Biodegradable MoNx@Mo-foil electrodes for human-friendly supercapacitors†
Hongjia
Ren‡
a,
Hongru
Zhao‡
a,
Muhammad Sufyan
Javed
 *a,
Sajid Hussain
Siyal
*a,
Sajid Hussain
Siyal
 b,
Xinze
Zhang
a,
Xiaofeng
Zhang
a,
Awais
Ahmad
cd,
Iftikhar
Hussain
b,
Xinze
Zhang
a,
Xiaofeng
Zhang
a,
Awais
Ahmad
cd,
Iftikhar
Hussain
 e,
Mohamed A.
Habila
e,
Mohamed A.
Habila
 f and
Weihua
Han
f and
Weihua
Han
 *a
*a
aSchool of Physical Science and Technology, Lanzhou University, Lanzhou 730000, China. E-mail: safisabri@gmail.com; hanwh@lzu.edu.cn
bDepartment of Metallurgy and Materials Engineering, Dawood University of Engineering and Technology, Karachi 74800, Sindh, Pakistan
cDepartamento de Quimica Organica, Universidad de Cordoba, EdificioMarie Curie (C-3), Ctra Nnal IV-A, Km 396, E14014 Cordoba, Spain
dDepartment of Chemistry, The University of Lahore, Lahore, 54590, Pakistan
eDepartment of Mechanical Engineering, City University of Hong Kong, 83 Tat Chee Avenue, Hong Kong
fDepartment of Chemistry, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia
First published on 8th May 2024
Abstract
With the advancement in the field of biomedical research, there is a growing demand for biodegradable electronic devices. Biodegradable supercapacitors (SCs) have emerged as an ideal solution for mitigating the risks associated with secondary surgeries, reducing patient discomfort, and promoting environmental sustainability. In this study, MoNx@Mo-foil was prepared as an active material for biodegradable supercapacitors through high-temperature and nitridation processes. The composite electrode exhibited superior electrochemical performance in both aqueous and solid-state electrolytes. In the case of the solid-state electrolyte, the MoNx@Mo-foil composite electrode-based device demonstrated excellent cycling stability and electrochemical performance. Additionally, the composite electrode exhibited rapid and complete biodegradability in a 3% H2O2 solution. Through detailed experimental analysis and performance testing, we verified the potential application of the MoNx@Mo-foil composite electrode in biodegradable supercapacitors. This work provides a new choice of degradable material for developing biomedical electronic devices.
Introduction
In recent years, the increasing severity of the aging population issue and the continuous development in the field of biomedicine have led to a steady growth in demand for energy storage devices, implantable medical devices, and other electronic components.1–3 These devices can be used for monitoring physiological parameters, drug delivery, etc.4–7 This trend has spurred the development of biodegradable biomedical devices, presenting new challenges for the flexibility, portability, biocompatibility, and degradability of energy storage devices in temperature-dependent environments.8,9 However, providing a stable and efficient energy supply for these devices has become a significant challenge for scientists. Flexible supercapacitors have attracted considerable interest owing to their impressive characteristics such as elevated energy density, fast charge–discharge efficiency, and robust cycling stability.10,11 However, the energy density of supercapacitors is lower than that of batteries.12 Recently, new kinds of batteries have emerged to compensate for the long life with high energy density, such as divalent and trivalent ion batteries.13,14 Furthermore, dual-ion batteries and supercapacitors are also proposed to boost the energy and power densities of the devices.15,16 Researchers are dedicated to selecting biodegradable materials to fabricate biodegradable supercapacitors, serving as novel bioenergy storage devices for implantable electronic devices within organisms. This innovation provides a solution to the energy supply challenge for bio-devices.17,18 After completing the energy supply tasks, these biodegradable electronic devices can autonomously degrade inside the biological body, thereby avoiding the risk of secondary surgeries, enhancing safety, and being environmentally friendly, representing a technology in line with the concept of sustainable development.19–22 Le et al.23 reported a CsPbBr3 electrode composed of CsPbBr3 nanocrystals synthesized via a hot injection method, exhibiting a specific capacitance of 528 mF g−1. In a symmetric supercapacitor fabricated using TBAPF6 as the electrolyte, the specific capacitance reached 119.8 mF g−1, indicating its potential as an electrode material in supercapacitors. However, it has been noted that the charge/discharge time is short, and the potential window is narrow, leaving room for further improvement in power density and energy density. On the other hand, He et al.24 reported the continuous preparation of hollow poly(3,4-ethylenedioxythiophene):polystyrene sulfonate thin-walled fibers (HPFs) via coaxial wet-spinning. These HPF electrodes exhibited a specific capacitance of 115.2 mF cm−2 at a current density of 0.3 mA cm−2. Moreover, at a power density of 0.112 mW cm−2, the energy density of the HPFs reached 9 μW h cm−2, demonstrating promising prospects for application in smart wearable devices. In addition, Li et al.25 reported a symmetric supercapacitor using biomass-derived activated carbon as electrodes, which can operate under alkaline acidic electrolyte, with stable operating voltage and high cycling performance, and the energy density is able to reach 36.9 W h kg−1, which is an excellent aqueous supercapacitor. Compared to previously reported works, the MoNx@Mo-foil electrode exhibits certain advantages while maintaining considerable energy density and power density. It achieves a balance between cycling stability and stability. Furthermore, it demonstrates superior biocompatibility as it can undergo stable degradation in H2O2 solution.Flexible solid-state biodegradable supercapacitors represent the primary research focus in this direction currently. Their extensibility, portability, biocompatibility, and degradability make them applicable to a wide range of biomedical applications, including wearable electronic devices, implantable medical devices, and various other biologically related electronic applications.17,26 In the ongoing quest to find suitable electrode materials for biodegradable supercapacitors, molybdenum, and its compounds have demonstrated a range of superior properties, including excellent conductivity, chemical stability, and mechanical strength. Furthermore, molybdenum, as a soluble metal, can be metabolized, decomposed, or excreted by organisms under certain conditions, rendering it an excellent biocompatible material.27,28 Consequently, molybdenum-based materials emerge as ideal candidate electrodes for biodegradable supercapacitors.
In this study, we employed pure molybdenum foil as the substrate. Following cleaning with dilute hydrochloric acid and alcohol, the foil underwent high-temperature annealing in pure ammonia gas.29,30 This process facilitated the uniform growth of molybdenum nitride nanosheet arrays on the surface, providing more attachment sites for electrolyte ions, thereby enhancing their adsorption capacity and electrochemical performance. The MoNx@Mo-foil composite electrode was characterized using techniques such as XRD, XPS, and SEM.
1 M sodium chloride (NaCl) aqueous solution and PVA/NaCl gel were used as electrolytes. We assembled both aqueous hybrid supercapacitors and flexible solid-state supercapacitors for electrochemical testing. Compared to the original molybdenum foil electrode, the MoNx@Mo-foil composite electrode exhibited significantly improved electrochemical performance. In the NaCl aqueous electrolyte, MoNx@Mo-foil demonstrated superior electrochemical energy storage efficiency and discharge time. After undergoing 5000 cycles at a current density of 10 A g−1, it exhibited a capacitance retention of 88.55%, showcasing its robustness and longevity in electrochemical performance.
In solid-state supercapacitors employing the PVA/NaCl electrolyte, the MoNx@Mo-foil electrode demonstrated exceptional electrochemical performance, exhibiting remarkable energy and power density. Even after 5000 cycles, it sustained a capacitance retention rate of 77.51%, underscoring its enduring stability and efficacy.
Finally, to validate biocompatibility, biodegradation experiments were conducted in a 3% concentration of H2O2 solution. Both the MoNx@Mo-foil composite electrode and the MoNx@Mo//PVA/NaCl//MoNx@Mo assembly were found to completely degrade in the 3% H2O2 solution, demonstrating excellent biodegradability.
Fig. 1a illustrates the synthesis procedure of the MoNx@Mo-foil electrode. Initially, the molybdenum foil undergoes ultrasonic cleaning in ethanol and dilute hydrochloric acid, followed by rinsing with DI water to ensure cleanliness. Subsequently, the cleaned foils are subjected to annealing at 500 °C in a tubular furnace under an ammonia gas atmosphere for 3 hours, with a gradual heating rate of 3 °C min−1, and then naturally cooled to room temperature. This meticulous process leads to the formation of a layer comprising MoNx nanosheets on the surface of the molybdenum foil, a structural transformation confirmed through scanning electron microscopy analysis for surface alterations. Fig. 1b illustrates the surface morphology of the original molybdenum foil, which exhibits a smooth metallic surface. After high-temperature annealing under an ammonia gas atmosphere, a layer of smooth nanosheet arrays uniformly grew on the surface, forming the MoNx@Mo-foil composite electrode (Fig. 1c). Fig. 1d provides SEM images at higher resolutions, exposing the uniform sizes and distributions of MoNx nanoparticles, indicative of consistent morphological characteristics within the electrode material.
 | ||
| Fig. 1 (a) Schematic diagram of the molybdenum nitride synthesis process. (b) SEM image of the original Mo-foil. (c) SEM image of MoNx@Mo foil. (d) High-magnification SEM image of MoNx@Mo foil. | ||
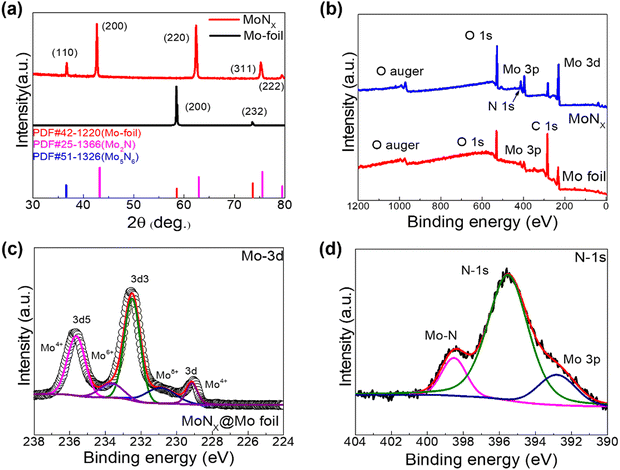
X-ray diffraction (XRD) was performed to examine the crystal structures of both the pristine Mo foil and the MoNx@Mo-foil, as depicted in Fig. 2a. The diffraction peak observed at 58.4° is attributed to the (200) crystal plane of metallic Mo, as per the PDF card, and the peak observed at 37° can be attributed to the (110) crystal plane of Mo5N6, delineating the presence of specific structural arrangements within the MoNx@Mo-foil. Additionally, peaks observed at 47°, 59°, 76°, and 79° correspond to the (200), (220), (311), and (222) crystal planes of Mo2N, respectively, according to the PDF card. These findings suggest the formation of a composite material comprising various molybdenum-based nitrides on the surface of the original molybdenum foil after high-temperature annealing under an ammonia gas atmosphere. For more comprehensive analysis, X-ray photoelectron spectroscopy (XPS) was utilized to explore the distribution of valence states in MoNx@Mo-foil. As depicted in Fig. 2b, the XPS survey spectrum of MoNx@Mo-foil validates the existence of Mo, O, N, and C elements, underscoring the elemental composition of the material under investigation. Fig. 2c displays the high-resolution XPS spectrum analysis of Mo 3d, delineating peaks observed at 235.65 eV, indicative of Mo4+, along with peaks detected at 233.69 eV and 230.9 eV, corresponding to Mo6+ and Moδ+ states, respectively, and a sub-peak at 229.13 eV corresponding to Mo4+. The high-resolution XPS spectrum of N-1s (depicted in Fig. 2d) reveals a peak at 398.44 eV, associated with the Mo–N bond, along with a peak at 395.55 eV representing N 1s, and another peak observed at 392.79 eV attributed to Mo 3p. Additionally, the XPS spectrum of C 1s illustrates peaks at 284.6 eV and 286.1 eV, corresponding to C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively, accompanied by a sub-peak at 288.7 eV attributed to the C
C bonds, respectively, accompanied by a sub-peak at 288.7 eV attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Through the comprehensive analysis of the X-ray photoelectron spectroscopy (XPS) spectra, the nitrogen content in the MoNx@Mo-foil electrode obtained after annealing was determined, as depicted in Fig. S1 (ESI†). XPS spectra were acquired for four elements: Mo, N, O, and C. Upon normalization, the nitrogen content was found to be approximately 32.77%, while the Mo content was approximately 10.74%. This finding corroborates the formation of a thin and dense MoNx layer on the surface of the original molybdenum foil after ammonia annealing and provides insight into the nitrogen content within it. After BET testing, the specific surface area and pore information of the pristine molybdenum foil electrode and MoNx@Mo-foil electrode were obtained. The experimental data are, respectively, presented in Fig. S2 and S3 (ESI†). Following annealing, the specific surface area of the MoNx@Mo-foil electrode increased from 2.31 m2 g−1 to 2.39 m2 g−1, while the pore diameter expanded from 31.00 Å to 3308.26 Å. The augmented specific surface area and pore size enable the MoNx@Mo-foil electrode to offer more insertion/deinsertion sites for cations during the electrochemical process, resulting in enhanced electron transfer rates and improved cycling stability.
O bond. Through the comprehensive analysis of the X-ray photoelectron spectroscopy (XPS) spectra, the nitrogen content in the MoNx@Mo-foil electrode obtained after annealing was determined, as depicted in Fig. S1 (ESI†). XPS spectra were acquired for four elements: Mo, N, O, and C. Upon normalization, the nitrogen content was found to be approximately 32.77%, while the Mo content was approximately 10.74%. This finding corroborates the formation of a thin and dense MoNx layer on the surface of the original molybdenum foil after ammonia annealing and provides insight into the nitrogen content within it. After BET testing, the specific surface area and pore information of the pristine molybdenum foil electrode and MoNx@Mo-foil electrode were obtained. The experimental data are, respectively, presented in Fig. S2 and S3 (ESI†). Following annealing, the specific surface area of the MoNx@Mo-foil electrode increased from 2.31 m2 g−1 to 2.39 m2 g−1, while the pore diameter expanded from 31.00 Å to 3308.26 Å. The augmented specific surface area and pore size enable the MoNx@Mo-foil electrode to offer more insertion/deinsertion sites for cations during the electrochemical process, resulting in enhanced electron transfer rates and improved cycling stability.
 | ||
| Fig. 2 (a) XRD analysis of the Mo foil, MoNx@Mo foil, and labeled PDF cards. (b) XPS full spectrum of the Mo foil and MoNx@Mo foil. (c) XPS spectra of Mo-3d. (d) XPS spectra of N-1s. | ||
To evaluate the electrochemical performance of the MoNx@Mo-foil in three electrode system. The three-electrode setup comprised an Ag/AgCl electrode as the reference electrode and a platinum foil with an area of approximately 2 cm2 was employed as the counter electrode, operating within a defined voltage window of −1.0 to 0 V. Fig. 3a and b depicts the cyclic voltammetry (CV) curves obtained for both the original molybdenum foil and the MoNx@Mo-foil electrode. The analysis reveals that the MoNx@Mo-foil electrode exhibits a significantly larger area under the CV curve compared to the original molybdenum foil electrode across various scan rates. This observation suggests a marked enhancement in electrochemical performance with the integration of MoNx onto the Mo-foil substrate. Furthermore, as the scan rate increases, the morphology of the CV curve for the MoNx@Mo-foil electrode remains stable and maintains a characteristic rectangular shape.31 This behavior indicates consistent double-layer electrochemical behavior, underscoring the electrode's stability and suitability for high-performance energy storage applications.
Moreover, the charge storage mechanisms of both electrodes were meticulously calculated (as depicted in Fig. 3c and d). At a scan rate of 10 mV s−1, the original molybdenum foil electrode exhibited approximately 20.26% charge storage through the capacitive process, with the remaining 79.74% stored via the diffusion process. In contrast, the capacitive charge storage of the MoNx@Mo-foil composite electrode notably increased to 35.15%, representing a substantial enhancement.
Furthermore, as the scan rate escalated from 1 mV s−1 to 75 mV s−1, the capacitive charge storage capacity of the MoNx@Mo-foil electrode exhibited a remarkable surge from 14.63% to 59.75%, surpassing the original molybdenum foil electrode, which increased from 7.43% to 41.03%. This observation underscores the outstanding charge storage capability of the MoNx@Mo-foil composite electrode, primarily characterized by capacitive charge storage, particularly at elevated scan rates.
Additionally, constant current charge–discharge tests (GCD) were performed on both electrodes within a potential window of −1.0 V to 0 V. As shown in Fig. 4a, the discharge time of the MoNx@Mo-foil electrode at a current density of 0.3 A g−1 is significantly longer compared to that of the original molybdenum foil electrode, indicating superior electrochemical performance, consistent with the conclusion drawn from the CV curves. Fig. 4b and c showcases the galvanostatic charge–discharge (GCD) curves of the original molybdenum foil and MoNx@Mo-foil electrodes under varying current densities. Notably, the MoNx@Mo-foil electrode exhibits prolonged discharge times compared to the original molybdenum foil electrode across different current densities, indicative of stable electrochemical performance. The specific capacitance of both electrodes was computed based on the discharge time obtained from the GCD curves (Fig. 4d). At a current density of 0.1 A g−1, the MoNx@Mo-foil electrode achieved a notable capacitance of 27 F cm−2, significantly surpassing that of the original molybdenum foil electrode. Subsequently, as the current density increased to 2.0 A g−1, the capacitance of the MoNx@Mo-foil electrode decreased to 8 F cm−2, with a capacitance retention rate of approximately 24.49%. This marks a substantial improvement compared to the 10.58% capacitance retention rate of the original molybdenum foil. Furthermore, Fig. 4e illustrates the cycling stability test conducted on both electrode materials. Following 5000 cycles, the MoNx@Mo-foil electrode demonstrated an outstanding capacitance retention rate of 88.55%, surpassing the 82.64% capacitance retention rate of the original molybdenum foil electrode, thus underscoring its superior capacitance retention characteristics.
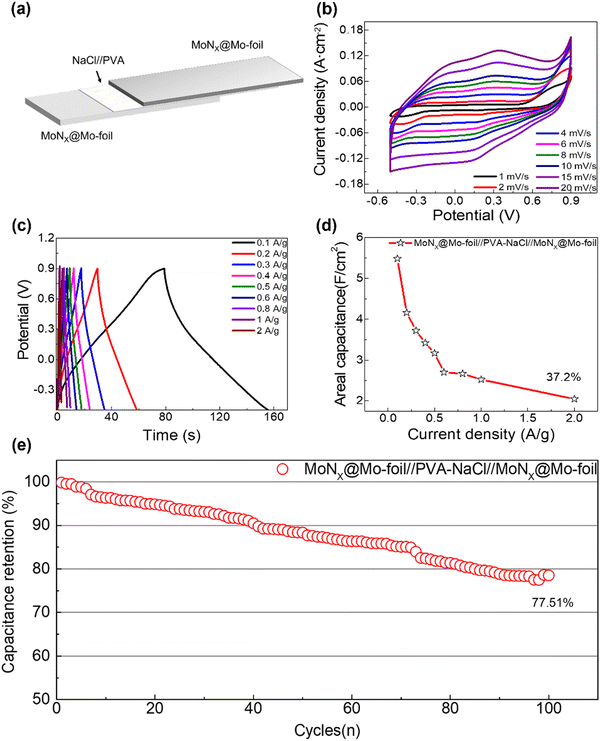
To assess the practical performance of the solid-state flexible biodegradable supercapacitor (MoNx@Mo//NaCl/PVA//MoNx@Mo-SBSC), we assembled the device using two identical MoNx@Mo-foil electrodes and a NaCl/PVA solid-state electrolyte, as depicted in Fig. 5a. The capacitor structure comprises two MoNx@Mo-foil composite electrodes of identical size, separated by a blend of 0.9% NaCl and PVA gel, which also serves as a binder. CV tests were conducted at varying scan rates (1–20 mV s−1) within a voltage range of −0.5 to 0.9 V using an electrochemical workstation, as depicted in Fig. 5b. The increasing area under the CV curve with scan rate signifies the robust electrochemical performance of the assembled solid-state supercapacitor. Furthermore, Fig. 5c exhibits the GCD curves of the solid-state flexible biodegradable supercapacitor at different current densities (0.1–2.0 A g−1). The symmetric distribution of the GCD curve shapes indicates excellent Coulombic efficiency. As shown in Fig. 5d, the specific capacitance was computed based on the discharge time, and even at a current density of 2 A g−1, it retained a capacitance retention rate of 37.2%, underscoring its remarkable performance. Moreover, cycling stability tests were conducted on the solid-state flexible biodegradable supercapacitor (Fig. 5e). After 5000 charge–discharge cycles, it retained 77.51% of its initial capacitance, demonstrating commendable electrochemical performance. Compared to previously published results for biodegradable supercapacitors, the MoNx@Mo-foil electrode exhibits certain advantages while maintaining considerable energy density and power density. It achieves a balance between cycling stability and stability. Furthermore, it demonstrates superior biocompatibility as it can undergo stable degradation in H2O2 solution, which has an objective prospect in the field of implantable electronic devices and other fields.
To verify their biodegradability, biodegradation experiments were conducted on the MoNx@Mo-foil electrode and the MoNx@Mo//NaCl/PVA//MoNx@Mo-SBSC. The samples were immersed in a 3% H2O2 solution, and changes in their appearance in the solution were observed using an optical camera and an optical microscope. Fig. 6a and b illustrate the degradation process of the MoNx@Mo-foil electrode. Initially, the surface was smooth upon immersion in the solution. After 2 hours, the electrode began to dissolve, showing wrinkling and cracking on the surface. Throughout 2 to 6 hours, the electrode gradually dissolved, with cracks on the surface contracting inward, and the overall area gradually decreasing. Partial debris started to detach after 8 hours, and after 10 hours, internal fractures appeared, gradually dispersing into particles. By 12 hours, the entire electrode had dissolved into particles and gradually disappeared. Upon further magnification with an optical microscope, the dissolved electrode was observed as fine particles and flake-like structures dispersed in the solution. The scanning electron microscopy (SEM) characterization of the MoNx@Mo-foil electrode after 6 hours of degradation, as depicted in Fig. 6b, reveals that compared to the dense array of MoNx nanosheets observed in Fig. 1, the nanosheet array on the electrode surface has largely disappeared after degradation, with some debris adhering to the surface. Additionally, the molybdenum foil substrate has fractured, forming multiple cracks, consistent with observations under optical microscopy. Fig. 6c displays the degradation process of the MoNx@Mo//NaCl/PVA//MoNx@Mo-SBSC. Apart from the fixed area, both MoNx@Mo-foil composite electrodes lost their metallic luster after 2 hours of degradation, deforming and shrinking gradually after 8 hours. By 12 hours, the structure of the supercapacitor started to disintegrate. After 24 hours, most of the electrode had dissolved, and after 36 hours, complete dissolution was observed. Only the membrane, which is harmless to the organism, remained intact and could not degrade. It is mainly organic material, which can be naturally expelled from the body's circulatory system.
Conclusion
In summary, we synthesized a MoNx@Mo-foil electrode through annealing under an ammonia atmosphere, resulting in a composite electrode with a unique morphology characterized by uniform growth of MoNx nanoplates on the surface of the molybdenum foil. XRD and XPS confirmed the formation of molybdenum-based nitride on the electrode surface, indicating the successful synthesis of the sample. Electrochemical tests revealed the improved performance of the MoNx@Mo-foil electrode compared to the original molybdenum foil, with significant enhancements in charge storage mechanisms, specific capacitance, and cycling stability. Additionally, the solid-state flexible and biodegradable supercapacitor assembled using the MoNx@Mo-foil electrode exhibited robust electrochemical performance and good capacitance retention properties. Biodegradation experiments confirmed the biodegradability of the MoNx@Mo-foil electrode and supercapacitor, highlighting their potential for environmentally friendly applications. Overall, the synthesized MoNx@Mo-foil electrode demonstrates enhanced electrochemical performance and biodegradability, making it promising for various energy storage and biodegradable electronic applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Research Fund for International Young Scientists of the National Natural Science Foundation of China (NSFC) under grant no. 52250410342 and a Scientific research start-up grant for Youth Researchers at Lanzhou University (China). This work was funded by the Researchers Supporting Project Number (RSP2024R441), King Saud University, Riyadh, Saudi Arabia.References
- C. Zhu, Y. Hao, H. Wu, M. Chen, B. Quan, S. Liu, X. Hu, S. Liu, Q. Ji, X. Lu and J. Qu, Self-Assembly of Binderless MXene Aerogel for Multiple-Scenario and Responsive Phase Change Composites with Ultrahigh Thermal Energy Storage Density and Exceptional Electromagnetic Interference Shielding, Nano-Micro Lett., 2023, 16(1), 57 CrossRef PubMed.
- T. Wei, C. Sun, X. Guo, Y. Zhou, M. Wang, X. Qiu, Q. Wang and Y. Tang, Petaloid bimetallic metal-organic frameworks derived ZnCo2O4/ZnO nanosheets enabled intermittent lithiophilic model for dendrite-free lithium metal anode, J. Colloid Interface Sci., 2024, 664, 596–606 CrossRef CAS PubMed.
- X. Zhang, M. S. Javed, H. Ren, X. Wei, X. Zhang, S. Khan, A. Ahmad, A. M. Tighezza, A. M. Hassan and W. Han, Human friendly biodegradable supercapacitors utilizing water-soluble MoOx@Mo-foil as electrode and normal saline as electrolyte, Appl. Phys. Lett., 2023, 123(21), 213905 CrossRef CAS.
- H.-J. Kim, W. Sritandi, Z. Xiong and J. S. Ho, Bioelectronic devices for light-based diagnostics and therapies, Biophys. Rev., 2023, 4, 1 Search PubMed.
- M. Mariello, I. Eş and C. M. Proctor, Soft and Flexible Bioelectronic Micro-Systems for Electronically Controlled Drug Delivery, Adv. Healthcare Mater., 2023, 2302969 CrossRef PubMed.
- M. Sang, K. Kim, J. Shin and K. J. Yu, Ultra-Thin Flexible Encapsulating Materials for Soft Bio-Integrated Electronics, Adv. Sci., 2022, 9(30), 2202980 CrossRef CAS PubMed.
- T. A. Baldo, L. F. de Lima, L. F. Mendes, W. R. de Araujo, T. Paixao and W. K. T. Coltro, Wearable and Biodegradable Sensors for Clinical and Environmental Applications, ACS Appl. Electron. Mater, 2021, 3(1), 68–100 CrossRef CAS.
- G.-H. Lee, H. Moon, H. Kim, G. H. Lee, W. Kwon, S. Yoo, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Multifunctional materials for implantable and wearable photonic healthcare devices, Nat. Rev. Mater., 2020, 5(2), 149–165 CrossRef PubMed.
- J. Zhu, R. Chaturvedi, Y. Fouad, I. Albaijan, N. Juraev, L. H. Alzubaidi, I. Mahariq, A. Afandi and H. A. L. Garalleh, A numerical modeling of battery thermal management system using nano-enhanced phase change material in hot climate conditions, Case Stud. Therm. Eng., 2024, 58, 104372 CrossRef.
- L. Wu, X. Y. Shi and Z. S. Wu, Recent Advancements and Perspectives of Biodegradable Polymers for Supercapacitors, Adv. Funct. Mater., 2023, 33(16), 35 Search PubMed.
- H. C. Zhang, J. Y. Zhang, X. L. Gao, L. Wen, W. X. Li and D. W. Zhao, Advances in materials and structures of supercapacitors, Ionics, 2022, 28(2), 515–531 CrossRef CAS.
- L.-Y. Tian, P.-B. Huang, J.-Q. Lv, Y.-H. Zhang, L.-N. Zhao, L. Liu, P.-F. Wang, Y.-H. Wu, Q. Shi and F.-N. Shi, Metal-organic frameworks based on ternary transition metal ions for high-performance lithium ion batteries, J. Solid State Chem., 2024, 335, 124717 CrossRef CAS.
- M. Wang, C. Jiang, S. Zhang, X. Song, Y. Tang and H.-M. Cheng, Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage, Nat. Chem., 2018, 10(6), 667–672 CrossRef CAS PubMed.
- X. Zhang, Y. Tang, F. Zhang and C.-S. Lee, A novel aluminum–graphite dual-ion battery, Adv. Energy Mater., 2016, 6(11), 1502588 CrossRef.
- Y. Su, J. Shang, X. Liu, J. Li, Q. Pan and Y. Tang, Constructing π–π Superposition Effect of Tetralithium Naphthalenetetracarboxylate with Electron Delocalization for Robust Dual-Ion Batteries, Angew. Chem., Int. Ed., 2024, e202403775 CAS.
- M. Zhang, W. Zhang, F. Zhang, C.-S. Lee and Y. Tang, Anion-hosting cathodes for current and late-stage dual-ion batteries, Sci. Chin. Chem., 2024, 67(5), 1–25 Search PubMed.
- H. T. Das, E. Balaji T, S. Mohapatra, S. Dutta, N. Das and M. A. Assiri, Advance Technologies in Biodegradable Flexible Solid-State Supercapacitors: A Mini Review on Clean and Sustainable Energy, Chem. Rec., 2023, 24, e202300226 CrossRef PubMed.
- A. Ben Amar, A. B. Kouki and H. Cao, Power Approaches for Implantable Medical Devices, Sensors, 2015, 15(11), 28889–28914 CrossRef PubMed.
- Y. Cao and K. E. Uhrich, Biodegradable and biocompatible polymers for electronic applications: A review, J. Bioact. Compat. Polym., 2019, 34(1), 3–15 CrossRef CAS.
- W. H. Lee, G. D. Cha and D. H. Kim, Flexible and biodegradable electronic implants for diagnosis and treatment of brain diseases, Curr. Opin. Biotechnol, 2021, 72, 13–21 CrossRef CAS PubMed.
- K. J. Yu, D. Kuzum, S.-W. Hwang, B. H. Kim, H. Juul, N. H. Kim, S. M. Won, K. Chiang, M. Trumpis, A. G. Richardson, H. Cheng, H. Fang, M. Thompson, H. Bink, D. Talos, K. J. Seo, H. N. Lee, S.-K. Kang, J.-H. Kim, J. Y. Lee, Y. Huang, F. E. Jensen, M. A. Dichter, T. H. Lucas, J. Viventi, B. Litt and J. A. Rogers, Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex, Nat. Mater., 2016, 15(7), 782–791 CrossRef CAS PubMed.
- G. D. Cha, D. Kang, J. Lee and D. H. Kim, Bioresorbable Electronic Implants: History, Materials, Fabrication, Devices, and Clinical Applications, Adv. Healthc. Mater, 2019, 8(11), 20 Search PubMed.
- L. Pang, M. T. Hoang, A. O’Mullane and H. Wang, Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors, Energy Mater., 2023, 3(2), 300012 CAS.
- C. He, J. Cheng, Y. Liu, X. Zhang and B. Wang, Thin-walled hollow fibers for flexible high energy density fiber-shaped supercapacitors, Energy Mater., 2022, 1, 1 Search PubMed.
- C. Li, W. Wu, P. Wang, W. Zhou, J. Wang, Y. Chen, L. Fu, Y. Zhu, Y. Wu and W. Huang, Fabricating an Aqueous Symmetric Supercapacitor with a Stable High Working Voltage of 2 V by Using an Alkaline-Acidic Electrolyte, Adv. Sci., 2019, 6(1), 1801665 CrossRef PubMed.
- Q. Liu, L. Liu, Y. Zheng, M. Li, B. Ding, X. Diao, H.-M. Cheng and Y. Tang, On-demand engineerable visible spectrum by fine control of electrochemical reactions, Natl. Sci. Rev., 2023, 11, 3 Search PubMed.
- C. Fernandes and I. Taurino, Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants, Sensors, 2022, 22(8), 22 CrossRef PubMed.
- C. Redlich, P. Quadbeck, M. Thieme and B. Kieback, Molybdenum - A biodegradable implant material for structural applications?, Acta Biomater., 2020, 104, 241–251 CrossRef CAS PubMed.
- V. Orlov, R. Osaulenko and V. Y. J. I. M. Kuznetsov, Synthesis of Molybdenum Nitrides, Inorg. Mater., 2020, 56, 1113–1121 CrossRef CAS.
- Y. J. Ting, K. Lian and N. Kherani, In Fabrication of Titanium Nitride and Molybdenum Nitride for Supercapacitor Electrode Application, Symposium on Batteries and Energy Technology Joint General Session/219th Meeting of the Electrochemical-Society (ECS), Montreal, CANADA, May 01-06, Electrochemical Soc Inc: Montreal, Canada, 2011, pp 133–139 Search PubMed.
- X. Zhang, M. S. Javed, H. Ren, X. Zhang, S. Ali, K. Han, A. Ahmad, A. M. Tighezza, W. Han and K.-Q. Peng, Human-friendly flexible solid-state biodegradable supercapacitor based on Ti3C2Tx MXene film without adhesive structure, Mater. Today Energy, 2024, 40, 101496 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb00649f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |