O62 shows an initial Coulombic efficiency of 93.8% and large reversible capacity of 272 mA h g−1 at 0.1C. This practical capacity of HfNb24O62 is >100 mA h g−1 larger than that of Li4Ti5O12 and surpasses the majority of the reported intercalation-type niobium-based oxide anode materials (Fig. 2c).3,4,6,9,10,14–16,22,23 The high initial Coulombic efficiency can be due to the fact that little SEI layers formed on the HfNb24O62 particle surfaces above 0.8 V.
The rate performance of HfNb24O62 was investigated at various current rates for every ten cycles, as shown in Fig. 2d and e. HfNb24O62 delivers large reversible capacities of 223, 195, 174, 138 and 105 mA h g−1 at 0.5, 1, 2, 5 and 10C, respectively. When the current rate gradually returns from 10C to 0.5C, the capacity is recovered to its original value. When cycled at 1C, HfNb24O62 shows a small capacity change from 193 to 182 mA h g−1 after 100 cycles, giving 94.3% capacity retention (Fig. 2f and Fig. S4, ESI†). When cycled at 10C, HfNb24O62 shows 87.1% capacity retention even after 500 cycles during the prolonged cycling (Fig. 2g and Fig. S5, ESI†). Such superior cyclability can be due to the stable crystal structure and good Li+-transport kinetics of HfNb24O62.
To interpret the high rate performance of HfNb24O62, its Li+-storage kinetics was analyzed by additional CV experiments recorded at different sweep rates (Fig. 2h). It was found that the cathodic/anodic CV peak current exhibited power law dependence on the sweep rate with an exponent of 0.67/0.74 (Fig. 2i),7,24 suggesting that the pseudocapacitive behaviour significantly contributed to the fast charge storage of HfNb24O62 since it is well known that exponents of 0.5 and 1 indicate diffusion-controlled and pseudocapacitive charge storage, respectively.
A galvanostatic intermittent titration technique (GITT) with a current pulse at 0.1C for 10 min between rest intervals for 20 min was applied to elucidate the Li+ diffusion coefficient (DLi) of HfNb24O62 during the initial two cycles. Based on Fick's second law (see ESI†),25 the evolving DLi of HfNb24O62 at each potential in the discharging (lithiation) and charging (delithiation) processes is determined and plotted in Fig. 2j. The DLi values show a similar variation trend in the two cycles, and minor values appear in the plateau region (∼1.7 V), where the Li+ interaction with the host matrix is strong during the two-phase transition reaction. During the first lithiation/delithiation processes, the DLi value varies from 1.51 × 10−13 to 2.91 × 10−12 cm2 s−1, averaging at 1.61 × 10−12 cm2 s−1. The average DLi value in the second lithiation/delithiation processes (1.70 × 10−12 cm2 s−1) is slightly larger than that of the initial cycle, but the curves are similar to those in the initial cycle. It is noteworthy that the Li+ diffusion coefficient of HfNb24O62 is significantly larger than those of the reported niobium-based oxide anode materials (Table S2, ESI†), which can be ascribed to its open Wadsley–Roth shear crystal structure with an enlarged unit-cell volume. Clearly, the fast Li+ diffusivity together with the significant pseudocapacitive behaviour of HfNb24O62 greatly contributes to its superior Li+ storage (especially its rate performance).
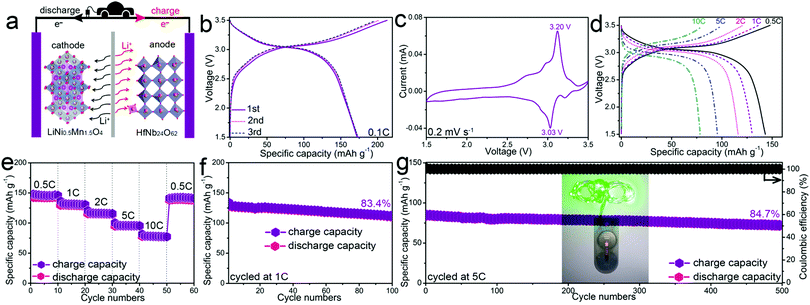
To demonstrate the practical application of HfNb24O62, a full cell with a HfNb24O62 anode and a LiNi0.5Mn1.5O4 cathode was fabricated (Fig. 3a). The LiNi0.5Mn1.5O4//HfNb24O62 full cell affords a large charge capacity of 213 mA h g−1 with an average operating voltage of ∼3.0 V at 0.1C (Fig. 3b). This high voltage agrees with the CV result (Fig. 3c). As the current rate gradually increases to 0.5, 1, 2, 5 and even 10C, the reversible capacity retains 143, 130, 116, 95 and 78 mA h g−1, respectively (Fig. 3d). When the current rate returns from 10C to 0.5C, the obtained capacity of 141 mA h g−1 indicates good electrochemical reversibility (Fig. 3e). The full cell also presents outstanding cyclability at both 1C (83.4% capacity retention after 100 cycles, Fig. 3f) and 5C (84.7% capacity retention after 500 cycles, Fig. 3g). The full cell can power a green light-emitting diode (LED) after being cycled 500 times at 5C (Fig. 3g inset).
In summary, highly-Li+-conductive HfNb24O62 is demonstrated as a novel anode material to realize superior Li+ storage. Both the open Wadsley–Roth shear crystal structure and enlarged unit-cell volume lead to favorable Li+ conduction. Benefiting from the robust host framework and fast ion diffusion pathways, HfNb24O62 exhibits prominent electrochemical properties. At 0.1C, it displays a large capacity of 272 mA h g−1 with a relatively safe operating potential of ∼1.66 V and a high initial Coulombic efficiency of 93.8%. Even at 10C, a remarkable reversible capacity of 105 mA h g−1 is preserved. Furthermore, it exhibits good long-term cyclability, as indicated by 87.1% capacity retention after 500 cycles at 10C. More importantly, a LiNi0.5Mn1.5O4//HfNb24O62 full cell also exhibits high rate performance with 213 mA h g−1 at 0.1C and 78 mA h g−1 at 10C, and good long-term cyclability with 84.7% capacity retention after 500 cycles at 5C. This work can benefit the future designs of highly-Li+-conductive and fast-charging electrode materials.
This work was supported by the National Natural Science Foundation of China (51762014) and China Postdoctoral Science Foundation (2019M652316).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. T. Sun, L. Mei and J. F. Liang, et al. , Science, 2017, 356, 599 CrossRef CAS; (b) Y. Zhang, Y. An and L. Wu, et al. , J. Mater. Chem. A, 2019, 7, 19668 RSC; (c) C. Hou, J. Wang and W. Du, et al. , J. Mater. Chem. A, 2019, 7, 13460 RSC.
- (a) S. R. Sivakkumar, J. Y. Nerkar and A. G. Pandolfo, Electrochim. Acta, 2010, 55, 3330 CrossRef CAS; (b) M. Idrees, S. Batool and J. Kong, et al. , Electrochim. Acta, 2019, 296, 925 CrossRef CAS; (c) R. Li, C. Lin and N. Wang, et al. , Adv. Compos. Hybrid Mater., 2018, 1, 440 CrossRef CAS.
- (a) C. F. Lin, X. Y. Fan, Y. L. Xin, F. Q. Cheng, M. O. Lai, H. H. Zhou and L. Lu, J. Mater. Chem. A, 2014, 2, 9982 RSC; (b) M. Idrees, L. Liu and S. Batool, et al. , Eng. Sci., 2019, 6, 64 Search PubMed.
- L. Hu, L. J. Luo, C. F. Lin, R. J. Li and Y. J. Chen, J. Mater. Chem. A, 2018, 6, 9799 RSC.
- C. F. Lin, S. Yu, S. Q. Wu, S. W. Lin, Z. Z. Zhu, J. B. Li and L. Lu, J. Mater. Chem. A, 2015, 3, 8627 RSC.
- C. Yang, S. J. Deng, C. F. Lin, S. W. Lin, Y. J. Chen, J. B. Li and H. Wu, Nanoscale, 2016, 8, 18792 RSC.
- S. F. Lou, X. Q. Cheng and Y. Zhao, et al. , Nano Energy, 2017, 34, 15 CrossRef CAS.
- X. Y. Wu, J. Miao, W. Z. Han, Y. S. Hu, D. F. Chen, J. Lee, J. Kim and L. Q. Chen, Electrochem. Commun., 2012, 25, 39 CrossRef CAS.
- J. L. Gao, X. Q. Cheng, S. F. Lou, Y. L. Ma, P. J. Zuo, C. Y. Du, Y. Z. Gao and G. P. Yin, J. Alloys Compd., 2017, 728, 534 CrossRef CAS.
- W. L. Wang, B. Oh, J. Park, H. Ki, J. Jang, G. Lee, H. Hu and M. Ham, J. Power Sources, 2015, 300, 272 CrossRef CAS.
- C. F. Lin, S. Yu, H. Zhao, S. Q. Wu, G. Z. Wang, L. Yu, Y. F. Li, Z. Z. Zhu, J. B. Li and S. W. Lin, Sci. Rep., 2015, 5, 17836 CrossRef CAS PubMed.
- R. T. Zheng, S. S. Qian, X. Cheng, H. X. Yu, N. Peng, T. T. Liu, J. D. Zhang, M. T. Xia, H. J. Zhu and J. Shu, Nano Energy, 2019, 58, 399 CrossRef CAS.
- Q. F. Fu, X. Liu, J. R. Hou, Y. R. Pu, C. F. Lin, L. Yang, X. Z. Zhu, L. Hu, S. W. Lin, L. J. Luo and Y. J. Chen, J. Power Sources, 2018, 397, 231 CrossRef CAS.
- X. M. Lou, C. F. Lin, Q. Luo, J. B. Zhao, B. Wang, J. B. Li, Q. Shao, X. K. Guo, N. Wang and Z. H. Guo, ChemElectroChem, 2017, 4, 3171 CrossRef CAS.
- X. M. Lou, Q. F. Fu, J. Xu, X. Liu, C. F. Lin, J. X. Han, Y. P. Luo, Y. J. Chen, X. Y. Fan and J. B. Li, ACS Appl. Nano Mater., 2018, 1, 183 CrossRef CAS.
- K. J. Griffith, K. M. Wiaderek, G. Cibin, L. E. Marbella and C. P. Grey, Nature, 2018, 559, 556 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Found. Crystallogr., 1976, 32, 751 CrossRef.
- Y. Wang, W. D. Richards, S. P. Ong, L. J. Miara, J. C. Kim, Y. Mo and G. Ceder, Nat. Mater., 2015, 14, 1026 CrossRef CAS.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210 CrossRef CAS.
- A. N. Mansour, Surf. Sci. Spectra, 1994, 3, 221 CrossRef CAS.
- X. Lu, Z. L. Jian, Z. Fang, L. Gu, Y. S. Hu, W. Chen, Z. X. Wang and L. Q. Chen, Energy Environ. Sci., 2011, 4, 2638 RSC.
- F. M. Ran, X. Cheng, H. X. Yu, R. T. Zheng, T. T. Liu, X. F. Li, N. Ren, M. Shui and J. Shu, Electrochim. Acta, 2018, 282, 634 CrossRef CAS.
- G. Li, X. L. Wang and X. M. Ma, J. Mater. Chem. A, 2013, 1, 12409 RSC.
- J. P. Yue, C. Suchomski and P. Voepel, et al. , J. Mater. Chem. A, 2017, 5, 1978 RSC.
- W. Weppner and R. A. Huggins, J. Electrochem. Soc., 1977, 124, 1569 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc07447c |
| This journal is © The Royal Society of Chemistry 2020 |