An efficient synthesis of sulfonated quinoline diones by copper catalyzed sulfonylation of activated alkenes with sulfonylhydrazides†
Shucheng
Wang
,
Xuhu
Huang
,
Qi
Wang
,
Zemei
Ge
,
Xin
Wang
* and
Runtao
Li
*
State Key Laboratory of National and Biomimetic Drugs, School of Pharmaceutical Science, Peking University, Beijing 100191, P. R. China. E-mail: xinwang@bjmu.edu.cn; lirt@bjmu.edu.cn; Fax: +86 10 82805954; Tel: +86 10 82805954
First published on 22nd January 2016
Abstract
A novel and highly efficient cascade synthesis of sulfonated quinoline dione derivatives from N-(2-cyanoaryl) methylacrylamides and sulfonylhydrazides in good yields is described. The reaction proceeds through radical addition, cyclization, and imine hydrolysis, in which one new C–C bond and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond were formed.
O bond were formed.
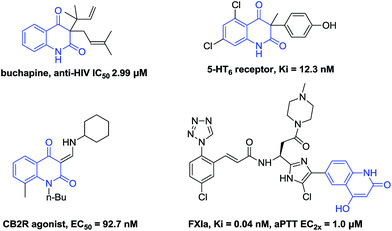
Quinoline diones are important heterocycles found in a wide range of natural products and pharmaceuticals (Fig. 1).1 Meanwhile, they are widely used as key intermediates in the preparation of biological and related structures.2 However, a few methods are reported for the synthesis of quinoline diones compounds. Generally, two strategies have been employed to achieve the targets including intramolecular cyclization of N-acylanthranilates1e,f and oxidation of 4-hydroxyquinolin-2-one.3 However, both methods could only be used for synthesizing specific compounds. Hence there is a great demand for the development of novel and atom-economical synthetic method for the synthesis of quinolone dione derivatives.
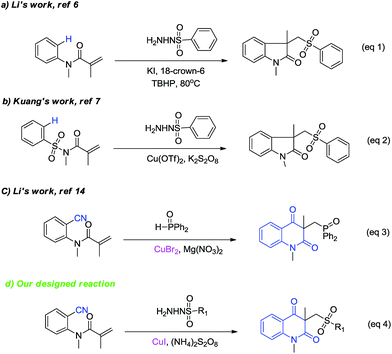
Sulfone groups represent one of the most important classes of organic functionalities, which are widely found in a number of biologically active compounds, drug moleculars, and versatile synthetic intermediates.4 Sulfonyl radicals are useful intermediates for the synthesis of sulfone compounds. Various methods for the generation of sulfonyl radicals and the addition reaction to C–C multiple bonds providing a particularly useful contribution to organosulfone synthesis have been extensively explored. For example, Wang et al. reported a copper catalyzed direct oxysulfonylation of alkenes with dioxygen and sulfonylhydrazides to access β-ketosulfones.5 Li and coworkers disclosed that sulfonated oxindoles could be synthesized from N-arylacrylamide with sulfonylhydrazides using the KI–TBHP system (Scheme 1, eqn (1)).6 Later, Kuang and coworkers provided an alternative strategy for the synthesis of sulfonated oxindoles from N-arylsulfonyl-acrylamides and sulfonylhydrazides (Scheme 1, eqn (2)).7 Very recently, Zhou's group succeeded in construction of isoquinoline dione framework via N-alkyl-N-methacryloyl benzamides with ethers and benzenesulfonohydrazides.8 However, to the best of our knowledge, there is no report on the synthesis of sulfonated quinoline dione.
Nitriles are versatile building blocks and precursors in organic synthesis,9 and the cyano group itself is ubiquitous in medicines and functional materials. Radical additions to nonpolar C–C multiple bonds have been extensively employed for making C–C bonds.10 On the other hand, radical additions to polar multiple bonds such as nitrile groups are not generally efficient because of the formation of unfavorable iminyl radicals. However, the reaction might be successful if the unstable radical intermediates are effectively trapped. With this in mind, a number of transition metal complex such as Ti,11 Sm,12 and Ag13 were successfully employed for the radical addition of nitrile. Very recently, Li's group reported a novel Cu-catalyzed access to phosphonylated quinoline dione from o-cyanoarylacrylamide and diphenylphosphine oxide (Scheme 1, eqn (3)).14 Inspired by these elegant works and our interest in developing efficient methodologies for synthesize heterocyclic compounds,15 we report herein a novel Cu-catalyzed construction of sulfonated quinoline dione derivatives, where the cyclization was accomplished by an intramolecular addition of the carbon radical to the nitrile, in which one new C–C bond and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond were formed (Scheme 1, eqn (4)).
O bond were formed (Scheme 1, eqn (4)).
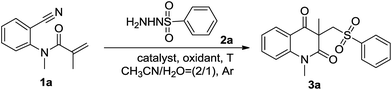
Initially, N-(2-cyanophenyl)-N-methylacrylamide 1a and phenylsulfonohydrazides 2a were chosen as the model substrates to optimize the reaction conditions, and the results were summarized in Table 1. To our delight, the desired product 3a was isolated in 76% yield in the presence of a catalytic amount of Cu(OAc)2 and K2S2O8 (3 equiv.) as the oxidant at 80 °C for 12 h (Table 1, entry 1).7 Other copper salts, such as CuBr, CuI, Cu(OAc)2, CuCl were then examined. The results showed that CuI was more efficient than other copper salts, and the desired product 3a was isolated in 91% yield (Table 1, entries 2–5). Next, a variety of oxidants, including PhI(OAc)2, TBHP, H2O2 and (NH4)2S2O8, were examined in the presence of CuI in CH3CN/H2O system, and (NH4)2S2O8 gave the best yield (Table 1, entries 6–9). By lowering the temperature to 70 °C, the yield of 3a was slightly decreased (Table 1, entry 10). In addition, reducing the amounts of oxidant (Table 1, entry 11) or catalyst (Table 1, entry 13) would dramatically reduce the reaction efficiency, whereas the yield was not reduced with lower amounts of phenylsulfonohydrazides (Table 1, entry 12).
| Entry | Catalyst | Oxidant | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol, 1 eq.), 2a (0.6 mmol, 2 eq.), catalyst (0.06 mmol, 0.2 eq.), oxidant (0.9 mmol, 3 eq.), solvent (5 mL), 12 h. b Isolated yields. c NR = no reaction. d Oxidant (2.0 eq.). e 2a (1.5 eq.). f CuI (0.03 mmol, 0.1 eq.). | ||||
| 1 | Cu(OTf)2 | K2S2O8 | 80 | 76 |
| 2 | CuBr | K2S2O8 | 80 | 82 |
| 3 | CuI | K2S2O8 | 80 | 91 |
| 4 | Cu(OAc)2 | K2S2O8 | 80 | 84 |
| 5 | CuCl | K2S2O8 | 80 | 65 |
| 6 | CuI | PhI(OAc)2 | 80 | NRc |
| 7 | CuI | TBHP | 80 | 45 |
| 8 | CuI | H2O2 | 80 | 40 |
| 9 | CuI | (NH4)2S2O8 | 80 | 95 |
| 10 | CuI | (NH4)2S2O8 | 70 | 85 |
| 11d | CuI | (NH4)2S2O8 | 80 | 88 |
| 12 e | CuI | (NH4)2S2O8 | 80 | 95 |
| 13f | CuI | (NH4)2S2O8 | 80 | 75 |
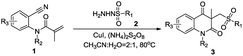
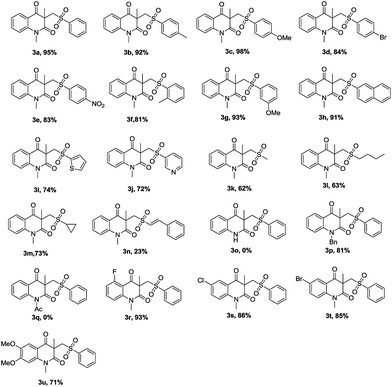
With the optimal reaction conditions in hand, we next examined the scope and limitations of this di-functionalization process, in which various N-(2-cyanophenyl) acrylamides 1 and various sulfonohydrazides 2 have been employed (Table 2). Generally, the reactions could proceed well by using diverse arylsulfonohydrazides with electro-donating group (Me, OMe) or electro-withdrawing group (Br, NO2) on the aromatic ring so as to afford the corresponding products in excellent yields (3a–3g). When the benzene ring of the substrates 2 was changed to a naphthalene ring, the reaction successfully provided the desired product 3h in 91% yield.
| a Reaction conditions: 1 (0.3 mmol, 1 eq.), 2 (0.45 mmol, 1.5 eq.), catalyst (0.06 mmol, 0.2 eq.), oxidant (0.90 mmol, 3.0 eq.), solvent (5 mL) at 80 °C for 12 hours under Ar. |
|---|
|
Furthermore, heteroaryl sulfonohydrazides 2i and 2j reacted also well with 1a and gave the desired products 3i and 3j in 74% and 72% yields, respectively. It is worth noting that this method could be extended to aliphatic sulfonohydrazides, including methyl, butyl and cyclopropyl sulfonohydrazide, to afford the corresponding products (3k, 3l, 3m) in 62%, 63% and 73% yields, respectively. In addition, the desired product 3n was obtained in only 23% yield when the reaction of phenylethenesulfonohydrzide 2n with performed 1a was conducted through the standard procedure due to the poor activity of 2n. Next, the effect of N-protecting groups was examined. The reaction underwent only for methyl and benzyl group (products 3a and 3p), whereas acetyl and unprotected N-(2-cyanophenyl)-N-acrylamides did not work at all (products 3q and 3o). Finally, the property and position of substituents R3 bearing both electron-donating and electro-withdrawing groups on the aromatic moieties were compatible with this reaction to give the corresponding products in good yields (3r–3u, 71–93%).
To confirm that this chemistry proceeds through radical-based mechanism, the reaction of 1a and 2a was repeated in the presence of 1.5 equiv. radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) under the standard reaction conditions, the reaction progress was remarkably suppressed, and only trace amounts of the desired product 3a was detected.
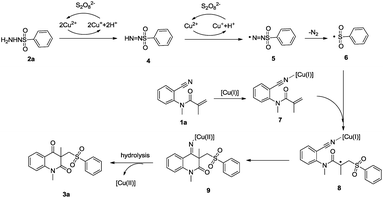
Based on these results and previous observations,6,7,14 a possible mechanism is proposed in Scheme 2. Initially, the sulfonyl radical 6 was generated from 2avia single electron transfer and the deprotonation process. Then the Cu(I) species coordinate with N-(2-cyanophenyl)-N-methylacrylamide 1a to form intermediate 7, the sulfonyl radical 6 attacks the carbon–carbon double bond of 7 to afford the nucleophilic carbon-centered radical 8. Next, an intramolecular addition of the alkyl radical to the coordinated nitrile afford an imine 9, which is hydrolyzed by H2O to generate the final product 3a.
Conclusions
In summary, a simple and environmentally entry to sulfonated quinoline dione derivatives from N-(2-cyanoaryl) methylacrylamides and sulfonylhydrazides has been developed. The transformation proceeded in good yields and tolerated a wide range of functional groups. This tandem reaction was accomplished by an intramolecular addition of the carbon radical to the nitrile. The present protocol provided a new avenue to formation of potentially bioactive sulfonated quinoline dione derivatives, and it would be gained more attention in synthetic and pharmaceutical chemistry. Further application to other heterocyclic systems is underway.Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 81172915).Notes and references
- For selected examples of quinoline diones, see: (a) J. L. McCormick, T. C. Mckee, J. H. Cardellina II and M. R. Boyd, J. Nat. Prod., 1996, 59, 469 CrossRef CAS; (b) T. S. Wu, Y. L. Leu, Y. Y. Chan, F. W. Lin, C. Y. Li, L. S. Shi, S. C. Kuo, C. F. Chen and Y. C. Wu, Phytochemistry, 1998, 49, 1467 CrossRef CAS; (c) S. S. Yang, G. M. Cragg, D. J. Newman and J. P. Bader, J. Nat. Prod., 2001, 64, 265 CrossRef CAS; (d) N. Ahmed, K. G. Brahmbhatt, S. Sabde, D. Mitra and I. P. Singh, Bioorg. Med. Chem., 2010, 18, 2872 CrossRef CAS; (e) C. M. Seong, W. K. Park, C. M. Park, J. Y. Kong and N. S. Park, Bioorg. Med. Chem. Lett., 2008, 18, 738 CrossRef CAS; (f) W. S. Hamama, A. El-Din, E. Hassanien and H. H. Zoorob, Synth. Commun., 2014, 44, 1833 CrossRef CAS; (g) Z. L. Hu, P. C. Wong, P. J. Gilligan, W. Han, K. B. Pabbisetty, J. M. Bozarth, E. J. Crain, T. Harper, J. M. Luettgen, J. E. Myers, V. Ramamurthy, K. A. Rossi, S. Sheriff, C. A. Watson, A. Z. Wei, J. J. Zheng, D. A. Seiffert, R. R. Wexler and M. L. Quan, ACS Med. Chem. Lett., 2015, 6, 590 CrossRef CAS PubMed.
- (a) M. Sankaran, C. Kumarasamy, U. Chokkalingam and P. S. Mohan, Bioorg. Med. Chem. Lett., 2010, 20, 7147 CrossRef CAS; (b) C. D. Haffner, J. D. Becherer, E. E. Boros, R. Cadilla, T. Carpenter, D. Cowan, D. N. Deaton, Y. Guo, W. Harrington, B. R. Henke, M. R. Jeune, I. Kaldor, N. Milliken, K. G. Petrov, F. Preugschat, C. Schulte, B. G. Shearer, T. Shearer, T. L. Smalley and J. C. Ulrich, J. Med. Chem., 2015, 58, 3548 CrossRef CAS.
- (a) R. Kumabe and H. Nishino, Tetrahedron Lett., 2004, 45, 703 CrossRef CAS; (b) S. Kafka, K. Proisl, V. Kasparkova, D. Urankar, R. Kimmel and J. Kosmrlj, Tetrahedron, 2013, 69, 10826 CrossRef CAS.
- (a) N. S. Simpkins, Sulfones in Organic Synthesis, Pergamon Press, Oxford, 1993 Search PubMed; (b) C. Napier, M. Stewart, H. Melrose, B. Hopkins, A. McHarg and R. Wallis, Eur. J. Pharmacol., 1999, 375, 61 CrossRef; (c) K. G. Petrov, Y. Zhang, M. Carter, G. S. Cockerill, S. Dickerson, C. A. Gauthier, Y. Guo, R. A. Mook, D. W. Rusnak, A. L. Walker, E. R. Wood and K. E. Lackey, Bioorg. Med. Chem. Lett., 2006, 16, 4686 CrossRef CAS; (d) J. N. Desrosiers and A. B. Charette, Angew. Chem., Int. Ed., 2007, 46, 5955 CrossRef CAS.
- W. Wei, C. L. Liu, D. S. Yang, J. W. Wen, J. M. You, Y. R. Suo and H. Wang, Chem. Commun., 2013, 49, 10239 RSC.
- X. Q. Li, X. S. Xu, P. Z. Hu, X. Q. Xiao and C. Zhou, J. Org. Chem., 2013, 78, 7343 CrossRef CAS.
- Q. S. Tian, P. He and C. X. Kuang, Org. Biomol. Chem., 2014, 12, 6349 RSC.
- M. Zhang, P. Xie, W. N. Zhao, B. Niu, W. Wu, Z. G. Bian Jr, C. U. Pittman and A. H. Zhou, J. Org. Chem., 2015, 80, 4176 CrossRef CAS.
- (a) J. S. Miller and J. L. Manson, Acc. Chem. Res., 2001, 34, 563 CrossRef CAS; (b) F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902 CrossRef CAS; (c) T. Wang and N. Jiao, Acc. Chem. Res., 2014, 47, 1137 CrossRef CAS.
- (a) J. Fossey, D. Lefort and J. Sorba, Free Radicals in Organic Chemistry, John Wiley & Sons, New York, 1995 Search PubMed; (b) D. P. Curran, N. A. Porter and B. Giese, Stereochemistry of Radical Reactions, VCH, Weinheim, 1995 CrossRef; (c) Y. M. Li, M. Sun, H. L. Wang, Q. P. Tian and S. D. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS; (d) J. Xie, P. Xu, H. M. Li, Q. C. Xue, H. M. Jin, Y. X. Cheng and C. J. Zhu, Chem. Commun., 2013, 49, 5672 RSC; (e) S. C. Wang, X. H. Huang, B. W. Li, Z. M. Ge, X. Wang and R. T. Li, Tetrahedron, 2015, 71, 1869 CrossRef CAS.
- (a) Y. Yamamoto, D. Matsumi, R. Hattori and K. Itoh, J. Org. Chem., 1999, 64, 3224 CrossRef CAS; (b) A. Fernandez-Mateos, P. H. Teijon, L. M. Buron, R. R. Clemente and R. R. Gonzalez, J. Org. Chem., 2007, 72, 9973 CrossRef CAS.
- G. A. Molander and C. N. Wolfe, J. Org. Chem., 1998, 63, 9031 CrossRef CAS.
- W. X. Zhao and J. Montgomery, Angew. Chem., Int. Ed., 2015, 54, 12683 CrossRef CAS.
- Y. M. Li, S. S. Wang, F. C. Fu, Y. H. Shen and K. J. Chang, Org. Biomol. Chem., 2015, 13, 5376 RSC.
- (a) J. F. Wang, Q. Li, C. Qi, Y. Liu, Z. M. Ge and R. T. Li, Org. Biomol. Chem., 2010, 8, 4240 RSC; (b) L. C. Luo, L. L. Meng, Q. Sun, Z. M. Ge and R. T. Li, RSC Adv., 2014, 4, 6845 RSC; (c) S. C. Wang, X. H. Huang, Y. Z. Wen, Z. M. Ge, X. Wang and R. T. Li, Tetrahedron, 2015, 71, 8117 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of the 1H and 13C NMR spectra. See DOI: 10.1039/c5ra27878c |
| This journal is © The Royal Society of Chemistry 2016 |