Emerging microelectronic microneedles (eMN) for biomedical applications
Shu
Zhou
,
Qian
Zhou
,
Xin
Li
* and
Bingbing
Gao
 *
*
School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing 211816, China. E-mail: lixincc@njtech.edu.cn; gaobb@njtech.edu.cn
First published on 3rd June 2024
Abstract
Microneedles, an emerging medical tool, have attracted significant attention for their superior ability to puncture the skin noninvasively and painlessly, facilitating tasks such as physiological monitoring, disease diagnosis, and transdermal drug delivery. However, if microneedles are used alone, the efficiency of drug delivery or physiological substance extraction is still not very efficient. In recent years, the rapid development of microelectronic microneedle (eMN) devices, integrating microneedle technology with microelectronics, has solidified the foundation of MN-based percutaneous disease diagnosis and treatment platforms. However, comprehensive review reports on this topic remain scarce. This paper systematically reviews the research progress in the manufacture, processing and biomedical applications of eMN devices. Initially, we present an overview of the materials used in eMNs, including MN-fabrication materials and substrate materials that enhance electrical conductivity. Subsequently, we delve into the various eMN preparation techniques, including micromolding, etching, laser cutting, and 3D printing, along with suitable power sources such as batteries adaptable to diverse materials. Additionally, we elaborate on the mechanism of action of these eMN devices, including ionophoresis, electroporation, thermal stimulation, and ultrasound. Finally, we summarize the application progress of some typical eMN devices in drug delivery and physiological monitoring. By perusing this article, readers can gain valuable insights into the design of eMN medical devices, thereby catalyzing the advancement of intelligent and personalized medicine.
1. Introduction
Microneedles (MNs) were initially proposed as groundbreaking medical tools for drug delivery in 1976.1 These minuscule needles, which typically range from 150 μm to 1000 μm in length, possess the remarkable ability to penetrate the skin barrier, creating channels within the epidermis. Initially, designed for drug delivery systems, their advantages of noninvasiveness, painless application, and low infection rates following skin penetration have led to their broadened application. Currently, MNs are utilized not only for drug delivery but also for extracting biomarkers from interstitial fluid (ISF) for physiological monitoring, disease diagnosis, and various other research areas, including wound healing.2–4 ISF, derived from blood filtered through capillaries, exhibits a high degree of similarity to blood components, thus emerging as a promising alternative to blood as a source for biomarker detection.5 However, the sole reliance of MNs on the free diffusion of drug molecules and their limited drug-carrying capacity has constrained the broader application of MN technology in drug delivery. Fortunately, the recent emergence of devices that integrate microneedles with microelectronic technologies has effectively addressed this challenge, paving the way for innovative and efficient drug delivery systems.These microneedle-based microelectronic (eMN) devices, which often employ physical or chemical means such as iontophoresis, electroporation, thermotherapy, and ultrasound, are designed to collaborate with microneedles. This collaboration accelerates both drug delivery and the extraction of target molecules.6–8 These methods, which are already widely used in clinical settings, effectively aid in the rapid delivery of drugs to deeper tissues. By harnessing the precision of these eMN devices, we can significantly enhance the local delivery of drugs, thereby minimizing systemic side effects. This targeted delivery is particularly beneficial in diseases that require chemotherapeutic agents, such as cancer, as it allows direct application to lesions while sparing healthy organs from harmful effects.4 Moreover, the integration of MNs with microelectronic medical devices can significantly improve the efficiency of extracting target substances. This is achieved through the utilization of techniques such as reverse iontophoresis and electroosmotic flow.9 As a result, the combination of MNs with these advanced microelectronic devices is poised to revolutionize the development of wearable medical technology, ushering in a new era of personalized and efficient healthcare.
Despite the plethora of reviews on microneedles, focusing on their preparation methods, needle designs, and applications, there remains a gap in systematically discussing their integration with microelectronic devices. In this article, we present a comprehensive review dedicated to eMN devices. We delve into the materials and preparation techniques suitable for microneedles in microelectronic applications, explore the power sources of these microelectronic devices, and meticulously outline the physiological mechanisms underlying their effectiveness.
By elucidating these physiological mechanisms, we aim to enhance the reader's understanding of why these eMN medical devices are pivotal in drug delivery and ISF extraction. Additionally, we present illustrative examples and highlight the advantages of these cases, hoping to serve as a valuable reference for fellow researchers. We anticipate the future directions and prospects of eMN medical devices, expecting them to revolutionize healthcare delivery (Fig. 1).
2. Materials for the eMN devices
A diverse array of materials, including metal and ceramic MNs, have been employed in the fabrication of MNs. However, polymeric MNs have garnered significant attention from researchers due to their exceptional biocompatibility and biodegradability.10,11 The selection of materials for MNs is highly dependent on the intended application. Factors such as biocompatibility, solubility, mechanical strength, and electrical conductivity must be carefully considered for different targets. Currently, a broad range of materials are utilized for various MN applications. For instance, hard and highly biocompatible materials are frequently used in the fabrication of hollow MNs for drug delivery, while soluble materials are preferred for painless drug delivery through dissolvable MNs loaded with drugs.12 Given the integration of MNs with microelectronics, materials with high electrical conductivity are essential for minimizing microcurrent losses. Therefore, we provide a summary of commonly used MN materials and delve into conductive polymer materials suitable for eMN applications. The materials discussed in this paper for MN fabrication are outlined in Table 1.| Material | Advantages | Limitations | Application for MN | Ref. |
|---|---|---|---|---|
| AuNPs | Conductivity, antibacterial, low toxicity | Differences in size distribution may lead to differences in properties | AuNPs deposited microneedles biosensor for glucose sensing | 13 |
| PPy | High conductivity, low cytotoxicity | Poor dispersion | Microneedles for electronically controlled drug release | 14 |
| PEDOT:PSS | High conductivity, excellent processability | Poor mechanical properties | Microneedles based on electrical stimulation to promote dental anesthesia | 15 |
| PANI | Conductivity, processability stability | Infusibility | Microneedle sensor for monitoring multiple metabolites in sweat | 16 |
| SWCNTs | Conductivity, formability, biocompatibility | High cost of production | Real-time monitoring and early warning of cytokine storms in the body with microneedle patches | 17 |
| MXene | Conductivity, biocompatibility, acid/base resistance | Difficult to synthesize | Microneedles for monitoring muscle contraction and electrical stimulation therapy | 18 |
| PU/SF | Flexible, biocompatibility | Low toughness | Flexible microneedle dressing for wound management | 19 |
| Si | Conductivity, processability | Low conductivity | Microneedle platform system for glucose monitoring and insulin release | 20 |
| PLA | Thermoplastic, biocompatible | Poor toughness low degradation rate | Closed loop intelligent glucose control mini system | 21 |
| Stainless steel | Conductivity, stiffness | Difficult to process | Use of electroporation to promote vaccination | 22 |
| Mg | Conductivity, biocompatibility | Easy to degrade | Implantable absorbable microneedles for radiotherapy and drug delivery | 23 |
2.1 Substrate materials for MNs
In prior scientific inquiries, metal-based materials, polymers, silicon, hydrogels, along with ceramics, were the principal materials of choice. In particular, metals and certain polymers have garnered significant attention. Metals and alloys exhibit superior mechanical properties, stability, and corrosion resistance, rendering them suitable for fabricating MNs that possess robust dermal resistance and enduring durability. For instance, gold and gold nanoparticles, with a conductivity reaching approximately 4.1 × 107 S m−1, are frequently employed as coatings to enhance the conductivity of MN electrodes.24–26 As depicted in Fig. 2(a), the initial high-density Au–Si MN sensors coated with gold to enhance conductivity exhibited a sensitivity of 0.1622 μA mM−1 cm−2 within the detection range of 1–9 mM glucose. This advanced MN-sensing device holds promise as a replacement for traditional glucose monitoring methods.25Stainless steel, which is renowned for its mechanical robustness and biocompatibility, is a preferred material for fabricating metal MNs. The Young's modulus of stainless steel is about 200 GPa, depends on its elemental composition. Fig. 2(b) illustrates the application of stainless steel MNs in electroporation for efficient and cost-effective DNA vaccination.27 Additionally, metal MNs loaded with drugs combined with electrical stimulation have been reported to enhance subcutaneous drug delivery for inflammatory skin diseases. Briefly, stainless steel is extensively utilized in the preparation of MNs for iontophoresis, electrochemical sensing, and various other functionalities.22,28–33
Despite their utility, most metal MNs suffer from limited biocompatibility, which can lead to inflammatory reactions. Their preparation is also challenging due to the hardness of metals. Consequently, liquid metals and polymers have garnered significant attention in recent years. Zhang et al. developed a liquid metal (LM)-encapsulated MN patch combined with electrical stimulation (ES) to accelerate wound healing.3 This liquid metal MN is depicted in Fig. 2(c). Polymers are extensively used for MN fabrication due to their excellent biocompatibility, cost-effectiveness, and ease of manufacturing. Materials such as polylactic acid (PLA), polymethyl methacrylate (PMMA), poly(lactic-co-glycolic acid) (PLGA) (as shown in Fig. 2(d) for a PLGA microneedle), polyurethane (PU), silk fibroin (SF), hyaluronic acid (HA), and resins are commonly employed.23,34–36 These polymers are often blended with other materials to achieve desired outcomes. Herein, we primarily focus on PLA and PU/SF resins.
Polylactic acid (PLA), a thermoplastic derived from lactic acid, exhibits exceptional biocompatibility, mechanical strength (with a Young's modulus ranging from 1 to 6 GPa), biodegradability, and processing ease. Its versatility has led to widespread applications in agriculture, textiles, biomedicine, and beyond.37 Given these advantages, PLA has become a popular choice for fabricating MNs, as exemplified by the well-formed PLA MNs depicted in Fig. 2(e). Diabetes mellitus, a prevalent condition that can be effectively managed, can lead to severe sequelae such as retinopathy and nephropathy if not appropriately treated. Recently, a study introduced an innovative closed-loop system for glucose monitoring and insulin delivery, leveraging PLA as the MN material.21 The selection of PLA was based on its robust mechanical properties, low solubility in aqueous solutions, and favorable processing characteristics. In this system, PLA MNs serve dual functions: facilitating blood glucose detection through the dermis and enabling painless insulin administration beneath the skin. This closed-loop setup enables real-time blood glucose control, offering diabetic patients additional management options. Additionally, PLA is frequently combined with other conductive materials to enhance the electrical conductivity of MNs, further broadening its applications in this field.19,26Fig. 2(f) illustrates a PU/SF-based flexible MN dressing designed for wound management. The experimental data demonstrated its robust skin adhesion and promising applications in biochemical analysis and motion sensing. Biocompatible photosensitive resins, a specialized category of resins, possess both biocompatibility and photosensitivity, enabling their utilization in 3D printing or Wiener manufacturing to fabricate miniature medical devices that require intimate interaction with the body. These resins facilitate high-precision 3D printing, enabling the creation of intricate, miniature structures. As depicted in Fig. 2(g), a microneedle equipped with a microchannel structure was fabricated using a biocompatible photosensitive resin to facilitate efficient drug delivery.4 While the aforementioned polymers are prime candidates for MN selection, the integration of other materials with excellent conductivity is necessary to confer high conductivity to the MNs for medical applications such as electrostimulation, electroporation, and iontophoresis. In the subsequent section, we delve into the realm of (semi)conducting materials.
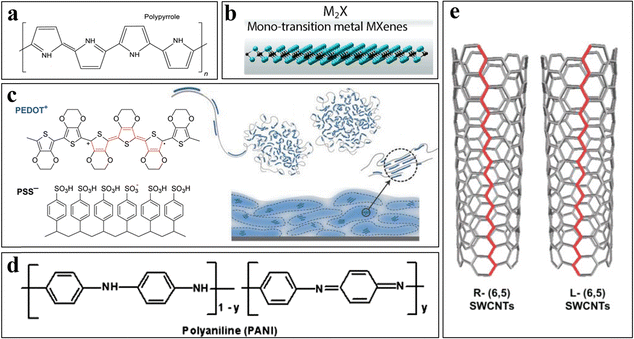
2.2 Electrically enhanced materials for eMN
When selecting materials for MNs, conductivity is paramount, particularly for biosensing applications or any medical requirements involving electrical signals. Conductive materials are crucial for eMN due to their ability to efficiently detect, amplify, and transmit electrical signals with minimal loss, enabling accurate health status monitoring. Additionally, these materials facilitate electrical stimulation, accelerating disease healing by activating specific physiological processes.3,23,38 Furthermore, in brain–computer interface research, conductive MNs enable direct interactions between artificial devices and the brain's electrical signals, which is crucial for exploring novel treatment options.39,40 Typically, these materials are not employed exclusively in the fabrication of eMN but are frequently blended with a variety of conventional substances to fine-tune their conductivity properties. Among the materials commonly utilized to enhance the conductivity of MN are polypyrrole (PPy), MXene, PEDOT:PSS, polyaniline (PANI), and SWCNTs. This approach ensures that the desired electrical characteristics are achieved, thus optimizing the performance of the eMN for various applications.PPy, an organic polymer renowned for its exceptional electrical conductivity, attributes this property to its conjugated double bond structure within its molecular framework (Fig. 3(a)).41 Moreover, PPy has widespread application in medical devices, primarily due to its robust chemical stability and biocompatibility.14,26,42Fig. 2(h) shows an MN with an impeccable morphology fabricated through the integration of dopamine and PPy. Notably, dopamine (DA) plays a pivotal role in enhancing the dispersion of PPy.43 In the context of spinal cord injury, nerve cells often undergo inflammatory responses. Intriguingly, PPy microneedles have been demonstrated to suppress these inflammatory responses through the electronically modulated release of the steroid dexamethasone.14 This discovery offers novel insights into the potential applications of PPy MN arrays. Nevertheless, despite its promising medical applications, further investigation and optimization of the biostability and durability of PPy remain crucial.
MXenes, initially discovered by Drexel University in 2011, constitute a class of two-dimensional (2D) materials primarily composed of transition metal carbides (TMCs), nitrides (TMNs), and carbonitrides (TMCNs).44 Their chemical formula, Mn+1XnTχ, encapsulates the essence of their composition, with M representing a transition metal, χ denoting carbon or nitrogen, T standing for surface functional groups, and n indicating the number of atomic layers. Fig. 3(b) shows a vivid depiction of the structural configuration of M2X, a prototypical MXene. Owing to their exceptional electrical conductivity, biocompatibility, expansive surface area, and robust mechanical and physical properties, MXenes have garnered widespread attention in diverse fields, including materials technology, biomedicine, and agriculture.3,45,46 Notably, MXenes have been seamlessly integrated with MNs for biosensing and electrostimulation applications, as shown in Fig. 2(i). This innovative system, capable of capturing muscular signals and regulating drug delivery, represents a promising therapeutic avenue for patients suffering from neuromuscular disorders.18
PEDOT:PSS, a blend of poly(3,4-ethylenedioxythiophene) and poly(4-methylthiophene-3-sulfonic acid), exhibits remarkable properties, as shown in Fig. 3(c). PEDOT, an electrically conductive monomer, possesses a conjugated structure that enables it to withstand bending and deformation, rendering it an ideal candidate for the fabrication of flexible electronic devices. Conversely, PSS serves as a dispersant, effectively counteracting the aggregation tendencies of PEDOT, thereby enhancing the overall performance of the material. Furthermore, PSS contributes to improving the stability of PEDOT, ensuring the durability and chemical resilience of the device.47 Despite its mechanical limitations, PEDOT:PSS has been widely applied in the production of MNs due to its exceptional electrical conductivity, biocompatibility, processability, and flexibility. These MNs have been utilized in the preparation of various devices associated with electrical signals.15,48,49 In a study exploring the use of electrically conductive MNs for dental anesthesia via iontophoresis (Fig. 2(j)), the integration of PEDOT:PSS significantly increased the electrical conductivity of the MNs by twofold and reduced skin resistance in mice by 30%.15 Additionally, PEDOT:PSS was successfully combined with PLGAs, which are known for their mechanical robustness, to fabricate MNs capable of monitoring electrophysiological signals in plants.49
Polyaniline (PANI), an organic polymer with remarkable conductivity, chemical stability, and mechanical durability, is extensively employed in MN fabrication, as exemplified in Fig. 2(k). Its redox properties distinguish it from those of other conductive materials, making it promising for widespread applications in electrochemical biosensing.50,51 Furthermore, the cost-effectiveness of PANI, which is 80% less than that of PEDOT:PSS, facilitates its mass production. Its conductivity and pH responsiveness have been exploited in the creation of MNs for electrochemical sensing, achieving a pH detection range of 4.25–10, which is suitable for clinical use.16 In addition, PANI has been used in iontophoresis-assisted conductive MNs for the percutaneous delivery of dextran.52
Carbon nanotubes (CNTs), which are tubular structures composed of carbon atoms arranged in a honeycomb pattern, can be classified into two primary types: single-walled CNTs (SWCNTs) and multiwalled CNTs (MWCNTs). SWCNTs exhibit optical heterogeneity, as demonstrated in Fig. 3(e). These nanotubes possess exceptional electrical conductivity due to their superior electron transport properties, while MWCNTs are renowned for their remarkable toughness. SWCNTs have garnered widespread application in nanomaterials, biosensors, and wearable medical devices due to their exceptional electrical and thermal conductivity.53–56 Consequently, they are ideal candidates for the fabrication of eMNs, as illustrated in Fig. 2(l). A recent study on sensing MNs for real-time monitoring and early warning of cytokine storms in vivo utilized SWCNTs to capture cytokines and facilitate electrical transduction. Unlike previous wearable sensors that primarily detect small biomarkers, this study introduced a novel, efficient, and convenient method for monitoring macromolecular biomarkers, specifically cytokines, which play pivotal roles in pathological and physiological processes such as inflammation, immune response, and cellular development.17,57 This advancement holds significant promise for enhancing immune status monitoring in sepsis patients.
Furthermore, silicon, a prevalent and versatile semiconductor material, holds a pivotal position within the electronics industry. Our group has previously conducted a comprehensive review on the utilization of silicon for MN fabrication, which will not be duplicated in this current review.58
3. Fabrication strategies of eMN deveices
Various methods have been employed in the fabrication of MNs, including micromolding, injection molding, hot embossing, 3D printing, and two-photon polymerization. The selection of a manufacturing method hinges upon factors such as the chosen material, intended application, and specific requirements. In this review, we offer insights into four MN fabrication techniques endorsed by our peers. Alongside detailing each method, we delineate the compatible materials and target applications. Furthermore, we thoroughly evaluate the merits and demerits of each approach.3.1 Micromolding
The mold method stands out as a conventional approach for fabricating MNs, enjoying widespread adoption in MN preparation. As illustrated in Fig. 4(a), the core process unfolds as follows: (1) Initially, a negative mold is crafted by embedding a well-formed MN model procured from a reputable institution within a semisolid or molten mold material. The commonly employed polydimethylsiloxane (PDMS) is prized for its thermal stability, reproducibility, minimal adhesion, facile processing, and cost-effectiveness.59 Additionally, our research group reported promising outcomes utilizing Ecoflex as a mold material.60 (2) The negative mold, featuring slots mirroring the MN model, emerges after the removal of the MN model postdrying. Alternatively, a laser is utilized to directly perforate holes into the solidified negative mold, yielding negative mold slots embodying the MN morphology. (3) Subsequently, the mold is infused with the chosen MN material-liquid or molten-suitable for flowing into and occupying the negative slots, followed by vacuum treatment to ensure complete filling. (4) Final solidification involves various methods tailored to the specific materials employed, such as ultraviolet radiation or thermal treatment. Postsolidification, the fabricated MNs detached from the mold, mirroring the initial MN model. This method is ideal for fabricating MNs when the manufacturing material is in a liquid or molten state, particularly when accommodating intricate structures. For instance, the fabrication of microneedles containing microvias necessitates the incorporation of a porogenic agent or additional treatment of the formed microneedles.6,61 Various common polymers, including PU/SF, PPy, MXene, and other electrically conductive materials, as discussed earlier, are well suited for MN fabrication via this method.19,62–64 For example, Li et al. utilized the micromolding technique to fabricate mesoporous MNs. Initially, a negative mold was created. Subsequently, poly(glycidyl) methacrylate (PGMA) and polyethylene glycol (PEG) (as a porogen) were mixed in 2-methoxyethanol and poured into a PDMS mold. After UV irradiation and peeling, the desired mesoporous MNs were successfully obtained.6Furthermore, the microneedles produced by this laboratory method are typically fabricated in batches, resulting in a relatively time-consuming process. Nevertheless, we believe that the efficiency of this method can be significantly enhanced through industrialized design and production. Despite this limitation, this method remains the most widely utilized for MN preparation because of its simplicity and low cost.
3.2 Etching
Etching methods include chemical etching and physical etching, which are suitable for some hard materials, such as stainless steel and metal, as well as silicon for the manufacture of MNs. Chemical etching entails leveraging chemical reactions to selectively corrode designated regions of the material while preserving specific sections, whereas physical etching involves the removal of material segments through mechanical impact and cutting, thereby sculpting the desired morphology.65 The procedural essence of chemical etching can be distilled into several pivotal steps: initially, a protective agent is applied to shield areas slated for preservation; subsequently, the material is immersed in an etching solution, effectuating the removal of unprotected regions and thus delineating the rudimentary framework of the MN structure; ultimately, the specimens undergo meticulous cleansing to eradicate residual etchant and the protective layer, followed by surface refinement through subsequent processing iterations. However, notwithstanding its efficacy, chemical etching may encounter challenges stemming from the nonuniform distribution of the protective and etching agents, thereby compromising the homogeneity of the MN surface roughness, rendering it suitable only for select material substrates. Conversely, physical etching, characterized by mechanical ablation, entails the translation of a preconceived MN pattern into computer-aided design (CAD) software, which is then executed through a precision cutting apparatus. Although this technique accommodates a broader spectrum of materials, such as stainless steel and silicon, the process is susceptible to inducing morphological inconsistencies in comparison with the target owing to residual stresses inherent in the substrate. Residual stresses are internal stresses in an object caused by cutting, machining and other processes. The presence of residual stress may cause deformation of the surface of the object, changes in the internal structure and thus changes in physical and chemical properties. In the case of MNs, since their substrate layer is generally very thin, they are more susceptible to deformation and changes in properties due to residual stress during etching.66–68 Moreover, the burgeoning domain of deep reactive ion etching (DRIE) has garnered considerable attention as a nascent micro- and nanofabrication paradigm. DRIE entails etching substrates via an ion beam to sculpt intricate three-dimensional structures, distinguished by a prodigious aspect ratio.69,70 The inherent merits of DRIE include unparalleled precision and controllability, albeit tempered by the potential deleterious effects of ion aggressiveness toward the substrate. As depicted in Fig. 4(b), researchers have adeptly harnessed physical etching to construct an MN-incorporated microelectrical sensor array platform for multimodal detection of protein biomarkers in vivo. By employing physical etching initially to fashion the microneedles, they mitigated residual stress effects by opting for a thicker substrate (initial thickness: 750 μm), culminating in the fabrication of the final microneedle elements through DRIE technology.713.3 Laser cutting
Laser cutting epitomizes a precision material processing modality, wherein a laser beam, laden with ultrahigh energy, swiftly sculpts a material into the desired configuration. Widely embraced in the manufacture of microneedles (MNs), laser cutting boasts exceptional precision and efficiency, mitigating the thermal deformation and residual stress inherent in conventional mechanical cutting methods. Its ubiquity extends across diverse sectors, encompassing medical devices, electronics, and aerospace endeavors.72 The underlying principle of laser cutting resides in focusing the laser beam's energy onto a designated point of the workpiece, precipitating rapid temperature elevation to the point of material liquefaction or sublimation. Controlled by a numerical control system, the trajectory of the laser beam orchestrates targeted and precise incisions on the workpiece.73 Laser engraving, contingent upon the laser type, predominantly features fiber lasers, CO2 lasers, and UV lasers. In this discourse, we accentuate the salient attributes of fiber lasers and CO2 lasers, both of which are pivotal in MN fabrication.3.4 3D Print
Three-dimensional (3D) printing, an additive manufacturing technique, facilitates the transformation of digital designs into physical models. This versatile technology encompasses five main types: fused deposition modeling (FDM), stereolithography (SLA), continuous liquid interface (CLIP), digital light projection (DLP), and two-photon polymerization (2PP).79 Each method has distinct advantages and limitations; for instance, while FDM is cost effective and simple, it suffers from lower resolution. In contrast, emerging methods such as 2PP exhibit remarkably high resolution, which is ideal for intricate processing tasks.80 Given the exigencies of manufacturing MNs with precise and intricate structures, 3D printing has emerged as a highly efficient and customizable solution. The process typically involves several key steps: initially, the desired MN model is meticulously designed using CAD software, meticulously considering parameters such as shape, needle length, diameter, and specialized features such as cavities or micro-orifices. Subsequently, suitable composite materials, tailored to the specific printing method, are meticulously selected. Common materials include photosensitive resins and acrylates.80 The final step entails the utilization of 3D printers to actualize the predesigned MN morphology. Widely embraced across various research endeavors, 3D printing has revolutionized MN fabrication.4,14,42,81,82 For instance, Chang et al. leveraged high-precision 3D printing to construct microneedles featuring microchannels for drug delivery in cancer treatment (Fig. 4(e)). This innovative approach facilitated highly efficient localized drug delivery through electrophoresis and triggered cellular electroporation via an applied concentrated electric field, effectively mitigating potential drug toxicity to normal tissues.4In this part, we explore four prominent microneedle (MN) fabrication techniques: micromolding, etching, laser cutting, and 3D printing. Each method has distinct advantages and limitations that inform their application based on material choice and specific requirements. Micromolding is a traditional method known for its simplicity and cost-effectiveness, ideal for producing MNs with intricate structures, though it is time-consuming and best for batch production. Etching, including chemical and physical methods, is suitable for hard materials like stainless steel and silicon but can lead to nonuniform surfaces and residual stress issues. Laser cutting offers high precision and efficiency, minimizing thermal deformation, and is widely used in medical devices and electronics, with fiber and CO2 lasers being particularly effective. 3D printing stands out for its high efficiency and customization capabilities, suitable for complex MN structures, though it can be more expensive. In summary, micromolding and 3D printing are expected to lead MN manufacturing due to their cost and complexity advantages, respectively. Hybrid approaches combining these methods could optimize production efficiency and quality. For example, combining CO2 lasers with micromolding technology enables better fabrication of structurally complex microneedles. The choice of method should be guided by application needs, material properties, and production scale.
4. Power supplying for the eMN devices
In the design of a microelectronic medical device, the power supply of the device serves as a pivotal factor that deserves utmost attention. A compact, high-energy, and portable power supply can effectively aid in the seamless functioning of microelectronic medical devices, thereby enhancing their overall performance and reliability. The choice of power source depends on the device's design, function, and target requirements. Given their diminutive size and weight, these devices demand wearable configurations with optimal performance, thereby mandating power supply modules that minimize space and weight occupation. Presently, the prevalent power sources for microelectronic medical devices include button batteries and external power supplies. Additionally, the integration of MNs with microelectronic devices allows in vivo enzymatic reactions with the help of MNs pierced under the skin and power generation via triboelectric nanogenerator (TENG) technology. This article introduces four distinct power sources and their respective applications.Capacitors and batteries constitute distinct electronic components: capacitors store energy via charge accumulation between electrodes, facilitating rapid charging and discharging, which is ideal for scenarios requiring quick response and short-term energy storage. Conversely, batteries convert chemical energy into electrical energy through chemical reactions, offering larger energy storage suitable for stable, long-term power supply needs. However, the emergence of supercapacitors, electrochemical capacitors, and two-dimensional material-based capacitors has blurred the traditional boundaries between batteries and capacitors. Consequently, the field of microelectronic medical devices increasingly relies on these evolving capacitors (batteries) as energy sources.83–85Fig. 5(a) depicts a self-charging supercapacitor employing glucose oxidase, featuring a structure comprising a PDMS platform, a PDMS microneedle, an electrolyte gel, and rGO electrodes. (Reduced graphene oxide (rGO): a material obtained by oxidizing and reducing graphene. rGO has good electrical conductivity, thermal conductivity, and mechanical properties and has a wide range of applications in electronic devices and energy storage materials.86,87) Glucose oxidase-coated MNs, augmented with PEDOT:PSS and PVA, catalyze glucose oxidation in interstitial fluid, initiating self-charging. This innovative approach combines MN glucose sensing with self-charging supercapacitors, offering continuous power for diabetic glucose monitoring.64
Conventional power sources typically include batteries and external power supplies. Fig. 5(b) illustrates a glucose sensor that incorporates reverse iontophoresis, utilizing a coin battery as the primary source of electrical energy for this process.28 The primary advantage of batteries lies in their convenience and accessibility. Nevertheless, their disadvantages are more pronounced. For instance, their limited capacity necessitates frequent replacements, which can potentially compromise the stability and reliability of microelectronic medical devices. Additionally, their size and weight constrain the flexibility and comfort of the device, while the hazardous substances contained within the device may pose risks to the environment and human health. Researchers have also explored the utilization of smartphones as a power source for iontophoresis in insulin delivery, as depicted in Fig. 5(c). However, microelectronic devices are susceptible to damage due to the need for connection to an external power source, along with the inherent disadvantages of discontinuity and inconvenience. These limitations highlight the need for alternative, reliable, and sustainable power sources for microelectronic medical devices.35
Nanogenerators, devices adept at transforming minute amounts of mechanical energy into electrical power, leverage piezoelectric and friction-induced phenomena, rendering them pivotal in self-powered systems. Among these, triboelectric nanogenerators (TENGs) stand out as a burgeoning power generation paradigm pioneered in 2012. TENGs harness friction or relative motion between diverse materials to generate electrical energy. Typically, composed of dual layers featuring disparate electrophilic properties, TENGs operate through the transfer of electrostatic charges during friction, where electrons gravitate toward the more electrophilic material due to horizontal stratification, culminating in a potential difference. Upon integration into an external circuit, this disparity yields a current flow,88 as shown in Fig. 5(d). TENGs boast formidable energy conversion capabilities, facilitating mechanical energy harvesting below 3 Hz and yielding high voltage and low current outputs, all of which are achieved via a straightforward, cost-effective fabrication process. Their inherent simplicity, cost efficiency, flexibility, and eco-friendliness render them ubiquitous in the realm of microelectronic medical devices.89 Numerous applications, particularly in microneedling and microfluidics, rely on TENGs as primary energy sources.26,27,78,90 For example, Feng et al. used polytetrafluoroethylene (PTFE) and polyamide 66 (PA66) films, which have opposite abilities to gain and lose electrons, as friction layers to construct a TENG to generate a voltage as an energy source for electrical stimulation in combination with MN for psoriasis treatment. With a maximum power density of 28 mW m−2, remarkable mechanical–electrical energy conversion efficiency, and enduring voltage stability after 5000 contact-detachment cycles,27 this endeavor underscores the profound practicality of TENGs in microelectronic medical devices. Overall, the integration of innovative power sources such as self-charging supercapacitors and TENGs holds significant promise for advancing microelectronic medical devices, offering enhanced performance, sustainability, and patient comfort.
5. Physiological mechanisms of eMN devices
5.1 Iontophoresis and reverse iontophoresis
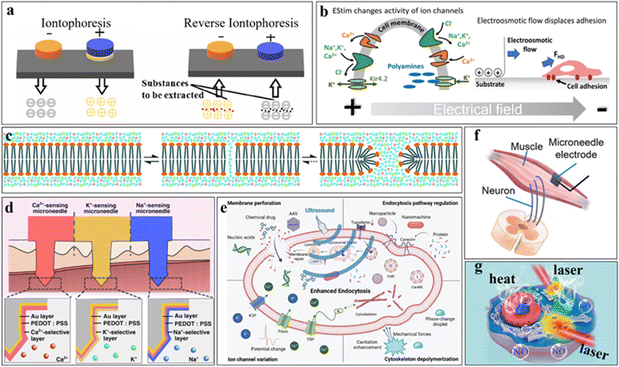
Iontophoresis and reverse iontophoresis represent innovative approaches to facilitate transdermal delivery of drugs and biomolecules through the application of an electric field. This technology, characterized by its noninvasive and safe nature, holds significant promise in the realm of medical therapeutics. The underlying principle involves the directed movement of charged molecules from an electrode sharing the same polarity as the molecule toward an electrode of opposite polarity (Fig. 6(a)). In contrast to reverse iontophoresis, which uses an applied electric field to assist in the extraction of ions or molecules in the ISF under the skin, ionontophoresis is commonly used for the percutaneous delivery of already ionized drugs into the body.Iontophoresis has emerged as a highly effective modality for noninvasive transdermal drug delivery, garnering widespread utilization in the treatment of localized ailments such as pain, inflammation, infection, and cancer.6,35,62,63 This technique leverages an applied electric field to facilitate the penetration of drugs into deep tissues, enabling precise and targeted delivery. Fundamentally, negatively charged drug ions are repelled by the cathode, traversing into subcutaneous tissues and subsequently migrating toward the anode. Conversely, positively charged drug ions are repelled by the anode, penetrating the subcutaneous tissue and migrating toward the cathode. The initiation and cessation of drug release can be meticulously regulated by alternating between the cathode and anode. The efficacy of iontophoresis hinges upon several factors, including the properties of the drug, the nature of the current, the intensity thereof, the conductivity and composition of the electrodes, and the thickness of the skin's stratum corneum.91 The drug of choice is usually water soluble and readily ionizable, as the drug is usually dissolved in water in the form of an ionic compound to be easily introduced into subcutaneous tissues to facilitate its introduction before iontophoresis is performed. Direct current is widely used in iontophoresis, but there are also cases where alternating current has been used and shown to be more effective.92 Typically, water-soluble and readily ionizable drugs are preferred because they can be easily introduced into subcutaneous tissues prior to iontophoresis. While direct current has extensive applications in iontophoresis, there are instances where alternating current has demonstrated superior effectiveness. Notably, this approach allows for the direct delivery of drugs to target tissues without necessitating systemic circulation, thereby mitigating systemic side effects. Moreover, precise and controlled drug delivery can be achieved by modulating the strength and duration of the electric current. In the context of facilitating drug introduction, careful consideration must be given to selecting appropriate iontophoresis conditions and target sites. A comprehensive review by Tomasz M systematically delineated the optimal iontophoresis conditions for various drugs.93 Additionally, a significant barrier to transdermal drug delivery lies in the impermeability of the stratum corneum to drug molecules. However, iontophoresis has been shown to transiently disrupt the lipid bilayer of the stratum corneum, thereby augmenting its permeability and enhancing the efficiency of molecular delivery.94 Microneedles, which serve as adept tools capable of breaching the stratum corneum of the skin, hold promise as electrodes for iontophoresis, facilitating enhanced transdermal drug delivery to deeper tissues for more efficacious treatments.63
Reverse iontophoresis is a process in which an applied electric field aids in the outward transport of small charged or neutral molecules from skin tissues. By functioning as a noninvasive modality for biological sample collection, reverse iontophoresis has been applied for monitoring the physiological parameters of biomarkers and drug metabolism within organisms by capturing sweat, ISF, and other analytes.91,95,96 Furthermore, the utility of reverse iontophoresis extends to the extraction of vital compounds from plants. For instance, Richard H. et al. successfully extracted phytochemicals from intact pomegranates under optimal conditions (3 mA current for 1 h in a pH 7.4 buffer) utilizing reverse iontophoresis, confirming the nondestructive extraction of these compounds from plants. This study underscores the feasibility of noninvasively extracting phytochemicals from plants via reverse iontophoresis.97 The underlying principle of reverse iontophoresis parallels that of iontophoresis, wherein ions or small molecules within an organism undergo directed movement in response to an externally applied electric field. Electromigration governs the directional migration of ions under the influence of an electric field, whereas electroosmosis drives the flow of liquid, thereby facilitating the movement of uncharged small molecules alongside ions. It is worth mentioning that pH can have a direct effect on the effectiveness of reverse iontophoresis; for example, it has been reported that the concentration of glucose extracted increases with increasing pH, which is an important insight for medical devices that extract glucose by reverse iontophoresis. Individual differences in epidermal pH should be taken into account when extracting glucose.96 Reverse iontophoresis significantly contributes to portable wearable medical devices that can safely and efficiently extract biomarkers from the body, providing important information for the diagnosis and prevention of certain diseases or real-time adjustments to drug treatment regimens. In addition, the monitoring of drug metabolites with the help of reverse iontophoresis can also greatly contribute to the development of drug efficacy assessment and drug safety.
5.2 Electrical stimulation
Electrical stimulation, a therapeutic modality, entails the application of an electric field to biological tissues to modulate physiological processes. This technique operates at the molecular and cellular levels, influencing ion channels, membrane proteins, cell migration, and organelle functions, thereby altering cellular activities such as migration and proliferation.98 It has extensive applications in neurological disorders, pain management, wound healing, and muscle rehabilitation. The initial applications of electrical stimulation in the medical field encompassed the utilization of electrocardiography (ECG) for directly monitoring cardiac activity, as well as its integration in pacemakers and defibrillators for treating cardiac dysfunction. As technology has progressed, the scope of electrical stimulation has gradually expanded to encompass diverse areas, such as tissue engineering and regenerative medicine. Its application has also been extended to neuromuscular electrical stimulation for sports medicine, neurostimulation for managing pain, treating Parkinson's disease, addressing psychiatric disorders, and diagnosing and treating numerous other diseases.38,99 Cellular responses to electrical stimulation involve various mechanisms, including voltage-gated channel opening, asymmetric ionic current generation, electroosmotic currents affecting cell proliferation and differentiation through directional movement and intracellular molecule transport, mechanotransduction modulation, and membrane structural alterations, as depicted in Fig. 6(b). In the presence of an applied electric field, the behavior of voltage-gated channels within a cell undergoes modulation owing to the disparate potentials across its membrane. This disparity precipitates the generation of asymmetric ionic currents, which are pivotal in governing various phenotypic physiological phenomena within the cell. Additionally, the application of an electric field induces electroosmotic flow, which exerts forces on the cell surface, notably in the form of hydrodynamic drag force (FHD), thus propelling the cell in a directional manner.98 The utilization of electrical stimulation to facilitate wound healing represents a burgeoning area of research interest. Over a century ago, the German physiologist Emil Du-Bois Reymond elucidated that the human epidermis maintains a negative charge,100 known as the skin battery or transepithelial potential (TEP), which typically ranges between 10 and 60 mV mm−1 and is attributed to the asymmetric distribution of ion channels.38,101 Upon skin injury, a low-intensity ionic current, primarily composed of Cl− and Na+ ions, termed the wound potential, flows from intact to injured skin due to the potential decrease at the wound site resulting from short circulation of TEPs. Evidence suggests that the presence of a wound potential fosters wound healing, thereby implying the potential for pharmacological interventions to augment this potential or direct electrical stimulation to mimic it directly, thus accelerating wound healing.100 The simulation of wound potentials through electrical stimulation holds promise for directing the migration of immune cells and cytokines, fostering cell proliferation, differentiation, and antimicrobial properties, and enhancing blood circulation near the wound.38,98,101 Moreover, studies have demonstrated that electrical stimulation can activate Ca2+ signaling, consequently facilitating the formation of osteoblasts or enhancing their activity, thereby offering novel therapeutic avenues for osteoporosis treatment.102 Overall, electrical stimulation represents a versatile tool in the medical armamentarium, furnishing innovative modalities for managing diverse ailments.5.3 Electroporation
Perforation of the cell membrane of bovine adrenomedullary cells under the application of an electric field was first observed in 1972.103 Electroporation, also referred to as electropermeabilization, is a technique aimed at generating transient or permanent micropores, typically with nanometer-scale radii, within cell membranes. This process facilitates the ingress of macromolecules such as drugs, DNA, RNA, or proteins into cells by inducing alterations in membrane permeability.104 The fundamental principle underlying electroporation involves the application of a transient high-voltage electric field to the cell membrane, thereby inducing a shift in its permeability and allowing macromolecules to traverse into the cytoplasm. As illustrated in Fig. 6(c), the process initiates with water molecule penetration through the lipid bilayer, followed by the reorientation of adjacent phospholipid molecules’ hydrophilic heads toward these water molecules, ultimately leading to the formation of microporous channels within the cell membrane's lipid bilayer.105 Factors influencing the formation of such micropores include the magnitude of voltage, the duration of its application, and the cell type.106 When the pores swiftly close upon the entry of molecules into the cell, excessively intense electric fields may hinder or delay pore closure, thus jeopardizing cellular activity. Electroporation has significantly advanced medicine by addressing challenges such as the poor immunogenicity inherent in DNA vaccines. Its application has demonstrated efficacy in enhancing immunogenicity, thereby bolstering drug efficacy. A multitude of DNA vaccines, including those developed for combating COVID-19, now leverage electroporation.22 With ongoing technological advancements and extensive clinical investigations, electroporation is poised to assume a more prominent role in clinical settings, fostering further innovation and breakthroughs in the medical domain. In addition to medicine, electroporation serves as a cornerstone biotechnological technique in molecular biology, facilitating cell transfection for purposes such as gene transfer, transformation, or gene editing. Moreover, its utility extends to diverse fields, including wastewater treatment and food sterilization, where the induction of irreversible cell membrane damage by electric currents has proven instrumental in microorganism inactivation.107However, conventional electroporation presents certain challenges, notably, the risk of irreversible cell inactivation due to excessive voltages and the potential loss of cell viability induced by Joule heat generated from high currents traversing through tissue. To address these challenges, contemporary approaches integrate electroporation with microfluidic or microelectronic devices, enabling precise control over the electric field through externally applied microelectrodes positioned in close proximity. This integration facilitates operation at lower voltages, thereby enhancing the uniformity, reliability, and controllability of electroporation processes.22,108
5.4 Electrochemical sensors
Over the past decade, there has been an influx of research focused on electrochemical technology, which is widely used in the development of wearable devices aimed at the direct detection of biological biomarkers in real time.109 Electrochemical sensors exploit biomolecular interactions with electrode surfaces to detect the presence or quantity of target molecules, typically comprising a working electrode (WE), a reference electrode (RE), and a counter electrode (CE).25,110 The pivotal role of the WE lies in its ability to interact with the target molecule, thereby eliciting changes in electrochemical signals detectable on its surface. Concurrently, the RE provides a known potential for measuring the potentiometric disparity between itself and the working electrode,6 while the CE serves to balance reactions at the working electrode, ensuring a stable current path for the sensor. For example, oxidation/reduction at the WE means reduction/oxidation at the CE. Microneedles prepared using conductive materials have emerged as favorable electrodes for electrochemical sensors owing to their noninvasive, painless nature, and excellent electrical conductivity.25,58,111,112 The underlying mechanism of electrochemical sensing involves surface modification of the working electrode with biomolecules (e.g., enzymes and antibodies) capable of specific binding to the target molecule, thereby influencing electrochemical parameters such as current or impedance and consequently generating electrochemical signals. Techniques such as potentiometry, voltammetry, and impedimetry are commonly employed to detect alterations in electrode surface characteristics, subsequently amplifying, converting, and digitizing these data into readable formats for determining the presence or concentration of the target molecule.110In addition to biomolecular modifications, alternative approaches have been explored to enable specific binding of target substances to working electrodes. As shown in Fig. 6(d), Xie et al. devised an electrochemical MN sensor for simultaneous monitoring of multiple ions within the body. By coating electrode surfaces with distinct ion-selective membranes, each interacting with a different ion, they devised a sensor capable of painlessly monitoring real-time changes in Ca2+, Na+, and K+ concentrations in vivo.30 The integration of electrochemical technologies with microfluidics, microelectronics, and microneedles has substantially enhanced the efficiency, maneuverability, portability, and sensitivity of electrochemical sensors. This convergence has facilitated the development of point-of-care testing (POCT), which has broad-ranging applications across various domains.17,31,113,114
5.5 Ultrasound
In recent years, the role of ultrasound in clinical diagnosis and therapy has garnered significant attention because of its ability to treat diseases without harming human tissues or causing serious adverse reactions. Based on these advantages, ultrasound has a promising future and application in drug delivery and therapy.115 This approach aims to enhance drug delivery and absorption through ultrasound, leveraging mechanisms such as enhancing cell permeability,116 dilating cellular interstitial spaces, increasing vascular wall permeability,117 and augmenting blood flow and microcirculation.118 The effects of ultrasound on cells and their underlying mechanisms, including cell membrane perforation, endocytosis regulation, ion channel modulation, and cytoskeletal depolymerization,116 are delineated in Fig. 6(e). Ultrasound induces reversible physiological changes in cells, bolstering cellular endocytosis and facilitating the entry of drug molecules, chemicals, and nanoparticles into cells. Consequently, ultrasound significantly enhances drug absorption efficiency, enabling dose and frequency reductions and mitigating drug-related side effects and toxicity. Particularly advantageous for tumor treatment and scenarios necessitating high drug concentrations, ultrasound-promoted drug delivery enhances drug penetration into lesion sites, elevating local drug concentrations and therapeutic efficacy.117Traditionally, ultrasound-facilitated drug delivery involves complex operations, specialized equipment, and demanding operator expertise. However, recent studies integrating ultrasound with microelectronic devices have led to the development of portable, cost-effective ultrasound microdevices, markedly enhancing the accessibility and practicality of ultrasound technology.21 With ongoing technological advancements and extensive research endeavors, ultrasound-promoted drug delivery technology is poised for further refinement and application in the future.
5.6 Potentiometric recordings
Potentiometric recording serves as a straightforward biomedical technique for gauging potential fluctuations stemming from bioelectrical activity within an organism or on its surface, notably in tissues such as muscle, heart, and brain. However, conventional electrophysiological monitoring devices, which are cumbersome and intricate to operate, necessitate specialized physicians to dedicate substantial time to electrode installation and removal. Additionally, patients are burdened with an array of complex wires, significantly impeding convenience and mobility.Attracting attention is the emergence of portable, user-friendly microelectronic devices that have revolutionized electrophysiological monitoring, enabling real-time tracking of body activity irrespective of time or location.48 Presently developed wearable electrophysiological recording devices primarily monitor key physiological indices: (1) electrocardiography (ECG): this technique records cardiac potential differences, facilitating monitoring of heart rhythm, conduction, and functionality. (2) Electroencephalogram (EEG): EEG data capture potential differences in the scalp, allowing the assessment of brain electrical activity and aiding in the diagnosis of conditions such as epilepsy and sleep disorders. (3) Electromyography (EMG): EMG measures muscle potential differences and evaluates muscle function, neuromuscular connectivity, and motor control. Electrooculogram (EOG): EOG records eye potential differences to track eye movements, notably during rapid eye movement (REM) sleep stages.119,120 Given the typically faint nature of these surface physiological potentials, the fabrication of wearable electrophysiological recording devices necessitates sensitive electrodes and effective reduction of skin resistance. MNs constructed from high-conductivity materials exhibit remarkable efficacy in electrode applications due to their sensitivity to subtle potential changes and their ability to penetrate the epidermis, significantly reducing skin resistance.48Fig. 6(f) illustrates the transmission of bioelectric signals from a neuron to an MN electrode. Analyzing and diagnosing physiological functions entails capturing potential differences and converting them into graphical or data representations. Furthermore, integrating this electrophysiological recording capability with electrical stimulation, drug delivery, and other therapeutic modalities within a unified microsystem holds promise for efficient and timely disease diagnosis and treatment.
5.7 Thermotherapy
Heat therapy, a practice rooted in ancient traditions, primarily utilizes hot packs to alleviate pain and enhance blood circulation. Its contemporary applications extend to diverse fields, including local drug delivery facilitation, tissue engineering, and regenerative medicine (TERM).121 The main treatment modalities include hot packs, saunas, hot tubs, and infrared therapy, all aimed at increasing tissue temperature.122,123 The physiological effects of heat therapy include augmented blood circulation, muscle tension or pain relief, reduced inflammation, and the promotion of tissue repair and healing. Its mechanisms include increased blood flow to diseased areas and enhanced delivery of proteins, oxygen, and nutrients, which are crucial for wound healing. Each 1 °C increase in tissue temperature correlates with a 10% to 15% increase in tissue metabolism.123 Moreover, repeated heat therapy sessions increase the levels of endothelial nitric oxide synthase (eNOS), which is pivotal for vascular remodeling, thus accelerating this process.124 Following intense exercise, heat therapy counteracts decreased GLUT4 levels and transient insulin resistance, facilitating glucose uptake and glycogen synthesis.125 Additionally, thermotherapy promotes mitochondrial biosynthesis via the mitochondrial biogenesis pathway.In cancer treatment, thermotherapy shows promise, as exemplified by Youshui Gao et al.'s multifunctional therapeutic platform for bone tumor treatment. This platform employs near-infrared (NIR) radiation to induce heat generation, promoting nitric oxide (NO) production (Fig. 6(g)). NO, a vital biomolecule, induces endothelial cell proliferation and migration at low concentrations and exhibits direct anticancer effects at higher concentrations.123
Precision in temperature and treatment duration is paramount for effective heat therapy, as excessive heat can compromise normal cellular functions, including DNA and protein inactivation.121 Consequently, precise temperature control can be achieved through the utilization of microelectronic devices. The integration of thermotherapy with wearable microelectronic devices has the potential to enhance the effectiveness of transdermal drug delivery and pain management while simultaneously improving the safety of diagnosis and treatment. This innovative approach offers patients convenient and efficient treatment alternatives.
6. Clinical examples of eMNs devieces
Telemedicine, miniaturized diagnostic and treatment systems, and wearable sensors, which primarily extract subcutaneous ISF rather than blood, have made significant efforts in the diagnosis and monitoring of diseases. However, the high impedance of the stratum corneum (SC), exceeding 105 Ω, and its thickness ranging from approximately 10 to 40 μm pose significant challenges to ISF extraction.126 Microneedles offer a solution by easily penetrating the SC, thereby finding diverse applications in the medical realm, including painless sampling, transdermal drug delivery, topical therapy, and real-time physiological monitoring. In tandem with microelectronic devices, microneedles crafted from conductive materials serve as electrodes to facilitate the passage of electrical currents through the skin. These MN electrodes enable iontophoresis or reverse iontophoresis, electrical stimulation, and physiological electrical signal monitoring. Currently, microneedle-based microelectronic devices that show good prospects for clinical applications are mainly used for drug delivery, extraction of target molecules in vivo, and monitoring of physiological signals, of which drug delivery is the most concerning.6.1 The eMN devices for drug delivery
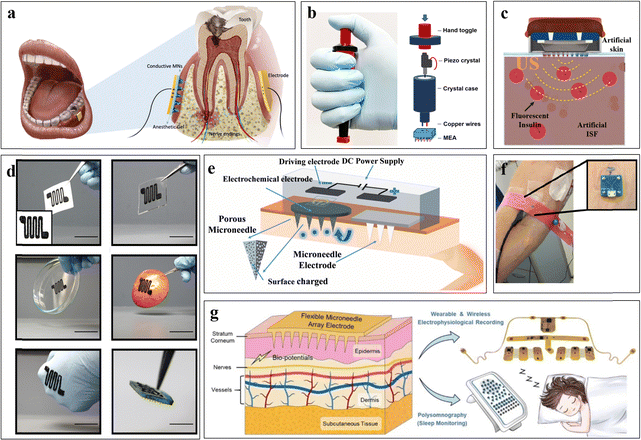
In contemporary medical practice, traditional syringes remain a common choice for drug administration in hospitals. However, this method often leads to intensely unpleasant experiences, particularly pain, especially among patients requiring frequent injections and pediatric populations with needle phobia. Microneedles, which typically range from 150 μm to 1000 μm in length, have emerged as promising alternatives for drug delivery. When applied to the skin, they create microscopic pores, facilitating drug penetration through the epidermis. Nonetheless, micron-sized MNs have limited drug-carrying capacity, and passive drug diffusion from the epidermis to deeper skin layers is slow.To address these limitations, physical stimulation methods such as iontophoresis, electroporation, thermal stimulation, and ultrasound can significantly enhance drug absorption. Conductive MNs facilitate iontophoresis by creating micrometer-sized pores on the skin surface and reducing the electrical resistance of the skin, aiding the flow of electrical charge. Fig. 7(a) shows a schematic diagram of MN-assisted iontophoresis for dental anesthesia. Microneedles fabricated from polymeric materials of PEDOT:PSS and HA polymers exhibit good electrical conductivity and mechanical strength, enabling successful molecular delivery of lidocaine to bone tissue via synergistic treatment with iontophoresis. This approach achieves anesthesia effects comparable to those of traditional injectable anesthesia, demonstrating excellent drug delivery efficacy.15
Moreover, combining microneedles with electroporation enhances cellular drug uptake by creating microporous channels in the cell membrane, facilitating intracellular drug transport.4Fig. 7(b) depicts a handheld device for macromolecular vaccine delivery, featuring a piezoelectric pulse generator and an array of stainless steel microneedles. These microneedles, with a length of 650 μm, effectively penetrate the skin stratum corneum during application. When operating at field strengths ranging from 2 kV cm−1 to 3 kV cm−1 in microseconds, the device surpasses the threshold for millisecond electroporation, effectively enhancing the cellular immune response to DNA vaccines.127 This straightforward ePatch device requires no additional power and significantly improves DNA vaccination efficiency, even reducing the required vaccine dosage by tenfold, as evidenced by experimental results.90
The integration of ultrasound with MNs also offers a promising approach for targeted drug delivery. While ultrasound is commonly used in imaging applications, its utility extends to targeted drug delivery and genetic engineering.128 Ultrasound enhances cell membrane permeability, facilitating the uptake of external drug molecules, and heat generation promotes local blood circulation. Therefore, combining ultrasound with MNs effectively assists in targeted drug delivery, offering a small and user-friendly microdevice.129 However, attention must be given to the potential adverse effects of high-frequency vibrations from the ultrasound generator on MN morphology. Consequently, when selecting MN materials, emphasis should be placed on their tensile properties and mechanical strength. Fig. 7(c) shows a schematic of a microelectronic device for ultrasound-assisted transdermal delivery of insulin based on MNs. This system comprises a piezoelectric (PZT) ring and a stainless steel plate-based ultrasonic pump with stainless steel microneedles. The resonant frequency of the PZT ring of 100 kHz induces reversible cellular perforation and stimulates endocytosis, reducing resistance to drug entry across cell membranes. The experimental results demonstrated the effectiveness of the device in promoting insulin diffusion deeper into tissue and accelerating the diffusion rate.21
Additionally, heat therapy combined with microneedling offers another avenue for drug delivery. Localized heat increases molecular diffusion rates and provides direct pain relief. While few studies have explored their joint application, challenges include the bulky nature of heating devices compared to the small size of microelectronic devices and the requirement for material flexibility in wearable microelectronics. Nevertheless, the microheater shown in Fig. 7(d) overcomes these challenges. Fabricated via 3D printing using an ink material of polydimethylsiloxane (PDMS) and high-purity multiwalled carbon nanotubes (MWCNTs), this material exhibits excellent fitting properties and stretchability, which are crucial for wearable devices. When powered, the microheater generates heat, promoting drug release and diffusion through the MN patch and accelerating drug diffusion from the epidermis to deeper tissues. Additionally, increased temperature enhances blood circulation, further facilitating drug diffusion into deeper tissues.81 This study provides insights into the integration of thermotherapy with MNs for enhanced drug delivery.
In conclusion, the integration of microelectronic devices with MNs represents a promising avenue for enhancing drug delivery in contemporary medical practice. Microneedles offer a viable alternative to traditional syringes, addressing the challenges of pain and limited drug-carrying capacity. Physical stimulation methods such as iontophoresis, electroporation, thermal stimulation, and ultrasound further augment drug absorption, demonstrating significant potential for improving patient outcomes. The clinical examples in Fig. 7(a)–(d) underscore the efficacy of these approaches in facilitating drug delivery, ranging from anesthesia to DNA vaccination and insulin administration. While challenges remain, such as potential adverse effects of ultrasound vibrations and the need for material mechanical strength in wearable devices, innovative solutions such as new resilient materials offer promising avenues for overcoming these obstacles. Overall, this study provides valuable insights into the future of drug delivery technology, paving the way for more efficient and patient-friendly treatment modalities.
6.2 The eMN for physiological monitoring
Furthermore, in addition to their role in drug delivery, MNs have been extensively applied for extracting target molecules in vivo and monitoring physiological parameters. Integrated with microelectronic devices, microneedles serve as biosensors that penetrate the skin, enabling the collection of body fluid samples such as ISF for subsequent analysis and diagnosis. These biosensors offer real-time monitoring of biomarker levels, providing valuable health status information to patients or physicians. Additionally, microneedles effectively reduce skin-electrode impedance when coupled with certain biosensing devices, offering a novel solution for monitoring electrophysiological signals.Iontophoresis not only facilitates the delivery of drug molecules into the epidermis but can also assist in the percutaneous extraction of biomarker molecules in vivo, a process known as reverse iontophoresis. By combining MNs with reverse iontophoresis, small and even large biomolecules such as DNA can be effectively extracted from organisms.130 The mechanisms underlying reverse iontophoresis, primarily electromigration and electro-osmosis, enhance the efficiency of biomolecule extraction when combined with MNs. When combined with MNs, MNs can effectively penetrate the epidermis to act as electrodes for reverse iontophoresis, which results in the directed movement of biomolecules in the ISF toward the MN. Fig. 7(e) illustrates a wearable MN patch for glucose extraction based on reverse iontophoresis. The device operates by selectively transferring glucose to the MN electrode surface via reverse iontophoresis, utilizing preimmobilized glucose oxidase for chemical reactions and subsequent electrical signal generation for biosensing. The device demonstrated a sensitivity of 0.0859 ± 0.0028 μA mM−1 within the detection limit range of 3–15 mM, along with excellent selectivity and stability under experimental conditions.9 This study not only advances the development of novel glucose sensing devices but also serves as a valuable reference for extracting other biomarkers from ISF using reverse iontophoresis.
Furthermore, among microelectronic devices incorporating MNs, the integration of MNs with electrochemical sensors has garnered significant attention. MNs serve as electrodes for electrochemical sensors, offering several advantages. First, they require low sample volumes due to their high specific surface area and unique structures, such as porous MNs, enabling the efficient extraction of biomarkers from ISF. Second, microneedles can be tailored in various shapes, sizes, and structures, allowing researchers to select materials, structures, and needle lengths according to the application site. Third, MNs exhibit biocompatibility, causing minimal trauma to the skin and supporting continuous wear due to the use of highly biocompatible materials. The mechanism of electrochemical sensors involves the reaction of target substances on electrodes, generating weak electrical signals that are amplified, transmitted, digitally converted, and sent in real time for continuous monitoring of physiological indicators. MNs have been widely employed as electrodes in electrochemical sensors, enabling real-time ISF extraction and physiological monitoring. For instance, Anthony et al. developed an electrochemical microneedle (MN) sensor for lactate monitoring, as depicted in Fig. 7(f). A notable advantage of this sensor compared to previous electrochemical sensors lies in the utilization of direct electron transfer (DET) enzymes. Unlike conventional sensors that generate redox products on the electrode surface, DET enzymes directly produce electrons for electrical signal acquisition.111 The DET lactase enzyme is preimmobilized on the MN electrode, facilitating a rapid reaction upon encountering lactate molecules, resulting in the generation of a weak electric current.131 Despite its excellent performance, the only drawback is that the device currently relies on USB wired transmission to send electrical signals to the outside, which indeed needs to be optimized. In future upgraded versions, we can explore wireless transmission technologies such as Bluetooth to further enhance the portability and ease of use of the device. Additionally, the use of highly conductive materials for MN fabrication minimizes signal loss, facilitating sensitive and rapid feedback of physiological information.
The utilization of MNs for electrophysiological signal sensors represents a burgeoning area of research. Traditional recording methods for electrophysiological signals necessitate the use of conductive gels as wet electrodes to establish low-impedance channels between the skin and the electrodes. However, the application of conductive gels requires skin preparation techniques such as shaving or dermabrasion, which can cause skin damage and trigger immune responses to the gel.132 Moreover, the gel tends to dry over time, compromising the sustainability and wearability of electrophysiological recordings. In contrast, employing conductive MN arrays as electrodes for potential recording devices allows for direct sensing of electrophysiological signals without the need for conductive gel, thereby improving device wearability and user friendliness. This advancement is particularly beneficial for individuals with skin sensitivities, infants, and elderly individuals. For instance, Zhihong Li et al. investigated a flexible MN electrophysiological signal sensor, as illustrated in Fig. 7(g). Polyimide was selected as the flexible substrate and primary MN material due to its thermal and chemical stability. The surface layer of the MN was modified with metallic materials such as titanium and gold, along with PEDOT:PSS for high electrical conductivity, enabling efficient transmission of weak electrical signals from the body surface. Furthermore, the incorporation of a wireless transmission device enables real-time data transmission from the microneedle electrophysiology signal recorder to external devices. After 44 nights of recording from numerous healthy and sleep-disordered volunteers, the experimental results demonstrated that the mini-wearable system could effectively replace bulky and complex electrophysiological recording devices.48 This user-friendly device allows patients to easily don and doff it, thereby saving healthcare workers significant energy and time compared to traditional devices.
eMN devices are transitioning from single-functionality to multifunctionality and intelligence. Instead of serving a singular purpose, these devices are now equipped with multiple functions concurrently. For example, they can facilitate real-time drug administration while simultaneously monitoring physiological indicators. Additionally, self-powered and wireless signal-transmitting devices can complement MN microelectronics to realize intelligent medicine. In conclusion, the integration of MNs and microelectronics holds promise for drug delivery, in vivo extraction of target molecules, and physiological indicator monitoring, paving the way for innovative medical diagnostics and treatments and fostering the advancement of personalized medicine.
6.3 Evaluation of current clinical examples
Although eMNs show bright applications in drug delivery and physiological monitoring, there are still several challengs and potential drawbacks. First, limitations on material selection: the materials used in eMN fabrication, such as metals and polymers, must be biocompatible to avoid adverse reactions. Some materials might elicit allergic responses or other negative reactions if not carefully selected and tested. The tensile properties and mechanical strength of eMN are critical, especially when integrated with technologies like ultrasound or heat therapy. eMN must maintain their structural integrity without breaking or deforming, which could otherwise lead to incomplete drug delivery or remnants being left in the skin. Sencond, skin irritation and infection risk: although MNs are designed to be minimally invasive, repeated or prolonged use can lead to skin irritation, redness, and inflammation. Penetrating the skin barrier with MNs, even on a microscopic level, can introduce pathogens if the device is not properly sterilized. This increases the risk of infections, particularly with repeated use or in immunocompromised patients. Third, adverse effects of physical stimulation: techniques like iontophoresis, electroporation, and ultrasound, while enhancing drug delivery, can have side effects. For instance, high-frequency ultrasound can generate heat, potentially causing thermal damage to the skin and underlying tissues. At last, cost and accessibility: advanced MN devices incorporating microelectronics, ultrasound, or heat therapy can be costly to produce, potentially limiting their accessibility, especially in low-resource settings. This could widen the gap between high-tech medical solutions and the needs of underserved populations. Addressing these challenges requires ongoing research and development to enhance the biocompatibility, mechanical stability, and overall safety of MN devices, while also ensuring they remain cost-effective and user-friendly for widespread clinical adoption.7. Conclusion and perspectives
As a new type of medical tool, microneedles, which are invasive and painless, have shown great potential in the field of skin puncture and are widely used in physiological monitoring, disease diagnosis, and transdermal drug delivery. In recent years, with the rapid development of microelectronic technology, the combination of microneedles and microelectronic devices has provided new possibilities for the systematic diagnosis and treatment of diseases. In this paper, we review the application of eMN devices in microelectronic medical and provide a comprehensive introduction to various aspects of eMN materials, preparation methods, power supplies, and mechanisms of action. In terms of materials, we discuss in detail the materials used to prepare the eMN matrix and the conductive materials used to improve the conductivity of the eMN surface. By modifying the surface of eMN matrix materials with conductive materials, the conductivity of eMN materials required for microelectronic devices can be greatly improved. In terms of preparation methods, we present a variety of techniques, such as micromolding, etching, laser cutting, and 3D printing, which provide a wealth of options for the preparation of eMN from different materials. In addition, we discuss the types of power sources suitable for these eMN devices, such as batteries, to meet the needs of different application scenarios. In terms of the mechanism of action, this paper discusses in depth a variety of technological tools, such as ionic introduction, electroporation, thermal stimulation, and ultrasound, which, by combining with eMN, achieve efficient transdermal drug delivery and precise detection of physiological signals. Finally, we also list some typical cases of eMN devices to demonstrate the practical application results in this field.The integration of microneedles and microelectronic medical devices, as an innovative technology in the medical field, still faces many challenges to be solved in the actual advancement process, despite its broad application prospects.
First, miniaturization and integration of the device is a major challenge. To be effectively integrated with eMN patches for use in the human body, these devices need to be made as small as possible. Then, technical bottlenecks will be encountered in the process of device miniaturization, such as a decrease in device performance and an increase in manufacturing difficulty.
Second, the stability and reliability of the device is also a concern. The integration of microneedles with microelectronic medical devices involves a number of complex technical links, such as power supply and signal transmission. Failure of any one of these links may lead to the failure of the whole device. Therefore, a circuit system that can be stabilized over time is needed. For example, the emergence of triboelectric nanogenerators (TENGs), enzymatic biobatteries, supercapacitors, etc., has created a new way of thinking about the power supply problem.62,64,133 In addition, the cost of the devices is an important constraint to their widespread use. The relatively high manufacturing and development costs of MNs and microelectronic medical devices limit their widespread use in areas with limited medical resources. To promote the widespread use of this technology, it is necessary to continuously reduce manufacturing costs and improve production efficiency. For example, in studies related to electroporation, researchers have used a very low-cost household stovetop lighter as a piezoelectric generator.134 Finally, legal and ethical issues cannot be ignored. As microneedling and microelectronic medical devices are widely used in the medical field, related legal and ethical issues are gradually emerging. Issues such as ensuring the privacy and rights of patients and regulating the development and use of devices need to be properly addressed. Only by overcoming the above challenges can further development of this technology be promoted.
In the future, the combination of microneedles and microelectronic medical devices will have broader application prospects. With the continuous progress of materials science, new eMN materials will continue to emerge to improve the mechanical properties and biocompatibility of MN materials. At the same time, with the continuous innovation of microelectronic technology, the performance of eMN devices will be further improved to achieve higher precision and more personalized medical services. In addition, the integration of microneedle (MN) devices with artificial intelligence (AI) and machine learning (ML) holds significant promise for advancing medical diagnostics, treatment, and personalized healthcare. By utilizing these technologies, enhanced date analysis and interpretation can be realized. AI and ML can improve the diagnostic accuracy as their ability to intelligently process large amounts of data. The development of these technologies is expected to promote the widespread application of eMN devices in the field of healthcare and make great contributions to the health and well-being of humankind. In conclusion, the combination of MNs and microelectronic medical devices has revolutionized the modern medical field. With the continuous progress of related technologies and the expansion of application scenarios, we have reason to believe that this field will show more brilliant prospects for development in the future.
Author contributions
Writing – original draft preparation: Mr S. Zhou and Dr X. Li. Writing – revision: Dr Q. Zhou. Writing – review and editing: Prof. B. B. Gao.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (32371435, 32101118), the National Students' Platform for Innovation and Entrepreneurship Training Program (202310291098Z), Nanjing Tech University Teaching Reform Project (20230248), Jiangsu Provincial Government Scholarship Program (Bingbing Gao), the Discipline Fund of Nanjing Tech University School of Pharmaceutical Sciences (2023) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_1537, KYCX24_1553).References
- J. Yang, X. Liu, Y. Fu and Y. Song, Acta Pharm. Sin. B, 2019, 9, 469–483 CrossRef PubMed
.
- Y. Yang, R. Luo, S. Chao, J. Xue, D. Jiang, Y. H. Feng, X. D. Guo, D. Luo, J. Zhang, Z. Li and Z. L. Wang, Nat. Commun., 2022, 13, 6908 CrossRef CAS PubMed
.
- X. Zhang, G. Chen, L. Sun, F. Ye, X. Shen and Y. Zhao, Chem. Eng. J., 2021, 406, 126741 CrossRef CAS
.
- L. Lin, Y. Wang, M. Cai, X. Jiang, Y. Hu, Z. Dong, D. Yin, Y. Liu, S. Yang, Z. Liu, J. Zhuang, Y. Xu, C. F. Guo and L. Chang, Adv. Funct. Mater., 2021, 32, 2109187 CrossRef
.
- L. Zheng, D. Zhu, Y. Xiao, X. Zheng and P. Chen, Biosens. Bioelectron., 2023, 237, 115506 CrossRef CAS PubMed
.
- X. Li, X. Huang, J. Mo, H. Wang, Q. Huang, C. Yang, T. Zhang, H. J. Chen, T. Hang, F. Liu, L. Jiang, Q. Wu, H. Li, N. Hu and X. Xie, Ad. Sci., 2021, 8, 2100827 CrossRef CAS PubMed
.
- D. Terutsuki, R. Segawa, S. Kusama, H. Abe and M. Nishizawa, J. Controlled Release, 2023, 354, 694–700 CrossRef CAS PubMed
.
- T. Sawaira, A. Jamil, S. Aziz, A. Mujahid, T. Hussain and A. Afzal, Composites Commun., 2023, 37, 101398 CrossRef
.
- Q. Y. He, J. H. Zhao, S. M. Du, D. G. Li, Z. W. Luo, X. Q. You and J. Liu, Talanta, 2024, 267, 125156 CrossRef CAS PubMed
.
- P. Singh, B. Youden, A. Carrier, K. Oakes, M. Servos, R. Jiang, S. Lin, T. D. Nguyen and X. Zhang, J. Controlled Release, 2023, 353, 1050–1067 CrossRef CAS PubMed
.
- M. Ali, S. Namjoshi, H. A. E. Benson, Y. Mohammed and T. Kumeria, J. Controlled Release, 2022, 347, 561–589 CrossRef CAS PubMed
.
- Y. Zhi, J. Che, H. Zhu, R. Liu and Y. Zhao, Adv. Funct. Mater., 2023, 33, 2304353 CrossRef CAS
.
- B. L. Zhang, Y. Yang, Z. Q. Zhao and X. D. Guo, Electrochim. Acta, 2020, 358, 136917 CrossRef CAS
.
- J. Huang, N. Yap, M. Walter, A. Green, C. Smith, J. Johnson and R. Saigal, ACS Biomater. Sci. Eng., 2022, 8, 1544–1553 CrossRef CAS PubMed
.
- R. Z. Seeni, M. Zheng, D. C. S. Lio, C. Wiraja, M. F. B. Mohd Yusoff, W. T. Y. Koh, Y. Liu, B. T. Goh and C. Xu, Adv. Funct. Mater., 2021, 31, 2105686 CrossRef CAS
.
- S. M. Mugo, S. V. Robertson and W. Lu, Talanta, 2023, 259, 124531 CrossRef CAS PubMed
.
- J. Xu, B. Yang, J. Kong, Y. Zhang and X. Fang, Adv. Healthcare Mater., 2023, 12, 2203133 CrossRef CAS PubMed
.
- Y.-C. Yang, Y.-T. Lin, J. Yu, H.-T. Chang, T.-Y. Lu, T.-Y. Huang, A. Preet, Y.-J. Hsu, L. Wang and T.-E. Lin, ACS Appl. Nano Mater., 2021, 4, 7917–7924 CrossRef CAS
.
- M. Guo, Y. Wang, B. Gao and B. He, ACS Nano, 2021, 15, 15316–15327 CrossRef CAS PubMed
.
- O. Heifler, E. Borberg, N. Harpak, M. Zverzhinetsky, V. Krivitsky, I. Gabriel, V. Fourman, D. Sherman and F. Patolsky, ACS Nano, 2021, 15, 12019–12033 CrossRef CAS PubMed
.
- X. Luo, Q. Yu, L. Yang and Y. Cui, ACS Sens., 2023, 8, 1710–1722 CrossRef CAS PubMed
.
- D. Xia, R. Jin, G. Byagathvalli, H. Yu, L. Ye, C.-Y. Lu, M. S. Bhamla, C. Yang and M. R. Prausnitz, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2110817118 CrossRef CAS PubMed
.
- Y. Huang, H. Li, T. Hu, J. Li, C. K. Yiu, J. Zhou, J. Li, X. Huang, K. Yao, X. Qiu, Y. Zhou, D. Li, B. Zhang, R. Shi, Y. Liu, T. H. Wong, M. Wu, H. Jia, Z. Gao, Z. Zhang, J. He, M. Zheng, E. Song, L. Wang, C. Xu and X. Yu, Nano Lett., 2022, 22, 5944–5953 CrossRef CAS PubMed
.
- M. Dervisevic, E. Dervisevic, L. Esser, C. D. Easton, V. J. Cadarso and N. H. Voelcker, Biosens. Bioelectron., 2023, 222, 114955 CrossRef CAS PubMed
.
- M. Dervisevic, M. Alba, L. Yan, M. Senel, T. R. Gengenbach, B. Prieto-Simon and N. H. Voelcker, Adv. Funct. Mater., 2021, 32, 2009850 CrossRef
.
- Y. Yang, L. Xu, D. Jiang, B. Z. Chen, R. Luo, Z. Liu, X. Qu, C. Wang, Y. Shan, Y. Cui, H. Zheng, Z. Wang, Z. L. Wang, X. D. Guo and Z. Li, Adv. Funct. Mater., 2021, 31, 2104092 CrossRef CAS
.
- L. Qian, F. Jin, Z. Wei, T. Li, Z. Sun, C. Lai, J. Ma, R. Xiong, X. Ma, F. Wang, F. Sun, W. Zheng, W. Dong, K. Sun, T. Wang and Z. Q. Feng, Adv. Funct. Mater., 2023, 33, 2209407 CrossRef CAS
.
- Y. Cheng, X. Gong, J. Yang, G. Zheng, Y. Zheng, Y. Li, Y. Xu, G. Nie, X. Xie, M. Chen, C. Yi and L. Jiang, Biosens. Bioelectron., 2022, 203, 114026 CrossRef CAS PubMed
.
- A. M. Downs, A. Bolotsky, B. M. Weaver, H. Bennett, N. Wolff, R. Polsky and P. R. Miller, Biosens. Bioelectron., 2023, 236, 115408 CrossRef CAS PubMed
.
- X. Huang, S. Zheng, B. Liang, M. He, F. Wu, J. Yang, H.-J. Chen and X. Xie, Microsyst. Nanoeng., 2023, 9, 1–16 CrossRef PubMed
.
- X. Huang, B. Liang, S. Zheng, F. Wu, M. He, S. Huang, J. Yang, Q. Ouyang, F. Liu, J. Liu, H.-J. Chen and X. Xie, Bio-Des. Manuf., 2023, 7, 14–30 CrossRef
.
- Y. Zheng, R. Ye, X. Gong, J. Yang, B. Liu, Y. Xu, G. Nie, X. Xie and L. Jiang, Microsyst. Nanoeng., 2023, 9, 1–14 CrossRef PubMed
.
- V. R. Jayaneththi, K. Aw, M. Sharma, J. Wen, D. Svirskis and A. J. McDaid, Sens. Actuators, B, 2019, 297, 126708 CrossRef CAS
.
- Y. Liu, R. Menon, A. Putcha, K. Huang, L. Bonilla, R. Vora, J. Li, L. Zhang, Y. Wang, L. Fletcher, A. Lassiter, C. Huang, J. B. Buse, K. Cheng and W. Bai, Adv. Mater. Technol., 2022, 7, 2200468 CrossRef CAS
.
- J. Yang, Y. Li, R. Ye, Y. Zheng, X. Li, Y. Chen, X. Xie and L. Jiang, Microsyst. Nanoeng., 2020, 6, 1–14 CrossRef PubMed
.
- S. M. Ajmal Mokhtar, M. Yamada, T. W. Prow, M. Moore, X. L. Strudwick and D. R. Evans, J. Mater. Chem. B, 2023, 11, 5021–5031 RSC
.
- R. Ilyas, M. Zuhri, H. Aisyah, M. Asyraf, S. Hassan, E. Zainudin, S. Sapuan, S. Sharma, S. Bangar, R. Jumaidin, Y. Nawab, A. Faudzi, H. Abral, M. Asrofi, E. Syafri and N. Sari, Polymers, 2022, 14, 202 CrossRef CAS PubMed
.
- Y. J. Cheah, M. R. Buyong and M. H. Mohd Yunus, Polymers, 2021, 13, 3790 CrossRef CAS PubMed
.
- S. Liang, H. Guan, G. Yang, W. Lin, Z. Long, T. Zhong, R. Lin, L. Xing, Y. Zhang, G. Li, M. Chen, X. Xue and Y. Zhan, Nano Energy, 2023, 115, 108764 CrossRef CAS
.
- C.-T. Lin and T.-T. N. Do, IEEE Trans. Systems, Man, Cybernetics: Syst., 2021, 51, 298–312 Search PubMed
.
- J. Stejskal, Chem. Pap., 2019, 74, 1–54 Search PubMed
.
- A. Keirouz, Y. L. Mustafa, J. G. Turner, E. Lay, U. Jungwirth, F. Marken and H. S. Leese, Small, 2023, 19, 2206301 CrossRef CAS PubMed
.
- X. Zhang, Z. Wang, H. Jiang, H. Zeng, N. An, B. Liu, L. Sun and Z. Fan, Sci. Adv., 2023, 9, eadh1415 CrossRef CAS PubMed
.
- Y. Gogotsi and B. Anasori, ACS Nano, 2019, 13, 8491–8494 CrossRef CAS PubMed
.
- N. Ahmad, S. Rasheed, A. Mohyuddin, B. Fatima, M. I. Nabeel, M. T. Riaz, M. Najam-ul-Haq and D. Hussain, Chemosphere, 2024, 352, 141280 CrossRef CAS PubMed
.
- D. Johnson, Z. Qiao, E. Uwadiunor and A. Djire, Small, 2022, 18, 2106129 CrossRef CAS PubMed
.
- L. V. Kayser and D. J. Lipomi, Adv. Mater., 2019, 31, 1806133 CrossRef PubMed
.
- J. Li, Y. Ma, D. Huang, Z. Wang, Z. Zhang, Y. Ren, M. Hong, Y. Chen, T. Li, X. Shi, L. Cao, J. Zhang, B. Jiao, J. Liu, H. Sun and Z. Li, Nano-Micro Lett., 2022, 14, 132 CrossRef CAS PubMed
.
- L. Kong, H. Wen, Y. Luo, X. Chen, X. Sheng, Y. Liu and P. Chen, ACS Appl. Mater. Interfaces, 2023, 15, 43515–43523 CrossRef CAS PubMed
.
- S. Bhadra, D. Khastgir, N. K. Singha and J. H. Lee, Prog. Polym. Sci., 2009, 34, 783–810 CrossRef CAS
.
- S. M. Mugo, W. Lu and S. Lemieux, Microchim. Acta, 2022, 189, 206 CrossRef CAS PubMed
.
- T. Hu, Z. Zhang and C. Xu, J. Mater. Chem. B, 2022, 10, 8075–8081 RSC
.
- A. S. R. Bati, L. Yu, M. Batmunkh and J. G. Shapter, Adv. Funct. Mater., 2019, 29, 1902273 CrossRef
.
- T. Zheng, P. Pour Shahid Saeed Abadi, J. Seo, B.-H. Cha, B. Miccoli, Y.-C. Li, K. Park, S. Park, S.-J. Choi, R. Bayaniahangar, D. Zhang, S.-H. Lee, C.-K. Lee, A. Khademhosseini and S. R. Shin, ACS Appl. Mater. Interfaces, 2019, 11, 20615–20627 CrossRef CAS PubMed
.
- S. Wei, Y. Zhang, H. Lv, L. Deng and G. Chen, Chem. Eng. J., 2022, 428, 131137 CrossRef CAS
.
- Y. Chen, M. Lyu, Z. Zhang, F. Yang and Y. Li, ACS Cent. Sci., 2022, 8, 1490–1505 CrossRef CAS PubMed
.
- G. Lippi and M. Plebani, Clin. Chem. Lab. Med., 2021, 59, 637–639 CrossRef CAS PubMed
.
- C. Cai, Y. Liu, Z. Zhang, T. Tian, Y. Wang, L. Wang, K. Zhang and B. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 4895–4902 CrossRef CAS PubMed
.
- A. K. Maparu, P. Singh, B. Rai, A. Sharma and S. Sivakumar, J. Colloid Interface Sci., 2024, 659, 629–638 CrossRef CAS PubMed
.
- Y. Wang, H. Lu, M. Guo, J. Chu, B. Gao and B. He, Adv. Healthcare Mater., 2021, 11, 2101659 CrossRef PubMed
.
- X. Luo, Q. Yu, Y. Liu, W. Gai, L. Ye, L. Yang and Y. Cui, ACS Sens., 2022, 7, 1347–1360 CrossRef CAS PubMed
.
- S. Kusama, K. Sato, Y. Matsui, N. Kimura, H. Abe, S. Yoshida and M. Nishizawa, Nat. Commun., 2021, 12, 658 CrossRef CAS PubMed
.
- Y. Li, J. Yang, Y. Zheng, R. Ye, B. Liu, Y. Huang, W. Zhou and L. Jiang, Acta Biomater., 2021, 121, 349–358 CrossRef CAS PubMed
.
- H.-J. Kil, S.-R. Kim and J.-W. Park, ACS Appl. Mater. Interfaces, 2022, 14, 3838–3848 CrossRef CAS PubMed
.
- N. Kashaninejad, A. Munaz, H. Moghadas, S. Yadav, M. Umer and N.-T. Nguyen, Chemosensors, 2021, 9, 9040083 CrossRef
.
- L. A. Denguir, J. C. Outeiro and G. Fromentin, Corrosion Eng., Sci. Technol., 2020, 56, 189–198 CrossRef
.
- E. Mirkoohi, P. Bocchini and S. Y. Liang, J. Manuf. Process., 2019, 38, 462–471 CrossRef
.
- C. R. Chighizola, C. R. D’Elia, J. E. Jonsson, D. Weber, B. Kirsch, J. C. Aurich, B. S. Linke and M. R. Hill, Exp. Mech., 2022, 62, 1437–1459 CrossRef
.
- H. Roh, Y. J. Yoon, J. S. Park, D.-H. Kang, S. M. Kwak, B. C. Lee and M. Im, Nano-Micro Lett., 2021, 14, 24 CrossRef PubMed
.
- N. M. Kommanaboina, T. S. Yallew, A. Bagolini and M. F. Pantano, Microsyst. Nanoeng., 2024, 10, 5 CrossRef CAS PubMed
.
- N. Harpak, E. Borberg, A. Raz and F. Patolsky, ACS Nano, 2022, 16, 13800–13813 CrossRef CAS PubMed
.
- W. Shi, A. Schulzgen, R. Amezcua, X. Zhu and S.-U. Alam, J. Opt. Soc. Am. B, 2017, 34, FLA1 CrossRef CAS
.
- Y. Hu, E. Chatzilakou, Z. Pan, G. Traverso and A. K. Yetisen, Adv. Sci., 2024, 11(12), 2306560 CrossRef CAS PubMed
.
- M. Parrilla, U. Detamornrat, J. Domínguez-Robles, R. F. Donnelly and K. De Wael, Talanta, 2022, 249, 123695 CrossRef CAS PubMed
.
- M.-H. Lai, K.-S. Lim, D. S. Gunawardena, Y.-S. Lee and H. Ahmad, IEEE Sens. J., 2017, 17, 2961–2974 CAS
.
- Y.-W. Chen, M.-C. Chen, K.-W. Wu and T.-Y. Tu, Biomedicines, 2020, 8, 427 CrossRef CAS PubMed
.
- G. Anbazhagan, S. B. Suseela and R. Sankararajan, Drug Delivery Transl. Res., 2023, 13, 1813–1827 CrossRef CAS PubMed
.
- K. H. Ke and C. K. Chung, Small, 2020, 16, 2001209 CrossRef CAS PubMed
.
- P. Makvandi, A. Maleki, M. Shabani, A. R. J. Hutton, M. Kirkby, R. Jamaledin, T. Fang, J. He, J. Lee, B. Mazzolai, R. F. Donnelly, F. R. Tay, G. Chen and V. Mattoli, Matter, 2022, 5, 390–429 CrossRef CAS
.
- S. R. Dabbagh, M. R. Sarabi, R. Rahbarghazi, E. Sokullu, A. K. Yetisen and S. Tasoglu, iScience, 2021, 24, 102012 CrossRef CAS PubMed
.
- M. Yin, L. Xiao, Q. Liu, S. Y. Kwon, Y. Zhang, P. R. Sharma, L. Jin, X. Li and B. Xu, Adv. Healthcare Mater., 2019, 8, 1970093 CrossRef
.
- B. Ciui, A. Martin, R. K. Mishra, B. Brunetti, T. Nakagawa, T. J. Dawkins, M. Lyu, C. Cristea, R. Sandulescu and J. Wang, Adv. Healthcare Mater., 2018, 7, 1701264 CrossRef PubMed
.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. X. Shen, Adv. Sci., 2017, 5, 1700322 CrossRef PubMed
.
- Y. Zhou, L. Yin, S. Xiang, S. Yu, H. M. Johnson, S. Wang, J. Yin, J. Zhao, Y. Luo and P. K. Chu, Adv. Sci., 2023, 11, 2304874 CrossRef PubMed
.
- A. K. Shukla, A. Banerjee, M. K. Ravikumar and A. Jalajakshi, Electrochim. Acta, 2012, 84, 165–173 CrossRef
.
- S. J. Rowley-Neale, E. P. Randviir, A. S. Abo Dena and C. E. Banks, Appl. Mater. Today, 2018, 10, 218–226 CrossRef
.
- J. Yang, J. Zhang, X. Li, J. Zhou, Y. Li, Z. Wang, J. Cheng, Q. Guan and B. Wang, Nano Energy, 2018, 53, 916–925 CrossRef CAS
.
- Z. L. Wang, Mater. Today, 2017, 20, 74–82 CrossRef
.
- Y. Zi, H. Guo, Z. Wen, M.-H. Yeh, C. Hu and Z. L. Wang, ACS Nano, 2016, 10, 4797–4805 CrossRef CAS PubMed
.
- Y. Wang, T. Ying, J. Li, Y. Xu, R. Wang, Q. Ke, S. G. F. Shen, H. Xu and K. Lin, Chem. Eng. J., 2020, 402, 126273 CrossRef CAS
.
- H. Zheng, Z. Pu, H. Wu, C. Li, X. Zhang and D. Li, Biosens. Bioelectron., 2023, 223, 115036 CrossRef CAS PubMed
.
- T. Shibaji, Y. Yasuhara, N. Oda and M. Umino, J. Controlled Release, 2001, 73, 37–47 CrossRef CAS PubMed
.
- T. Karpiński, Pharmaceutics, 2018, 10, 10040204 Search PubMed
.
- T. H. Kim, N. Y. Kim, H. U. Lee, J. W. Choi, T. Kang and B. G. Chung, J. Controlled Release, 2023, 364, 383–392 CrossRef CAS PubMed
.
- K. Moore, S. Grégoire, J. Eilstein, M. B. Delgado-Charro and R. H. Guy, Mol. Pharmaceutics, 2023, 21, 234–244 CrossRef PubMed
.
- W. Zhu, H. Yu, Z. Pu, Z. Guo, H. Zheng, C. Li, X. Zhang, J. Li and D. Li, Biosens. Bioelectron., 2023, 235, 115406 CrossRef CAS PubMed
.
- K. Moore, S. B. Reeksting, V. Nair, S. T. Pannakal, N. Roy, J. Eilstein, S. Grégoire, M. B. Delgado-Charro and R. H. Guy, RSC Adv., 2023, 13, 11261–11268 RSC
.
- S. Zhao, A. S. Mehta and M. Zhao, Cell. Mol. Life Sci., 2020, 77, 2681–2699 CrossRef CAS PubMed
.
- S. Bestmann and V. Walsh, Curr. Biol., 2017, 27, R1258–R1262 CrossRef CAS PubMed
.
- M. Zhao, Semin. Cell Dev. Biol., 2009, 20, 674–682 CrossRef CAS PubMed
.
- R. Luo, J. Dai, J. Zhang and Z. Li, Adv. Healthcare Mater., 2021, 10, 2100557 CrossRef CAS PubMed
.
- J. Tian, R. Shi, Z. Liu, H. Ouyang, M. Yu, C. Zhao, Y. Zou, D. Jiang, J. Zhang and Z. Li, Nano Energy, 2019, 59, 705–714 CrossRef CAS
.
- E. Neumann and K. Rosenheck, J. Membr. Biol., 1972, 10, 279–290 CrossRef CAS PubMed
.
- F. Wang, S. Lin, Z. Yu, Y. Wang, D. Zhang, C. Cao, Z. Wang, D. Cui and D. Chen, Lab Chip, 2022, 22, 2624–2646 RSC
.
- T. Kotnik, W. Frey, M. Sack, S. Haberl Meglič, M. Peterka and D. Miklavčič, Trends Biotechnol., 2015, 33, 480–488 CrossRef CAS PubMed
.
- G. Byagathvalli, S. Sinha, Y. Zhang, M. P. Styczynski, J. Standeven and M. S. Bhamla, PLoS Biol., 2020, 18, 3000589 CrossRef PubMed
.
- Z.-Y. Huo, H. Liu, C. Yu, Y.-H. Wu, H.-Y. Hu and X. Xie, Chem. Eng. J., 2019, 369, 1005–1013 CrossRef CAS
.
- S.-E. Choi, H. Khoo and S. C. Hur, Chem. Rev., 2022, 122, 11247–11286 CrossRef CAS PubMed
.
- P. C. Ferreira, V. N. Ataíde, C. L. Silva Chagas, L. Angnes, W. K. Tomazelli Coltro, T. R. Longo Cesar Paixão and W. Reis, de Araujo, TrAC, Trends Anal. Chem., 2019, 119, 115622 CrossRef CAS
.
- H. A. Saputra, Monatsh. Chem., 2023, 154, 1083–1100 CrossRef CAS
.
- D. M. E. Freeman, D. K. Ming, R. Wilson, P. L. Herzog, C. Schulz, A. K. G. Felice, Y.-C. Chen, D. O’Hare, A. H. Holmes and A. E. G. Cass, ACS Sens., 2023, 8, 1639–1647 CrossRef CAS PubMed
.
- S. Odinotski, K. Dhingra, A. GhavamiNejad, H. Zheng, P. GhavamiNejad, H. Gaouda, D. Mohammadrezaei and M. Poudineh, Small, 2022, 18, 2200201 CrossRef CAS PubMed
.
- J. J. García-Guzmán, C. Pérez-Ràfols, M. Cuartero and G. A. Crespo, TrAC, Trends Anal. Chem., 2021, 135, 116148 CrossRef
.
- M. V. Chinnamani, A. Hanif, P. K. Kannan, S. Kaushal, M. J. Sultan and N.-E. Lee, Biosens. Bioelectron., 2023, 237, 115468 CrossRef CAS PubMed
.
- Z. Zhao, Q. Saiding, Z. Cai, M. Cai and W. Cui, Mater. Today, 2023, 63, 210–238 CrossRef CAS
.
- Z. Wen, C. Liu, Z. Teng, Q. Jin, Z. Liao, X. Zhu and S. Huo, Nanoscale, 2023, 15, 13532–13545 RSC
.
- D. Omata, L. Munakata, K. Maruyama and R. Suzuki, J. Med. Ultrasonics, 2022, 2001, DOI:10.1007/s10396-022-01201-x
.
- A. K. Kösters, B. Ganse, B. Gueorguiev, K. Klos, A. Modabber, S. Nebelung, B.-S. S. Kim and M. Knobe, Int. Orthop., 2017, 41, 2067–2074 CrossRef PubMed
.
- M. A. Yokus and J. S. Jur, IEEE Trans. Biomed. Eng., 2016, 63, 423–430 Search PubMed
.
- L. Cheng, J. Li, A. Guo and J. Zhang, npj Flexible Electron., 2023, 7, 39 CrossRef
.
- L. Li, X. Zhang, J. Zhou, L. Zhang, J. Xue and W. Tao, Small, 2022, 18, 2107705 CrossRef CAS PubMed
.
- D. I. Moon, G. Plečkaitytė, T. Choi, M. L. Seol, B. Kim, D. Lee, J. W. Han and M. Meyyappan, Adv. Healthcare Mater., 2020, 9, 1901575 CrossRef CAS PubMed
.
- Q. Yang, H. Yin, T. Xu, D. Zhu, J. Yin, Y. Chen, X. Yu, J. Gao, C. Zhang, Y. Chen and Y. Gao, Small, 2020, 16, 1906814 CrossRef CAS PubMed
.
- K. Kim, J. C. Monroe, T. P. Gavin and B. T. Roseguini, J. Appl. Physiol., 2020, 128, 1635–1642 CrossRef CAS PubMed
.
- K. Kim, J. C. Monroe, T. P. Gavin and B. T. Roseguini, Exercise Sport Sci. Rev., 2020, 48, 163–169 CrossRef PubMed
.
- F. Lu, C. Wang, R. Zhao, L. Du, Z. Fang, X. Guo and Z. Zhao, Biosensors, 2018, 8, 31 CrossRef PubMed
.
- V. Novickij, A. Zinkevičienė, V. Malyško, J. Novickij, J. Kulbacka, N. Rembialkowska and I. Girkontaitė, J. Photochem. Photobiol., B, 2020, 213, 112066 CrossRef CAS PubMed
.
- C. Rabut, S. Yoo, R. C. Hurt, Z. Jin, H. Li, H. Guo, B. Ling and M. G. Shapiro, Neuron, 2020, 108, 93–110 CrossRef CAS PubMed
.
- A. Del Campo Fonseca and D. Ahmed, Adv. Drug Delivery Rev., 2024, 205, 115164 CrossRef CAS PubMed
.
- B. Yang, X. Fang and J. Kong, Adv. Funct. Mater., 2020, 30, 2000591 CrossRef CAS
.
- M. Parrilla, A. Vanhooydonck, M. Johns, R. Watts and K. De Wael, Sens. Actuators, B, 2023, 378, 133159 CrossRef CAS
.
- O. P. Singh, A. Bocchino, T. Guillerm, Y. Hu, F. Stam and C. O’Mahony, Adv. Mater. Technol., 2023, 9, 2301606 CrossRef
.
- J. Sun, L. Zhang, Z. Li, Q. Tang, J. Chen, Y. Huang, C. Hu, H. Guo, Y. Peng and Z. L. Wang, Adv. Mater., 2021, 33, 2102765 CrossRef CAS PubMed
.
- Z.-H. Li, Y. Chen, Y. Sun and X.-Z. Zhang, ACS Nano, 2021, 15, 5189–5200 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |