 Open Access Article
Open Access ArticleMain advances in the application of scorpionate-based catalytic systems for the preparation of sustainable polymers
Luis F.
Sánchez-Barba
 *a,
Andrés
Garcés
*a,
Andrés
Garcés
 *a,
Agustín
Lara-Sánchez
*a,
Agustín
Lara-Sánchez
 *b,
Marta
Navarro
*b,
Marta
Navarro
 ab and
David
González-Lizana
a
ab and
David
González-Lizana
a
aUniversidad Rey Juan Carlos, Departamento de Biología y Geología, Física y Química Inorgánica, Móstoles, 28933, Madrid, Spain. E-mail: luisfernando.sanchezbarba@urjc.es; andres.garces@urjc.es
bUniversidad de Castilla-La Mancha, Departamento de Química Inorgánica, Orgánica y Bioquímica-Centro de Innovación en Química Avanzada (ORFEO-CINQA), Campus Universitario, 13071-Ciudad Real, Spain. E-mail: agustin.lara@uclm.es
First published on 6th December 2024
Abstract
Scorpionate ligands have emerged as pivotal components in the field of coordination chemistry and catalysis since the seminal work by Trofimenko in the late 1960s. These species have demonstrated an extraordinarily rich tridentate coordination chemistry, enhancing the stability of metal complexes. In addition, they offer the possibility of modifying the chemical and electronical features as κ3-ligands, providing a wide variety of potential substrates with multiple donor atoms. Furthermore, this type of ligand has shown wide versatility in its coordination mode and can adopt different binding arrangements, expanding its potential as a universal ligand. This review provides a comprehensive overview of the main advances in exploring scorpionate complexes based on the tris(1H-pyrazol-1-yl)borate and bis(1H-pyrazol-1-yl)methane moieties, which have been recently reported as efficient catalysts for the synthesis of sustainable polymers. Specifically, this work focuses on the preparation of biorenewable polylactides (PLAs), other polyesters and polycarbonates (PCs), derived from cyclohexene carbonate, polylactide-co-polycarbonate copolymers and alternative sustainable polymeric materials. Thus, we have faced this challenge by selecting and classifying the most well-performed scorpionate catalyst system, including divalent (magnesium, calcium, zinc and iron) and other metals (rare-earth metals and zirconium), for each of the catalytic processes mentioned above. This review represents the first contribution that summarises and illustrates the current state of the art related to the use of scorpionate-based systems as efficient catalysts for the preparation of sustainable polymer materials. This account finally aims to guide future research towards the development of more eco-friendly catalytic processes in promoting sustainable polymers to achieve relevant commodities.
Introduction
Since the pioneering work by Trofimenko in the late 1960s1,2 and later,3–5 scorpionate ligands, particularly poly(1H-pyrazol-1-yl)-borates (B-scorpionates) and methanes (C-scorpionates) (see Chart 1), have emerged as a highly popular class of ligands with an extraordinarily rich coordination chemistry.In addition, these ligands have proved to be highly efficient in a wide variety of applications in several hot topics.6–18 In this sense, relevant examples include homogeneous and supported catalysis, organic transformations, template synthesis, metalloenzyme modelling and advanced materials.
The main feature of these ligands is their tridentate coordination mode that resembles, as Trofimenko described for the tris(1H-pyrazol-1-yl)borates, “the action of a scorpion catching and stinging its prey with its pincers and tail.” These similarities gave, as a result, the so-called designation of “scorpionate ligands”. These platforms have also sufficiently demonstrated remarkable versatility and stability in binding to various metal centres,6–19 facilitating their use in numerous catalytic transformations.20
For instance, B-scorpionate coordination chemistry (see Chart 1) has achieved spectacular success.3–5,8,9,16–22 It began with the coordination of tris(1H-pyrazol-1-yl)borates to metal centres, combining features of cyclopentadienyl and β-diketonate ligands.2 Furthermore, a large number of substituted pyrazoles have been synthesised through straightforward procedures,8 given as a result one of the most versatile classes of ancillary ligands. This possibility allows different steric and electronic effects at the central atom by simply altering the nature, number or position of substituents on the pyrazolyl rings (see Chart 1). As a result, the reactivity of the corresponding metal complexes can be fine-tuned.
On the other hand, in 1937 Huckel et al.23 reported for the first time the preparation of potential C-scorpionate ligands (see Chart 1), which have been clearly less popular than their boron anionic counterparts. However, synthetic advancements in the past decades introduced by Elguero et al.24 and Reger et al.25 led to the development of countless novel functionalised C-scorpionates,6–15 which definitively highlighted their potential in coordination chemistry. Currently, there are focused investigations to prepare more sustainable synthetic pathways as well as the tuning of steric and electronic properties of C-scorpionates. These advantages are critical for specific applications and are consistently contributing to substantial advancements across multiple domains of chemistry.
Scorpionate-based complexes have also confirmed their ability to facilitate a variety of catalytic transformations. Very interesting examples include the oxidation of light alkanes or more reactive alkenes or alcohols,26–29 C–X (X = C, N, O or S) bond formation,17,30,31 CO2 activation,32,33 ring-opening (co) polymerisation34–36 or olefin polymerisation reactions,9,37–39 among others. All these examples operate with high selectivities and efficiencies, as well as under mild conditions.
More importantly, the current linear model of plastic production and consumption is unsustainable due to its important contribution to waste, pollution, and carbon dioxide emissions. This model follows a “take–make–dispose” approach, resulting in large amounts of unnecessary and mismanaged wastes, causing significant pollution, and harming natural environments.40 This unsustainable plastic lifecycle undermines global climate targets and the sustainable development goals (SDGs), with a special impact on SDG 12 (responsible consumption and production), SDG 13 (climate action), and SDG 14 (life below water).
In this context, polylactic acids (PLAs)41 and polycarbonates (PCs),42 and their copolymers, especially those derived from renewable resources, represent very exciting improvements in the search for sustainable materials. Thus, scorpionate-based catalytic systems have emerged as promising candidates for these applications, offering great potential to produce efficiently sustainable polymers. These features will be evidenced in detail in this account.
This feature article illustrates for the first time a comprehensive overview of the main advances in the application of all-known scorpionate-based systems as efficient catalysts in advancing sustainable polymers.
The review initially focuses on the uses of catalytic scorpionate-based systems, containing divalent (Mg, Ca, Zn, and Fe), trivalent (rare-earth), as well as tetravalent (Zr) metals. These systems were applied for the ring-opening polymerisation (ROP) of lactides (LA) and the ring-opening co-polymerisation (ROCoP) of cyclic anhydrides and epoxides to produce homopolymers of PLAs, and other related polyesters, respectively. Secondly, the main advances in the preparation of polycarbonates derived from the cyclohexene oxide,43 as well as copolymers of poly(lactide) or related polyesters, and polycarbonates (PLA/polyesters-b-PCs) are also described. Finally, the preparation of alternative sustainable materials reported in the literature is also addressed. Moreover, certain catalytic and microstructural features in the resulting polymeric materials are also highlighted for each selected example. These examples are presented as activity performance with increasing temperature. This information is presented in detail in the Data availability section.
This feature article ends in an Outlook and Perspectives to guide future directions. The work particularly addresses potential improvements in catalyst design, as well as broader applications and new opportunities to be explored in the preparation of more sustainable polymers to produce commodity materials.
Preparation of poly(lactide)s, poly(carbonate)s and related copolymers mediated by scorpionate-based catalytic systems
Scorpionate-based catalytic systems including main group divalent metals (magnesium, calcium, zinc and iron) for the preparation of poly(lactide)s and other polyesters
Over the last few years, great attention has been devoted to the ring-opening polymerisation (ROP) of cyclic esters44 (see Scheme 1). These sustainable materials allow very promising applications, including the controlled release of drugs,45 regenerative medicine,46 and wound healing,47 as well as packaging and agriculture.48 Additional reasons for their interest include their relatively facile production from renewable agricultural sources, as well as the biodegradability and biocompatibility that these materials can offer. | ||
| Scheme 1 Preparation of poly(lactides)s via ring-opening polymerisation of rac-lactide, showing possible tacticities of the poly(lactides)s produced. | ||
In this framework, very interesting contributions have been reported in the literature in the recent years. For instance, Cui et al.49 reported the preparation of zwitterionic bis(pirazol-1-yl)methane-based κ3-NNN scorpionate calcium complexes of the type [Ca L1N(SiMe3)2(THF)] (1) and [CaL2N(SiMe3)2] (2), [L1 = (3,5-Me2Pz)2CHP(Ph)2NPh, L2 = (3,5-Me2Pz)2CHP(Ph)2NPh(2-OMe), Pz = pyrazole] (see Fig. 1, complexes 1 and 2). Complex 2 included an additional coordination side arm, leading to the asymmetry of the molecular structure. Complex 2 proved to be very active for the ROP of rac-LA with high monomer feeds at 25 °C (TOF up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 h−1), affording atactic PLAs with medium molecular weights (Mn > 25
700 h−1), affording atactic PLAs with medium molecular weights (Mn > 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da). Interestingly, decreased temperature (−75 °C) gave a heterotactic sequence enriched polylactides (Ps up to 0.84) in the case of 1, whereas an isotactic enriched polymer in the case of 2 (Pi up to 0.78) (see Table 1, entries 1 and 2). The coordination of the additional side arm in 2 proved to be the key factor to enhance activity and isoselectivity. Both materials showed medium-high dispersity values (Đ = 1.40–1.80).
000 Da). Interestingly, decreased temperature (−75 °C) gave a heterotactic sequence enriched polylactides (Ps up to 0.84) in the case of 1, whereas an isotactic enriched polymer in the case of 2 (Pi up to 0.78) (see Table 1, entries 1 and 2). The coordination of the additional side arm in 2 proved to be the key factor to enhance activity and isoselectivity. Both materials showed medium-high dispersity values (Đ = 1.40–1.80).
 | ||
| Fig. 1 Selected scorpionate-based catalytic systems including magnesium, calcium, and zinc for the preparation of poly(lactide)s. | ||
| Entry | Complex | Monomer | [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat] |
T (°C) | Conv. (%)b | TOF (h−1)c | M n,exp (Da) | P s | P i | Đ |
|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator, time and solvent specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c TOF (turnover frequency) = number of moles of starting material consumed/(moles of catalyst × time of reaction). d Determined by GPC relative to polystyrene standards in tetrahydrofuran. Experimental Mn was calculated considering Mark–Houwink's corrections50 for Mn [Mn(obsd.) = 0.58 × Mn(GPC)]. e P s is the probability of racemic linkages (s = syndiotactic) between monomer units and is determined from the relative intensity in the tetrads obtained in the decoupled 1H NMR.51 f The parameter Pi (i = isotactic) is the probability of forming a new i-dyad. The Pi values were calculated from the tetrad probabilities based on enantiomorphic site control statistics.52 | ||||||||||
| 1 | 1 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−75 | 79 | 17.56 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.84 | — | 1.40 |
| 2 | 2 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−75 | 62 | 13.78 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
— | 0.78 | 1.80 |
| 3 | 3 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−25 | 97 | 48.50 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.85 | — | 1.25 |
| 4 | 4 | rac-LA | 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 93 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 718 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.71 | — | 1.12 |
| 5 | 5 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 50 | 1200 | 6800 | 0.79 | — | 1.01 |
| 6 | 6 | L-LA | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 92 | 1577 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
— | — | 1.09 |
| 7 | 7 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 38 | 15.20 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.78 | — | 1.10 |
| 8 | 8 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 96 | 96.00 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.85 | — | 1.09 |
| 9 | 9 | L-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 93 | 3.90 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
— | — | 1.53 |
In addition, the in situ generated alkyl tridentate derivative [ZnLCl2]/MeLi53 (3) (L = 1-(3,5-dimethyl-1H-pyrazol-1-yl)-N-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-N-(furan-2-ylmethyl)methanamine) (see Fig. 1, complex 3) produced PLA from rac-LA at 25 °C (TOF up to 48.50 h−1). Maintenance of the TOF value was also observed at low temperature (−25 °C), reaching lower dispersion values (Đ = 1.25), despite superior heterotactic bias (Ps = 0.85) (see Table 1, entry 3).
Several divalent metal-based scorpionate catalysts have been reported in the literature to be very active even at 20 °C. For instance, a sterically hindered scorpionate magnesium complex [Mg(κ3-pbptamd)(CH2SiMe3)] (4) (pbptamd = N,N′-diisopropylbis(3,5-di-tert-butylpyrazol-1-yl)acetamidinate) (see Fig. 1, complex 4),54 bearing bulky tert-butyl groups as substituents on the pyrazole rings, exhibited high activity for the ROP of rac-LA at 20 °C (TOF = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 h−1) (see Table 1, entry 4). Substituents on the amidinate fragment had a significant influence on both the degree of stereoselectivity and the rate of polymerisation. Thus, the analog [Mg(κ3-tbptamd)(CH2SiMe3)] (5) [tbptamd = N-ethyl-N′-tert-butylbis(3,5-di-tert-butylpyrazol-1-yl)acetamidinate)] (see Fig. 1, complex 5) reduced the activity value, whereas exerted enhanced levels of heteroselectivity during the propagation. Complex 5 produced enriched-heterotactic PLAs at low temperature (0 °C), with a Ps value of up to 0.79 (see Table 1, entry 5). Materials produced by complexes 4 and 5 showed narrow and very narrow dispersity values, respectively (Đ = 1.12–1.01).
718 h−1) (see Table 1, entry 4). Substituents on the amidinate fragment had a significant influence on both the degree of stereoselectivity and the rate of polymerisation. Thus, the analog [Mg(κ3-tbptamd)(CH2SiMe3)] (5) [tbptamd = N-ethyl-N′-tert-butylbis(3,5-di-tert-butylpyrazol-1-yl)acetamidinate)] (see Fig. 1, complex 5) reduced the activity value, whereas exerted enhanced levels of heteroselectivity during the propagation. Complex 5 produced enriched-heterotactic PLAs at low temperature (0 °C), with a Ps value of up to 0.79 (see Table 1, entry 5). Materials produced by complexes 4 and 5 showed narrow and very narrow dispersity values, respectively (Đ = 1.12–1.01).
Very interestingly, it has also been established55 that the reaction of low sterically hindered mononuclear scorpionates, containing smaller substituents such as methyl groups on the pyrazole rings (as opposed to the bulky tert-butyl groups), with Mg(CH2SiMe3)Cl in tetrahydrofuran gave rise to the dinuclear complex [{Mg(CH2SiMe3)}(κ3-pbpamd){MgCH2SiMe3(THF)}] (6) [pbpamd = N,N′-diisopropylbis(3,5-dimethylpyrazol-1-yl)acetamidinate] (see Fig. 1, complex 6). However, when the reaction was carried out in a mixture of tetrahydrofuran/dioxane with the same stoichiometry, a new tetranuclear species [{{Mg(CH2SiMe3)}(κ3-tbpamd){Mg(CH2SiMe3)}}2{μ-O,O-(C4H8)}] (7) [tbpamd = N-ethyl-N′-tert-butylbis(3,5-dimethylpyrazol-1-yl)-acetamidinate] (see Fig. 1, complex 7) was obtained. In addition, complex 6 was much more active for lactide polymerisation, affording PLAs with medium molecular weights at 20 °C for L-LA (TOF = 1577 h−1) (see Table 1, entry 6), and at 50 °C for rac-LA propagations. More importantly, microstructural analysis of the poly(rac-lactide) materials revealed that the tetranuclear 7 exerted enhanced levels of heteroselectivity on the PLAs under mild conditions, with Ps values of up to 0.78 (see Table 1, entry 7). All PLAs produced showed narrow dispersity values (Đ = 1.09–1.10). Moreover, the sterically bulky mononuclear [Mg(tBu)(κ3-phbptamd)]56 (8) [phbpamd = N,N′-di(p-tolyl)bis(3,5-dimethylpyrazol-1-yl)acetamidinate] (see Fig. 1, complex 8) exhibited good performances in the ROP of rac-LA at 20 °C, exerting very high levels of heteroselectivity on the PLAs produced (Ps = 0.85) (see Table 1, entry 8).
Furthermore, other very interesting divalent metal-based scorpionates have been also reported in the literature to behave successfully for the production of sustainable polymers at room temperature. For instance, amide magnesium complexes57 of the type [TpyiPrMg(HMDS)] (9) (see Fig. 1, complex 9), bearing a site-isolating tris(2-pyridyl)borate ligand, has been recently described. This species showed good performance for the ROP at 20 °C of L-LA (see Table 1, entry 9) and trimethylenecarbonate (TMC) for the production of PLAs and poly(trimethylenecarbonate)s (PTMC)s, respectively.
In addition, binuclear bis(pyrazol-1-yl)methane-based chiral N,N,O-scorpionate zinc alkyl complexes, such as [(ZnEt)2(bpzbe)]58 (10) (bpzbe = 1-bis(3,5-dimethylpyrazol-1-yl)-3,3-di-methyl-2-butanoxide) (see Fig. 2, complex 10), have been reported. This initiator demonstrated well-controlled ROP of rac-LA at 20 °C (TOF = 11.25 h−1), although the heteroselectivity in the resulting PLAs afforded low values (Ps = 0.66) (see Table 2, entry 1).
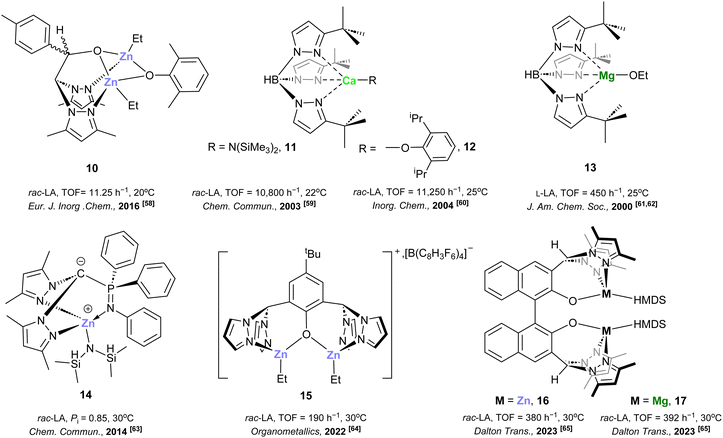 | ||
| Fig. 2 Selected scorpionate-based catalytic systems including magnesium, calcium and zinc for the preparation of poly(lactide)s. | ||
| Entry | Complex | Monomer | [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat] |
T (°C) | Conv. (%)b | TOFc (h−1) | M n,exp (Da) | P s | P i | Đ |
|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator, time and solvent specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c TOF (turnover frequency) = number of moles of starting material consumed/(moles of catalyst × time of reaction). d Determined by GPC relative to polystyrene standards in tetrahydrofuran. Experimental Mn was calculated considering Mark–Houwink's corrections50 for Mn [Mn(obsd.) = 0.58 × Mn(GPC)]. e P s is the probability of racemic linkages (s = syndiotactic) between monomer units and is determined from the relative intensity in the tetrads obtained in the decoupled 1H NMR.51 f The parameter Pi (i = isotactic) is the probability of forming a new i-dyad. The Pi values were calculated from the tetrad probabilities based on enantiomorphic site control statistics.52 | ||||||||||
| 1 | 10 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 45 | 11.25 | 7000 | 0.66 | — | 1.03 |
| 2 | 11 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
22 | 90 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>0.9 | — | 1.74 |
| 3 | 12 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | >90 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.9 | — | 1.68 |
| 4 | 13 | L-LA | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | 90 | 450 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | — | 1.25 |
| 5 | 14 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 96 | 24.00 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 0.85 | 1.37 |
| 6 | 15 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 95 | 190 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 044 044 |
— | — | 1.05 |
| 7 | 16 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 95 | 380 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.63 | — | 1.08 |
| 8 | 17 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 98 | 392 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.58 | — | 1.45 |
Excitingly, Chisholm et al. reported calcium complexes supported by bulky trispyrazolylborate of the type [Ca{η3-HB(3-tBupz)3}R] (pz = pyrazole, R = amide58 (11), aryl oxide58,59 (12)) (see Fig. 2, complexes 11 and 12). These complexes exhibited remarkable activities for the ROP of rac-LA at 22–25 °C, showing heteroselectivity higher than 90% in the PLAs (see Table 2, entries 2–3). This research group previously reported related tris(3-tert-butylpyrazolyl)borate magnesium complexes of the type [Mg{HB(Butpz)3}(OEt)] (pz = pyrazole), (13) (see Fig. 2, complex 13).60,61 This complex exhibited good activity at a similar temperature (25 °C) for the fast ROP of L-LA (TOF = 450 h−1) (see Table 2, entry 4).
Furthermore, Cui62et al. communicated achiral scorpionate zwitterionic zinc silylamido complexes such as [Zn{(3,5-Me2pz)2-C-P(Ph)2NPh}{N(SiHMe2)2}] (pz = pyrazole), (14) (see Fig. 2, complex 14). This species was very efficient for the isoselective ROP of rac-LA at 30 °C, producing a highly isotactic PLA stereoblock (Pi = 0.85), via a chain-end control mechanism (see Table 2, entry 5).
In addition, Comito et al. has reported discrete cationic dialkyldizinc bis-scorpionate complexes,63 bearing two bis(pyrazolyl)alkane units linked by a phenol of the type [PDHZn2Et2][B(C8H3F6)4] (15) (PDH = 2,6-bis(pyrazol-1-ylmethane)-4-tert-butylphenoxide) (see Fig. 2, complex 15). High control over the polymerisation of rac-LA at 30 °C (TOF = 190 h−1) was achieved by this complex (Đ = 1.05) (see Table 2, entry 6).
Very recently, the same group described homobimetallic complexes [LM2(HDMS)2] (M = Zn 16, Mg 17; L = binaphthol-derived unit) in situ generated via the reaction between [M(HDMS)2] [HDMS = N(SiMe3)2] and a binaphthol-derived bis-scorpionate ligand (see Fig. 2, complexes 16 and 17).64 These initiators reported metal–metal cooperativity, achieving enhanced polymerization rates relative to their monometallic counterparts, with very low heteroselectivity values (Ps = 0.63–0.58) (see Table 2, entries 7 and 8).
An enantiopure NNO-scorpionate zinc amide complex, [Zn{N(SiMe}2){κ3-(R,R)-bpzmm}] (18) {(R,R)-bpzmmH = (1R)-1-[(1R)-6,6-dimethylbicyclo[3.1.1]-2-hepten-2-yl]-2,2-bis(3,5-dimethylpyrazol-1-yl)ethanol} (see Fig. 3, complex 18) has also been reported.65 Demonstrating high efficiency, complex 18 facilitated the ROP of rac-LA producing enriched-heterotactic PLAs with a Ps value of up to 0.79 under mild conditions (at 50 °C) (see Table 3, entry 1). At this temperature, we published66 a highly sterically demanding NNN′-scorpionate alkyl zinc mononuclear catalyst [ZnMe(κ3-phbptamd)] (19) [phbptamd = N,N′-di-p-tolylbis(3,5-di-tert-butylpyrazole-1-yl)acetamidinate] (see Fig. 3, complex 19). This complex achieved enhanced levels of heteroselectivity (Ps = 0.74) (see Table 3, entry 2). Also, we have very recently reported36,67 an enantiopure NNN-scorpionate zinc complex [ZnMe(κ3-(−)-cis-bpmy)] (20) {bpmyH = 3,3-bis(3,5-dimethyl-1H-pyrazol-1-yl)-N-(((1S,2R,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-yl)-methyl)-1,1-diphenylprop-1-en-2-amine} (see Fig. 3, complex 20), which exhibited enhanced levels of isoselectivity in the materials produced (Pi = 0.88) at 50 °C (see Table 3, entry 3).36
 | ||
| Fig. 3 Selected scorpionate-based catalytic systems including magnesium, calcium, zinc and iron for the preparation of poly(lactide)s and other polyesters. | ||
| Entry | Complex | Monomer | [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat] |
T (°C) | Conv. (%)b | TOFc (h−1) | M n,exp (Da) | P s | P i | Đ |
|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator, time and solvent specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c TOF (turnover frequency) = number of moles of starting material consumed/(moles of catalyst × time of reaction). d Determined by GPC relative to polystyrene standards in tetrahydrofuran. Experimental Mn was calculated considering Mark–Houwink's corrections50 for Mn [Mn(obsd.) = 0.58 × Mn(GPC)]. e P s is the probability of racemic linkages (s = syndiotactic) between monomer units and is determined from the relative intensity in the tetrads obtained in the decoupled 1H NMR.51 f The parameter Pi (i = isotactic) is the probability of forming a new i-dyad. The Pi values were calculated from the tetrad probabilities based on enantiomorphic site control statistics.52 | ||||||||||
| 1 | 18 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 79 | 31.60 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.79 | — | 1.09 |
| 2 | 19 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 95 | 25.30 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.74 | — | 1.13 |
| 3 | 20 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 37 | 14.80 | 4900 | — | 0.88 | 1.07 |
| 4 | 21 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 89 | 118.70 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
— | — | 1.45 |
| 5 | 22 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 59 | 14.75 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.5 | — | 1.12 |
| 6 | 19 | rac-LA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
125 | 92 | 5520 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.68 | — | 1.28 |
| 7 | 23 | rac-LA | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 74 | 12.40 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.5 | — | 1.5 |
[CHO]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PA] [PA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PPNCl] [PPNCl] |
||||||||||
| 8 | 24 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 96 | 12.20 | 2870 | — | — | 1.49 | |
| 9 | 25 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
65 | 99 | 8.40 | 5100 | — | — | 1.1 | |
Moreover, Mountford68et al. prepared a sterically demanding N2N′-scorpionate bis(pyrazol-1-yl)methane-derived magnesium amide of the type [Mg{HC(tBu2pz)2SiMe2NPh}{N(SiMe3)2}] (21) (see Fig. 3, complex 21), which proved to be an active initiator for the ROP of rac-LA. Regrettably, this species showed poor control in the polymerization at 70 °C with no tacticity details in the PLA materials presented (see Table 3, entry 4). A higher temperature (90 °C) was necessary to observe polymerization activity in the ROP of rac-LA mediated by the hybrid scorpionate/cyclopentadienyl magnesium [Mg(CH2SiMe3)(κ2-η5-bpzcp)] (22) (see Fig. 2, complex 22) reported previously,69 resulting in atactic PLAs (see Table 3, entry 5). Notably, the highly sterically demanding acetamidinate zinc complex 19 described earlier in this article66 behaved as a very active initiator for the ROP of rac-LA under melt (125 °C) conditions (TOF = 5520 h−1) (see Table 3, entry 6). Additionally, this complex resulted a very robust initiator since it was capable of polymerising this monomer non-completely purified as melt twice-sublimed (TOF = 504 h−1). Very interestingly, Herres-Pawlis et al.70 reported a highly efficient iron catalyst, [Fe{HC(Pz)2Bipy}(CF3CO2)2] (pz = pyrazole; py = pyridine), supported by an N,N,N,N tetradentate bis-(pyrazolyl)bipyridinylmethane ligand (see Fig. 3, complex 23). This catalyst demonstrated exceptional performance by catalysing the melt ROP of sublimed and unsublimed technical-grade rac-lactide at 150 °C, reaching molecular weights near 30 kDa after 30 h (see Table 3, entry 7).
Finally, other polymeric sustainable polyesters have been also prepared using M(II)-based scorpionate catalysts. For instance, a bimetallic scorpionate acetate zinc complex [{Zn(κ3-bpzbdeape)}(μ-O2CCH3)3{Zn(HO2CCH3)}] (24) (bpzbdeape = 1,1-bis(4-(diethylamino)phenyl)-2,2-bis(3,5-dimethyl-1H-pyrazol-1-yl)ethan-1-olate) (see Fig. 3, complex 24), have been recently developed.71 This complex was capable of catalysing selectively and efficiently the ROCoP of cyclohexene oxide (CHO)43 with several anhydrides (see Scheme 2). Particularly, phthalic (PA), maleic (MA), succinic (SA) and naphthalic (NA) anhydrides were employed in the presence of bis(triphenylphosphine)iminium (PPNCl) as a cocatalyst at 80 °C to afford the corresponding polyester materials (Table 3, entry 8). In addition, very recently we have reported the novel dinuclear calcium complex [CaI{(κ3-bpzbdeape)(μ-O)}(THF)]272 (25) (bpzbdeape = 1,1-bis(4-(diethylamino)phenyl)-2,2-bis(3,5-dimethyl-1H-pyrazol-1-yl)ethan-1-oxide) (see Fig. 3, complex 25). This catalyst behaved as a bifunctional catalyst for the ROCoP of epoxides and cyclic anhydrides under mild reaction conditions and in the absence of a cocatalyst (see Table 3, entry 9). Complex 25 demonstrated tolerance to a variety of bio-sourced epoxides/phthalic anhydrides, providing access to a range of potentially functionalisable polyesters.
 | ||
| Scheme 2 Preparation of polyesters via ring-opening Copolymerisation of cyclohexene oxide and a variety of anhydrides. | ||
To summarise, in this section we can highlight that the divalent metal-based scorpionates calcium-based 11 and 12 showed the highest activities at 20 °C in the ROP of rac-LA (TOF = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–11
800–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 h−1). Interestingly, the robust zinc-based complex 19 was capable of polymerising non-completely purified twice-sublimed rac-LA in the bulk (TOF 504 h−1), showing a medium–broad dispersity (Đ = 1.35). Additionally, complex 11 and the zinc complex 20 exerted enhanced levels of hetero- and isoselectivity in the PLAs produced, respectively (Ps > 0.9, 11, Pi = 0.88, 20). Finally, the zinc complex 24 and the calcium complex 25 demonstrated the ability to produce alternative polyesters via a ROCoP process of CHO and sustainable anhydrides.
250 h−1). Interestingly, the robust zinc-based complex 19 was capable of polymerising non-completely purified twice-sublimed rac-LA in the bulk (TOF 504 h−1), showing a medium–broad dispersity (Đ = 1.35). Additionally, complex 11 and the zinc complex 20 exerted enhanced levels of hetero- and isoselectivity in the PLAs produced, respectively (Ps > 0.9, 11, Pi = 0.88, 20). Finally, the zinc complex 24 and the calcium complex 25 demonstrated the ability to produce alternative polyesters via a ROCoP process of CHO and sustainable anhydrides.
Scorpionate-based catalytic systems including non-divalent metals (rare earths and zirconium) for the preparation of poly(lactide)s
Rare-earth complexes have been identified as highly effective catalysts for the synthesis of high-molecular-weight PLAs73 (see Scheme 1). However, the large ionic radii of the metal centres in these complexes require the presence of sterically hindered ligands to prevent their resulting coordination unsaturation, and therefore, low selectivity. In this context, Cui et al.74 presented a series of rare-earth-metal zwitterionic complexes supported by oxophosphine and iminophosphine scorpionate ligands bearing various bulky substituent groups that act as active and selective initiators for the ROP of rac-LA. Particularly, the bis(alkyl) oxophosphine lutetium complex [Lu(CH2SiMe3)2{(3,5-Me2Pz)2CPPh2O}] (26) (Pz = pyrazole), (see Fig. 4, complex 26) was able to achieve full conversion of high rac-LA loading (400 to 1000 eq.) at 20 °C in just 1 hour. Moreover, complex 26 also demonstrated remarkable heterotactic selectivity (Ps = 0.85–0.87) (see Table 4, entry 1). This performance was attributed to the cage-like geometry of the scorpionate ligand. | ||
| Fig. 4 Selected scorpionate-based catalytic systems including rare earth and zirconium for the preparation of poly(lactide)s. | ||
| Entry | Complex | Monomer | [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat] |
T (°C) | Conv.b (%) | TOFc (h−1) | M n,exp (Da) | P s | P i | Đ |
|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator and solvent specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c TOF (turnover frequency) = number of moles of starting material consumed/(moles of catalyst × time of reaction). d Determined by GPC relative to polystyrene standards in tetrahydrofuran. Experimental Mn was calculated considering Mark–Houwink's corrections57 for Mn [Mn(obsd.) = 0.58 × Mn(GPC)]. e P s is the probability of racemic linkages (s = syndiotactic) between monomer units and is determined from the relative intensity in the tetrads obtained in the decoupled 1H NMR.51 f The parameter Pi (i = isotactic) is the probability of forming a new i-dyad. The Pi values were calculated from the tetrad probabilities based on enantiomorphic site control statistics.52 | ||||||||||
| 1 | 26 | rac-LA | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 100 | 1000 | 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.85 | — | 1.8 |
| 2 | 27 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 | 88 | 176 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.98 | — | 1.28 |
| 3 | 28 | rac-LA | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 97 | 40.40 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
— | — | 1.6 |
| 4 | 29 | rac-LA | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
110 | 44 | 110 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
— | — | 1.4 |
| 5 | 30 | rac-LA | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 52 | 6.9 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 0.29 | 1.09 |
| 6 | 31 | rac-LA | 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 93 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.57 | — | 1.29 |
Remarkably, the same authors later reported a new family of rare-earth oxo- and iminophosphine scorpionate complexes with various bulky substituents.75 They demonstrated that introducing an inert σ-bonded ligand into these mixed halogen/alkyl or halogen/amido complexes significantly improved heteroselectivity. For example, the complex [YCl(N(SiHMe2)2)(THF)]{(3,5-Me2Pz)2CPPh2NPh}] (Pz = pyrazol) (27) (see Fig. 4, complex 27) exhibited lower activity (TOF 150–190 h−1) compared to its bis-alkyl counterparts but endowed the metal centre with excellent heteroselectivity, achieving a Ps value of up to 0.98 at 25 °C (see Table 4, entry 2). The authors attributed this greater control to the presence of the σ-halogen group on the active centre, which directs monomer coordination and insertion. More recently, additional oxophosphine scorpionate complexes have been reported, although they have shown only modest activity and control. For instance, the dimeric lanthanum amide complex {La[(3,5-Me2Pz)2CP(O)Ph2][N(SiMe3)2](μ2-OP(O)Ph2)}2, (28) (see Fig. 4, complex 28), recently prepared by Trifonov et al., demonstrated quantitative conversion of 500 equivalents of rac-LA (TOF 40.40 h−1) at room temperature to yield high molecular weight atactic materials (see Table 4, entry 3).76
In recent years, other examples of rare-earth catalysts supported by scorpionate ligands have proven to be efficient initiators for the ROP of LA, though they operate effectively only at above-ambient temperatures. Thus, Scrivanti et al.77 observed that the presence of hard donor atoms in these ligands is particularly appropriate due to the greater stabilisation effect in group 3 and lanthanide metals. Thus, the authors observed that medium-high molecular weight PLA samples (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da) can be prepared in moderate yields by heating rac-LA to melt in the presence of the yttrium scorpionate amido-complex [Y(BTSA)(bpza)2] (29) (BTSA = bis(trimethylsilyl)amide; bpza = bis(pyrazol-1-yl)acetate) (see Fig. 4, complex 29) (see Table 4, entry 4).
400 Da) can be prepared in moderate yields by heating rac-LA to melt in the presence of the yttrium scorpionate amido-complex [Y(BTSA)(bpza)2] (29) (BTSA = bis(trimethylsilyl)amide; bpza = bis(pyrazol-1-yl)acetate) (see Fig. 4, complex 29) (see Table 4, entry 4).
In addition, our research group has also contributed to this field by searching for novel rare-earth complexes supported by scorpionate ligands. In 2009, we presented the synthesis and characterization of a neutral enantiopure scorpionate neodymium complex of the type [Nd((S)-mbpam)(N(SiMe2H)2]2 (30) [(S)-mbpamH = (S)-(−)-N-α-methylbenzyl-2,2-bis(3,5-dimethylpyrazol-1-yl)acetamide] (see Fig. 4, complex 30).78 Complex 30 represented the first example of activation of the C–H methine bridging group, leading to a dimeric species in which two neodymium atoms are linked by two oxygen and two nitrogen atoms from the acetamidate groups.
Complex 30 demonstrated good control in the ROP of rac-LA under mild conditions (50 °C), exhibiting a preference for homosteric polymerisation of the L-LA component at low conversion levels (10%), with a Pi value reaching up to 0.61. At higher conversion (52%), medium-molecular-weight PLA materials (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) with a low dispersity index (Đ = 1.09) were obtained, though a decrease in the meso linkages was observed (Pi = 0.29) (see Table 4, entry 5).
000 Da) with a low dispersity index (Đ = 1.09) were obtained, though a decrease in the meso linkages was observed (Pi = 0.29) (see Table 4, entry 5).
On the other hand, the use of aluminium complexes as catalysts for the polymerisation of rac-LA has gathered significant attention in recent years.79 Notably, complexes with tridentate ligands are of particular interest. They provide a less coordinatively saturated environment to the metal compared to those with commonly used tetradentate ligands, such as SALEN and derived. However, to the best of our knowledge, there are no examples in the literature describing an aluminium complex supported by a scorpionate ligand maintaining a κ3-coordination in solution. Therefore, none has successfully proven to initiate the ROP of L- or rac-LA and other sustainable polyesters.
Thus, aside from the previously mentioned contributions involving rare-earth metals, there is only one additional example of a scorpionate complex with a non-divalent metal that can be cited in this context. Specifically, the preparation and structural characterisation of an enantiopure alkoxide zirconium derivative, [Zr(OCHMeEt)3(κ3-R,R-fbpza)] (31) (R,R-fbpzaH = N-p-fluorophenyl-(1R)-1-[(1R)-6,6-dimethylbicyclo[3.1.1]-2-hepten-2-yl]-2,2-bis(3,5-dimethylpyrazol-1-yl)ethylamine) (see Fig. 4, complex 31) has been reported.80 This complex demonstrated exceptional thermal stability and robustness as an initiator for the ROP of rac-LA. Complex 31 exhibited remarkable activity under melt conditions by consuming almost quantitatively 300 equivalents of monomer feed in just one minute (see Table 4, entry 6). This achieved a TOF value of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 h−1, which was significantly higher than any previously reported zirconium complex by then. Interestingly, complex 31 maintained stability and activity even under less rigorously anhydrous conditions, remaining effective with non-fully purified rac-LA (TOF 588 h−1).
740 h−1, which was significantly higher than any previously reported zirconium complex by then. Interestingly, complex 31 maintained stability and activity even under less rigorously anhydrous conditions, remaining effective with non-fully purified rac-LA (TOF 588 h−1).
In conclusion, scorpionate rare-earth complexes, particularly those bearing sterically hindered ligands, such as 26 and 27, have proven to be among the most effective catalysts for polyester synthesis, including high-molecular-weight PLAs. Complex 26 is currently the most active rare-earth scorpionate complex, achieving a rapid ROP of high monomer loading at room temperature (TOF = 1000 h−1). Interestingly, the inclusion of halides in the metal centre significantly improved polymerization control by directing monomer coordination and insertion, leading to higher tacticity values (Ps = 0.98) in complex 27. Other rare-earth complexes operated effectively under melt conditions, achieving moderate yields and producing medium-to-high molecular weight PLAs (e.g., 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da) as complex 29. On the other hand, the enantiopure rare-earth complex 30 showed an isotactic preference in the ROP of rac-LA through an enantiomorphic site control mechanism, reaching Pi values up to 0.61 at low conversions. Finally, the zirconium complex 31 showed promising activity (TOF = 16
400 Da) as complex 29. On the other hand, the enantiopure rare-earth complex 30 showed an isotactic preference in the ROP of rac-LA through an enantiomorphic site control mechanism, reaching Pi values up to 0.61 at low conversions. Finally, the zirconium complex 31 showed promising activity (TOF = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 h−1) and stability for this process, highlighting the potential of alternative non-divalent metal catalysts in this field.
740 h−1) and stability for this process, highlighting the potential of alternative non-divalent metal catalysts in this field.
Scorpionate-based catalytic systems for the preparation of poly(carbonate)s
The synthesis of polycarbonates is of great interest to the plastic industry due to the wide range of properties and applications of polycarbonates as materials for medical devices, 3D printing or thermoplastics.42 Unfortunately, the industrial scale production of these polymers comes from petroleum-based sources requiring toxic and non-sustainable reagents. Therefore, the synthesis of polycarbonates through the ring-opening co-polymerisation (ROCoP) of CO2 with epoxides, such as cyclohexene oxide (CHO, which is not considered renewable, but it can be synthesized from bioderived resources)43 to produce poly(cyclohexene carbonate) (PCHC), is an interesting approach to reach this sustainable goal. This strategy has recently received great attention in the scientific community40 (see Scheme 3). | ||
| Scheme 3 Preparation of poly(cyclohexene carbonate) via ring-opening co-polymerisation of cyclohexene oxide and carbon dioxide. | ||
In this context, reports on scorpionate-based catalytic systems for the preparation of sustainable polycarbonates are scarce, with some of the contributions in this area coming from our group. Thus, rare-earth complexes have demonstrated to be excellent candidates to afford polycarbonates via ROCoP employing substituted epoxides, due to the role of these species to behave as very good Lewis acids, and their ability to activate the corresponding monomers. For instance, we have reported81 the synthesis of a bis(pirazol-1-yl)methane-based scorpionate alkyl yttrium complex of the type [Y(CH2SiMe3)2(κ3-bpzbdmape)(THF)] (32) (bpzbdmape = (3,5-Me2Pz)2CHCO(4-NMe2-Ph)2) (see Fig. 5, complex 32). Complex 32 demonstrated good catalytic activity and very high selectivity (99%) at 60 °C for the ROCoP of 1000 equiv. of cyclohexene oxide (CHO) and 40 bar of CO2 pressure for the preparation of poly(cyclohexene carbonate) (PCHC) (TOF up to 39.38 h−1) (see Table 5 entry 1). No appearance of ether linkages was observed during polymerization, exhibiting medium–low dispersity values (Đ = 1.30).
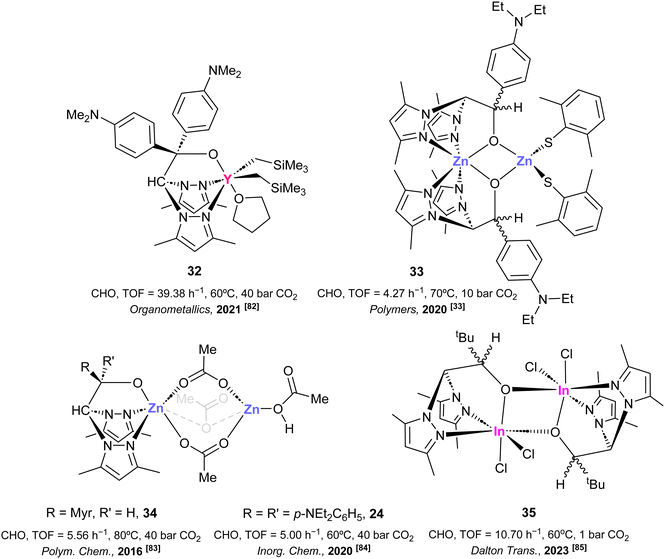 | ||
| Fig. 5 Selected examples of scorpionate-based catalytic systems for the preparation of poly(cyclohexene)carbonate. | ||
| Entry | Complex | [CHO]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat] |
PCO2 (bar) | T (°C) | Conv. (%)b | Selectivity PCHC (%)c | TOFd (h−1) | M n,exp (Da) | Đ |
|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator and time specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c Percentage selectivity of the carbonate linkages is determined by 1H-NMR spectroscopy of the crude reaction mixture. d TOF (turnover frequency) = number of moles of starting material consumed/(moles of catalyst × time of reaction). e Determined by SEC relative to polystyrene standards in tetrahydrofuran. | |||||||||
| 1 | 32 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 | 60 | 60 | 99 | 39.38 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.30 |
| 2 | 33 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 70 | 72 | 95 | 4.27 | 9500 | 1.09 |
| 3 | 34 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 | 80 | 89 | 99 | 5.56 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 061 061 |
1.08 |
| 4 | 24 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 | 60 | 75 | 98 | 5.00 | 5220 | 1.45 |
| 5 | 35 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 60 | 32 | >99 | 10.70 | 1800 | 1.20 |
In addition, a chiral dinuclear thioalkoxide derivative κ2-NN-μ-O scorpionate zinc complex of the type [Zn(bpzaepe)2{(2,6-Me2C6H3S)2}](33) [bpzaepe = (3,5-Me2Pz)2CHCO(4-NEt2Ph)] (see Fig. 5, complex 33), in a dimer structure with a rhomboidal (ZnO)2 core, has been reported.33 This complex 33 exhibited significant catalytic activity in the ROCoP process of 100 eq. of cyclohexene oxide and low CO2 pressure (10 bar) at 70 °C (TOF = 4.27 h−1) (see Table 5, entry 2). This dinuclear zinc complex 33 exhibited high selectivity for PCHC production with high selectivity (95%, compared to the 5% of cyclohexene carbonate formation). This selectivity was possibly due to the hemilability of the thioalkoxyde ligand, resulting in materials with low dispersity values (Đ = 1.09) (see Table 5, entry 2).
Furthermore, alternative examples have been developed of biocompatible zinc-based scorpionate catalysts, which demonstrated activity for poly(cyclohexene carbonate) production via a ROCoP process. Thus, in 2016, we also presented a bimetallic zinc scorpionate complex of the type [{Zn(κ3-(R,R)-bpzmm)}(μ-O2CCH3)3{Zn(HO2CCH3)}],82 (34) [(R,R)-bpzmm = (1R)-1-{(1R)-6,6-dimethyl-bicyclo[3.1.1]-2-hepten-2-yl}-2,2-bis(3,5-dimethylpyrazol-1-yl)ethoxide] (see Fig. 5, complex 34). This complex 34 presented one zinc centre in an octahedral environment with three bridging acetate ligands connected with the second zinc centre, which showed a tetrahedral coordination sphere with one terminal acetic acid molecule. Notably, this bimetallic complex 34 displayed high catalytic activity in the ROCoP of 100 eq. of CHO at 80 °C (TOF = 5.56 h−1) at 40 bar of CO2 pressure, affording a 89% conversion with high content of carbonate linkages (>99%), with very low value of dispersity (Đ = 1.08) (see Table 5, entry 3). More recently, in 2020, analog bimetallic zinc acetate complexes, such as 24, were applied for the successful ROCoP of CHO and CO2 (see Fig. 3, complex 24).83 However, a slight decrease in catalytic activity was observed under 60 °C and 40 bar of pressure, resulting in a 75% conversion of CHO, with a slight decrease in polycarbonate selectivity (98%) (see Fig. 5, complex 24; Table 5, entry 4).
Recently, in 2023, a dinuclear indium scorpionate complex [InCl2{(κ3-bpzbe)(μ-O)}]2 (35) (bpzbe = 1,1-bis(3,5-dimethyl-1H-pyrazol-1-yl)-3,3-dimethylbutan-2-oxide) (see Fig. 5, complex 35) has also been reported as an active catalyst for the selective production of PCHC via the ROCoP process.84 This homobimetallic complex presents each indium centre in an octahedral geometry, with two additional chlorides in a cis configuration, finally completed by the oxygen of a second scorpionate ligand. Notably, complex 35 demonstrated high catalytic activity and excellent selectivity (>99%) for PCHC synthesis. Specifically, complex 35 achieved a TOF value of 10.70 h−1 at 60 °C requiring only just one bar of CO2 pressure (see Table 5, entry 5).
In summary, we can highlight in this section that complexes 32, 34 and 24 showed good activities and selectivities for the ROCoP process at high CO2 pressures (40 bar). Particularly, complex 32 polymerised 1000 equiv. of CHO and demonstrated the highest TOF (of up to 39.38 h−1) leading to high molecular weight polymers (42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 Da) with very high selectivity. Additionally, complex 33 revealed good efficiency (72% conv.) at a lower CO2 pressure (10 bar). Furthermore, complex 35 exhibited the highest selectivity in the production of poly(cyclohexene carbonate) (>99%) under ambient CO2 pressure conditions.
800 Da) with very high selectivity. Additionally, complex 33 revealed good efficiency (72% conv.) at a lower CO2 pressure (10 bar). Furthermore, complex 35 exhibited the highest selectivity in the production of poly(cyclohexene carbonate) (>99%) under ambient CO2 pressure conditions.
Scorpionate-based catalytic systems for the preparation of polyester-co-polycarbonate copolymers
The physical (mainly thermal and mechanical) properties of individual materials such as PLAs41 and PCHCs42 and restrict their further applications. Thus, one strategy to improve these features is to incorporate both materials into block polymers, resulting in hybrid materials that will finally present combined properties of both original polymers. Thus, the incorporation of a polycarbonate unit into a polyester matrix holds substantial relevance due to the superior material properties in conjunction with the sustainability advantages that the PC affords. For instance, polycarbonates, such as poly(cyclohexene carbonate) (PCHC), synthesised through the ring-opening co-polymerisation (ROCoP) of carbon dioxide and epoxides, as described above (see Scheme 3), exhibit desirable attributes such as high glass transition temperature and excellent tensile modulus.42 However, the brittleness and low thermal decomposition temperature of PCHC limit its standalone applications. By incorporating polycarbonate into polyester chains as block copolymers via the ring opening co-polymerisation of cyclohexene oxide, lactide and carbon dioxide (see Scheme 4) provides a promising solution to these limitations. This approach allows materials with very interesting and enhanced features, such as increase in flexibility, better thermal stability, and superior mechanical strength to be obtained, which definitively mitigate several of the inherent limitations of pure PLA.85 | ||
| Scheme 4 Preparation of poly(lactide)-co-poly(cyclohexene carbonate) copolymers via ROCoP of cyclohexene oxide, lactide and carbon dioxide. | ||
In this context, the first dinuclear indium scorpionate complexes were recently reported as efficient catalysts for the preparation of hybrid polyester-co-polycarbonate copolymers under mild conditions.84 Particularly, complex [InCl2{(κ3-bpzbe)(μ-O)}]2 (35) (see Fig. 5, complex 35) was highly active and versatile for the preparation of PCHC. Thus, complex 35 was also capable of producing hybrid polylactide-co-polycyclohexene carbonate copolymers in a one-pot polymerisation procedure at different CHO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-LA monomer ratios (up to 700
L-LA monomer ratios (up to 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) at 60 °C and 10 bar of CO2 pressure (see Table 6, entry 1). Under these conditions, complex 35 converted CHO in 27% and L-lactide completely, affording a terpolymer with the highest content of PCHC of 60% and 40% of PLA.
100) at 60 °C and 10 bar of CO2 pressure (see Table 6, entry 1). Under these conditions, complex 35 converted CHO in 27% and L-lactide completely, affording a terpolymer with the highest content of PCHC of 60% and 40% of PLA.
| Entry | Complex | [CHO]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [M] [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DMAP] [DMAP] |
PCO2 (bar) | T (°C) | Conv. CHOb (%) | Conv. Mb (%) | Selectivity PCHCc (%) | M n,exp (Da) | Đ |
|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator and time specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c Percentage selectivity of the carbonate linkages is determined by 1H-NMR spectroscopy of the crude reaction mixture. d Determined by SEC relative to polystyrene standards in tetrahydrofuran. | |||||||||
| 1 | 35 | 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100(L-LA) 100(L-LA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
10 | 60 | 27 | 100(L-LA) | 60 | 9100 | 1.60 |
| 2 | 24 | 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100(PA) 100(PA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
40 | 80 | 22 | 22(PA) | 47 | 3977 | 1.53 |
In addition, the ring opening co-polymerisation of epoxides, cyclic anhydrides and carbon dioxide represents an excellent methodology to synthesize these hybrid polyester-co-polycarbonate materials, offering a wide range of epoxides and anhydrides in their composition (see Scheme 5). In this context, the bimetallic scorpionate zinc acetate catalyst [{Zn(κ3-bpzbdeape)}(μ-O2CCH3)3{Zn(HO2CCH3)}] (24) (see Fig. 3, complex 24) has also been reported for the preparation of these polyester-co-polycarbonate materials (see Scheme 5).71 Remarkably, complex 24 resulted highly active and versatile in the terpolymerisation catalysis of cyclohexene oxide, CO2 and phthalic anhydride to produce the corresponding hybrid materials using DMAP as the cocatalyst. Then, with a molar ratio 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for CHO, PA, 24 and DMAP respectively, we afforded a controlled terpolymer closer to a 50
2 for CHO, PA, 24 and DMAP respectively, we afforded a controlled terpolymer closer to a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, formed by 47% of polycarbonate and 53% of polyester, under 40 bar of CO2 at 80 °C (see Table 6, entry 2). Moreover, copolymer materials were obtained as block copolymers with narrow dispersity (Đ = 1.53).
50 ratio, formed by 47% of polycarbonate and 53% of polyester, under 40 bar of CO2 at 80 °C (see Table 6, entry 2). Moreover, copolymer materials were obtained as block copolymers with narrow dispersity (Đ = 1.53).
 | ||
| Scheme 5 Preparation of polycarbonate-co-polyester copolymers via a ROCoP of cyclohexene oxide, carbon dioxide and phthalic anhydride. | ||
Scorpionate-based catalytic systems for the preparation of other sustainable materials
In the recent years, scorpionate-based systems have been also employed for catalytic processes to produce alternative biosustainable polymers. These sustainable materials aim to replace certain long-shelf-life packaging and produce interesting flexible and rigid rubbers.86For instance, in 2021, we reported a novel strategy to produce non-isocyanate poly(hydroxy) urethanes (NIPUs) by reaction with the corresponding fatty acid based bis(cyclic carbonate)s and 1,4-diaminobutane (see Scheme 6). For this purpose, the synthesis of a bis(silylamide) lanthanum complex of the type [La{N(SiHMe2)2}2{κ3-bpzcp}] (36) [bpzcp = (3,5-Me2Pz)2C(Cp)] (see Fig. 6, complex 36),87 with a hybrid cyclopentadienyl-scorpionate ligand coordinated in a κ3-fashion, has been described.
 | ||
| Scheme 6 Preparation of non-isocyanate poly(hydroxy) urethanes (NIPUs) via initial cycloaddition of CO2 to bis-epoxides derived from waste fatty acids, and subsequent reaction with 1,4-diaminobutane. | ||
 | ||
| Fig. 6 Selected examples of scorpionate-based catalytic systems for the preparation of alternative sustainable materials. | ||
This rare-earth complex 36, assisted by TBAC as the co-catalyst, displayed high efficiency for synthesising a wide variety of cyclic carbonates derived from waste fatty acid bis epoxides by reaction with CO2. An outstanding result was achieved with 100 eq. of the fatty acid derived bis-epoxide n-pentyl ester, at 80 °C (TOF = 40 h−1) at 20 bar of CO2 pressure, with 100% of selectivity for the corresponding bis-cyclic carbonate (see Table 7, entry 1). Consequently, we applied these bis(cyclic carbonates) as bioderived starting materials for successful NIPU production via ring-opening reaction with one equivalent of 1,4-diaminobutane in acetonitrile at 80 °C for 16 hours (see Scheme 6).
| Entry | Complex | [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] [cat] |
PCO2 (bar) | T (°C) | Conv. Mb (%) | TOFc (h−1) | M n,exp (Da) | Đ |
|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: moles of initiator and time specified on the Data availability document. b Percentage conversion of the monomer [(weight of polymer recovered/weight of monomer) × 100]. c TOF (turnover frequency) = number of moles of starting material consumed/(moles of catalyst × time of reaction). d Determined by SEC relative to polystyrene standards in tetrahydrofuran. | ||||||||
| 1 | 36 | 100(bis-epoxide)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 80 | 95 | 40 | — | — |
| 2 | 37 | 200(HMF)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200(MPS) 200(MPS)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 40 | >99 | — | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.02 |
On the other hand, very interestingly Cui et al.88 reported in 2019 an unprecedented step-growth copolymerization of 5-hydroxymethyl furfural (HMF), as a renewable feedstock monomer, with dihydrosilanes to afford biosustainables linear poly(silylether)s (see Scheme 7). To achieve this aim, Cui et al. developed a zwitterionic κ3-NNN scorpionate zinc hydride complex of the type [ZnLH], [L = (3,5-Me2Pz)2CP(Ph)2NPh]. However, the addition of B(C6F5), very well-known as a highly active group for hydro-silylation of aldehydes, formed the corresponding zwitterionic hydride adduct species [ZnL(μ-H){B(C6F5)3}]88 (37) (see Fig. 6, complex 37). Complex 37 exhibited synergistic catalysis in the copolymerisation of 200 eq. of HMF and methylphenylsilane (MPS), obtaining poly(silylether) (PMPS) in >99% conversion (see Table 7, entry 2). The low molecular weight materials (Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 Da) produced showed an unprecedented dispersity value for a step-growth copolymerisation (Đ = 1.02). Therefore, this represents the first example to use asymmetric multi-functionalised HMF as the polymerisation unit and step-growth polymerisation mediated by a coordination mechanism.
800 Da) produced showed an unprecedented dispersity value for a step-growth copolymerisation (Đ = 1.02). Therefore, this represents the first example to use asymmetric multi-functionalised HMF as the polymerisation unit and step-growth polymerisation mediated by a coordination mechanism.
 | ||
| Scheme 7 Preparation of linear poly(silylethers) via copolymerisation of 5-hydroxymethyl furfural with dihydrosilanes and subsequent reaction with 1,4-diaminobutane. | ||
Conclusions
In summary, a variety of scorpionate catalytic systems, based on the bis(1H-pyrazol-1-yl)methane and tris(1H-pyrazol-1-yl)borate units, have been rationally selected. These species include divalent (Mg, Ca, Zn and Fe), trivalent (rare-earth), and tetravalent (Zr) metals. These scorpionates have successfully proved to behave efficiently to produce a wide range of sustainable materials, confirming beyond doubt, their high potential and versatility. In this sense, we selected complexes 1–31 (Fig. 1–4) as the most interesting catalysts for the preparation of poly(lactide)s (Scheme 1) and other polyesters (Scheme 2), and included their most relevant catalytic features (Tables 1–4). In addition, we have indicated complexes 32–35 (Fig. 5) as the most relevant catalysts for the production of poly(cyclohexene carbonate)s (Scheme 3), and presented their catalytic performances (Table 5). Moreover, we have denoted complexes 24 and 35 for the preparation of poly(lactide)-co-poly(cyclohexene carbonate) copolymers (Schemes 4 and 5), and reported their catalytic behaviours (Table 6). Finally, complexes 36–37 (Fig. 6) have been selected for the preparation of other sustainable materials (Schemes 6 and 7), and described their catalytic features (Table 7).It is notable that complexes 24 and 35 are very versatile. For instance, 24 can operate efficiently in the preparation of polyesters and polycarbonates. In addition, complex 35 resulted in being very active and selective to obtain polycarbonates and polyester-co-polycarbonate hybrid materials. Moreover, complexes 11 and 12 exhibited the highest activities for the production of PLAs at room temperature (TOF = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–11
800–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 h−1). Finally, in terms of control of tacticity in the polyester backbone, complexes 20 and 27 exerted enhanced level of iso- and hetero-tacticity, respectively, in the PLAs produced (Pi = 0.88, 20; Ps = 0.98, 27).
250 h−1). Finally, in terms of control of tacticity in the polyester backbone, complexes 20 and 27 exerted enhanced level of iso- and hetero-tacticity, respectively, in the PLAs produced (Pi = 0.88, 20; Ps = 0.98, 27).
From a future perspective, society has made clear the need for the transition from a linear production model to promote the circular economy. This change will imply the challenging aim to consume renewable resources to produce sustainable materials in the coming decades. This also means the adaptation and redesign of the chemical industry. Therefore, the scientific community must generate adequate knowledge to enable chemical processes, mostly catalytic, with high atomic economy, benefiting on accessible and renewable raw materials (feedstocks). In addition, scientists need the commitment to perform with waste and energy consumption near to zero, in alignment with several of the Sustainable Development Goals (SDGs, 12, 13 and 14).
In this context, bio-based (co)-polymers derived from bio-sourced feedstocks have recently attracted considerable interest. These materials represent one of the most promising alternatives to replace non-renewable fossil fuel-based polymers to address the growing concerns of fossil fuels dependence and plastic waste. In addition, modification via post-polymerization reactions will be essential to create commercially renewable and viable materials that will be also potentially biodegradable and biocompatible.
To address these challenging targets, it is necessary to design more efficient and selective catalytic systems, based on Earth-abundant metals supported by non-innocent ligands that could tune the electronic and steric features of the catalysts. A right balance in this complex combination will result in catalytic systems competent in numerous catalytic processes, being scorpionate complexes excellent candidates as it has been already demonstrated in this account. These demanding objectives will probably mark our future efforts, opening new avenues towards this promising research area of designing more sustainable polymeric materials.
Author contributions
All authors contributed to the conceptualization and writing on the manuscript and helped in the critical reading of the final version of the work.Data availability
Data for this article includes the selection and organization of the most relevant catalytic details for the main complexes reported in all references consulted in the preparation of this feature article. They are available at ecienciaDATOS at https://doi.org/10.21950/MVYQII.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the following financial support: grant PID2020-117788RB-I00 funded by MCIN/AEI/10.13039/501100011033, grant RED2022-134287-T funded by MCIN/AEI, grants SBPLY/21/180501/000132 and SBPLY/22/180502/000059 funded by Junta de Comunidades de Castilla-La Mancha and by the EU through “Fondo Europeo de Desarrollo Regional” (FEDER), and grant 2021-GRIN-31240 funded by Universidad de Castilla-La Mancha. L. F. S.-B., A. L-S. and A. G. thank Prof. Antonio Otero and Dr Juan Fernández Baeza for their contributions to the development of the “scorpionates” in our group during their active scientific careers.References
- S. Trofimenko, J. Am. Chem. Soc., 1966, 88, 1899–1904 CrossRef CAS.
- S. Trofimenko, J. Am. Chem. Soc., 1967, 89, 3170–3177 CrossRef CAS.
- S. Trofimenko, J. R. Long, T. Nappier and S. G. Shore, Inorg. Synth., 1970, 12, 99–107 CrossRef CAS.
- S. Trofimenko, Chem. Rev., 1993, 93, 943–980 CrossRef CAS.
- S. Trofimenko, Scorpionates: The Coordination Chemistry Of Polypyrazolylborate Ligands, World Scientific Publishing Company, 1999 Search PubMed.
- A. Otero, J. Fernández-Baeza, A. Lara-Sánchez and L. F. Sánchez-Barba, Coord. Chem. Rev., 2013, 257, 1806–1868 CrossRef CAS.
- L. M. D. R. S. Martins and A. J. L. Pombeiro, Coord. Chem. Rev., 2014, 265, 74–88 CrossRef CAS.
- J. Reglinski and M. D. Spicer, Coord. Chem. Rev., 2015, 297–298, 181–207 CrossRef CAS.
- C. Pettinari, R. Pettinari and F. Marchetti, in Advances in Organometallic Chemistry, ed. P. J. Pérez, Academic Press, 2016, vol. 65, pp. 175–260 Search PubMed.
- L. M. D. R. S. Martins and A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2016, 2236–2252 CrossRef CAS.
- C. J. Carrano, Eur. J. Inorg. Chem., 2016, 2377–2390 CrossRef CAS.
- R. F. Semeniuc and D. L. Reger, Eur. J. Inorg. Chem., 2016, 2253–2271 CrossRef CAS.
- L. M. D. R. S. Martins, Catalysts, 2017, 7, 12 CrossRef.
- I. Alkorta, R. M. Claramunt, E. Díez-Barra, J. Elguero, A. de la Hoz and C. López, Coord. Chem. Rev., 2017, 339, 153–182 CrossRef CAS.
- L. M. D. R. S. Martins, Coord. Chem. Rev., 2019, 396, 89–102 CrossRef CAS.
- J. M. Muñoz-Molina, T. R. Belderrain and P. J. Pérez, Coord. Chem. Rev., 2019, 390, 171–189 CrossRef.
- J. M. Muñoz-Molina, T. R. Belderrain and P. J. Pérez, Dalton Trans., 2019, 48, 10772–10781 RSC.
- D. Ottaviani, V. Van-Dúnem, A. P. Carvalho, A. Martins and L. M. D. R. S. Martins, Catal. Today, 2020, 348, 37–44 CrossRef CAS.
- A. M. Andrade and M. D. R. S. L. Martins, Curr. Med. Chem., 2019, 26, 7452–7475 CrossRef PubMed.
- L. M. D. R. S. Martins, Inorg. Chim. Acta, 2022, 541, 121069 CrossRef CAS.
- P. J. Fischer, in Comprehensive Coordination Chemistry III, ed. E. C. Constable, G. Parkin and L. Que Jr, Elsevier, Oxford, 2021, pp. 428–504 Search PubMed.
- C. Hossack, C. Cahill and C. Besson, Dalton Trans., 2023, 52, 17656–17665 RSC.
- W. HücKel and H. B. Chneider, Ber. Dtsch. Chem. Ges., 1937, 70, 2024–2026 CrossRef.
- S. Juliá, J. M. del Mazo, L. Ávila and J. Elguero, Org. Prep. Proced. Int., 1984, 16, 299–307 CrossRef.
- D. L. Reger, T. C. Grattan, K. J. Brown, C. A. Little, J. J. S. Lamba, A. L. Rheingold and R. D. Sommer, J. Organomet. Chem., 2000, 607, 120–128 CrossRef CAS.
- A. de Palo, G. La Ganga, F. Nastasi, M. Guelfi, M. Bortoluzzi, G. Pampaloni, F. Puntoriero, S. Campagna and F. Marchetti, Dalton Trans., 2020, 49, 3341–3352 RSC.
- M. A. Andrade, A. S. Mestre, A. P. Carvalho, A. J. L. Pombeiro and L. M. D. R. S. Martins, Catal. Today, 2020, 357, 46–55 CrossRef CAS.
- D. Schäfer, F. Fink, D. Kleinschmidt, K. Keisers, F. Thomas, A. Hoffmann, A. Pich and S. Herres-Pawlis, Chem. Commun., 2020, 56, 5601–5604 RSC.
- S. Gabrielli, M. Pellei, I. Venditti, I. Fratoddi, C. Battocchio, G. Iucci, I. Schiesaro, C. Meneghini, A. Palmieri, E. Marcantoni, L. Bagnarelli, R. Vallesi and C. Santini, Dalton Trans., 2020, 49, 15622–15632 RSC.
- J.-C. Castillo, N.-F. Bravo, L.-V. Tamayo, P.-D. Mestizo, J. Hurtado, M. Macías and J. Portilla, ACS Omega, 2020, 5, 30148–30159 CrossRef CAS PubMed.
- C. Sun, G. Skorupskii, J.-H. Dou, A. M. Wright and M. Dincă, J. Am. Chem. Soc., 2018, 140, 17394–17398 CrossRef CAS PubMed.
- A. P. C. Ribeiro, P. Goodrich and L. M. D. R. S. Martins, Molecules, 2021, 26, 1089 CrossRef CAS PubMed.
- S. Sobrino, M. Navarro, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Lara-Sánchez, A. Garcés, J. A. Castro-Osma and A. M. Rodríguez, Polymers, 2020, 12, 2148 CrossRef CAS.
- A. Otero, J. Fernández-Baeza, L. F. Sánchez-Barba, S. Sobrino, A. Garcés, A. Lara-Sánchez and A. M. Rodríguez, Dalton Trans., 2017, 46, 15107–15117 RSC.
- A. Garcés, L. F. Sánchez-Barba, J. Fernández-Baeza, A. Otero, I. Fernández, A. Lara-Sánchez and A. M. Rodríguez, Inorg. Chem., 2018, 57, 12132–12142 CrossRef PubMed.
- M. Honrado, S. Sobrino, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, A. Lara-Sánchez and A. M. Rodríguez, Chem. Commun., 2019, 55, 8947–8950 RSC.
- J.-E. Park, S. K. Kang, J. O. Woo and K.-S. Son, Dalton Trans., 2015, 44, 9964–9969 RSC.
- S. V. Zubkevich, V. A. Tuskaev, S. C. Gagieva, A. S. Kayda, V. N. Khrustalev, A. A. Pavlov, D. N. Zarubin and B. M. Bulychev, Appl. Organomet. Chem., 2020, 34, e5873 CrossRef CAS.
- S. V. Zubkevich, V. A. Tuskaev, S. C. Gagieva, A. A. Pavlov, V. N. Khrustalev, O. V. Polyakova, D. N. Zarubin, D. A. Kurmaev, N. A. Kolosov and B. M. Bulychev, New J. Chem., 2020, 44, 981–993 RSC.
- F. Vidal, E. R. van der Marel, R. W. F. Kerr, C. McElroy, N. Schroeder, C. Mitchell, G. Rosetto, T. T. D. Chen, R. M. Bailey, C. Hepburn, C. Redgwell and C. K. Williams, Nature, 2024, 626, 45–57 CrossRef CAS.
- X. Zhang, M. Fevre, G. O. Jones and R. M. Waymouth, Chem. Rev., 2018, 118, 839–885 CrossRef CAS PubMed.
- S. Fukuoka, I. Fukawa, T. Adachi, H. Fujita, N. Sugiyama and T. Sawa, Org. Process Res. Dev., 2019, 23, 145–169 CrossRef CAS.
- Different alkenes are available from renewable resources that are the main raw materials for the preparation of bio-based epoxides. Cyclohexene oxide (CHO) is one of the most used epoxide substrates for the synthesis of different bio-based polymers such as polyesters and polycarbonates, via ROCoP with cyclic anhydrides or CO2, respectively. For this reason, while CHO is not considered renewable, it can be synthesized from bioderived resources. See also references: (a) B. M. Stadler, C. Wulf, T. Werner, S. Tin and J. G. de Vries, ACS Catal., 2019, 9, 8012–8067 CrossRef CAS; (b) F. De La Cruz-Martínez, J. A. Castro-Osma and A. Lara-Sánchez, Adv. Catal., 2022, 70, 189–236 CrossRef.
- M. L. Di Lorenzo and R. Androsch, Synthesis, Structure and Properties of Poly (lactic acid), Springer, 2018 Search PubMed.
- J. Qi, Y. Zhang, X. Liu, Q. Zhang and C. Xiong, New J. Chem., 2020, 44, 14632–14641 RSC.
- B. Tyler, D. Gullotti, A. Mangraviti, T. Utsuki and H. Brem, Adv. Drug Delivery Rev., 2016, 107, 163–175 CrossRef CAS.
- H. Bi, T. Feng, B. Li and Y. Han, Polymers, 2020, 12, 839 CrossRef CAS.
- F. Wu, M. Misra and A. K. Mohanty, Prog. Polym. Sci., 2021, 117, 101395 CrossRef CAS.
- N. Liu, D. Liu, B. Liu, H. Zhang and D. Cui, Polym. Chem., 2021, 12, 1518–1525 RSC.
- S. Inoue, J. Polym. Sci., Part A:Polym. Chem., 2000, 38, 2861–2871 CrossRef CAS.
- F. Drouin, P. O. Oguadinma, T. J. J. Whitehorne, R. E. Prud’homme and F. Schaper, Organometallics, 2010, 29, 2139–2147 CrossRef CAS.
- C. Bakewell, G. Fateh-Iravani, D. W. Beh, D. Myers, S. Tabthong, P. Hormnirun, A. J. P. White, N. Long and C. K. Williams, Dalton Trans., 2015, 44, 12326–12337 RSC.
- S. Choe, H. Lee and S. Nayab, RSC Adv., 2021, 11, 18840–18851 RSC.
- L. F. Sánchez-Barba, A. Garcés, J. Fernández-Baeza, A. Otero, C. Alonso-Moreno, A. Lara-Sánchez and A. M. Rodríguez, Organometallics, 2011, 30, 2775–2789 CrossRef.
- A. Garcés, L. F. Sánchez-Barba, J. Fernández-Baeza, A. Otero, M. Honrado, A. Lara-Sánchez and A. M. Rodríguez, Inorg. Chem., 2013, 52, 12691–12701 CrossRef.
- A. Garcés, L. F. Sánchez-Barba, J. Fernández-Baeza, A. Otero, A. Lara-Sánchez and A. M. Rodríguez, Organometallics, 2017, 36, 884–897 CrossRef.
- J. Qian and R. J. Comito, Inorg. Chem., 2022, 61, 10852–10862 CrossRef CAS PubMed.
- M. H. Chisholm, J. Gallucci and K. Phomphrai, Chem. Commun., 2003, 48–49 RSC.
- M. H. Chisholm, J. C. Gallucci and K. Phomphrai, Inorg. Chem., 2004, 43, 6717–6725 CrossRef CAS PubMed.
- M. H. Chisholm and N. W. Eilerts, Chem. Commun., 1996, 853–854 RSC.
- M. H. Chisholm, N. W. Eilerts, J. C. Huffman, S. S. Iyer, M. Pacold and K. Phomphrai, J. Am. Chem. Soc., 2000, 122, 11845–11854 CrossRef CAS.
- Z. Mou, B. Liu, M. Wang, H. Xie, P. Li, L. Li, S. Li and D. Cui, Chem. Commun., 2014, 50, 11411–11414 RSC.
- Z. Gu and R. J. Comito, Organometallics, 2022, 41, 1911–1916 CrossRef CAS.
- M. Tansky and R. J. Comito, Dalton Trans., 2023, 52, 8784–8791 RSC.
- M. Honrado, A. Otero, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, A. Lara-Sánchez and A. M. Rodríguez, Dalton Trans., 2014, 43, 17090–17100 RSC.
- M. Navarro, A. Garcés, L. F. Sánchez-Barba, F. de la Cruz-Martínez, J. Fernández-Baeza and A. Lara-Sánchez, Polymers, 2021, 13, 2356 CrossRef CAS.
- M. Navarro, S. Sobrino, I. Fernández, A. Lara-Sánchez, A. Garcés and L. F. Sánchez-Barba, Dalton Trans., 2024, 53, 13933–13949 RSC.
- M. Luna Barros, M. G. Cushion, A. D. Schwarz, Z. R. Turner and P. Mountford, Dalton Trans., 2019, 48, 4124–4138 RSC.
- A. Garcés, L. F. Sánchez-Barba, C. Alonso-Moreno, M. Fajardo, J. Fernández-Baeza, A. Otero, A. Lara-Sánchez, I. López-Solera and A. M. Rodríguez, Inorg. Chem., 2010, 49, 2859–2871 CrossRef.
- U. Herber, K. Hegner, D. Wolters, R. Siris, K. Wrobel, A. Hoffmann, C. Lochenie, B. Weber, D. Kuckling and S. Herres-Pawlis, Eur. J. Inorg. Chem., 2017, 1341–1354 CrossRef CAS.
- F. de la Cruz-Martínez, M. Martínez de Sarasa Buchaca, A. del Campo-Balguerías, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, C. Alonso-Moreno, J. A. Castro-Osma and A. Lara-Sánchez, Polymers, 2021, 13, 1651 CrossRef.
- E. Francés-Poveda, M. Martínez de Sarasa Buchaca, C. Moya-López, I. J. Vitorica-Yrezabal, I. López-Solera, J. A. Castro-Osma, F. de la Cruz-Martínez and A. Lara-Sánchez, Polym. Chem., 2024, 15, 3238–3245 RSC.
- D. M. Lyubov, A. O. Tolpygin and A. A. Trifonov, Coord. Chem. Rev., 2019, 392, 83–145 CrossRef CAS.
- Z. Zhang and D. Cui, Chem. – Eur. J., 2011, 17, 11520–11526 CrossRef CAS.
- Z. Mou, B. Liu, X. Liu, H. Xie, W. Rong, L. Li, S. Li and D. Cui, Macromolecules, 2014, 47, 2233–2241 CrossRef CAS.
- N. Y. Rad’kova, A. V. Cherkasov and A. A. Trifonov, Russ. J. Coord. Chem., 2023, 49, 710–717 CrossRef.
- A. Scrivanti, M. Bortoluzzi and M. Gatto, Chem. Pap., 2016, 70, 53–60 CAS.
- A. Otero, J. Fernández-Baeza, A. Lara-Sánchez, C. Alonso-Moreno, I. Márquez-Segovia, L. F. Sánchez-Barba and A. M. Rodríguez, Angew. Chem., Int. Ed., 2009, 48, 2176–2179 CrossRef CAS PubMed.
- R. Jianming, X. Anguo, W. Hongwei and Y. Hailin, Des. Monomers Polym., 2014, 17, 345–355 CrossRef.
- A. Otero, J. Fernández-Baeza, A. Garcés, L. F. Sánchez-Barba, A. Lara-Sánchez, J. Martínez-Ferrer, M. P. Carrión and A. M. Rodríguez, Dalton Trans., 2017, 46, 6654–6662 RSC.
- F. d la Cruz-Martínez, M. M. d Sarasa Buchaca, J. Fernández-Baeza, L. F. Sánchez-Barba, A. M. Rodríguez, C. Alonso-Moreno, J. A. Castro-Osma and A. Lara-Sánchez, Organometallics, 2021, 40, 1503–1514 CrossRef.
- J. Martínez, J. A. Castro-Osma, A. Lara-Sánchez, A. Otero, J. Fernández-Baeza, J. Tejeda, L. F. Sánchez-Barba and A. Rodríguez-Diéguez, Polym. Chem., 2016, 7, 6475–6484 RSC.
- F. de la Cruz-Martínez, M. Martínez de Sarasa Buchaca, J. Martínez, J. Tejeda, J. Fernández-Baeza, C. Alonso-Moreno, A. M. Rodríguez, J. A. Castro-Osma and A. Lara-Sánchez, Inorg. Chem., 2020, 59, 8412–8423 CrossRef PubMed.
- M. Martínez de Sarasa Buchaca, F. de la Cruz-Martínez, L. F. Sánchez-Barba, J. Tejeda, A. M. Rodríguez, J. A. Castro-Osma and A. Lara-Sánchez, Dalton Trans., 2023, 52, 3482–3492 RSC.
- A. Nag, A. E. Arifianti, A. Khankhuean and H. Ajiro, Eur. Polym. J., 2024, 214, 113146 CrossRef CAS.
- J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef.
- J. Martínez, F. de la Cruz-Martínez, M. Martínez de Sarasa Buchaca, J. Fernández-Baeza, L. F. Sánchez-Barba, M. North, J. A. Castro-Osma and A. Lara-Sánchez, ChemPlusChem, 2021, 86, 460–468 CrossRef.
- C. Li, L. Wang, M. Wang, B. Liu, X. Liu and D. Cui, Angew. Chem., Int. Ed., 2019, 58, 11434–11438 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |






