 Open Access Article
Open Access ArticleOptically active chiral photonic crystals exhibiting enhanced fluorescence and circularly polarized luminescence†
Qilin
Guo
,
Xingye
Huang
,
Huateng
Li
,
Jia
Guo
 and
Changchun
Wang
and
Changchun
Wang
 *
*
State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science and Laboratory of Advanced Materials, Fudan University, Shanghai 200433, China. E-mail: ccwang@fudan.edu.cn
First published on 4th March 2025
Abstract
Photonic crystals with advanced, unique and well-defined functional nanostructures demonstrate exquisite controllable modulation in light harvesting and emission for unrivalled optical performance. Herein, through ingeniously integrating aggregation-induced emission (AIE) luminogens and chiral helical media into ordered periodic structures, the resulting optically active photonic crystal films exhibit an enhanced photoluminescence (PL) characteristic (increased to 2.2 times the original value) and distinctive emerging circular dichroism (CD) responses near the photonic bandgap (PBG) of the photonic crystal. The modulation of the PL intensity and CD signal peak position is precisely achieved by regulating the PBG by facilely tuning the size of the colloidal nanoparticles. Such an interesting phenomenon is mainly the consequence of the PBG edge enhancement effect (including the slow photon effect) and bandgap separation arising from chirality. Remarkably, the boosted fluorescence facilitates the synergistic effect of valid chirality transfer among achiral AIEgens and chiroptical media in a photonic matrix, which effectively contributes to the enhanced circularly polarized luminescence (CPL) activity, thereby expanding the potential applications of CPL-based optically active photonic materials in circularly polarizing emitting devices.
Introduction
Natural creatures with a hierarchical complex and sophisticated macro-/nano-structure at multiple length scales exhibit tremendous manipulability of exceptional photonic characteristics, enabling effective light propagation and harvesting for a variety of optimized optical multifunctional applications, including vision detection, information storage, photosynthesis, and signal transduction.1–10 A striking example is the wing of Morpho butterflies, which features brilliant blue structural color due to the constructive interference of multilayer reflected light from the unique and well-defined periodic lamellar structure that is formed by densely arranged nanoscale ridges.11,12 Besides, the elytron of beetles consists of chiral and cuticular ordered periodic photonic nanostructures with helicoidal orientation, contributing to their exceptional ability to selectively filter left circularly polarized light.13 Inspired by these natural designs, a diverse range of progressing artificial photonic materials with naturally engineered structures have been developed, promising novel next-generation smart optical devices for desirable and sustainable practical applications.14–21Photonic crystals (PCs), a class of structurally colored materials, feature highly multiscale, well-ordered periodic dielectric architectures that are comparable to the wavelengths of visible light. These structures are created using distinct components with varying refractive indices, which induce specific photonic bandgaps (PBGs) that manipulate the orientation of light propagation within the crystal lattice, resulting in iridescent reflections.22–31 In particular, the photonic bandgap allows light to exist as a standing wave mode that resonates with the photonic lattice, in which light travels with a group velocity approaching zero at wavelengths corresponding to the edges of these stopbands.32–36 This phenomenon is referred to as “slow photons”.37 Therefore, when incorporating various luminescent materials such as organic molecules or luminogens with an aggregation-induced emission (AIE) character (AIEgens) into photonic crystals, the developed light–matter interactions are significantly enhanced, thus improving absorption and emission properties.38–44 In addition, chiral helical copolymers with intrinsic handedness have been introduced into a photonic crystal medium to explore the unique capability of the PBG in modulating the light polarization mode, endowing composite photonic crystal materials with distinctive optical activity.45,46 Furthermore, it is well known that the bonding assembly of luminescent substances with chiral media tends to induce an incredibly effective chirality transfer, which results in luminescent systems emitting left- and right-handed circularly polarized light (LCP/RCP) with different emission intensities, in turn producing circularly polarized luminescence (CPL).47–55 Consequently, integrating chiral helical media and fluorescent emitters within photonic crystals is expected to yield intriguing optical properties, including fluorescence enhancement, modulation of chiral signals, and the generation of circularly polarized luminescence. These advancements present considerable and promising prospects for optically active photonic crystal materials in potential applications, such as three-dimensional optical displays,56 asymmetric syntheses and catalysis,57 optoelectronic devices58 or circularly polarizing beamsplitter.59
In this work, we demonstrate the constructive fabrication of optically active photonic crystal materials with typical CPL performance based on effective chirality transfer in a photonic matrix comprising AIE units and chiral helical copolymers. The PBG edge enhancement effect and slow photon effect of PCs contribute to the remarkably enhanced photoluminescence (PL) property of the tetraphene (TPE) element in the confined rigid cross-linked polymer network, in which the restriction of intramolecular motions (RIM) is intensely manipulated to inhibit the non-radiative decay pathway of excitons and promote the radiative channels. What is more, the composite photonic films exhibit emerging circular dichroism (CD) responses with mirror-image CD signals approaching the PBG of PCs attributed to the bandgap separation in the chiral photonic structures. Owing to the efficient chiral-transfer process between the AIE character and chiral helical copolymers, the emission efficiency of the system is improved for displaying the enhanced CPL activity, providing remarkable tunability and robustness in promisingly the creation of CPL-based photonic crystal materials.
Results and discussion
Learned from nature, there are various sophisticated optical and structural designs for exceptional photonic functions in different luminescent organisms. For instance, the fireflies wondrously display intense CPL effects (see Fig. 1a) owing to the light polarization that arises from the composited interaction between periodic cuticle layer stacking and chiral medium.60 To create such multi-functional photonic luminescent material, a novel biomimetic optically active photonic crystal is established (Fig. 1b). During our design, core/shell colloidal nanoparticles are employed for the fabrication of well-ordered periodic photonic structures. AIEgens and chiral helical copolymers in the photonic matrix are conducted to produce effective chirality transfer to endow photonic materials with optical activity. Initially, four kinds of monodisperse PS@P(EA-co-AA) core/shell nanoparticles with various diameters ranging from 145 to 204 nm were fabricated by semi-continuous emulsion polymerization. The related characterization information of the as-prepared nanoparticles is shown in the ESI.† The growth of particle diameters at each step of polymerization was obtained from the TEM images shown in Fig. S1.† In addition, the size distribution histogram of the PS core based on TEM analysis further demonstrates the great monodispersity of uniform colloidal nanoparticles (Fig. S2†), which is a prerequisite for constructing long-range ordered structures as physical blocking units. Afterwards, the chiral helical copolymer poly(N-propargyl acrylamide)-random-poly(N-propargyl-(R/S)-phenylpropanoamide) (PM1-r-PM2) was synthesized through a solution copolymerization reaction in the presence of an rhodium catalyst (Fig. S3–S6†).61 The obtained chiral copolymers could be further photopolymerized with other vinyl-containing monomers such as (2-(4-vinylphenyl)ethene-1,1,2-triyl)tribenzene (TPEE) or polymer matrix (AA) of photonic crystals for constructing stable and functional optically active photonic films. Owing to the superior features of the molecule-mediated shear-induced assembly technique (MSAT),62 multiple building units can be perfectly integrated without affecting the ordered arrangement of colloids, thus forming homogeneous structural color materials. As illustrated in Fig. 1c, 2D ultrasmall-angle X-ray scattering (USAXS) was employed to further verify the evolution of the arrayed structure before and after shear-induced ordering assembly. The apparent diffraction spots were observed in the composite photonic films, indicating typical long-range ordered periodic structures, while no clear spots were present in the blending slurry. Finally, this distinct scheme results in an expected composite optically active photonic crystal film, which comprises tunable structural color, chirality and enhanced fluorescence property, even featuring active CPL response (Fig. 1d).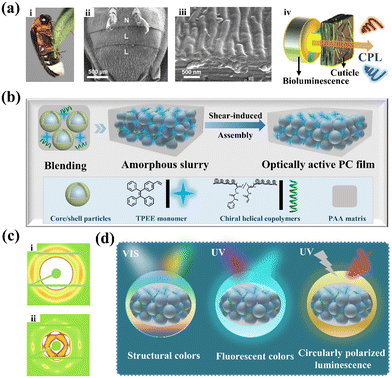 | ||
| Fig. 1 Biomimetic optically active photonic crystal design. (a) Illustrative image of a firefly light organ: (i) optical image of a firefly (L. lateralis Motschulsky) in males; (ii) surface and (iii) cross-sectional SEM images of the abdominal segments of a firefly, including normal (N) and lantern (L) cuticles; and (iv) schematic diagram of the bioluminescence emitted from firefly lanterns passing through the ordered cuticle to convert it into circularly polarized light. Adapted with permission.60 Copyright 2012, National Academy of Sciences. (b) Schematic illustration of the shear-induced assembly for preparing composite photonic crystal films. (c) 2D USAXS patterns of (i) particle slurry and (ii) photonic films. (d) Schematic of the PC films enabling multilevel optical performance. | ||
To investigate in more detail the impact of individual components on the composite optically active photonic crystal films, fluorescence performance was first explored, as illustrated in Fig. 2. Owing to the powerful tool of MSAT, the tailoring of monodispersed colloidal nanoparticles enables precise arrangement engineering for generating well-ordered periodic nanostructures. Consequently, highly saturated colored photonic films with gradual change from blue to red were obtained through the shearing assembly of colloidal particles with varied diameters (Fig. 2a), which is evidenced by the fact that four representative single peaks with reflection maximum varied at 456, 508, 565 and 607 nm for blue, green, yellow and red, respectively (Fig. 2b). Additionally, the surface structure characterization further confirmed the formation of closely packing long-range ordered arrays utilized by Scanning Electron Microscopy (SEM), which is also verified by the apparent sharp diffraction spot patterns in 2D fast Fourier-transform (FFT) diagrams (Fig. S7†). Such a periodic photonic structure can create coherent light scattering to yield sparkling structural colors, which is consistent with their optical appearance under white light (Fig. 2a). As expected, a brilliant blue fluorescent emission with a maximum wavelength of around 495 nm at an excitation wavelength of 370 nm was observed in the composite photonic films because the TPE molecules were embedded in the photonic crystal matrix (Fig. 2c and d). It is widely known that TPE monomers, as a kind of AIEgen, can induce effective fluorescence emission by the aggregate formation with AIE property.63,64 When incorporating these TPE units into the photonic crystal matrix, the confined rigid polymer network possesses a more efficient restriction of intramolecular motions (RIMs) of AIEgens,65 producing typically vivid blue fluorescent color (Fig. 2c). Besides, the collected reflection spectrum creates more clarity on the distinctive dual optical state in the CIE diagrams under white light or UV irradiation (Fig. 2e and f).
Interestingly, the composite photonic crystal films exhibit great fluorescence enhancement compared to neat AIE films with the same TPE concentration, as shown in Fig. 2d and S8.† We attribute this phenomenon to two factors: the photonic bandgap edge enhancement effect and the slow photon effect of the photonic crystal. Owing to the overlapping of fluorescence emission wavelength with the PBG edge of colored films, the fluorescence can be amplified.36 Pronouncedly, the green film displays the highest boosted fluorescence intensity (increased to 2.2 folds) owing to the emission peak position of the TPE, which matches well with the PBG edge of the green PC film, exhibiting intense fluorescence coupling. Notably, prior to exhaustively exploring the impact of PBG on AIE fluorescence emission intensity, the thicknesses of different composite photonic films were excluded to avoid potential external influences (Fig. S9†).
Another two composite photonic films with quasi-ordered and amorphous structures were employed to affirm that the fluorescence enhancement is mainly contributed by the PBG edge effect. The photonic glass (PG) film with short-range ordered arrays and a similar green reflected color was observed, as indicated by the SEM image, digital picture and reflection wavelength in Fig. S10a and S10b.† Although PG films can trigger the pseudo PBG matching the emission wavelength of AIEgens to effectively boost the PL intensity, the degree of enhancement is relatively weak compared with that of photonic crystals (Fig. S10e†). This suggests that only the true photonic bandgap in the photonic crystal could maximize enhanced fluorescence emission. For amorphous films, no structural color was obtained owing to the disordered nanostructures for multiple incoherent scattering, as confirmed via the reflection spectrum and SEM image (Fig. S10c and S10d†), that is, no effective PBG appears in the amorphous film. Eventually, the PL intensity of amorphous film remains almost constant with pure AIE film (Fig. S10e†). All these results indicate that the existence of the PBG effect can significantly enhance fluorescence emission, which provides an effective platform to facilely introduce photonic crystals, thus endowing AIEgens with high luminescence and facilitating the enormous optical applications of active PL-enhanced photonic crystal materials.
Subsequently, we focus on attempting to elucidate the PL enhancement mechanism from the perspective of the “slow photons” effect, which could contribute to a reduced group velocity of light propagation for strongly improving the relaxation processes.33,37 The TPE lifetimes of different films were examined by the time-correlated single-photon counting. As illustrated in Fig. 2g, the exciton lifetimes were 1.71 ns for neat AIE film, 2.28 ns for blue film, 2.48 ns for green film, 2.16 ns for yellow film and 2.04 ns for red film. According to the expression of the intensity of excited state fluorescent molecules decaying: It = I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ), where I0 represents the initial PL intensity, It represents the intensity detected at a certain time, and τ represents the fluorescence lifetime. Thus, the significant increase in PL lifetime leads to the corresponding enhanced PL intensity because other factors remain almost constant in different photonic films. This indicates that the intramolecular rotations of the TPE monomer embedded in the photonic crystal matrix are intensely restricted owing to the involved physical polymer network constraint, which greatly strengthens the radiative relaxation of excited states while blocking the non-radiative decay channel.
exp(−t/τ), where I0 represents the initial PL intensity, It represents the intensity detected at a certain time, and τ represents the fluorescence lifetime. Thus, the significant increase in PL lifetime leads to the corresponding enhanced PL intensity because other factors remain almost constant in different photonic films. This indicates that the intramolecular rotations of the TPE monomer embedded in the photonic crystal matrix are intensely restricted owing to the involved physical polymer network constraint, which greatly strengthens the radiative relaxation of excited states while blocking the non-radiative decay channel.
To further clarify that it is the intrinsic slow photon effect of the PC film that improves the PL emission, neat photonic films were placed on top of or beneath the pure AIE film to demonstrate the regulation of fluorescence intensity at varied positions. As shown in Fig. S11a and 11b,† when the PC film was placed beneath the neat AIE film (AIE/PC), the improved PL emission was observed owing to the enhancement of the 3D PC surface effect on fluorescent molecules.66 However, the modulation of PL intensity is slightly inferior to that of the composite AIE PC film (AIE-PC). What is more, when the PC film was placed above the AIE film (PC/AIE), obvious inhibition of PL intensity occurred (Fig. S11c and 11d†), which is derived from the reflected dissipation of light by photonic crystals reducing the luminescence efficiency of fluorescent monomers. The corresponding lifetime is illustrated in Fig. 2h, Fig. S11e and S11f,† which further demonstrates the “slow photon” effect only existing inside photonic crystals, thus increasing the interaction between photons and AIEgens to improve radiation efficiency. Moreover, the fluorescence lifetimes of TPE in PG films (2.38 ns) and amorphous films (1.77 ns) were measured, as depicted in Fig. S10f,† clearly indicating that the improved fluorescence emission is mainly responsible for the selective enhancement at the bandgap edge of the true PBG in the photonic crystal owing to the slow photon effect. What is more, finite-difference time-domain (FDTD) simulation was used to further analyse the electric field distributions of neat AIE film, composite AIE-PG film and AIE-PC film to demonstrate the fluorescence enhancement effect. As shown in Fig. 2i, the uniform electric field distributes on the whole AIE film, implying no increased fluorescence emission in the homogenous neat AIE system. However, the introduction of a short-range ordered structure for photonic glass contributes to the apparent near-field enhancement located in the mixing matrix domains, and the maximum value of the near-field enhancement reaches the greatest value in the photonic crystal with long-range ordered arrays. Generally, the local electric field intensity distribution of photonic crystals has been confirmed to be a positive function of fluorescence intensity.67 In this case, the raised electric field intensity distribution dictates that the periodic structure of photonic crystals has a promoting effect on enhancing AIE fluorescence emission.
Circular dichroism and UV–vis absorption spectroscopies were employed to fully characterize the chiral properties of composite photonic crystal films. For neat AIE film, the intense mirror-image CD signals with an opposite sign of around 375 nm originating from chiral helical polymer chains were measured, in which the plus Cotton effect depends on the AIE film with R-copolymers, while the minus Cotton effect is found in the presence of AIE film with S-copolymers (Fig. 3a). Excitingly, composite PC films are provided with inherent chiral signs derived from chiral polymers, and new CD response signals are also depicted near the PBG of photonic crystals (Fig. 3b–e). In addition, UV–vis absorption spectra were used to evaluate the respective peak positions of chiral copolymers and photonic crystals (Fig. 3b–e). What is more, the tailored CD signals can be headily tuned by simply controlling the variety of relative PBG of the photonic crystal films, in which the resultant CD signal peaks nearly scale linearly with the diameter of colloidal nanoparticles (Fig. 3f). We suggest that the simultaneous presence of photonic bandgaps and chiral media gives rise to this phenomenon and is attributed to the new CD signal peaks generated to the band gap separation in photonic crystals composed of chiral helical polymer medium.46,68,69 For pure PC films without chiral media, no specific polarization state of light (multiple states or overlapping situations) emerges through the homogeneous PC owing to the left-hand/right-hand circularly polarized light possessing the same bandgap structure. This implies that the optical mode exhibited by the photonic crystal film in this situation is degenerate. Consequently, neat PC films perform silent CD signals, as shown in Fig. S12a.† However, the introduction of chiral copolymers into the PC matrix contributes to LCP and RCP with different bandgap structures stemming from the existence of varied refraction indexes in mixture media, which greatly makes the polarization of light uniquely determined and displays a nondegenerate state for new CD signals close to the PBG of PC. The reported results, such as FDTD simulation, further confirmed that introducing chirality into PCs may break the degeneracy, resulting in the spectra for LCP and RCP light being separated in opposite directions, with changes in both the bandgap widths and the positions of the defect modes.69 Additionally, the composite PG film with pseudo PBG and amorphous film with no PBG reveals that the emerging CD signals are almost zero value except for the inherent optical activity of chiral copolymers (Fig. S12b and c†), which discloses the critical role of PBG of the photonic crystal in inducing the emerging CD responses. It turns out that only the presence of chiral helical polymers and true PBG can allow for the formation of optically active photonic crystal films with tunable CD signals approaching the PBG by readily regulating the lattice constant of PC film fabricated by different-sized nanoparticles as the template.
Considering that the integration of combining the AIEgens and chiroptical materials into the photonic crystal matrix may give rise to the promotion of chirality transform between the fluorescent units and chiral helical polymer chains, and functional photonic crystal films are inclined to generate remarkable circularly polarized luminescence emissions. As demonstrated in Fig. 4a–e, a pair of mirror-image CPL signals emerge at approximately 500 nm, in which the peak position is located near the PL emission peaks of the TPE monomer with a positive sign for left handedness and a negative sign for right handedness. This indicates that the effective chirality transform occurs under this condition, clearly elucidating the synergistic effect of chiroptical helical polymers, and aggregate-state AIE molecules greatly facilitate the generation of emitting mirror-symmetric CPL responses. However, the generation of CPL performance can be induced from the overlapping area of the chiral ingredient's CD spectra and the fluorescent ingredient's PL spectra, which is called the “matching rule”.70 As shown in Fig. S13,† no CPL signals are observed in the neat PC, AIE or chiral PC films, supporting the absence of chirality transfer and inconsistent with the “matching rule”, which firmly illustrates that the combination of fluorescence and handedness is the essential preconditions for triggering CPL performance. In addition, benefiting from the boosted fluorescent substances, emission propels amplified luminous efficiency for concurrently advancing CPL strength. Fig. 4f depicts the enhanced CPL-active effect in the composite PC films, as evidenced by the obvious increased CPL signal intensity (Fig. 4a–e). This can be explained by the polymerized PC polymer matrix restricting the motions of chiral and fluorescent polymer chains, resulting in multidimensional interchain entanglement, which significantly strengthens the RIM effect and chirality fixation, contributing to the enhanced chirality transfer between the bonding luminophores and chiral molecules to enhance the CPL activity.
What is more, the luminescence dissymmetry factor (glum) was employed to evaluate the relative CPL performance of chiral fluorescent PC films.71,72 As shown in Fig. 4f, the maximum dissymmetry factor in composite films without ordered periodic colloidal arrays (|glum| = 5 × 10−3) is inferior to that of composite green PC films (|glum| = 22 × 10−3), which suggests that the incorporation of long-range ordered nanostructure into the chiral fluorescent media could manipulate the dissymmetry of light emission even amplifying CPL response of optically active materials. The boosted CPL and |glum| value are mainly attributed to the strengthened PL intensity, that is, the improved efficiency of AIE fluorescence emission is favorable for chirality transfer efficiency and capability. Consequently, integrating chiral fluorescent substances into a photonic crystal matrix encapsulated with a confined rigid polymer network can achieve better chirality fixation and fluorescence enhancement, facilitating strengthened CPL performance in the optically active photonic crystal emitters.
Conclusion
In summary, we demonstrated the successful fabrication of optically active photonic crystal films based on the integrating system of “AIEgens + chiral helical copolymers”. The enhanced fluorescence properties are obtained from the photonic bandgap edge effect and slow photon effect, which turns out to be the effective induction of radiative pathways by greatly inhibiting the non-radiative decay channel in the confined rigid photonic matrix domains. Interestingly, a pair of emerging mirror-CD signals appear to approach the PBG owing to the band gap separation in the composite photonic crystals composed of chiroptical media. The PL intensity and new CD signal position are highly modulated through precisely tailored PBG of PC by regulating the diameters of colloidal building units. Moreover, the synergistic effect between AIEgens and the chiral helical medium is devoted to the valid chirality transfer to promote emission efficiency, affording the apparent enhanced CPL-active characteristic. This work is envisioned to enrich the foundational comprehension of multi-functional photonic crystals in manipulating light–matter interactions (including AIE luminogens and chirality) for constructing high-performance chirality- or CPL-based optical advanced materials.Author contributions
C. Wang and Q. Guo conceived the study and designed the experiments. Q. Guo carried out the experiments under the supervision of J. Guo and C. Wang. X. Huang and H. Li helped with measurements. Q. Guo and C. Wang wrote and revised the manuscript. All authors contributed to this work and have given approval to the final version of the manuscript.Data availability
Data are available upon request from the authors.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported financially by National Natural Science Foundation of China (Grant No. 52131308, 52373131), MOST (2022YFA1203002), and Key Research and Development Project of Guangdong Province (Grant No. 2020B010190003).References
- P. Singh, G. Dosovitskiy and Y. Bekenstein, ACS Nano, 2024, 18, 14029–14049 CrossRef CAS PubMed.
- R. Xiong, J. Luan, S. Kang, C. Ye, S. Singamaneni and V. V. Tsukruk, Chem. Soc. Rev., 2020, 49, 983–1031 RSC.
- P. Wu, J. Wang and L. Jiang, Mater. Horiz., 2020, 7, 338–365 RSC.
- S. Tadepalli, J. M. Slocik, M. K. Gupta, R. R. Naik and S. Singamaneni, Chem. Rev., 2017, 117, 12705–12763 CrossRef CAS PubMed.
- D. Gur, B. A. Palmer, S. Weiner and L. Addadi, Adv. Funct. Mater., 2017, 27, 1603514 CrossRef.
- G. England, M. Kolle, P. Kim, M. Khan, P. Muñoz, E. Mazur and J. Aizenberg, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 15630–15634 CrossRef CAS PubMed.
- S. Vignolini, P. J. Rudall, A. V. Rowland, A. Reed, E. Moyroud, R. B. Faden, J. J. Baumberg, B. J. Glover and U. Steiner, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15712–15715 CrossRef CAS PubMed.
- S. Caffarri, K. Broess, R. Croce and H. van Amerongen, Biophys. J., 2011, 100, 2094–2103 CrossRef CAS PubMed.
- V. Saranathan, C. O. Osuji, S. G. J. Mochrie, H. Noh, S. Narayanan, A. Sandy, E. R. Dufresne and R. O. Prum, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 11676–11681 CrossRef CAS PubMed.
- P. Vukusic and J. R. Sambles, Nature, 2003, 424, 852–855 CrossRef CAS PubMed.
- S. Yoshioka and S. Kinoshita, Proc. R. Soc. London, Ser. B, 2004, 271, 581–587 Search PubMed.
- P. Vukusic, J. R. Sambles, C. R. Lawrence and R. J. Wootton, Proc. R. Soc. London, Ser. B, 1999, 266, 1403–1411 Search PubMed.
- V. Sharma, M. Crne, J. O. Park and M. Srinivasarao, Science, 2009, 325, 449–151 CrossRef CAS PubMed.
- X. Zhang, T. Yin and J. Ge, Adv. Mater., 2023, 36, 2309344 CrossRef PubMed.
- J. Fonseca, L. Meng, P. Moronta, I. Imaz, C. López and D. Maspoch, J. Am. Chem. Soc., 2023, 145, 20163–20168 CrossRef CAS PubMed.
- Z. Wang, Y. Wang, Z. Ge, Y. Tian, M. Ai, S. Cao, M. Wang, S. Wang and J. Ma, Matter, 2022, 5, 4060–4075 CrossRef CAS.
- Y. L. Shi, Q. Lv, Y. C. Tao, Y. X. Ma and X. D. Wang, Angew. Chem., Int. Ed., 2022, 61, e202208768 CrossRef CAS PubMed.
- R. Lin, Y. Qi, D. Kou, W. Ma and S. Zhang, Adv. Funct. Mater., 2022, 32, 2207691 CrossRef CAS.
- Z. Li, X. Wang, L. Han, C. Zhu, H. Xin and Y. Yin, Adv. Mater., 2022, 34, e2107398 CrossRef PubMed.
- J. Zhang, J. Zhang, Y. Ou, Y. Qin, H. Wen, W. Dong, R. Wang, S. Chen and Z. Yu, Small, 2021, 17, e2007426 CrossRef PubMed.
- D. P. Song, T. H. Zhao, G. Guidetti, S. Vignolini and R. M. Parker, ACS Nano, 2019, 13, 1764–1771 CAS.
- X. Yu, J. Wu, J. Wang, N. Zhang, R. Qing, G. Li, Q. Li and S. Chen, Adv. Mater., 2024, 36, 2312879 CrossRef CAS PubMed.
- J. Wu, X. Yu, G. Li and S. Chen, Chin. Chem. Lett., 2024, 35, 109234 CrossRef CAS.
- D. Pociecha, N. Vaupotič, M. Majewska, E. Cruickshank, R. Walker, J. M. D. Storey, C. T. Imrie, C. Wang and E. Gorecka, Adv. Mater., 2021, 33, 2103288 CrossRef CAS PubMed.
- Z. Cai, Z. Li, S. Ravaine, M. He, Y. Song, Y. Yin, H. Zheng, J. Teng and A. Zhang, Chem. Soc. Rev., 2021, 50, 5898–5951 RSC.
- Y. Wang, W. Li, M. Li, S. Zhao, F. De Ferrari, M. Liscidini and F. G. Omenetto, Adv. Mater., 2018, 31, 1805312 CrossRef PubMed.
- E. S. A. Goerlitzer, R. N. Klupp Taylor and N. Vogel, Adv. Mater., 2018, 30, e1706654 CrossRef PubMed.
- Y. Liu and A. A. Houck, Nat. Phys., 2016, 13, 48–52 Search PubMed.
- M. Kuang, J. Wang and L. Jiang, Chem. Soc. Rev., 2016, 45, 6833–6854 RSC.
- E. Yablonovitch, Phys. Rev. Lett., 1987, 58, 2059–2062 CrossRef CAS PubMed.
- S. John, Phys. Rev. Lett., 1987, 58, 2486–2489 CrossRef CAS PubMed.
- L. Ondič, M. Varga, K. Hruška, J. Fait and P. Kapusta, ACS Nano, 2017, 11, 2972–2981 CrossRef PubMed.
- J. Liu, H. Zhao, M. Wu, B. Van der Schueren, Y. Li, O. Deparis, J. Ye, G. A. Ozin, T. Hasan and B. L. Su, Adv. Mater., 2017, 29, 1605349 CrossRef PubMed.
- K. Chen, S. Schünemann and H. Tüysüz, Angew. Chem., Int. Ed., 2017, 56, 6548–6552 CrossRef CAS PubMed.
- H. Li, J. Wang, F. Liu, Y. Song and R. Wang, J. Colloid Interface Sci., 2011, 356, 63–68 CrossRef CAS PubMed.
- Y. Zhang, P. Han, H. Zhou, N. Wu, Y. Wei, X. Yao, J. Zhou and Y. Song, Adv. Funct. Mater., 2018, 28, 1802585 CrossRef.
- J. I. L. Chen, G. von Freymann, S. Y. Choi, V. Kitaev and G. A. Ozin, J. Mater. Chem., 2008, 18, 369–373 RSC.
- Z. Chen, H. Jia, Z. Liu, Y. Chen and J. Wei, Chem. Eng. J., 2024, 480, 148185 CrossRef CAS.
- S. Liu, Y. Cheng, Y. Li, M. Chen, J. W. Y. Lam and B. Z. Tang, ACS Nano, 2020, 14, 2090–2098 CrossRef CAS PubMed.
- V. V. Vipin, P. R. Chandran, A. M. Ramachandran, A. P. Mohamed and S. Pillai, New J. Chem., 2019, 43, 16264–16272 RSC.
- L. Zhang, J. Wang, S. Tao, C. Geng and Q. Yan, Adv. Opt. Mater., 2018, 6, 1701344 CrossRef.
- Z. Yin, H. Li, W. Xu, S. Cui, D. Zhou, X. Chen, Y. Zhu, G. Qin and H. Song, Adv. Mater., 2016, 28, 2518–2525 CrossRef CAS PubMed.
- S. H. Kim, K.-S. Kim, K. Char, S. I. Yoo and B.-H. Sohn, Nanoscale, 2016, 8, 10823–10831 RSC.
- H. Li, J. Wang, H. Lin, L. Xu, W. Xu, R. Wang, Y. Song and D. Zhu, Adv. Mater., 2010, 22, 1237–1241 CrossRef CAS PubMed.
- J. Lv, D. Ding, X. Yang, K. Hou, X. Miao, D. Wang, B. Kou, L. Huang and Z. Tang, Angew. Chem., Int. Ed., 2019, 58, 7783–7787 CrossRef CAS PubMed.
- K. Hou, W. Ali, J. Lv, J. Guo, L. Shi, B. Han, X. Wang and Z. Tang, J. Am. Chem. Soc., 2018, 140, 16446–16449 Search PubMed.
- H. Zhong, B. Zhao and J. Deng, Adv. Opt. Mater., 2023, 11, 2202787 CrossRef CAS.
- H. Yan, X. Yin, D. Wang, T. Han and B. Z. Tang, Adv. Sci., 2023, 10, 2305149 CrossRef CAS PubMed.
- H. Yan, Y. He, D. Wang, T. Han and B. Z. Tang, Aggregate, 2023, 4, e331 CrossRef CAS.
- X. Yang, J. Lv, J. Zhang, T. Shen, T. Xing, F. Qi, S. Ma, X. Gao, W. Zhang and Z. Tang, Angew. Chem., Int. Ed., 2022, 61, e202201674 CrossRef CAS PubMed.
- X. Gao, B. Zhao and J. Deng, Macromolecules, 2022, 55, 10618–10627 CrossRef CAS.
- N. Lu, X. Gao, M. Pan, B. Zhao and J. Deng, Macromolecules, 2020, 53, 8041–8049 CrossRef CAS.
- M. Khorloo, X. Yu, Y. Cheng, H. Zhang, S. Yu, J. W. Y. Lam, M. Zhu and B. Z. Tang, ACS Nano, 2020, 15, 1397–1406 Search PubMed.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2019, 32, 1900110 Search PubMed.
- J. Han, D. Yang, X. Jin, Y. Jiang, M. Liu and P. Duan, Angew. Chem., Int. Ed., 2019, 58, 7013–7019 Search PubMed.
- A. K. Srivastava, W. Zhang, J. Schneider, A. L. Rogach, V. G. Chigrinov and H. S. Kwok, Adv. Mater., 2017, 29, 1701091 Search PubMed.
- C. He, G. Yang, Y. Kuai, S. Shan, L. Yang, J. Hu, D. Zhang, Q. Zhang and G. Zou, Nat. Commun., 2018, 9, 5117 Search PubMed.
- J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- M. D. Turner, M. Saba, Q. Zhang, B. P. Cumming, G. E. Schröder-Turk and M. Gu, Nat. Photonics, 2013, 7, 801–805 CrossRef CAS.
- J.-J. Kim, Y. Lee, H. G. Kim, K.-J. Choi, H.-S. Kweon, S. Park and K.-H. Jeong, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 18674–18678 Search PubMed.
- X. Du, J. Liu, J. Deng and W. Yang, Polym. Chem., 2010, 1, 1030–1038 Search PubMed.
- Q. Guo, S. Chen, H. Li, X. Wang, J. He, J. Chu, J. Guo and C. Wang, Chem. Eng. J., 2024, 488, 150969 Search PubMed.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 Search PubMed.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- S. Wu, H. Xia, J. Xu, X. Sun and X. Liu, Adv. Mater., 2018, 30, 1803362 Search PubMed.
- D. Li, D. Zhou, W. Xu, X. Chen, G. Pan, X. Zhou, N. Ding and H. Song, Adv. Funct. Mater., 2018, 28, 1804429 CrossRef.
- C. He, M.-H. Lu, R.-C. Yin, T. Fan and Y.-F. Chen, J. Appl. Phys., 2010, 108, 073103 Search PubMed.
- L. Junqing, J. Lei, L. Li and L. Chunfei, IEEE Photonics Technol. Lett., 2006, 18, 1261–1263 Search PubMed.
- B. Zhao, K. Pan and J. Deng, Macromolecules, 2018, 52, 376–384 Search PubMed.
- C. Zhang, S. Li, X. Y. Dong and S. Q. Zang, Aggregate, 2021, 2, c48 CrossRef.
- M. Hu, H.-T. Feng, Y.-X. Yuan, Y.-S. Zheng and B. Z. Tang, Coord. Chem. Rev., 2020, 416, 213329 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental materials and characterization methods, details on nanoparticles. See DOI: https://doi.org/10.1039/d4nr05442c |
| This journal is © The Royal Society of Chemistry 2025 |



