A novel small molecule based on naphtho[1,2-b:5,6-b′]dithiophene benefits both fullerene and non-fullerene solar cells†
Huan
Li
abc,
Jin
Fang
 a,
Jianqi
Zhang
a,
Ruimin
Zhou
ac,
Qiong
Wu
ac,
Dan
Deng
a,
Muhammad
Abdullah Adil
ac,
Kun
Lu
a,
Jianqi
Zhang
a,
Ruimin
Zhou
ac,
Qiong
Wu
ac,
Dan
Deng
a,
Muhammad
Abdullah Adil
ac,
Kun
Lu
 *a,
Xuefeng
Guo
b and
Zhixiang
Wei
*a,
Xuefeng
Guo
b and
Zhixiang
Wei
 a
a
aCAS Key Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: lvk@nanoctr.cn
bAcademy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 13th November 2017
Abstract
Small molecule solar cells have made great progress in recent years. Herein, we synthesized a novel small molecule donor NDTR with naphtho[1,2-b:5,6-b′]dithiophene (NDT) units as the building blocks. NDTR exhibited complementary absorption for both fullerene acceptor PC71BM and non-fullerene acceptor IDIC. Meanwhile, NDTR possessed a HOMO energy level of −5.23 eV and a LUMO energy level of −3.50 eV, which matched well with these two kinds of acceptors. When mixed with PC71BM, a high power conversion efficiency (PCE) of 7.75% was obtained, while the NDTR:IDIC system presented a PCE of 6.60%. The results indicated that NDTR was an all-round small molecule donor which can work well in both fullerene and non-fullerene systems.
1. Introduction
As an effective way to utilize solar energy, organic solar cells (OSCs) have been rapidly developed owing to the advancements made in the design of materials and the structures of devices in recent years.1–6 Now, various systems can achieve a high power conversion efficiency (PCE) over 10%, and the best performance of single-junction solar cells has exceeded 13% which indicates a bright future for OSCs.7–15 Generally, the donor materials for OSCs consist of conjugated polymers and small molecules (SMs). Although the progress of SM donors fell far behind that of polymers in the early stages, the highest PCE of SM-OSCs has reached 11.53% which is comparable to that of polymers.7 Compared with polymers, SMs possess better crystallinity, accurate molecular weight, and high reproducibility without batch-to-batch variation.16–20 Therefore, the design and synthesis of SM donors for OSCs has been a promising research topic and attracted more and more attention.21From the reported results, the symmetrical donor–acceptor (D–A) structural frameworks have been proved to be a successful strategy for the design of SM donors.22 Owing to the push–pull effect between the donor and acceptor units, π electrons can delocalize to the whole molecule effectively. Among the many kinds of D–A constructions, A–D–A type SMs exhibit great potential.23 Through the change of donor and acceptor units, the energy levels and absorption of the SM can be effectively tuned. Oligothiophenes,8 benzodithiophene (BDT),24–27 and dithienosilole (DTS)28 have been widely used as donor units in the construction of A–D–A SMs, which always show good photovoltaic performance when mixed with traditional fullerene acceptors. For instance, Chen et al.8,29 reported a series of oligothiophenes and BDT-based SM donors, and PCEs of nearly 10% were obtained which greatly promoted the development of SM donors. After that, Wei et al. synthesized a novel A–D–A SM named BTID-2F with BDT as a donor unit, and a remarkable efficiency of 11.3% was acquired, which was the first time that an SM donor had presented a PCE over 11%.9 From the successful examples, it is easy to find that SMs possess suitable energy levels for high open-circuit voltage (Voc) and efficient charge transfer, good crystallinity for the generation of appropriate phase separation with PC71BM and efficient charge transportation.
In addition, with the rapid development of non-fullerene acceptors,30–33 a few A–D–A SM donors also exhibit good performance in non-fullerene systems. However, because most small molecule donors can mix well with the non-fullerene acceptors, it is difficult to achieve considerable phase separation for SM non-fullerene systems leading to low fill factors (FF).34 Thus, thermal annealing (TA) and solvent vapor annealing (SVA) methods are always used to modulate the phase separation in the active layer.35,36 Meanwhile, in order to work well with the non-fullerene acceptors, extra donor units were introduced into the SMs which effectively improved the molecular ordering and charge carrier mobility through the increased planarity and intermolecular interactions. Hou et al.36 and Lee et al.37 separately reported two A–D–A SMs with BDT-trimers as donor units, both of which provided a high PCE over 7% with non-fullerene acceptors. However, because of the increased intermolecular interactions, the PCEs of these SMs decreased when blended with fullerene acceptors compared with the corresponding non-fullerene system. Therefore, A–D–A SMs that can work well both in fullerene and non-fullerene systems deserve to be developed and further investigated to clarify the key factor affecting the performance.
For the design of all-round SMs, the building blocks are supposed to be smaller than the BDT trimer and also possess good planarity. On the other hand, the absorption edge of the SM is expected to cover the range from 650 nm to 700 nm, which is conducive to making full use of the sunlight for both the fullerene and non-fullerene systems. Therefore, the naphtho[1,2-b:5,6-b′]dithiophene (NDT)38,39 unit was chosen as the donor unit in this work, which effectively expands the plane compared with the BDT unit. Meanwhile, NDT-based SMs have been proven to have good crystallinity in the previous work. And in order to obtain suitable absorption, strong acceptor units, 3-ethylrhodanines, were used as terminal groups. According to this guide line, a novel SM named NDTR was synthesized and the structure is shown in Fig. 1. The NDTR presented a medium optical bandgap of 1.73 eV and a low highest occupied molecular orbital (HOMO) level of −5.23 eV. As expected, when blended with fullerene acceptor PC71BM, a high PCE of 7.75% was achieved which is close to that of the same kinds of BDT-based SMs. Furthermore, the non-fullerene system of NDTR with IDIC31 as an acceptor also showed a PCE of 6.60%, which fully indicates that NDTR is an all-round SM and good candidate for all-small-molecule solar cells.
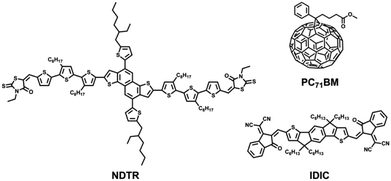 | ||
| Fig. 1 The structures of small molecule donor NDTR, fullerene acceptor PC71BM, and non-fullerene acceptor IDIC. | ||
2. Result and discussion
The synthesis route of NDTR is shown in Fig. S1 (ESI†). NDT was synthesized according to the previous work of our group40 and the π-bridge 3T-CHO was acquired referring to the literature.41 And NDTCHO was obtained by a Stille coupling reaction between NDT and 3T-CHO. The final small molecule (SM) NDTR was prepared with a high yield over 80% through a Knoevenagel condensation of 3-ethylrhodanine with NDTCHO. The detailed processes of synthesis and purification are provided in the ESI.† The chemical structures were identified by 1H-NMR analysis and mass spectra, which are also afforded in the ESI.† In addition, due to the appropriate number and length of the side chains, NDTR was soluble in common solvents, such as chloroform and chlorobenzene, which guaranteed the easy fabrication of the photovoltaic devices.2.1 Optical and electrochemical properties
The absorption spectra of NDTR in a chloroform solution and thin film are shown in Fig. 2a. From the solution to the film, a red shift of nearly 100 nm was observed suggesting enhanced intermolecular electronic interactions in the solid state. And the shoulder peak that emerged at 621 nm in the film also verified the strong interactions, which originated from ordered SM π–π packing.42 The absorption band edge of the NDTR film was located at 700 nm, corresponding to an optical bandgap of 1.73 eV. As is known, fullerene acceptors always possess absorption in the short wavelength range while low bandgap non-fullerene acceptors show good absorption in the long wavelength range, so NDTR can match well with both of the two kinds of acceptors in theory. Moreover, the absorption of the blend films also was measured. As expected, the blend film of NDTR:PC71BM exhibited good absorption in the range from 300 to 700 nm as shown in Fig. 2b, which is similar to most of the A–D–A SMs. In contrast, the blend film of NDTR:IDIC presented weak absorption in the short wavelength range, but the absorption edge was shifted to 800 nm which is beneficial to achieve high short circuit current density (Jsc) values. On the other hand, it is interesting to find that the absorption peaks of NDTR in the blend films changed a lot compared with that of the pure NDTR film, implying strong interaction between NDTR and the acceptors.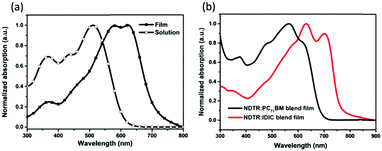 | ||
| Fig. 2 The (a) absorption spectra of NDTR in a chloroform solution and thin film, and (b) absorption spectra of the blend films. | ||
Cyclic voltammetry (CV) with Ag/Ag+ as a reference electrode was employed to measure the energy levels of NDTR (Fig. S4, ESI†). Through the equation of EHOMO = −e (Eox − E1/2(Fc/Fc+) + 4.8) (eV), the highest occupied molecular orbital (HOMO) energy level of NDTR was calculated to be −5.23 eV from the onset oxidation potential.43 Calculated from the HOMO level and optical bandgap, the lowest unoccupied molecular orbital (LUMO) level of NDTR was determined to be 3.50 eV. And the deep HOMO energy level of NDTR was considered to result from the weak electron donating ability of the NDT unit. Thus, a high Voc was expected for the NDTR-based devices. Meanwhile, as shown in Fig. 3a, the IDIC and PC71BM acceptors presented nearly the same LUMO energy levels which were obtained from the reported literatures.31,44 And the big differences of the LUMO energy levels between NDTR and two acceptors are conducive to efficient charge separation and transport in the active layer.45 Therefore, in addition to the absorption spectra, the energy levels of NDTR also can match well with that of the two kinds of acceptors.
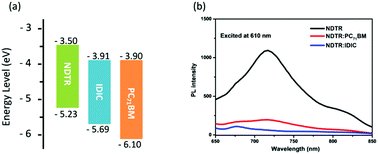 | ||
| Fig. 3 (a) The HOMO and LUMO energy levels of NDTR, IDIC, and PC71BM; (b) the PL spectra of pure NDTR and blend films excited at 610 nm. | ||
Furthermore, photoluminescence (PL) measurements were employed to investigate the charge separation in the fullerene and non-fullerene systems. When excited at 610 nm, a strong emission peak of pure NDTR was observed as shown in Fig. 3b. And after being blended with two acceptors respectively, the photoluminescence spectra were quenched obviously. Calculated from the ratio of intergrated emission intensity between the blend film and pure NDTR, the quenching effiency was 80% for the NDTR:PC71BM system and 91% for the NDTR:IDIC system, indicating that the charge separation of the NDTR non-fullerene system might be much more efficient.
2.2 Photovoltaic performance
To investigate the photovoltaic performance of NDTR in the fullerene and non-fullerene systems, conventional devices were fabricated with a structure of ITO/PEDOT:PSS/active layer/Ca/Al. The current density–voltage (J–V) curves of the best performance devices under 100 mW cm−2 AM 1.5G illumination are shown in Fig. 4, and the corresponding detailed parameters are listed in Table 1. Owing to the matched energy levels between NDTR and two acceptors, good photovoltaic performance was expected for both systems. When mixed with PC71BM, the as-cast device only exhibited a PCE of 4.02% induced by the low short circuit current density (Jsc). And then, the performances were further optimized through varying the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A weight ratios and processing additives which can effectively regulate the morphology of the active layer. Finally, a high PCE of 7.75% was achieved for the NDTR:PC71BM systems with an Voc of 0.93 V, Jsc of 11.42 mA cm−2, and fill factor (FF) of 72.79% after 0.2% diphenyl ether (DPE) was added. On the other hand, when blended with non-fullerene acceptor IDIC, the as-cast devices provided a low PCE of 3.08%. After thermal annealing (TA) and optimizing the D
A weight ratios and processing additives which can effectively regulate the morphology of the active layer. Finally, a high PCE of 7.75% was achieved for the NDTR:PC71BM systems with an Voc of 0.93 V, Jsc of 11.42 mA cm−2, and fill factor (FF) of 72.79% after 0.2% diphenyl ether (DPE) was added. On the other hand, when blended with non-fullerene acceptor IDIC, the as-cast devices provided a low PCE of 3.08%. After thermal annealing (TA) and optimizing the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios, the best performance of NDTR:IDIC increased to 6.60% which was a relatively high value for SM non-fullerene solar cells. Although both of the two NDTR-based systems presented good photovoltaic performance, apparent distinctions existed between the detailed parameters. From Table 1, it is easy to find that the FF value of the fullerene system is much higher than that of the non-fullerene system with NDTR as a donor, whereas the Jsc of the non-fullerene system is a little higher. And the reason is supposed to be the different interactions between NDTR and the two acceptors which affected the morphology of the blend films and led to different phase separation.
A ratios, the best performance of NDTR:IDIC increased to 6.60% which was a relatively high value for SM non-fullerene solar cells. Although both of the two NDTR-based systems presented good photovoltaic performance, apparent distinctions existed between the detailed parameters. From Table 1, it is easy to find that the FF value of the fullerene system is much higher than that of the non-fullerene system with NDTR as a donor, whereas the Jsc of the non-fullerene system is a little higher. And the reason is supposed to be the different interactions between NDTR and the two acceptors which affected the morphology of the blend films and led to different phase separation.
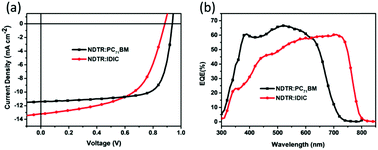 | ||
| Fig. 4 (a) J–V curves and (b) EQE spectra of the optimized devices for NDTR:PC71BM and NDTR:IDIC blends. | ||
| Active layer | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) | J sc (mA cm−2) | μ h (cm2 V−1 s−1) | μ e (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|---|
| a The calculated Jsc values from EQE results. | |||||||
| NDTR:PC71BM | 0.93 | 11.42 | 72.79 | 7.75 | 11.38 | 5.87 × 10−4 | 1.58 × 10−3 |
| NDTR:IDIC | 0.89 | 13.20 | 56.60 | 6.60 | 13.04 | 4.86 × 10−5 | 3.36 × 10−5 |
The external quantum efficiency (EQE) spectra of the two systems are shown in Fig. 4b. The fullerene system exhibited higher absorption in the short wavelength range compared with the NDTR:IDIC system which was mainly ascribed to the PC71BM absorption. Despite the weak absorption in the short wavelength range, the NDTR:IDIC non-fullerene system provided a much broader absorption from 300 nm to 800 nm owing to the introduction of IDIC, which should be one of the major reasons for the higher Jsc. Furthermore, the Jsc values calculated from the EQE spectra agreed well with those acquired from the J–V measurements as listed in Table 1.
In addition, the space charge limited current (SCLC) method was utilized to measure the hole and electron mobilities which were relative to the FF of photovoltaic devices.46 The hole-only devices were fabricated with a structure of ITO/PEDOT:PSS/active layer/MoOx/Ag, whereas the electron-only devices were fabricated with a structure of ITO/ZnO/active layer/Al. And the hole mobilities of the NDTR:PC71BM and NDTR:IDIC systems were calculated to be 5.87 × 10−4 cm2 V−1 s−1 and 4.86 × 10−5 cm2 V−1 s−1 separately from the J–V curves of these devices as shown in Fig. S5 (ESI†). The higher hole mobility should be the direct reason for the high FF of the fullerene system. Moreover, the electron mobility of the NDTR:PC71BM blend film was calculated to be 1.58 × 10−3 cm2 V−1 s−1, while that of NDTR:IDIC was 3.36 × 10−5 cm2 V−1 s−1. A big difference can be seen between the electron mobilities of these two systems, which was supposed to be caused by the different electron transport ability of the acceptors.
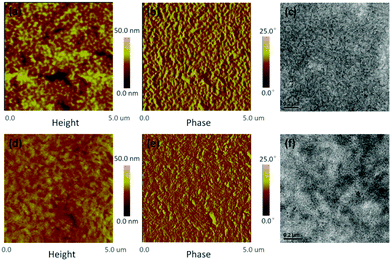
2.3 Morphology and crystallinity analysis
The morphologies of the two blend films were investigated through atomic force microscopy (AFM) and transmission electron microscopy (TEM), and the resulting images are displayed in Fig. 5, Fig. S7 and S8 (ESI†). Towards the NDTR:PC71BM system, the additive of DPE could effectively decrease the aggregation of PC71BM, and suitable phase separation was obtained leading to better photovoltaic performance. On the other hand, the thermal annealing (TA) method appropriately increased the domain size of the NDTR:IDIC system, resulted in the improved Jsc and FF.From Fig. 5, the optimal blend films of the two systems exhibited different phase separation and morphology. NDTR:PC71BM presented a much more rough surface than NDTR:IDIC, while the roughnesses were 5.39 nm and 2.49 nm respectively indicating that IDIC possessed good miscibility with NDTR. Meanwhile, compared with NDTR:PC71BM, the phase separation of NDTR:IDIC was smaller, which was beneficial for the charge separation and consistent with the PL results. Although the domain size of NDTR:PC71BM was bigger, the phase separation was appropriate to charge transport as for most efficient small molecule fullerene systems, which should be the main reason for the high values of FF.9,38,44 Therefore, in order to further improve the FF of NDTR non-fullerene systems, the phase separation should be increased properly.
To analyze the crystallinity and molecular stacking mode of the molecules in the active layer, Grazing-incidence wide-angle X-ray scattering (GIWAXS) was employed. From the two-dimensional GIWAXS results as shown in Fig. 6, obvious lamellar peaks including a first-order diffraction peak (100, qz ≈ 0.3 A−1), second-order diffraction peak (200, qz ≈ 0.7 A−1), and third-order diffraction peak (300, qz ≈ 1.0 A−1) in the out-of-plane direction can be seen in the blend film of NDTR:PC71BM, suggesting the good crystallinity and highly ordered structure of NDTR.47 Meanwhile, these characteristic peaks of NDTR also can be seen in the blend film of NDTR:IDIC, indicating that the introduction of IDIC will not disrupt the crystallization of NDTR. Moreover, the two systems both exhibited a (010) peak in the in-plane direction, which meant NDTR possessed a typical edge-on orientation in the active layer. On the other hand, in addition to the characteristic peaks of NDTR, the NDTR:IDIC blend film also presented extra peaks in the out of plane direction in comparison with NDTR:PC71BM, which should belong to IDIC. Thus, owing to good crystallinity of IDIC, appropriate phase separation can be generated in the active layer of NDTR:IDIC which was always difficult to realize for SM non-fullerene systems. Although PC71BM showed weak crystallinity, the strong aggregation ability led to appropriate phased separation, resulting in a high value of FF for the photovoltaic devices.
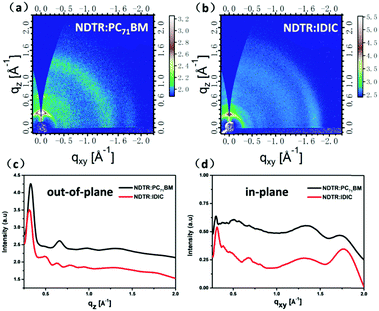 | ||
| Fig. 6 Two-dimensional GIWAXS patterns of the two blend films. And the corresponding out-of-plane and in-plane cuts of the GIWAXS patterns. | ||
3. Conclusions
In summary, a novel A–D–A small molecule donor NDTR with a naphtho[1,2-b:5,6-b′]dithiophene (NDT) unit as the core was designed, synthesized, and fully characterized. Resulting from the expanded planarity of the NDT unit, NDTR exhibited good crystallinity and highly ordered structure in the active layer. Meanwhile, NDTR can work well with both fullerene acceptor PC71BM and non-fullerene acceptor IDIC. When mixed with PC71BM, a high PCE of 7.75% was obtained with a high FF resulting from the high charge mobility. On the contrary, the NDTR:IDIC system presented smaller phase separation leading to a PCE of 6.60% with a higher value of Jsc. It is clear that the big difference between the fullerene and non-fullerene systems based on the NDTR donor was mainly caused by the morphologies in the active layer. But there is no doubt that the medium bandgap and suitable energy levels made NDTR an all-round small molecule donor and good candidate for small molecule solar cells. Moreover, the design and synthesis of all-round small molecules is also significant for the development of small molecule ternary solar cells which is an effective strategy to further improve the PCEs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the National Natural Science Foundation of China (Grant No. 21474022, 21125420, and 91427302), the Ministry of Science and Technology of China (No. 2016YFA0200700, 2016YFF0203803), the Beijing Nova Program, the Youth Innovation Promotion Association CAS, and the Chinese Academy of Sciences.Notes and references
- H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397–7457 CrossRef CAS PubMed.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2015, 49, 175–183 CrossRef PubMed.
- A. J. Heeger, Adv. Mater., 2014, 26, 10–28 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- R. F. Service, Science, 2011, 332, 293 CrossRef CAS PubMed.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- J. Wan, X. Xu, G. Zhang, Y. Li, K. Feng and Q. Peng, Energy Environ. Sci., 2017, 10, 1739–1745 CAS.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang and H. Feng, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- D. Deng, Y. J. Zhang, J. Q. Zhang, Z. Y. Wang, L. Y. Zhu, J. Fang, B. Z. Xia, Z. Wang, K. Lu, W. Ma and Z. X. Wei, Nat. Commun., 2016, 7, 13740 CrossRef CAS PubMed.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganäs, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734–4739 CrossRef CAS PubMed.
- S. Dai, F. Zhao, Q. Zhang, T.-K. Lau, T. Li, K. Liu, Q. Ling, C. Wang, X. Lu and W. You, J. Am. Chem. Soc., 2017, 139, 1336–1343 CrossRef CAS PubMed.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148–7151 CrossRef CAS PubMed.
- H. Bin, L. Gao, Z.-G. Zhang, Y. Yang, Y. Zhang, C. Zhang, S. Chen, L. Xue, C. Yang and M. Xiao, Nat. Commun., 2016, 7, 13651 CrossRef CAS PubMed.
- B. Z. Xia, L. Yuan, J. Q. Zhang, Z. Y. Wang, J. Fang, Y. F. Zhao, D. Deng, W. Ma, K. Lu and Z. X. Wei, J. Mater. Chem. A, 2017, 5, 9859–9866 CAS.
- J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821–3838 CrossRef CAS PubMed.
- L. Yuan, K. Lu, B. Z. Xia, J. Q. Zhang, Z. Wang, Z. Y. Wang, D. Deng, J. Fang, L. Y. Zhu and Z. X. Wei, Adv. Mater., 2016, 28, 5980–5985 CrossRef CAS PubMed.
- D. Deng, Y. J. Zhang, L. Yuan, C. He, K. Lu and Z. X. Wei, Adv. Energy Mater., 2014, 4, 1400538 CrossRef.
- L. Yuan, Y. F. Zhao, J. Q. Zhang, Y. J. Zhang, L. Y. Zhu, K. Lu, W. Yan and Z. X. Wei, Adv. Mater., 2015, 27, 4229–4233 CrossRef CAS PubMed.
- X.-X. Shen, G.-C. Han and Y.-P. Yi, Chin. Chem. Lett., 2016, 27, 1453–1463 CrossRef CAS.
- S. D. Collins, N. A. Ran, M. C. Heiber and T. Q. Nguyen, Adv. Energy Mater., 2017, 7, 1602242 CrossRef.
- Q. Wu, D. Deng, K. Lu and Z.-X. Wei, Chin. Chem. Lett., 2017, 28, 2065–2077 CrossRef CAS.
- Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645–2655 CrossRef CAS PubMed.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529–15532 CrossRef CAS PubMed.
- Z. Wang, X. Xu, Z. Li, K. Feng, K. Li, Y. Li and Q. Peng, Adv. Electron. Mater., 2016, 2, 1600061 CrossRef.
- C. Cui, X. Guo, J. Min, B. Guo, X. Cheng, M. Zhang, C. J. Brabec and Y. Li, Adv. Mater., 2015, 27, 7469–7475 CrossRef CAS PubMed.
- K. Sun, Z. Xiao, S. Lu, W. Zajaczkowski, W. Pisula, E. Hanssen, J. M. White, R. M. Williamson, J. Subbiah and J. Ouyang, Nat. Commun., 2015, 6, 6013 CrossRef CAS PubMed.
- W. Ni, M. Li, F. Liu, X. Wan, H. Feng, B. Kan, Q. Zhang, H. Zhang and Y. Chen, Chem. Mater., 2015, 27, 6077–6084 CrossRef CAS.
- M. Li, W. Ni, X. Wan, Q. Zhang, B. Kan and Y. Chen, J. Mater. Chem. A, 2015, 3, 4765–4776 CAS.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C.-J. Su, T. Li, J. Wang and J. Zhu, J. Am. Chem. Soc., 2016, 138, 2973–2976 CrossRef CAS PubMed.
- A. Gupta, A. Rananaware, P. S. Rao, D. D. La, A. Bilic, W. Xiang, J. Li, R. A. Evans, S. V. Bhosale and S. V. Bhosale, Mater. Chem. Front., 2017, 1, 1600–1606 RSC.
- A. Rananaware, A. Gupta, G. Kadam, D. D. La, A. Bilic, W. Xiang, R. A. Evans and S. V. Bhosale, Mater. Chem. Front., 2017 10.1039/C7QM00355B.
- O. K. Kwon, J. H. Park, D. W. Kim, S. K. Park and S. Y. Park, Adv. Mater., 2015, 27, 1951–1956 CrossRef CAS PubMed.
- H. Bin, Y. Yang, Z.-G. Zhang, L. Ye, M. Ghasemi, S. Chen, Y. Zhang, C. Zhang, C. Sun and L. Xue, J. Am. Chem. Soc., 2017, 139, 5085–5094 CrossRef CAS PubMed.
- L. Yang, S. Zhang, C. He, J. Zhang, H. Yao, Y. Yang, Y. Zhang, W. Zhao and J. Hou, J. Am. Chem. Soc., 2017, 139, 1958–1966 CrossRef CAS PubMed.
- S. Badgujar, C. E. Song, S. Oh, W. S. Shin, S.-J. Moon, J.-C. Lee, I. H. Jung and S. K. Lee, J. Mater. Chem. A, 2016, 4, 16335–16340 CAS.
- X. Zhu, B. Xia, K. Lu, H. Li, R. Zhou, J. Zhang, Y. Zhang, Z. Shuai and Z. Wei, Chem. Mater., 2016, 28, 943–950 CrossRef CAS.
- X. W. Zhu, K. Lu, H. Li, R. M. Zhou and Z. X. Wei, Chin. Chem. Lett., 2016, 27, 1271–1276 CrossRef CAS.
- X. Zhu, J. Fang, K. Lu, J. Zhang, L. Zhu, Y. Zhao, Z. Shuai and Z. Wei, Chem. Mater., 2014, 26, 6947–6954 CrossRef CAS.
- R. J. Kumar, J. M. MacDonald, T. B. Singh, L. J. Waddington and A. B. Holmes, J. Am. Chem. Soc., 2011, 133, 8564–8573 CrossRef CAS PubMed.
- S. Badgujar, G. Y. Lee, T. Park, C. E. Song, S. Park, S. Oh, W. S. Shin, S. J. Moon, J. C. Lee and S. K. Lee, Adv. Energy Mater., 2016, 6, 1600228 CrossRef.
- Q. Sun, H. Wang, C. Yang and Y. Li, J. Mater. Chem., 2003, 13, 800–806 RSC.
- Y. Zhang, D. Deng, K. Lu, J. Zhang, B. Xia, Y. Zhao, J. Fang and Z. Wei, Adv. Mater., 2015, 27, 1071–1076 CrossRef CAS PubMed.
- A. A. Bakulin, A. Rao, V. G. Pavelyev, P. H. van Loosdrecht, M. S. Pshenichnikov, D. Niedzialek, J. Cornil, D. Beljonne and R. H. Friend, Science, 2012, 335, 1340–1344 CrossRef CAS PubMed.
- P. W. Blom, M. De Jong and J. Vleggaar, Appl. Phys. Lett., 1996, 68, 3308–3310 CrossRef CAS.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, 1H NMR spectra, J1/2–V curves for hole-only and electron-only devices and other device data. See DOI: 10.1039/c7qm00397h |
| This journal is © the Partner Organisations 2018 |