Bartłomiej Szarłan, Jakub Robaszkiewicz, Piotr Pawluć, Maciej Kubicki and Maciej Zaranek
Dalton Trans., 2025,54, 7088-7094
DOI:
10.1039/D4DT03257H,
Paper
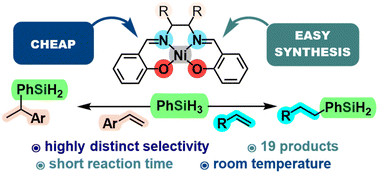
We present a reaction system based on simple nickel(II) salen-type complexes as efficient precatalysts for selective hydrosilylation of olefins with selectivity dependent on the type of substrate used. The system is activated by lithium triethylborohydride and it operates under mild conditions, i.e., at room temperature, and full conversion of the substrates is achieved within just one hour. To demonstrate the substrate scope, 19 exemplary compounds were synthesised and isolated. Aromatic alkenes were transformed into α-adducts while aliphatic olefins yielded nearly exclusively β-addition products. Our findings highlight the potential for sustainable catalysis with simple, cost-effective ligand systems.