Open Access Article
Open Access ArticleThe odd–even effect in n-carboxyalkylammonium-containing organic–inorganic hybrids of Mn(II) halides: structural and magnetic characterisation†
Shalene N.
Bothma
 a,
Charles J.
Sheppard
a,
Charles J.
Sheppard
 b,
Mark M.
Turnbull
b,
Mark M.
Turnbull
 c,
Christopher P.
Landee
d and
Melanie
Rademeyer
c,
Christopher P.
Landee
d and
Melanie
Rademeyer
 *a
*a
aDepartment of Chemistry, University of Pretoria, Pretoria, 0002, South Africa. E-mail: melanie.rademeyer@up.ac.za
bCr Research Group, Department of Physics, University of Johannesburg, Auckland Park, Johannesburg, 2006, South Africa
cCarlson School of Chemistry and Biochemistry, Clark University, 950 Main St., Worcester, Massachusetts 01610, USA
dDepartment of Physics, Clark University, 950 Main St., Worcester, Massachusetts 01610, USA
First published on 9th November 2023
Abstract
The understanding of magnetic properties of hybrid compounds is important for the design of magnetic devices. In this contribution a prominent odd–even effect in both the structural characteristics and magnetic properties of four new hybrid compounds comprised of n-carboxyalkylammonium cations and perchloridomanganate anions is reported. When the n-carboxyalkylammonium cations contain an even number of carbon atoms, compounds of the formula (NH3(CH2)nCOOH)2[MnCl4], with n = 3 and 5, are formed, which display the two-dimensional (2D) hybrid halide perovskite structure in which bridging chlorido ligands link Mn2+ ions. Compounds containing n-carboxyalkylammonium cations with an odd number of carbon atoms have the formula (NH3(CH2)nCOOH)2[MnCl4(H2O)2], with n = 2 and 4, and display a zero-dimensional (0D) structure with hydrogen bonding interactions linking neighbouring [MnCl4(H2O)2]2− anions. The odd–even effect is also evident in the magnetic properties of the compounds, which are linked to the structural differences observed in these compounds. Compounds containing an even number of carbon atoms show antiferromagnetic (AFM) interactions and spin canting at temperature TN, with 2JK = −8.28(5) K and TN = 45.0(5) K when n = 3 and 2JK = −7.72(4) K and TN = 43(1) K when n = 5. Much weaker AFM interactions and no spin canting is observed in compounds containing an odd number of carbon atoms, with 2JK = −0.14(2) K when n = 2 and 2JK = −0.14(2) K when n = 4.
Introduction
In recent years, three-dimensional (3D) organic–inorganic hybrid halide perovskites of Pb2+ have been of interest because of their application in photovoltaic devices.1–3 However, it has been shown that two-dimensional (2D) hybrid halide perovskites of Pb2+ offer certain advantages over their 3D counterparts.4 2D hybrid compounds containing metal ions other than Pb2+ often exhibit the 2D hybrid halide perovskite structure,5 and these compounds also have interesting properties, with the resulting material typically combining the properties of the organic and inorganic components comprising the hybrid material.5,6 Of specific interest in the current study are 2D hybrid halide perovskites containing the divalent metal ion Mn2+, which imparts magnetic properties to the hybrid material.7,8 Mn2+ has a low toxicity as well as low cost due to its natural abundance, making it a useful component for such materials.92D hybrid halide perovskites containing Mn2+ and n-alkylammonium or n-alkyldiammonium cations have been reported to exhibit interesting properties such as catalytic activity,10 reversible barocaloric effects,11 broadband emission for potential use in light emitting diodes,12–14 potential use as solid–solid phase change materials15 and ferroelastic properties.16 Of specific interest in the current study, is the magnetic properties of these materials, with a number of reports on this topic in the literature.17,18
The magnetic properties exhibited by a material may be influenced by, among other things, the dimensionality of the structure, where structural dimensionality is determined by covalent or coordination bonding.19 In the case of n-alkylammonium or n-alkyldiammonium-containing 2D hybrid halide perovskites of Mn2+, a layered, 2D structure is typically formed, consisting of alternating organic and inorganic layers, making them low-dimensional magnetic materials.20 The organic layers contain the organic cation, and the inorganic layers are comprised of corner sharing [MX6]4− octahedra, with no Jahn–Teller distortion.20 Mn2+ is a S = 5/2 ion,21 and in the 2D hybrid halide perovskite structure the magnetic metal ions are linked by halido ligands to form approximately linear, symmetrical Mn–X–Mn bonds, with X = Cl− or Br−.20 According to the Goodenough–Kanamori rules,22,23 this linear magnetic superexchange is antiferromagnetic (AFM) in nature, resulting in compounds exhibiting quasi-2D AFM behaviour.24,25
A number of 2D Mn2+ hybrid chloride perovskites containing n-alkylammonium, n-alkyldiammonium or aryl-ammonium cations have been reported to show rich magnetic behaviour, including spin canting and spin–flop transitions.26,27 The 2D perovskite (CH3NH3)2[MnCl4] is a canted antiferromagnet below 47 K, and exhibits a spin–flop transition.18,24,25,28 Canted antiferromagnetism has also been reported for the perovskite (C6H5C2H4NH3)2[MnCl4] below 44.3 K, attributed to a spin–flop transition.29 The perovskite (C3H7NH3)2[MnCl4] was reported to exhibit spin canting.27 A study of a series of compounds with the formula (C6H5CxH2xNH3)2[MnCl4], with x = 0 to 4, showed that all the compounds exhibit 2D antiferromagnetism, and spin canting below ordering temperatures ranging from 42–46 K.30 In addition, work by Asensio et al.26 showed that spin canting and a spin–flop transition are observed for the compounds (C6H5CH2CH2NH3)2[MnCl]4 and (C2H5NH3)2[MnCl]4, but no spin canting, and a weak spin–flop transition is observed for the compound (NH3C2H2NH3)[MnCl]4. The authors attributed the difference in behaviour to the relative orientations of neighbouring inorganic layers of the compounds, because of the presence of either a mono-cation or a dication. Spin canting has also been observed in the 2D perovskite (C6H5C2H3FNH3)2[MnCl]4.31
The spin canting observed in these Mn2+ perovskites is attributed to the tilting of the [MnCl6]4− octahedra in the inorganic layer, since spin canting depends on the symmetry of the structure.27,32 A weak net moment is present in the inorganic plane, due to the spin canting. There are two sources of spin canting in these types of compounds. The first is anisotropy, while the second occurs as a result of antisymmetric Dzyaloshinskii–Moriya exchange interactions, because of the tilted [MnCl6]4− octahedra in the inorganic layer.25 Single crystal studies showed anisotropy in the magnetisation of the in-plane and the out-of-plane directions.26,28–30 In the these studies26,28–30 the magnetic susceptibility was shown to approach zero with a decrease in temperature when measured out-of-plane, and it was shown to increase when measured in-plane, indicating that the easy-axis lies perpendicular to the inorganic plane.
In the current study, the structural characteristics and magnetic properties of compounds formed through the combination of MnCl2 and n-carboxyalkylamines (NH2(CH2)nCOOH) in the presence of HCl have been investigated. The n-carboxyalkylammonium cation, (NH3(CH2)nCOOH)+, that is formed through the protonation of a n-carboxyalkylamine molecule by an acid, is related to n-alkylammonium and n-alkyldiammonium cations since it also contains a terminal ammonium group and an alkyl portion, and it has a similar linear shape. However, the n-carboxyalkylammonium cation differs in terms of its hydrogen bonding capability compared to that of an n-alkylammonium or n-alkyldiammonium cation.8,33,34 An n-alkylammonium cation has only one hydrogen bond donor group in the form of a single terminal ammonium group, while the n-alkyldiammonium cation has two hydrogen bonding terminal ammonium groups. In the n-carboxyalkylammonium cation, both a terminal ammonium group, which can act as a hydrogen bonding donor, as well as a terminal carboxylic acid group, are present. The terminal carboxylic acid group can act as both a hydrogen bond donor or acceptor, thus, two hydrogen bonding donors (the ammonium group and the carboxylic acid group), and one hydrogen bonding acceptor (the carboxylic acid group) are present in an n-carboxyalkylammonium cation, while the n-alkylammonium and n-alkyldiammonium cations only contain hydrogen bonding donor groups. n-Carboxyalkylammonium cations may form supramolecular dimers that mimic long chain n-alkyldiammonium cations.33
Of specific interest is the type of structure formed for perchloridomanganate hybrid compounds containing n-carboxyalkylammonium cations, and whether these compounds exhibit the 2D hybrid halide perovskite structure like their n-alkylammonium or n-alkyldiammonium counterparts, or not. Moreover, the effect of the number of carbon atoms in the n-carboxyalkylammonium cation on the type of structure formed, as well as on the magnetic behaviour of the compound, is of interest, since an odd–even structural effect is often observed in compounds containing alkyl chains.35–38
A search of the Cambridge Structural Database (CSD) (Version 5.43, June 2022 update)39 showed that no structures of compounds comprised of n-carboxyalkylammonium cations and perchloridomanganate anions have been reported in the literature to date, hence the focus of the current study on the investigation of the structural aspects and magnetic properties of these materials.
The n-carboxyalkylammonium compounds and their structures will be abbreviated as CxMnCl, where x indicates the number of carbon atoms in the n-carboxyalkylammonium cation. For example, when the cation is 2-carboxyethylammonium, (NH3(CH2)2COOH)+, x = 3. In the case where aqua ligands are present, the abbreviation CxMnClH2O will be used.
Experimental
Chemicals and reagents
All chemicals were used as purchased without further purification: MnCl2·4H2O (Sigma-Aldrich, ≥99%), 3-aminopropanoic acid (Sigma-Aldrich, 99%), 4-aminobutyric acid (Sigma, 99%), 5-aminopentanoic acid (Sigma-Aldrich, 99%), 6-aminohexanoic acid (Sigma-Aldrich, ≥99%), HCl (Sigma-Aldrich, 37%) and chloroform (Sigma-Aldrich, ≥99.8%).Synthesis
All reactions followed a similar synthetic procedure carried out in excess HCl with a stoichiometric L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MnCl2 ratio of 2
MnCl2 ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where L indicates the n-carboxyalkylamine. Good quality single crystals were harvested from the reaction vessels and washed using chloroform. No attempt was made to optimise the yields of the reactions.
1, where L indicates the n-carboxyalkylamine. Good quality single crystals were harvested from the reaction vessels and washed using chloroform. No attempt was made to optimise the yields of the reactions.
Instrumental studies
Single crystal X-ray diffraction data for the C6MnCl structure were collected on a Rigaku XtaLAB Synergy R diffractometer, with a HyPix CCD detector, at 150 K, using ω scans and a rotating-anode X-ray source supplying monochromatic Mo-Kα radiation of wavelength 0.71073 Å. Cooling was achieved using an Oxford Cryogenics Cryostat. Data reduction and absorption corrections were carried out using the CrysAlisPro (Version 1.171.40.23a) software package.43
All the crystal structures were solved either by direct methods or intrinsic phasing using SHELX-2014/744 as part of the WinGX suite.45 Structure refinements were done using SHELXL46 in WinGX45 as GUI. Graphics and publication material were generated using Mercury 2022.2.0.47
All non-hydrogen atoms for all structures were refined anisotropically. In structures C3MnClH2O and C4MnCl the hydrogen atom on the carboxylic acid group was placed as observed in the difference map, and its position refined, while the rest of the hydrogen atoms were placed in calculated positions and allowed to ride on the parent atoms. In structure C5MnClH2O the hydrogen atoms on the carbon atoms were placed in calculated positions and allowed to ride on the parent atoms, while the rest were all placed as observed in the difference map and their positions refined. One of the hydrogen atoms on one carboxylic acid group of structure C6MnCl was placed as observed in the difference map and its position refined, while the rest of the hydrogen atoms were placed in calculated positions and allowed to ride on the parent atoms.
Results and discussion
Crystallographic discussion of structures
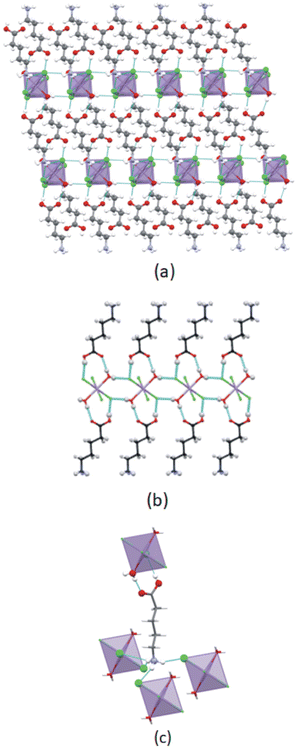
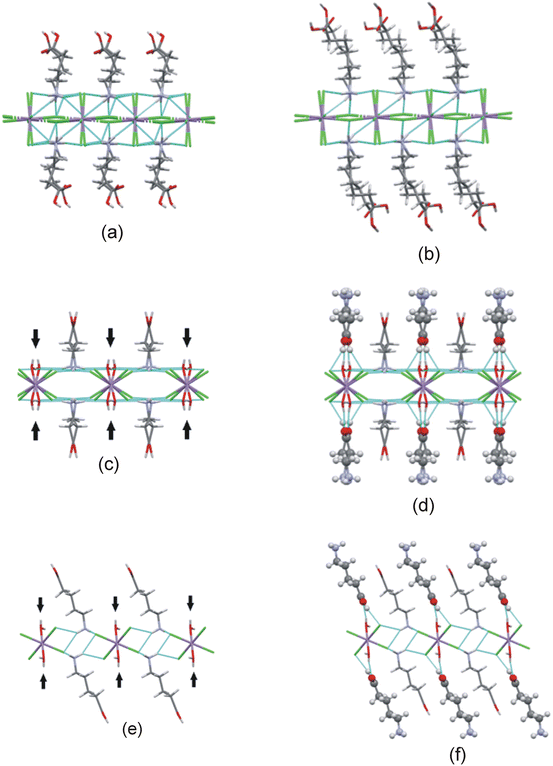
In this study two types of structures were determined, as illustrated in Scheme 1. Firstly the structures of two bis-(n-carboxyalkylammonium)diaquatetrachloridomanganate compounds of the formula (NH3(CH2)nCOOH)2[MnCl4(H2O)2], with n = 2 and 4, abbreviated C3MnClH2O and C5MnClH2O were determined. In addition, the structures of two bis-(n-carboxyalkylammonium)tetrachloridomanganate compounds of the formula (NH3(CH2)nCOOH)2[MnCl4], with n = 3 and 5, abbreviated C4MnCl and C6MnCl, were determined.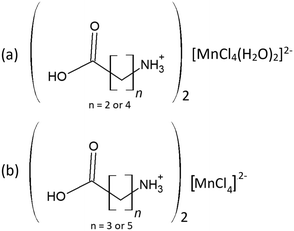 | ||
| Scheme 1 Compounds structurally characterised in this study. (a) 0D organic–inorganic hybrids. (b) 2D hybrid halide perovskites. | ||
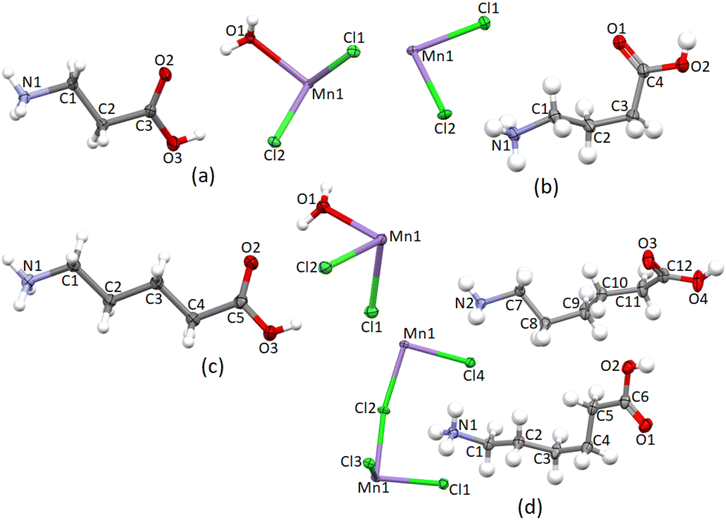
The crystallographic parameters are listed in Table 1, the asymmetric units are shown in Fig. 1, and selected bond lengths, angles, torsion angles, hydrogen bonding parameters and other structural descriptors are listed in Table 2.
| Abbreviation | C3MnClH2O | C4MnCl | C5MnClH2O | C6MnCl |
|---|---|---|---|---|
| Empirical formula | (C3H8O2N+)2[MnCl4(H2O)2]2− | (C4H10O2N+)2[MnCl4]2− | (C5H12O2N+)2[MnCl4(H2O)2]2− | (C6H14O2N+)2[MnCl4]2− |
| Formula weight (g mol−1) | 412.98 | 405.00 | 469.08 | 461.10 |
| Temperature (K) | 150(2) | 150(2) | 150(2) | 150(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Triclinic |
| Space group | C2/c | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 21.3208(14) | 16.1956(4) | 5.9046(4) | 7.1370(1) |
| b (Å) | 7.0794(4) | 7.1780(6) | 7.8188(5) | 7.2664(1) |
| c (Å) | 11.1440(6) | 7.2126(6) | 11.5638(7) | 19.6318(4) |
| α (°) | 90 | 90 | 91.740(2) | 85.695(2) |
| β (°) | 107.916(2) | 100.097(2) | 99.872(2) | 87.366(2) |
| γ (°) | 90 | 90 | 104.651(2) | 88.660(2) |
| Volume (Å3) | 1600.49(16) | 825.49(4) | 507.33(6) | 1013.96(3) |
| Z | 4 | 2 | 1 | 2 |
| Density calculated (g cm−3) | 1.714 | 1.629 | 1.535 | 1.510 |
| Absorption coefficient (mm−1) | 1.511 | 1.455 | 1.202 | 1.195 |
| F(000) | 844 | 414 | 243 | 478 |
| Crystal size (mm3) | 0.265 × 0.326 × 0.606 | 0.050 × 0.104 × 0.232 | 0.021 × 0.263 × 0.347 | 0.053 × 0.121 × 0.263 |
| Reflections collected | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 457 457 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 955 955 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
| Unique reflections | 1665 [R(int) = 0.0334] | 2239 [R(int) = 0.0268] | 2113 [R(int) = 0.0302] | 4138 [R(int) = 0.0315] |
| Completeness | 99.9 | 99.9 | 99.9 | 99.9 |
| Data/restraints/parameters | 1665/1/125 | 2239/0/93 | 2114/0/130 | 4138/0/214 |
| Goodness-of-fit F2 | 1.160 | 1.129 | 1.077 | 1.110 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0182 | R 1 = 0.0199 | R 1 = 0.0192 | R 1 = 0.0256 |
| wR2 = 0.0458 | wR2 = 0.0502 | wR2 = 0.0459 | wR2 = 0.0616 | |
| R indices (all data) | R 1 = 0.0206 | R 1 = 0.0220 | R 1 = 0.0233 | R 1 = 0.0288 |
| wR2 = 0.0467 | wR2 = 0.0512 | wR2 = 0.0476 | wR2 = 0.0626 |
| Structure | C3MnClH 2 O | C4MnCl | C5MnClH 2 O | C6MnCl |
|---|---|---|---|---|
| Symmetry operators for hydrogen bonding acceptors: i: −x + ½, y − ½, −z + ½; ii: x − ½, y − ½, z; iii: −x + ½, y + ½, −z + ½; iv: −x + 1, −y + 1, −z + 1; v: −x + 1, y, −z + ½; vi: x, −y + 3/2, z − ½; vii: x, −y + ½, z − ½; viii: x, y, z − 1; ix: −x + 2, −y + 2, −z + 1; x: −x + 1, −y + 2, −z + 1; xi: x, y, z + 1; xii: x − 1, y, z; xiii: x + 1, y, z; xiv: −x + 1, −y + 1, −z + 2; xv: −x, −y + 2, −z + 2; xvi: −x + 2, −y, −z + 1. | ||||
| Perovskite layer orientation | Staggered | Staggered | Staggered | Staggered |
| M–Cl bond length (Å) | 2.4732(4) | 2.5571(3) | 2.5034(4) | 2.4920(4) |
| 2.5656(4) | 2.4853(3) | 2.5672(4) | 2.5040(4) | |
| 2.5632(3) | 2.5419(4) | |||
| 2.6025(4) | ||||
| 2.5903(4) | ||||
| 2.5376(4) | ||||
| M–O bond length (Å) | 2.2248(9) | — | 2.1872(11) | — |
| M⋯M distance (Å) | 5.572 | 5.088 | 5.905 | 5.033 |
| 5.152 | ||||
| Area of interstitials (Å2) | — | 25.89 | — | 25.93 |
| Penetration depth (Å) | — | 0.153 | — | 0.168 |
| Mn–Cl⋯Cl–Mn contact distance (Å) | 6.990 | 11.005 | 8.327 | 14.614 |
| Layer repeat distance (Å) | 10.143 | 15.945 | 11.358 | 19.557 |
| Supramolecular cation length (Å) | — | 12.189 | — | 17.211 |
| 17.412 | ||||
| Mn–Cl⋯Cl–Mn bridging angle (°) | — | 167.097(13) | — | 164.430(2) |
| 165.590(2) | ||||
| ψ angle (°) | 46.83 | 7.43 | 46.31 | 7.63 |
| 7.59 | ||||
| β angle (°) | 83.22 | 78.71 | 88.28 | 69.28 |
| 63.74 | ||||
| ϕ angle (°) | 62.80 | 84.78 | 76.64 | 68.02 |
| 68.42 | ||||
| N–C–C–C torsion angle (°) (gauche bond) | 174.58(11) | — | −173.10(12) | — |
| C–C–C–C torsion angle (°) (gauche bond) | — | −73.24(18) | — | −66.27(7) |
| 70.41(7) | ||||
| Hydrogen bonding configuration | — | t 2 b | — | t 2 b |
| N–H+⋯−Cl–Mn (D⋯A) distance (Å) | 3.1742(12)i | 3.1973(11)vi | 3.1612(13)x | 3.2473(16) |
| 3.3211(12)ii | 3.2748(11) | 3.2178(13)x | 3.2512(17)xiii | |
| 3.4484(11)vii | 3.3033(13)xi | 3.2679(18) | ||
| 3.4710(12)viii | 3.4316(14)v | 3.2705(17)xiv | ||
| 3.2978(18) | ||||
| 3.3973(17)xv | ||||
| 3.2506(17)v | ||||
| N–H+⋯O(aqua)–Mn (D⋯A) distance (Å) | 2.9677(15)iii | — | 3.1396(11)x | — |
| C–O–H⋯−Cl–Mn (D⋯A) distance (Å) | 3.1003(11)iv | — | 3.0835(12) | — |
| Mn–O–H(aqua)⋯−Cl–Mn (D⋯A) distance (Å) | 3.1666(10)v | — | 3.1799(11)xii | — |
Mn–O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (D⋯A) distance (Å) C (D⋯A) distance (Å) |
2.7335(14) | — | 2.7486(15) | — |
O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (D⋯A) distance (Å) C (D⋯A) distance (Å) |
— | 2.6330(14)v | — | 2.615(2)xvi |
| 2.639(2) | ||||
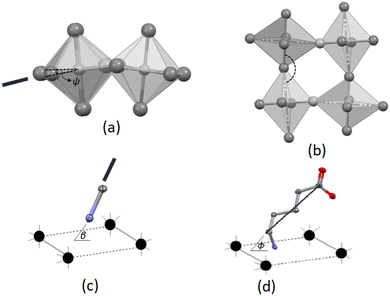
The Mn–Cl–Mn bridging angle describes the rotational tilt of the polyhedra about the plane perpendicular to the inorganic plane, as illustrated in Fig. 2(b). The Mn–Cl–Mn bridging angle is applicable only to the 2D hybrid halide perovskite structures and not the 0D hybrid structures, as no bridging halido ligands are present between neighbouring metal ions in the 0D structures.
The β angle indicates the angle between the C–N bond and the inorganic plane, as shown in Fig. 2(c). This geometric parameter is used for both the 2D and 0D structures. The angle ϕ is defined as the angle formed between the inorganic plane and the vector connecting the carbon atom bonded to the nitrogen atom and the carboxy carbon atom, as shown in Fig. 2(d). Thus, the ϕ angle gives an average tilt of a single cation relative to the inorganic plane. A ϕ angle close to 90° is indicative of cations that pack approximately perpendicular to the inorganic plane. The ϕ angle is used as geometric parameter in both 2D and 0D structures.
The layer repeat distance refers to the distance between two neighbouring inorganic planes, as defined by the perpendicular distance between the mean planes through the metal ions of consecutive inorganic layers. The inter-layer Mn–Cl−⋯−Cl–Mn contact distance indicates the shortest distance between two terminal halido ligands in consecutive inorganic sheets in the 2D hybrid halide perovskite structures. The Mn–O⋯O–Mn contact distance refers to the shortest distance between two oxygen atoms of aqua ligands coordinated to metal ions in neighbouring inorganic layers in 0D hybrid structures.
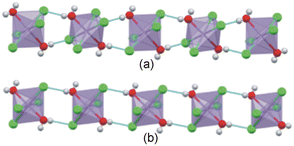
Based on the hydrogen atom geometries put forth by Mitzi,53 the hydrogen atom configurations can be described as either bridging (b) or terminal (t), as shown in Fig. 4(a) and (b). Only the strong hydrogen bonding interactions, namely charge assisted N–H+⋯−X–M interactions, are considered when determining the hydrogen bonding configuration, and when bifurcated or trifurcated interactions are present, the shortest N–H+⋯−X–M interaction is considered. The nomenclature bmtn, m + n = 3,53 is adopted, which describes the number of bridging (m) and terminal (n) halido ligand acceptors per hydrogen bonding configuration. Where more than three hydrogen bonding interactions are present, only the three shortest D⋯A hydrogen bond distances are considered. If the number of bridging hydrogen bonding acceptors outnumber terminal hydrogen bonding acceptors, the overall configuration is bridging, and the reverse is true if there are more terminal hydrogen bonding acceptors.
Structural results
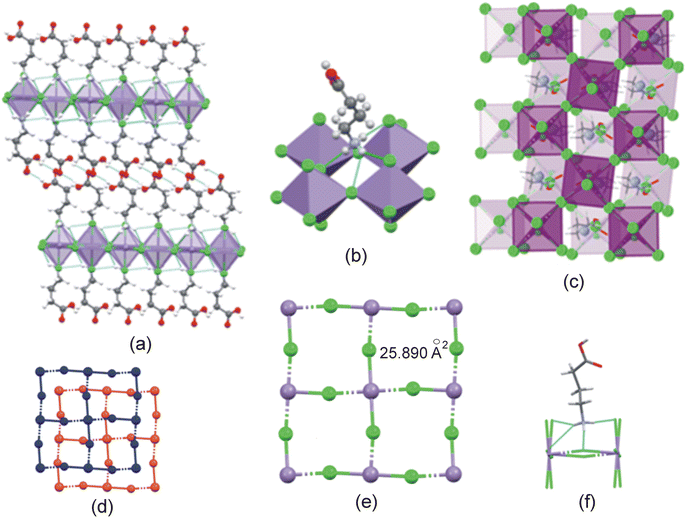
The main structural features of the four structures are discussed below, with additional information on the structures included in ESI† Section S2.The cation exhibits an all-trans conformation. A layered structure is formed, which is comprised of alternating organic and inorganic layers, as illustrated in Fig. 5(a). The organic layer is formed by interdigitated 2-carboxyethylammonium cations, while isolated [MnCl4(H2O)2]2− anions comprise the inorganic layer. Adjacent inorganic layers adopt a staggered arrangement, whereby neighbouring inter-layer metal ions are off-set. The layer repeat distance is 10.143 Å and the Mn–O⋯O–Mn inter-layer distance is 7.873 Å.
Different types of hydrogen bonding interactions form a 3D hydrogen bonded network, as illustrated in Fig. 5(b). These include N–H+⋯−Cl–Mn, C–O–H⋯−Cl–Mn, Mn–O–H⋯−Cl–Mn, N–H+⋯O–Mn, and Mn–O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding interactions.
C hydrogen bonding interactions.
In the inorganic layer, individual [MnCl4(H2O)2]2− anions are cross-linked through Mn–O–H⋯−Cl–Mn hydrogen bonding interactions to form 1D hydrogen bonded anionic ribbons, as shown in Fig. 5(b). The ψ angle is 46.83°, indicating that the polyhedra are tilted. The ammonium group and carboxylic acid group of a cation are hydrogen bonded to different inorganic layers with the chlorido and aqua ligands of the anions acting as hydrogen bonding acceptors, and the ammonium groups and carboxylic acid groups of the cations as hydrogen bond donors.
Cations hydrogen bond to both sides of the anionic hydrogen bonded ribbon through their carboxylic acid groups, as illustrated in Fig. 5(b), with the carboxylic acid groups acting as hydrogen bonding donors to chlorido ligands, while they accept hydrogen bonds from aqua ligands, to form a flat ribbon extending along the c-direction. Neighbouring ribbons are further linked by hydrogen bonding ammonium groups, with each ammonium group forming two classical, charge assisted N–H+⋯−Cl–Mn hydrogen bonds to one ribbon, as well as a classical, charge-assisted N–H+⋯−Cl–Mn hydrogen bond to a neighbouring ribbon, thereby linking the ribbons. The β angle of the ammonium group is 84.89° and the ϕ angle is 62.80°.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and forms a 0D hybrid structure, with isolated organic cations and inorganic anions comprising the structure.19 The asymmetric unit, as illustrated in Fig. 1, comprises one 4-carboxybutylammonium cation and one [MnCl2(H2O)]− moiety, with the metal ion located on an inversion centre, which generates the rest of the [MnCl4(H2O)2]2− anion.
, and forms a 0D hybrid structure, with isolated organic cations and inorganic anions comprising the structure.19 The asymmetric unit, as illustrated in Fig. 1, comprises one 4-carboxybutylammonium cation and one [MnCl2(H2O)]− moiety, with the metal ion located on an inversion centre, which generates the rest of the [MnCl4(H2O)2]2− anion.
The 4-carboxybutylammonium cation adopts the all-trans conformation, with all torsion angles close to 180°. The β angle equals 88.28°, indicating the tilting of the cations relative to the inorganic plane and the ϕ angle is 76.64°.
The ψ angle is 46.31°, and is indicative of the tilt of the octahedra.
Packing of the cations and anions results in the formation of a layered structure, comprised of alternating organic and inorganic layers, as illustrated in Fig. 6(a). Consecutive inorganic sheets are staggered as metal ions of adjacent inorganic layers do not align.
The inter-layer Mn–O⋯O–Mn contact distance is 8.327 Å and the layer repeat distance is 11.358 Å. The inorganic layer comprises hexa-coordinated metal ions. In each [MnCl4(H2O)2]2− anion, two aqua ligands and four chlorido ligands are coordinated to the Mn2+ ion, trans to each other, resulting in an octahedral geometry.
Different types of hydrogen bonding interactions are present in the structure to form a 3D hydrogen bonded network, as shown in Fig. 6(a) to (c). These include N–H+⋯−Cl–Mn, C–O–H⋯−Cl–Mn, Mn–O–H⋯−Cl–Mn and Mn–O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding interactions. Unlike what was observed for structure C3MnClH2O, no N–H+⋯O–Mn hydrogen bonds are present, making the hydrogen bonding network in structure C5MnClH2O different from that formed in structure C3MnClH2O.
C hydrogen bonding interactions. Unlike what was observed for structure C3MnClH2O, no N–H+⋯O–Mn hydrogen bonds are present, making the hydrogen bonding network in structure C5MnClH2O different from that formed in structure C3MnClH2O.
The [MnCl4(H2O)2]2− anions in the inorganic layer are cross-linked through Mn–O–H⋯−Cl–Mn hydrogen bonding interactions to form a 1D hydrogen bonded ribbon, as shown in Fig. 6(b). The ammonium group and carboxylic group of each cation is hydrogen bonded to consecutive inorganic layers, with the chlorido and aqua ligands of the anions accepting the hydrogen bonds, while the ammonium and carboxylic acid groups are hydrogen bonding donors.
The carboxylic acid group forms one C–O–H⋯−Cl–Mn interaction to an anion and accepts a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O–Mn hydrogen bond from the same anion, while the ammonium group forms two classical and one bifurcated, charge-assisted N–H+⋯−Cl–Mn hydrogen bonding interactions to three different anions in two anionic hydrogen bonded ribbons, meaning that the ammonium groups straddle neighbouring anionic hydrogen bonded ribbons, to form a 3D hydrogen bonded network.
O⋯H–O–Mn hydrogen bond from the same anion, while the ammonium group forms two classical and one bifurcated, charge-assisted N–H+⋯−Cl–Mn hydrogen bonding interactions to three different anions in two anionic hydrogen bonded ribbons, meaning that the ammonium groups straddle neighbouring anionic hydrogen bonded ribbons, to form a 3D hydrogen bonded network.
The 3-carboxypropylammonium cation deviates from the all-trans conformation, with a gauche orientation present in the cation, displaying a C1–C2–C3–C4 torsion angle of −73.25(18)°, as shown in Fig. 7(b).
The Mn–Cl−⋯−Cl–Mn contact distance between consecutive inorganic layers is 11.005 Å, with a layer repeat distance of 15.945 Å. Consecutive inorganic layers are offset by (0.36,0.36), resulting in this structure being classified as a near-RP (nRP)52 perovskite, since the offset is close to the offset of (½,½) expected for an ideal RP structure. The staggered consecutive layers are illustrated in Fig. 7(c) and (d). The coordination sphere of the Mn2+ metal ion comprises six chlorido ligands forming the [MnCl6]4− polyhedra. The Mn–Cl–Mn bridging angle is 167.097(13)°, and the ψ angle is 7.43°. The Mn2+ metal ions are uniformly separated along the ab-plane at 5.088 Å, and the metal ions, together with the bridging chlorido ligands create a 2D inorganic grid comprised of “squares” with approximate areas of 25.890 Å2, calculated using the M⋯M distances, as illustrated in Fig. 7(e). This 2D grid, together with the terminal halide ions, form a cavity into which the ammonium group penetrates slightly, as shown in Fig. 7(f), while it forms hydrogen bonding interactions to the terminal and/or bridging halides. The positive penetration depth of the ammonium group is 0.153 Å.
Two types of hydrogen bonding interactions are present in the structure. Firstly, the cations are connected to the inorganic layer via charge-assisted N–H+⋯−Cl–Mn hydrogen bonding interactions, with each ammonium group forming two classical and one bifurcated hydrogen bonds to two terminal and two bridging chlorido acceptors, as shown in Fig. 7(b), resulting in a 2D hydrogen bonding network. The β angle is 78.71°, the ϕ angle is 84.78°, and the hydrogen bonding configuration is t2b. In addition, hydrogen bonded carboxylic acid dimers of the type O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C are formed between carboxylic acid groups of adjacent cations in the organic layer, to form supramolecular cations of length 12.189 Å.
C are formed between carboxylic acid groups of adjacent cations in the organic layer, to form supramolecular cations of length 12.189 Å.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and forms a 2D hybrid halide perovskite structure.19 The asymmetric unit comprises two crystallographically independent 5-carboxypentylammonium cations and one [Mn2Cl4]2− unit, as shown in Fig. 1. The crystallographically independent metal ions will be labelled Mn1 and Mn2. Both metal ions lie on inversion centres, and thus contribute only half their charge to the asymmetric unit, hence the charge of 2− for the [Mn2Cl4]2− unit. The two crystallographically independent cations will be distinguished based on their nitrogen atoms, with cation 1 containing atom N1, and cation 2 atom N2. Both cation 1 and cation 2 deviate from the all-trans conformation and display gauche bonds, however, the gauche bonds are in different positions in the two cations. In cation 1, the C2–C3–C4–C5 torsion angle deviates from 180°, with a value of −66.27(7)°, while in cation 2, the C7–C8–C9–C10 torsion angle has a value of 70.41(7)°.
, and forms a 2D hybrid halide perovskite structure.19 The asymmetric unit comprises two crystallographically independent 5-carboxypentylammonium cations and one [Mn2Cl4]2− unit, as shown in Fig. 1. The crystallographically independent metal ions will be labelled Mn1 and Mn2. Both metal ions lie on inversion centres, and thus contribute only half their charge to the asymmetric unit, hence the charge of 2− for the [Mn2Cl4]2− unit. The two crystallographically independent cations will be distinguished based on their nitrogen atoms, with cation 1 containing atom N1, and cation 2 atom N2. Both cation 1 and cation 2 deviate from the all-trans conformation and display gauche bonds, however, the gauche bonds are in different positions in the two cations. In cation 1, the C2–C3–C4–C5 torsion angle deviates from 180°, with a value of −66.27(7)°, while in cation 2, the C7–C8–C9–C10 torsion angle has a value of 70.41(7)°.
Repetition of the inorganic part of the asymmetric unit results in the formation of an inorganic sheet consisting of corner sharing [MnCl6]4− octahedra. The Mn⋯Mn distances between metal ions bridged by halido ligands in the 2D inorganic layer are 5.152 Å and 5.033 Å in the ab-plane. The bridging Mn–Cl–Mn angles are 165.590(2)° (Mn1–Cl2–Mn3) and 164.430(2)° (Mn1–Cl2–Mn2), indicating the tilting of the octahedra relative to each other. The ψ angles are 7.63° for the octahedron containing ion Mn1 and 7.59° for the octahedron containing ion Mn2, resulting in corrugation of the inorganic layer.
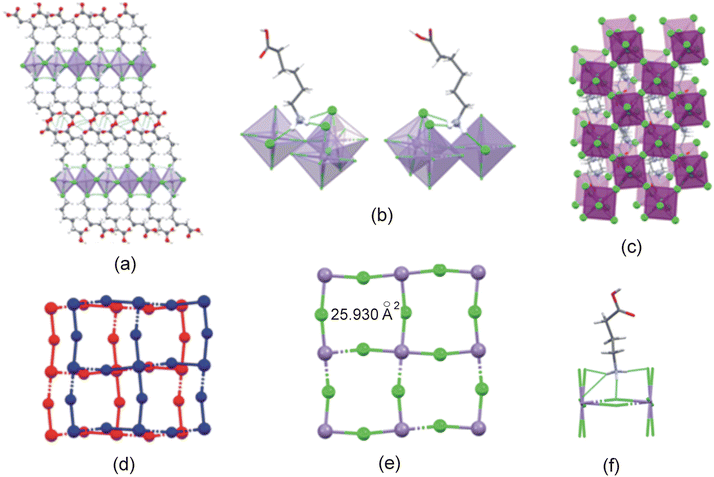
The C6MnCl structure is layered, as illustrated in Fig. 8(a). The Mn–Cl−⋯−Cl–Mn contact distance between neighbouring inorganic layers is 14.636 Å and the layer repeat distance is 19.624 Å.
The ammonium groups from cations 1 and 2 anchor to the inorganic layer through strong, charge-assisted hydrogen bonding interactions of the type N–H+⋯−Cl–Mn. Both cations from three classical hydrogen bonds to one bridging and two terminal chlorido ligands, as shown in Fig. 8(b). The hydrogen bonding configuration adopted is t2b for both cations. The β angles are 69.28° and 69.56° for cation 1 and 2 and the ϕ angles are 68.02° and 68.42° for cation 1 and 2.
Adjacent cations in the organic layer are hydrogen bonded through O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding interactions between carboxylic acid groups, to form supramolecular cations. The supramolecular cation chain lengths are 17.449 Å (cation 1 to cation 1) and 17.300 Å (cation 2 to cation 2).
C hydrogen bonding interactions between carboxylic acid groups, to form supramolecular cations. The supramolecular cation chain lengths are 17.449 Å (cation 1 to cation 1) and 17.300 Å (cation 2 to cation 2).
Neighbouring inorganic layers are staggered, as illustrated in Fig. 8(c) and (d), with consecutive layers offset by (0.32,0.03). As such, the structure can be classified as a near-DJ (nDJ) perovskite,52 since this offset is close to the offset of (½, 0) found for the ideal DJ perovskite.
The metal ions, together with the bridging chlorido ligands, create a 2D inorganic grid comprised of “squares” with areas of 25.930 Å2, as illustrated in Fig. 8(e). The ammonium groups penetrate into the inorganic cavity, as shown in Fig. 8(f), forming hydrogen bonding interactions to the terminal and/or bridging halides. The positive penetration depths of the ammonium groups into the cavities are 0.168 Å and 0.166 Å for cations 1 and 2, respectively.
Structural comparison and discussion
In this section, the new structures determined in this study will be compared, structural trends identified, and comparisons made to structures reported in the literature, where applicable.The tilt angles of the polyhedra are similar in the C3MnClH2O and C5MnClH2O structures, at approximately 46°. Both compounds show staggered inorganic layers and a 1D hydrogen bonded ribbon is formed between the [MnCl4(H2O)2]2− anions. However, in structure C3MnClH2O, neighbouring anions in the ribbon alternate with respect to the orientation of their chlorido ligands, while they show the same orientation in structure C5MnClH2O, as shown in Fig. 9(a) and (b). In both structures the carboxylic acid group forms a hydrogen bond to a chlorido ligand of an anion and accepts a hydrogen bond from a coordinated water molecule on the same anions, as illustrated in Fig. 5(c) and 6(c). However, they differ in terms of the hydrogen bonding interactions involving the ammonium group. In structure C3MnClH2O, each ammonium group forms one hydrogen bond to a coordinated water molecule, and two hydrogen bonds to two different chlorido ligands, as shown in Fig. 5(c). In structure C5MnClH2O each ammonium group forms three hydrogen bonds, one of which is bifurcated, to four different chlorido ligands, shown in Fig. 6(c). Hence, the overall hydrogen bonding networks formed in the two structures are different.
The ammonium group of each cation is hydrogen bonded to one inorganic layer, while its carboxylic acid group is hydrogen bonded to a neighbouring inorganic layer, and cation adopts an all-trans conformation in both structures.
A search of the CSD (Version 5.43, June 2022 update)39 showed that only two structures containing only one type of organic cation and a [MnCl4(H2O)2]2− anion have been reported in the literature. These structures are EHIWIC,54 which contains an adamantanylammonium cation and TAKZOV,55 that contains a carbamolguanidinium cation. In both literature structures the [MnCl4(H2O)2]2− anion adopts a trans conformation, and the same 1D hydrogen bonded ribbon that is observed in structures C3MnClH2O and C5MnClH2O, is formed between the anions. As such, the formation of the [MnCl4(H2O)2]2− anion is not very common.
The positioning of the ammonium ends of the cations is determined by the size of the square grid created by the metal ions and halide ions in the inorganic layer, with cations anchoring to neighbouring cavities created by these ions. Gauche bonds are introduced in the alkyl portions of the cations to increase their average thickness or diameter when the area of the square grid is larger than the average diameter of the all-trans cation, allowing the cation pack more efficiently in the organic layer. The average diameter of an all-trans alkyl chain is approximately 18–20 Å2.56 In structures C4MnCl and C6MnCl the areas of the square grids created in the inorganic layer are 25.890 Å2 and 25.930 Å2 respectively, which are larger than the average diameter of an all-trans alkyl chain, hence this leads to the introduction of gauche bonds in the cation to allow for better close packing of the alkyl portion of the n-carboxyalkylammonium cations in the organic layer.
The Mn–Cl–Mn bridging angle of structure C4MnCl (167.097(13)°) is slightly larger than those of structure C6MnCl (164.430(2)° and 165.590(2)°), indicating that the polyhedra are more tilted relative to each other in structure C6MnCl. The penetration depth of the cation in structure C4MnCl (0.153 Å) is slightly smaller than that of the cations in structure C6MnCl (0.166 Å and 0.168 Å), which agrees with reports in the literature that an increase in penetration depth of the cation is correlated with a smaller M–X–M angle.52 The cations in the C6MnCl structure (β angles of 69.28° and 69.56°) are more tilted relative to the inorganic layer than the cations in the C4MnCl structure (β angle of 78.71°). Tilting of the cations also improves the close packing of the alkyl chains in the organic bilayer.
In both structures, the classic N–H+⋯−Cl–Mn hydrogen bonding interactions adopt the t2b configuration, and a carboxylic acid dimer hydrogen bond is present in the organic layer. The carboxylic acid dimer hydrogen bond links two n-carboxyalkylammonium cations into what can be viewed as a supramolecular cation. The supramolecular cation in the C4MnCl structure stretches athwart by one [MnCl6]4− octahedron in the neighbouring inorganic layer, and the supramolecular cation in the C6MnCl structure by two octahedra.
The C4MnCl structure exhibits an offset of (0.36,0.36) of the Mn⋯Mn distance between consecutive inorganic layers, making it a nRP perovskite, while structure C6MnCl displays an offset of (0.32,0.03), hence it can be classified as a nDJ perovskite structure.
A search of the Cambridge Structural Database (CSD) (Version 5.43, June 2022 update)39 was conducted to find structures for n-alkylammonium or n-alkyldiammonium-containing hybrid chloride 2D Mn2+ perovskites reported in the literature, with the search focussing in particular on structures containing either n-alkylammonium or n-alkyldiammonium cation and a Mn–Cl unit. Manual sifting of the structures was done to find only those structures exhibiting a 2D hybrid halide perovskite structure, with the formulae either (NH3(CH2)nCH3)2[MnCl4] or (NH3(CH2)nNH3)[MnCl4]. Only those with atomic coordinates reported were considered. The search revealed that the following compounds exhibit 2D hybrid halide perovskite structures, with the CSD39 reference codes (refcodes) and the organic cation indicated: methylammonium: MATCMN01,57MATCMN04,58MATCMN05,59MATCMN07,60MATCMN12;61n-ethylammonium: EAMNCL,62 and EAMNCL11,63n-propylammonium: PAMMNC01,64PAMMNC10,65PAMMNC13,8PAMMNC36;66n-decylammonium: DECACM,67DECACM01,11DECACM02,11 and n-undecylammonium: TAZQUJ.15
n-Alkyldiammonium cation containing structures include: 1,2-ethyldiammonium: ENDAMN1068 and ENDAMN11;69 1,3-propyldiammonium: PYDAMN,70 and PYDAMN03;71 1,4-butyldiammonium: BUCLMN72 and BUCLMN01;72 1,5-pentyldiammonum: LUHCUO,73LUHCUO0174 and LUHCUO0275 and 1,6-hexyldiammonium: POCJOJ.76
In addition to the n-alkylammonium or n-dialkylammonium-containing hybrid halide perovskite structures of Mn2+, two other structure types with a Mn–Cl-containing anion and n-alkylammonium cation have been reported. One structure containing methylammonium cations combined with a 1D halide-bridged polymeric chain of the form (Mn(H2O)2Cl3)∞ has been reported (ZZZAXM01,77ZZZAXM0278). In the polymer, chlorido ligands share corners, and pairs of ligands of the same type (chlorido or aqua) alternate along opposite sides of the chain. In addition, a structure comprised of n-decylammonium cations and isolated, tetrahedral [MnCl4]2− anions has been reported (TAZQOD).15
However, for all the structures containing n-alkyldiammonium cations and a perchloridomanganate anion, only the 2D hybrid halide perovskite structure has been reported. In summary, the CSD search revealed that the 2D hybrid halide perovskite structure is by far the preferred structure formed on combination of n-alkylammonium or n-alkyldiammonium cations and perchloridomanganate anions.
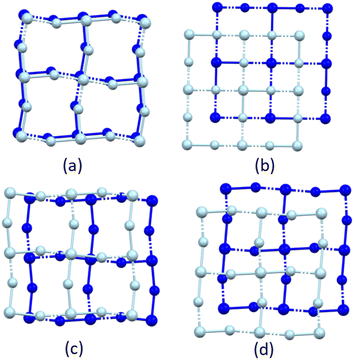
For comparison purposes, the offset in structures of 2D hybrid perchloridomanganate perovskites containing n-alkylammonium or n-alkyldiammonium cations reported in the literature were determined, and they were classified as DJ, RP, nDJ or nRP perovskites.52 The results of the analysis are tabulated in Table 3.
| CSD Refcode | Cation | Space group and temperature | Offset | Staggered/eclipsed | Classification | Ref. |
|---|---|---|---|---|---|---|
| n-Alkyldiammonium cations | ||||||
| ENDAMN10 | 1,2-Ethyldiammonium | P21/b (295 K) | (0,0) | Eclipsed | Ideal DJ | 68 |
| ENDAMN11 | P21/c (100 K) | 79 | ||||
| PYDAMN | 1,3-Propyldiammonium | Imma (295 K) | (0,0) | Eclipsed | Ideal DJ | 70 |
| PYDAMN03 | Pnma (295 K) | 71 | ||||
| BUCLMN | 1,4-Butyldiammonium | P21/b (295 K) | (0,0) | Eclipsed | Ideal DJ | 72 |
| BUCLMN01 | Pnmb (404 K) | 72 | ||||
| LUHCUO | 1,5-Pentyldiammonium | I212121 (298 K) | (0,0) | Eclipsed | Ideal DJ | 73 |
| LUHCUO01 | Imma (333 K) | 74 | ||||
| LUHCUO02 | Pnma (173 K) | 75 | ||||
| POCJOJ | 1,6-Hexyldiammonium | P21/c (295 K) | (0,0) | Eclipsed | Ideal DJ | 76 |
| n-Alkylammonium cations | ||||||
|---|---|---|---|---|---|---|
| MATCMN01 | Methylammonium | Abma (295 K) | (½,½) | Staggered | Ideal RP | 57 |
| MATCMN04 | Pccn (188 K) | 58 | ||||
| MATCMN05 | I4/m (404 K) | 59 | ||||
| MATCMN07 | P42/ncm (188 K) | 60 | ||||
| MATCMN12 | Bmab (295 K) | 61 | ||||
| EAMNCL | n-Ethylammonium | Abma (295 K) | (½,½) | Staggered | Ideal RP | 62 |
| EAMNCL11 | Pbca (126 K)63 | 63 | ||||
| PAMMNC01 | n-Propylammonium | Abma (182 K) | (0,0) | Eclipsed | Ideal DJ | 64 |
| PAMMNC10 | n-Propylammonium | Cmca (295 K) | (½,½) | Staggered | Ideal RP | 65 |
| PAMMNC13 | P21/b (8 K) | 8 | ||||
| PAMMNC36 | Abma (365 K) | 66 | ||||
| DECACM | n-Decylammonium | P21/a (283 K) | (0.35,0.35) | Staggered | Ideal RP | 67 |
| DECACM01 | P21/a (283 K) | (0.35,0.35) | 11 | |||
| DECACM02 | C2/m (330 K) | (½,½) | 11 | |||
| TAZQUJ | n-Undecylammonium | P21/c (293 K) | (0.26,0.42) | Staggered | nRP | 15 |
| n-Carboxyalkylammonium cations | ||||||
|---|---|---|---|---|---|---|
| C4MnCl | 3-Carboxylpropylammonium | P21/c (150 K) | (0.36,0.36) | Staggered | nRP | This study |
| C6MnCl | 5-Carboxylpentylammonium |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (150 K) (150 K) |
(0.32,0.03) | Staggered | nDJ | This study |
As can be seen from the results in Table 3, all the n-alkyldiammonium-containing perchloridomanganate hybrid halide perovskite structures display a (0,0) offset of their inorganic layers, hence the ideal DJ structure is adopted, as expected for dication-containing perovskites.
Most n-alkylammonium-containing perchloridomanganate hybrid halide perovskite structures display a (½,½) offset of the inorganic layers, thus forming the ideal RP structure, as expected for monocation-containing perovskites, however, there are some exceptions.
Firstly, two types of structures have been reported for the n-decylammonium containing analogue. These structures were determined at different temperatures and represent thermotropic polymorphs. One of the structures, DECACM02,68 determined at 330 K, displays the expected RP structure with an (½,½) offset. The second structure (DECACM67 and DECACM0168), determined at 283 K, exhibits an offset of (0.35,0.35), which indicates a nRP perovskite. The structure TAZQUJ,15 containing n-undecylammonium cations, displays an offset of (0.26,0.26), hence it is a nRP perovskite. It is noteworthy that both structures that display nRP perovskite structures contain long-chain n-alkylammonium cations. A third exception is structure PAMMNC01,64 determined at 182 K, which contains an n-propylammonium cation, and displays a (0,0) offset, making it an ideal DJ perovskite, despite the presence of a monocation in the structure. However, three ideal RP structures have also been reported for this compound, determined at three different temperatures, spanning a wide temperature range, namely PAMMNC1065 (determined at 295 K), PAMMNC138 (determined at 8 K) and PAMMNC3666 (determined at 365 K). It is unclear why the compound exhibited an ideal RP structure over such a wide temperature range, but an ideal DJ structure at 182 K.
In summary, all the n-alkyldiammonium-containing perchloridomanganate structures considered form ideal DJ structures, and most of the n-alkylammonium-containing structures form ideal RP structures, as expected. Two of the exceptions can be ascribed to the presence of long-chain n-alkylammonium cations, and increased flexibility in the longer cationic chains. Thus, as the length of the n-alkylammonium chain increases, a nRP instead of an ideal RP structure is formed.
Structure C4MnCl also forms a nRP perovskite structure, while structure C6MnCl exhibits a nDJ perovskite structure. Both structures contain n-carboxyalkylammonium monocations, and based on this, a RP structure is expected. However, since a supramolecular cation is formed in these structures via hydrogen bonded carboxylic acid groups, the supramolecular cation may be viewed as a diammonium dication, in which case a DJ structure is expected.
However, neither the ideal DJ or ideal RP perovskite structure is formed for C4MnCl or C6MnCl. This means that the change of one terminal functional group from an ammonium group in an n-alkyldiammonium cation, or a methyl group in an n-alkylammonium cation, to a carboxylic acid group, impacts on the relative orientation of the inorganic layers in the structures C4MnCl and C6MnCl, and a results in a deviation from the ideal RP or ideal DJ structures to form either a nRP or nDJ structure, and may be used as a structure manipulation tool.
Structural types
A prominent odd–even effect is displayed in the structures under investigation. When the number of carbon atoms in the n-carboxyalkylammonium cation is odd, i.e., structures C3MnClH2O and C5MnClH2O, a 0D organic–inorganic hybrid structure is formed, with water molecules forming part of the coordination sphere of the Mn2+ ion, resulting in isolated [MnCl4(H2O)2]2− anions. Even though water was not used specifically as a reagent in the reaction, water molecules were present as part of the concentrated HCl used in the reaction. However, when an even number of carbon atoms are present in the n-carboxyalkylammonium cation, i.e., structures C4MnCl and C6MnCl, the hybrid halide perovskite structure is formed, and no water molecules are incorporated into the structure, even though water molecules were available as part of the concentrated acid.
Both structure types are layered but differ in terms of the type of inorganic anion formed, the packing of ions and hydrogen bonding interactions. A 2D inorganic layer consisting of corner-sharing [MnCl6]4− octahedra is formed in the 2D hybrid halide perovskite structures, C4MnCl and C6MnCl, while the inorganic layer comprises isolated 0D [MnCl4(H2O)]2− anions in the odd-chain containing compounds C3MnClH2O and C5MnClH2O.
In the 2D hybrid halide perovskite-type structures, a bilayer of non-interdigitated organic cations is formed, with the ammonium groups hydrogen bonding to the inorganic layer, and the carboxylic acid groups forming a separate hydrogen bonded layer comprised of hydrogen bonded carboxylic acid dimers as part of the organic layer. However, in the 0D structures, the cations are interdigitated, and all the functional groups capable of hydrogen bonding (i.e., the carboxylic acid group, ammonium group and anion), form a single hydrogen bonded network.
In order to understand the preference for a certain structural type based on the number of carbon atoms in the n-carboxyalkylammonium chain, it is informative to consider some related structures reported in the literature.
As noted previously, a search of the CSD39 indicated that compounds of this type containing n-alkyldiammonium chains all form the 2D hybrid halide perovskite structure. For compounds containing n-alkylammonium chains, the 2D hybrid perovskite structure is by far the preferred structure type, with only two exceptions reported in the literature, namely one with a tetrahedral [MnCl4]2− anion (TAZQOD15) and one with a 1D polymeric [Mn(H2O)2Cl3]∞ anion (ZZZAXM01 (ref. 77) and ZZZAXM02 (ref. 78).
The question can be asked if it is possible to form a 2D hybrid halide perovskite structure containing the odd numbered cations 2-carboxyethylammonium or 4-carboxybutylammonium. A search of the CSD (Version 5.43, June 2022 update)39 reveals that 2D hybrid halide perovskite structures containing these cations have indeed been reported in the literature for a range of metal ions and halido ligands, as listed in Table 4.
| CSD Refcode | Cation | Metal ion | Halido ligand | Ref. |
|---|---|---|---|---|
| BEHXIV | 2-Carboxyethylammonium | Cu2+ | Cl− | 80 and 81 |
| BEHXIV01 | ||||
| CAYPOH | 2-Carboxyethylammonium | Cu2+ | Br− | 82 |
| KETHID | 4-Carboxybutylammonium | Pd2+ | Cl− | 83 |
| VUHVIG | 4-Carboxybutylammonium | Pb2+ | Cl− | 84–86 |
| VUHVIG01 | ||||
| VUHVIG02 | ||||
| VUHVUS | 4-Carboxybutylammonium | Pb2+ | Br− | 84, 85 and 87 |
| VUHVUS01 | ||||
| VUHVUS02 |
Thus, it is possible to incorporate these cations containing an odd number of carbon atoms into the perovskite structure, however, this does not occur when the metal ion is Mn2+ and the halido ligand is a chlorido ligand. Rather than a 2D perovskite structure forming, where only halide ions are coordinated to the Mn2+ ion, isolated [MnCl4(H2O)2]2− anions are formed via the coordination of water molecules to the Mn2+ ion. This process can be seen as dimensional reduction from the 2D hybrid perovskite structure, which contains a [MnCl4]2− repeat unit, to form a [MnCl4(H2O)2]2− anion, through replacement of two corner sharing chlorido ligands with terminal aqua ligands and changing two other corner sharing chlorido ligands to terminal chlorido ligands.
Anions of the formula [MCl4(H2O)2]2−, in either the cis- or trans conformation, where M is any metal ion, are relatively rare. A search of the CSD (Version 5.43, June 2022 update)39 was conducted to find any structures containing either cis- or trans anions of this type containing any metal ion, in combination with any other species, with the results of the search listed in ESI† Section S3. The results from the search revealed that only 32 structures containing this type of anion have been reported. Trans-[MCl4(H2O)2]2− anions have been reported for the following metal cations, with the number of structures reported in the CSD39 indicated in parenthesis: Mn2+ (5), Sn4+ (2), Fe3+ (2), Ca2+ (1), Cu2+ (1), Fe2+ (1), In3+ (1), Co2+ (1), Rh3+ (1), Cd2+ (1). The cis analogues of the [MCl4(H2O)2]2− anions have been reported for the following metal ions, with the number of structures containing this anion indicated in parenthesis: Sn4+ (8), In3+ (3), Rh3+ (1), Pt4+ (1), Tc4+ (1), Mn2+ (1), Ru3+ (1). Thus, the formation of this type of anion is quite rare. The reason for the formation of this specific anion in the structures in which the cation contains an odd number of carbon atoms is unclear, and it can only be noted. The odd–even effect is well known for compounds containing n-alkyl portions, where compounds with an even number of carbon atoms display a different type of structure compared to those with an odd number of carbon atoms, with each family forming a homologous structural series.36–39
Thus, even though n-alkylammonium- and n-alkyldiammonium-containing perchloridomanganate compounds show a clear preference for the hybrid halide perovskite structure, this structure is only preferred for the n-carboxyalkylammonium containing perchloridomanganate compounds with an even number of carbon atoms in the chain.
A study33 considering salts of n-carboxylalkylammonium cations containing an even number of carbon atoms showed that, depending on the type of anion present, either an organic bilayer structure, similar to the organic layer in the 2D hybrid halide perovskite structures reported here, or an interdigitated organic layer, similar to the organic layer of the 0D structures reported here, may be formed. It was found the structures containing chloride and bromide anions showed interdigitated n-carboxyalkylammonium chains, and a single hydrogen bonding network, similar to the cation packing in structures C3MnClH2O and C5MnClH2O. However, the structures containing nitrate and perchlorate anions formed non-interdigitated, bilayer structures, with carboxylic acid dimers forming in the organic layer, similar to what is observed in structures C4MnCl and C6MnCl. Thus, the specific anion present in the structure plays a role in whether a structure with interdigitated n-carboxyalkylammonium chains, like the 0D structures reported here, or a non-interdigitated structure, similar to the 2D perovskite type structures reported here, is formed. This observation was attributed to the hydrogen bonds formed between the cations and anions in these structures, and the effect of anion size on the hydrogen bonding network.
In the current series the interdigitation or non-interdigitation of the cations can also be explained by considering the hydrogen bonding network formed in the two structural types. Etter88 stated that the strongest hydrogen bond donor will hydrogen bond to the strongest hydrogen bond acceptor. In the structures under investigation, the strongest hydrogen bonding donor is the ammonium group, and the strongest hydrogen bonding acceptor is the coordinated chlorido ligands, due to the charge assisted nature of the N–H+⋯−Cl–Mn hydrogen bonds that can form between these species.89 The rule provided by Etter88 can be applied to define a primary hydrogen bonding network in both the 0D and the 2D structure types, i.e. the hydrogen bonding network formed between the ammonium groups and the chlorido ligands represent the primary network. Other hydrogen bonding species that form weaker hydrogen bonds can then also form or accept hydrogen bonds to or from this primary network, provided there is space accessible on the primary network for additional hydrogen bonding interactions to occur. If no space is available, the weaker groups will form a separate hydrogen bonding network.
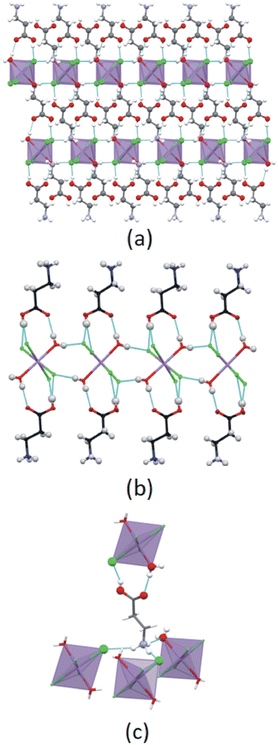
The primary hydrogen bonding network formed between ammonium groups and halido ligands in structure C4MnCl is shown in Fig. 11(a), viewed down an arbitrary axis to show the ammonium groups anchoring to the cavities created in the inorganic layer. From this figure it is clear that all available hydrogen bonding acceptor sites on the inorganic layer are occupied by ammonium groups, hence no sites are available for carboxylic acid groups to form hydrogen bonds to the inorganic layer. As a result, the carboxylic acid groups cannot hydrogen bond to the primary hydrogen bonding layer, and instead form carboxylic acid dimers in the organic layer due to their donor and acceptor character,33 with is the only option available to satisfy the hydrogen bonding capability of the carboxylic acid functional groups, and a non-interdigitated, organic bilayer is formed. The same argument holds for structure C6MnCl, with the primary network illustrated in Fig. 11(b), resulting in the formation of a non-interdigitated organic bilayer.
The primary hydrogen bonding network formed in structure C3MnClH2O between the ammonium group donors and chlorido acceptors is illustrated in Fig. 11(c). As indicated by the arrows in Fig. 11(c), the sites above the coordinated water molecules in this network are unoccupied and are available to accept and donate hydrogen bonds to the carboxylic acid groups of cations of which the ammonium groups form part of a neighbouring primary network. Fig. 11(d) shows the cations hydrogen bonded to the primary network via their carboxylic acid groups. The same is true for structure C5MnClH2O, as illustrated in Fig. 11(e) and (f). As a result, cations are hydrogen bonded to one primary network via their ammonium groups and to a neighbouring primary network via their carboxylic acid groups, resulting in interdigitated structures. Hence, the nature of the primary hydrogen bonding network and the type of inorganic layer formed play an important role in determining whether an interdigitated or non-interdigitated structure is formed.
In both structure types, the effect of the hydrogen bonds involving the carboxylic acid groups is to “zipper” neighbouring primary networks together, either through the formation of an interdigitated structure where the carboxylic acid groups hydrogen bond to the primary hydrogen bonding network, or through the formation of a non-interdigitated bilayer structure, where carboxylic acid dimers are formed in the organic layer.
Magnetic characterisation
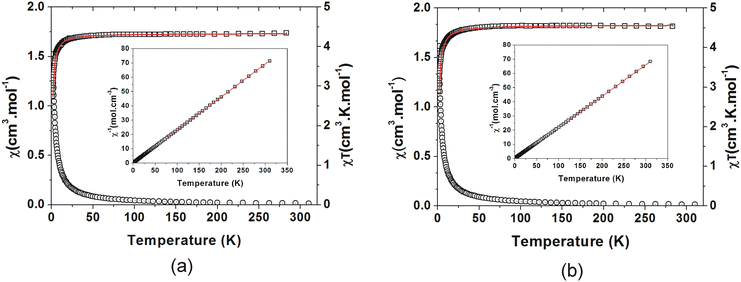
All four compounds structurally characterised were also characterised magnetically by SQUID magnetometry.Paramagnetic behaviour is observed at higher temperatures, with a χT value of 4.26 cm3 K mol−1 for C3MnClH2O and 4.57 cm3 K mol−1 for C5MnClH2O at 310 K. These values are close to the expected value of 4.375 cm3 K mol−1 for a Mn2+ ion of S = 5/2. Below 25 K for C3MnClH2O and 36 K for C5MnClH2O, the χT values show gradual decrease followed by a sharper decrease, which is indicative of AFM interactions between the Mn2+ ions or possibly single ion anisotropy (SIA) due to the polyhedra not being truly octahedral.
A Curie–Weiss analysis of the χ−1(T) plots (Fig. 12(a) and (b)) was done for C3MnClH2O between 77 K and 310 K, and for C5MnClH2O between 122 K and 310 K. Due to the plots not being perfectly linear, approximate Curie constants of 4.34(2) cm3 K mol−1 and 4.52(2) cm3 K mol−1 were obtained for C3MnClH2O and C5MnClH2O, with negative Weiss temperatures, θ, of −0.98(3) K and −0.83(3) K, indicating AFM behaviour.
Structures C3MnClH2O and C5MnClH2O contain 1D hydrogen bonded chains, with neighbouring Mn2+ ions linked via Mn–O–H⋯−Cl–Mn hydrogen bonds. The experimental susceptibility data was fitted to a model for a S = 5/2 AFM chain.90 This model is based on the Hamiltonian H = −∑nn2JKSiSj with ∑nn running over all possible pairs of nearest-neighbour spins i and j, with a negative JK value indicating AFM interactions. The model is given by eqn (1):
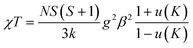 | (1) |
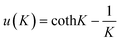 and K = −2JKS(S + 1)/kT.
and K = −2JKS(S + 1)/kT.
Here, N is Avogadro's constant, β the Bohr magneton, k the Boltzmann's constant, JK the exchange parameter, g = 2 and S = 5/2. On substitution of the constants, eqn (2) is obtained:
 | (2) |
| Compound | C3MnClH 2 O | C5MnClH 2 O | C4MnCl | C6MnCl |
|---|---|---|---|---|
| a Curie constant obtained from fitting to model. b Curie constant obtained from Curie–Weiss analysis. | ||||
| 2J K (K) | −0.14(2) | −0.14(2) | −8.28(5) | −7.72(4) |
| Exchange | AFM | AFM | AFM | AFM |
| g | — | — | 1.96(8) | 1.94(6) |
| Curie constant (mol cm−3)a | 4.33(1) | 4.55(3) | — | — |
| Curie constant (mol cm−3)b | 4.34(2) | 4.52(2) | 5.16(2) | 4.53(2) |
| θ (K) | −0.98(3) | −0.83(3) | −190(5) | −175(5) |
| Model | 1D AFM chain90 | 1D AFM chain90 | AFM square lattice94 | AFM square lattice94 |
| T N (K) | — | — | 45.0(5) | 43(1) |
| T(χmax) calc (K) | — | — | 83 | 89 |
| T(χmax) exp (K) | — | — | 83 | 87 |
The fits of the χT(T) data of C3MnClH2O and C5MnClH2O to this model are shown in Fig. 12(a) and (b), and the values obtained through the fits are listed in Table 5. A value of 2JK equal to −0.14(2) K and a Curie constant of 4.33(1) cm3 K mol−1 were obtained for C3MnClH2O, and 2JK equal to −0.14(2) K and a Curie constant of 4.55(3) cm3 K mol−1 was found for C5MnClH2O, with the negative 2JK values indicating AFM interactions or SIA interactions. The small values of the exchange parameters indicate weak magnetic exchange interactions via the Mn–O–H⋯−Cl–Mn hydrogen bonding interactions linking Mn2+ ions.
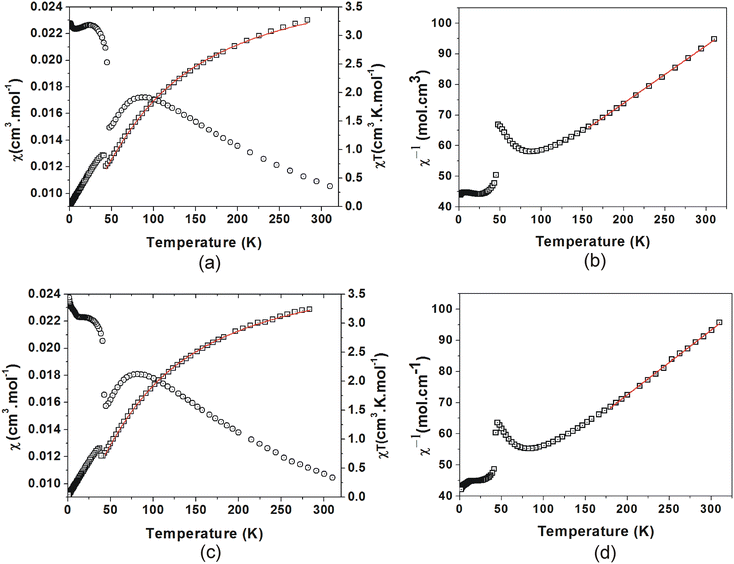
The χ(T) plots (Fig. 13(a) and (c)) both show a gradual increase from 0.011 cm3 mol−1 at 310 K, with a broad maximum of 0.017 cm3 mol−1 at 87 K for C4MnCl and 0.018 cm3 mol−1 at 83 K for C6MnCl. On further cooling, the susceptibility of both compounds shows a decrease followed by a sharp increase at the Neél temperature, TN, and a steep rise below TN, which signifies spontaneous magnetisation as a result of long range 3D AFM ordering due to spin canting,91 with TN = 45.0(5) K for C4MnCl and TN = 43(1) K for C6MnCl, respectively. The spin canting is attributed to antisymmetric Dzyaloshinskii–Moriya interactions that occur due to the tilting of the [MnCl6]4− octahedra in the inorganic plane.26,92 Further cooling results in a continued increase in susceptibility.
The χT(T) plots (Fig. 13(a) and (c)) show values of 3.35 cm3 K mol−1 and 3.32 cm3 K mol−1 for C4MnCl and C6MnCl respectively at 310 K, which is lower than the expected value of 4.375 cm3 K mol−1 for Mn2+. On cooling from 310 K, the χ(T) data show a gradual decrease to reach a minimum at TN = 45.0(5) K for C4MnCl and TN = 43(1) K for C6MnCl, where after there is a sharp increase. This is followed by a further decrease on cooling, approaching zero, for both compounds.
A Curie–Weiss analysis of the χ−1(T) plots was done for C4MnCl between 157 K and 310 K, and for C6MnCl between 180 K and 310 K. At high temperatures there is no region where χT(T) is constant, thereby completely obeying Curie–Weiss law. Thus, the Curie–Weiss values obtained serve merely as an approximation. From the Curie–Weiss plots (Fig. 13(b) and (d)), approximate Curie constants of 5.16(2) mol cm−3 and 4.53(2) mol cm−3 were obtained for C4MnCl and C6MnCl, with approximate Weiss temperatures, θ, of −190(5) K and −175(5) K, with the negative Weiss temperatures indicating AFM behaviour.
C4MnCl and C6MnCl adopt the 2D perovskite structure and since they contain Mn2+ ions, are expected to behave as 2D S = 5/2 Heisenberg antiferromagnets at low temperature. The intra-planar AFM exchange interactions occur through the single-halide, Mn–Cl–Mn superexchange pathway where bridging halido ligands link neighbouring Mn2+ ions to form a square lattice. The magnetic d orbitals of neighbouring metal ions overlap via the common orbital of the bridging halido ligand. Inter-layer magnetic coupling occurs at low temperature and is expected to be much smaller than intra-layer coupling, by at least a factor (104),93 and is seen as negligible.
Since the C4MnCl and C6MnCl systems can be treated as AFM square lattices, the magnetic susceptibility data was fit to a model for a S = 5/2 square lattice of classical spins, based on the 2JK Hamiltonian H = −∑nn2JKSiSj, with ∑nn running over all possible pairs of nearest-neighbours spins i and j, with a negative 2JK value indicating an AFM interaction, given by eqn (3):94
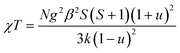 | (3) |
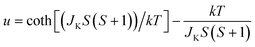 where, N is Avogadro's constant, g the g factor, β the Bohr magneton, k the Boltzmann's constant, JK the magnetic exchange parameter and S = 5/2.
where, N is Avogadro's constant, g the g factor, β the Bohr magneton, k the Boltzmann's constant, JK the magnetic exchange parameter and S = 5/2.
On substitution of the constants, eqn (4) is obtained:
 | (4) |
The values of JK and g are allowed to vary, with g constrained between 1.5 and 2.1.
The χT(T) data of C4MnCl and C6MnCl were fit to the model from temperature TN to 310 K. The fits are shown in Fig. 13(a) and (c), and Table 5 lists the values obtained. A value of 2JK equal to −8.28(5) K and a g value of 1.96(8) were obtained for C4MnCl, and 2JK equal to −7.72(4) K and a g value of 1.94(6) was found for C6MnCl, with the negative 2JK values indicating AFM interactions. Even though the g-factors are slightly smaller than the expected value of 2, g-factors smaller than 2 have been reported for related compounds in the literature.26,30
Eqn (5), which is used to predict the temperature at which the magnetic susceptibility has a maximum value, has also been reported:94
| kT(χ)max = −1.2625(2J)S(S + 1) | (5) |
A very good agreement is obtained between the predicted and experimentally observed temperatures of χmax for both C4MnCl and C6MnCl, as listed in Table 5.
The magnetic behaviour of the C4MnCl and C6MnCl compounds clearly indicates the presence of a magnetic phase transition(s), structural phase transition(s), or both. Detailed studies of the magnetic properties of these compounds, including FC/ZFC measurements at multiple fields, full hysteresis sweeps at multiple temperatures and low temperature X-ray diffraction studies will be required to fully understand the nature of the samples. Such measurements are in progress, but are beyond the scope of the current study.
Discussion of magnetic results
As expected, the magnetic behaviour of the two types of compounds observed in the study is markedly different due to the difference in structures and magnetic exchange interactions.From the parameters obtained by fitting the data of C3MnClH2O and C5MnClH2O, it can be deduced that the magnetic exchange along the 1D hydrogen bonded anionic chain involves very weak AFM interactions at low temperature with Weiss temperatures, θ, of −0.98(3) K and −0.83(3) K for C3MnClH2O and C5MnClH2O, respectively. Weak AFM exchange parameters, 2JK, of −0.14(2) K for both C3MnClH2O and C5MnClH2O are obtained, with a negative JK value indicating AFM exchange. For these compounds the magnetic exchange pathway between Mn2+ ions involve hydrogen bonding interactions through coordinated water molecules and chlorido ligands, which results in very weak magnetic exchange. Magnetic coupling through a hydrogen bonding pathway was reported in a series of trinuclear and dinuclear Mn3+/Fe3+ complexes, where weak AFM interactions exchange, with JK < 1 K was reported for the Mn3+ complex.95 The hydrogen bonding magnetic exchange pathway has been proposed in many studies, although the mechanism of exchange is not well understood.96
For the even-chain containing compounds, C4MnCl and C6MnCl, which display the 2D hybrid halide perovskite structure, a magnetic superexchange pathway involving a bridging halido ligand result in more effective magnetic exchange, with 2JK values of −8.28(5) K and −7.72(4) K respectively.
The results of magnetic studies of a number of related Mn2+ hybrid chloride perovskites have been reported in the literature, which allows for the comparison of the results obtained in this study with the literature results. As mentioned previously, interesting magnetic phenomena are typically observed in these types of compounds, with AFM interactions observed at higher temperatures, and spin canting observed at lower temperatures. A literature survey was performed, and a number of reports on the magnetic behaviour of compounds containing n-alkylammonium- or aryl-ammonium cations and perchloridomanganate anions with the perovskite structure were identified, however, the magnetic behaviour of only one n-alkyldiammonium-containing compound has been reported. The results of the literature survey are summarised in Table 6. Information on the onset temperature of spin canting, TN, values of magnetic exchange constants, JK, Weiss temperatures, θ, and whether the structure can be classified as RP or DJ are tabulated. It should be noted that not all the information listed above was available for all the compounds listed in Table 6. In certain cases, the crystal structures have not been reported, hence the classification as RP or DJ could not be performed. Certain studies did not report either the magnetic exchange constant or the Weiss temperature or neither of the two. In some cases, more than one study investigated the same compound, and it should be noted that there is some variability in the values reported. In addition, year of publication of the magnetic studies are included in Table 6. It is interesting to note that there has been renewed interest in the magnetic properties of these compounds, with no less than seven magnetic studies reported in the period 2020 to 2022. Prior to that, publications on the magnetic properties of these perovskites were scant.
| Compound | T N (K) | 2J K (K) | θ (K) | RP or DJ | CSD Refcode and reference | Magnetic study reference | Year of publication of magnetic study |
|---|---|---|---|---|---|---|---|
| a Note that a negative JK value indicates an AFM interaction, and values indicated with an asterisk are double the values reported in the original publications. b H⊥ indicates an applied magnetic field perpendicular to the inorganic layer, and H|| an applied magnetic field parallel to the inorganic layer. | |||||||
| (CH3NH3)2[MnCl]4 | 47(3) | −10.0(2)* | — | RP | MATCMN01 59 | 18 | 1972 |
| 45.3(5) | −10.0(2)* | — | MATCMN04 60 | 27 | 1979 | ||
| 44 | ≈−8 K* | — | MATCMN05 61 | 17 | 1995 | ||
| — | ≈−7.25 | — | MATCMN07 62 | 32 | 2000 | ||
| 47 | — | −207.8 | 28 | 2021 | |||
| 44 | — | −23 | 99 | 2021 | |||
| (CH3CH2NH3)2[MnCl]4 | 43.1(2) | −9.20(5)* | — | RP | EAMNCL 64 | 27 | 1979 |
| 44 | ≈−8* | — | EAMNCL11 65 | 17 | 1995 | ||
| — | ≈−7.2 | — | 32 | 2000 | |||
| 45 | −8.8 | — | 26 | 2022 | |||
| (CH3(CH2)2NH3)2[MnCl]4 | 39.2(2) | −8.90(5)* | — | RP | PAMMNC01 64 | 27 | 1979 |
| 40 | ≈−8* | — | PAMMNC10 65 | 17 | 1995 | ||
| — | ≈−7.25 | — | PAMMNC13 8 | 32 | 2000 | ||
| PAMMNC36 66 | |||||||
| (CH3(CH2)4NH3)2[MnCl]4 | 37 | ≈−8* | — | — | — | 17 | 1995 |
| (CH3(CH2)6NH3)2[MnCl]4 | 39 | ≈−8* | — | — | — | 17 | 1995 |
| (CH3(CH2)7NH3)2[MnCl]4 | — | ≈−7.2 | — | — | — | 32 | 2000 |
| (CH3(CH2)8NH3)2[MnCl]4 | 40 | ≈−8* | — | — | — | 17 | 1995 |
| (CH3(CH2)9NH3)2[MnCl]4 | — | ≈−7.4 | — | — | — | 32 | 2000 |
| (CH3(CH2)11NH3)2[MnCl]4 | — | ≈−6.25 | — | — | — | 32 | 2000 |
| (C6H5NH3)2[MnCl]4 | 42.6 | −6.19 | −126 | nRP | ZZZBMJ01 30 | 30 | 2021 |
| (C6H5CH2NH3)2[MnCl]4 | 44.6 | −7.47 | −135 | RP | ZZZBMG01 30 | 30 | 2021 |
| ZZZBMG02 97 | |||||||
| (C6H5(CH2)2NH3)2[MnCl]4 | 44.3 | — | −171.4 | RP | ZZZBMD01 29 | 29 | 2012 |
| 44.0 | — | ZZZBMD02–04 98 | 100 | 2020 | |||
| 45.7 | −7.65 | −162 | 30 | 2021 | |||
| 47 | −9.9 | — | 26 | 2022 | |||
| (C6H5(CH2)3NH3)2[MnCl]4 | 41.8 | −7.23 | −148 | DJ | ZZZBMA01 30 | 30 | 2021 |
| (C6H5(CH2)4NH3)2[MnCl]4 | 37 | — | −153.9 | RP | ZZZBLY01 97 | 7 | 2022 |
| (NH3(CH2)2NH3)[MnCl]4 | No spin canting | −10.2 | — | DJ | ENDAMN10 68 | 26 | 2022 |
| ENDAMN11 69 | |||||||
| (C6H5C2H3FNH3)2[MnCl]4 | 45 | — | −272.67 (H⊥)b | RP | 31 | 31 | 2022 |
| −273.01 (H||)b | |||||||
| (HOOC(CH2)3NH3)2[MnCl]4 (C4MnCl) | 47 | −8.28(5) | −190(5) | nRP | This study | This study | |
| (HOOC(CH2)5NH3)2[MnCl]4 (C6MnCl) | 45 | −7.72(4) | −175(5) | nDJ | This study | This study | |
Since the S = 5/2 AFM square lattice model developed by Curély and Rouch94 is based on a 2JK Hamiltonian, and the parameter being varied is JK, some reports in the literature give JK instead of 2JK. These JK values were multiplied by two before being included in Table 6, and are indicated by an asterisk next to the value of 2JK.
The 2JK values in Table 6 range from −6.2 K to −10.2 K. The magnetic exchange parameter values of C4MnCl and C6MnCl of −8.28(5) K and −7.72(4) K are comparable with these values. The relatively similar 2JK values are attributed to the similar superexchange Mn–Cl–Mn pathways found in both the C4MnCl and C6MnCl compounds, and related compounds in the literature. The largest 2JK value of 10.2 K was reported for (NH3(CH2)2NH3)[MnCl]4,26 which is the only n-alkyldiammonium-containing compound listed, and the only compound exhibiting an ideal DJ structure. To determine if compounds exhibiting the ideal DJ structure generally exhibit larger magnetic exchange parameters, additional studies are required.
Considering the information in Table 6, the TN values reported in the literature fall within the range of 37 K to 47 K. The lowest TN values were reported for (C6H5(CH2)4NH3)2[MnCl4]97 and (CH3(CH2)4NH3)2[MnCl4],18 while the highest value was reported for (CH3NH3)2[MnCl4].18 The TN values of C4MnCl and C6MnCl compare well with those reported in the literature, at 45.0(5) K and 43(1) K, respectively. It was reported that for (CH3(CH2)nNH3)2[MnCl4] compounds, as the inter-layer spacing is increased, a decrease in the transition temperature, TN, occurs.32 However, this behaviour was seen for short chain lengths only, with n < 4.32 For the longer chain lengths the change in transition temperature is independent of chain length.32 In this current study, the slight decrease in TN of 45.0(5) K for C4MnCl to 43(1) K for C6MnCl is probably within experimental error and therefore the transition temperature for the C4MnCl and C6MnCl is independent of cation chain length.
The only compound reported in the literature that did not display spin canting was (NH3(CH2)2NH3)[MnCl]4,26 and the authors attributed it to the fact that the compound exhibited a DJ structure, while the rest of the compounds in the family they investigated fell into the category of RP structures. It was proposed that the perfect alignment of neighbouring inorganic layers prevented spin canting. The classification of the compounds according to RP or DJ, as indicated in Table 6, indicate that all the compounds that exhibit the onset of spin canting at TN can indeed be categorised as RP or nRP structures, except for C6MnCl and (C6H5(CH2)3NH3)2[MnCl4],30 which are nDJ structures. Hence, it appears as if at least some degree of staggering of neighbouring inorganic layers is required for spin canting to occur, but more ideal DJ compounds should be studied to confirm this.
A very small Weiss temperature of −23 K was reported for (CH3NH3)2[MnCl4] by Zhao et al.,99 while Kim et al.28 reported a Weiss temperature of −207.8 K for the same compound. Ignoring the Weiss temperature reported by Zhao et al.,99 which seems to be an outlier, the Weiss temperatures reported in the literature fall in the range of −126 K to −273 K. The Weiss temperatures of −190(5) K for C4MnCl and −175(5) K for C6MnCl fall within this temperature range.
In summary, the magnetic behaviour of C4MnCl and C6MnCl agrees well with the magnetic behaviour of related Mn2+ perovskite compounds reported in the literature. This is expected, since the main difference between C4MnCl and C6MnCl and the compounds reported in the literature is the identity of the organic cation, while the inorganic portion consisting of corner sharing [MnCl6]4− octahedra is similar in all the compounds. It should, however, be kept in mind that the organic cation indirectly influences the structure in that compounds containing n-alkyldiammonium cations exhibit DJ structures, which are not expected to show spin canting.
Conclusions
The even-odd effect is distinctly displayed by compounds comprised of n-carboxyalkylammonium cations and perchloridomanganate anions investigated in this study. Compounds C4MnCl and C6MnCl, containing an even number of carbon atoms, exhibit the layered 2D hybrid halide perovskite structure, with corner sharing octahedra comprising the inorganic layer, and non-interdigitated n-carboxyalkylammonium cations forming the organic layer. When an odd number of carbon atoms is present in the n-carboxyalkylammonium chain, i.e. compounds C3MnClH2O and C5MnClH2O, a 0D hybrid structure is formed, where interdigitated n-carboxyalkylammonium cations form the organic layer, and isolated [MnCl4(H2O)2]2− anions the inorganic layer. This family of compounds represent a striking example of how a change in one component of a hybrid compound, in this case the addition of single carbon atom to the n-carboxyalkylammonium cation chain, can drastically change the type, crystal structure and dimensionality of the hybrid compound formed. Interestingly, this is only observed in the case where the metal ion is Mn2+, while the 2D hybrid halide perovskite structure is always formed, regardless of the number of carbon atoms in the n-carboxyalkylammonium cation, for other metal ions.The interdigitation or non-interdigitation of the cations was explained by considering the formation of a primary hydrogen bonding network comprised of the strongest hydrogen bonding donors, and whether space is available for additional hydrogen bonds to form to the primary network.
The relative orientation of consecutive inorganic layers in structures C4MnCl and C6MnCl were compared with those of related n-alkylammonium- and n-alkyldiammonium-containing hybrid halide perovskite perchloridomanganates reported in the literature, and indicated that, while n-alkyldiammonium-containing structures of this type always exhibit ideal DJ structures, n-alkylammonium-containing structures often, but not exclusively, adopt the ideal RP structure. Structures C4MnCl and C6MnCl were classified as nRP and nDJ respectively, showing the effect of a change in the cation, and the hydrogen bonding ability of the cation on the relative orientations of the inorganic layers.
It was shown through a literature survey that [MnCl4(H2O)2]2− anions are rare, and that compounds containing 2-carboxyethylammonium or 4-carboxybutylammonium can indeed form the 2D hybrid halide perovskite structure with metal ions other than Mn2+. As such, structures C3MnClH2O and C5MnClH2O are quite unique, in that they contain a rare anion, and do not form the 2D hybrid halide perovskite structure as was found for compounds of this type containing other metal ions.
Weak magnetic exchange between Mn2+ ions is observed compounds C3MnClH2O and C5MnClH2O which is ascribed to the fact that the exchange occurs via hydrogen bonding interactions in a 1D hydrogen bonded chain.
Stronger magnetic interactions are observed in compounds C4MnCl and C6MnCl, which occur via a halide-bridged exchange pathway. In addition, spin canting observed in compounds C4MnCl and C6MnCl is ascribed to the tilting of the octahedra in the inorganic layer. A literature survey of reports of the magnetic properties of related 2D hybrid halide perovskite compounds was conducted and highlighted the magnetic behaviour of these types of compounds. It also showed that the magnetic exchange parameters as well as spin canting onset temperatures of C4MnCl and C6MnCl correlate well with those of related compounds reported in the literature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Dr. F. Malan and Mr. D. Liles for assistance with crystallographic aspects. MR acknowledges financial support from the University of Pretoria, SASOL and the National Research Foundation (Grant No.: CSUR13090533011 and SRUG210427597644). SNB acknowledges financial support from SASOL and the National Research Foundation (Grant No.: 106452). MMT and CPL are grateful to the National Science Foundation (USA) for grants to purchase the SQUID magnetometer (IMR-0314773) and toward the purchase and construction of a helium recycling system (NSF DMR-1905950).References
- H. Do Kim, H. Ohkita, H. Benten and S. Ito, Adv. Mater., 2016, 28, 917–922 CrossRef PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. Il Seok, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed.
- C. Ortiz-Cervantes, P. Carmona-Monroy and D. Solis-Ibarra, ChemSusChem, 2019, 12, 1560–1575 CrossRef CAS PubMed.
- L. Mao, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2019, 141, 1171–1190 CrossRef CAS PubMed.
- Q. A. Akkerman and L. Manna, ACS Energy Lett., 2020, 604–610 CrossRef CAS PubMed.
- B. Huang, P. Wang, W.-X. Zhang and X.-M. Chen, Mater. Adv., 2022, 3, 9103–9110 RSC.
- P. Harris, F. K. Larsen, B. Lebech and N. Achiwa, Acta Crystallogr., Sect. B: Struct. Sci., 1994, 50, 676–684 CrossRef.
- Y. P. Lin, L. C. Rao, M. J. Zhao, X. Y. Huang and K. Z. Du, Dalton Trans., 2021, 50, 2001–2006 RSC.
- P. Mondal, S. K. Abdel-Aal, D. Das and S. M. Islam, Catal. Lett., 2017, 147, 2332–2339 CrossRef CAS.
- J. Li, M. Barrio, D. J. Dunstan, R. Dixey, X. Lou, J. L. Tamarit, A. E. Phillips and P. Lloveras, Adv. Funct. Mater., 2021, 31, 1–8 Search PubMed.
- M. Bochalya and S. Kumar, J. Appl. Phys., 2020, 127, 1–8 CrossRef.
- S. Wang, X. Han, T. Kou, Y. Zhou, Y. Liang, Z. Wu, J. Huang, T. Chang, C. Peng, Q. Wei and B. Zou, J. Mater. Chem. C, 2021, 9, 4895–4902 RSC.
- G. Zhou, J. Ding, X. Jiang, J. Zhang, M. S. Molokeev, Q. Ren, J. Zhou, S. Li and X. M. Zhang, J. Mater. Chem. C, 2022, 10, 2095–2102 RSC.
- W. W. Zhong, Y. Y. Di, Y. X. Kong, D. F. Lu and J. M. Dou, J. Chem. Thermodyn., 2014, 72, 100–107 CrossRef CAS.
- S. J. Lee, M. Y. Choi and A. R. Lim, ACS Omega, 2021, 6, 15392–15399 CrossRef CAS PubMed.
- S. Flandrois, N. B. Chanh, R. Duplessix, T. Maris and P. Négrier, Phys. Status Solidi A, 1995, 149, 697–710 CrossRef CAS.
- W. D. van Amstel and L. J. de Jongh, Solid State Commun., 1972, 11, 1423–1429 CrossRef CAS.
- Y. Jing, Y. Yoshida, T. Komatsu and H. Kitagawa, Angew. Chem., Int. Ed., 2023, 1–6 CAS.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS PubMed.
- T. Vasilchikova, V. Nalbandyan, I. Shukaev, H. J. Koo, M. H. Whangbo, A. Lozitskiy, A. Bogaychuk, V. Kuzmin, M. Tagirov, E. Vavilova, A. Vasiliev and E. Zvereva, Phys. Rev. B, 2020, 101, 1–12 CrossRef.
- J. B. Goodenough and A. L. Loeb, Phys. Rev., 1955, 98, 391–408 CrossRef CAS.
- J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87–98 CrossRef CAS.
- G. Heger, E. Henrich and B. Kanellakopulos, Solid State Commun., 1973, 12, 1157–1165 CrossRef CAS.
- N. Achiwa, T. Matsuyama and T. Yoshinari, Phase Transitions, 1990, 28, 79–97 CrossRef CAS.
- Y. Asensio, S. Marras, D. Spirito, M. Gobbi, M. Ipatov, F. Casanova, A. Mateo-Alonso, L. E. Hueso and B. Martín-García, Adv. Funct. Mater., 2022, 2207988, 1–13 Search PubMed.
- H. A. Groenendijk, A. J. van Duyneveldt and R. D. Willett, Physica B+C, 1979, 98, 53–59 CrossRef CAS.
- Y. H. Kim and N. Hur, J. Appl. Phys., 2021, 129, 1–6 Search PubMed.
- S. H. Park, I. H. Oh, S. Park, Y. Park, J. H. Kim and Y. D. Huh, Dalton Trans., 2012, 41, 1237–1242 RSC.
- L. Septiany, D. Tulip, M. Chislov, J. Baas and G. R. Blake, Inorg. Chem., 2021, 60, 15151–15158 CrossRef CAS PubMed.
- Z. Hu, L. Li, Y. Han, J. Zhang, J. Li, Z. Chen, S. Wu, Y. Zhang, H. Ye and Y. Song, Aggregate, 2022, 1–6 Search PubMed.
- K. W. Lee, C. H. Lee, C. E. Lee and J. Kang, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 95–98 CrossRef CAS.
- M. Rademeyer and B. van der Westhuizen, CrystEngComm, 2017, 19, 6821–6836 RSC.
- H. Arend, K. Tichy, K. Baberschke and F. Rys, Solid State Commun., 1976, 18, 999–1003 CrossRef CAS.
- R. Boese, H. C. Weiss and D. Bläser, Angew. Chem., Int. Ed., 1999, 38, 988–992 CrossRef CAS PubMed.
- V. R. Thalladi, R. Boese and H.-C. Weiss, Angew. Chem., Int. Ed., 2000, 39, 918–922 CrossRef CAS PubMed.
- A. D. Bond, New J. Chem., 2004, 28, 104–114 RSC.
- E. Badea, G. Della Gatta, D. D'Angelo, B. Brunetti and Z. Rečková, J. Chem. Thermodyn., 2006, 38, 1546–1552 CrossRef CAS.
- C. R. Groom and F. H. Allen, Angew. Chem., Int. Ed., 2014, 53, 662–671 CrossRef CAS PubMed.
- Bruker, SAINT+, Bruker AXS Inc., Madison, Wisconsin, USA Search PubMed.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany Search PubMed.
- Bruker, APEX, Bruker AXS Inc., Madison, Wisconsin, USA Search PubMed.
- Agilent, CrysAlisPRO, Agilent Technologies Ltd., Yarnton, Oxfordshire, England, 2014 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- V. Vreshch, J. Appl. Crystallogr., 2011, 44, 219–220 CrossRef CAS.
- R. L. Carlin, Magnetochemistry, Springer-Verlag, Berlin, Heidelberg, Germany, 1986 Search PubMed.
- F. Lichtenberg, A. Herrnberger, K. Wiedenmann and J. Mannhart, Prog. Solid State Chem., 2001, 29, 1–70 CrossRef CAS.
- F. Lichtenberg, A. Herrnberger and K. Wiedenmann, Prog. Solid State Chem., 2008, 36, 253–387 CrossRef CAS.
- M. H. Tremblay, J. Bacsa, B. Zhao, F. Pulvirenti, S. Barlow and S. R. Marder, Chem. Mater., 2019, 31, 6145–6153 CrossRef CAS.
- D. B. Mitzi, Prog. Inorg. Chem., 1999, 48, 1–121 CAS.
- M. M. Zhao, J. Z. Ge and Z. R. Qu, Inorg. Chem. Commun., 2010, 13, 1152–1155 CrossRef CAS.
- C. A. Bremner and W. T. A. Harrison, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2003, 59, 596–598 CrossRef.
- D. M. Small, J. Lipid Res., 1984, 25, 1490–1500 CrossRef CAS.
- G. Heger, D. Mullen and K. Knorr, Phys. Status Solidi B, 1975, 31, 455–462 CrossRef CAS.
- I. Mikhail, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 1317–1321 CrossRef.
- I. Mikhail, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 1321–1325 CrossRef.
- G. Heger, D. Mullen and K. Knorr, Phys. Status Solidi B, 1976, 35, 627–637 CrossRef CAS.
- G. Chapuis, G. Brunisholz, C. Javet and R. Roulet, Inorg. Chem., 1983, 22, 455–458 CrossRef CAS.
- W. Depmeier, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1976, 32, 303–305 CrossRef.
- W. Depmeier, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 3713–3718 CrossRef.
- W. Depmeier and S. A. Mason, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1978, 34, 920–922 CrossRef.
- E. R. Peterson and R. D. Willet, J. Chem. Phys., 1972, 56, 1879–1882 CrossRef CAS.
- M. Meyer, W. A. Paciorek, K. J. Schenk, G. Chapuis and W. Depmeier, Acta Crystallogr., Sect. B: Struct. Sci., 1994, 50, 333–343 CrossRef.
- M. R. Ciajolo, P. Corradini and V. Pavone, Gazz. Chim. Ital., 1976, 106, 807–855 CAS.
- K. Tichý, J. Beneš, W. Halg and A. Arend, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1978, 34, 2970–2981 CrossRef.
- M. Gabro and R. A. Lalancette, CSD Private Communication, 2006 Search PubMed.
- R. D. Willett and E. F. Riedel, Chem. Phys., 1975, 8, 112–122 CrossRef CAS.
- J. C. Crowley, H. W. Dodgen and R. D. Willet, J. Phys. Chem., 1982, 86, 4046–4055 CrossRef CAS.
- K. Tichý, J. Beneš, R. Kind and H. Arend, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 1355–1367 CrossRef.
- S. K. Abdel-Aal, CSD Private Communication, 2015 Search PubMed.
- X.-H. Lv, W.-Q. Liao, P.-F. Li, Z.-X. Wang and C.-Y. Mao, J. Mater. Chem. C, 2016, 4, 1881–1885 RSC.
- X.-H. Lv, CSD Private Communication, 2015 Search PubMed.
- T. Maris, R. Zouari, R. Duplessix, J. Leger and N. B. Chan, PhD Thesis, University of Bordeaux, 1996 Search PubMed.
- W. Depmeier and K.-H. Klaska, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 1065–1068 CrossRef.
- R. E. Caputo and R. D. Willett, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37, 1616–1617 CrossRef.
- M. Gabro and R. A. Lalancette, CSD Private Communication, 2006 Search PubMed.
- R. D. Willett, F. H. Jardine, I. Rouse, R. J. Wong, C. P. Landee and M. Numata, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 24, 5372–5381 CrossRef CAS.
- A. Kaiba, M. H. Geesi, P. Guionneau, T. A. Aljohani, L. Bih, H. Bih and S. Kassou, J. Mol. Struct., 2020, 1204, 1–19 CrossRef.
- R. D. Willett, R. J. Wong and M. Numata, Inorg. Chem., 1983, 22, 3189–3194 CrossRef CAS.
- L. Efimenko, I. A. Churakov, A. V. Ivanova, N. A. Erofeeva and O. S. Demina, Zh. Neorg. Khim., 2017, 62, 1476–1478 CrossRef.
- H. Hillebrecht, CSD Private Communication, 2020 Search PubMed.
- M. Krummer, B. Zimmermann, P. Klingenberg, M. Daub and H. Hillebrecht, Eur. J. Inorg. Chem., 2020, 4581–4592 CrossRef CAS.
- W. Yang, X. Xiao, H. He, G. Tong, J. Hu, X. Xiao, J. Chen, M. Li and Y. He, Cryst. Growth Des., 2021, 21(10), 5731–5739 CrossRef CAS.
- L. Li, CSD Private Communication, 2020 Search PubMed.
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120–126 CrossRef CAS.
- X. Li, J. M. Hoffman and M. G. Kanatzidis, Chem. Rev., 2021, 121, 2230–2291 CrossRef CAS PubMed.
- R. Dingle, M. E. Lines and S. L. Holt, Phys. Rev., 1969, 187, 643–648 CrossRef CAS.
- B. Huang, P. Wang, W.-X. Zhang and X.-M. Chen, Mater. Adv., 2022, 3, 9103–9110 RSC.
- I. Dzyaloshinsky, J. Phys. Chem. Solids, 1958, 4, 241–255 CrossRef CAS.
- L. J. De Jongh and A. R. Miedema, Adv. Phys., 2001, 50, 947–1170 CrossRef.
- J. Curély and J. Rouch, Phys. B, 1998, 254, 298–321 CrossRef.
- I. Nemec, R. Herchel, T. Šilha and Z. Trávniček, Dalton Trans., 2014, 43, 15602–15616 RSC.
- V. Zeleňák, A. Orendáčová, I. Cısařová, J. Černák, O. V. Kravchyna, J.-H. Park, M. Orendáč, A. G. Anders, A. Feher and M. W. Meisel, Inorg. Chem., 2006, 45, 1774–1782 CrossRef PubMed.
- S. He, S. Hao, J. Lin, N. Wang, J. Cao, Z. Guo, C. Wolverton, J. Zhao and Q. Liu, Inorg. Chem., 2022, 11973–11980 CrossRef CAS PubMed.
- M. E. Kamminga, R. Hidayat, J. Baas, G. R. Blake and T. T. M. Palstra, APL Mater., 2018, 66106 CrossRef.
- H. Zhao, H. Fu, Z. Hu, Q. Fu, H. Tao, J. Weng, L. Xiong and Z. Cheng, CrystEngComm, 2021, 23, 5208–5213 RSC.
- K.-Y. Kim, G. Park, J. Cho, J. Kim, J.-S. Kim, J. Jung, K. Park, C.-Y. You and I.-H. Oh, Small, 2020, 16, 1–12 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2281554–2281557. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ce00855j |
| This journal is © The Royal Society of Chemistry 2023 |