Open Access Article
Open Access ArticleChemical recycling of imine-linked covalent organic frameworks
Yimiao Jing,
Jie Wang,
Yu Fang and
Zhongshan Liu
and
Zhongshan Liu *
*
MOE Key Laboratory of Applied Surface and Colloid Chemistry, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an, 710119, China. E-mail: zhongshan.liu@snnu.edu.cn
First published on 11th February 2026
Abstract
Imine-linked covalent organic frameworks (COFs) have been explored for various applications; however, chemical recycling of end-of-life COFs is an undeveloped area of research. Here, we report closed-loop recycling methods for imine-linked COFs, realizing their chemical depolymerization and reconstruction through dynamic imine chemistry. An alkyl monoamine with a stronger nucleophilicity is adopted to attack aromatic imine linkages of COFs at room temperature, depolymerizing the crosslinked frameworks into small molecular alkyl-imines and aromatic amine monomers. To accelerate depolymerization rates, we further combine the monoamine with Sc(OTf)3 to shorten depolymerization time by a factor of 8. The alkyl-substituted imines are unstable in the presence of acetic acid and easily revert back to the aromatic imines via reversible transamination, enabling in situ regeneration of COFs without tedious monomer purification and with recoveries up to 92%. As evidenced by powder X-ray diffraction and nitrogen and benzene vapor adsorption measurements, the recovered COFs retain their crystallinity, characteristic pore size and adsorption performance. The successful recycling of imine-linked COFs offers a promising strategy for the sustainable development of porous organic materials.
Introduction
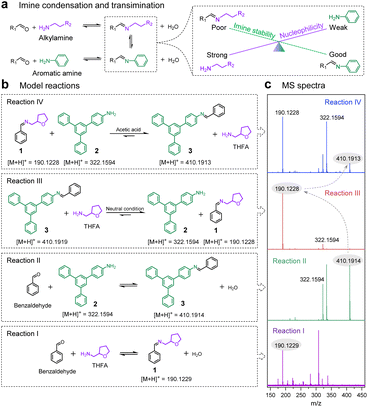
Covalent organic frameworks (COFs), featuring customizable topologies and permanent porosities,1 have demonstrated potential applications in adsorption,2–5 separation,6–8 catalysis,9–11 and sensing.12–15 Despite their functional superiority, the high cost of building blocks is one of the limitations for COF production on a large scale and their commercial applications. Especially for imine-linked COFs, end-of-life materials may release toxic compounds, such as anilines that can serve as precursors of disinfection byproducts in water.16 Unlike booming synthetic methods and applications, the chemical recycling of end-of-life COFs remains undeveloped. But this should be of significance for environmental and economic sustainability and for practical applications of COF materials. Several studies have attempted physical recovery strategies by means of adsorption–desorption or magnetic separation.17–19 However, these methods struggle to retain the structural integrity and performance stability of the recovered COFs and also fail to recycle physically inseparable COF moieties from composite materials. Therefore, developing a chemical recycling strategy that can regenerate high-quality COFs is highly desired.Dynamic imine chemistry, which is classified as reversible imine condensation, transimination and imine metathesis, has evolved from the study of small molecular reaction mechanisms to the synthesis of functional supermolecules and polymers.20–25 Typically, reactions of aldehydes with amines generate imine compounds that can also revert back to starting monomers (Fig. 1a). This reversibility between imine condensation and hydrolysis enables polymeric network self-correction under suitable conditions and formation of crystalline imine-linked COFs.26 Compared to imine condensation, transamination based on amine–imine exchange shows a controllable reaction process, which has emerged as a versatile tool to produce single-crystal COFs.27,28
Most imine-linked COFs are made with aromatic amines because rigid conjugated networks contribute to framework formation and good stability. However, aromatic amines have an inferior nucleophilicity compared to alkylamines. This difference would drive the hydrolysis of aromatic imines and formation of alkyl-substituted imines (Fig. 1a). Paradoxically, preferentially formed alkyl-imines possess relatively poorer stability than conjugated aromatic imines, especially under acidic conditions. Inspired by such transimination, here we present a closed-loop recycling method for imine-linked COFs, including two steps of chemical depolymerization and regeneration. An alkyl monoamine is used as a nucleophilic agent to depolymerize imine-linked frameworks into small molecular alkyl-imines and aromatic amine monomers. Then we leverage the instability of alkyl-imines to regenerate crystalline COFs in the presence of acetic acid.
Although the transimination reactions of small molecules in dilute solutions were well studied using the NMR technique,23,29–31 the path toward the solid COF recovery poses several challenges: (i) unlike model reactions with small molecules, the heterogeneous depolymerization of crosslinked COFs involves a complex dynamic process, but it is poorly understood. (ii) The amount of monoamine that is required for complete depolymerization of solid COFs is uncertain. (iii) The kinetic limitations between liquid reagents and solid COFs take more time. (iv) Reverting back to aromatic imine-linked amorphous polymers may easily occur, but reconstruction of original properties of COFs is difficult. For instance, their crystallinity and porosity are two necessary criteria to define frameworks. To tackle these problems, we need to optimize the ratios of monoamine to COFs and use an NMR instrument and a mass spectrometer (MS) to identify the depolymerization products. After building depolymerization protocols with monoamine, we introduce a catalyst of scandium triflate (Sc(OTf)3) to accelerate the transimination process,24 shortening depolymerization time by a factor of 8. Leveraging the instability of alkyl-substituted imines, we propose two recovery routes under acidic conditions. The crystallinity, porosity and adsorption capacity of recovered COFs are confirmed by powder X-ray diffraction (PXRD) and physical adsorption measurements.
Results and discussion
Model reactions
Fig. 1b depicts that the transimination direction depends on reaction conditions. We chose an alkyl monoamine of tetrahydrofurfurylamine (THFA), a primary amine featuring a cyclic ether moiety that has strong nucleophilicity with good solubility in either water or organic solvents, to synthesize an alkyl-imine 1 (Reaction I). An aromatic monoamine of 3′,5′-diphenylbiphenyl-4-amine (2) was used to produce a compound 3 that contains a typical linkage of imine-linked COFs (Reaction II). Their formation and transimination were studied using MS (Fig. 1c) and liquid chromatography (Fig. S1). In neutral solvents (e.g., methanol and dichloromethane), THFA possessing a stronger nucleophilicity than monomer 2 enabled the transformation of aromatic imine 3 into an alkyl-substituted imine 1 (Reaction III). But in the presence of acetic acid, more basic THFA made itself preferentially protonated, leaving the monomer 2 to revert back to aromatic imine 3 (Reaction IV). Generally, the aromatic imine 3 with a conjugated structure possesses relatively good chemical stability, which is the essential prerequisite for various applications of imine-linked COFs. Our initial proposal was to use an aqueous solution of HCl or NaOH to depolymerize crosslinked COFs, but it was unfeasible (Fig. S2). Based on the amine–imine exchange reaction results, we employed THFA to chemically depolymerize imine-linked COFs.Room-temperature depolymerization of TAPB-TPA COF
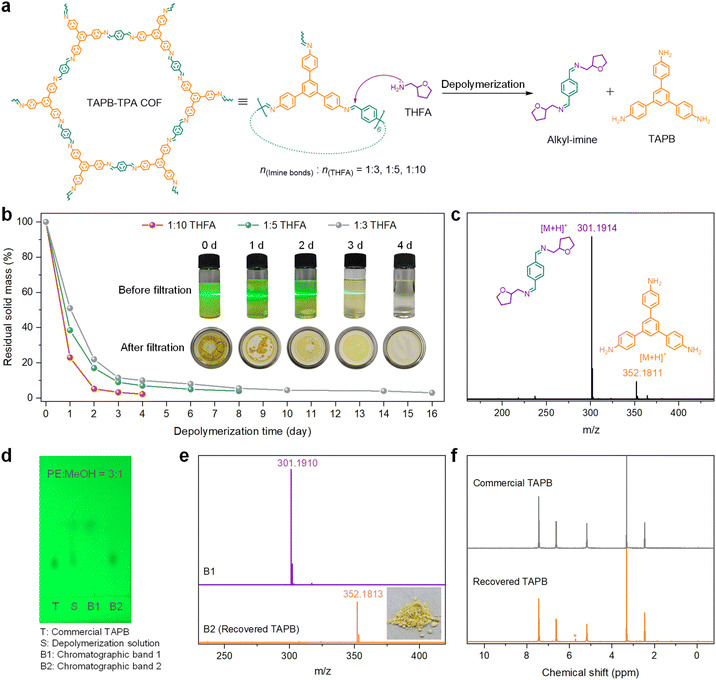
As a demo, TAPB-TPA COF was synthesized through the condensation of 1,3,5-tri(4-aminophenyl)benzene (TAPB) with terephthalaldehyde (TPA) according to our reported method32 (Fig. 2a, Scheme S1, Fig. S3 and S4, SBET = 1778 m2 g−1 and pore width = 2.95 nm). The theoretical content of imine bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) was calculated to be 6 mmol per gram of TAPB-TPA COF solid. The depolymerization reaction was carried out at room temperature by mixing THFA and TAPB-TPA COF powder in dichloromethane (Fig. 2a). We set molar ratios of imine bonds to THFA at 1
N) was calculated to be 6 mmol per gram of TAPB-TPA COF solid. The depolymerization reaction was carried out at room temperature by mixing THFA and TAPB-TPA COF powder in dichloromethane (Fig. 2a). We set molar ratios of imine bonds to THFA at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively, to investigate the depolymerization process. As shown in Fig. 2b, the TAPB-TPA COF dispersed in dichloromethane declined along with the depolymerization time when the 1
10, respectively, to investigate the depolymerization process. As shown in Fig. 2b, the TAPB-TPA COF dispersed in dichloromethane declined along with the depolymerization time when the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA condition was adopted. An obvious change from a turbid yellow suspension to a transparent colloidal dispersion (Fig. S5) was observed. Residual solids in depolymerization mixtures were filtered using a 0.1 µm filter (Fig. 2b, inset, and Fig. S6), weighed, and characterized using a scanning electron microscope (SEM, Fig. S7). Percentages of residual solids regarding to the mass of pristine TAPB-TPA COF were calculated to be 23.0% (1 day), 5.2% (2 days), 3.3% (3 days) and 2.2% (4 days). In contrast, 1
10 THFA condition was adopted. An obvious change from a turbid yellow suspension to a transparent colloidal dispersion (Fig. S5) was observed. Residual solids in depolymerization mixtures were filtered using a 0.1 µm filter (Fig. 2b, inset, and Fig. S6), weighed, and characterized using a scanning electron microscope (SEM, Fig. S7). Percentages of residual solids regarding to the mass of pristine TAPB-TPA COF were calculated to be 23.0% (1 day), 5.2% (2 days), 3.3% (3 days) and 2.2% (4 days). In contrast, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA and 1
3 THFA and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 THFA conditions took longer times of 8 and 16 days, respectively, for complete depolymerization (Fig. 2b, S8 and S9). It should be noted that the sharp decline of the TAPB-TPA COF amount occurred within 4 days, corresponding to a residual solid mass of 10.0% for 1
5 THFA conditions took longer times of 8 and 16 days, respectively, for complete depolymerization (Fig. 2b, S8 and S9). It should be noted that the sharp decline of the TAPB-TPA COF amount occurred within 4 days, corresponding to a residual solid mass of 10.0% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA and 7.0% for 1
3 THFA and 7.0% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 THFA. These results proved that THFA can disassemble imine-linked frameworks of TAPB-TPA COF.
5 THFA. These results proved that THFA can disassemble imine-linked frameworks of TAPB-TPA COF.
Products in a four-day depolymerization mixture obtained with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA were confirmed by MS and NMR techniques. As shown in Fig. 2c, m/z peaks at 301.1914 and 352.1811 were assigned to a THFA-substituted imine compound (denoted as alkyl-imine) and TAPB monomer, respectively. They were purified through silica flash column chromatography with a mobile phase of petroleum ether/methanol (PE/MeOH, 3
10 THFA were confirmed by MS and NMR techniques. As shown in Fig. 2c, m/z peaks at 301.1914 and 352.1811 were assigned to a THFA-substituted imine compound (denoted as alkyl-imine) and TAPB monomer, respectively. They were purified through silica flash column chromatography with a mobile phase of petroleum ether/methanol (PE/MeOH, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Two chromatographic bands of B1 and B2 were observed and assigned to alkyl-imine and TAPB monomer as evidenced in the thin-layer chromatogram (Fig. 2d), MS spectra (Fig. 2e) and 1H NMR spectra (Fig. 2f). With respect to the mass of pristine TAPB-TPA COF, 46% TAPB was recovered. We utilized the recovered TAPB and commercial TPA monomer to synthesize a fresh TAPB-TPA COF. Its diffraction peaks at 2.48°, 4.56°, 5.25°, 7.15° and 9.54° were in accordance with the pristine material (Fig. S10). The nitrogen adsorption isotherm indicated a Brunauer–Emmett–Teller (BET) surface area of 1178 m2 g−1 and characteristic pore width of 2.95 nm for the fresh TAPB-TPA COF (Fig. S11).
1, v/v). Two chromatographic bands of B1 and B2 were observed and assigned to alkyl-imine and TAPB monomer as evidenced in the thin-layer chromatogram (Fig. 2d), MS spectra (Fig. 2e) and 1H NMR spectra (Fig. 2f). With respect to the mass of pristine TAPB-TPA COF, 46% TAPB was recovered. We utilized the recovered TAPB and commercial TPA monomer to synthesize a fresh TAPB-TPA COF. Its diffraction peaks at 2.48°, 4.56°, 5.25°, 7.15° and 9.54° were in accordance with the pristine material (Fig. S10). The nitrogen adsorption isotherm indicated a Brunauer–Emmett–Teller (BET) surface area of 1178 m2 g−1 and characteristic pore width of 2.95 nm for the fresh TAPB-TPA COF (Fig. S11).
Two in situ recycling routes for TAPB-TPA COF
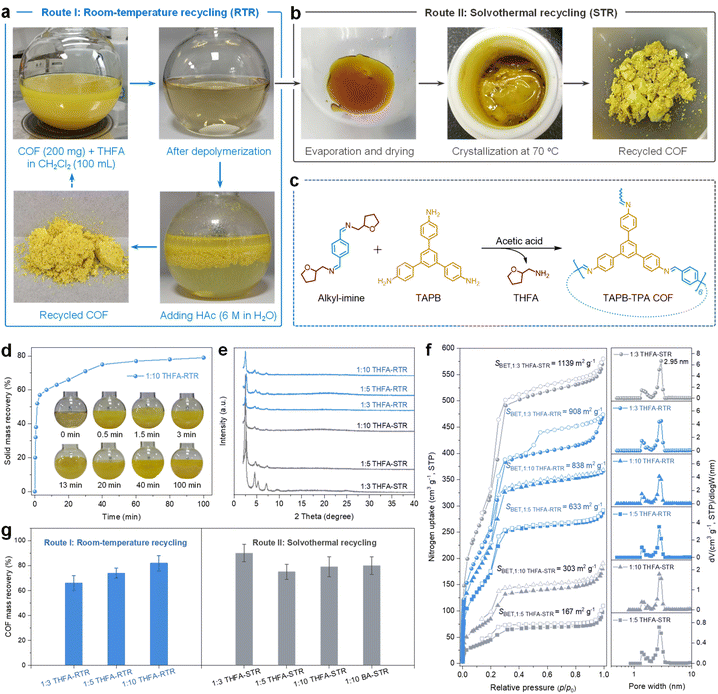
Inspired by the transamination reversibility in acetic acid solution (Fig. 1b), we proposed two in situ recycling methods for TAPB-TPA COF, including room-temperature recycling (RTR, Route I, Fig. 3a) and solvothermal recycling (STR, Route II, Fig. 3b). Both recycling routes did not require tedious monomer purifications. The depolymerization products underwent an in situ reversion back to the crosslinked TAPB-TPA COF (Fig. 3c). As a demonstration, we scaled up the depolymerization mixture by dispersing 200 mg of TAPB-TPA COF in 100 mL of THFA-containing dichloromethane solution. For Route I, aqueous acetic acid (18 mL, 6 mol L−1) was added to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA depolymerization mixture, accompanied by the precipitation of 58% solid (with respect to the theoretical solid mass) at 5 min and an approximate plateau within 40 min (Fig. 3d). In order to achieve the crystalline TAPB-TPA COF, reaction mixtures were stirred at ambient pressure and temperature for 24 h. Route II was built on the success of using the solvothermal method for synthesizing TAPB-TPA COF. Briefly, the depolymerization mixtures were evaporated and dried, to which solvents of 1,4-dioxane, 1,3,5-trimethylbenzene, glacial acetic acid and water were added. The resulting mixture was transferred to a Teflon-sealed autoclave and treated at 70 °C for 24 h, regenerating crystalline TAPB-TPA COF (Fig. S12). In the following discussion, TAPB-TPA COFs recycled using different conditions were named 1
10 THFA depolymerization mixture, accompanied by the precipitation of 58% solid (with respect to the theoretical solid mass) at 5 min and an approximate plateau within 40 min (Fig. 3d). In order to achieve the crystalline TAPB-TPA COF, reaction mixtures were stirred at ambient pressure and temperature for 24 h. Route II was built on the success of using the solvothermal method for synthesizing TAPB-TPA COF. Briefly, the depolymerization mixtures were evaporated and dried, to which solvents of 1,4-dioxane, 1,3,5-trimethylbenzene, glacial acetic acid and water were added. The resulting mixture was transferred to a Teflon-sealed autoclave and treated at 70 °C for 24 h, regenerating crystalline TAPB-TPA COF (Fig. S12). In the following discussion, TAPB-TPA COFs recycled using different conditions were named 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA-RTR (Route I) and THFA-STR (Route II), respectively.
10 THFA-RTR (Route I) and THFA-STR (Route II), respectively.
We examined crystallinities, porosities and mass recoveries of the recycled TAPB-TPA COFs. PXRD patterns indicated that all recycled COFs had typical diffraction peaks (Fig. 3e), in accordance with the pristine TAPB-TPA COF (Fig. S3). Nitrogen adsorption isotherms confirmed that the amount of THFA used at the depolymerization stage affected BET surface areas of recycled COFs, but their pore width was united at 2.95 nm (Fig. 3f). For instance, TAPB-TPA COFs recycled from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA depolymerization mixtures showed the highest surface areas of 908 m2 g−1 (1
3 THFA depolymerization mixtures showed the highest surface areas of 908 m2 g−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-RTR) and 1139 m2 g−1 (1
3 THFA-RTR) and 1139 m2 g−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-STR). The 1
3 THFA-STR). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA depolymerization condition, which took the least depolymerization time (4 days), in tandem with two recycling routes, generated surface areas of 838 m2 g−1 (1
10 THFA depolymerization condition, which took the least depolymerization time (4 days), in tandem with two recycling routes, generated surface areas of 838 m2 g−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA-RTR) and 303 m2 g−1 (1
10 THFA-RTR) and 303 m2 g−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA-STR). The TAPB-TPA COF mass recoveries ranged from 66% to 90% (Fig. 3g), which were higher than the TAPB monomer recovery (46%). Besides THFA, we proved that another alkylamine, n-butylamine (BA), can depolymerize TAPB-TPA COF in dichloromethane when the molar ratio of imine bonds to BA was 1
10 THFA-STR). The TAPB-TPA COF mass recoveries ranged from 66% to 90% (Fig. 3g), which were higher than the TAPB monomer recovery (46%). Besides THFA, we proved that another alkylamine, n-butylamine (BA), can depolymerize TAPB-TPA COF in dichloromethane when the molar ratio of imine bonds to BA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. S12). Using Route II, 80% crystalline TAPB-TPA COF was recycled (1
10 (Fig. S12). Using Route II, 80% crystalline TAPB-TPA COF was recycled (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 BA-STR, Fig. 3g and S13), which possessed a BET surface area of 442 m2 g−1 and pore width of 2.95 nm (Fig. S14). Similar compositions and morphologies of all recycled TAPB-TPA COFs were confirmed using FT-IR spectra (Fig. S15) and SEM images (Fig. S16), respectively.
10 BA-STR, Fig. 3g and S13), which possessed a BET surface area of 442 m2 g−1 and pore width of 2.95 nm (Fig. S14). Similar compositions and morphologies of all recycled TAPB-TPA COFs were confirmed using FT-IR spectra (Fig. S15) and SEM images (Fig. S16), respectively.
Considering that the recycled TAPB-TPA COF showed superior porosity with BA compared to THFA (442 vs. 303 m2 g−1), we further tested BA under the optimized THFA recycling conditions (imine/amine = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, STR method, Fig. S12). A highly crystalline TAPB-TPA COF with a BET surface area of 735 m2 g−1 was successfully obtained (Fig. S17 and S18), but, unfortunately, its surface area was lower than that obtained using THFA under identical conditions (1139 m2 g−1, Fig. 3f). The difference in BET surface areas among the recycled and pristine COFs was likely attributed to the loss of crystallinity, as evidenced by the full width at half maximum (FWHM) of the (100) diffraction peak (Table S1). Besides, the residual monoamines in depolymerization mixtures might interfere with framework reconstruction, introducing structural defects and increasing the likelihood of pore collapse during drying.
3, STR method, Fig. S12). A highly crystalline TAPB-TPA COF with a BET surface area of 735 m2 g−1 was successfully obtained (Fig. S17 and S18), but, unfortunately, its surface area was lower than that obtained using THFA under identical conditions (1139 m2 g−1, Fig. 3f). The difference in BET surface areas among the recycled and pristine COFs was likely attributed to the loss of crystallinity, as evidenced by the full width at half maximum (FWHM) of the (100) diffraction peak (Table S1). Besides, the residual monoamines in depolymerization mixtures might interfere with framework reconstruction, introducing structural defects and increasing the likelihood of pore collapse during drying.
Depolymerization method generality
After developing the depolymerization method for TAPB-TPA COF, we further examined its generality for the structurally diverse COFs, including TFB-N2H4 COF (Scheme S2 and Fig. S19–S21), ETTA-TPA COF (Scheme S3 and Fig. S22–S24), COF-LZU1 (Scheme S4 and Fig. S25–S27), COF-300 (Scheme S5 and Fig. S28–S30), [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TAPB-DMTA COF (Scheme S6 and Fig. S31–S33), TAPB-TT COF (Scheme S7 and Fig. S34–S36) and ETTA-TT COF (Scheme S8 and Fig. S37–S39). These frameworks vary in imine linkages and topologies, and some of them contain heteroatom-based functional groups or building blocks with side chains. Their depolymerization kinetics curves are plotted in Fig. 4. As expected, THFA cannot depolymerize TFB-N2H4 COF due to its weaker nucleophilicity than hydrazine. A highly efficient depolymerization process was observed for ETTA-TPA COF, [HC
C]0.17-TAPB-DMTA COF (Scheme S6 and Fig. S31–S33), TAPB-TT COF (Scheme S7 and Fig. S34–S36) and ETTA-TT COF (Scheme S8 and Fig. S37–S39). These frameworks vary in imine linkages and topologies, and some of them contain heteroatom-based functional groups or building blocks with side chains. Their depolymerization kinetics curves are plotted in Fig. 4. As expected, THFA cannot depolymerize TFB-N2H4 COF due to its weaker nucleophilicity than hydrazine. A highly efficient depolymerization process was observed for ETTA-TPA COF, [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TAPB-DMTA COF and COF-LZU1. COF-300 having N-fold interpenetrated structures required relatively long time for complete depolymerization. For TAPB-TT COF and ETTA-TT COF, the electron-rich thiophene unit likely reduced the electrophilicity of carbon in imine bonds, resulting in a slow depolymerization rate.
C]0.17-TAPB-DMTA COF and COF-LZU1. COF-300 having N-fold interpenetrated structures required relatively long time for complete depolymerization. For TAPB-TT COF and ETTA-TT COF, the electron-rich thiophene unit likely reduced the electrophilicity of carbon in imine bonds, resulting in a slow depolymerization rate.
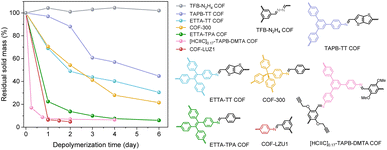 | ||
Fig. 4 Depolymerization kinetics under the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA condition for COFs with different imine linkages and topologies. 10 THFA condition for COFs with different imine linkages and topologies. | ||
To validate the feasibility of our chemical recycling method across structurally diverse COFs, we selected the [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TAPB-DMTA COF as an example for recovery study. Under the 1
C]0.17-TAPB-DMTA COF as an example for recovery study. Under the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA-RTR condition (Fig. S40), 73% of the crystalline COF was successfully recycled (Fig. S41). The recycled COF possessed a BET surface area of 722 m2 g−1 and pore width of 2.73 nm (Fig. S42), which notably exceeded the value of the pristine [HC
10 THFA-RTR condition (Fig. S40), 73% of the crystalline COF was successfully recycled (Fig. S41). The recycled COF possessed a BET surface area of 722 m2 g−1 and pore width of 2.73 nm (Fig. S42), which notably exceeded the value of the pristine [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TAPB-DMTA COF (215 m2 g−1, Fig. S32).
C]0.17-TAPB-DMTA COF (215 m2 g−1, Fig. S32).
Using Sc(OTf)3 to accelerate the depolymerization process
Despite our depolymerization method demonstrating a good versatility for imine-linked COFs, its practical application is hampered by the trade-off between the depolymerization rate and THFA consumption (Fig. 2a). Based on the abovementioned results, we introduced Sc(OTf)3, a catalyst for the fast amine–imine exchange reaction,24 to accelerate the depolymerization rates. Fig. 5a shows that the residual solids were calculated to be 12.0% (6 h), 7.0% (18 h), 5.5% (36 h) and 5.0% (48 h) when 4% Sc(OTf)3, with respect to the molar amount of THFA, was adopted in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA depolymerization mixture. The depolymerization time was shortened by a factor of 8 compared to the 16 days required in the absence of the catalyst (Fig. S43). Even though the Sc(OTf)3 amount decreased to 0.5% under the 1
3 THFA depolymerization mixture. The depolymerization time was shortened by a factor of 8 compared to the 16 days required in the absence of the catalyst (Fig. S43). Even though the Sc(OTf)3 amount decreased to 0.5% under the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA condition, complete depolymerization can be achieved within 96 h (Fig. S44). This efficiency was comparable to that of the 1
3 THFA condition, complete depolymerization can be achieved within 96 h (Fig. S44). This efficiency was comparable to that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA condition without the use of the catalyst. To make the discussion clearer, the depolymerization conditions are denoted as 1
10 THFA condition without the use of the catalyst. To make the discussion clearer, the depolymerization conditions are denoted as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x THFA-y% ScIII, where 1
x THFA-y% ScIII, where 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x indicates the molar ratio of THFA to imine bonds in TAPB-TPA COF and y is the molar percentage of Sc(OTf)3 relative to THFA. With 4% ScIII, the TAPB-TPA COF was largely depolymerized within 48 hours even at lower imines/THFA ratios (1
x indicates the molar ratio of THFA to imine bonds in TAPB-TPA COF and y is the molar percentage of Sc(OTf)3 relative to THFA. With 4% ScIII, the TAPB-TPA COF was largely depolymerized within 48 hours even at lower imines/THFA ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with a depolymerization efficiency of 94.0%, 89.7% and 73.2%, respectively (Fig. 5b and S45).
1), with a depolymerization efficiency of 94.0%, 89.7% and 73.2%, respectively (Fig. 5b and S45).
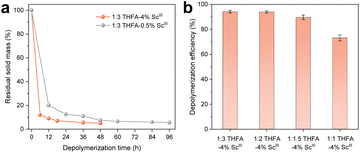 | ||
Fig. 5 (a) Depolymerization time-dependent residual solid mass under 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA in the presence of Sc(OTf)3 to THFA. (b) Effect of THFA amount on depolymerization efficiency. 3 THFA in the presence of Sc(OTf)3 to THFA. (b) Effect of THFA amount on depolymerization efficiency. | ||
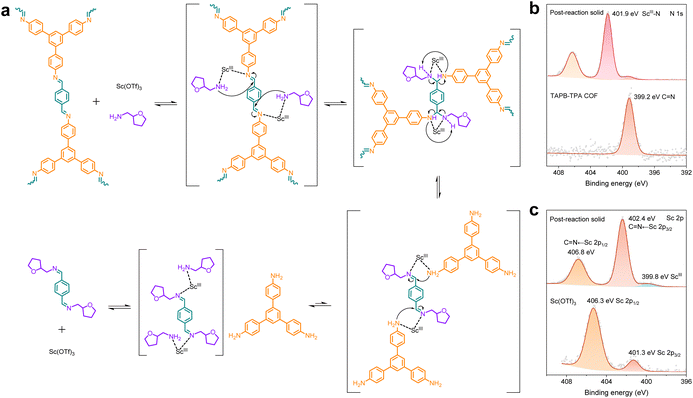
As depicted in Fig. 6a, the proposed catalyzed transamination mechanism involves the simultaneous coordination of the ScIII center to the nitrogen atoms of both the imine bond and THFA. A key ternary intermediate polarized the imine and facilitated the nucleophilic attack of THFA. Then the resulting gem-diamino intermediate collapsed, completing the amine–imine exchange. The subsequent dissociation of Sc(OTf)3 from the new alkyl-imine enables its regeneration, simultaneously releasing the TAPB monomer. To confirm the ScIII–N coordination, X-ray photoelectron spectroscopy (XPS) analysis was performed (Fig. S46). The residual solid sample was collected using a 0.1 µm filter from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-4% ScIII depolymerization mixture, followed by washing with water to remove uncombined Sc(OTf)3. This sample, likely representing a ScIII–N coordinated complex, was compared with the pristine TAPB-TPA COF and pure Sc(OTf)3. As evidenced in N 1s spectra (Fig. 6b), the characteristic imine peak at 399.2 eV shifted to a higher binding energy of 401.9 eV, indicating a significant loss of electron density on nitrogen atoms. This shift was attributed to the formation of the ScIII–N coordinated complex, which was also confirmed using Sc 2p spectra (Fig. 6c).
3 THFA-4% ScIII depolymerization mixture, followed by washing with water to remove uncombined Sc(OTf)3. This sample, likely representing a ScIII–N coordinated complex, was compared with the pristine TAPB-TPA COF and pure Sc(OTf)3. As evidenced in N 1s spectra (Fig. 6b), the characteristic imine peak at 399.2 eV shifted to a higher binding energy of 401.9 eV, indicating a significant loss of electron density on nitrogen atoms. This shift was attributed to the formation of the ScIII–N coordinated complex, which was also confirmed using Sc 2p spectra (Fig. 6c).
We found that excessive amounts of Sc(OTf)3 adversely affected the subsequent reconstruction of COFs (Fig. S47–S49). For instance, using the room-temperature recycling route, we recovered crystalline TAPB-TPA COF from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-4% ScIII depolymerization products. The TAPB-TPA COF recovered from filtrates showed high crystallinity and BET surface area. Therefore, a filtration step was performed to remove excess Sc(OTf)3 in depolymerization prior to the in situ recycling. To identify the optimal balance between the COF and THFA-Sc(OTf)3 amounts, we recorded the room-temperature depolymerization and reconstruction processes in Fig. S50 and summarized recoveries, structural integrities and porosities of recovered TAPB-TPA COF materials. For all depolymerization conditions in tandem with the RTR route, mass recoveries ranged from 79% to 92% (Fig. S51). PXRD patterns confirmed that all recycled COFs displayed typical diffraction peaks. The COFs obtained from 4% Sc(OTf)3 depolymerization mixtures showed stronger intensity peaks compared to 0.5% Sc(OTf)3 counterparts (Fig. S52). Nitrogen adsorption isotherms revealed their pore widths at 2.73 nm and 2.95 nm (Fig. S53). The recovered TAPB-TPA COF using the 1
3 THFA-4% ScIII depolymerization products. The TAPB-TPA COF recovered from filtrates showed high crystallinity and BET surface area. Therefore, a filtration step was performed to remove excess Sc(OTf)3 in depolymerization prior to the in situ recycling. To identify the optimal balance between the COF and THFA-Sc(OTf)3 amounts, we recorded the room-temperature depolymerization and reconstruction processes in Fig. S50 and summarized recoveries, structural integrities and porosities of recovered TAPB-TPA COF materials. For all depolymerization conditions in tandem with the RTR route, mass recoveries ranged from 79% to 92% (Fig. S51). PXRD patterns confirmed that all recycled COFs displayed typical diffraction peaks. The COFs obtained from 4% Sc(OTf)3 depolymerization mixtures showed stronger intensity peaks compared to 0.5% Sc(OTf)3 counterparts (Fig. S52). Nitrogen adsorption isotherms revealed their pore widths at 2.73 nm and 2.95 nm (Fig. S53). The recovered TAPB-TPA COF using the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-4% ScIII condition exhibited the highest specific surface area of 709 m2 g−1, while the 1
3 THFA-4% ScIII condition exhibited the highest specific surface area of 709 m2 g−1, while the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-0.5% ScIII condition in tandem with the RTR route generated only a surface area of 145 m2 g−1.
3 THFA-0.5% ScIII condition in tandem with the RTR route generated only a surface area of 145 m2 g−1.
To further evaluate the potential application of our recycling strategy for end-of-life COFs, the pristine TAPB-TPA COF was intentionally damaged by stirring in 1 mol L−1 HCl for 12 h. The treated COF showed severe losses of crystallinity (Fig. S54) and BET surface area, from 1778 m2 g−1 to 167 m2 g−1 (Fig. S55). As evidenced in Fig. S56 and S57, the end-of-life TAPB-TPA COF was successfully restored using our recycling method of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-4% ScIII-RTR, whose BET surface area increased from 167 m2 g−1 to 747 m2 g−1. The mass recovery was calculated to be 88%.
3 THFA-4% ScIII-RTR, whose BET surface area increased from 167 m2 g−1 to 747 m2 g−1. The mass recovery was calculated to be 88%.
Comparison of depolymerization and recycling routes
The depolymerization conditions and recycling methods (e.g., processing time, mass recovery, crystallization, and BET surface area) are summarized in Tables S2 and S3. Without the use of Sc(OTf)3, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 THFA depolymerization condition (4 days) in tandem with the room-temperature recycling route (1.5 days) is time-saving, regenerating the TAPB-TPA COF with a comparable surface area. The 1
10 THFA depolymerization condition (4 days) in tandem with the room-temperature recycling route (1.5 days) is time-saving, regenerating the TAPB-TPA COF with a comparable surface area. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA condition consumed fewer reagents, but took more time, 16 days, for complete depolymerization. If we can accept the compromise of 90% depolymerization within 4 days, either using 1
3 THFA condition consumed fewer reagents, but took more time, 16 days, for complete depolymerization. If we can accept the compromise of 90% depolymerization within 4 days, either using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-RTR or 1
3 THFA-RTR or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-STR as an alternative strategy is recommended for the recycling of high-quality TAPB-TPA COF. In contrast, the introduction of Sc(OTf)3 remarkably accelerated depolymerization rates. This allows for not only shortening depolymerization time from 16 to 2 days (1
3 THFA-STR as an alternative strategy is recommended for the recycling of high-quality TAPB-TPA COF. In contrast, the introduction of Sc(OTf)3 remarkably accelerated depolymerization rates. This allows for not only shortening depolymerization time from 16 to 2 days (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-4% ScIII), but also efficient depolymerization even at lower THFA/COF ratios. Additionally, we plotted BET surface areas versus depolymerization time in Fig. S58. Among the evaluated conditions, 1
3 THFA-4% ScIII), but also efficient depolymerization even at lower THFA/COF ratios. Additionally, we plotted BET surface areas versus depolymerization time in Fig. S58. Among the evaluated conditions, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 THFA-4% ScIII-RTR is recommended as the optimal strategy for recycling high-quality TAPB-TPA COF.
3 THFA-4% ScIII-RTR is recommended as the optimal strategy for recycling high-quality TAPB-TPA COF.
We found that it was difficult to reconstruct crystallinity and porosity as good as those of the pristine TAPB-TPA COF. As we know, the preparation of COFs is very sensitive to the synthesis conditions (e.g., solvents, catalyst, temperature, and time). Unlike synthesizing COFs starting with pure monomers, residual THFA, catalyst and solvents in the depolymerization mixtures would affect their reconstructions. As such, two recycling routes depicted in Fig. 3a and b were built on a number of attempts.
To functionally assess the pore accessibility of the recycled COFs, we performed benzene vapor adsorption measurements. Benzene has attracted much attention among volatile organic compounds due to its high toxicity. Our previous study has shown the potential application of COFs for benzene removal.4 Here, we utilized benzene as a probe to give an insight into changes in available pores and adsorption capacity between pristine and recycled TAPB-TPA COFs. Consequently, due to the difference in BET surface area between recovered and pristine TAPB-TPA COFs, the benzene vapor adsorption decreased from 14.68 to 5.94 mol per gram of COF (Fig. S59).
Conclusions
In conclusion, we have demonstrated chemical recycling methods of TAPB-TPA COF through dynamic imine chemistry. As evidenced in this work, alkyl monoamines can attack the aromatic imine linkages of COFs in neutral organic solvents, depolymerizing the crosslinked frameworks into small molecules. Moreover, it was found that the combination of monoamines with Sc(OTf)3 remarkably accelerates the depolymerization process, offering a time-saving protocol. Without tedious monomer purifications, we have realized the in situ regeneration of crystalline COFs in complex depolymerization mixtures. Given that most imine-linked COFs are made using aromatic amine building blocks, we anticipate that these findings on their closed-loop recycling open up an avenue in the sustainable development of porous organic materials. Last but not least, we have proved the versatility of a monoamine–imine exchange-based depolymerization strategy for aromatic imine-linked COFs and demonstrated a case study for the reconstruction of TAPB-TPA COF. But we must honestly point out that the optimal reconstruction conditions vary for each building block and framework. Additionally, for COFs that were synthesized by post-modifications or suffered from imine linkage damage, the chemical recycling method in this work needs to be further studied.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc09502f.Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (22372097 to Z. Liu; 22132002 to Y. Fang) and the Fundamental Research Funds for the Central Universities (GK202302001).References
- Y. Liu, X. Liu, A. Su, C. Gong, S. Chen, L. Xia, C. Zhang, X. Tao, Y. Li, Y. Li, T. Sun, M. Bu, W. Shao, J. Zhao, X. Li, Y. Peng, P. Guo, Y. Han and Y. Zhu, Chem. Soc. Rev., 2024, 53, 502–544 RSC.
- X. Liu, H. Pang, X. Liu, Q. Li, N. Zhang, L. Mao, M. Qiu, B. Hu, H. Yang and X. Wang, Innovation, 2021, 2, 100076 CAS.
- S. Karak, K. Dey, A. Torris, A. Halder, S. Bera, F. Kanheerampockil and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 7572–7581 CrossRef CAS PubMed.
- Y. Su, M. Qin, J. Kong, Q. Zhai, D. Yuan, Z. Liu and Y. Fang, Adv. Funct. Mater., 2024, 34, 2400433 CrossRef CAS.
- W. Ji, L. Xiao, Y. Ling, C. Ching, M. Matsumoto, R. P. Bisbey, D. E. Helbling and W. R. Dichtel, J. Am. Chem. Soc., 2018, 140, 12677–12681 CrossRef CAS PubMed.
- R. Wang, Y. Zhou, Y. Zhang, J. Xue, J. Caro and H. Wang, Adv. Mater., 2022, 34, 2204894 CrossRef CAS PubMed.
- Z. Guo, H. Wu, Y. Chen, S. Zhu, H. Jiang, S. Song, Y. Ren, Y. Wang, X. Liang, G. He, Y. Li and Z. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202210466 CrossRef CAS PubMed.
- J. Liu, G. Han, D. Zhao, K. Lu, J. Gao and T.-S. Chung, Sci. Adv., 2020, 6, eabb1110 CrossRef CAS PubMed.
- C. Jin, N. Li, E. Lin, X. Chen, T. Wang, Y. Wang, M. Yang, W. Liu, J. Yu, Z. Zhang and Y. Chen, ACS Catal., 2022, 12, 8259–8268 CrossRef CAS.
- C. Krishnaraj, H. Sekhar Jena, L. Bourda, A. Laemont, P. Pachfule, J. Roeser, C. V. Chandran, S. Borgmans, S. M. J. Rogge, K. Leus, C. V. Stevens, J. A. Martens, V. Van Speybroeck, E. Breynaert, A. Thomas and P. Van Der Voort, J. Am. Chem. Soc., 2020, 142, 20107–20116 CrossRef CAS PubMed.
- H. S. Sasmal, S. Bag, B. Chandra, P. Majumder, H. Kuiry, S. Karak, S. Sen Gupta and R. Banerjee, J. Am. Chem. Soc., 2021, 143, 8426–8436 CrossRef CAS PubMed.
- X. Liu, R. Huang, L. Peng, J. Yang, J. Yan, B. Zhai, Y. Luo, C. Zhang, S. Tan, X. Liu, L. Ding and Y. Fang, Angew. Chem., Int. Ed., 2025, 64, e202414472 CrossRef CAS PubMed.
- G. Das, B. P. Biswal, S. Kandambeth, V. Venkatesh, G. Kaur, M. Addicoat, T. Heine, S. Verma and R. Banerjee, Chem. Sci., 2015, 6, 3931–3939 RSC.
- A. Jrad, G. Das, N. Alkhatib, T. Prakasam, F. Benyettou, S. Varghese, F. Gándara, M. Olson, S. Kirmizialtin and A. Trabolsi, Nat. Commun., 2024, 15, 10490 CrossRef CAS PubMed.
- Z. Li, N. Huang, K. H. Lee, Y. Feng, S. Tao, Q. Jiang, Y. Nagao, S. Irle and D. Jiang, J. Am. Chem. Soc., 2018, 140, 12374–12377 CrossRef CAS PubMed.
- Z. T. Kralles, P. K. Deherikar, C. A. Werner, X. Hu, E. P. Kolodziej and N. Dai, Environ. Sci. Technol., 2024, 58, 17497–17509 CrossRef CAS PubMed.
- Q. Sun, B. Aguila, L. D. Earl, C. W. Abney, L. Wojtas, P. K. Thallapally and S. Ma, Adv. Mater., 2018, 30, 1705479 CrossRef PubMed.
- S. He, T. Zeng, S. Wang, H. Niu and Y. Cai, ACS Appl. Mater. Interfaces, 2017, 9, 2959–2965 CrossRef CAS PubMed.
- L. Zhu, L. Ding, J. Kong, Y. Fang and Z. Liu, Cell Rep. Phys. Sci., 2025, 6, 102813 CrossRef CAS.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- G. Melchiorre, L. Visieri, M. Valentini, R. Cacciapaglia, A. Casnati, L. Baldini, J. A. Berrocal and S. Di Stefano, J. Am. Chem. Soc., 2025, 147, 11327–11335 CrossRef CAS PubMed.
- M. Ciaccia and S. Di Stefano, Org. Biomol. Chem., 2015, 13, 646–654 RSC.
- M. Ciaccia, R. Cacciapaglia, P. Mencarelli, L. Mandolini and S. Di Stefano, Chem. Sci., 2013, 4, 2253–2261 RSC.
- N. Giuseppone, J.-L. Schmitt, E. Schwartz and J.-M. Lehn, J. Am. Chem. Soc., 2005, 127, 5528–5539 CrossRef CAS PubMed.
- J. Wang, R. Wen, J. Kong, J. Liu, Z. Liu and Y. Fang, Angew. Chem., Int. Ed., 2025, 64, e202502020 CrossRef CAS PubMed.
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570–4571 CrossRef CAS PubMed.
- T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L.-H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Science, 2018, 361, 48–52 CrossRef CAS PubMed.
- J. Han, J. Feng, J. Kang, J.-M. Chen, X.-Y. Du, S.-Y. Ding, L. Liang and W. Wang, Science, 2024, 383, 1014–1019 CrossRef CAS PubMed.
- M. Ciaccia, S. Pilati, R. Cacciapaglia, L. Mandolini and S. Di Stefano, Org. Biomol. Chem., 2014, 12, 3282–3287 RSC.
- Y. Zhang, S. Xie, M. Yan and O. Ramström, Chem.–Eur. J., 2017, 23, 11908–11912 CrossRef CAS PubMed.
- D. Schultz and J. R. Nitschke, J. Am. Chem. Soc., 2006, 128, 9887–9892 CrossRef CAS PubMed.
- L. Zhu, Y. Su, Z. Liu and Y. Fang, Small, 2023, 19, 2205501 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |