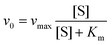Pt74Ag26 nanoparticle-decorated ultrathin MoS2 nanosheets as novel peroxidase mimics for highly selective colorimetric detection of H2O2 and glucose†
Shuangfei
Cai
,
Qiusen
Han
,
Cui
Qi
,
Zheng
Lian
,
Xinghang
Jia
,
Rong
Yang
* and
Chen
Wang
*
CAS Key Lab for Biological Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 100190, P. R. China. E-mail: yangr@nanoctr.cn; wangch@nanoctr.cn
First published on 15th January 2016
Abstract
To extend the functionalities of two-dimensional graphene-like layered compounds as versatile materials, the modification of transition metal dichalcogenide nanosheets such as MoS2 with metal nanoparticles is of great and widespread interest. However, few studies are available on the preparation of bimetallic nanoparticles supported on MoS2. Herein, a facile and efficient method to synthesize MoS2–PtAg nanohybrids by decorating ultrathin MoS2 nanosheets with octahedral Pt74Ag26 alloy nanoparticles has been reported. The as-prepared MoS2–Pt74Ag26 nanohybrids were investigated as novel peroxidase mimics to catalyze the oxidation of classical peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2, producing a blue colored reaction and exhibiting typical Michaelis–Menten kinetics. MoS2–Pt74Ag26 has a higher affinity for H2O2 than horseradish peroxidase (HRP) and a higher vmax value with TMB as the substrate than MoS2. The improved catalytic activity of hybrids for colorimetric reactions could be attributed to the synergistic effects of octahedral Pt74Ag26 nanoparticles and ultrathin MoS2 nanosheets as supports. Meanwhile, the generation of active oxygen species (˙OH) by H2O2 decomposition with MoS2–Pt74Ag26 was responsible for the oxidation of TMB. On the basis of these findings, a colorimetric method based on MoS2–Pt74Ag26 nanohybrids that is highly sensitive and selective was developed for glucose detection. Lower values of the limit of detection (LOD) were obtained, which is more sensitive than MoS2 nanosheets.
1. Introduction
Since the pioneering discovery of monolayer graphene in 2004,1 increasing attention has been paid to other two-dimensional (2D) layered materials, such as layered transition metal dichalcogenides (LTMDs).2–4 One of the typical materials is mono- and few-layer molybdenum disulfide (MoS2), which has received significant attention because of its tunable energy bandgap and natural richness.5–7Owing to abundant active edges and a large specific surface area, graphene-like MoS2 provides a promising support material for potential catalytic performance. To potentially extend the functionalities of 2D MoS2 as versatile materials, modifying MoS2 with 0D metal nanoparticles (NPs) to form 2D–0D multifunctional hybrid materials is of great and widespread interest. Many monometallic NPs, such as Au,8 Ag,9 Pd,10 Pt,11 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and Co13 NPs have been successfully decorated on 2D MoS2 nanosheets. These MoS2–NP hybrid materials not only enhance the intrinsic properties of the materials but also bring novel properties and functions, thus providing great opportunities in developing novel optical biosensors and advanced electrocatalysts for energy conversion. So far, however, the studies on the preparation of bimetallic NPs (BNPs) supported on MoS2 are extremely rare.
12 and Co13 NPs have been successfully decorated on 2D MoS2 nanosheets. These MoS2–NP hybrid materials not only enhance the intrinsic properties of the materials but also bring novel properties and functions, thus providing great opportunities in developing novel optical biosensors and advanced electrocatalysts for energy conversion. So far, however, the studies on the preparation of bimetallic NPs (BNPs) supported on MoS2 are extremely rare.
BNPs consist of two metals and often display enhanced catalytic performance than their monometallic counterparts because of a synergistic effect. Among them, Pt-based BNPs are presently being widely used as essential catalytic substances in various fields owing to the modified geometric and electronic structures of Pt. For example, PtAg NPs have been used in methanol oxidation reactions due to their high electrocatalytic activity. Recently, many groups have started to work on MoS2–BNP nanostructures. Ma's group has reported improved hydrazine oxidation activities using a NiFe alloy supported on MoS2 by electrodeposition and electroplating approaches.14 In spite of effectiveness of an in situ growth method of BNPs on MoS2, to develop a simple method for dispersing BNPs on MoS2 is highly appealing. Fan's group has prepared MoS2 nanosheet supported AuPd BNPs with a core–shell structure by a co-reduction method and the resulting MoS2–AuPd exhibited excellent peroxidase-like activity.15 Although this method is facile, the lack of a stabilizer often leads to aggregation of the products. Most recently, Wang's group employed a seeded growth strategy to achieve the controllable synthesis of Au@Pt core–shell nanodendrites on MoS2 nanosheets, which displayed much higher electrocatalytic activity and stability than MoS2–Pt or the commercial Pt/C catalyst.16 Until now, the facile preparation of MoS2–BNP hybrids with high-quality and different heterostructures still remains a challenge.
The accurate detection of H2O2 and glucose is of critical significance in many fields involving food, chemistry, biology, clinical control, and environmental protection.17 Presently, colorimetric methods for H2O2 and glucose detection are particularly attractive for point-of-care applications because of their low cost, simplicity, and practicality.18 In this contribution, we report a facile and efficient method to synthesize MoS2–PtAg nanohybrids by decorating ultrathin MoS2 nanosheets with octahedral Pt74Ag26 alloy NPs. MoS2 nanosheets prepared by liquid exfoliation were functionalized with polyallylamine hydrochloride (PAH), and then the MoS2–PtAg nanohybrids were obtained through a hydrothermal synthesis. This approach may represent a significant step toward the missing link between the synthesis of high-quality MoS2–BNP nanohybrids and their multiple promising applications, as firstly exemplified by the detection of H2O2 and glucose in this study.
2. Results and discussion
2.1 Preparation and characterization of MoS2–PtAg hybrid nanomaterials
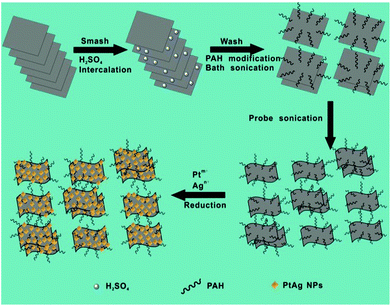
A schematic illustration of the synthesis of MoS2–PtAg hybrid nanomaterials is illustrated in Fig. 1 and detailed in the Experimental section. In a typical experiment, the MoS2 nanosheets were prepared via a liquid exfoliation method. Briefly, commercial MoS2 powder was first ground and then intercalated with concentrated H2SO4, which breaks the van der Waals interaction forces in bulk MoS2. Subsequently, the H2SO4-intercalated intermediate was collected by centrifugation and repeatedly washed with ultrapure water to remove residual H2SO4. After that, the obtained intermediate was functionalized with a highly hydrophilic surfactant PAH through physical adsorption in water under bath sonication conditions, followed by probe sonication treatment to give MoS2–PAH nanosheets.Among the various known strategies for the fabrication of ultrathin MoS2 nanosheets, the mechanical exfoliation19 process is simple, but limited by the low yield; chemical vapour deposition (CVD)20 yields large flake sizes but often at the cost of crystalline quality and thickness control; the chemical exfoliation21 process gives large quantities of nanosheets but requires relatively higher reaction temperature (e.g. 100 °C), long duration (e.g. 3 days) and a lack of controllability over the degree of lithium insertion; the electrochemical exfoliation22 process requires sophisticated instrumentation, that is, a lithium ion battery in an Ar-filled glove box connected to a battery test system. Moreover, the lithium intercalation–exfoliation process often results in a crystalline structure transformation from 2H-MoS2 (hexagonal, semiconducting) to 1T-MoS2 (octahedral, metallic). Specifically, the lithium intercalated compound LixMoS2 is extremely sensitive to air, flammable, and the metallic Li violently reacts with H2O to produce H2 gas during the exfoliation process, which is dangerous. A relatively new approach is the liquid exfoliation23 process by direct sonication in commonly used solvents involving N-methylpyrrolidone (NMP) and isopropyl alcohol (IPA), which was initially proposed by Coleman et al. in 2011. Unlike the lithium intercalation–exfoliation process, the crystalline structure of MoS2 can be well retained by liquid exfoliation. Herein, we used a modified liquid exfoliation method to obtain MoS2 nanosheets. Compared to the reported routes for preparing ultrathin MoS2 nanosheets, the procedure presented here is simple, economical and safe. Thus, it could potentially be scaled up to produce large quantities of exfoliated MoS2 nanosheets.
The obtained MoS2–PAH nanosheets were first characterized by atomic force microscopy (AFM). The AFM image in Fig. S1a† and the line profile in the inset of Fig. S1a† reveal that they are very thin (below 2 nm), which is less than 3 layers. The scanning electron microscopy (SEM) image in Fig. S1b† indicates the size of MoS2–PAH nanosheets is at the nanoscale. The morphology of the obtained MoS2–PAH nanosheets was further characterized by high-resolution transmission electron microscopy (HRTEM). The corresponding fast Fourier transform (FFT) pattern (inset in Fig. S1c†) illustrates the hexagonal lattices of MoS2, which is consistent with the typical hexagonal atomic structure of 2H-MoS2.24 Additionally, the magnified image (Fig. S1d†) of a representative mono-layer MoS2–PAH clearly shows the hexagonal lattices of the obtained nanosheets with a lattice constant of 0.315 nm.25 The crystalline structure of the as-prepared MoS2–PAH nanosheets was further measured by powder X-ray diffraction (XRD). All the peaks shown in Fig. S1e† exhibit the characteristics of the hexagonal MoS2 (JCPDS no. 03-065-7025) and no other impurities were found, revealing the high purity of the as-obtained product. The surface groups of the as-prepared MoS2–PAH nanosheets were detected by Fourier transform infrared (FT-IR) spectroscopy. As shown in Fig. S1f,† the N–H stretch (around 3400 cm−1), CH2 vibrations (about 2900 cm−1), N–H asymmetric bending (about 1610 cm−1) and C–H bending (around 1510 cm−1) confirm the successful modification of PAH molecules on MoS2.
The composition and the chemical state of the as-prepared MoS2–PAH nanosheets were confirmed by X-ray photoelectron spectroscopy (XPS). A typical XPS survey spectrum of the as-prepared MoS2–PAH nanosheets shows the peaks attributed to O, N, C, Mo, Cl and S elements (Fig. S2a†). The calculated atomic ratio between Mo and S from XPS is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4, which is close to the stoichiometric MoS2. The high-resolution Mo 3d XPS spectra can be fitted well into two individual doublets (Fig. S2b†). The two peaks at 232.7 eV and 229.5 eV are attributed to the Mo 3d3/2 and Mo 3d5/2 binding energies for Mo4+, respectively.26,27 Meanwhile, the S 2p spectrum shows two peaks located at 163.5 eV and 162.3 eV (Fig. S2c†), which are characteristic of the S 2p1/2 and S 2p3/2 components of MoS2, respectively.26,27 The as-prepared MoS2–PAH nanosheets exhibited good dispersibility in ultra-pure water. The zeta potential measurement of MoS2–PAH nanosheets indicates they are highly positively charged when dispersed in ultrapure water below pH 8 (Fig. S2d†).
2.4, which is close to the stoichiometric MoS2. The high-resolution Mo 3d XPS spectra can be fitted well into two individual doublets (Fig. S2b†). The two peaks at 232.7 eV and 229.5 eV are attributed to the Mo 3d3/2 and Mo 3d5/2 binding energies for Mo4+, respectively.26,27 Meanwhile, the S 2p spectrum shows two peaks located at 163.5 eV and 162.3 eV (Fig. S2c†), which are characteristic of the S 2p1/2 and S 2p3/2 components of MoS2, respectively.26,27 The as-prepared MoS2–PAH nanosheets exhibited good dispersibility in ultra-pure water. The zeta potential measurement of MoS2–PAH nanosheets indicates they are highly positively charged when dispersed in ultrapure water below pH 8 (Fig. S2d†).
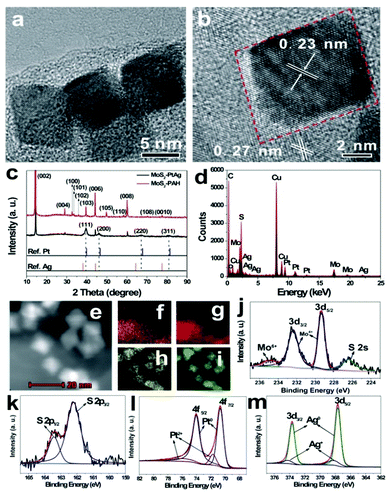
Hydrothermal synthesis for BNPs has significant economic and environmental advantages, and is the most common “bottom-up” method. To prepare the MoS2–PtAg hybrid nanomaterials, H2PtCl6·6H2O and AgNO3 as metal precursors were reduced by HCHO under hydrothermal conditions and PtAg alloys were formed on the MoS2–PAH nanosheets. The as-prepared MoS2–PtAg nanohybrids were characterized in detail. Fig. 2a shows a typical TEM image of the as-prepared MoS2–PtAg nanohybrids with a Pt/Ag feeding molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. One can see that PtAg NPs are regularly octahedral and well dispersed on the MoS2–PAH nanosheets (Fig. S3a–e†). The average size of PtAg NPs is about 13 nm with narrow size distribution (Fig. S3f†). As one of the simplest Platonic solids, the octahedron is one end of the growth morphologies of Platonic solids with 8 equilateral triangle faces.28 The HRTEM image in Fig. 2b shows the lattice fringes with an inter-fringe distance of 0.23 nm, which is between the distance of the face-centered cubic (fcc) Pt (0.226 nm, JCPDS no. 04-0802) and Ag (0.236 nm, JCPDS no. 04-0783) in the (111) plane. The result indicates the formation of a fcc PtAg nano-octahedron, which has high symmetry with the {111} lattice facet as the basal surface.29–31 Owing to different radii, Ag and Pt atoms are compressed and stretched during the formation process of a fcc PtAg nano-octahedron, respectively, resulting in the corresponding change in the interatomic distance, thus the inter-fringe distance of PtAg is between Pt and Ag in the (111) plane. The lattice spacing of 0.27 nm belongs to the (100) plane of MoS2
1. One can see that PtAg NPs are regularly octahedral and well dispersed on the MoS2–PAH nanosheets (Fig. S3a–e†). The average size of PtAg NPs is about 13 nm with narrow size distribution (Fig. S3f†). As one of the simplest Platonic solids, the octahedron is one end of the growth morphologies of Platonic solids with 8 equilateral triangle faces.28 The HRTEM image in Fig. 2b shows the lattice fringes with an inter-fringe distance of 0.23 nm, which is between the distance of the face-centered cubic (fcc) Pt (0.226 nm, JCPDS no. 04-0802) and Ag (0.236 nm, JCPDS no. 04-0783) in the (111) plane. The result indicates the formation of a fcc PtAg nano-octahedron, which has high symmetry with the {111} lattice facet as the basal surface.29–31 Owing to different radii, Ag and Pt atoms are compressed and stretched during the formation process of a fcc PtAg nano-octahedron, respectively, resulting in the corresponding change in the interatomic distance, thus the inter-fringe distance of PtAg is between Pt and Ag in the (111) plane. The lattice spacing of 0.27 nm belongs to the (100) plane of MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 and is also observed in the same image. As shown in Fig. 2c, the crystalline structure of the MoS2–PtAg nanohybrids was further examined by XRD. Diffraction peaks at 2θ values of 39.6°, 46.1°, 67.2° and 81.0° can be assigned to the (111), (200), (220) and (311) crystal planes of a PtAg fcc structure, respectively. The diffraction peaks positioned between the standard peaks of Pt (JCPDS no. 04-0802) and Ag (JCPDS no. 04-0783), demonstrating the formation of a bimetallic phase of Pt and Ag. The energy dispersive X-ray (EDX) analysis of MoS2–PtAg shows that both the signals of Mo, S, Pt and Ag can be easily detected from these samples (Fig. 2d). The analysed atomic ratio (Pt/Ag) by inductively coupled plasma optical emission spectroscopy (ICP-OES) is 74
32 and is also observed in the same image. As shown in Fig. 2c, the crystalline structure of the MoS2–PtAg nanohybrids was further examined by XRD. Diffraction peaks at 2θ values of 39.6°, 46.1°, 67.2° and 81.0° can be assigned to the (111), (200), (220) and (311) crystal planes of a PtAg fcc structure, respectively. The diffraction peaks positioned between the standard peaks of Pt (JCPDS no. 04-0802) and Ag (JCPDS no. 04-0783), demonstrating the formation of a bimetallic phase of Pt and Ag. The energy dispersive X-ray (EDX) analysis of MoS2–PtAg shows that both the signals of Mo, S, Pt and Ag can be easily detected from these samples (Fig. 2d). The analysed atomic ratio (Pt/Ag) by inductively coupled plasma optical emission spectroscopy (ICP-OES) is 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26, which is very close to the Pt/Ag feeding ratio of 3
26, which is very close to the Pt/Ag feeding ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. According to the analysed ICP result, the loading amount of Pt74Ag26 NPs on MoS2–PAH nanosheets is 29.0 wt%, which is slightly lower than the calculated value owing to the loss during the repeated washing and dissolving process. To further investigate the nanostructure of MoS2–PtAg, the element distributions of Mo, S, Pt and Ag in the hybrid material were studied by using a high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM). From the representative STEM image (Fig. 2e) and its corresponding Mo, S, Pt and Ag elemental maps (Fig. 2f–i), it can be seen that both Pt and Ag are evenly distributed in each individual NP. The chemical state of the as-prepared MoS2–PtAg was confirmed by XPS. The high-resolution Mo 3d XPS spectrum can be deconvoluted into four single peaks (Fig. 2j). The two peaks at 232.4 eV (3d3/2) and 229.3 eV (3d5/2) binding energies are attributed to Mo4+, while the shoulder at 235.0 eV are likely assigned to Mo6+.27,33 Meanwhile, a peak at 226.2 eV corresponding to the S 2s line of MoS2 can be found. Additionally, the S 2p spectrum shows two peaks located at 163.4 eV and 162.2 eV (Fig. 2k), which are characteristic of the S 2p1/2 and S 2p3/2 components of MoS2, respectively.26,27 The XPS spectrum of Pt 4f (Fig. 2l) displays two peaks at 74.0 eV (4f5/2) and 70.7 eV (4f7/2), which is indicative of elemental Pt0.34 From the fitting curves, the calculated percentage of Pt0 species is about 83.4%. The Ag 3d spectrum of the sample (Fig. 2m) consists of two individual peaks at 373.6 eV (3d3/2) and 367.6 eV (3d5/2), which can be assigned to the Ag0.35 The calculated percentage of Ag0 species is about 84.8% from the fitting curves. The above results indicate that the ultrathin MoS2–PAH nanosheets are successfully decorated with octahedral Pt74Ag26 alloy NPs.
1. According to the analysed ICP result, the loading amount of Pt74Ag26 NPs on MoS2–PAH nanosheets is 29.0 wt%, which is slightly lower than the calculated value owing to the loss during the repeated washing and dissolving process. To further investigate the nanostructure of MoS2–PtAg, the element distributions of Mo, S, Pt and Ag in the hybrid material were studied by using a high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM). From the representative STEM image (Fig. 2e) and its corresponding Mo, S, Pt and Ag elemental maps (Fig. 2f–i), it can be seen that both Pt and Ag are evenly distributed in each individual NP. The chemical state of the as-prepared MoS2–PtAg was confirmed by XPS. The high-resolution Mo 3d XPS spectrum can be deconvoluted into four single peaks (Fig. 2j). The two peaks at 232.4 eV (3d3/2) and 229.3 eV (3d5/2) binding energies are attributed to Mo4+, while the shoulder at 235.0 eV are likely assigned to Mo6+.27,33 Meanwhile, a peak at 226.2 eV corresponding to the S 2s line of MoS2 can be found. Additionally, the S 2p spectrum shows two peaks located at 163.4 eV and 162.2 eV (Fig. 2k), which are characteristic of the S 2p1/2 and S 2p3/2 components of MoS2, respectively.26,27 The XPS spectrum of Pt 4f (Fig. 2l) displays two peaks at 74.0 eV (4f5/2) and 70.7 eV (4f7/2), which is indicative of elemental Pt0.34 From the fitting curves, the calculated percentage of Pt0 species is about 83.4%. The Ag 3d spectrum of the sample (Fig. 2m) consists of two individual peaks at 373.6 eV (3d3/2) and 367.6 eV (3d5/2), which can be assigned to the Ag0.35 The calculated percentage of Ag0 species is about 84.8% from the fitting curves. The above results indicate that the ultrathin MoS2–PAH nanosheets are successfully decorated with octahedral Pt74Ag26 alloy NPs.
To understand the formation mechanism of MoS2–PtAg nanohybrids, a series of control experiments were carried out. Under the standard reaction conditions, the use of Pt precursor alone gave spherical Pt NPs supported on the MoS2 nanosheets (Fig. S4a†). However, replacing the Pt precursor with a Ag precursor failed to synthesize the corresponding MoS2–Ag nanohybrids, suggesting that a Ag precursor alone cannot be reduced under the present conditions. Moreover, using different Pt/Ag feeding molar ratios, various nanohybrids of MoS2–PAH nanosheet-supported octahedral PtAg NPs (Fig. S4b–4d†) can be obtained. Obviously, the introduction of Ag+ species into the reaction system can favor the formation of octahedral PtAg NPs. It is noted that the use of a large amount of Ag precursors in excess of Pt precursors brings about a low yield of the final products, leading to a low loading of octahedral PtAg NPs on MoS2 nanosheets (Fig. S4d†). It is known that the standard reduction potential (E) for Pt2+/Pt (1.18 V) is more positive than that of the Ag+/Ag (0.80 V) pair in the same coordination environment, Pt ions with high standard reduction potential could be reduced first. Meanwhile, the overlapped elemental maps of Pt and Ag (Fig. S5d†) reveal these two elements are uniformly distributed in each individual NP, suggesting the continuous addition of Pt and Ag atoms during the formation of a PtAg nano-octahedron. In addition, the EDX line scanning profile across a single Pt-Ag NP (Fig. S5e†) clearly shows Pt and Ag content change synchronously, verifying the structure of the alloy. The selected PtAg NP contains 74% of Pt and 26% of Ag, which is in good agreement with the ICP-OES result. Based on the above result and discussion, we speculate that the Pt nanocrystals as seeds were first formed on the MoS2 nanosheets. With the quick diffusion of Ag+ ions at high temperature, they were adsorbed strongly on the Pt {100} facets, followed by the reduction by the preformed Pt seeds. As more and more Ag atoms continuously deposited on the Pt {100} facets,36 active Ag atoms from the corners of the reaction intermediates were selectively removed by the oxidative etching process of dissolved oxygen in the reaction system.37,38 With the periodic deposition of Pt and Ag atoms as well as the re-oxidation of the Ag atoms on the growing intermediates, an octahedral PtAg NP enclosed by {111} facets was formed.
2.2 Peroxidase-like activity of MoS2–PtAg hybrid nanomaterials
To investigate the catalytic activity of the as-prepared hybrid nanomaterials, 3,3′,5,5′-tetramethylbenzidine (TMB), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and o-phenylenediamine (OPD) are chosen as chromogenic substrates to study the oxidase-like or peroxidase-like activities of MoS2–Pt74Ag26. In the presence of H2O2, MoS2–Pt74Ag26 nanohybrids can catalyze the oxidation of TMB, ABTS and OPD, producing the typical blue color for TMB, green color for ABTS and yellow color for OPD (Fig. 3), while the control experiments without MoS2–Pt74Ag26 or without H2O2 showed negligible color variation (Fig. S6†). This result clearly indicates that MoS2–Pt74Ag26 has a peroxidase-like activity.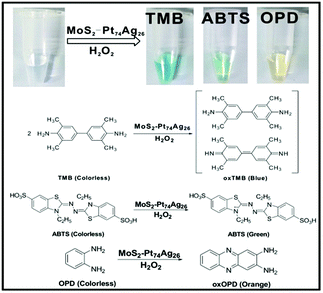 | ||
| Fig. 3 The color change and corresponding reaction schemes for the oxidation of TMB, ABTS and OPD catalyzed by the as-prepared MoS2–Pt74Ag26. | ||
To study the interaction between the as-prepared ultrathin MoS2 nanosheets and PtAg NPs, several control experiments were performed. Compared to the MoS2 nanosheets and unsupported Pt73Ag27 NPs (Fig. S7†), MoS2–Pt74Ag26 hybrids display enhanced catalytic activity with the example of TMB oxidation (Fig. S8a†). Different from MoS2 alone, MoS2–Pt74Ag26 not only has larger surface area, but also provides abundant active sites for the adsorption of TMB molecules. Accordingly, lone-pair electrons can be readily transferred from the amino groups of TMB molecule to the hybrids, which gives rise to an increase in electron density and mobility in the hybrids for electron transfer from hybrids to H2O2, leading to the reduction of H2O2 to H2O and the reaction rate of TMB oxidation by H2O2 increases. The effect of Pt/Ag ratios of MoS2–PtAg on their catalytic oxidation capability is also investigated. Fig. S8b† shows that MoS2–Pt74Ag26 has the highest activity with the maximum absorbance at 652 nm. Thus, MoS2–Pt74Ag26 is chosen as a typical enzyme mimetic for further studies. It has been found that MoS2–Pt74Ag26 has good storage stability. When it has been stored in water at room temperature for 2 months, no obvious decrease in response to H2O2 is observed (Fig. S8c†). MoS2–Pt74Ag26 also has good reusability after repeated cycles of H2O2 sensing as shown in Fig. S8d.† There is no apparent difference in morphology and size between the as-prepared hybrids and the recycled hybrids (Fig. S9†), indicating the stability of hybrids. The good stability and reusability of MoS2–Pt74Ag26 makes it suitable and practical for a broad range of applications.
2.3 Reaction mechanism
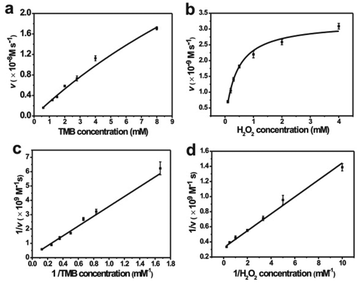
Similar to HRP, the catalytic activities of MoS2–PtAg show temperature, pH and H2O2 concentration dependence (Fig. S10†). MoS2–Pt74Ag26 displays high catalytic activity at different pH values (3.0–6.0) and a wide range of temperatures (20–70 °C), while the catalytic activity of HRP was largely inhibited after incubation at pH below 4.0 or at temperature higher than 50 °C.39 Thus, the robustness of MoS2–Pt74Ag26 makes it potentially applicable under harsh conditions. For the catalytic oxidation of TMB by MoS2–Pt74Ag26, the optimal pH is 4.0 and an optimal temperature is 50 °C. The peroxidase-like activity MoS2–Pt74Ag26 is further investigated by the steady-state kinetic experiments. By monitoring the absorbance change at 652 nm for 30 min, typical Michaelis–Menten curves with TMB and H2O2 are obtained, respectively (Fig. 4a and b). Michaelis–Menten constant (Km) and maximum initial rate (vmax) are obtained using the Lineweaver–Burk plot (Fig. 4c and d).The apparent Km value indicates the affinity of the substrates to enzymes and a lower value means a higher affinity. Table 1 lists the Km and vmax values for MoS2–Pt74Ag26, HRP and MoS2 nanosheets. Compared to HRP, the Km value for MoS2–Pt74Ag26 with H2O2 as the substrate is lower, suggesting MoS2–Pt74Ag26 has higher affinity for H2O2 than HRP. This can be understood since a HRP molecule contains only one iron ion which decomposes H2O2, while the surface of the mono-layer MoS2 structure is decorated with lots of Pt74Ag26 NPs. The lower affinity for TMB can also guarantee more active sites would be available for H2O2. Meanwhile, the vmax value for MoS2–Pt74Ag26 with TMB as the substrate was higher than MoS2 alone. The synergistic effects of octahedral Pt74Ag26 NPs and ultrathin MoS2 nanosheets as catalytic supports resulted in the improved catalytic activity of hybrids for colorimetric reactions.
The hydroxyl radical (˙OH) as an important intermediate in peroxidase mimic-catalyzed colorimetric detection of H2O2 was studied extensively.42,43 Here, the ˙OH is assessed by adding terephthalic acid (TA) as a fluorescent probe into the H2O2/MoS2–Pt74Ag26 system, where TA easily reacts with ˙OH forming highly fluorescent 2-hydroxy terephthalic acid.44 As shown in Fig. S11,† there is negligible fluorescence intensity in the absence of H2O2 or MoS2–Pt74Ag26, while an emission peak at about 440 nm appears after MoS2–Pt74Ag26 is added in the TA solution in the presence of H2O2, which indicates the production of ˙OH after the interaction between MoS2–Pt74Ag26 and H2O2. The result shows that MoS2–Pt74Ag26 could decompose H2O2 to generate the ˙OH radical. To further evaluate the effects of MoS2–Pt74Ag26 on ˙OH signal intensity, a series of electron spin resonance (ESR) experiments in the 5,5-dimethyl-1-pyrroline N-oxide (DMPO) spin trap system have been carried out. As shown in Fig. S12,† one can see that there are no apparent ˙OH signals in the buffer/TMB/H2O2 system (curve a in Fig. S12†) while, the intensity of ˙OH increases in the system of buffer/H2O2/MoS2–Pt74Ag26 as time increases (curves b, c). However, after the addition of TMB to this solution, the signal of ˙OH disappeared. Instead, a strong TMB cation radical45 signal can be detected immediately (curve d in Fig. S12†). The above results indicate that MoS2–Pt74Ag26 possesses peroxidase-like activity.
2.4 Detection of H2O2 and glucose
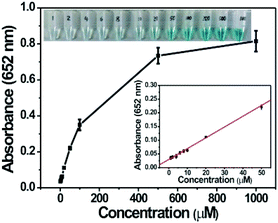
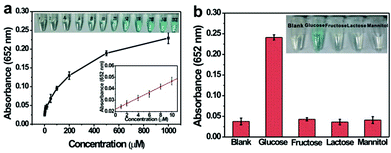
Based on the intrinsic peroxidase property of MoS2–Pt74Ag26, the detection of H2O2 and glucose is designed using the blue color reaction catalyzed by MoS2–Pt74Ag26. Fig. 5 shows the dependence of the absorbance at 652 nm on the concentration of H2O2, revealing that the intensity of absorption peak at 652 nm increases with increased H2O2 concentration from 1 μM to 1 mM. The upper and lower insets of Fig. 5 show the corresponding photographs of different solutions and the linear calibration plot, respectively. The estimated linear detection range and limit of detection (LOD) at a signal-to-noise ratio of 3 are listed in Table 2.| Peroxidase mimics | Linear range (μM) for | LOD (μM) for | Ref. | ||
|---|---|---|---|---|---|
| H2O2 detection | Glucose detection | H2O2 detection | Glucose detection | ||
| MoS2–Pt74Ag26 | 1–50 | 1–10 | 0.4 | 0.8 | This work |
| MoS2 nanosheets | 5–100 | 5–150 | 1.5 | 1.2 | 41 |
We also designed a method for the detection of glucose using the MoS2–Pt74Ag26-based sensing system (see Experimental section). The dependence of the absorbance at 652 nm on the concentration of glucose from 1 μM to 1 mM is shown in Fig. 6a. One can see that the intensity of absorbance peak at 652 nm increases with increased glucose concentration. To examine the selectivity of the present assay toward glucose, detection experiments have been performed in the presence of glucose analogue substances including glucose, fructose, lactose and mannitol. A blue color solution was obtained in the presence of glucose, however, no obvious blue color is observed for other compounds although their concentration is 5-fold higher than that of glucose (Fig. 6b, inset). The above observations indicate that our sensing system exhibits excellent selectivity for glucose.
The linear detection range and LOD for the detection of H2O2 and glucose are listed in Table 2. Lower LOD values are obtained with MoS2–Pt74Ag26 as a peroxidase mimic, which indicates more sensitivity than the MoS2 nanosheets.
3. Conclusions
In summary, novel MoS2–Pt74Ag26 hybrid nanomaterials composed of octahedral Pt74Ag26 NP decorated ultrathin MoS2 nanosheets have been successfully prepared by a facile and efficient method and investigated as peroxidase mimics. The obtained MoS2–Pt74Ag26 hybrids have long-term stability and good reusability after repeated cycles of H2O2 sensing. MoS2–Pt74Ag26 has higher affinity for H2O2 than HRP. Meanwhile, the vmax value for MoS2–Pt74Ag26 with TMB was higher than MoS2. The enhanced catalytic activity of hybrid nanomaterials for colorimetric reactions could be attributed to the synergistic effects of octahedral Pt74Ag26 NPs and ultrathin MoS2 nanosheets as catalytic supports. With MoS2–Pt74Ag26 nanohybrids, the production of active oxygen species (˙OH) by H2O2 decomposition was responsible for the oxidation of TMB. On the basis of these findings, a colorimetric method with high sensitivity and selectivity for H2O2 and glucose detection has been developed. The detection of H2O2 and glucose are in a linear range from 1 × 10−6 to 5 × 10−5 mol L−1 and 1 × 10−6 to 1 × 10−5 mol L−1, respectively, with the detection limit down to 4 × 10−7 mol L−1 for H2O2 and 8 × 10−7 mol L−1 for glucose. Lower LOD values have been obtained with MoS2–Pt74Ag26 nanohybrids as peroxidase mimics, indicating that nanohybrids are more sensitive than MoS2 nanosheets. Our approach can be potentially extended to the preparation of a series of other MoS2–PtM nanohybrids (M = Pd, Rh, Au, Cu, Zn, Fe, Co, Ni, etc.) as peroxidase mimics. The present study provides a new avenue for developing highly sensitive sensors for biological and clinical diagnosis applications.4. Experimental section
4.1 Materials
Polyallylamine hydrochloride (molecular weight 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 200
000 to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), silver nitrate, o-phenylenediamine (OPD), D-mannitol, L-cysteine and MoS2 powder were supplied from Alfa Aesar. Chloroplatinic acid hexahydrate (H2PtCl6·6H2O) and 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS) were provided by J&K Scientific Ltd (Beijing, China). 3,3′,5,5′-Tetramethylbenzidine (TMB) was obtained from Acros. Terephthalic acid was bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Formaldehyde solution (40%) was purchased from Xilong Chemical Co., Ltd (Shantou, China). H2O2 was provided by Beijing Chemical Works (Beijing, China). β-D-Glucose, α-lactose, D-fructose, and glucose oxidase (GOx, 50 KU, from Aspergillus niger) were purchased from Sigma-Aldrich. Other reagents and chemicals were at least analytical reagent grade. Ultrapure water was obtained by using a Milli-Q ultrapure system (18 MΩ cm).
000), silver nitrate, o-phenylenediamine (OPD), D-mannitol, L-cysteine and MoS2 powder were supplied from Alfa Aesar. Chloroplatinic acid hexahydrate (H2PtCl6·6H2O) and 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS) were provided by J&K Scientific Ltd (Beijing, China). 3,3′,5,5′-Tetramethylbenzidine (TMB) was obtained from Acros. Terephthalic acid was bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Formaldehyde solution (40%) was purchased from Xilong Chemical Co., Ltd (Shantou, China). H2O2 was provided by Beijing Chemical Works (Beijing, China). β-D-Glucose, α-lactose, D-fructose, and glucose oxidase (GOx, 50 KU, from Aspergillus niger) were purchased from Sigma-Aldrich. Other reagents and chemicals were at least analytical reagent grade. Ultrapure water was obtained by using a Milli-Q ultrapure system (18 MΩ cm).
4.2 Preparation of PAH-functionalized MoS2 nanosheets
PAH-functionalized MoS2 nanosheets (MoS2–PAH) were prepared from commercial MoS2 flakes via a simple liquid exfoliation method. Briefly, commercial MoS2 powder (20 mg) was first ground with NaCl in a grinding miller for 2 h. Subsequently, the ground MoS2 flakes were separated by dissolving NaCl with ultrapure water and then centrifuged. After being dried completely, the obtained MoS2 flakes were dispersed in 20 mL of H2SO4 (95.0–98.0%) for intercalation at 90 °C overnight. Then the products were collected by centrifugation, repeatedly washed with ultrapure water to remove the residual H2SO4, and dispersed into 20 mL of ultrapure water. After that, 80 mg of PAH was added to the obtained dispersion under bath-sonication for 30 min. Then the solution was further probe-sonicated at a power of 325 W for 1 h and centrifuged at 2000 rpm for 10 min. Finally, the supernatant was collected and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to obtain MoS2–PAH nanosheets.
000 rpm for 10 min to obtain MoS2–PAH nanosheets.
4.3 Preparation of MoS2–PtAg
Take the preparation of MoS2–Pt74Ag26 hybrids for example. After 3 mL of MoS2–PAH solution (about 0.1 mg mL−1) was dispersed in 1 mL of ultrapure water, the solution was sonicated for 10 min, followed by addition of 0.3 μmol of AgNO3, 0.9 μmol of H2PtCl6 and 10 μL of aqueous solution of HCHO solution (40%). Then the resulting mixture was transferred to 10 mL Teflon-lined stainless-steel autoclave and was heated at 180 °C for 4 h. After being cooled to room temperature, the obtained hybrid material was separated by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, washed with ultrapure water, and then dispersed into ultrapure water.
000 rpm for 10 min, washed with ultrapure water, and then dispersed into ultrapure water.
4.4 Characterization
Transmission electron microscopy (TEM) images were obtained using a FEI Tecnai G2 20 S-TWIN transmission electron microscope operating at an accelerating voltage of 200 kV. Scanning electron microscopy (SEM) images were collected on a Hitachi S-4800 field-emission scanning electron microscope. X-ray powder diffraction (XRD) was performed using a Bruker D8 focus with Cu Kα radiation (λ = 1.5406 Å). High-resolution transmission electron microscopy (HRTEM) images were collected with a FEI Tecnai G2 F20 U-TWIN. Partial HRTEM images were collected on a JEOL JEM 2100F transmission electron microscope. Atomic force microscopy (AFM) characterization was performed on a Shimadzu SPM-9600 microscope. The composition of the products was measured by using a Thermo Scientific iCAP 6300 inductively coupled plasma optical emission spectrometer (ICP-OES). Zeta potential was measured using a Malvern Zetasizer Nano system. UV-Vis absorption spectroscopic measurements were carried out using a PerkinElmer Lambda 950 UV/VIS spectrophotometer. FT-IR spectra were recorded on a PerkinElmer Spectrum One FT-IR spectrometer in the transmission mode using CaF2. X-ray photoelectron spectra (XPS) were recorded using a Thermo Fisher ESCALAB 250Xi X-ray photoelectron spectrometer. Binding energies (BE) were calibrated by setting the measured BE of C 1s to 284.8 eV. Electron spin resonance (ESR) spectra were recorded at room temperature on a JEOL JES-FA200 ESR spectrometer. DMPO (5,5-dimethyl-1-pyrroline N-oxide) was employed as the radical trap. Fluorometric measurements were carried out by using a Hitachi F-4600 fluorescence spectrophotometer.4.5 Peroxidase-like activity of MoS2–PtAg
In the presence of H2O2, the activity of MoS2–Pt74Ag26 was investigated through the oxidation of TMB, ABTS and OPD to generate a colorimetric reaction. The reactions were carried out at room temperature using 18 nmol of MoS2–Pt74Ag26 and 10 μL of H2O2 (33 mM) in 300 μL of NaAc buffer (pH 4.0) with 10 μL of substrate (50 mM).4.6 Steady-state kinetic assays
The steady-state kinetic assays of the catalytic reactions were carried out at 50 °C in the TMB/H2O2/MoS2–Pt74Ag26 reaction system. With TMB as the substrate, the kinetic analysis of MoS2–Pt74Ag26 was performed with constant H2O2 concentration (1 mM) but varied TMB concentrations (0.6 to 8 mM). Meanwhile, with H2O2 as the substrate, the kinetic measurement was carried out with constant TMB concentration (1 mM) but varied H2O2 concentrations (0.1 to 4 mM).Absorbance values monitored at 652 nm for all reactions were back-converted to TMB concentration derived oxidation products by the Beer–Lambert Law, A = εbC; in which the molar absorption coefficient ε was 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, vitric cuvettes of path length b was 1 cm. Kinetic parameters were fitted based on the Michaelis–Menten equation:
000 M−1 cm−1, vitric cuvettes of path length b was 1 cm. Kinetic parameters were fitted based on the Michaelis–Menten equation:
 was also used to fit kinetic parameters.
was also used to fit kinetic parameters.
4.7 Detection of H2O2 and glucose
For detection of H2O2, measurements were carried out by monitoring the absorbance change at 652 nm. In a typical run, 18 nmol of MoS2–Pt74Ag26 was added into 300 μL of NaAc buffer solution (pH 4.0), followed by addition of 10 μL of TMB solution (50 mM in DMSO). After reaction for 30 min at 50 °C, the UV-vis spectra were recorded.Glucose detection was performed according to the following three steps: (1) 100 μL of GOx (1 mg mL−1) and 100 μL of glucose with different concentrations in 200 μL of Na2HPO4 buffer (10 mM, pH 7.0) were incubated at 37 °C for 1 h; (2) 10 μL of TMB (50 mM) and 18 nmol of MoS2–Pt74Ag26 were added into the above solution; (3) the resulting mixture was incubated at 50 °C for 30 min before measurements.
Acknowledgements
This work was supported by National Natural Science Foundation of China (21261130090, 21501034, 21503053, 21573050) and Chinese Academy of Sciences (XDA09030303). Financial support from CAS Key Laboratory of Biological Effects of Nanomaterials and Nanosafety was gratefully acknowledged.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- M. Chhowalla, H. S. Shin, G. Eda, L. J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- X. Huang, Z. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS PubMed.
- G. Du, Z. Guo, S. Wang, R. Zeng, Z. Chen and H. Liu, Chem. Commun., 2010, 46, 1106–1108 RSC.
- X. Huang, Z. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC.
- S. S. Singha, D. Nandi and A. Singha, RSC Adv., 2015, 5, 24188–24193 RSC.
- A. J. Cheah, W. S. Chiu, P. S. Khiew, H. Nakajima, T. Saisopa, P. Songsiriritthigul, S. Radimane and M. A. A. Hamid, Catal. Sci. Technol., 2015, 5, 4133–4143 CAS.
- L. Yuwen, F. Xu, B. Xue, Z. Luo, Q. Zhang, B. Bao, S. Su, L. Weng, W. Huang and L. Wang, Nanoscale, 2014, 6, 5762–5769 RSC.
- J. Deng, H. Li, J. Xiao, Y. Tu, D. Deng, H. Yang, H. Tian, J. Li, P. Ren and X. Bao, Energy Environ. Sci., 2015, 8, 1594–1601 CAS.
- F. Cheng, J. Chen and X. Gou, Adv. Mater., 2006, 18, 2561–2564 CrossRef CAS.
- Z. Xiang, Z. Zhang, X. Xu, Q. Zhang, Q. Wang and C. Yuan, Phys. Chem. Chem. Phys., 2015, 17, 15822–15828 RSC.
- X. Zhong, H. Yang, S. Guo, S. Li, G. Gou, Z. Niu, Z. Dong, Y. Lei, J. Jin, R. Li and J. Ma, J. Mater. Chem., 2012, 22, 13925–13927 RSC.
- Z. Sun, Q. Zhao, G. Zhang, Y. Li, G. Zhang, F. Zhang and X. Fan, RSC Adv., 2015, 5, 10352–10357 RSC.
- S. Su, C. Zhang, L. Yuwen, X. Liu, L. Wang, C. Fan and L. Wang, Nanoscale, 2016, 8, 602–608 RSC.
- C. Hou, Q. Xu, L. Yin and X. Hu, Analyst, 2012, 137, 5803–5808 RSC.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- Y.-H. Lee, X.-Q. Zhang, W. Zhang, M.-T. Chang, C.-T. Lin, K.-D. Chang, Y.-C. Yu, J. T.-W. Wang, C.-S. Chang, L.-J. Li and T.-W. Lin, Adv. Mater., 2012, 24, 2320–2325 CrossRef CAS PubMed.
- H. S. S. R. Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. R. Rao, Angew. Chem., Int. Ed., 2010, 49, 4059–4062 CrossRef CAS PubMed.
- Z. Zeng, T. Sun, J. Zhu, X. Huang, Z. Yin, G. Lu, Z. Fan, Q. Yan, H. Hoon Hng and H. Zhang, Angew. Chem., Int. Ed., 2012, 51, 9052–9056 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- F. Cheng, J. Chen and X. Gou, Adv. Mater., 2006, 18, 2561–2564 CrossRef CAS.
- J. P. Shi, M. Liu, J. Wen, X. Ren, X. Zhou, Q. Ji, D. Ma, Y. Zhang, C. Jin, H. Chen, S. Deng, N. Xu, Z. Liu and Y. Zhang, Adv. Mater., 2015, 27, 7086–7092 CrossRef CAS PubMed.
- Y. Shi, J.-K. Huang, L. Jin, Y.-T. Hsu, S. F. Yu, L.-J. Li and H. Y. Yang, Sci. Rep., 2013, 3, 1839 Search PubMed.
- L. Yang, W. Zhou, D. Hou, K. Zhou, G. Li, Z. Tang, L. Lia and S. Chen, Nanoscale, 2015, 7, 5203–5208 RSC.
- C. Li, K. L. Shuford, Q.-H. Park, W. Cai, Y. Li, E. J. Lee and S. O. Cho, Angew. Chem., Int. Ed., 2007, 46, 3264–3268 CrossRef CAS PubMed.
- Y. Wu, S. Cai, D. Wang, W. He and Y. Li, J. Am. Chem. Soc., 2012, 134, 8975–8981 CrossRef CAS PubMed.
- J. G. Zhang, Y. Gao, R. A. Alvarez-Puebla, J. M. Buriak and H. Fenniri, Adv. Mater., 2006, 18, 3233–3237 CrossRef CAS.
- C.-C. Chang, H.-L. Wu, C.-H. Kuo and M. H. Huang, Chem. Mater., 2008, 20, 7570–7574 CrossRef CAS.
- W. Zhou, Z. Yin, Y. Du, X. Huang, Z. Zeng, Z. Fan, H. Liu, J. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- Z. Chen, D. Cummins, B. N. Reinecke, E. Clark, M. K. Sunkara and T. F. Jaramillo, Nano Lett., 2011, 11, 4168–4175 CrossRef CAS PubMed.
- B. Zhou, Z. Sun, D. Li, T. Zhang, L. Deng and Y.-N. Liu, Nanoscale, 2013, 5, 2669–2673 RSC.
- L. Yuwen, F. Xu, B. Xue, Z. Luo, Q. Zhang, B. Bao, S. Su, L. Weng, W. Huang and L. Wang, Nanoscale, 2014, 6, 5762–5769 RSC.
- M. E. Grass, Y. Yue, S. E. Habas, R. M. Rioux, C. I. Teall, P. Yang and G. A. Somorjai, J. Phys. Chem. C, 2008, 112, 4797–4804 CAS.
- M. Liu, Y. Zheng, L. Zhang, L. Guo and Y. Xia, J. Am. Chem. Soc., 2013, 135, 11752–11755 CrossRef CAS PubMed.
- M. Jin, H. Zhang, Z. Xie and Y. Xia, Energy Environ. Sci., 2012, 5, 6352–6357 CAS.
- J. Mu, Y. Wang, M. Zhao and L. Zhang, Chem. Commun., 2012, 48, 2540–2542 RSC.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Y. Yan, Nat. Nanotechnol., 2007, 2, 577–283 CrossRef CAS PubMed.
- T. Lin, L. Zhong, L. Guo, F. Fu and G. Chen, Nanoscale, 2014, 6, 11856–11862 RSC.
- T. Zhang, Y. Lu and G. Luo, ACS Appl. Mater. Interfaces, 2014, 6, 14433–14438 CAS.
- L. Su, J. Feng, X. Zhou, C. Ren, H. Li and X. Chen, Anal. Chem., 2012, 84, 5753–5758 CrossRef CAS PubMed.
- K. Ishibashi, A. Fujishima, T. Watanabe and K. Hashimoto, Electrochem. Commun., 2000, 2, 207–210 CrossRef CAS.
- P. D. Josephy, T. Eling and R. P. Mason, J. Biol. Chem., 1982, 257, 3669–3675 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr08038j |
| This journal is © The Royal Society of Chemistry 2016 |