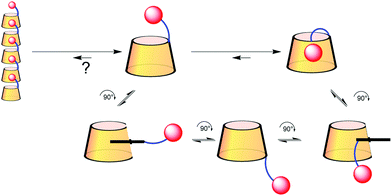
Cyclodextrin-adamantane conjugates, self-inclusion and aggregation versus supramolecular polymer formation†‡
Diem Ngan
Tran
ab,
Dmitri
Colesnic
ab,
Ségolène
Adam de Beaumais
ab,
Gaëlle
Pembouong
ab,
François
Portier
ab,
Álvaro Antelo
Queijo
c,
José
Vázquez Tato
c,
Yongmin
Zhang
ab,
Mickaël
Ménand
ab,
Laurent
Bouteiller
*ab and
Matthieu
Sollogoub
*ab
aSorbonne Universités, UPMC Univ Paris 06, Institut Universitaire de France, UMR CNRS 8232, IPCM, F-75005 Paris, France. E-mail: laurent.bouteiller@upmc.fr; matthieu.sollogoub@upmc.fr
bCNRS, UMR 8232, Institut Parisien de Chimie Moléculaire (IPCM), F-75005 Paris, France
cUniversidad de Santiago de Compostela, Campus de Lugo, Facultad de Ciencias, Avda Alfonso X el Sabio s/n, 27002 Lugo, Spain
First published on 6th June 2014
Abstract
Cyclodextrin-adamantane conjugates have been prepared and their ability to form supramolecular polymers was tested. It appears that they are either insoluble when the link is too rigid or they form self-included derivatives inhibiting the formation of the polymer.
Introduction
Cyclodextrins (CDs) are hydrosoluble cyclic oligosaccharides presenting a lamp-shade shape delineating a hydrophobic cavity. Due to this shape they can form inclusion complexes in water with hydrophobic guests. Consequently, CDs conjugated to a hydrophobic moiety have been widely studied for their ability to form supramolecular oligomeric assemblies.1 In these assemblies the hydrophobic moiety linked to a given CD is included in the cavity of the next. The size of the assembly is generally governed by the affinity of the hydrophobic guest for the CD cavity, in an isodesmic model, according to the Carothers equation.2 In this context, the well-known high affinity of adamantane for the β-CD cavity should have been exploited and studied in detail to form supramolecular polymers. But, rather surprisingly, only a few examples have been reported in the literature. This might be due to the report by Lincoln on a β-CD-adamantane conjugate with a flexible linker in which the adamantane moiety is included in the cavity of the CD bearing it.3 The self-included adamantane complex was not displaced by 2 equivalents of adamantane carboxylate illustrating the high stability of this complex. The authors actually question the reason for this high stability that might be due to the synthesis where the arm could be already self-included and its functionalization with adamantane would form a stable “slip-knot”. This report prompted Tato to use a very short and rigid linker to conjugate β-CD and adamantane to prevent self-inclusion, which afforded a linear polymer described in the solid state.4 Harada conjugated α-CD with adamantane through its secondary rim, no self-inclusion was reported here.5 Finally, Ritter reported a series of triazol-linked β-CDs with various hydrophobic groups6 including adamantane.7 The link here is semi-rigid and the association in water was characterized using dynamic light scattering (DLS). This technique showed that the conjugate produced assemblies with higher hydrodynamic radii than in the absence of adamantane. However, those radii increased with time, which was attributed to some association through hydrogen bonding rather than inclusion.Results and discussion
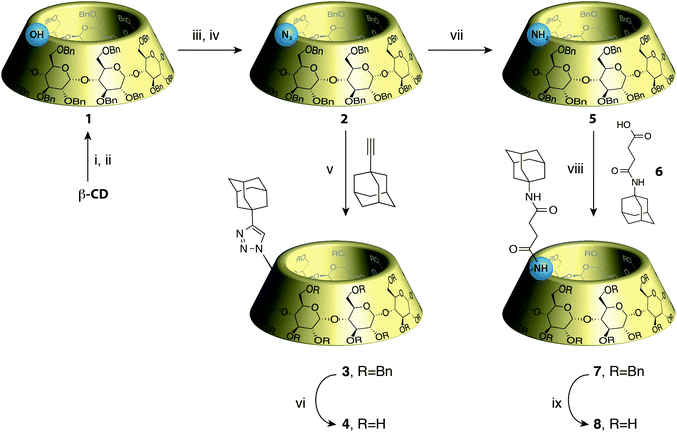
Based on those observations, we designed a CD-adamantane conjugate 4 with a very rigid triazol link. The synthesis started by the classical benzylation/monodebenzylation sequence on β-CD to give alcohol 1,8 which was then converted into the azido-CD 29 through mesylation followed by nucleophilic azidation.10 Copper assisted azide–alkyne cycloaddition of 2 with 1-adamantylacetylene under micro-waves gave the protected conjugate 3 in 86% yield, which was deprotected through hydrogenolysis to give the desired conjugate 4 (Scheme 1). Unfortunately, compound 4 appeared to be insoluble in water rendering the study of its self-assembly impossible. This might also explain why Tato's conjugate assembly was only described in the solid phase. We then turned our attention to a more flexible and potentially more soluble linker, and inspired by another work by Tato11 we oriented our study toward CD 8. Azido CD 2 was therefore reduced to the amine using LiAlH4 to give CD 5 in 65% yield.9 Peptide coupling of 5 with adamantane linked to an acid 6 was carried out to give 7 in 56% yield, which in turn was deprotected to give the desired CD conjugate 8 in 81% yield (Scheme 1).CD 8 is soluble in water and the study of its self assembly could be performed by DLS, NMR, viscometry and ITC. First, it has to be said that the solubility of CD 8 is not very high as the solution becomes turbid above 5 mM concentration. At this concentration, in an aqueous solution containing NaN3, DLS showed formation of species with a hydrodynamic radius of 145 nm, corresponding to the previously reported assembly sizes. Such a hydrodynamic radius is actually much larger than what is expected if the only interaction involved is host–guest complexation. Indeed, the association constant between adamantane derivatives and β-CD is at best on the order of K = 105 L mol−1, which translates into a number average degree of polymerization DPn ∼ (KC)0.5∼20 (at a concentration C = 5 mM), based on a classical mass action law model.12 Therefore, the curvilinear end-to-end distance of the chains should be on the order of 20 nm. The one order of magnitude larger hydrodynamic radius measured is therefore a clear indication that other interactions than simple host–guest complexation are driving the assembly.
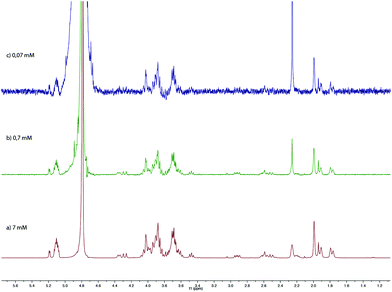
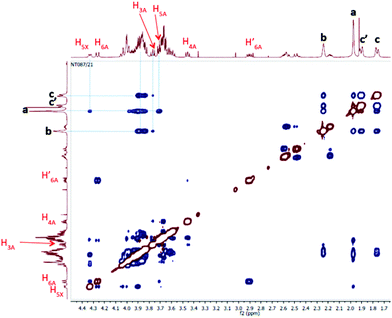
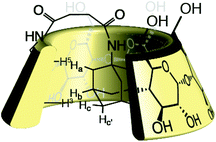
We further studied our system by NMR. In a standard study, we performed a dilution, hoping to observe shifts and sharpening of signals. However, no chemical shift changes could be observed, nor sharpening of the signals upon 10 and 100 times dilutions starting from a 7 mM solution of CD 8 was observed (Fig. 1). We then operated a detailed assignment of the 1H NMR spectrum and we were lucky enough to observe that two H-5s and two H-3s were clearly identifiable (see ESI Fig. SI9–SI16‡). The ROESY experiment then showed that adamantane was indeed inside the cavity but upside down. The 2D spectrum clearly shows cross-correlations only between H-5s of the CD and Ha of the adamantane, when the H-3s of the CD cross-correlate with Hb and Hc of the adamantane moiety (Fig. 2 and 3). Consequently compound 8 is in a self included form. We next performed DOSY experiments depending on the concentration of CD 8, and observed that whatever the concentration the diffusion coefficient remains in the same range as that for monomeric CD13 (Fig. 4).
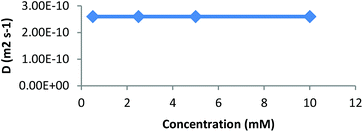 | ||
| Fig. 4 Diffusion coefficient (D) obtained by DOSY experiments (D2O, 600 MHz) as a function of the concentration of CD 8. | ||
We next performed capillary viscosity measurements, and observed no difference in flow time between the blank and the 5 mM solution of CD-adamantyl 8. Finally, we probed intermolecular interactions by isothermal titration calorimetry (ITC). Dilution of a 2.5 mM solution of CD-adamantyl 8 in water produced a weak and concentration independent heat exchange (Fig. 5). In contrast, dilution of an equimolar solution of β-CD and succinic acid linked adamantane 6 produced a strong endothermic effect that can be quantitatively fitted with a simple 1 to 1 association model, yielding an association constant K = 3.5 × 104 L mol−1 and a favourable association enthalpy ΔHassoc = −4.4 kcal mol−1. This means that in the concentration range probed (0.01 to 2.5 mM) CD-adamantyl 8 is not affected by dilution, which rules out the presence of a supramolecular polymer at 2.5 mM, and is in agreement with the presence of self-included monomers and/or very stable aggregates. This result was also confirmed by NMR through successive additions of adamantylcarboxylate to a 5 mM solution of CD 8 in D2O. It is only when we added between 2.8 and 4.5 equivalents of adamantylcarboxylate that chemical shifts of the CD started to be modified (see Fig. SI17‡). The self-included complex is therefore particularly stable.
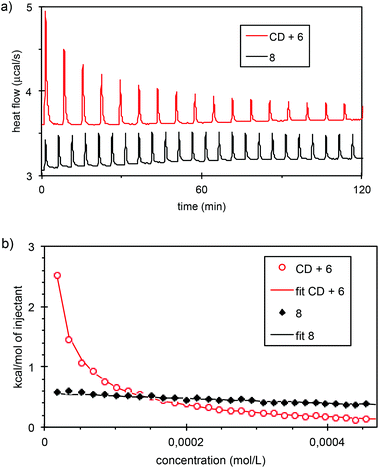 | ||
| Fig. 5 (a) Heat flow curves obtained by dilution of a 2.5 mM solution of CD 8 or by dilution of a 2.5 mM equimolar mixture of β-CD and adamantyl derivative 6. (b) The corresponding ITC enthalpograms. The continuous curves are fits according to ref. 14. | ||
From all those results, we tend to conclude that CD 8 does not form supramolecular polymers because the self-included complex is highly stable. DOSY also did not detect any association whatever the concentration, and no viscosity enhancement could be observed. That leaves us with the DLS results that showed the presence of large species. We believe that this signal is due to the well-known aggregation15 of CDs undetectable by NMR because the aggregates are too large. Furthermore, we can answer to Lincoln interrogation whether the self-included compound was in equilibrium with the empty one, or if the self-included compound was obtained in the conjugation step.3 In our case the conjugation is operated on a benzylated CD 5 excluding self-inclusion at that stage. The self-inclusion, therefore, most probably uses the inversion or tumbling mechanism, which has been mainly observed on CD-dimers16 so far, but which seems to be a rather general mechanism (Scheme 2). In our case, this mechanism could also be favoured by the 12 kJ mol−1 free energy difference in favour of the complexation of adamantane from the secondary rim over that from the primary rim.17
Conclusions
In conclusion, the conjugation of a hydrophobic moiety to β-CD is a rather arduous task: if the linker is too rigid the conjugate tends to aggregate and be insoluble, if the linker is more flexible it can form self-included complexes inhibiting the self-association to yield polymers.Acknowledgements
This work was supported by the LabEx MiChem part of French state funds managed by the ANR within the Investissements d'Avenir programme under reference ANR-11-IDEX-0004-02, the Agence Nationale de la Recherche (Supra-HierArchi Project ANR-Blanc SIMI 7-2012) and the programme Emergence-UPMC.Notes and references
- A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875–882 RSC
.
-
Supramolecular Polymers, ed. A. Ciferri, Taylor & Francis, New York, 2nd edn, 2005, 397 Search PubMed
.
- B. L. May, P. Clements, J. Tsanaktsidis, C. J. Easton and S. F. Lincoln, J. Chem. Soc., Perkin Trans. 1, 2000, 463–469 RSC
.
- V. H. Soto Tellini, A. Jover, L. Galantini, F. Meijide and J. Vazquez Tato, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 204–210 Search PubMed
.
- M. Miyauchi and A. Harada, J. Am. Chem. Soc., 2004, 126, 11418–11419 CrossRef CAS PubMed
; A. Miyawaki, M. Miyauchi, Y. Takashima, H. Yamaguchi and A. Harada, Chem. Commun., 2008, 456–458 RSC
.
- M. Munteanu, U. Kolb and H. Ritter, Macromol. Rapid Commun., 2010, 31, 616–618 CrossRef CAS PubMed
; A. Maciollek, H. Ritter and R. Beckert, Beilstein J. Org. Chem., 2013, 9, 827–831 CrossRef PubMed
.
- M. Munteanu, S. Choi and H. Ritter, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 197–202 CrossRef CAS
.
- T. Lecourt, A. Herault, A. J. Pearce, M. Sollogoub and P. Sinaÿ, Chem. – Eur. J., 2004, 10, 2960–2971 CrossRef CAS PubMed
; S. Guieu and M. Sollogoub, J. Org. Chem., 2008, 73, 2819–2828 CrossRef PubMed
.
- S. Guieu and M. Sollogoub, Angew. Chem., Int. Ed., 2008, 47, 7060–7063 CrossRef CAS PubMed
.
- S. Hanessian, A. Benalil and C. Laferriere, J. Org. Chem., 1995, 60, 4786–4797 CrossRef CAS
.
-
Á. A. Queijo, PhD thesis, Santiago de Compostela, may 5th, 2008
.
- V. Simic, L. Bouteiller and M. Jalabert, J. Am. Chem. Soc., 2003, 125, 13148–13154 CrossRef CAS PubMed
.
- Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520–554 CrossRef CAS PubMed
.
- A. Arnaud and L. Bouteiller, Langmuir, 2004, 20, 6858–6863 CrossRef CAS PubMed
.
- A. W. Coleman, I. Nicolis, N. Keller and J. P. Dalbiez, J. Inclusion Phenom. Macrocyclic Chem., 1992, 13, 139–143 CrossRef CAS
; M. Bonini, S. Rossi, G. Karlsson, M. Almgren, P. Lo Nostro and P. Baglioni, Langmuir, 2006, 22, 1478–1484 CrossRef PubMed
; Y. He, P. Fu, X. Shen and H. Gao, Micron, 2008, 39, 495–516 CrossRef PubMed
.
- T. Yamada, G. Fukuhara and T. Kaneda, Chem. Lett., 2003, 32, 534–535 CrossRef CAS
; K. Yamauchi, A. Miyawaki, Y. Takashima, H. Yamaguchi and A. Harada, Org. Lett., 2010, 12, 1284–1286 CrossRef PubMed
; S. Menuel, N. Azaroual, D. Landy, N. Six, F. Hapiot and E. Monflier, Chem. – Eur. J., 2011, 17, 3949–3955 CrossRef PubMed
; V. Legros, C. Vanhaverbeke, F. Souard, C. Len and J. Désiré, Eur. J. Org. Chem., 2013, 2583–2590 CrossRef
; J. Potier, S. Menuel, N. Azaroual, E. Monflier and F. Hapiot, Eur. J. Org. Chem., 2014, 1547–1556 CrossRef
.
- J. Carrazana, A. Jover, F. Meijide, V. H. Soto and J. V. Tato, J. Phys. Chem. B, 2005, 109, 9719–9726 CrossRef CAS PubMed
.
Footnotes |
| † Dedicated to Professor Max Malacria on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental section, NMR analysis, ITC experimental and adamantine carboxylate addition experiments. See DOI: 10.1039/c4qo00104d |
| This journal is © the Partner Organisations 2014 |