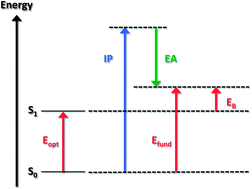
The energy gap between the highest occupied and lowest unoccupied electronic levels is a critical parameter determining the electronic, optical, redox, and transport (electrical) properties of a material. However, the energy gap comes in many flavors, such as the band gap, HOMO–LUMO gap, fundamental gap, optical gap, or transport gap, with each of these terms carrying a specific meaning. Failure to appreciate the distinctions among these different energy gaps has caused much confusion in the literature, which is manifested by the frequent use of improper terminology, in particular, in the case of organic molecular or macromolecular materials. Thus, it is our goal here to clarify the meaning of the various energy gaps that can be measured experimentally or evaluated computationally, with a focus on π-conjugated materials of interest for organic electronics and photonics applications.