 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formaldehyde and its surrogates as a C1 platform for defossilised modern societies
Andrea Rodil
a,
Jan Deska
 *a and
Martin H. G. Prechtl
*a and
Martin H. G. Prechtl
 *bcd
*bcd
aDepartment of Chemistry, University of Helsinki, Chemicum, A. I. Virtasen aukio 1, 00560 Helsinki, Finland. E-mail: jan.deska@helsinki.fi; Web: https://www.deskalab.com
bCentro de Química Estrutural (CQE) and Centro de Recursos Naturais e Ambiente (CERENA), Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais 1, 1049-001, Lisboa, Portugal. E-mail: martin.prechtl@tecnico.ulisboa.pt; Web: https://tecnico.ulisboa.pt Web: https://www.h2.bio
cInstituto Superior de Engenharia de Lisboa, Instituto Politécnico de Lisboa, 1959-007 Lisboa, Portugal
dAlbert Hofmann Institute for Physiochemical Sustainability, Akademie zur Förderung Physiochemischer Nachhaltigkeit e.V., Albert-Schweitzer-Str. 22, 32602 Vlotho, Germany Web: https://www.a-h.institute
First published on 10th November 2025
Abstract
This tutorial serves as an accessible introduction for researchers and students interested in the multifaceted chemistry of formaldehyde and its potential in shaping a more sustainable future. We explore its roles in renewable energy storage in the form of liquid organic hydrogen carriers (LOHCs) and renewable fuels, as well as carbon capture, utilization and storage (CCUS), and biomass valorisation. Furthermore, the relevance of these applications to several United Nations Sustainable Development Goals (UNSDGs 6, 7, 9, 12, and 13) is examined. Beyond the energy and environmental aspects, we discuss the use of formaldehyde and related surrogates in synthetic chemistry, focusing on innovative catalytic strategies to make use of this versatile and abundant C1 building block. Given formaldehyde's central role as an intermediate in both synthetic and biological C1-H2 reaction networks, the tutorial additionally offers discussion points on related small molecules, including methane, methanol, formic acid, CO, and CO2.
Key learning points1. Biological and anthropogenic sources of formaldehyde and their relevance in nature and for modern societies.2. Formaldehyde and its surrogates – production and utilisation in industries. 3. The formaldehyde toolbox: opportunities in R&D for synthetic transformations. 4. Current developments and opportunities for hydrogen storage with C1-molecules coupled with CCUS. 5. Future opportunities and challenges for sustainable chemistry and the potential for the energy transition. |
Introduction
Formaldehyde (H2CO) is the simplest aldehyde and one of the most abundant molecules in the universe, where it is believed to play a crucial role in the origin of life on this planet.1–6 Its formation traces back to 13.7 billion years ago when it was among the first molecules to exist in a universe under abiotic conditions.1 Formaldehyde, albeit not bioaccumulative, acts as a reactive intermediate for the formation of various biologically relevant molecules such as carbohydrates and amino acids, thus serving as a key molecule in the development of nucleobases and other complex biomolecules like ribose and other sugars.2,4,6,7 In the interface between the living and the inanimate world where abiotic reaction pathways in the presence of inorganic bases or minerals lead to complex organic matter,8–11 the Formose reaction is a general pathway to convert formaldehyde into higher carbohydrates (Scheme 1).2,4,6,7,10,12–20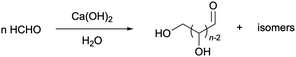 | ||
| Scheme 1 Formose reaction, discovered by Butlerov (1861)14 and reaction pathways further clarified by Breslow (1959).12 | ||
Formaldehyde also represents an intermediate in the pathways of biological carbon fixation, CO2 reduction, through photosynthesis as part of the Calvin–Benson cycle,21–24 with ribulose-1,5-bisphosphate carboxylase/oxygenases (RuBisCO) as central enzymes for the CO2 conversion and O2 evolution.25 Other sources of formaldehyde originate from the decomposition of biological and anthropogenic organic matter,26–29 where the emission of formaldehyde to the atmosphere, soil and water takes us to the more problematic aspects of our lead actor in this tutorial.
In an aqueous environment, formaldehyde exists almost exclusively in its hydrated form methanediol (H2C(OH)2) (>99%), along with some higher oligomers and only trace amounts of free non-hydrated formaldehyde.27,29,30
As such formaldehyde hydrate is involved in biological energy conversion processes, such as methylotropic cycles wherein it acts both as a hydride source for NAD(P)H generation and as C1-entity for the formation of carbohydrates.31 Moreover, formaldehyde is a key intermediate in biological detoxification processes.32,33 In the aforementioned pathways for decomposition, energy conversion and detoxification, the terminal product is carbon dioxide and formaldehyde is therefore not bioaccumulative. These processes are essential for the life of mammals and other species. Albeit formaldehyde is often only mentioned with regard to its toxicity, actually, a human being has an estimated natural turnover of formaldehyde of 878–1310 mg kg−1 bodyweight per day with an estimated half-life of approx. 1–1.5 min. Here, only a fraction of <2 mg kg−1 is related to the intake through daily nutrition,34 underlining its central role as a transitory puzzle piece in both anabolic and catabolic processes in not just the human body, but across all kingdoms of life.32,35,36 At the same time, one needs to take into account the early conclusion by Paracelsus that “the dose makes the poison”, a fact also true for formaldehyde. Health-related concerns of anthropogenic formaldehyde emissions impact not only the wellbeing of employees in formaldehyde industries, but more generally the broader population and end-users.29,37–43 This relates, not only to emissions of gaseous formaldehyde to the atmosphere, but also to water contamination, with the necessity for the removal of formaldehyde from wastewater streams down to a very low, safe level of <40 ppm.27,28,30,44–49
Taking the above considerations into account, the natural energy conversion and detoxification pathways for formaldehyde formation and decomposition may serve as inspiration for artificial procedures in industries and R&D activities to create processes that do not consume large amounts of energy and eliminate toxic substances from the waste streams and supply chains. While it seems unlikely that we would see major efforts to intervene in natural processes related to formaldehyde emissions, one can certainly improve the anthropogenic formaldehyde production processes, utilisation and emissions in industries and products in a multi-billion dollar market which surpassed >30 megatons annually, and a forecast for an annual growth of 4%, to enable the production of various formaldehyde based-resins (>75%) and other bulk chemicals established and used in modern societies.27,50,51 Notably, more than 35% of the global annual production of methanol is used in order to fulfil the formaldehyde global demands. In the 1880s, formaldehyde was initially used as a medical preservative and has since been established for the production of synthetic materials, such as Bakelite (1907, phenol-formaldehyde resin invented by the Belgian chemist Baekeland). Now, formaldehyde is a crucial building block and cross-linker in >50 industrial processes, for the production of daily life commodities.27 This includes applications in the healthcare (germicides/disinfectants) and pharmaceutical areas, but also for the production of other chemicals relevant, for many sectors such as paints, inks, cosmetics, resins, and polymers/adhesives, besides many others for the production of furniture, construction, automotives, airplanes and textiles, to cite a few.52–54 In this context, one can distinguish between the requirements in some industries, where formaldehyde is unavoidable for the production of certain substances, and the urgent need to defossilise industry. A balance might be achieved by considering greener chemicals, or even maybe, new applications for the energy transition, energy storage (e-fuels, hydrogen storage) and the use of renewable sources whenever possible.
Limitations of reaction conditions and mitigations
Three major application pathways can be identified when it comes to embracing formaldehyde's potential new roles and opportunities for energy storage and as a versatile synthetic intermediate: (i) enzymatically catalysed formation of formaldehyde during carbon fixation, (ii) its release during biomass fractionation and decomposition, (iii) formation catalysed by inorganic species under ambient, thermal or photo-induced conditions. These pathways need to take into account the properties, reactivity and stability (vide infra) of formaldehyde and its surrogates in comparison to other C1-molecules, namely methanol and formic acid, which can be also produced from renewables such as carbon dioxide directly or indirectly.51,53,55–58Reaction temperature and pressure
The relatively high reactivity of formaldehyde in comparison to methanol is interesting for both synthesis and low-temperature energy conversion processes, allowing for mild reaction conditions. For example, the dehydrogenation of methanol to carbon dioxide is endergonic while the dehydrogenation of formaldehyde and formic acid is exergonic (Table 1;59 for more discussion refer to the section ‘hydrogen evolution and energy storage’). Moreover, low temperature conversion processes can be advantageous over for example high temperature methanol dehydrogenation where the intermediate formaldehyde may suffer terminal decarbonylation rather than dehydrogenative decarboxylation (Table 1).| Reaction | ΔHr [kJ mol−1] | ΔG0 [kJ mol−1] |
|---|---|---|
| CH3OH + H2O → CO2 + 3 | 38.8 | 8.8 |
| H2CO + H2O → CO2 + 2H2 | −35.8 | −47.4 |
| HCO2H → CO2 + H2 | −14.9 | −31.8 |
| H2CO + H2O → H2C(OH)2 → HCO2H + H2 | −20.9 | −15.5 |
| H2CO → CO + H2 | +5.4 | −27.2 |
| CO + H2O → CO2 + H2 | −41.2 | −20.2 |
Thus, the utilisation of formaldehyde might become promising for applications where moderate temperatures are required as for example in the preparation of thermo-sensitive organic products or in energy storage solutions for mobile electronic devices where higher temperatures would be a disadvantage or a risk for the end-user. A limitation related to the high reactivity of formaldehyde needs to be considered in isochoric processes under dehydrogenative conditions. On the one hand, while it is beneficial to enable transfer hydrogenation reactions with appropriate hydrogen acceptor molecules under mild conditions (vide infra),59–61 in the absence of hydrogen acceptors the remaining formaldehyde may undergo reduction in the presence of hydrogen and form methanol. In this context, paraformaldehyde can be considered as a methanol surrogate (i.e. Ir- or Ru-cat., 25–60 °C, yields 93–95%).62–65 Thus, for efficient hydrogen generation it is important to release the hydrogen from the reaction vessel to increase the hydrogen yield.28,30,66–71
pH-sensitivity
In addition to the thermo-sensitivity, an often underestimated issue with regards to formaldehyde conversion processes is the pH-sensitivity. Under relatively basic conditions (i.e. pH > 9.5), a shift of the reaction pathway may occur which results in the Cannizzaro reaction (Scheme 2),72,73 and at pH > 11, the formaldehyde is disproportionate to methanol and formic acid and it becomes the dominant pathway (refer to the section: Utilisation of formaldehyde and surrogates).65 If pH sensitivity is not considered, this phenomenon can result in misinterpretation of experimental data, where for example the generated hydrogen is assigned to aq. formaldehyde dehydrogenation, when the generated hydrogen is in fact solely derived from in situ formed formic acid. Thus, to prevent a classical base-promoted Cannizzaro reaction, the pH needs to be accurately buffered.65 A competing reaction pathway under basic conditions needs to be considered in the presence of trace amounts of carbohydrates, which may shift the reaction in the direction of the Formose reaction.7,15–20 | ||
| Scheme 2 Base-promoted Cannizzaro reaction of formaldehyde which takes place as a background reaction at pH >9.5 and becomes dominant under more basic conditions (pH > 11).65,72,73 | ||
Notably, the pH-sensitivity of formaldehyde might also be relevant for the reaction pathway of complete methanol dehydrogenation under strongly basic conditions at low dehydrogenation catalyst loadings through ligand dissociation of the coordinated formaldehyde which then subsequently undergoes the Cannizzaro reaction in the presence of base. Nevertheless, the overall H2 generation likely remains unchanged, since the in situ formed formaldehyde feeds the Cannizzaro reaction again with methanol which is subsequently converted via HCO2H into CO2 and H2 (Scheme 3). Therefore, only the discussed mechanism might be different as originally proposed for MeOH reforming under basic conditions (i.e. pH 14), but the outcome is likely unchanged.
 | ||
| Scheme 3 Possibility of a shift to the Cannizzaro reaction as a competing pathway during methanol dehydrogenation under strongly basic conditions. | ||
Water content
Formaldehyde, a volatile organic compound (VOC; b.p. 254 K) is commonly used as aqueous solution (formalin or formol) containing 37 wt% of formaldehyde. The formaldehyde hydrate (FAH), methanediol (H2C(OH)2) concentration in equilibrium is the dominated form (K ≈ 2 × 103) in water and free formaldehyde is only present in low concentration (0.1%). Other species present in equilibrium in aqueous solution are oligomeric mixtures of poly(oxymethylene) glycols [HO–(CH2O)n–H, n = 1–8]. In the commercial solutions, methanol is usually added to promote stabilisation through acetal formation. The degree of polymerisation of these oligomers depends on the water content/concentration, temperature, and pH. Hence, diluted solutions likely contain lower amounts of oligomers, and in contrast, the evaporation of water from formalin results in the formation of white solid paraformaldehyde (PFA), an easy-to-handle solid formaldehyde surrogate (m.p. 120 °C, [HO–(CH2O)n–H, n = 8–100]; refer also to the section ‘production of formaldehyde and its surrogates’). PFA can be converted into the monomeric FAH through heating or under slightly acidic conditions (e.g. pH 5–6), where the polyacetal releases monomeric methanediol (H2C(OH)2) in aqueous solution. In organic solvents, the polyacetal opening yields non-hydrated H2CO for utilisation as a C1-building block in organic synthesis (i.e. synthesis of imidazolium salts, N-heterocyclic carbenes).74 Thus, depending on the targeted utilisation the water content in the reaction system should be considered to prevent undesired reaction pathways (Scheme 4). | ||
| Scheme 4 Exemplary hydration/dehydration and dehydrogenation reaction pathways of formaldehyde depending on the reaction conditions incl. water content and pH. For the FORMOX process refer to Scheme 5. | ||
Methods, limitations and mitigations
Considering the above mentioned parameters in applications and planning of experiments (see also the sections below about HCHO formation and degradation), one should carefully select appropriate analytical tools for the qualitative and quantitative determination regarding formation, consumption, degradation, cross-sensitivity and selectivity of (aq.) HCHO and the respective reagents (in particular H2, CO2 and CO). For the determination for the source of HCHO or the derived products, one should consider isotope-labelling (2H, 13C, 18O) and analyse the samples with isotope-sensitive methods such as NMR, MS or IR for example. Isotope-labelling helps to determine the source and to exclude misinterpretation of results. Classical methods for quantification likely include titration such as Sørensen formol titration and titration with EDTA.75,76 And, commercial field test indicators,77 or digital colorimetry with smartphones (<1250 μg L−1) might also be suitable tools.78 These are examples in the analytical portfolio to complement i.e. the qualitative and quantitative composition of the gas phase (H2, CO2, CO, and O2).28,30 The methods for the quantitative analysis of the gas phase may include gas burettes and mass flow meters in combination with GC-TCD and mass spectrometry for further qualitative and quantitative analysis. Note here that helium as a carrier gas should be avoided for GC-TCD due to the similar conductivity to H2. As a carrier gas nitrogen appears to be more suitable since it allows the analysis of hydrogen and carbon dioxide accurately. In contrast, argon is relatively similar to carbon dioxide in terms of conductivity and molecular weight and therefore the separation is more challenging. For mass spectrometry, one needs to consider the low molecular weight of the gases present in the gaseous samples, as not all MS detectors are suitable for such small molecules. In case one does not have access to a GC with a TCD detector, but to a GC equipped with a FID, one should consider coupling the setup with a methaniser to enable carbon dioxide and carbon monoxide detection, since these gases are among those gases which cannot be directly analysed with a standard FID, alternatively an electron capture detector (ECD) might be considered.79–82 A sensitive method for formaldehyde detection in solution may include spectroscopy (i.e. UV-VIS) in combination with enzymatic sensors.83 For sample analysis and reaction monitoring, NMR and IR are also suitable but often less sensitive. Ideally the instrumental tools (i.e. GC; MS, IR, gas sensors flow meter, gas burette) are implemented for online reaction monitoring.Last but not least, the experimental conditions should be well planned with the chemicals in use, and this holds true also for gaseous formaldehyde and formaldehyde solutions and the herein mentioned toxicity of formaldehyde. Therefore, experimental precautions should be well planned to avoid risks for the experimentalist. This includes experimentation in well-ventilated fume hoods, use of gloves, lab coat and safety glasses.
Experimental and analytical methodologies should consider mitigations in terms of miscibility/solubility and reactivity of the used reagents in the presence of formaldehyde. Thus, the concentration of water or other polar-protic solvents should be assessed to stabilise intermediates and control the reaction pathways. Additionally, the pH should be buffered to shift the reaction in the direction of the target product. The same holds true for the reaction temperature and pressure.
Formaldehyde (surrogates) – production and utilisation in industries
The state of the art industrial production of formaldehyde through the FORMOX process (Scheme 5) with Mo/Fe-catalysts, iron molybdates,84 and related processes (with silver catalysts), is directly dependent on the methanol production (98 Mt per year in 2023).85 Likewise, formaldehyde, its derivatives and its surrogates rely on the methanol industries. Hence, about 50% of the methanol is used for the formaldehyde production.27,59,86 Details on process parameters and further properties can be found in the reviews under ref. 87 and further details are provided in ref. 27. | ||
| Scheme 5 FORMOX process for the production of formaldehyde.27,87 | ||
Formaldehyde is widely used for the production of resins (i.e. urea-formaldehyde (UF, 3), melamine formaldehyde (MF, 5), phenol formaldehyde (PF, 7) >70% use), dyes, inks, varnishes, cosmetics, disinfectants, and other fine chemicals, as well as some hydrogen-rich surrogates such as 1,3,5-trioxane (8), paraformaldehyde (PFA) or hexamethylenetetramine (HMTA, 9; Scheme 6).27,87 Trioxane and PFA are formed from aq. formaldehyde through condensation and acidic dehydration respectively, while PFA may precipitate already from saturated formalin solutions by simple cooling (vide supra). These surrogates might also serve as an anhydrous formaldehyde source in organic synthesis.52,88 HMTA is readily formed by reacting aq. ammonia with aq. formaldehyde89 and it finds synthetic application as a base, polydentate ligand, formylation reagent, and resin hardener, to name a few. It can also be used in medicine given to its antiseptic properties and as a preservative additive (E239) in the food industries.
 | ||
| Scheme 6 Exemplary current synthetic applications of formaldehyde for the production of formaldehyde surrogates, solid combustion fuels and resins.29 HMTA = hexamethylenetetramine; MF = melamine formaldehyde resin; PF = phenolic formaldehyde resins; PFA = paraformaldehyde; UF = urea formaldehyde resin. | ||
Albeit HMTA (i.e. Esbit™) and trioxane serve as solid combustion fuels in a niche market for camping or military stoves, a broader application of formaldehyde and its surrogates as an energy storage platform in the global energy sector is not significant so far. A more promising potential utilisation of formaldehyde derivatives in the energy sector targets renewable combustion fuels to complement and substitute diesel and aviation fuels (kerosene; C8–C16 aliphatic alkanes).58,90–92 For example, it is possible to synthesise dialkoxymethanes (oxymethylene ethers, OMEs) from the corresponding (bio-derived) alcohols and formaldehyde or CO2. Apart from the energy sector, formaldehyde and the before mentioned surrogates are common reagents in organic synthesis and catalysis as C1 building blocks and hydrogen donating molecules (more details are provided in the section ‘utilisation of formaldehyde and surrogates in R&D’).27,29,52,59,74,86,88,93 Owing to the practical aspects of solids for lab scale synthetic experimentation, PFA in particular has been frequently studied (vide infra).27,52,59–62,64,66,67,69,88,94–121
The perspectives for the carbon, capture, utilisation and storage (CCUS) framework
Catalytic HCHO formation and degradation
In ongoing efforts towards defossilisation and decarbonisation of the chemical industries, it is likely that the conventional production process of formaldehyde from methanol through the FORMOX process will be continued during the transition period, whilst the production of renewable methanol and renewable syngas from CO2 and biomass will be implemented in the first stage,122 and will likely continuously serve as a major feedstock for the formaldehyde industries. Nonetheless, a direct synthesis of formaldehyde from methanol, CO2 or syngas under milder enzymatic, thermo-, photo- or electrochemical conditions is the focus of current research.27,51,53,59,86,123–132 Despite the high reactivity of formaldehyde and the limiting selectivity, thermodynamics and kinetics for gas phase conversions in the case of syngas26,51,53 or CO2,58 it has been demonstrated that the reduction can be achieved and stabilised on the formaldehyde-level by conducting the reaction in the liquid phase in polar protic solvents such as water or alcohols,27,53,58,86 enabling the formation of aq. formaldehyde/formalin (methanediol) or dialkoxymethanes/oxymethylene ethers (OME), as formaldehyde surrogates. The protic polar environment is essential to shift the equilibrium of the in situ formed formaldehyde to the corresponding diol or acetal respectively, for the prevention of reduction to methanol.Tanksale and co-workers demonstrated with a bimetallic NiRu catalyst that the endergonic (ΔG > +34 [kJ mol−1]; K = <10 × 10−7 mol−1) gas phase conversion of syngas to formaldehyde turns exergonic (ΔG <0 [kJ mol−1]; K = >1.5 mol−1) in water at low temperature (25–100 °C; Scheme 7) and aq. formaldehyde/methanediol is formed with 100% selectivity at a conversion level of 19% at 80 °C.53 Higher temperatures have been reported to disfavour the reaction in agreement with the thermodynamics. In a subsequent study, the authors reported that the efficiency can be improved by conducting the reaction in methanol as solvent and stabiliser (yield: 15.6 mmol L−1 gcat−1 HCHO; T = 90 °C; p = 100 bar) without affecting the excellent selectivity.51 The high selectivity seems favourable for flow reactions, and underlines the promising aspects to conduct the reaction under mild conditions in protic polar solvents in the absence of highly acidic or basic conditions which likely would lower the selectivity for formaldehyde. Reports on selective gas phase conversion of dry carbon dioxide to formaldehyde are scarce while the thermodynamics can be shifted significantly with water (PtC@Al2O3; dry: ΔG = 59.8 [kJ mol−1]; with water: ΔG = 4.91 [kJ mol−1]).128,133,134 Therefore, the ongoing research for carbon dioxide hydrogenation under enzymatic, thermo-, photo- and electrocatalytic conditions considers these limitations and tries to stabilise formaldehyde as methanediol or as oxymethylene ethers rather than free formaldehyde.
 | ||
| Scheme 7 Aq. formaldehyde formation from syngas in water.53 | ||
Furthermore, albeit homogeneous metal-catalysed low-temperature formation of HCHO from methanol is rare, a notable recent study on methanol oxidation to HCHO evaluated an iron complex with a hexadentate nitrogen–sulphur ligand (N3S3-tripod type ligand),135 which yielded a 1.3 mM concentration with 2.2 μmol catalyst under an O2 atmosphere at r.t. within 24 h.
Oxymethylene ethers
Oxymethylene ethers based on methanol (DMM: dimethoxymethane, 10a) or pentanol (dipentoxymethane, 10e) for example are considered as potential complementary renewable fuels and fuel additives. These are interesting, due to their potential accessibility through biomass, CCUS and green hydrogen and to their comparable high energy density (OME: 20–23 MJ L−1; MeOH: ca. 18 MJ L−1) and specific energy (OME: 20–23 MJ kg−1; MeOH: 23 MJ kg−1). Also of interest, is their cleaner combustion in comparison to conventional diesel fuels and competitive production costs.90,91,136–142 OMEs can be synthesised through acetalisation under acidic conditions (Brønsted or Lewis acids) from: (a) alcohols and formaldehyde (surrogates such as PFA or trioxane; 2 MeOH + HCHO → H2C(OMe)2 + H2O), (b) through partial oxidation of methanol in alcohol (3 MeOH + 0.5 O2 → H2C(OMe)2 + 2 H2O), (c) reduction of carbon dioxide in alcohol (2 MeOH + 2 H2 + CO2 → H2C(OMe)2 + 2 H2O) or (d) reduction of carbon monoxide/syngas in alcohol (2 MeOH + H2 + CO → H2C(OMe)2 + H2O). The direct acetalisation of HCHO appears attractive since no metal catalysts are required for oxidation/reduction, however it strongly depends on the formaldehyde supply and the conventional FORMOX process. Albeit the direct approach with PFA and methanol proceeds in a short reaction time (equilibrium reached within less than 2 h at 60–80 °C with i.e. amberlyst 36), and it appears quite straightforward at low temperatures, the reaction results in product mixtures of OME, hemiacetal and trioxane, and large fractions of methanol and formaldehyde remained unreacted.143,144 Liquid phase extraction for the separation of the OME from the mixture is challenging due to the good solubility in both polar (water) and non-polar solvents (diesel).143Hence, OME synthesis is an extension of the FORMOX process coupled with acetalisation. Current research focuses on the development of bi-catalytic solid state catalysts consisting of mixed metal and metal oxides (i.e. Sb, Re, V2O5, MoO3, Fe2O3) which combine the properties required for the oxidation of methanol and acetalisation of formaldehyde.137 The selectivities for OMEs are in general good (>90%) at comparable low temperatures in relation to the FORMOX process, but the conversion rates (<60%) could still be improved. In addition, Palkovits and co-workers reported on silver and copper catalysts for dehydrogenative methanol conversion in the gas phase to DMM with high selectivities (>70%) at elevated temperature (240–250 °C; i.e. Ag-cat.: 2 mmolMeOH,conv h−1 gcat−1).145–147
Considering the feedstock requirements for OME production either from formaldehyde or coupled with methanol oxidation, the integration of such a process into the facilities of methanol and FORMOX plants is the logical consequence to extend the production capacities and the product portfolio.138,148
A report by Klankermayer, Leitner and co-workers58 demonstrated the reduction of carbon dioxide to the formaldehyde-level in alcohols (C1–C10) enabling access to dimethoxymethane (DMM 10a) and other oxymethylene ethers (OME 10b–10j). In their study, a molecular ruthenium-triphos catalyst in the presence of a Lewis acid (Al(OTf)3) was active to realise the formation of the OME at 80 °C within 18 h (CO2 = 20 bar; H2 = 60 bar; Scheme 8).
 | ||
| Scheme 8 OME synthesis from carbon dioxide in alcohols (TON: turnover number).58 | ||
Further ongoing studies by Tanksale and co-workers149–151 evaluate ruthenium-based catalysts on β-zeolite for the hydrogenation of carbon dioxide and carbon monoxide to DMM. The highest yield for the carbon monoxide reduction to DMM was achieved with a RuNi catalyst (5.11 mmol gcat−1·LMeOH−1) at 120 °C (ptot = 75 bar) within 48 h.151 While for carbon dioxide hydrogenation with a ruthenium catalyst they reported DMM yields of up to 7.42 mmol gcat−1·LMeOH−1) at 150 °C (ptot = 75 bar),150 respectively 13.2 mmol gcat−1·LMeOH−1 with 100% selectivity for DMM with a recyclable ruthenium catalyst.149
Other early reports on the catalytic reduction of carbon dioxide to the formaldehyde-level in the presence of hydrogen donor molecules considered boranes (pinacolborane) for hydroborylation ((R2B-O)2CH2; Bontemps/Sabo-Etienne)152 and silanes (trimethylsilane) for hydrosilylation of CO2 ((R3Si-O)2CH2; Oestreich/Metsänen;153 Serrano/Rodriguez;154 Berke155) with the target of addressing mechanistic studies and more complex transformations in synthesis and energy conversion processes which potentially also opens perspectives for the preparation of inorganic–organic hybrid materials.156 These heterooxomethylene molecules may release formaldehyde upon hydrolysis for example.
Photocatalysis for HCHO formation and degradation
The photocatalytic synthesis and formation of HCHO has been studied for several decades inspired by the role of formaldehyde in photosynthesis and the Calvin cycle.21–24,157,158 In addition, owing to its long known toxicity159 together with anthropogenic and natural HCHO emissions,41–43,92,160–163 there is also interest in HCHO degradation processes for the purification of exhaust gases and aqueous waste streams to improve air and water quality.28,38,43–49,130,163–186 However, the reports on photocatalytic formaldehyde formation from carbon dioxide or methanol are limited. Early studies, since the 1970s, reported formaldehyde as minor species along with methane, methanol or formic acid as major products.27,187–189 An approach with a Ru(II) dye-sensitised TiO2 film for carbon dioxide reduction gave at pH 10 (hν >420 nm for 5 h) a mixture of HCO2H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCHO
HCHO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (40
MeOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 with >120 μmol cm−2 of HCHO).190 The best selectivities with a photoelectrocatalytic approach to date have been obtained with a bismuth vanadium oxide anode and copper cathode, resulting in a faradaic efficiency (FE) of 85.1% (−0.9 V) for HCHO with simulated sunlight irradiation (Xe lamp, 300 W).191
100 with >120 μmol cm−2 of HCHO).190 The best selectivities with a photoelectrocatalytic approach to date have been obtained with a bismuth vanadium oxide anode and copper cathode, resulting in a faradaic efficiency (FE) of 85.1% (−0.9 V) for HCHO with simulated sunlight irradiation (Xe lamp, 300 W).191
The studies on oxidative formaldehyde formation from methanol under photocatalytic conditions give further insights about the required conditions and limitations.132,192 With a photocatalyst consisting of zinc indium sulfide nanocrystals (ZIS NCs) and nickel formaldehyde can be produced with 99% selectivity with high nickel loadings (6 wt% Ni precursor; 70.4 mmol g−1 h−1 HCHO), and whereas with low nickel loadings glycol (<0.5 wt 6.5–13.8 mmol g−1 h−1) is formed with 88% selectivity under UV light irradiation (365 nm).132 A study on the evaluation of bimetallic palladium and platinum photocatalysts PtPd@TiO2 under direct sunlight reported the formation of up to 0.2 μmol mg−1 h−1 formaldehyde and 44 μmol mg−1 h−1 hydrogen from methanol.192 The catalysts remained active over 100 h (over 20 days; 5 h sun light per day; mean value 17.5 μmol mg−1 h−1 hydrogen production).
The formaldehyde degradation to carbon dioxide may proceed under hydrogen evolution,30 or release of water in a gas phase reaction.193 PdAg@Ag core–shell nanoparticles (NPs 5 mg, dye eosin Y: 35 mg) were reported to be active for hydrogen generation yielding 0.2 mmol h−1 hydrogen from formalin (HCHO 0.75 mol L−1) under visible light and basic conditions.194 Using a photocatalyst consisting of NiO/BiVO4 ZnIn2S4 (0.3 g) and 1.5 M aq. formaldehyde under visible light (350 W Xe lamp) and basic conditions, 9 mmol g−1 h−1 of hydrogen is generated.169 The decomposition of gaseous formaldehyde has been studied with platinum TiO2 nanowires in a flow reactor under visible light irradiation with degradation rates of 86–92% of up to 50 degradation cycles in the flow system (HCHO 0.5 mg m−3, flow rate 50 mL min−1).193 The evaluation of the hydrogen generation from aq. formalin with CuNi@C/TiO2 nanoparticle photocatalysts under basic conditions yielded 254.6 mmol g−1 h−1 hydrogen.129
Electrocatalysis and fuel cells (FC) for HCHO formation and degradation
Highly selective electrochemical reduction of carbon dioxide to formaldehyde remains a rarity due to the stability and reactivity of formaldehyde.27,126,128,195,196 Nakata and Einaga studied carbon dioxide reduction to formaldehyde with boron-doped diamond electrodes and platinum counter electrodes (−1.0 V to −1.9 V vs. Ag/Ag+) with a maximum faradaic efficiency for formaldehyde of 74% at −1.7 V over 20 h in a methanol electrolyte.195 In these studies, they also used seawater as an electrolyte yielding 36% aq. formaldehyde (7.5 mM h−1) and 62% when 0.1 M aq. NaCl was used as an electrolyte. In a study by Nam and co-workers, Sn and Pt electrodes together with Lewis acids were evaluated for carbon dioxide reduction and methanol oxidation with a focus on the formation of formaldehyde and its derivatives DMM and PFA.126 They demonstrated faradaic efficiencies of 50% (cathode) and 90% (anode) with a charge of 200C, for the combined production PFA and DMM.In contrast to the studies mentioned above in this section, Robert and co-workers197 explored the electrochemical reduction of carbon monoxide. In their studies, they used electrodes modified with multiwalled carbon nanotubes (MW-CNTs) and an immobilised cobalt phthalocyanine complex. Optimised conditions in this study were found to be pH 12 and 10 °C, while the Cannizzaro reaction does not play a role under these conditions (potential, pH and time scale) and the formate concentration remained below 1%. The best selectivity for HCHO (FE 17.5%; TON = 349; TOF = 0.2 s−1) was obtained with a maximum partial current of E = −0.650 VRHE and current density of 0.64 mA cm−2.
Electrocatalytic oxidation of dry methanol to formaldehyde with platinum electrodes have been reported by Sasaki and Nagaura with Faraday efficiencies to formaldehyde of 70% (0.1 M NaOMe in MeOH; 2 h, current density of 3.3 mA cm−2),198 respectively, by Mechler and co-workers with a Faraday efficiency of 90% for a current density of 100 mA cm−2.123 The product concentration of the latter conditions is equal to 7.7 g L−1 after 30 min (production rate of 17 mol h−1 m−2). In a different approach, Garcia and co-workers125 studied the electrochemical oxidation of methanol to HCHO with a polymer-bound 2,2,6,6-tetramethylpiperidinyloxyl radical (TEMPO) which is known for selective oxidation of alcohols to aldehydes. The polymer-modified glassy carbon electrode contains a TEMPO with a pyrrole backbone. The reactions were conducted with methanol as the solvent and reactant in the presence of different bases where among the tested bases, lutidine (5 mM concentration) gave the best results at a potential of 1.0 V with a maximum FE of 97.5% and a TON of 1250.
In addition to the demonstrations for electrocatalytic formaldehyde formation from carbon dioxide and methanol, it has been also evaluated for the decomposition under hydrogen release and in formaldehyde-fed fuel cells.44,59,181,186,199–217 Formally the oxidative electrochemical degradation of formaldehyde proceeds at the anode (HCHO + H2O → CO2 + 4 H+ + 4 e−) and the sacrificial component is reduced at the cathode (i.e. aerial O2: O2 + 4 H+ + 4 e− → 2 H2O). Typical studied electrocatalysts for such fuel cells include metals, alloys and metal oxides (i.e. Ru, CuPd, Ce, Cu, CuO, NiO, and TiO2) or enzymes/bacteria for formaldehyde degradation in microbial fuel cells.44,186,202–204,206–208,210,212,214,215 Even though the HCHO degradation in microbial fuel cells is interesting, so far the efficiency is limited with reports and samples with 200 ppm of HCHO requiring 152 h to reduce the concentration by 89%.204 An example for the evaluation of a fuel cell for formaldehyde degradation under CO2 evolution with a TiO2-photoanode and a Cu2O photocathode in a fuel cell,206 reached a current of 1.2 mA cm−2 with a voltage of 0.58 V under sunlight illumination (100 mW cm−2) at room temperature. The setup used a proton exchange membrane (PEM), 0.5 M sodium sulphate as the electrolyte, and 1 M aq. formaldehyde and pure oxygen for the oxygen reduction reaction (ORR). In a more recent study by Fu and co-workers, it was demonstrated that a formaldehyde fuel cell is capable of operating without carbon dioxide evolution.202 The terminal oxidation product is formate in this case and the electrochemical oxidation of formaldehyde to formate (53 mol) proceeds under hydrogen evolution (0.62 N m3 with a faradaic efficiency of 100% between 0–0.3 VRHE) per 1 kWh of generated electricity (voltage: 1 V, peak power density: 350 mW cm−2). The study used an anion exchange membrane (AEM), metallic copper foam/nanosheets as anodes, platinum on a carbon cathode and 1 M KOH as the electrolyte. The authors analysed the role of a possible Cannizzaro reaction, and concluded that it is negligible under these conditions. In a follow-up study on aq. formaldehyde, the same group reported higher hydrogen yields along with a lower amount of formate and generated electricity.186 A study by Chen and co-workers203 used a PdCu alloy as the catalyst material. The oxidation of formaldehyde at 0.2 V results in a current density of 50 mA cm−2 and the current remains stable over 100 h and the best results reached an open-circuit voltage of 0.9 V and a peak power density of 100 mW mgCu−1. In a different context, an HCHO FC electrode with amorphous nickel tungstate nanoparticles was evaluated as a sensor for residue HCHO,207 and a detection limit of 3.6 μM was determined.
Bioremediation & biomanufacturing
In addition to chemical means that deal with issues related to formaldehyde as a pollutant, tailor-made biological systems can also offer an attractive approach to the selective decomposition for formaldehyde. Despite the general cytotoxicity of the C1 aldehyde, a number of microorganisms have been identified that facilitate the metabolic oxidation to CO2 and certain strains can be employed for rather effective removal of the pollutant.171,218 While some species tolerate impressive concentrations of the toxic aldehyde,219 typically, bioremediation is performed with immobilised whole cell systems.220–222 The functional principles of the overall metabolic pathways in formaldehyde-degrading bacteria and archaea goes beyond the scope of this tutorial, yet knowledge of the key enzymes such as formaldehyde dismutase223 or formaldehyde dehydrogenase (FAldDH),224,225 which are directly involved in the conversion of the aldehyde substrate,226 can inform the design of in vitro enzymatic,227 biomimetic,65 or chemoenzymatic C1 conversion pathways.228,229Besides, the same knowledge of crucial C1-converting enzymes can essentially also form the basis for the biomanufacturing of formaldehyde as a future production platform of this indispensable chemical building block. Particularly, CO2 represents naturally a highly attractive raw material where formate dehydrogenases as well established enzymes enable the reductive activation of the greenhouse gas.230–232 While in vitro multi-enzyme cascades for CO2 fixation have so far mainly focused on methanol production,233–235 recently the first successful formaldehyde biomanufacturing reports have appeared. Liu and co-workers established a photo-enzymatic CO2 fixation leading to formalin concentrations up to 4.1 mM. The recycling of the NADH cofactor that both FDH and FaldDH require is accomplished through a complex UV light-driven electron transfer chain including a homogenous rhodium complex and TiO2 as a photocatalyst where EDTA acts as terminal reductant.236 A purely biocatalytic cascade on the other hand was described by Huang and co-workers, which could be employed with NADH as a stoichiometric reducing agent or with a phosphite/phosphite dehydrogenase recycling system.225 The formaldehyde yields of this in vitro reduction, however, remained significantly lower.
In addition to these reductive pathways based on CO2 fixation, methanol can also serve as a raw material for formaldehyde production. As a consequence of the diversity of natural methylotrophs, a range of methanol-oxidizing enzymes is available, including NAD-dependent methanol dehydrogenases (MDH), PQQ-dependent MDHs (PQQ: pyrroloquinoline quinine), and O2-dependent alcohol oxidases (AOx).237 While the former enzymes play central roles in the pathway optimization for microbial methanol valorisation, alcohol oxidases are particularly attractive enzymes as they operate without the need for any cofactor recycling systems and air as the sole oxidant. The aerobic dehydrogenation delivers aqueous formaldehyde alongside hydrogen peroxide, the latter being typically decomposed in situ by the addition of catalase in order to reduce the risk for oxidative deactivation of the oxidase enzyme.238,239
Utilisation of formaldehyde and surrogates
Organic synthesis with formaldehyde
In the context of organic synthesis, formaldehyde exhibits the same kind of electrophilic carbonyl reactivity as its higher homologues, while lacking the nucleophilic alpha-reactivity of other, more complex carbonyl compounds. The characteristic aldehyde reactivity can lead to a number of different species, some stable, some reactive intermediates. The reaction with C-nucleophiles and primary amines proceeds through addition to the formaldehyde's carbon under the formation of a hydroxymethylated product (Scheme 9(a)), that, depending on the electronic nature of the nucleophile, can be isolated or will further eliminate water to yield a methylenated product (i.e. a terminal olefin or imine).On the other hand, the reaction with other heteroatom nucleophiles such as secondary amines or alcohols, typically in the presence Brønsted or Lewis acids, will take a similar addition–elimination pathway that leads to highly electrophilic iminium or oxonium species, respectively (Scheme 9(b)). These intermediates are readily attacked by other nucleophiles so that formaldehyde acts eventually as a methylene bridge. Lastly, in the presence of nucleophilic species such as cyanide or N-heterocyclic carbenes, formaldehyde can undergo an umpolung reaction where a carbanionic intermediate is formed which functions as a nucleophilic formyl transfer agent (Scheme 9(c)).240 While the umpolung pathways have found only very limited application in traditional organic synthesis beyond formose-type formaldehyde oligomerizations (mainly due to Cannizzaro disproportionation side reactions,16 we will revisit this reaction mode later during the discussion of organocatalytic methodologies.
The synthetic application of the hydroxymethylation reactivity can be widely observed with a broad range of C-nucleophiles. Like most other aldehydes, formaldehyde also readily engages in aldol-type C–C bond forming reactions. Due to the relatively high carbonyl reactivity of the C1 aldehyde, however, the selective reaction with only one equivalent of formaldehyde is challenging,241 as sparsely substituted ketones, esters and other enolate precursors often suffer from exhaustive per-hydroxymethylation.242 Hence, direct hydroxymethylation with aqueous formalin is mostly limited to α,α-disubstituted carbonyl compounds such as α-methylindanone 11 where this selectivity issue is non-existent, which delivers the β-hydroxy products 12 in generally high yields (Scheme 10, top left).
 | ||
| Scheme 10 Hydroxymethylation of carbon-centred nucleophiles Brønsted base-catalysed (top left and bottom left); Lewis acid catalysed (top right); Lewis base-catalysed (bottom right). | ||
In order to achieve a selective mono-hydroxymethylation of less substituted ketones, the Mukaiyama protocol based on the Lewis acid activation of aqueous formalin in the presence of silyl enol ethers (13) poses a valid alternative (Scheme 10, top right).243 Also non-sp3 carbon nucleophiles such as phenols (15) or enones (17) can be employed. The former is for example accomplished by means of metaborate as a reaction mediator (Scheme 10, bottom left), facilitating selective ortho-hydroxymethylation (16) without the risk of polymerization.104 The Morita–Baylis–Hillman reaction between enones/enoates and formalin proceeds in the presence of a stoichiometric amount of Lewis-basic N-heterocycles such as 1,4-diazabicyclo[2.2.2]octane (DABCO) or 3-hydroxyquinuclidine (Scheme 10, bottom right).244
With a slightly different set of C-nucleophiles, the reaction with formaldehyde can also be diverted to α-methylenations. Pre-nucleophiles carrying a CH2 moiety between two activating groups such as carbonyls, nitriles, arenes and/or nitro groups, typically do not stop on the hydroxymethylation stage but rather react in Knoevenagel-type condensation (20) with paraformaldehyde under basic conditions (Scheme 11, left).245
 | ||
| Scheme 11 Olefinations and imine synthesis with paraformaldehyde. Brønsted base-catalysed (left and centre), thermal olefination under neat conditions (right). | ||
Without the intrinsic reactivity of electron-withdrawing functionalities, simple unsaturated terminal alkenes (22) are also accessible with paraformaldehyde when phosphonium ylides (21) are employed as nucleophiles in Wittig olefinations (Scheme 11, centre).246 In addition to the polymeric anhydrous formaldehyde, surrogates such as hydroxymethyl triphenylphosphonium salts or N-hydroxymethyl phthalimide have also found application in transformations where strong bases and anhydrous conditions are required.247
Different from the hydroxymethylation that is generally not employed with nitrogen nucleophiles other than the reagent preparation of these surrogates such as the above mentioned N-hydroxymethyl phthalimide, formaldehyde condensation has been observed and the corresponding C1 imines could be isolated in cases where the nitrogen nucleophile (23) provides sufficient steric protection of the highly reactive Schiff base (Scheme 11, right).
While formaldehyde as one of the smallest organic molecules usually does not contribute much to the complexity of the synthetic products, the generation of formaldehyde-derived iminium/oxonium intermediates in particular offers highly interesting pathways for cyclisation reactions and especially those in multi-component reactions. Both alcohol-derived oxonium intermediates as well as amine-derived iminium salts have been successfully employed to intermolecularly link the heteroatom functionalities through a methylene bridge with carbon-centred nucleophiles. Thus, oxygen-heterocycles are obtained through reaction of formaldehyde with simple alkenes248 or homoallylic alcohols (25) through Prins-type cyclizations (Scheme 12(a)).249 In a similar fashion, biogenic amines such as epinephrine (27) can be used in Pictet–Spengler cyclizations where the formaldehyde-derived iminium species engages in electrophilic aromatic substitutions to yield tetrahydroisoquinoline derivatives (28) (Scheme 12(b)).250
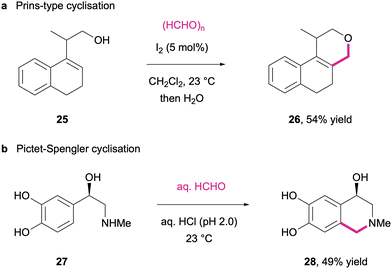 | ||
| Scheme 12 Representative cyclisation reactions via formaldehyde-derived oxonium (a) and iminium (b) intermediates. | ||
The high carbonyl reactivity and ease of formation of oxonium intermediates, iminium salts or olefinic Knoevenagel products in complex reaction mixtures has led to a high popularity of formaldehyde and its surrogates as a critical element in multicomponent reactions.251 The application scope is very wide and has been nicely compiled in a critical review by Jérôme and co-workers.252 Particularly, the reaction between formaldehyde-derived imines/iminium intermediates and C-nucleophiles has been a popular target of organic method development, including Petasis and Mannich reactions.253 For example, nucleophilic N-heterocycles such as indole, pyrrole and the likes find frequent application in Mannich-type reactions where secondary amines and formaldehyde in the presence of a Lewis acid deliver aminomethylated products (31) in high yields (Scheme 13(a)).254 But also Knoevenagel condensations with formaldehyde as an electrophile offer interesting opportunities for multicomponent reactions. Jayakanthan et al. used in situ condensation between formalin and methyl nitroacetate (32) to generate the corresponding nitroacrylate in the presence of a sugar-derived diene (33). Spontaneous [4+2]-cycloaddition trapped the reactive enoate and delivered bicyclic carbohydrate derivatives (34) in high yield and excellent diastereoselectivity (Scheme 13(b)).255 Moreover, a formaldehyde-based multicomponent reaction was also established as a final synthetic step in the preparation of the marine natural product exigurin (37).256 In a four component Ugi reaction, a terpenoid isonitrile (35) is reacted with the iminium intermediate resulting from the condensation of sarcosine (36) with formalin, and ring opening of the thus formed cyclic mixed anhydride by the solvent methanol delivers the sponge metabolite in 53% yield (Scheme 13(c)).
 | ||
| Scheme 13 Representative multi-component reactions using formaldehyde as the C1 linker element. (a) Mannich reaction; (b) Diels–Alder cycloaddition; (c) Ugi-type four component reaction. | ||
Organocatalysis with formaldehyde
The previously discussed basic application patterns of formaldehyde in organic synthesis fall right within the core application of many organocatalytic methodologies that often deal with the activation of carbonyl compounds. Hence, many of these methods were also found to be useful when it comes to reactions between a range of carbonyl compounds and formaldehyde. Here, organocatalytic activation was proven to provide certain benefits over traditional methods as exceptionally mild conditions help to overcome challenges related to selectivity posed by the high reactivity of the C1 aldehyde.The hydroxymethylation pathway illustrates these issues well, as the small footprint of formaldehyde renders it more challenging than other electrophiles in asymmetric aldol additions while also being prone to elimination and double hydroxylation side reactivities.257 Maruoka and co-workers reported on a system employing phase transfer catalysis for the enantioselective hydroxymethylation of α-nitroesters where aqueous formalin was utilized in a biphasic reaction medium. With the aid of an axially chiral C2 symmetric ammonium salt under base-free conditions, good optical purities were achieved, and the products were further transformed into α,α-disubstituted serine derivatives (Scheme 14).258
 | ||
| Scheme 14 Asymmetric hydroxymethylation of α-nitroesters through phase transfer catalysis (PTC).258 | ||
Similarly, the enamine catalysis pioneered by List and MacMillan was also examined as a tool for the selective aldol chemistry of formaldehyde. In a study by Takabe and co-workers, the amino acid threonine was identified as the most effective catalyst, and the selective mono-hydroxymethylation of cyclic ketones (40 to 41) was accomplished in moderate to high enantioselectivities (42 to 43; Scheme 15(a)).259 Slight modification of the reaction conditions on the other hand leads to an aldol condensation pathway where secondary amine catalysts in presence of a Brønsted acid cocatalyst facilitate the α-methylenation of aldehydes (Scheme 15(b)),260 or the γ-methylenation of enals.261
 | ||
| Scheme 15 Aldol addition (a) and aldol condensation (b) reactions between enolisable carbonyl compounds and formaldehyde.259,260 | ||
The amino acid-mediated activation of ketones and enones can further be extended to multicomponent reactions. Sundén et al. illustrated this approach in an asymmetric aza-Diels–Alder cycloaddition, where proline-derived dienamines recombine with in situ formed methylene imines, leading to the formation of optically pure bicyclic N-heterocycles (46; Scheme 16).262
 | ||
| Scheme 16 Proline-catalyzed three component enantioselective aza-Diels–Alder cycloaddition.262 | ||
Organocatalysis has so far also proven to be the best solution for any attempts to conduct umpolung of formaldehyde or its surrogates in order to transfer formyl groups in a nucleophilic fashion. However, whenever direct umpolung of formaldehyde was attempted through the use of nucleophilic N-heterocyclic carbenes (NHCs), either self-condensation to higher carbohydrates was observed,263 or cross acyloin reactions took place where a secondary alcohol was selectively converted to the nucleophilic Breslow intermediate.264 The latter leads to an alternative hydroxymethylation pathway where aldehydes are activated by thiazolium-derived nucleophilic NHCs and add to the electrophilic formaldehyde, furnishing α-hydroxyketones (48) (Scheme 17(a)).265
 | ||
| Scheme 17 NHC-catalysed C–C bond formation through acyl anion equivalents. (a) shows the hydroxymethylation of umpoled aldehydes and (b) the use of glucose as a formaldehyde surrogate for an NHC-catalysed formylation.265,266 | ||
So far, the only way to effectively generate nucleophilic Breslow intermediates of formaldehyde utilises higher carbohydrates as formaldehyde surrogates. Here, the stepwise NHC-catalysed degradation of sugars reversibly leads to the nucleophilic formyl anion equivalents. As demonstrated by Chi and co-workers, glucose can be employed as “formaldehyde hexamer” and in the presence of simple trimethylthiazolium salts and K2CO3, formyl transfer through Stetter reaction with enones (49) is achieved in high yields (Scheme 17(b)).266
Formaldehyde and biocatalysis
The presence of formaldehyde in biocatalytic reactions is rich and varied, as this most simple aldehyde can be found steadily both in living systems and in laboratory-based environments – with dramatically different effects and applications.Routes to and from formaldehyde
Formaldehyde is abundantly found in biological pathways, being introduced by external sources or endogenously produced in vivo.267 Not only HCHO formation is the result of catabolic oxidation of methanol,268 but it is also involved in crucial pathways, such as the ribulose monophosphate (RuMP) and the xylulose monophosphate (XuMP)269 or the serine cycles.270 These natural routes have served as an inspiration to expand the role of formaldehyde for hydrogen storage and production through biomimetics.228,229 Alternatively, some bacteria are also known to produce formaldehyde from methylamine through methylamine dehydrogenase (MADH)271 or from trimethylamine oxide through a trimethylamine-N-oxidase (TMAOase),272 amongst others. From a man-made perspective, the conversion of CO2 to formaldehyde has been exploited both chemically and enzymatically;27,273 and the CO2-derived formaldehyde could also be further condensed to generate C3 compounds.274 Most of these processes, especially those which are part of larger and more intricate pathways, are – or can be made – reversible; rendering this C1-aldehyde both a product of anabolic construction and a substrate for catabolic degradation (Scheme 18).65,269,270,275Endogenous formaldehyde (“formaldehyde in vivo”)
Given its ubiquitous presence in nature, it is of no surprise that formaldehyde rises with a key physiological position: that of a metabolic regulator. In this sense, FA has been proven to be of crucial importance for epigenetic dysregulation and genome disability, by inhibiting the biosynthesis of S-adenosyl methionine (SAM) in cells – a development that is of relevance for serious conditions such as fatty liver disease and cancer.276,277This key role as a regulator is partly because HCHO is highly toxic and carcinogenic,159,278 having been established that formaldehyde and its metabolism are directly linked to DNA damage repair and genotoxicity279,280 and to protein–DNA crosslinking.281 In this line, many studies have been conducted to establish the chemistry of formaldehyde with amino acids and proteins.282–284 Formaldehyde's high toxicity in vivo has also led to the development of natural detoxification systems285 and fuels the establishment of these highly valorisable cycles,286 as different metabolic pathways have evolved to circumvent such eventuality, and in turn, transform it into more valuable substrates.287,288
Exogenous formaldehyde (“formaldehyde in vitro”)
In truth, formaldehyde has been gaining a more prominent role in the biosynthesis of fine chemicals.289 Its unique reactivity and versatility set it apart from other C1 scaffolds, as is the fact that FA is an intermediate of methylotrophic microorganisms. In this way, the exploration of biocatalytic routes to exploit (chiral) transformations of formaldehyde has been mostly defined in three ways: C–C aldolase bond formation (Scheme 19(a)),290 umpolung activation by thiamine diphosphate dependent enzymes (ThDPs; Scheme 19(b)),291 and a formolase reaction (Scheme 19(c)).292–294 Indirectly speaking, formaldehyde is also used by thymidylate synthase (ThyX) as a direct methylene donor of deoxyuridylate (dUMP) in folate-dependent reactions, which are a critical step toward DNA biosynthesis (Scheme 19(d)).295 | ||
| Scheme 19 Formaldehyde in biocatalysis. (a) 3-methyl-2-oxobutanoate hydroxymethyltransferase (KPHMT) is able to incorporate formaldehyde to produce 2-oxolactones; (b) benzaldehyde lyase (BAL); (b) ThDP-dependant enzyme, catalyses C–C bond formation to yield 2-hydroxy-1-phenyl-ethan-1-one; (c) formolase (FLS) is a computationally-designed enzyme that allows the development of a novel one-carbon assimilation pathway; (d) reductive methylation of deoxyuridylate (dUMP) into deoxythymidylate (dTMP) from formaldehyde, using thymidylate synthase (ThyX).290–294 | ||
Photoredox catalysis
More recently, photoredox catalysis has experienced a veritable boom as a versatile new tool in the organic chemist's toolbox.296 The development of organic or organometallic dyes has opened up reaction pathways that in many ways complement the other catalytic approaches through mild light-induced generation of reactive radical species.297 Formaldehyde itself has not yet been a major focus in the methodology exploration based on photoredox catalysis, however, the C1-aldehyde occasionally finds recognition as part of substrate scope investigations as illustrated by the reductive aminomethylation of butyl acrylate (61) in the presence of an iridium dye (Scheme 20(a)).298 So far, two studies have explicitly taken a closer look at formaldehyde as a C1 building block, both in combination with alkyl halides (63) and triphenylphosphine. Leonori and co-workers reported on the use of the isophthalonitrile dye as a mediator in a broadly applicable dehalogenative hydroxymethylation (Scheme 20(b)),299 while Jiang et al. observed under slightly different conditions, using a ruthenium-based photoredox catalyst and an inorganic base, the conversion of benzylic bromides (65) into the corresponding styrene derivatives (66) (Scheme 20(c)).300 | ||
| Scheme 20 Aminomethylation (a), hydroxymethylation (b) and olefination (c) based on visible light-driven activation modes. | ||
Metal-catalysed introduction of CH, CH2 and CH3 entities
As outlined above, formalin and PFA are well established in classical organic synthesis for the introduction of C1-entities (i.e. in aldol condensation, Baeyer diarylmethane synthesis, and Blanc Mannich, Petasis, Prins–Kriewitz, Tiffeneau reactions). In time, such protocols and substrate scopes were extended through metal catalysis to enable new protocols or give new insights for methylation (excerpts in Table 2), hydroxymethylation, alkynylation, and synthesis of allenes among others.101,119,301–312Notably, early examples for methylation reactions with rhodium and cobalt catalysts, performed the reactions under CO pressure to enable the formylation followed by reduction to the methyl entity.307–309 The more recent study on N-methylation with ruthenium catalysts allowed for the conversion through subsequent imine formation followed by transfer-hydrogenation of the in situ formed N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 entity to N–CH3. The protocol is also applicable in a biphasic solvent system which allows catalyst recycling and product separation.119 Interestingly, allylic amines are accessible through Pd-catalysed coupling of alkenes with amines in the presence of PFA.313 Allylic alcohols are accessible in moderate to good yields (39–86%) for example through Ru-, Ni- or Ir-catalysed hydroxymethylation of dienes, allenes and alkynes with PFA as the C1-donor usually in the presence of protic solvents or additional terminal reductants to improve the yields (i.e. water, alcohol, formic acid).95,109,116,314–318 And, propargylic alcohols can be synthesised in good yields from terminal alkynes and PFA with silver or copper catalysts.97,305 The hydroxymethylation of substituted 1,4-dienes gives access to the corresponding coupling products on the C1 (≤63%) or C4 (≤71%) position applying a Ni-catalyst, while with ruthenium the coupling occurs at the C2 (≤74%) or C3 (≤80%) position.314 The hydroxymethylation of substituted alkynes occurs preferably at the less hindered side (≤81%) with nickel while with ruthenium the isomer with the bulkier substituent (≤85%) is accessible.95 Moreover, hydroxymethylation of aromatic ring systems with PFA has been demonstrated with azaindoles in the presence of a ruthenium catalyst giving the products in good yields (up to 83%).101 The ortho-selective ruthenium-catalysed hydroxymethylation of benzoic acid amides with PFA yields phthalides after subsequent lactonisation (up to 72%).121 Interestingly, the treatment of pyridinium salts with PFA, magnesium methoxide and potassium iodide in the presence of a ruthenium catalyst leads to dearomatisation and regioselective hydroxymethylation.319 In a different context, hydroxymethylation with PFA of α-cyanocarboxylates occurs with (chiral) rhodium and palladium catalysts, while with gold catalysts dihydrooxazoles are formed in moderate to good yields and enantioselectivities.320–322
CH2 entity to N–CH3. The protocol is also applicable in a biphasic solvent system which allows catalyst recycling and product separation.119 Interestingly, allylic amines are accessible through Pd-catalysed coupling of alkenes with amines in the presence of PFA.313 Allylic alcohols are accessible in moderate to good yields (39–86%) for example through Ru-, Ni- or Ir-catalysed hydroxymethylation of dienes, allenes and alkynes with PFA as the C1-donor usually in the presence of protic solvents or additional terminal reductants to improve the yields (i.e. water, alcohol, formic acid).95,109,116,314–318 And, propargylic alcohols can be synthesised in good yields from terminal alkynes and PFA with silver or copper catalysts.97,305 The hydroxymethylation of substituted 1,4-dienes gives access to the corresponding coupling products on the C1 (≤63%) or C4 (≤71%) position applying a Ni-catalyst, while with ruthenium the coupling occurs at the C2 (≤74%) or C3 (≤80%) position.314 The hydroxymethylation of substituted alkynes occurs preferably at the less hindered side (≤81%) with nickel while with ruthenium the isomer with the bulkier substituent (≤85%) is accessible.95 Moreover, hydroxymethylation of aromatic ring systems with PFA has been demonstrated with azaindoles in the presence of a ruthenium catalyst giving the products in good yields (up to 83%).101 The ortho-selective ruthenium-catalysed hydroxymethylation of benzoic acid amides with PFA yields phthalides after subsequent lactonisation (up to 72%).121 Interestingly, the treatment of pyridinium salts with PFA, magnesium methoxide and potassium iodide in the presence of a ruthenium catalyst leads to dearomatisation and regioselective hydroxymethylation.319 In a different context, hydroxymethylation with PFA of α-cyanocarboxylates occurs with (chiral) rhodium and palladium catalysts, while with gold catalysts dihydrooxazoles are formed in moderate to good yields and enantioselectivities.320–322
In the broader field of organic synthesis, aq. formaldehyde and PFA find diverse applications in the synthesis of heterocycles and functionalisation of aromatic compounds. Prominent examples include the synthesis of imidazolium salts which are of interest for the generation of stable Arduengo-type carbenes (NHC), and imidazolium ionic liquids.74,323–327 For example, cycloaminomethylation of indole with PFA gives access to 3-alkyl(phenyl)-3,4-dihydro-2H-1,5-(metheno)[1,3]benzodiaze-pines328 and quinazoline derivatives are accessible through condensation of anilines in the presence of HCHO, derived from PFA, in the presence of a Schiff-base catalyst.96 Macrocycles, such as diaza tetraacetylenes, can be synthesised from α,ω-diacetylenes with PFA as the HCHO source in the presence of copper chloride as a catalyst.329 Other studies include the catalytic in situ formation of formaldehyde from methanol for the synthesis of aminals,330 or benzimidazoles.331 In a different context annulations of carbo- and N-heterocycles with formaldehyde under metal-catalysed or metal-free conditions were also demonstrated.332–334
Last but not least, metal hydroxides of i.e. calcium or lead in the presence of trace amounts of carbohydrates form enediol metal complexes which then induce the Formose reaction in the presence of formaldehyde leading to the formation of mixtures of carbohydrates such as glycolaldehyde, tetrose, and glyceraldehydes.7,15–20
Formaldehyde as a surrogate for carbonylation
Similar to the above mentioned reactions, FAH and PFA are also convenient alternatives to gaseous reagents (i.e. CO, syngas) for carbonylation reactions. In this context, these reagents serve as CO-surrogates that offer the possibility to perform reactions on the labscale without the specific infrastructure required for highly hazardous reagents based on carbon monoxide. Thus, for example hydroformylation, aryloxy/alkoxycarbonylation or N-formylation can be easily performed using such CO-surrogates to introduce functional groups like aldehydes, acids, esters or amides, enable the synthesis of heterocycles, introduce chiral centres or isotope-labelled entities (Table 3).52,54,98,112,113,335–339 An interesting study in the field of carbonylation reactions involving paraformaldehyde, showed that PFA can react with syngas (70 bar, 100 °C, 20 h) in the presence of a rhodium pincer complex and potassium formate forming ethylene glycol in moderate yields (40%).340| Entry | Catalyst | T [°C] | Reagent | Product | [%] | Ref. |
|---|---|---|---|---|---|---|
| a Typically with a high region-selectivity for terminal carbonylation of olefins. | ||||||
| 1 | Rh | ≤120 | Alkene | Aldehydea | ≤96 | 110, 113, 114, 337 and 341–344 |
| 2 | Pd | 110 | Bromo-biphenyl | Ketone | ≤75 | 345 |
| 3 | Ru or Pd | 100–130 | Alkene + alcohol | Estera | ≤93 | 105, 106 and 117 |
| 4 | Rh | 110 | PhOH + PhI | Arylester | ≤86 | 94 |
| 5 | Cu, Ir or Ru | 25–60, 115, 150 | Amine | Amide | ≤99 | 100, 112 and 115 |
| 6 | Rh | 100 | Aniline + alkyne | Quinoline | ≤95 | 346 |
| 7 | Co + Ag | 120 | Aniline + ketone | Quinoline | ≤91 | 347 |
Hydrogen evolution and transfer hydrogenation reactions
Labscale synthesis often requires specific equipment and infrastructure to handle (hazardous) gases under safe conditions. In this regard, formaldehyde (6.7 wt% H2) and paraformaldehyde (per unit: 6.7 wt% H2) as liquid/solid organic hydrogen carrier (LOHC/SOHC) molecules are interesting as they can generate hydrogen on demand, in situ, with less sophisticated reactors and glassware. Nonetheless, gas sensors (leakage) and appropriate reaction vessels should be used for the corresponding reactions and the expected gas amount should be considered for the reaction setup and the reaction vessel.Aq. formaldehyde (formaldehyde hydrate (FAH), methanediol; H2C(OH)2)) and paraformaldehyde (PFA) in water have been studied for hydrogen evolution and transfer-hydrogenation reactions. The high hydrogen content of methanediol (8.4 wt% H2) and the exothermic and exergonic decomposition to hydrogen and carbon dioxide enables fairly mild conditions (<100 °C) with molecular metal catalysts (Tables 1–4).28,30,66–71,348,349
| Entry | Metal complex | T [°C] | pH | TON | TOF [h−1] | Ref. |
|---|---|---|---|---|---|---|
| a Immobilised on a covalent triazine framework (CTF). | ||||||
| 1 | Ru | 95 | 5.5–7 | 700 | 3142 | 28 and 30 |
| 2 | Ir | 95 | 11 | 178 | n.d. | 69 |
| 3 | Ir | 60 | 11 | 51 | n.d. | 70 |
| 4 | Ru | 60 | >12 | 1787 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
68 |
| 5 | Ru | 95 | 7 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8300 | 71 |
| 6 | Ru | 95 | n.d. | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 905 905 |
5715 | 66 |
| 7 | Ru | 90 | n.d. | n.d. | 685 | 67 |
| 8a | Ru | 90 | n.d. | n.d. | 1650 | 350 |
These promising results let then to further evaluation of FAH and PFA in water for metal catalysed transfer-hydrogenation enabling the reduction of functional groups (i.e. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C,103 C
C,103 C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C,61 C
C,61 C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N,60 C
N,60 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,108 excerpts in Table 5). Interestingly, the use of D2O or deuterated PFA gives access to the regioselectively isotope-labelled/deuterated products. Moreover, bi-catalytic systems were also developed for hydrogen evolution from methanol, wherein these approaches formaldehyde is generated in situ from initial enzymatic methanol activation in particular, and coupled with metal-catalysed hydrogen evolution (i.e. Ru, Ir) at low temperature.228,229,348,351,352 In these studies Ru-MOFs as catalysts were also successfully tested to achieve good catalyst-enzyme compatibility along with good stability in the presence of formaldehyde (H2 production: 106 mmol h−1 mol−1Ru; pH 7.5 at 35 °C).351 The latter is important, since enzymes can also undergo deactivation in the presence of formaldehyde.353
O,108 excerpts in Table 5). Interestingly, the use of D2O or deuterated PFA gives access to the regioselectively isotope-labelled/deuterated products. Moreover, bi-catalytic systems were also developed for hydrogen evolution from methanol, wherein these approaches formaldehyde is generated in situ from initial enzymatic methanol activation in particular, and coupled with metal-catalysed hydrogen evolution (i.e. Ru, Ir) at low temperature.228,229,348,351,352 In these studies Ru-MOFs as catalysts were also successfully tested to achieve good catalyst-enzyme compatibility along with good stability in the presence of formaldehyde (H2 production: 106 mmol h−1 mol−1Ru; pH 7.5 at 35 °C).351 The latter is important, since enzymes can also undergo deactivation in the presence of formaldehyde.353
Formaldehyde in relation to biomass
In essence, biomass is all around us: everything that is living and everything that once lived or pertained to a living organism; from fallen tree leaves, to algae or domestic biowaste.354 With such abundance, it is no wonder that biomass has become a widely popular choice for energy production.355Chemically, biomass is primarily composed of carbohydrates (cellulose and hemicellulose) and the complex organic polymer lignin.356,357 Lignin, of all the biomass components, has the highest energy density and is a great source for the large-scale production of aromatic compounds.358 However, the huge potential of lignin has been stagnant for decades, given the lack of high-yielding depolymerisation methods.359 It is common that ether bonds in lignin cleave with acids or high temperatures, forming stable C–C bonds leading to “condensed lignin” and poor monomer yields (70–74). Formaldehyde allows for the stabilisation of lignin during extraction by hindering the formation of these stable C–C bonds, a process that can happen in two ways: by forming stable six-membered acetals (67) with lignin's 1,3-diols and by blocking electron-rich positions in the aromatic rings (Scheme 21).360 This development could be further exploited in industrial biomass processing, given that lignin-borne formaldehyde is generated during the thermal processing of wood,160,361 or through lignin acidolysis.362 To improve the depolymerisation of lignin it has been also demonstrated that the addition of ethanol as a formaldehyde scavenger is beneficial and as a by-product diethoxymethane is formed which is interesting as a biofuel.363 In the ever-growing sector of biomanufacturing, lignocellulose (and other sugar-based) feedstocks, which require tedious pre-treatments, are being shunned in favour of C1 compounds.209 Of these, methane, methanol, COx and formate have been at the lead of fossil fuel replacement.364 Formaldehyde was far from being included in this list, and in fact, for years, the only reports available would be on formaldehyde as a primary pollutant from biomass burning.163,365
 | ||
| Scheme 21 Lignin is one of the key biopolymers in woody biomass. Formaldehyde (pink) – which can also be generated in lignin acidolysis (left side route) – stabilizes the process of reactive lignin extraction and hydrogenolysis and provides for higher yields in monomer production (dotted square, left); compared to the lack of formaldehyde (dotted square, right). These studies used veratrylglycerol-b-guaiacyl (in blue in the lignin polymer) as a model compound.360,361 | ||
Still, recent studies have showcased the potential of a symbiotic biomass exploitation. Thus, the catalytic oxidation of biomass-derived polyols, such as glycerol, constitutes a promising and sustainable alternative. Recent studies have showcased this possibility using different, high-yield strategies. In this way, formic acid and formaldehyde (15% over TiO2/O2;366 21% over Co@TiO2)367 have been successfully obtained through photocatalytic degradation of glycerol. The major product of polyol/glycerol degradation in photo-, thermo- and electrocatalytic methods from biomass or their hydroxylated derivatives remains formic acid with high selectivity.366–369 While formic acid production from biomass remains the more attractive option, overoxidation to CO2 can significantly limit the yields.370,371 By coupling a second reaction that converts methanol into valuable formaldehyde, researchers have been able to significantly improve yields by twofold by producing two valuable end products: formic acid and formaldehyde.371 To this end, formaldehyde is starting to be known as a very valuable sub-product.372
All in all, formaldehyde remains a supporting actor in the biomass industry, with other C1 compounds in the main roles. Yet, realistically, most of these single-carbon substrates are naturally converted into formaldehyde in the first step of their assimilation process,370,373 a fact that places formaldehyde in the ideal position for the creation of a highly energy efficient, highly valorisable cycle.374,375 Notably, biomass-derived formaldehyde, from biomethanol oxidation, is economically viable according a LCA from 2024.372 For the valorisation of the biomass lignin, formaldehyde will continue to play an important role in particular for the production of renewable lignin-phenol-formaldehyde or lignin-urea-formaldehyde resins to complement and substitute fossil-based formaldehyde resins for example (Scheme 6).376–394
Conclusion, perspectives and outlook: the role of formaldehyde in a defossilised society?
Formaldehyde and surrogates are under investigation in diverse fields of novel and established applications. Historically, this also covers the modernisation of conventional production methods such as fossil-based resins to lignin-based resins (UNSDG 9 and 12). Likewise, classical synthetic methods in organic chemistry are further explored along with new catalysts and biomass-derived substrates under thermo-, electro-, photo- and biochemical conditions. The formaldehyde industries together with the methanol sector have promising opportunities for defossilisation of the required feedstocks through CCUS and biomass valorisation. In this context, photocatalytic and electrocatalytic processes driven by renewable energies for the carbon dioxide reduction to formaldehyde and methanol are important keys to enable the defossilisation of these industrial branches. These production pathways will be likely complemented with more efficient and sustainable formaldehyde production processes with renewable methanol and CCUS-derived syngas as feedstocks. In the energy and transportation sectors renewable formaldehyde together with methanol are of interest owing to their relatively high energy and hydrogen content which is of relevance not only for both stationary and mobile energy storage for fuel cells, but also for synthetic fuels such as renewable oxymethylene ethers for combustion engines (UNSDG 7 and 13).The successful implementation of technologies for the production of renewable formaldehyde and methanol along with the substitution of fossil reagents by biomass derived reagents enables the opportunity to use existing chemical production infrastructures with defossilised C1-feedstocks (UNSDG 9). Furthermore, new technologies for formaldehyde degradation are of interest to improve the purity of waste streams, water, soil and air through formaldehyde detoxification (UNSDG 3, 6, 13–15).
For further advancement of formaldehyde-based production and utilisation technologies, the above discussed methodologies under development will require more techno-economic analysis (TEA) and life-cycle assessment (LCA) to enable higher technology readiness levels (TRL) and industrial applications. To date, TEA and LCA are very limited for new technologies with formaldehyde as a key molecule for i.e. DME/OME fuel production, renewable HCHO from biomass or HCHO degradation,372,395,396 TEA and LCA with a focus on HCHO for hydrogen storage and within the CCUS sector are still required for further advancement. In general, LCA and TEA can be powerful tools to determine whether a specific element, process or area is functioning satisfactorily or is beneficial for the producer and end-user. While this review does not address the economy from a monetary perspective, we offer chemical insights into the benefits of using formaldehyde and its surrogates as a C1 platform for a defossilised society. For the exploration and discovery of new methods for formaldehyde formation and utilisation one needs to consider also the limitations of the reaction conditions (i.e. temperature, pH sensitivity and water content) as summarised in this tutorial.
All these measures and advancements for the defossilisation of the chemical industries over the next decades will require and will lead to more innovation and simultaneously improve the level of education and knowledge (UNSDG 4). In this way, we can conclude that the potential of formaldehyde is high on different counts. First and foremost, as a defossilising agent, by greatly improving our society through its surroundings; but also by promoting innovation through sustainability, given that a great potential of formaldehyde comes from natural, recyclable and reused sources. For all these reasons, we consider that renewable formaldehyde is an excellent asset to develop greener, richer and more sustainable societies.
Author contributions
Concept: MHGP; writing – original draft: MHGP; writing, review & editing: MHGP, JD and AR.Conflicts of interest
A patent has been filed under 10 2013 011 379.2 at the German Patent Office DMPA (WO2015/003680A003681; PCT/DE002014/000344).Data availability
All data included in this article are based on original data which are available through the cited literature in the references.Acknowledgements
The authors thank FCT for the projects 2022.02069.PTDC (DOI: 10.54499/2022.02069.PTDC), and EXPL/QUI-QOR/1079/2021, (DOI: 10.54499/EXPL/QUI-QOR/1079/2021) at the Centro de Química Estrutural, the Deutsche Forschungsgemeinschaft (DFG 411475421), the Centro de Química Estrutural/Institute of Molecular Sciences. JD and AR thank the European Research Council for financial support (ABIONYS, ERC CoG 865885). The COST Actions CYPHER (CA22151) and TransformERS (CA22156) are acknowledged for further support.Notes and references
- L. E. Snyder, D. Buhl, B. Zuckerman and P. Palmer, Phys. Rev. Lett., 1969, 22, 679–681 CrossRef CAS.
- M. E. Schrader, J. Geophys. Res.: Atmos., 2009, 114, D15305 CrossRef.
- J. W. Szostak, Nature, 2009, 459, 171–172 CrossRef CAS PubMed.
- S. Koyama, A. Kamada, Y. Furukawa, N. Terada, Y. Nakamura, T. Yoshida, T. Kuroda and A. C. Vandaele, Sci. Rep., 2024, 14, 2397 CrossRef CAS.
- S. Masuda, Y. Furukawa, T. Kobayashi, T. Sekine and T. Kakegawa, Astrobiology, 2021, 21, 413–420 CrossRef CAS PubMed.
- H. J. Cleaves, Precambrian Res., 2008, 164, 111–118 CrossRef CAS.
- H. J. Cleaves, in Encyclopedia of Astrobiology, ed. M. Gargaud, R. Amils, J. C. Quintanilla, H. J. Cleaves, W. M. Irvine, D. L. Pinti and M. Viso, Springer, Berlin Heidelberg, Editon edn, 2011, pp. 600–605 Search PubMed.
- M. Haas, S. Lamour, S. B. Christ and O. Trapp, Commun. Chem., 2020, 3, 140 CrossRef CAS PubMed.
- S. Lamour, S. Pallmann, A. Haas and O. Trapp, Life, 2019, 9, 52 CrossRef CAS.
- S. Pallmann, J. Steflová, M. Haas, S. Lamour, A. Henss and O. Trapp, New J. Phys., 2018, 20, 055003 CrossRef.
- S. Peters, D. A. Semenov, R. Hochleitner and O. Trapp, Sci. Rep., 2023, 13, 6843 CrossRef CAS PubMed.
- R. Breslow, Tetrahedron Lett., 1959, 1, 22–26 CrossRef.
- A. Eschenmoser, Tetrahedron, 2007, 63, 12821–12844 CrossRef CAS.
- A. Butlerow, Justus Liebigs Ann. Chem., 1861, 120, 295–298 CrossRef.
- A. N. Simonov, O. P. Pestunova, L. G. Matvienko and V. N. Parmon, Kinet. Catal., 2007, 48, 245–254 CrossRef CAS.
- H. Tambawala and A. H. Weiss, J. Catal., 1972, 26, 388–400 CrossRef CAS.
- A. H. Weiss, V. A. Seleznev, M. M. Sakharov, O. V. Krylov, Y. B. Gorokhovatsky and N. P. Evmenenko, J. Catal., 1977, 48, 354–364 CrossRef CAS.
- R. F. Socha, A. H. Weiss and M. M. Sakharov, J. Catal., 1981, 67, 207–217 CrossRef CAS.
- W. P. Huskey and I. R. Epstein, J. Am. Chem. Soc., 1989, 111, 3157–3163 CrossRef CAS.
- I. V. Delidovich, A. N. Simonov, O. P. Taran and V. N. Parmon, ChemSusChem, 2014, 7, 1833–1846 CrossRef CAS.
- T. D. Sharkey, Photosynth. Res., 2019, 140, 235–252 CrossRef CAS.
- M. Calvin, Angew. Chem., Int. Ed. Engl., 1962, 1, 65–75 CrossRef.
- A. A. Benson, S. Kawauchi, P. Hayes and M. Calvin, J. Am. Chem. Soc., 1952, 74, 4477–4482 CrossRef CAS.
- A. Benson and M. Calvin, Science, 1947, 105, 648–649 CrossRef CAS PubMed.
- L. Schulz, Z. Guo, J. Zarzycki, W. Steinchen, J. M. Schuller, T. Heimerl, S. Prinz, O. Mueller-Cajar, T. J. Erb and G. K. A. Hochberg, Science, 2022, 378, 155–160 CrossRef CAS.
- A. M. Bahmanpour, A. Hoadley and A. Tanksale, Green Chem., 2014, 30, 583–604 CAS.
- L. E. Heim, H. Konnerth and M. H. G. Prechtl, Green Chem., 2017, 19, 2347–2355 RSC.
- L. E. Heim, S. Vallazza, D. van der Waals and M. H. G. Prechtl, Green Chem., 2016, 18, 1469–1474 RSC.
- A. W. Franz, H. Kronemayer, D. Pfeiffer, R. D. Pilz, G. Reuss, W. Disteldorf, A. O. Gamer and A. Hilt, Ullmann's Encyclopedia of Industrial Chemistry, Editon edn, 2016, pp. 1–34 Search PubMed.
- L. E. Heim, N. E. Schloerer, J. H. Choi and M. H. G. Prechtl, Nat. Commun., 2014, 5, 3621 CrossRef.
- J. L. Cereghino and J. M. Cregg, FEMS Microbiol. Rev., 2000, 24, 45–66 CrossRef CAS.
- L. Liu, A. Hausladen, M. Zeng, L. Que, J. Heitman and J. S. Stamler, Nature, 2001, 410, 490–494 CrossRef CAS.
- D. P. Dixon, I. Cummins, D. J. Cole and R. Edwards, Curr. Opin. Plant Biol., 1998, 1, 258–266 CrossRef CAS PubMed.
- European Food Safety Authority, EFSA J., 2014, 12, 3550 Search PubMed.
- L. B. Pontel, I. V. Rosado, G. Burgos-Barragan, J. I. Garaycoechea, R. Yu, M. J. Arends, G. Chandrasekaran, V. Broecker, W. Wei, L. Liu, J. A. Swenberg, G. P. Crossan and K. J. Patel, Mol. Cell, 2015, 60, 177–188 CrossRef CAS PubMed.
- L. Ai, T. Tan, Y. Tang, J. Yang, D. Cui, R. Wang, A. Wang, X. Fei, Y. Di, X. Wang, Y. Yu, S. Zhao, W. Wang, S. Bai, X. Yang, R. He, W. Lin, H. Han, X. Cai and Z. Tong, Commun. Biol., 2019, 2, 446 CrossRef CAS.
- Formaldehyde: Exposure, Toxicity and Health Effects, ed. L. Zhang, The Royal Society of Chemistry, Editon edn, 2018 Search PubMed.
- A. Marcon, M. E. Fracasso, P. Marchetti, D. Doria, P. Girardi, L. Guarda, G. Pesce, V. Pironi, P. Ricci and R. de Marco, Environ. Health Perspect., 2014, 122, 639–645 CrossRef PubMed.
- A. Quintana, L. López-Doval, R. Rodríguez, J. A. Souto and J. J. Casares, WIT Trans. Ecol. Environ., 2006, 91, 33–41 Search PubMed.
- E. Priha, Regul. Toxicol. Pharmacol., 1995, 22, 243–249 CrossRef CAS.
- C. M. Cho, J. M. St Clair, J. Liao, G. M. Wolfe, S. Jeong, D. I. Kang, J. Choi, M. H. Shin, J. Park, J. H. Park, A. Fried, A. Weinheimer, D. R. Blake, G. S. Diskin, K. Ullmann, S. R. Hall, W. H. Brune, T. F. Hanisco and K. E. Min, Elementa: Sci. Anthrop., 2021, 9(1), 00015 CrossRef.
- G. D. Waters, Proceedings of the Twenty-Seventh Washington State University International Particleboard/Composite Materials Symposium, 1993, 201-205.
- G. Rosen, I. M. Andersson and L. Juringe, Ann. Occup. Hyg., 1990, 34, 293–303 CrossRef CAS PubMed.
- M. X. Dai, F. Li, J. Zhang, Q. N. Shi and Q. Kong, J. Water Proc. Eng., 2024, 59, 104984 CrossRef.
- M. B. Henda, S. H. Sadon, Z. Abdelmalek, Z. Li and Q. H. Le, Eng. Anal. Bound Elem., 2023, 151, 400–405 CrossRef.
- T. Baczynski, Przem. Chem., 2020, 99, 1753–1757 CAS.
- T. Baczynski, Przem. Chem., 2020, 99, 1749–1752 CAS.
- B. Xing, H. L. Chen and X. M. Zhang, Water Sci. Technol., 2017, 75, 1390–1398 CrossRef CAS PubMed.
- A. Talaiekhozani, M. Salari, M. R. Talaei, M. Bagheri and Z. Eskandari, J. Environ. Manage., 2016, 184, 204–209 CrossRef CAS PubMed.
- X. Tang, Y. Bai, A. Duong, M. T. Smith, L. Li and L. Zhang, Environ. Int., 2009, 35, 1210–1224 CrossRef CAS.
- A. M. Bahmanpour, A. Hoadley, S. H. Mushrif and A. Tanksale, ACS Sustainable Chem. Eng., 2016, 4, 3970–3977 CrossRef CAS.
- B. Sam, B. Breit and M. J. Krische, Angew. Chem., Int. Ed., 2015, 54, 3267–3274 CrossRef CAS PubMed.
- A. M. Bahmanpour, A. Hoadley and A. Tanksale, Green Chem., 2015, 17, 3500–3507 RSC.
- D. Roeda and C. Crouzel, Appl. Radiat. Isot., 2001, 54, 935–939 CrossRef CAS PubMed.
- S. Wesselbaum, V. Moha, M. Meuresch, S. Brosinski, K. M. Thenert, J. Kothe, T. V. Stein, U. Englert, M. Hölscher, J. Klankermayer and W. Leitner, Chem. Sci., 2015, 6, 693–704 RSC.
- S. Wesselbaum, T. vom Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 7499–7502 CrossRef CAS.
- K. Beydoun, K. Thenert, J. Wiesenthal, C. Hoppe and J. Klankermayer, ChemCatChem, 2020, 12, 1944–1947 CrossRef CAS.
- K. Thenert, K. Beydoun, J. Wiesenthal, W. Leitner and J. Klankermayer, Angew. Chem., Int. Ed., 2016, 55, 12266–12269 CrossRef CAS PubMed.
- M. Trincado, H. Grutzmacher and M. H. G. Prechtl, Phys. Sci. Rev., 2018, 3, 20170013 Search PubMed.
- G. Tavakoli and M. H. G. Prechtl, Catal. Sci. Technol., 2019, 9, 6092–6101 RSC.
- M. N. A. Fetzer, G. Tavakoli, A. Klein and M. H. G. Prechtl, ChemCatChem, 2021, 13, 1317–1325 CrossRef CAS.
- S. Patra, T. A. Kharde, K. Kalita and S. K. Singh, ChemCatChem, 2025, 17, e202401643 CrossRef CAS.
- A. J. M. Miller, D. M. Heinekey, J. M. Mayer and K. I. Goldberg, Angew. Chem., Int. Ed., 2013, 52, 3981–3984 CrossRef CAS PubMed.
- L. Wang, M. Z. Ertem, K. Murata, J. T. Muckerman, E. Fujita and Y. Himeda, ACS Catal., 2018, 8, 5233–5239 CrossRef CAS.
- D. van der Waals, L. E. Heim, S. Vallazza, C. Gedig, J. Deska and M. H. G. Prechtl, Chem. – Eur. J., 2016, 22, 11568–11573 CrossRef CAS PubMed.
- M. K. Awasthi and S. K. Singh, Sustainable Energy Fuelss, 2021, 5, 549–555 RSC.
- Y. B. Shen, C. Bai, Y. L. Zhan, F. D. Ning, H. H. Wang, G. J. Lv and X. C. Zhou, ChemPlusChem, 2020, 85, 1646–1654 CrossRef CAS.
- M. Trincado, V. Sinha, R. E. Rodriguez-Lugo, B. Pribanic, B. de Bruin and H. Grutzmacher, Nat. Commun., 2017, 8, 14990 CrossRef CAS.
- T. Suenobu, Y. Isaka, S. Shibata and S. Fukuzumi, Chem. Commun., 2015, 51, 1670–1672 RSC.
- K. Fujita, R. Kawahara, T. Aikawa and R. Yamaguchi, Angew. Chem., Int. Ed., 2015, 54, 9057–9060 CrossRef CAS PubMed.
- L. Wang, M. Z. Ertem, R. Kanega, K. Murata, D. J. Szalda, J. T. Muckerman, E. Fujita and Y. Himeda, ACS Catal., 2018, 8, 8600–8605 CrossRef CAS.
- T. A. Geissman, Organic Reactions, Editon edn, 2011, pp. 94–113 Search PubMed.
- S. Cannizzaro, Justus Liebigs Ann. Chem., 1853, 88, 129–130 CrossRef CAS.
- Nanocatalysis in Ionic Liquids, ed. M. H. G. Prechtl, Wiley-VCH, 2016 Search PubMed.
- L. Szekeres and Á. Kellner, Microchem. J., 1968, 13, 227–229 CrossRef CAS.
- S. P. L. Sørensen, Biochem. Z., 1907, 7, 407 Search PubMed.
- G. B. C. Martins, R. R. Sucupira and P. A. Z. Suarez, Quim. Nova, 2016, 40, 946–951 Search PubMed.
- A. F. S. Silva, I. C. Gonçalves and F. R. P. Rocha, Food Control, 2021, 125, 107956 CrossRef CAS.
- J. N. Arias, A. Bonmatí, F. X. Prenafeta-Boldú and M. Cerrillo, J. Chromatogr. A, 2025, 1745, 465750 CrossRef CAS.
- R. A. Shellie and W. S. Heng, in Encyclopedia of Forensic Sciences, ed. M. M. Houck, Elsevier, Oxford, 3rd edn, 2023, pp. 1–9 Search PubMed.
- L. Dabrowski, Separations, 2024, 11, 347 CrossRef CAS.
- M. Kamiński, R. Kartanowicz, D. Jastrzębski and M. M. Kamiński, J. Chromatogr. A, 2003, 989, 277–283 CrossRef.
- U. S. Akshath and P. Bhatt, RSC Adv., 2016, 6, 54777–54784 RSC.
- A. M. Beale, S. D. M. Jacques, E. Sacaliuc-Parvalescu, M. G. O’Brien, P. Barnes and B. M. Weckhuysen, Appl. Catal., A, 2009, 363, 143–152 CrossRef CAS.
- S. S. Tabibian and M. Sharifzadeh, Renewable Sustainable Energy Rev., 2023, 179, 113281 CrossRef CAS.
- L. E. Heim, H. Konnerth and M. H. G. Prechtl, ChemSusChem, 2016, 9, 2905–2907 CrossRef CAS PubMed.
- H. I. Mahdi, N. N. Ramlee, D. H. D. Santos, D. A. Giannakoudakis, L. H. de Oliveira, R. Selvasembian, N. I. W. Azelee, A. Bazargan and L. Meili, Mol. Catal., 2023, 537, 112944 CAS.
- A. M. M. F. Phillips, M. N. Kopylovich, L. H. Andrade and M. H. G. Prechtl, in Catalysis for a Sustainable Environment: Reactions, Processes and Applied Technologies, ed. A. J. L. Pombeiro, M. Sutradhar and E. C. B. A. Alegria, Wiley-VCH, Weinheim, 2024, vol. 3, pp. 785–818 Search PubMed.
- H. T. Clarke, H. B. Gillespie and S. Z. Weisshaus, J. Am. Chem. Soc., 1933, 55, 4571–4587 CrossRef CAS.
- R. Adam and J. R. Cabrero-Antonino, ChemCatChem, 2024, 16, e202400563 CrossRef CAS.
- J. Burger, M. Siegert, E. Ströfer and H. Hasse, Fuel, 2010, 89, 3315–3319 CrossRef CAS.
- X. Zhang, A. O. Oyedun, A. Kumar, D. Oestreich, U. Arnold and J. Sauer, Biomass Bioenergy, 2016, 90, 7–14 CrossRef CAS.
- T. Matsumoto, H. Yamamoto and S. Inoue, J. Am. Chem. Soc., 1984, 106, 4829–4832 CrossRef CAS.
- A. Abu Seni, L. Kollar, L. T. Mika and P. Pongracz, Mol. Catal., 2018, 457, 67–73 Search PubMed.
- C. C. Bausch, R. L. Patman, B. Breit and M. J. Krische, Angew. Chem., Int. Ed., 2011, 50, 5686–5689 CrossRef.
- A. Chakraborty, T. Chowdhury, M. I. Menendez and T. Chattopadhyay, ACS Appl. Mater. Interfaces, 2020, 12, 38530–38545 CrossRef CAS PubMed.
- X. Y. Dong, L. X. Gao, W. Q. Zhang, Y. M. Cui, K. F. Yang, Z. W. Gao and L. W. Xu, ChemistrySelect, 2016, 1, 4034–4043 CrossRef CAS.
- R. Geitner and B. M. Weckhuysen, Chem. – Eur. J., 2020, 26, 5297–5302 CrossRef CAS PubMed.
- Z. Q. Guo, J. L. Wu and X. H. Wei, Asian J. Org. Chem., 2024, 13, e202300553 CrossRef CAS.
- H. Lee, B. Kang, S. I. Lee and S. H. Hong, Synlett, 2015, 1077–1080 CAS.
- S. Q. Li, Y. Yu, Y. X. Yang and B. Zhou, Heterocycles, 2020, 100, 934–945 CrossRef CAS.
- W. Li and X.-F. Wu, Adv. Synth. Catal., 2015, 357, 3393–3418 CrossRef CAS.
- W. F. Li and X. F. Wu, Eur. J. Org. Chem., 2015, 331–335 CrossRef CAS.
- W. F. Li and X. F. Wu, J. Org. Chem., 2014, 79, 10410–10416 CrossRef CAS PubMed.
- Q. Liu, L. P. Wu, R. Jackstell and M. Beller, ChemCatChem, 2014, 6, 2805–2809 CrossRef CAS.
- Q. Liu, K. D. Yuan, P. B. Arockiam, R. Franke, H. Doucet, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2015, 54, 4493–4497 CrossRef CAS.
- C. C. Meyer and M. J. Krische, J. Org. Chem., 2022, 88, 4965–4974 CrossRef.
- K. Natte, W. F. Li, S. L. Zhou, H. Neumann and X. F. Wu, Tetrahedron Lett., 2015, 56, 1118–1121 CrossRef CAS.
- M. Y. Ngai, E. Rucas and M. J. Krische, Org. Lett., 2008, 10, 2705–2708 CrossRef CAS.
- T. Okano, T. Kobayashi, H. Konishi and J. Kiji, Tetrahedron Lett., 1982, 23, 4967–4968 CrossRef CAS.
- S. Patra, A. Kumar and S. K. Singh, Inorg. Chem., 2022, 61, 4618–4626 CrossRef CAS PubMed.
- M. C. Pichardo, G. Tavakoli, J. E. Armstrong, T. Wilczek, B. E. Thomas and M. H. G. Prechtl, ChemSusChem, 2020, 13, 882–887 CrossRef CAS.
- R. Pittaway, P. Dingwall, J. A. Fuentes and M. L. Clarke, Adv. Synth. Catal., 2019, 361, 4334–4341 CrossRef CAS.
- M. Rosales, A. Gonzalez, B. Gonzalez, C. Moratinos, H. Perez, J. Urdaneta and R. A. Sanchez-Delgado, J. Organomet. Chem., 2005, 690, 3095–3098 CrossRef CAS.
- O. Saidi, M. J. Bamford, A. J. Blacker, J. Lynch, S. P. Marsden, P. Plucinski, R. J. Watson and J. M. J. Williams, Tetrahedron Lett., 2010, 51, 5804–5806 CrossRef CAS.
- B. Sam, T. P. Montgomery and M. J. Krische, Org. Lett., 2013, 15, 3790–3793 CrossRef CAS.
- R. Sang, C. Schneider, R. Razzaq, H. Neumann, R. Jackstell and M. Beller, Org. Chem. Front., 2020, 7, 3681–3685 RSC.
- M. Subaramanian, S. S. Padhy, C. Gouda, T. Das, K. Vanka and E. Balaraman, Catal. Sci. Technol., 2024, 14, 2779–2793 RSC.
- D. van der Waals, L. E. Heim, C. Gedig, F. Herbrik, S. Vallazza and M. H. G. Prechtl, ChemSusChem, 2016, 9, 2343–2347 CrossRef CAS PubMed.
- J. J. Zhang, Q. L. Qian, M. Cui, C. J. Chen, S. S. Liu and B. X. Han, Green Chem., 2017, 19, 4396–4401 RSC.
- C. Zhou, J. Q. Zhao, W. K. Chen, M. Imerhasan and J. Wang, Eur. J. Org. Chem., 2020, 6485–6488 CrossRef CAS.
- G. A. Olah, Angew. Chem., Int. Ed., 2005, 44, 2636–2639 CrossRef CAS PubMed.
- F. Schwarz, E. Larenz and A. K. Mechler, Green Chem., 2024, 26, 4645–4652 RSC.
- F. Schwarz, E. Larenz and A. K. Mechler, Green Chem., 2024, 26, 8877 RSC.
- P. J. L. Broersen, J. J. N. Koning, G. Rothenberg and A. C. Garcia, ChemSusChem, 2024, 17, e202400582 CrossRef CAS PubMed.
- J. B. Yeo, J. H. Jang, Y. I. Jo, J. W. Koo and K. T. Nam, Angew. Chem., Int. Ed., 2024, 63, e202316020 CrossRef.
- J. Baessler, T. Oliveira, R. Keller and M. Wessling, ACS Sustainable Chem. Eng., 2023, 11, 6822–6828 CrossRef CAS.
- S. Zhao, H.-Q. Liang, X.-M. Hu, S. Li and K. Daasbjerg, Angew. Chem., Int. Ed., 2022, 61, e202204008 CrossRef CAS PubMed.
- Z. N. Zhou, M. Wei, G. X. Yang, W. B. Du, F. Peng, Y. P. Fang, Y. J. Liu, S. S. Zhang and R. L. Qiu, J. Alloys Compd., 2023, 936, 168360 CrossRef CAS.
- Q. G. Ma, P. C. Huo, K. S. Wang, Y. Yuan, S. J. Bai, C. T. Zhao and W. Z. Li, Molecules, 2024, 29, 3822 CrossRef CAS.
- A. Prasnikar, B. Hocevar, A. Jazbec, K. Ambrozic, L. Snoj and B. Likozar, Int. J. Hydrogen Energy, 2024, 92, 560–568 CrossRef CAS.
- J. Luo, C. Zhu, J. L. Li, J. B. Jin, N. E. Soland, P. W. Smith, Y. Shan, A. M. Oddo, A. L. Maulana, L. Jayasinghe, X. Y. Chen, T. L. Wang, J. A. Lin, E. M. Y. Lu, B. Schaefer, M. Schmalzbauer, R. Zhang, F. Seeler, C. Lizandara-Pueyo, J. H. Guo and P. D. Yang, J. Am. Chem. Soc., 2025, 147, 3428–3437 CrossRef CAS.
- D. K. Lee, D. S. Kim and S. W. Kim, Appl. Organomet. Chem., 2001, 15, 148–150 CrossRef CAS.
- A. Cárdenas-Acero, C. Álvarez-Romero, C. Daza, A. Álvarez and E. A. Baquero, Disc. Catal., 2024, 1, 4 CrossRef.
- M. Kubo, D. Nakane, Y. Funahashi, T. Ozawa, T. Inomata and H. Masuda, Chem. – Eur. J., 2024, 30, e202303955 CrossRef CAS PubMed.
- R. Geitner, T. Schuett, S. Zechel and U. S. Schubert, Chem. – Eur. J., 2024, 30, e202401570 CrossRef CAS.
- C. H. Gierlich, K. Beydoun, J. Klankermayer and R. Palkovits, Chem. Ing. Tech., 2020, 92, 116–124 CrossRef CAS.
- K. Hackbarth, P. Haltenort, U. Arnold and J. Sauer, Chem. Ing. Tech., 2018, 90, 1520–1528 CrossRef CAS.
- J. Burre, D. Bongartz and A. Mitsos, Ind. Eng. Chem. Res., 2019, 58, 5567–5578 CrossRef CAS.
- D. Bongartz, J. Burre and A. Mitsos, Ind. Eng. Chem. Res., 2019, 58, 4881–4889 CrossRef CAS.
- R. Sun, I. Delidovich and R. Palkovits, ACS Catal., 2019, 9, 1298–1318 CrossRef CAS.
- J. Burre, D. Bongartz, S. Deutz, C. Mebrahtu, O. Osterthun, R. Y. Sun, S. Völker, A. Bardow, J. Klankermayer, R. Palkovits and A. Mitsos, Energy Environ. Sci., 2021, 14, 3686–3699 RSC.
- D. Oestreich, L. Lautenschütz, U. Arnold and J. Sauer, Fuel, 2018, 214, 39–44 CrossRef CAS.
- F. Mantei, S. Kopp, A. Holfelder, E. Flad, D. Kloeters, M. Kraume and O. Salem, React. Chem. Eng., 2023, 8, 917–932 RSC.
- N. Simitsis, F. Egger, M. Zobel, C. Mebrahtu and R. Palkovits, Catal. Sci. Technol., 2025, 15, 4380–4385 RSC.
- N. Simitsis, C. Mebrahtu and R. Palkovits, ChemCatChem, 2024, 16, e202301704 CrossRef CAS.
- R. Sun, C. Mebrahtu, J. P. Hofmann, D. Bongartz, J. Burre, C. H. Gierlich, P. J. C. Hausoul, A. Mitsos and R. Palkovits, Sustainable Energy Fuels, 2021, 5, 117–126 RSC.
- M. Martín, J. Redondo and I. E. Grossmann, ACS Sustainable Chem. Eng., 2020, 8, 6496–6504 CrossRef.
- W. Ahmad, F. L. Chan, A. Shrotri, Y. N. Palai, H. T. Wang and A. Tanksale, J. Energy Chem., 2022, 66, 181–189 CrossRef CAS.
- W. Ahmad, F. L. Chan, A. Hoadley, H. Wang and A. Tanksale, Appl. Catal., B, 2020, 269, 118765 CrossRef CAS.
- W. Ahmad, F. L. Chan, A. L. Chaffee, H. Wang, A. Hoadley and A. Tanksale, ACS Sustainable Chem. Eng., 2020, 8, 2081–2092 CrossRef CAS.
- S. Bontemps and S. Sabo-Etienne, Angew. Chem., Int. Ed., 2013, 52, 10253–10255 CrossRef CAS.
- T. T. Metsanen and M. Oestreich, Organometallics, 2015, 34, 543–546 CrossRef.
- P. Rios, N. Curado, J. Lopez-Serrano and A. Rodriguez, Chem. Commun., 2016, 52, 2114–2117 RSC.
- Y. F. Jiang, O. Blacque, T. Fox and H. Berke, J. Am. Chem. Soc., 2013, 135, 7751–7760 CrossRef CAS PubMed.
- S. Desmons, J. Bonin, M. Robert and S. Bontemps, Chem. Sci., 2024, 15, 15023–15086 RSC.
- A. A. Benson, J. A. Bassham and M. Calvin, J. Am. Chem. Soc., 1951, 73, 2970 CrossRef CAS.
- A. A. Benson and M. Calvin, J. Exp. Bot., 1950, 1, 63–68 CrossRef.
- M. H. Fischer, J. Exp. Med., 1905, 6, 487–518 CrossRef CAS PubMed.
- Y. Fu, Y. Zhu, S. Q. Shi and B. Goodell, Green Chem., 2022, 24, 6631–6638 RSC.
- A. Marsal, S. Cuadros, L. Ollé, A. Bacardit, A. M. Manich and J. Font, J. Cleaner Prod., 2018, 186, 45–56 CrossRef CAS.
- C. Demirkir and G. Çolakoglu, Holz Roh-Werkst., 2007, 65, 483–484 CrossRef CAS.
- M. Lee, B. G. Heikes, D. J. Jacob, G. Sachse and B. Anderson, J. Geophys. Res.: Atmos., 1997, 102, 1301–1309 CrossRef CAS.
- X. X. Liu, Y. B. Xiang, L. Yi, J. P. Wang, L. S. Jiang, Y. B. Li and X. Y. Wu, Sep. Purif. Technol., 2024, 348, 127719 CrossRef CAS.
- X. Q. Wu, Z. Li, J. Y. Tao, J. Zhao, Y. F. Xie and S. J. Zhao, J. Environ. Chem. Eng., 2024, 12, 111982 CrossRef CAS.
- W. Y. Hao, H. M. Zhang, Y. R. Teng, A. O. Ibhadon and F. Teng, ACS Appl. Energy Mater., 2023, 7, 72–83 CrossRef.
- P. Ezhilkumar, V. M. Sivakumar and M. Thirumarimurugan, Desalin. Water Treat., 2021, 243, 206–210 CrossRef CAS.
- A. Hosseinzadeh, A. A. Najafpoor, A. A. Navaei, J. L. Zhou, A. Altaee, N. Ramezanian, A. Dehghan, T. Bao and M. Yazdani, Water-Sui, 2021, 13, 2754 CAS.
- R. J. Yang, Q. Q. Chen, Y. Y. Ma, R. S. Zhu, Y. Y. Fan, J. Y. Huang, H. N. Niu, Y. Dong, D. Li, Y. F. Zhang, L. Mei, B. Y. Chen and Z. Y. Zeng, Chem. Eng. J., 2021, 423, 130164 CrossRef CAS.
- L. Du, W. Q. Gao, Z. X. Li, W. Z. Jiao and Y. Z. Liu, Chem. Eng. Process., 2020, 155, 108053 CrossRef CAS.
- X. Mei, Z. W. Guo, J. Liu, S. Q. Bi, P. P. Li, Y. Wang, W. T. Shen, Y. Yang, Y. H. Wang, Y. Y. Xiao, X. Yang, Y. Liu, L. Zhao, Y. T. Wang and S. Hu, Chem. Eng. J., 2019, 372, 673–683 CrossRef CAS.
- C. J. Wang, J. Wang, Z. W. Li and Z. Y. Wang, Oxid. Commun., 2015, 38, 2238–2244 CAS.
- O. J. Prado, M. C. Veiga and C. Kennes, Chemosphere, 2008, 70, 1357–1365 CrossRef CAS PubMed.
- Yangyue, P. C. Hou, X. R. Zhang, Y. H. He, J. M. Zhou, Liangshan, Chenjun and Z. X. Song, Chem. Eng. J., 2025, 503, 158317 CrossRef CAS.
- S. Javadian, M. Saraji and A. Shahvar, Microchim. Acta, 2024, 191, 329 CrossRef CAS.
- S. Y. An, Z. H. Zhao, J. Bu, J. X. He, W. X. Ma, J. Lin, R. Bai, L. Shang and J. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202318989 CrossRef CAS PubMed.
- V. Mohammadi and M. Saraji, Microchim. Acta, 2024, 191, 66 CrossRef CAS PubMed.
- O. P. Tal, A. Mizrahi, N. Lerner, S. Gelfer, I. Columbus and D. Mizrahi, J. Water Proc. Eng., 2022, 45, 102448 CrossRef.
- X. Mei, M. Y. Ma, Z. W. Guo, W. T. Shen, Y. Wang, L. J. Xu, Z. M. Zhang, Y. Ding, Y. Y. Xiao, X. Yang, R. Jiang, Y. Zhang, C. Q. Yin and Y. X. Wang, J. Environ. Chem. Eng., 2021, 9, 105955 CrossRef CAS.
- X. L. Wang and L. Zhang, IOP Conf. Ser.: Earth Environ. Sci., 2021, 647, 012155 CrossRef.
- Z. Q. Zhou, L. L. Zeng, G. W. Xiong, L. J. Yang, H. F. Yuan, J. Y. Yu, S. J. Xu, D. F. Wang, X. L. Zhang, H. Liu and W. J. Zhou, Chem. Eng. J., 2021, 426, 129214 CrossRef CAS.
- S. Arsawiset and S. Teepoo, Anal. Chim. Acta, 2020, 1118, 63–72 CrossRef CAS.
- A. Akyol, B. Samuk, M. Kobya and E. Demirbas, Sep. Sci. Technol., 2020, 55, 2830–2843 CrossRef CAS.
- D. D. Yuan, L. Tian, D. Gu, X. Y. Shen, L. Y. Zhu, H. J. Wu and B. H. Wang, J. Cleaner Prod., 2017, 156, 310–316 CrossRef CAS.
- M. H. G. Prechtl, L. E. Heim and N. E. Schloerer, Wastewater treatment and hydrogen production (patent), WO2015/003680A003681 and PCT/DE002014/000344, 2013 Search PubMed.
- Y. Yang, J. W. Liu, M. Ahmad, X. Q. Sun, C. H. Liu, S. J. Chen, J. J. Zhang, J. L. Luo and X. Z. Fu, Chem. Eng. J., 2025, 505, 159653 CrossRef CAS.
- T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature, 1979, 277, 637–638 CrossRef CAS.
- B. Aurianblajeni, M. Halmann and J. Manassen, Sol. Energy, 1980, 25, 165–170 CrossRef CAS.
- M. Halmann, Nature, 1978, 275, 115–116 CrossRef CAS.
- G. H. Qin, Y. Zhang, X. B. Ke, X. L. Tong, Z. Sun, M. Liang and S. Xue, Appl. Catal., B, 2013, 129, 599–605 CrossRef CAS.
- C. W. Kim, M. J. Kang, S. Ji and Y. S. Kang, ACS Catal., 2018, 8, 968–974 CrossRef CAS.
- N. Nalajala, K. N. Salgaonkar, I. Chauhan, S. P. Mekala and C. S. Gopinath, ACS Appl. Energ Mater., 2021, 4, 13347–13360 CrossRef CAS.
- W. Y. Diao, H. Y. Cai, L. Wang, X. Rao and Y. P. Zhang, ChemCatChem, 2020, 12, 5420–5429 CrossRef CAS.
- H. X. Liu, M. Wang, X. Q. Zhang, J. T. Ma and G. X. Lu, Appl. Catal., B, 2018, 237, 563–573 CrossRef CAS.
- K. Nakata, T. Ozaki, C. Terashima, A. Fujishima and Y. Einaga, Angew. Chem., Int. Ed., 2014, 53, 871–874 CrossRef CAS.
- M. Girardi, S. Blanchard, S. Griveau, P. Simon, M. Fontecave, F. Bedioui and A. Proust, Eur. J. Inorg. Chem., 2015, 3642–3648 CrossRef CAS.
- A. Singh, A. Zamader, R. Khakpour, K. Laasonen, M. Busch and M. Robert, J. Am. Chem. Soc., 2024, 146, 22129–22133 CrossRef CAS.
- K. Sasaki and S. Nagaura, Bull. Chem. Soc. Jpn., 1965, 38, 649–653 CrossRef CAS.
- H. J. Yu, L. J. Zhang, S. J. Jiang, W. K. Liu, K. Deng, Z. Q. Wang, Y. Xu, H. J. Wang and L. Wang, Small, 2024, 20, 2406107 CrossRef CAS PubMed.
- V. Buravets, K. M. Macounová, R. Nebel, M. Zukalová, L. Kavan and P. Krtil, Electrocatalysis, 2021, 12, 15–25 CrossRef CAS.
- S. K. Hassaninejad-Darzi, M. Rahimnejad, F. Shajie and A. H. S. Kootenaei, Iran. J. Sci. Technol., Trans. A: Sci., 2018, 42, 1259–1268 CrossRef.
- Y. Yang, X. X. Wu, M. Ahmad, F. Z. Si, S. J. Chen, C. H. Liu, Y. Zhang, L. Wang, J. J. Zhang, J. L. Luo and X. Z. Fu, Angew. Chem., Int. Ed., 2023, 62, e202302950 CrossRef CAS PubMed.
- Y. Zhang, X. Zhu, J. Wu, Z. Liao, S. Song, Z. Ren and J. Chen, J. Colloid Interface Sci., 2025, 683, 964–972 CrossRef CAS.
- Z. Gao, P. Xue, H. Wang, Y. Wu and K. Shi, J. Electrochem. Soc., 2023, 170, 105501 CrossRef CAS.
- W. C. Liao, Y. W. Chen, Y. C. Liao, X. Y. Lin, S. Yau, J. J. Shyue, S. Y. Wu and H. T. Chen, Electrochim. Acta, 2020, 333, 135542 CrossRef CAS.
- Y. Wang, Y. Lan, D. Bu, B. Qian, Y. Wang, B. Wang, Q. Wu, S. Li, Y. Zhang and X.-M. Song, Electrochim. Acta, 2020, 352, 136476 CrossRef CAS.
- S. Daemi, M. Moalem-Banhangi, S. Ghasemi and A. A. Ashkarran, J. Electroanal. Chem., 2019, 848, 113270 CrossRef CAS.
- M. Rasmussen, S. Abdellaoui and S. D. Minteer, Biosens. Bioelectron., 2016, 76, 91–102 CrossRef CAS PubMed.
- P. Dürre and B. J. Eikmanns, Curr. Opin. Biotechnol, 2015, 35, 63–72 CrossRef.
- J. Filip and J. Tkac, Chem. Listy, 2014, 108, 442–450 CAS.
- S. K. Hassaninejad–Darzi, J. Electroceram., 2014, 33, 252–263 CrossRef.
- S. A. Neto, J. C. Forti and A. R. De Andrade, Electrocatalysis, 2010, 1, 87–94 CrossRef CAS.
- A. Boddien, B. Loges, H. Junge and M. Beller, ChemSusChem, 2008, 1, 751–758 CrossRef CAS.
- Q. Liu, X. H. Xu, G. L. Ren and W. Wang, Prog. Chem., 2006, 18, 1530–1537 CAS.
- W. R. Wolfe and K. B. Keating, J. Electrochem. Soc., 1974, 121, 1125 CrossRef CAS.
- K. J. Cathro, J. Electrochem. Soc., 1971, 118, 1523 CrossRef CAS.
- K. J. Cathro and C. H. Weeks, Energy Convers., 1971, 11, 133–141 CrossRef CAS.
- S. V. W. B. Oliveira, E. M. Moraes, M. A. T. Adorno, M. B. A. Varesche, E. Foresti and M. Zaiat, Water Res., 2004, 38, 1685–1694 CrossRef CAS PubMed.
- S. Mirdamadi, A. Rajabi, P. Khalilzadeh, D. Norozian, A. Akbarzadeh and F. A. Mohseni, World J. Microbiol. Biotechnol., 2005, 21, 1299–1301 CrossRef CAS.
- A. Habibi and F. Vahabzadeh, Biotechnol. Bioprocess Eng., 2013, 18, 455–464 CrossRef CAS.
- L. Qiu, W. Chen, L. Zhong, W. Wu, S. Wu, J. Chen, F. Zhang and W. Zhong, Bioprocess Biosyst. Eng., 2014, 37, 1377–1384 CrossRef CAS PubMed.
- Y. Zvulunov, Z. Ben-Barak-Zelas, A. Fishman and A. Radian, Chem. Eng. J., 2019, 374, 1275–1285 CrossRef CAS.
- N. Kato, K. Shirakawa, H. Kobayashi and C. Sakazawa, Agric. Biol. Chem., 1983, 47, 39–46 CAS.
- N. Tanaka, Y. Kusakabe, K. Ito, T. Yoshimoto and K. T. Nakamura, J. Mol. Biol., 2002, 324, 519–533 CrossRef CAS PubMed.
- B. X. Guo, X. L. Ji, Y. J. Xue and Y. H. Huang, Green Chem., 2024, 26, 11540–11547 RSC.
- N. Adroer, C. Casas, C. de Mas and C. Solá, Appl. Microbiol. Biotechnol., 1990, 33, 217–220 CrossRef CAS PubMed.
- Z. Zhai, Y. Li, Z. Li, J. Wang, Y. Li and Y.-H. P. J. Zhang, ChemSusChem, 2025, 18, e202402711 CrossRef CAS.
- L. E. Heim, D. Thiel, C. Gedig, J. Deska and M. H. G. Prechtl, Angew. Chem., Int. Ed., 2015, 54, 10308–10312 CrossRef CAS.
- G. Tavakoli, J. E. Armstrong, J. M. Naapuri, J. Deska and M. H. G. Prechtl, Chem. – Eur. J., 2019, 25, 6474–6481 CrossRef CAS PubMed.
- M. Moon, G. W. Park, J.-P. Lee, J.-S. Lee and K. Min, J. CO2 Util., 2020, 42, 101353 CrossRef CAS.
- A. Rodil, I. v Ossowski, M. Nyyssönen, Y. Tian, M. Hallamaa, J. Deska, M. Bomberg and S. Scheller, RSC Sustainability, 2024, 2, 3264–3275 RSC.
- Y. Amao, J. CO2 Util., 2018, 26, 623–641 CrossRef CAS.
- X. Wang, Z. Li, J. Shi, H. Wu, Z. Jiang, W. Zhang, X. Song and Q. Ai, ACS Catal., 2014, 4, 962–972 CrossRef CAS.
- R. Obert and B. C. Dave, J. Am. Chem. Soc., 1999, 121, 12192–12193 CrossRef CAS.
- Z. Zhang, B.-h Xu, J. Luo, N. V. Solms, H. He, Y. Zhang, M. Pinelo and S. Zhang, Catalysts, Editon edn, 2018, vol. 8 Search PubMed.
- M. Guo, F. Gu, L. Meng, Q. Liao, Z. Meng and W. Liu, Sep. Purif. Technol., 2022, 286, 120480 CrossRef CAS.
- T.-K. Le, Y.-J. Lee, G. H. Han and S.-J. Yeom, Front. Bioeng. Biotechnol., 2021, 9, 787791 CrossRef PubMed.
- R. Couderc and J. Baratti, Agric. Biol. Chem., 1980, 44, 2279–2289 CAS.
- J. A. Hughes and M. Jay, Nucl. Med. Biol., 1995, 22, 105–109 CrossRef CAS PubMed.
- B. Goldfuss and M. Schumacher, J. Mol. Model., 2006, 12, 591–595 CrossRef CAS PubMed.
- E. J. Corey and D. E. Cane, J. Org. Chem., 1971, 36, 3070 CrossRef CAS.
- M. S. Peters and C. R. Cupit, Chem. Eng. Sci., 1959, 10, 57–67 CrossRef CAS.
- F. Zhang, C. Liang, Z. Wang and H. Li, ChemCatChem, 2018, 10, 818–824 CrossRef CAS.
- V. K. Aggarwal, D. K. Dean, A. Mereu and R. Williams, J. Org. Chem., 2002, 67, 510–514 CrossRef CAS.
- J. Pietruszka and M. Schölzel, Adv. Synth. Catal., 2012, 354, 751–756 CrossRef CAS.
- E. A. Mash and T. M. Gregg, J. Org. Chem., 1995, 60, 6180–6182 CrossRef CAS.
- W. Huang and J. Xu, Synth. Commun., 2015, 45, 1777–1782 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, A. V. Hara Gopal, G. G. K. S. Narayana Kumar, C. Madavi and A. C. Kunwar, Tetrahedron Lett., 2008, 49, 4420–4423 CrossRef CAS.
- L. F. Silva and S. A. Quintiliano, Tetrahedron Lett., 2009, 50, 2256–2260 CrossRef CAS.
- H. A. Bates, J. Org. Chem., 1981, 46, 4931–4935 CrossRef CAS.
- C. I. Attorresi, J. A. Ramírez and B. Westermann, Beilstein J. Org. Chem., 2025, 21, 564–595 CrossRef CAS PubMed.
- C. Liu, W. Huang, J. Zhang, Z. Rao, Y. Gu and F. Jérôme, Green Chem., 2021, 23, 1447–1465 RSC.
- C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51–80 CrossRef CAS.
- H. G. Dai, J. T. Li and T. S. Li, Synth. Commun., 2006, 36, 1829–1835 CrossRef CAS.
- K. Jayakanthan and Y. D. Vankar, Org. Lett., 2005, 7, 5441–5444 CrossRef CAS PubMed.
- S. Hosokawa, K. Nakanishi, Y. Udagawa, M. Maeda, S. Sato, K. Nakano, T. Masuda and Y. Ichikawa, Org. Biomol. Chem., 2020, 18, 687–693 RSC.
- S. Meninno and A. Lattanzi, Chem. Rec., 2016, 16, 2016–2030 CrossRef CAS.
- S. Shirakawa, K. Ota, S. J. Terao and K. Maruoka, Org. Biomol. Chem., 2012, 10, 5753–5755 RSC.
- N. Mase, A. Inoue, M. Nishio and K. Takabe, Bioorg. Med. Chem. Lett., 2009, 19, 3955–3958 CrossRef CAS.
- A. Erkkilä and P. M. Pihko, J. Org. Chem., 2006, 71, 2538–2541 CrossRef.
- M. S. Kutwal, S. Dev and C. Appayee, Org. Lett., 2019, 21, 2509–2513 CrossRef CAS PubMed.
- H. Sundén, I. Ibrahem, L. Eriksson and A. Córdova, Angew. Chem., Int. Ed., 2005, 44, 4877–4880 CrossRef PubMed.
- J. Henrique Teles, J.-P. Melder, K. Ebel, R. Schneider, E. Gehrer, W. Harder, S. Brode, D. Enders, K. Breuer and G. Raabe, Helv. Chim. Acta, 1996, 79, 61–83 CrossRef.
- T. Matsumoto, M. Ohishi and S. Inoue, J. Org. Chem., 1985, 50, 603–606 CrossRef CAS.
- N. Kuhl and F. Glorius, Chem. Commun., 2010, 47, 573–575 RSC.
- J. Zhang, C. Xing, B. Tiwari and Y. R. Chi, J. Am. Chem. Soc., 2013, 135, 8113–8116 CrossRef CAS.
- R. Xiao and R. He, in Formaldehyde and Cognition, ed. R. He, Springer, Netherlands, Dordrecht, Editon edn, 2017, pp. 21–46 Search PubMed.
- M. R. Antoniewicz, Curr. Opin. Biotechnol, 2019, 59, 165–174 CrossRef CAS PubMed.
- S. N. Lindner and M. Ralser, PLoS Biol., 2025, 23, e3002996 CrossRef CAS.
- K. Schann and S. Wenk, Sustain. Microbiol., 2024, 1, qvae024 CrossRef.
- A. R. Pearson and C. M. Wilmot, Biochim. Biophys. Acta, Proteins Proteomics, 2003, 1647, 381–389 CrossRef CAS.
- S. Benjakul, W. Visessanguan and M. Tanaka, Food Chem., 2004, 84, 297–305 CrossRef CAS.
- S. Bierbaumer, M. Nattermann, L. Schulz, R. Zschoche, T. J. Erb, C. K. Winkler, M. Tinzl and S. M. Glueck, Chem. Rev., 2023, 123, 5702–5754 CrossRef CAS.
- H. Wu and D. Voiry, Nat. Catal., 2025, 8, 415–416 CrossRef CAS.
- M. Du, J. Zheng, L. Mei, Y. Zhang and C. Hou, Mol. Catal., 2022, 530, 112630 CAS.
- V. N. Pham, K. J. Bruemmer, J. D. W. Toh, E. J. Ge, L. Tenney, C. C. Ward, F. A. Dingler, C. L. Millington, C. A. Garcia-Prieto, M. C. Pulos-Holmes, N. T. Ingolia, L. B. Pontel, M. Esteller, K. J. Patel, D. K. Nomura and C. J. Chang, e, Scienc, 2023, 382, eabp9201 CrossRef CAS PubMed.
- G. Ramirez-Hernandez and N. Kory, Mol. Cell, 2024, 84, 20–22 CrossRef CAS.
- G. La Torre, T. Vitello, R. A. Cocchiara and C. Della Rocca, Public Health, 2023, 218, 186–196 CrossRef CAS PubMed.
- G. Burgos-Barragan, N. Wit, J. Meiser, F. A. Dingler, M. Pietzke, L. Mulderrig, L. B. Pontel, I. V. Rosado, T. F. Brewer, R. L. Cordell, P. S. Monks, C. J. Chang, A. Vazquez and K. J. Patel, Nature, 2017, 548, 549–554 CrossRef CAS PubMed.
- R. J. Hopkinson and C. J. Schofield, Biochemistry, 2018, 57, 904–906 CrossRef CAS.
- M. J. Solomon and A. Varshavsky, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 6470–6474 CrossRef CAS PubMed.
- B. Metz, G. F. A. Kersten, P. Hoogerhout, H. F. Brugghe, H. A. M. Timmermans, A. de Jong, H. Meiring, J. ten Hove, W. E. Hennink, D. J. A. Crommelin and W. Jiskoot, J. Biol. Chem., 2004, 279, 6235–6243 CrossRef CAS PubMed.
- J. J. A. G. Kamps, R. J. Hopkinson, C. J. Schofield and T. D. W. Claridge, Commun. Chem., 2019, 2, 126 CrossRef.
- T. Tayri-Wilk, M. Slavin, J. Zamel, A. Blass, S. Cohen, A. Motzik, X. Sun, D. E. Shalev, O. Ram and N. Kalisman, Nat. Commun., 2020, 11, 3128 CrossRef CAS PubMed.
- N. H. Chen, K. Y. Djoko, F. J. Veyrier and A. G. McEwan, Front. Microbiol., 2016, 7, 257 Search PubMed.
- H. Yurimoto, N. Kato and Y. Sakai, Chem. Rec., 2005, 5, 367–375 CrossRef CAS PubMed.
- W. Zhang, T. Zhang, S. Wu, M. Wu, F. Xin, W. Dong, J. Ma, M. Zhang and M. Jiang, RSC Adv., 2017, 7, 4083–4091 RSC.
- M. Jia, M. Liu, J. Li, W. Jiang, F. Xin, W. Zhang, Y. Jiang and M. Jiang, ACS Synth. Biol., 2024, 13, 3507–3522 CrossRef CAS PubMed.
- Y. Li, Y. Fan, P. Yao, Q. Wu and D. Zhu, ChemCatChem, 2025, 17, e202402005 CrossRef CAS.
- R. Marín-Valls, K. Hernández, M. Bolte, T. Parella, J. Joglar, J. Bujons and P. Clapés, J. Am. Chem. Soc., 2020, 142, 19754–19762 CrossRef.
- A. S. Demir, P. Ayhan, A. C. Igdir and A. N. Duygu, Tetrahedron, 2004, 60, 6509–6512 CrossRef CAS.
- S. Desmons, R. Fauré and S. Bontemps, ACS Catal., 2019, 9, 9575–9588 CrossRef CAS.
- J. B. Siegel, A. L. Smith, S. Poust, A. J. Wargacki, A. Bar-Even, C. Louw, B. W. Shen, C. B. Eiben, H. M. Tran, E. Noor, J. L. Gallaher, J. Bale, Y. Yoshikuni, M. H. Gelb, J. D. Keasling, B. L. Stoddard, M. E. Lidstrom and D. Baker, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 3704–3709 CrossRef CAS PubMed.
- J. Yang, S. Sun, Y. Men, Y. Zeng, Y. Zhu, Y. Sun and Y. Ma, Catal. Sci. Technol., 2017, 7, 3459–3463 RSC.
- C. Bou-Nader, F. W. Stull, L. Pecqueur, P. Simon, V. Guérineau, A. Royant, M. Fontecave, M. Lombard, B. A. Palfey and D. Hamdane, Nat. Commun., 2021, 12, 4542 CrossRef CAS PubMed.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688–6699 RSC.
- A. Trowbridge, D. Reich and M. J. Gaunt, Nature, 2018, 561, 522–527 CrossRef CAS PubMed.
- L. Caiger, C. Sinton, T. Constantin, J. J. Douglas, N. S. Sheikh, F. Juliá and D. Leonori, Chem. Sci., 2021, 12, 10448–10454 RSC.
- M. Jiang, H. Yang, Q. Lefebvre, J. Su and H. Fu, iScience, 2018, 6, 102–113 CrossRef CAS PubMed.
- G. W. Kabalka, L. L. Zhou, L. Wang and R. M. Pagni, Tetrahedron, 2006, 62, 857–867 CrossRef CAS.
- P. Crabbe, H. Fillion, D. Andre and J. L. Luche, J. Chem. Soc., Chem. Commun., 1979, 859–860 RSC.
- H. W. Luo and S. M. Ma, Eur. J. Org. Chem., 2013, 3041–3048 CrossRef CAS.
- S. C. Yu and S. M. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074–3112 CrossRef CAS PubMed.
- S. K. Kundu, K. Mitra and A. Majee, RSC Adv., 2015, 5, 13220–13223 RSC.
- M. Tramontini, Synthesis, 1973, 703–775 CrossRef CAS.
- Y. Watanabe, Y. Shimizu, K. Takatsuki and Y. Takegami, Chem. Lett., 1978, 215–216 CrossRef CAS.
- Y. Watanabe, M. Yamamoto, T. Mitsudo and Y. Takegami, Tetrahedron Lett., 1978, 1289–1290 CrossRef CAS.
- Y. Sugi, A. Matsuda, K. I. Bando and K. Murata, Chem. Lett., 1979, 363–364 CrossRef CAS.
- S. Sakai, Y. Kawashima, Y. Takahashi and Y. Ishii, Chem. Commun., 1967, 1073–1074 RSC.
- V. Paunović, P. Hemberger, A. Bodi, R. Hauert and J. A. van Bokhoven, ACS Catal., 2022, 12, 13426–13434 CrossRef.
- K. Natte, H. Neumann, R. V. Jagadeesh and M. Beller, Nat. Commun., 2017, 8, 1344 CrossRef PubMed.
- Y. J. Xie, J. H. Hu, Y. Y. Wang, C. G. Xia and H. M. Huang, J. Am. Chem. Soc., 2012, 134, 20613–20616 CrossRef CAS.
- A. Kopfer, B. Sam, B. Breit and M. J. Krische, Chem. Sci., 2013, 4, 1876–1880 RSC.
- M. Kimura and Y. Tamaru, Top. Curr. Chem., 2007, 279, 173–207 CrossRef CAS.
- S. Ogoshi, K. Tonomori, M. Oka and H. Kurosawa, J. Am. Chem. Soc., 2006, 128, 7077–7086 CrossRef CAS PubMed.
- T. Smejkal, H. Han, B. Breit and M. J. Krische, J. Am. Chem. Soc., 2009, 131, 10366–10367 CrossRef CAS PubMed.
- H. Han and M. J. Krische, Org. Lett., 2010, 12, 2844–2846 CrossRef CAS PubMed.
- B. Marinic, H. B. Hepburn, A. Grozavu, M. Dow and T. J. Donohoe, Chem. Sci., 2021, 12, 742–746 RSC.
- Y. Ito, M. Sawamura, M. Kobayashi and T. Hayashi, Tetrahedron Lett., 1988, 29, 6321–6324 CrossRef CAS.
- R. Kuwano, H. Miyazaki and Y. Ito, J. Organomet. Chem., 2000, 603, 18–29 CrossRef CAS.
- I. Fukuchi, Y. Hamashima and M. Sodeoka, Adv. Synth. Catal., 2007, 349, 509–512 CrossRef CAS.
- M. H. G. Prechtl, J. D. Scholten and J. Dupont, Molecules, 2010, 15, 3441–3461 CrossRef CAS PubMed.
- M. H. G. Prechtl, J. D. Scholten, B. A. D. Neto and J. Dupont, Curr. Org. Chem., 2009, 13, 1259–1277 CrossRef CAS.
- J. Dupont, B. C. Leal, P. Lozano, A. L. Monteiro, P. Migowski and J. D. Scholten, Chem. Rev., 2024, 124, 5227–5420 CrossRef CAS PubMed.
- P. Migowski, P. Lozano and J. Dupont, Green Chem., 2023, 25, 1237–1260 RSC.
- P. Migowski and J. Dupont, Chem. – Eur. J., 2007, 13, 32–39 CrossRef CAS PubMed.
- V. R. Akhmetova, E. M. Bikbulatova, N. S. Akhmadiev, N. F. Galimzyanova, R. V. Kunakova and A. G. Ibragimov, Chem. Heterocycl. Compd., 2018, 54, 520–527 CrossRef CAS.
- G. R. Khabibullina, F. T. Zaynullina, T. V. Tyumkina, V. M. Yanybin and A. G. Ibragimov, Russ. Chem. Bull., 2019, 68, 1407–1413 CrossRef CAS.
- B. A. L. Sacchelli, S. M. P. Onguene, R. S. M. Almeida, A. M. M. Antunes, D. S. Nesterov, L. H. Andrade, E. Alegria and M. H. G. Prechtl, Catal. Sci. Technol., 2024, 14, 1512–1523 RSC.
- Z. Sun, G. Bottari and K. Barta, Green Chem., 2015, 17, 5172–5181 RSC.
- S. Yu, C. Hong, Z. Liu and Y. Zhang, Org. Lett., 2021, 23, 5054–5059 CrossRef CAS PubMed.
- S. Yu, N. Lv, C. Hong, Z. Liu and Y. Zhang, Org. Lett., 2020, 22, 8354–8358 CrossRef CAS PubMed.
- H. D. Feng, H. H. Jia and Z. H. Sun, Adv. Synth. Catal., 2015, 357, 2447–2452 CrossRef CAS.
- W. F. Lia and X. F. Wu, Adv. Synth. Catal., 2015, 357, 3393–3418 CrossRef.
- S. Chakrabortty, A. A. Almasalma and J. G. de Vries, Catal. Sci. Technol., 2021, 11, 5388–5411 RSC.
- T. Morimoto, T. Fujii, K. Miyoshi, G. Makado, H. Tanimoto, Y. Nishiyama and K. Kakiuchi, Org. Biomol. Chem., 2015, 13, 4632–4636 RSC.
- D. U. Nielsen, K. T. Neumann, A. T. Lindhardt and T. Skrydstrup, J. Labelled Compd. Radiopharm., 2018, 61, 949–987 CrossRef CAS PubMed.
- R. Geitner, A. Gurinov, T. B. Huang, S. Kupfer, S. Grafe and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2021, 60, 3422–3427 CrossRef CAS PubMed.
- T. Meyer, R. Konrath, P. C. J. Kamer and X. F. Wu, Asian J. Org. Chem., 2021, 10, 245–250 CrossRef CAS.
- H. S. Ahn, S. H. Han, S. J. Uhm, W. K. Seok, H. N. Lee and G. A. Korneeva, J. Mol. Catal. A: Chem., 1999, 144, 295–306 CrossRef CAS.
- G. Makado, T. Morimoto, Y. Sugimoto, K. Tsutsumi, N. Kagawa and K. Kakiuchi, Adv. Synth. Catal., 2010, 352, 299–304 CrossRef CAS.
- E. Cini, E. Airiau, N. Girard, A. Mann, J. Salvadori and M. Taddei, Synlett, 2011, 199–202 CAS.
- M. Uhlemann, S. Doerfelt and A. Borner, Tetrahedron Lett., 2013, 54, 2209–2211 CrossRef CAS.
- T. Furusawa, T. Morimoto, N. Oka, H. Tanimoto, Y. Nishiyama and K. Kakiuchi, Chem. Lett., 2016, 45, 406–408 CrossRef CAS.
- S. P. Midya, M. K. Sahoo, V. G. Landge, P. R. Rajamohanan and E. Balaraman, Nat. Commun., 2015, 6, 8591 CrossRef.
- X. F. Xu, Y. R. Yang, X. Chen, X. Zhang and W. Yi, Org. Biomol. Chem., 2017, 15, 9061–9065 RSC.
- Y. B. Shen, Y. L. Zhan, S. P. Li, F. D. Ning, Y. Du, Y. J. Huang, T. He and X. C. Zhou, Chem. Sci., 2017, 8, 7498–7504 RSC.
- Y. B. Shen, Y. Xu and Y. L. Zhan, ChemPhysChem, 2023, 24, e202200695 CrossRef CAS.
- Y. L. Zhan, S. X. Zhou, Y. Xu and Y. B. Shen, Catal. Lett., 2024, 154, 808–815 CrossRef CAS.
- Y. B. Shen, L. Q. Wang, Z. W. Xu, F. D. Ning, Y. L. Zhan, C. Bai and X. C. Zhou, ChemSusChem, 2021, 14, 3867–3875 CrossRef CAS PubMed.
- Y. B. Shen, Z. W. Xu, L. Q. Wang and Y. L. Zhan, Green Chem., 2021, 23, 5618–5624 RSC.
- J. Duan, A. Veliju, O. Lampret, L. Liu, S. Yadav, U.-P. Apfel, F. A. Armstrong, A. Hemschemeier and E. Hofmann, J. Am. Chem. Soc., 2023, 145, 26068–26074 CrossRef CAS.
- S. V. Vassilev, D. Baxter, L. K. Andersen and C. G. Vassileva, Fuel, 2010, 89, 913–933 CrossRef CAS.
- J. Kumar and S. Vyas, Environ. Dev. Sustain., 2025, 27, 1–40 Search PubMed.
- L. Petrus and M. A. Noordermeer, Green Chem., 2006, 8, 861–867 RSC.
- H. Akinosho, K. Yee, D. Close and A. Ragauskas, Front. Chem., 2014, 2, 66 Search PubMed.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 8164–8215 CrossRef CAS PubMed.
- C. W. Lahive, P. J. Deuss, C. S. Lancefield, Z. Sun, D. B. Cordes, C. M. Young, F. Tran, A. M. Z. Slawin, J. G. de Vries, P. C. J. Kamer, N. J. Westwood and K. Barta, J. Am. Chem. Soc., 2016, 138, 8900–8911 CrossRef CAS.
- L. Shuai, M. T. Amiri, Y. M. Questell-Santiago, F. Héroguel, Y. Li, H. Kim, R. Meilan, C. Chapple, J. Ralph and J. S. Luterbacher, Science, 2016, 354, 329–333 CrossRef CAS.
- M. Tasooji and C. E. Frazier, ACS Sustainable Chem. Eng., 2021, 9, 207–215 CrossRef CAS.
- G. Wan and C. E. Frazier, ACS Sustainable Chem. Eng., 2017, 5, 4830–4836 CrossRef CAS.
- X. M. Huang, T. I. Koranyi, M. D. Boot and E. J. M. Hensen, Green Chem., 2015, 17, 4941–4950 RSC.
- W. Jiang, D. Hernández Villamor, H. Peng, J. Chen, L. Liu, V. Haritos and R. Ledesma-Amaro, Nat. Chem. Biol., 2021, 17, 845–855 CrossRef CAS.
- R. Holzinger, C. Warneke, A. Hansel, A. Jordan, W. Lindinger, D. H. Scharffe, G. Schade and P. J. Crutzen, Geophys. Res. Lett., 1999, 26, 1161–1164 CrossRef CAS.
- M. Liu, H. Liu, N. Li, C. Zhang, J. Zhang and F. Wang, ChemSusChem, 2022, 15, e202201068 CrossRef CAS.
- H. Fan, J. Su, E. Zhao, Y. Zheng and Z. Chen, J. Colloid Interface Sci., 2025, 684, 140–147 CrossRef CAS PubMed.
- W.-M. Zhang, K.-W. Feng, R.-G. Hu, Y.-J. Guo and Y. Li, Chem, 2023, 9, 430–442 CAS.
- H. Yan, B. Liu, X. Zhou, F. Meng, M. Zhao, Y. Pan, J. Li, Y. Wu, H. Zhao, Y. Liu, X. Chen, L. Li, X. Feng, D. Chen, H. Shan, C. Yang and N. Yan, Nat. Commun., 2023, 14, 4509 CrossRef CAS.
- O. Yishai, S. N. Lindner, J. Gonzalez de la Cruz, H. Tenenboim and A. Bar-Even, Curr. Opin. Chem. Biol., 2016, 35, 1–9 CrossRef CAS.
- Z. He, Y. Hou, J. Wei, S. Ren and W. Wu, Green Chem., 2024, 26, 2170–2182 RSC.
- B. Kazmi, R. Shareef, S. Noman, S. Saeed, T. Zehra, Z. Masood, G. Albasher and D. Juchelková, Process Saf. Environ. Prot., 2024, 186, 969–979 CrossRef CAS.
- G. J. Gregory, R. K. Bennett and E. T. Papoutsakis, Metab. Eng., 2022, 71, 99–116 CrossRef CAS PubMed.
- Y. Liu, F. M. Kirchberger, S. Müller, M. Eder, M. Tonigold, M. Sanchez-Sanchez and J. A. Lercher, Nat. Commun., 2019, 10, 1462 CrossRef PubMed.
- T. Wu, P. A. Gómez-Coronado, A. Kubis, S. N. Lindner, P. Marlière, T. J. Erb, A. Bar-Even and H. He, Nat. Commun., 2023, 14, 8490 CrossRef CAS PubMed.
- D. Zhao, W. L. Yang, G. Shen, W. H. Zhang and H. X. Feng, Colloid Polym. Sci., 2024, 302, 1815–1830 CrossRef CAS.
- Z. L. Li, Z. J. Zhao, X. J. Jiang, Y. J. Fu, G. Y. Tian and Z. J. Wang, Ceram. Int., 2024, 50, 34510–34518 CrossRef CAS.
- L. Zhao, W. J. Li, Y. Cheng, J. W. Zhao, D. Tian, M. Huang and F. Shen, Ind. Crops Prod., 2024, 211, 118168 CrossRef CAS.
- J. M. Pérez and A. Fernández, J. Appl. Polym. Sci., 2012, 123, 3036–3045 CrossRef.
- Z. Wang, J. W. Xue, J. B. Qu and W. X. Liu, Adv. Mater. Res., 2012, 560–561, 242–246 CAS.
- A. R. Mahendran, G. Wuzella and A. Kandelbauer, J. Adhes. Sci. Technol., 2010, 24, 1553–1565 CrossRef CAS.
- B. Danielson and R. Simonson, J. Adhes. Sci. Technol., 1998, 12, 941–946 CrossRef CAS.
- B. Danielson and R. Simonson, J. Adhes. Sci. Technol., 1998, 12, 923–939 CrossRef CAS.
- R. S. J. Piccolo, F. Santos and E. Frollini, J. Macromol. Sci., Part A: Pure Appl. Chem., 1997, A34, 153–164 CrossRef CAS.
- A. A. M. A. Nada, H. Elsaied, M. H. Fadl and M. A. Nasar, Polym.-Plast. Technol. Eng., 1994, 33, 515–536 CrossRef CAS.
- A. O. Barry, W. Peng and B. Riedl, Holzforschung, 1993, 47, 247–252 CrossRef CAS.
- B. Klasnja and S. Kopitovic, Holz Roh-Werkst., 1992, 50, 282–285 CrossRef CAS.
- C. Y. Hse and Q. Q. Hong, ACS Symp. Ser., 1989, 385, 96–109 CrossRef CAS.
- A. M. A. Nada, H. Elsaied, A. A. Ibrahem and M. A. Yousef, J. Appl. Polym. Sci., 1987, 33, 2915–2924 CrossRef CAS.
- H. Elsaied, A. M. Nada, A. A. Ibrahem and M. A. Yousef, Angew. Makromol. Chem., 1984, 122, 169–181 CrossRef CAS.
- P. C. Muller, S. S. Kelley and W. G. Glasser, Abstr. Pap. Am. Chem. Soc., 1984, 188, 78-Cell Search PubMed.
- R. C. Gupta and V. K. Sehgal, Holzforsch Holzverw, 1979, 31, 7–9 CAS.
- E. Roffael, W. Rauch and S. Beyer, Holz Roh-Werkst., 1974, 32, 469–472 CrossRef CAS.
- E. Roffael, W. Rauch and S. Beyer, Holz Roh-Werkst., 1974, 32, 225–228 CrossRef CAS.
- Y. Toenges, V. Dieterich, S. Fendt, H. Spliethoff and J. Burger, Fuels, 2023, 4, 1–18 CrossRef CAS.
- H. J. Liu, J. Fan, W. P. Liu, Y. Wang, Q. H. Ai and Y. L. Li, Separations, 2025, 12(1), 12 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |









