Open Access Article
Open Access ArticleB ← N Lewis pair fusion of [6]helicene: one way to integrate circularly polarized luminescence with two-photon absorption
Min Wangab,
Zhi-Qiang Liu *c and
Cui-Hua Zhao
*c and
Cui-Hua Zhao *a
*a
aSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China. E-mail: chzhao@sdu.edu.cn
bShandong Engineering Research Center of New Energy Materials and Devices, Weifang University of Science and Technology, Weifang 262700, China
cState Key Laboratory of Crystal Materials, Shandong University, Jinan 250100, China. E-mail: zqliu@sdu.edu.cn
First published on 23rd January 2026
Abstract
It is very challenging to integrate circularly polarized luminescence (CPL) and two-photon absorption (TPA) in a single organic molecule, which is of great interest for advanced optoelectronic and bioimaging applications. We here disclose the synthesis and properties of a new family of helicenes, in which [6]helicene is fused with six-membered B ← N heterocycles. Benefiting from the strong electron-affinity of the B ← N Lewis pair, the introduction of an electron-donating (para-diphenylamino)phenyl (p-Ph2NC6H4) group induces intramolecular charge transfer (CT) characteristics. For mono-fused derivatives, both molar absorption coefficients (ε) and fluorescence quantum yields (ΦF) progressively increase with enhancement of the intramolecular CT feature through introduction of p-Ph2NC6H4 and replacement of the boron-bound phenyl group with more electron-withdrawing perfluorophenyl. Moreover, the structural evolution from a mono-fused dipolar to a bis-fused quadrupolar architecture results in more than a twofold increase in ε and simultaneous significant enhancement of the TPA cross section (δTPA) and luminescence dissymmetry factor (glum). It thus became possible to achieve integration of excellent CPL and TPA properties in a single molecule for the bis-fused quadrupolar [6]helicene BiNFBPy-HC, with δTPA up to 1361 GM and CPL brightness (BCPL) reaching 13.2 M−1 cm−1, owing to its large ε (8.84 × 104 M−1 cm−1), good ΦF (0.41) and moderate |glum| (7.37 × 10−4).
Introduction
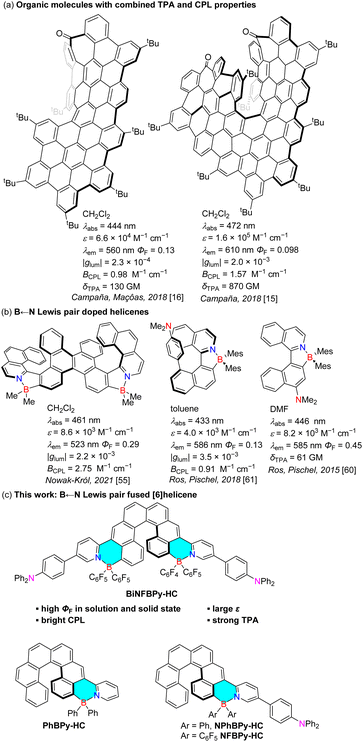
Organic materials exhibiting circularly polarized luminescence (CPL) have gained rapidly increasing attention in the last decade owing to their broad potential applications in 3D displays,1,2 optical data storage,3,4 biological probes and signatures.5,6 An ideal CPL-active material is expected to have not only a high fluorescence quantum yield (ΦF) but also a high luminescence dissymmetry factor (glum = 2(IL − IR)/(IL + IR)).7,8 More recently, CPL brightness (BCPL = ε × ΦF × |glum|/2), which also takes the molar extinction coefficient (ε) into account, has emerged as a more comprehensive parameter to evaluate the overall performance of CPL properties.9 Beyond achieving high BCPL, integrating CPL with additional optical functionalities, in particular, two-photon absorption (TPA), has become an important topic in this field. TPA allows excellent 3D resolution and absorption of lower-energy (near-infrared) light with reduced photodamage and deeper tissue penetration.10–12 The combination of CPL and TPA in a single molecule would not only lay a solid foundation for two-photon-excited CPL confocal microscopy in bioimaging but also open new avenues for other advanced optoelectronic applications.13,14 Nevertheless, examples that simultaneously exhibit high BCPL and large TPA cross section (δTPA) remain extremely rare (Fig. 1a).15–17 To achieve efficient TPA, one widely adopted strategy is to construct systems featuring intramolecular charge transfer (CT) characteristics by simultaneous incorporation of electron donor (D) and acceptor (A) groups.18,19 Three typical structures are generally employed, namely dipolar (D–A),20–22 quadrupolar (D–A–D or A–D–A),23,24 and octupolar [D–A3 or A–D3] architectures.25–27 Compared with dipolar systems, the multibranched quadrupoles and octupoles tend to display larger δTPA values due to the enhanced electron coupling among branches. In this context, it was envisioned that the construction of multipolar chiral systems may provide a promising route to achieve integrated CPL and TPA properties. Probably due to synthesis challenges, such examples have not been explored.Helicenes, characterized by an extended π-conjugation structure and inherent helical chirality, have long been recognized as promising skeletons for CPL-active molecules.28–30 However, parent helicenes suffer from very low ΦF values (ΦF < 0.05 for [n]helicenes, n ≥ 5), primarily due to the fast intersystem crossing (ISC) process.31,32 Herein, considerable efforts have been devoted to increasing ΦF of helicenes. One efficient strategy is to incorporate heteroatoms into helicene frameworks to perturb electronic structures.33–42 Another is to enlarge π-conjugation by fusion with polycyclic arenes or attachment of peripheral substituents.43–49 For the first strategy, one representative approach is to replace C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds with their isoelectronic B–N couple counterparts, which are typically formed via N-directed borylative cyclization of amino precursors.40–42 Instead of embedding the B–N couple into the parent helicene backbone, we have recently prepared B–N heterocycle-fused [6]helicenes using ortho-phenylamine-substituted precursors.50 It was found that the fusion with the B–N heterocycle helps extend π-conjugation and increase both the absorptivity and fluorescence efficiency of the first excited state.
C bonds with their isoelectronic B–N couple counterparts, which are typically formed via N-directed borylative cyclization of amino precursors.40–42 Instead of embedding the B–N couple into the parent helicene backbone, we have recently prepared B–N heterocycle-fused [6]helicenes using ortho-phenylamine-substituted precursors.50 It was found that the fusion with the B–N heterocycle helps extend π-conjugation and increase both the absorptivity and fluorescence efficiency of the first excited state.
Remarkably, a double [6]helicene derivative can show outstanding CPL performance with BCPL reaching 49.0 M−1 cm−1, owing to its very high ΦF (0.87), large ε (5.47 × 104 M−1 cm−1), and fairly good |glum| (2.06 × 10−3).
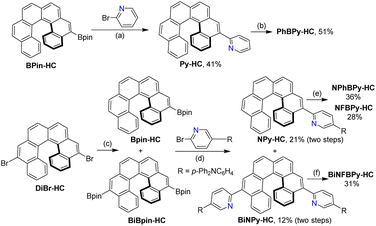
Inspired by the outstanding CPL performance of B–N heterocycle-fused [6]helicenes, we turned our attention to helicenes fused with heterocycles containing a B ← N Lewis pair, which remain unprecedented to date. Similar to the covalent B–N bond, intramolecular B ← N coordination bond formation can planarize and rigidify π-systems,51–53 thereby suppressing the nonradiative decay process and boosting ΦF, which have been well demonstrated by a number of highly emissive B ← N-doped helicenes (Fig. 1b).54–61 Moreover, the B ← N unit tends to increase the electron affinity of the system,62–64 which can endow the systems with intramolecular CT characteristics and thereby TPA properties when electron-donor groups are also introduced.60,61 Considering the facile accessibility of 5,12-dibromo[6]helicene and its easy transformation into a donor-substituted 2-pyridyl derivative, we envisioned that it was possible to prepare a quadrupole B ← N Lewis pair-fused [6]helicene. Following this strategy, we successfully synthesized a bis-B ← N Lewis pair-fused [6]helicene, BiNFBPy-HC (Fig. 1c), in which a strong electron-donating (para-diphenylamino)phenyl (p-Ph2NC6H4) group was introduced at the 4-position of the 2-pyridyl unit. It was fascinating to find that this molecule exhibits both excellent CPL and TPA properties with BCPL up to 13.2 M−1 cm−1 and δTPA up to 1361 GM. To further probe the influence of B ← N Lewis pair fusion and structural modification on photophysical properties, we also synthesized mono-fused derivatives, PhBPy-HC, NPhBPy-HC, and NFBPy-HC, which differ in the presence of electron donor groups and the substituent at the boron center. Herein, we report detailed results on the synthesis, structures and fascinating properties of these unprecedented B ← N Lewis pair-fused [6]helicenes.
Results and discussion
Synthesis and structures
The synthetic routes to the B ← N Lewis pair-fused [6]helicenes are depicted in Scheme 1. A typical method for the synthesis of stable B ← N Lewis pairs is N-directed electrophilic borylative cyclization, followed by trapping of the dihaloborane intermediate with organometallic reagents.65 Thus, the key point of the synthesis is the preparation of 2-pyridyl-substituted [6]helicene precursors. The precursor to PhBPy-HC, 5-(2-pyridyl)[6]helicene (Py-HC), was readily synthesized via a Pd-catalyzed Suzuki cross-coupling of 2-bromopyridine (Br-Py) with [6]helicene-5-boronic ester (BPin-HC), which we had previously prepared.50 To access 5-{5-[4-(N,N-diphenylamino)phenyl]-2-pyridyl}helicene (NPy-HC), the precursor for the mono-fused derivatives, NPhBPy-HC and NFBPy-HC, we initially attempted to adopt a similar Suzuki cross-coupling of BPin-HC with 2-bromo-4-[(4-N,N-diphenylamino)phenyl]pyridine (BrNPy), which was conveniently obtained through selective coupling of 2-bromo-5-iodopyridine with [(4-N,N-diphenylamino)phenyl]boronic acid (Scheme S1). However, challenges arose during the preparation of 5,12-bis{5-[4-(N,N-diphenylamino)phenyl]-2-pyridyl}[6]helicene (BiNPy-HC), the precursor for bis-fused BiNFBPy-HC. The planned route involved Suzuki coupling of 5,12-dibromo[6]helicene (DiBr-HC) with bis(pinacolato)-diboron to afford [6]helicene-5,12-diboronic ester (BiBPin-HC) and a second Suzuki coupling of BiBPin-HC with BrNPy. Unexpectedly, mono-debromination occurred during the borylation of DiBr-HC, resulting in the formation of BPin-HC along with the desired BiBPin-HC. In addition, BPin-HC and BiBPin-HC could not be separated through the usual purification techniques, such as flash column chromatography and recrystallization. Therefore, the resulting mixture was used directly in the subsequent coupling with BrNPy. Based on the comparison of 1H NMR spectra between the mixture and the pure BPin-HC (Fig. S1), the molar ratio of BPin-HC to BiBPin-HC was determined to be about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4. Fortunately, NPy-HC and BiNPy-HC could be separated by flash column chromatography. Consequently, both NPy-HC and BiNPy-HC were prepared simultaneously via two-step Suzuki coupling starting from DiBr-HC. With the 2-pyridyl-substituted [6]helicene precursors in hand, the final B ← N Lewis pair formation was accomplished through N-directed borylative cyclization with boron tribromide (BBr3) as the boron source and subsequent quenching of the dibromoborane intermediates with the corresponding arylmagnesium bromides.
1.4. Fortunately, NPy-HC and BiNPy-HC could be separated by flash column chromatography. Consequently, both NPy-HC and BiNPy-HC were prepared simultaneously via two-step Suzuki coupling starting from DiBr-HC. With the 2-pyridyl-substituted [6]helicene precursors in hand, the final B ← N Lewis pair formation was accomplished through N-directed borylative cyclization with boron tribromide (BBr3) as the boron source and subsequent quenching of the dibromoborane intermediates with the corresponding arylmagnesium bromides.
The chemical structures of all the B ← N fused [6]helicenes were fully characterized by 1H NMR, 13C NMR, and HR-MS. 11B NMR spectra were also characterized except for NPhBPy-HC, which has too poor solubility to afford a sufficiently strong signal. The signals observed in the range of −0.64––4.69 ppm in the 11B NMR spectra confirmed the presence of tetracoordinate boron atoms (Fig. S2). In the 1H NMR spectrum of PhBPy-HC, the presence of one singlet signal in the most downfield region (δ = 8.68 ppm) (Fig. S3), which corresponds to the proton at the 6-position of [6]helicene, indicates that the borylative cyclization occurs at the 4-position to form a six-membered ring rather than at the 6-position to generate a five-membered ring. Consistent with this result, two signals, a singlet and a doublet, with a very small coupling constant (J = 2.0 Hz) were observed in the 1H NMR spectra of derivatives containing Ph2NC6H4 (Fig. S4). The regioselectivity of borylative cyclization is attributed to the conjugative electronic effect of the 2-pyridyl substituent, which renders the 6,14-positions less reactive toward electrophilic substitution. All the final products are stable in air and water and can be purified by silica gel flash chromatography. Notably, the solubility is greatly affected by the substituent on the boron atom. Compounds PhBPy-HC and NPhBPy-HC bearing Ph groups show poor solubility in various solvents, whereas NFBPy-HC and BiNFBPy-HC with C6F5 substituents are highly soluble in common solvents such as CH2Cl2, CHCl3 and THF.
The presence of tetracoordinate boron atoms and the regioselectivity of borylative cyclization to form six-membered rings were further unambiguously confirmed by single-crystal X-ray diffraction analyses (Fig. 2). Single crystals of racemic PhBPy-HC and NFBPy-HC suitable for X-ray diffraction analysis were obtained by diffusion of hexane (PhBPy-HC) or methanol (NFBPy-HC) into their CHCl3 solutions. The B–N bond lengths of PhBPy-HC and NFBPy-HC were determined to be 1.64 Å and 1.62 Å, respectively, indicating strong Lewis pair interactions between the boron center and pyridyl N atom. It was noted that these two compounds adopt similar helical structures with very close dihedral angles (PhBPy-HC: 55.4°; NFBPy-HC: 57.9°) and centroid–centroid distances (PhBPy-HC: 4.43 Å; NFBPy-HC: 4.50 Å) between two terminal benzene rings (P1 and P2) in the [6]helicene skeleton. Owing to the formation of a B ← N Lewis pair, the pyridine ring (P4) is fixed with very high coplanarity to the adjacent P2 ring. Notably, the corresponding dihedral in NFBPy-HC (9.9°) is slightly smaller than that in PhBPy-HC (13.2°). Another difference in the X-ray structures is that the boron center of NFBPy-HC essentially lies within the six-membered ring (P3), while it protrudes in PhBPy-HC. The corresponding atom-plane distances are 0.11 Å for NFBPy-HC and 0.41 Å for PhBPy-HC, respectively. These structural differences may indicate more efficient conjugation between the pyridine ring and [6]helicene in NFBPy-HC. Regarding the structure of the triarylamine moiety in NFBPy-HC, it was noted that the nitrogen center adopts a perfect planar geometry with a sum of ∠C–N–C of 359.9°. In addition, the phenylene ring P5 of Ph2NC6H4is only slightly twisted relative to the pyridine ring (P4), indicating efficient electronic coupling between the electron donating amino group and the electron-accepting B ← N unit.
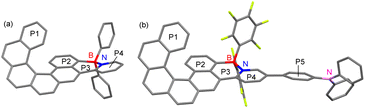 | ||
| Fig. 2 X-ray crystal structure of (a) PhBPy-HC and (b) NFBPy-HC (hydrogen atoms are omitted for clarity). | ||
Photophysical properties in solutions
The UV-vis absorption and fluorescence spectra of B ← N Lewis pair-fused [6]helicenes are shown in Fig. 3 with the corresponding data summarized in Table 1. In cyclohexane, the mono-B ← N fused [6]helicene PhBPy-HC shows the longest absorption at 455 nm, which was observed as a shoulder band and has a moderate intensity (ε = 6.8 × 103 M−1 cm−1). The fluorescence maximum is located at 489 nm with a fairly good efficiency (ΦF = 0.25). Notably, both the longest wavelength absorption and emission of PhBPy-HC are much stronger than those of the parent [6]helicene (ε = 0.3 × 103 M−1 cm−1 and ΦF = 0.05)66 and the recently reported mono-BN-naphthalene-fused [6]helicene (ε = 1.2 × 103 M−1 cm−1 and ΦF = 0.11).50 Introducing an electron-donating triphenylamine unit into NPhBPy-HC and further replacing the Ph group at the B center with a more electron-withdrawing C6F5 in NFBPy-HC lead to progressive enhancement of both the longest wavelength absorption and fluorescence intensities. Meanwhile, the longest-wavelength absorption undergoes a red shift to some extent. In contrast, the fluorescence maximum remains almost unchanged, except for the increased prominence of two distinct vibrational bands at 493 and 522 nm (ΦF = 0.49). The study on excited state dynamics suggests that the gradual increase of ΦF is mainly ascribed to the acceleration of the radiative decay process. Remarkably, from NFBPy-HC to BiNFBPy-HC, the further fusion of another B ← N Lewis pair resulted in a substantial increase in absorption intensity, with ε more than doubling to 8.84 × 104 M−1 cm−1, while ΦF slightly decreases to 0.41. Notably, BiNFBPy-HC exhibits a very narrow emission band in cyclohexane with a full width at half maximum (FWHM) of only 28 nm, indicating high molecular rigidity and suppressed excited-state structural relaxation. In view of the photophysical properties, another intriguing observation is that BiNPy-HC, the precursor of BiNFBPy-HC, shows much blue-shifted absorption (Δλ = 89 nm) and fluorescence (Δλ = 60 nm) relative to BiNFBPy-HC. In addition, its absorption is much weaker (ε = 2.85 × 104 M−1 cm−1) although ΦF remains nearly unchanged (0.40), highlighting the pronounced role of B ← N coordination in reinforcing absorption intensity. These findings clearly demonstrate that fusing [6]helicene with a B ← N Lewis pair, combined with judicious structural modifications, is effective in achieving intense absorption and strong fluorescence.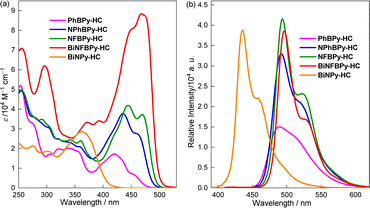 | ||
| Fig. 3 (a) UV/vis absorption (c = 2.0 × 10−5 M) and (b) fluorescence spectra (c = 1.0 × 10−5 M and λex = 400 nm) of B ← N Lewis pair-fused [6]helicenes in cyclohexane. | ||
| λabsa/nm (ε/104 M−1 cm−1) | λem/nm | ΦF | Δν/103 cm−1 | FWHM/nm | τ/ns | kr/108 s−1 | knr/108 s−1 | ||
|---|---|---|---|---|---|---|---|---|---|
| a Only the maxima of the longest wavelength band are shown.b Calculated using coumarin 307 as a standard.c Absolute quantum yields determined using an integrating sphere.d Average lifetime calculated using the equation Aiτi/ΣAiτi, where Ai is the preexponential for lifetime τi. | |||||||||
| PhBPy-HC | Cyclohexane | 419 (1.72), 455 (0.68) | 489 | 0.25b | 1.53 | 62 | 6.27 | 0.40 | 1.20 |
| Powder | — | 511 | 0.24c | — | 56 | 3.36d | 0.71 | 2.26 | |
| NPhBPy-HC | Cyclohexane | 435 (3.77), 463 (2.67)] | 493, 515 | 0.45b | 1.31 | 54 | 3.57 | 1.26 | 1.54 |
| Powder | — | 540 | 0.32c | — | 40 | 1.28d | 2.50 | 5.31 | |
| NFBPy-HC | Cyclohexane | 444 (4.19), 470 (3.72) | 493, 522 | 0.49b | 0.99 | 48 | 2.48 | 1.98 | 2.05 |
| Powder | — | 571 | 0.60c | — | 62 | 2.78d | 2.16 | 1.43 | |
| BiNFBPy-HC | Cyclohexane | 451 (8.09), 468 (8.84) | 496, 525 | 0.41b | 1.21 | 28 | 2.31 | 1.77 | 2.55 |
| Powder | — | 571 | 0.41c | — | 66 | 1.94d | 2.11 | 3.04 | |
| BiNPy-HC | Cyclohexane | 362 (2.85) | 436, 458 | 0.40b | 4.69 | 39 | 9.82 | 0.41 | 0.61 |
Considering the electron affinity of the B ← N Lewis pair and possible intramolecular CT characteristics in these [6]helicene derivatives, we further investigated solvent effects on their absorption and fluorescence (Fig. 4 and Table S4). It was found that the absorption spectra are barely affected by the solvent polarity for all the B ← N Lewis pair-fused [6]helicenes. From nonpolar cyclohexane to polar THF, the observed shifts in the longest wavelength absorption maxima are less than 9 nm, indicating that the electronic structure in the ground state (S0) is unaffected by the solvent environment. In contrast, the fluorescence solvatochromism is quite different depending on the presence of triphenylamine and the substituent at the boron center. For PhBPy-HC, which lacks the electron-donating Ph2NC6H4 group, the fluorescence emission remained essentially unchanged from cyclohexane to THF (Δλ ≤ 3 nm). However, NFBPy-HC, which contains C6F5 on B and triphenylamine on the pyridyl ring, exhibited a gradual red-shift in fluorescence from 493 nm in cyclohexane to 553 nm in THF. A more complex fluorescence solvatochromism was observed for NPhBPy-HC. From cyclohexane to CHCl3, the shift of fluorescence is minor (Δλ = 7 nm). Interestingly, an abrupt red-shift of 26 nm was observed when the solvent changed from CHCl3 to THF. Notably, the fluorescence maximum of NFBPy-HC in THF is 27 nm longer than that of NPhBPy-HC although they have nearly identical fluorescence maxima in cyclohexane. Regarding the fluorescence solvatochromism, another notable thing is that the bis-fused derivative BiNFBPy-HC exhibits fluorescence spectra that are very close to those of NFBPy-HC, albeit with slightly lower ΦF values. These fluorescence solvatochromism results suggest the first excited (S1) state of PhBPy-HC lacks significant CT character, while NPhBPy-HC displays partial CT nature due to the presence of the electron donating Ph2NC6H4 group. The CT character becomes even more pronounced in NFBPy-HC and BiNFBPy-HC as a result of the replacement of Ph with C6F5 at the boron center. These results hence confirm that the fusion of the B ← N Lewis pair helps enhance the electron affinity and tuning the substituent on the boron atom allows further modulation of this electron-accepting ability.
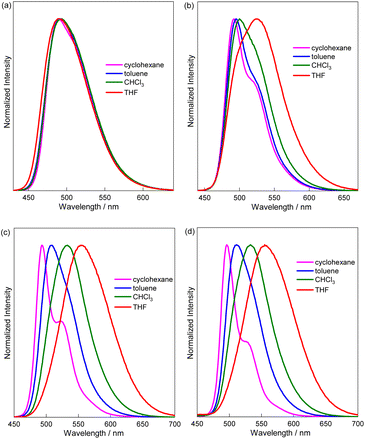 | ||
| Fig. 4 Fluorescence spectra (c = 1.0 × 10−5 M and λex = 400 nm) of (a) PhBPy-HC, (b) NPhBPy-HC, (c) NFBPy-HC, and (d) BiNFBPy-HC in various solvents. | ||
Theoretical calculations
To gain deeper insight into structure–property relationships, we conducted density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations at the B3LYP-D3(BJ)/6-31g(d) level. For all B ← N Lewis pair-fused [6]helicenes, the longest wavelength absorption band can be assigned to the S0 → S1 excitation process through comparison between the experimental and simulated absorption spectra (Fig. 5 and S10). For the three mono-fused [6]helicene derivatives, this excitation is predominantly composed of the HOMO → LUMO transition. Both the HOMO and the LUMO of PhBPy-HC are distributed over the pyridyl ring and the four adjacent benzene rings of the [6]helicene skeleton. Hence, the S0 → S1 excitation of PhBPy-HC is characterized by a π–π* transition process. Upon introduction of a Ph2NC6H4 group into NPhBPy-HC, the HOMO becomes further delocalized over the entire Ph2NC6H4 group while the LUMO remains almost unchanged. As a result, the introduction of triphenylamine imparts the S0 → S1 transition with a partial intramolecular CT feature. Moreover, the introduction of Ph2NC6H4 raises both HOMO and LUMO energy levels with a more pronounced increase for the HOMO (ΔE = 0.26 eV) than for the LUMO (ΔE = 0.06 eV). Although the aryl group on boron does not contribute to the frontier orbitals due to its tetracoordinated structure, the change of the aryl substituent on boron from phenyl in NPhBPy-HC to the more electron-withdrawing C6F5 in NFBPy-HC lowers both HOMO (ΔE = 0.15 eV) and LUMO (ΔE = 0.24 eV) energy levels with the LUMO decreased more greatly. Notably, the contribution of the [6]helicene core to the HOMO becomes less significant in NFBPy-HC, suggesting a stronger intramolecular CT character for S0 → S1 excitation of NFBPy-HC. Thus, NPhBPy-HC and NFBPy-HC can be regarded as D–A systems. Consistent with these electronic changes, the calculated absorption wavelength for S0 → S1 excitation gradually red shifts from PhBPy-HC to NPhBPy-HC and NFBPy-HC together with the progressive increase in the oscillator strength.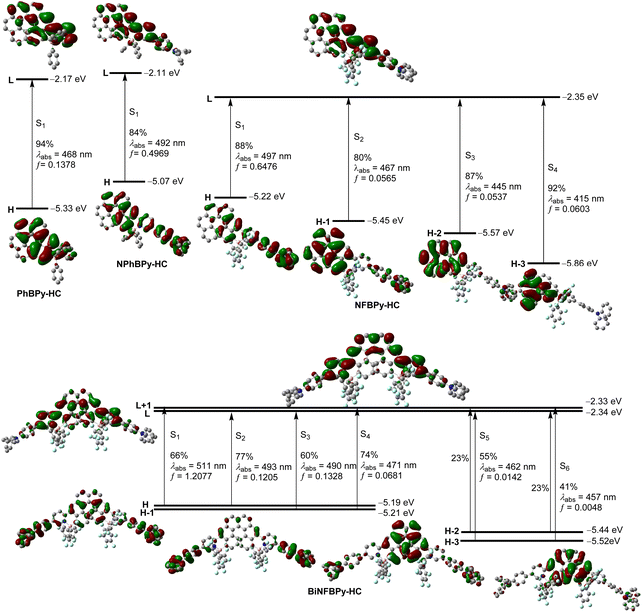 | ||
| Fig. 5 Kohn–Sham energy levels, pictorial drawing of frontier orbitals, and transitions of B ← N Lewis pair-fused [6]helicenes at the S0 geometries, calculated at the TD-B3LYP-D3(BJ)/6-31g(d) level. | ||
From mono-fused NFBPy-HC to bis-fused BiNFBPy-HC, the HOMO and LUMO energy levels remain nearly unchanged. However, the electronic distributions of the HOMO and LUMO change markedly with the fusion of additional B ← N Lewis pairs. In BiNFBPy-HC, the HOMO and LUMO are degenerate to HOMO−1 and LUMO+1, respectively. The HOMO is mainly distributed over two Ph2NC6H4 units and the central [6]helicene, while the HOMO−1 excludes the contribution from [6]helicene. In contrast, the LUMO and LUMO+1 are mainly localized on [6]helicene and two pyridyl rings. It was noted that HOMO−1 and LUMO+1 are C2-antisymmetric while the HOMO and LUMO are C2-symmetric. The S0 → S1 excitation of BiNFBPy-HC, which is dominated by HOMO → LUMO+1 (66%) and HOMO−1 → LUMO (19%) transitions, is symmetry permitted and exhibits much larger oscillator strength than NFBPy-HC. These results suggest the efficient exciton coupling between two fused moieties through the central [6]helicene skeleton in BiNFBPy-HC and this compound can be categorized as a quadrupolar D–A–D system. While the accuracy of calculated excitation energies is not sufficiently high at this computation level, these calculated results clearly demonstrate the CT feature of the S0 → S1 transition for three derivatives containing electron-donating Ph2NC6H4, which agrees well with their fluorescence solvatochromism. Furthermore, the simulated absorption spectra successfully reproduced the gradual red shift of absorption and increase of absorption intensity with increasing CT character from PhBPy-HC to NPhBPy-HC and NFBPy-HC, as well as the remarkable increase of absorption intensity from NFBPy-HC to BiNFBPy-HC as a result of effective exciton coupling.67
Fluorescence properties in the solid state
The high emission efficiency in the solid state is also a critical property for CPL emitters. Herein, the solid-state fluorescence properties of these B ← N Lewis pair-fused [6]helicenes were also investigated (Fig. 6, Table 1). For all the compounds, the fluorescence of powders appears at longer wavelengths even compared with their emission in THF solution, with red shifts within the range of 14–20 nm. The bathochromic shift indicates the presence of intermolecular π–π interactions. Notably, no significant fluorescence quenching was observed when going from cyclohexane to the powder form despite the presence of intermolecular π–π interactions in the solid state. From cyclohexane to the powder form, only NPhBPy-HC exhibits slight fluorescence quenching among the four B ← N Lewis pair-fused [6]helicenes. In contrast, the ΦF values of PhBPy-HC and BiNFBPy-HC remain nearly unchanged. Remarkably, the ΦF of NFBPy-HC in the powder form is even much higher than in cyclohexane, reaching a very high value of 0.60. Consequently, all these B ← N Lewis pair-fused [6]helicenes exhibit bright fluorescence in the solid state with moderate to high efficiency (ΦF = 0.24–0.60). Moreover, the emission colours can be facilely tuned from green (PhBPy-HC, λem = 511 nm) to yellow (NPhBPy-HC, λem = 540 nm) and then to orange (NFBPy-HC and BiNFBPy-HC, λem = 571 nm), highlighting the great utility of B ← N Lewis pair fusion with helicenes for achieving efficient and tunable solid-state emission.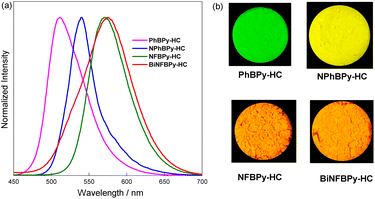 | ||
| Fig. 6 (a) Fluorescence spectra (λex = 400 nm) and (b) photographs under UV irradiation at 365 nm for the powder of B ← N Lewis pair-fused [6]helicenes. | ||
Two-photon absorption properties
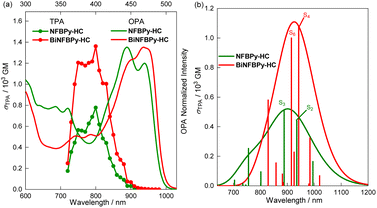
Considering the electron affinity endowed by B ← N Lewis pair fusion, we then investigated the TPA properties of these B ← N Lewis pair-fused [6]helicene compounds. The TPA spectra were measured in THF via a two-photon-excited fluorescence (TPEF) method using coumarin 485 as the standard compound with femtosecond laser excitation at wavelengths of 720–1000 nm (Fig. 7a). Unfortunately, PhBPy-HC decomposed during the measurement, as indicated by the complete disappearance of fluorescence. Interestingly, the other three compounds exhibit very strong TPA absorption. The highest δTPA values were determined to be 604 GM at 790 nm for NPhBPy-HC, 777 GM at 800 nm for NFBPy-HC, 1361 GM at 800 nm for BiNFBPy-HC, respectively. In addition, these three compounds can retain intense TPA absorption over a relatively broad region: 560–604 GM within 750–800 nm for NPhBPy-HC, 564–777 GM within 750−810 nm for NFBPy-HC and 1177–1360 GM within 750−800 nm for BiNFBPy-HC. Notably, the δTPA maximum of BiNFBPy-HC is nearly 1.8 times greater than that of NPhBPy-HC and NFBPy-HC and is among the largest values reported for the helicene derivatives.15,16,68,69The comparison between the TPA and the rescaled one-photon absorption (OPA) spectra revealed that the intense TPA bands of these three compounds correspond to dark transitions in the OPA and arise from excitations to a higher excited state. The theoretical calculations of TPA properties further supported this point (Fig. 7b, S11 and Table S7). The intense TPA band of BiNFBPy-HC primarily consists of S0 → S4 and S0 → S6 transitions with the S0 → S4 transition giving the highest theoretical δTPA. The S0 → S4 transition of BiNFBPy-HC is dominated by the HOMO−1 → LUMO+1 transition (74%), while the composition of S0 → S6 is more complex. The first two important components are HOMO−3 → LUMO+1 (41%) and HOMO−2 → LUMO (23%) transitions. Both HOMO−2 and HOMO−3 are distributed over the central [6]helicene core with HOMO−2 spreading to two Ph2N groups. It was noted that HOMO−1 → LUMO+1, HOMO−3 → LUMO+1, and HOMO−2 → LUMO transitions are symmetry prohibited, which is consistent with the low oscillator strengths of the corresponding OPA transitions (f = 0.0681 for of S0 → S4; 0.0048 for S0 → S6). For the mono-fused NPhBPy-HC and NFBPy-HC, which differ in the substituent at the boron center, the theoretical δTPA maxima originate from S0 → S2 and S0 → S3 excitations, respectively. Both transitions greatly contribute to TPA. In addition, the intense TPA band of NPhBPy-HC also involves the S0 → S4 excitation process. The S0 → S2, S0 → S3 and S0 → S4 excitations correspond to the transitions from HOMO−1, HOMO−2 and HOMO−3 to LUMO, respectively, for both compounds. Moreover, these two compounds show similar electronic distributions for HOMO−1, HOMO−2 and HOMO−3 orbitals. From HOMO−1 to HOMO−3, the electronic distribution gradually becomes more delocalized. The HOMO−1 is delocalized over the [6]helicene core skeleton and Ph2NC6H4 group, while HOMO−2 is only localized on the [6]helicene unit and HOMO−3 is located on a portion of the [6]helicene moiety. The theoretical calculations of TPA properties clearly indicate the significance of B ← N Lewis pair fusion for the promising TPA of the three amino-containing B ← N Lewis pair-fused [6]helicenes and the much more enhanced δTPA of bis-fused BiNFBPy-HC than the mono-fused NPhBPy-HC and NFBPy-HC. The highly symmetric charge transfer together with the extended π-conjugation in BiNFBPy-HC remarkably increases the probability of TPA transitions, thus leading to a much larger δTPA.18
Chiroptical properties
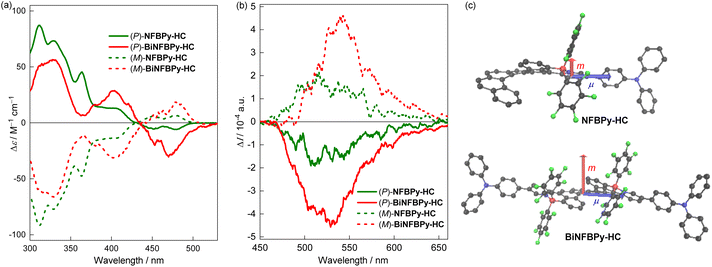
Finally, we proceeded to prepare their enantiomerically pure forms to investigate their chiroptical properties. Due to the extremely poor solubility of PhBPy-HC and NPhBPy-HC, no appropriate conditions for chiral resolution could be established. The enantiomeric pairs of NFBPy-HC and BiNFBPy-HC were successfully obtained through high-performance liquid chromatography (HPLC) with a chiral column (Fig. S7–S8 and Tables S2–S3). The experimental CD and CPL spectra in cyclohexane are shown in Fig. 8 with the corresponding data summarized in Table 2. The absolute configurations were assigned through the comparison between the experimental and theoretically simulated ECD spectra (Fig. S11b), which show the same signs for the longest and shortest wavelength bands above 300 nm. For both compounds, the (P)-isomer corresponds to the first eluted fraction.| CDa | CPLa | S0 → S1 transitionb | S1 → S0 transitionb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |gabs|/10−4 | |glum|/10−4 | BCPL/M−1 cm−1 | |µ|/10−17 esu cm | |m|/10−20 erg G−1 | θ/o | |gabs|cal/10−4 | |µ|/10−17 esu cm | |m|/10−20 erg G−1 | θ/o | |glum|cal/10−4 | |
| a Measured in cyclohexane solution.b Calculated by TD-DTF at the B3LYP-D3(BJ)/6-31g(d) level for M-enantiomers. | |||||||||||
| NFBPy-HC | 2.17 (480) | 3.31 (515) | 3.0 | 0.83 | 1.91 | 87.0 | 4.85 | 0.83 | 1.83 | 87.0 | 4.71 |
| BiNFBPy-HC | 5.00 (483) | 7.37 (543) | 13.2 | 1.15 | 4.85 | 87.0 | 9.04 | 1.08 | 4.45 | 86.7 | 9.32 |
In cyclohexane, nearly perfect mirror images were observed for the CD and CPL spectra of (P)- and (M)-isomers. The (P)-isomers of NFBPy-HC and BiNFBPy-HC display the longest wavelength CD bands at around 480 nm with a negative sign, while the strongest CD bands were observed at approximately 330 nm with a positive sign. The longest wavelength CD bands are assignable to the S0 → S1 transition and the corresponding CD dissymmetry factors (gabs, defined as Δε/ε) are determined to be −2.17 × 10−4 and −5.00 × 10−4 for (P)-NFBPy-HC and (P)-BiNFBPy-HC, respectively. The change of solvent from cyclohexane to THF caused negligible variations in the CD spectra, in terms of either position or intensity (Fig. S9). Remarkably, these two compounds display clearly mirror-imaged CPL signals in both nonpolar cyclohexane and polar THF, with the signs consistent with the corresponding longest CD bands. In cyclohexane, the |glum| values are up to 3.31 × 10−4 for NFBPy-HC and 7.37 × 10−4 for BiNFBPy-HC, respectively. Upon changing the solvent from cyclohexane to THF, the CPL spectra of both compounds are red-shifted by about 70 nm, which is consistent with their fluorescence solvatochromism. Notably, the bis-fused BiNFBPy-HC has significantly higher |gabs| for the longest wavelength band as well as larger |glum| compared to the mono-fused NFBPy-HC. Unfortunately, the CPL signals are nearly undetectable in both film and powder forms, although strong fluorescence emission is retained in the solid state.
To gain further insight into the chiroptical properties of the S1 state, the theoretical |gabs| and |glum| values for the S0 → S1 and S1 → S0 transitions were herein analysed (Table 2 and Fig. 8c and S13). Theoretically, the dissymmetry factors can be predicted using 4|µ||m|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/(|µ|2 + |m|2), where µ and m are the electronic and magnetic transition dipole moments, respectively, and θ is the angle between them. Because µ of organic molecules is hundreds of times larger than m, this expression can be simplified as 4|m|cos
θ/(|µ|2 + |m|2), where µ and m are the electronic and magnetic transition dipole moments, respectively, and θ is the angle between them. Because µ of organic molecules is hundreds of times larger than m, this expression can be simplified as 4|m|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/|µ|.70,71 For each compound, all the calculated parameters of S0 → S1 excitation are comparable to those of S1 → S0 deactivation. Notably, these two compounds show almost the same θ and close |µ|, whereas the |m| of BiNFBPy-HC is about 2.5 times that of NFBPy-HC. Consequently, the calculated |gabs| and |glum| values of BiNFBPy-HC are nearly two times those of NFBPy-HC, which is consistent with the experimental trend. These results suggest the structural evolution from a dipolar to a quadrupolar architecture helps enhance |m| and thereby increase transition dissymmetry factors. Another notable thing is that m is nearly perpendicular to µ for both compounds (θ ≈ 87°), giving a very small cos
θ/|µ|.70,71 For each compound, all the calculated parameters of S0 → S1 excitation are comparable to those of S1 → S0 deactivation. Notably, these two compounds show almost the same θ and close |µ|, whereas the |m| of BiNFBPy-HC is about 2.5 times that of NFBPy-HC. Consequently, the calculated |gabs| and |glum| values of BiNFBPy-HC are nearly two times those of NFBPy-HC, which is consistent with the experimental trend. These results suggest the structural evolution from a dipolar to a quadrupolar architecture helps enhance |m| and thereby increase transition dissymmetry factors. Another notable thing is that m is nearly perpendicular to µ for both compounds (θ ≈ 87°), giving a very small cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. It should be the small cos
θ. It should be the small cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ that results in unideal |gabs| and |glum| values. Although |glum| of BiNFBPy-HC is not quite high, its BCPL of BiNFBPy-HC can reach 13.2 M−1 cm−1 in cyclohexane, an excellent value among B ← N Lewis pair-functioned helicenes, due to its very high ε and good ΦF.58,59 Moreover, the BCPL of BiNFBPy-HC is more than four times that of NFBPy-HC (3.0 M−1 cm−1), highlighting the superior chiroptical properties of bis-fused quadrupolar BiNFBPy-HC over mono-fused dipolar NFBPy-HC.
θ that results in unideal |gabs| and |glum| values. Although |glum| of BiNFBPy-HC is not quite high, its BCPL of BiNFBPy-HC can reach 13.2 M−1 cm−1 in cyclohexane, an excellent value among B ← N Lewis pair-functioned helicenes, due to its very high ε and good ΦF.58,59 Moreover, the BCPL of BiNFBPy-HC is more than four times that of NFBPy-HC (3.0 M−1 cm−1), highlighting the superior chiroptical properties of bis-fused quadrupolar BiNFBPy-HC over mono-fused dipolar NFBPy-HC.
Conclusions
In summary, we have successfully synthesized a new family of [6]helicene derivatives, which are fused with six-membered B ← N heterocycles, via N-directed electrophilic borylation of 2-pyridyl-substituted [6]helicene precursors. Their photophysical properties were comprehensively investigated both experimentally and theoretically to elucidate the effect of structural modification. It was revealed that ε and ΦF increase progressively from parent [6]helicene to PhBPy-HC by fusion with the B ← N Lewis pair and are further enhanced in NPhBPy-HC due to the introduction of the electron-donor p-Ph2NC6H4 into pyridyl and thus the intramolecular CT nature. ε and ΦF of the mono-fused compounds reach maxima in NFBPy-HC, which displays more pronounced intramolecular CT characteristics as a result of the displacement of Ph on the boron atom with a stronger electron-withdrawing C6F5 group. The ε and ΦF NFBPy-HC are as high as 3.70 × 104 M−1 cm−1 and 0.49, respectively. From mono-fused dipolar NFBPy-HC to bis-fused quadrupolar BiNFBPy-HC, although ΦF slightly decreases to 0.41, ε more than doubles to 8.84 × 104. Notably, all three B ← N fused [6]helicenes, NPhBPy-HC, NFBPy-HC, and BiNFBPy-HC, which contain electron donating p-Ph2NC6H4, exhibit excellent TPA properties with δTPA maxima in the range of 604∼1361 GM. In particular, the bis-fused quadrupolar BiNFBPy-HC displays significantly improved TPA properties compared to mono-fused dipolar analogues due to the efficient electron coupling among its branches. Moreover, the enantiomerically pure forms of NFBPy-HC and BiNFBPy-HC emit notable CPL signals. Again, BiNFBPy-HC has significantly higher |gabs| for the longest wavelength band and larger |glum| compared to NFBPy-HC, which is ascribed to enhancement of magnetic transition dipoles. Hence, the structural evolution from a dipole to a quadrupole can simultaneously enhance absorptivity, TPA cross section and CPL dissymmetry. Owing to the large ε, good ΦF and moderate |glum|, the bis-fused quadrupolar BiNFBPy-HC achieves a remarkably high BCPL (13.2 M−1 cm−1), representing a very rare example of organic molecules that integrate excellent CPL and TPA properties. Regarding the photophysical properties, another notable thing is that all these B ← N fused [6]helicenes display intense fluorescence in the solid state (ΦF = 0.24–0.60 in powder form) with tunable emission colours from green to yellow and then to orange. Considering the facile preparation, great CPL performance, outstanding TPA properties, and efficient solid-state emission of BiNFBPy-HC, it is expected to be an excellent emitter. Furthermore, we believe that the valuable results about the structure–property relationships will lay a solid foundation for the rational design of new CPL-active molecules, especially those with integrated great CPL and TPA properties.Author contributions
C.-H. Zhao conceived the project. M. Wang synthesized the compounds and conducted all characterization and theoretical studies under the supervision of C.-H. Zhao and Z.-Q. Liu. C.-H. Zhao analyzed the data and wrote the manuscript. All authors commented on it.Conflicts of interest
There are no conflicts to declare.Data availability
2488475 (PhBPy-HC) and 2488477 (NFBPy-HC) contain the supplementary crystallographic data for this paper.72a,bSupplementary information (SI): preparation, photophysical properties, calculation details, and NMR spectra. See DOI: https://doi.org/10.1039/d5sc08697c.
Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of Shandong Province (Grant No. ZR2024MB033) and the National Natural Science Foundation of China (Grant No. 21971150 and U2330106). The HPC Cloud Platform of Shandong University is also sincerely acknowledged for its support of the theoretical calculations.Notes and references
- Y. Yang, R. C. da Costa, D. M. Smilgies, A. J. Campbell and M. J. Fuchter, Induction of Circularly Polarized Electroluminescence from an Achiral Light-Emitting Polymer via a Chiral Small-Molecule Dopant, Adv. Mater., 2013, 25, 2624–2628 CrossRef CAS PubMed.
- F. Furlan, J. M. Moreno-Naranjo, N. Gasparini, S. Feldmann, J. Wade and M. J. Fuchter, Chiral materials and mechanisms for circularly polarized light-emitting diodes, Nat. Photonics, 2024, 18, 658–668 CrossRef CAS.
- Y. Yang, R. C. da Costa, M. J. Fuchter and A. J. Campbell, Circularly polarized light detection by a chiral organic semiconductor transistor, Nat. Photonics, 2013, 7, 634–638 Search PubMed.
- Y. Chen, J. Gao and X. Yang, Chiral Grayscale Imaging with Plasmonic Metasurfaces of Stepped Nanoapertures, Adv. Optical Mater., 2019, 7, 1801467 CrossRef.
- R. Hassey, E. J. Swain, N. I. Hammer, D. Venkataraman and M. D. Barnes, Probing the chiroptical response of a single molecule, Science, 2006, 314, 1437–1439 CrossRef CAS PubMed.
- P. Stachelek, S. Serrano-Buitrago, B. L. Maroto, R. Pal and S. de la Moya, Circularly Polarized Luminescence Bioimaging Using Chiral BODIPYs: A Model Scaffold for Advancing Unprecedented CPL Microscopy Using Small Full-Organic Probes, ACS Appl. Mater. Interfaces, 2024, 16, 67246–67254 Search PubMed.
- E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Circularly Polarized Luminescence from Simple Organic Molecules, Chem.–Eur. J., 2015, 21, 13488–13500 CrossRef PubMed.
- G. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo and S. Abbate, Circularly Polarized Luminescence: A Review of Experimental and Theoretical Aspects, Chirality, 2016, 28, 696–707 Search PubMed.
- L. Arrico, L. Di Bari and F. Zinna, Quantifying the Overall Efficiency of Circularly Polarized Emitters, Chem.–Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed.
- F. Terenziani, C. Katan, E. Badaeva, S. Tretiak and M. Blanchard-Desce, Enhanced Two-Photon Absorption of Organic Chromophores: Theoretical and Experimental Assessments, Adv. Mater., 2008, 20, 4641–4678 CrossRef CAS.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Two-Photon Absorption and the Design of Two-Photon Dyes, Angew. Chem., Int. Ed., 2009, 48, 3244–3266 Search PubMed.
- H. M. Kim and B. R. Cho, Small-Molecule Two-Photon Probes for Bioimaging Applications, Chem. Rev., 2015, 115, 5014–5055 Search PubMed.
- P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactions, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed.
- B. Fabri, D. F. De Rosa, D. J. Black, R. Mucci, A. Krimovs, R. Pal and J. Lacour, Two-photon Excitation of Bright Diaza[4]Helicenes for Isotropic and Circularly Polarized Emission, Chem.–Eur. J., 2025, 31, e202501212 Search PubMed.
- C. M. Cruz, S. Castro-Fernández, E. Maçôas, J. M. Cuerva and A. G. Campaña, Undecabenzo[7]superhelicene: A Helical Nanographene Ribbon as a Circularly Polarized Luminescence Emitter, Angew. Chem., Int. Ed., 2018, 57, 14782–14786 CrossRef CAS PubMed.
- C. M. Cruz, I. R. Márquez, I. F. A. Mariz, V. Blanco, C. Sánchez-Sánchez, J. M. Sobrado, J. A. Martín-Gago, J. M. Cuerva, E. Maçôas and A. G. Campaña, Enantiopure distorted ribbon-shaped nanographene combining two-photon absorption-based upconversion and circularly polarized luminescence, Chem. Sci., 2018, 9, 3917–3924 Search PubMed.
- P. Reine, A. M. Ortuño, I. F. A. Mariz, M. Ribagorda, J. M. Cuerva, A. G. Campaña, E. Maçôas and D. Miguel, Simple Perylene Diimide Cyclohexane Derivative With Combined CPL and TPA Properties, Front. Chem., 2020, 8, 306 Search PubMed.
- M. Albota, D. Beljonne, J.-L. Bredas, J. E. Ehrlich, J.-Y. Fu, A. A. Heikal, S. E. Hess, T. Kogej, M. D. Levin and S. R. Marder, Design of organic molecules with large two-photon absorption cross sections, Science, 1998, 281, 1653–1656 Search PubMed.
- B. Jagatap and W. J. Meath, Contributions of permanent dipole moments to molecular multiphoton excitation cross sections, J. Opt. Soc. Am. B, 2002, 19, 2673–2681 Search PubMed.
- T.-C. Lin, G. S. He, P. N. Prasad and L.-S. Tan, Degenerate nonlinear absorption and optical power limiting properties of asymmetrically substituted stilbenoid chromophores, J. Mater. Chem., 2004, 14, 982–991 RSC.
- L. Beverina, J. Fu, A. Leclercq, E. Zojer, P. Pacher, S. Barlow, E. W. Van Stryland, D. J. Hagan, J.-L. Brédas and S. R. Marder, Two-photon absorption at telecommunications wavelengths in a dipolar chromophore with a pyrrole auxiliary donor and thiazole auxiliary acceptor, J. Am. Chem. Soc., 2005, 127, 7282–7283 Search PubMed.
- C.-L. Sun, Q. Liao, T. Li, J. Li, J.-Q. Jiang, Z.-Z. Xu, X.-D. Wang, R. Shen, D.-C. Bai, Q. Wang, S.-X. Zhang, H.-B. Fu and H.-L. Zhang, Rational design of small indolic squaraine dyes with large two-photon absorption cross section, Chem. Sci., 2015, 6, 761–769 Search PubMed.
- M. Rumi, J. E. Ehrlich, A. A. Heikal, J. W. Perry, S. Barlow, Z. Hu, D. McCord-Maughon, T. C. Parker, H. Röckel and S. Thayumanavan, Structure−property relationships for two-photon absorbing chromophores: bis-donor diphenylpolyene and bis (styryl) benzene derivatives, J. Am. Chem. Soc., 2000, 122, 9500–9510 Search PubMed.
- O. Mongin, L. Porrès, M. Charlot, C. Katan and M. Blanchard-Desce, Synthesis, Fluorescence, and Two-Photon Absorption of a Series of Elongated Rodlike and Banana-Shaped Quadrupolar Fluorophores: A Comprehensive Study of Structure–Property Relationships, Chem.–Eur. J., 2007, 13, 1481–1498 Search PubMed.
- Y. Jiang, Y. Wang, J. Hua, J. Tang, B. Li, S. Qian and H. Tian, Multibranched triarylamine end-capped triazines with aggregation-induced emission and large two-photon absorption cross-sections, Chem. Commun., 2010, 46, 4689–4691 Search PubMed.
- J. Shao, Z. Guan, Y. Yan, C. Jiao, Q.-H. Xu and C. Chi, Synthesis and Characterizations of Star-Shaped Octupolar Triazatruxenes-Based Two-Photon Absorption Chromophores, J. Org. Chem., 2011, 76, 780–790 CrossRef CAS PubMed.
- Y. M. Poronik, V. Hugues, M. Blanchard-Desce and D. T. Gryko, Octupolar Merocyanine Dyes: A New Class of Nonlinear Optical Chromophores, Chem.–Eur. J., 2012, 18, 9258–9266 CrossRef CAS PubMed.
- T. Mori, Chiroptical Properties of Symmetric Double, Triple, and Multiple Helicenes, Chem. Rev., 2021, 121, 2373–2412 Search PubMed.
- W.-L. Zhao, M. Li, H.-Y. Lu and C.-F. Chen, Advances in helicene derivatives with circularly polarized luminescence, Chem. Commun., 2019, 55, 13793–13803 Search PubMed.
- M. Cei, L. Di Bari and F. Zinna, Circularly polarized luminescence of helicenes: A data-informed insight, Chirality, 2023, 35, 192–210 CrossRef CAS PubMed.
- M. Sapir and E. Vander Donckt, Intersystem crossing in the helicenes, Chem. Phys. Lett., 1975, 36, 108–110 CrossRef CAS.
- J. Birks, D. Birch, E. Cordemans and E. Vander Donckt, Fluorescence of the higher helicenes, Chem. Phys. Lett., 1976, 43, 33–36 CrossRef CAS.
- H. Oyama, K. Nakano, T. Harada, R. Kuroda, M. Naito, K. Nobusawa and K. Nozaki, Synthetic Route to Highly Luminescent sila[7]helicene, Org. Lett., 2013, 15, 2104–2107 CrossRef CAS PubMed.
- T. Katayama, S. Nakatsuka, H. Hirai, N. Yasuda, J. Kumar, T. Kawai and T. Hatakeyama, Two-Step Synthesis of Boron-Fused Double Helicenes, J. Am. Chem. Soc., 2016, 138, 5210–5213 CrossRef CAS PubMed.
- K. Dhbaibi, L. Favereau and J. Crassous, Enantioenriched Helicenes and Helicenoids Containing Main-Group Elements (B, Si, N, P), Chem. Rev., 2019, 119, 8846–8953 Search PubMed.
- J.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A. C. H. Sue, X.-Y. Cao and X.-Y. Wang, B,N-Embedded Double Hetero[7]helicenes with Strong Chiroptical Responses in the Visible Light Region, J. Am. Chem. Soc., 2021, 143, 17958–17963 CrossRef CAS PubMed.
- F. Zhang, F. Rauch, A. Swain, T. B. Marder and P. Ravat, Efficient Narrowband Circularly Polarized Light Emitters Based on 1,4-B,N-embedded Rigid Donor–Acceptor Helicenes, Angew. Chem., Int. Ed., 2023, 62, e202218965 CrossRef CAS PubMed.
- A. Nowak-Król, P. T. Geppert and K. R. Naveen, Boron-containing helicenes as new generation of chiral materials: opportunities and challenges of leaving the flatland, Chem. Sci., 2024, 15, 7408–7440 RSC.
- F. Zhang, V. Brancaccio, F. Saal, U. Deori, K. Radacki, H. Braunschweig, P. Rajamalli and P. Ravat, Ultra-Narrowband Circularly Polarized Luminescence from Multiple 1,4-Azaborine-Embedded Helical Nanographenes, J. Am. Chem. Soc., 2024, 146, 29782–29791 CrossRef CAS PubMed.
- T. Hatakeyama, S. Hashimoto, T. Oba and M. Nakamura, Azaboradibenzo[6]helicene: Carrier Inversion Induced by Helical Homochirality, J. Am. Chem. Soc., 2012, 134, 19600–19603 CrossRef CAS PubMed.
- Y. Appiarius, S. Míguez-Lago, P. Puylaert, N. Wolf, S. Kumar, M. Molkenthin, D. Miguel, T. Neudecker, M. Juríček, A. G. Campaña and A. Staubitz, Boosting quantum yields and circularly polarized luminescence of penta- and hexahelicenes by doping with two BN-groups, Chem. Sci., 2024, 15, 466–476 RSC.
- Y. Jiao, Z. Sun, Z. Wang, Y. Fu and F. Zhang, Synthesis of Nonsymmetric NBN-Embedded [6]- and [7]Helicenes with Amplified Activities, Org. Lett., 2023, 25, 8766–8770 Search PubMed.
- T. Fujikawa, Y. Segawa and K. Itami, Synthesis, Structures, and Properties of π-Extended Double Helicene: A Combination of Planar and Nonplanar π-Systems, J. Am. Chem. Soc., 2015, 137, 7763–7768 CrossRef CAS PubMed.
- D. Reger, P. Haines, F. W. Heinemann, D. M. Guldi and N. Jux, Oxa[7]superhelicene: A π-Extended Helical Chromophore Based on Hexa-peri-hexabenzocoronenes, Angew. Chem., Int. Ed., 2018, 57, 5938–5942 CrossRef CAS PubMed.
- F. Saal, F. Zhang, M. Holzapfel, M. Stolte, E. Michail, M. Moos, A. Schmiedel, A.-M. Krause, C. Lambert, F. Würthner and P. Ravat, [n]Helicene Diimides (n = 5, 6, and 7): Through-Bond versus Through-Space Conjugation, J. Am. Chem. Soc., 2020, 142, 21298–21303 CrossRef CAS PubMed.
- Z. Qiu, C.-W. Ju, L. Frédéric, Y. Hu, D. Schollmeyer, G. Pieters, K. Müllen and A. Narita, Amplification of Dissymmetry Factors in π-Extended [7]- and [9]Helicenes, J. Am. Chem. Soc., 2021, 143, 4661–4667 CrossRef CAS PubMed.
- R. K. Dubey, M. Melle-Franco and A. Mateo-Alonso, Inducing Single-Handed Helicity in a Twisted Molecular Nanoribbon, J. Am. Chem. Soc., 2022, 144, 2765–2774 Search PubMed.
- F. Zhao, J. Zhao, H. Liu, Y. Wang, J. Duan, C. Li, J. Di, N. Zhang, X. Zheng and P. Chen, Synthesis of π-Conjugated Chiral Organoborane Macrocycles with Blue to Near-Infrared Emissions and the Diradical Character of Cations, J. Am. Chem. Soc., 2023, 145, 10092–10103 CrossRef CAS PubMed.
- X. Tian, K. Shoyama, B. Mahlmeister, F. Brust, M. Stolte and F. Würthner, Naphthalimide-Annulated [n]Helicenes: Red Circularly Polarized Light Emitters, J. Am. Chem. Soc., 2023, 145, 9886–9894 Search PubMed.
- M. Wang, M.-Y. Zhang and C.-H. Zhao, Brightening Up Circularly Polarized Luminescence of [6]Helicenes by Fusion with BN-Heterocycles, Org. Lett., 2025, 27, 1823–1828 Search PubMed.
- A. C. Shaikh, D. S. Ranade, S. Thorat, A. Maity, P. P. Kulkarni, R. G. Gonnade, P. Munshi and N. T. Patil, Highly emissive organic solids with remarkably broad color tunability based on N,C-chelate, four-coordinate organoborons, Chem. Commun., 2015, 51, 16115–16118 RSC.
- C. Zhu and L. Fang, Locking the Coplanar Conformation of π-Conjugated Molecules and Macromolecules Using Dynamic Noncovalent Bonds, Macromol. Rapid Commun., 2018, 39, 1700241 CrossRef PubMed.
- A. Sahoo, A. Patel, R. A. Lalancette and F. Jäkle, B ← N Lewis Pair Fusion of N,N-Diaryldihydrophenazines: Effect on Structural, Electronic, and Emissive Properties, Angew. Chem., Int. Ed., 2025, 64, e202503658 CrossRef CAS PubMed.
- C. Shen, M. Srebro-Hooper, M. Jean, N. Vanthuyne, L. Toupet, J. A. G. Williams, A. R. Torres, A. J. Riives, G. Muller, J. Autschbach and J. Crassous, Synthesis and Chiroptical Properties of Hexa-, Octa-, and Deca-azaborahelicenes: Influence of Helicene Size and of the Number of Boron Atoms, Chem.–Eur. J., 2017, 23, 407–418 CrossRef CAS PubMed.
- J. Full, S. P. Panchal, J. Götz, A. M. Krause and A. Nowak-Król, Modular Synthesis of Organoboron Helically Chiral Compounds: Cutouts from Extended Helices, Angew. Chem., Int. Ed., 2021, 60, 4350–4357 CrossRef CAS PubMed.
- F. Full, Q. Wölflick, K. Radacki, H. Braunschweig and A. Nowak-Król, Enhanced Optical Properties of Azaborole Helicenes by Lateral and Helical Extension, Chem.–Eur. J., 2022, 28, e202202280 CrossRef CAS PubMed.
- J. Full, M. J. Wildervanck, C. Dillmann, S. P. Panchal, D. Volland, F. Full and K. Meerholz, A. Nowak-Król, Impact of Truncation on Optoelectronic Properties of Azaborole Helicenes, Chem.–Eur. J., 2023, 29, e202302808 CrossRef CAS PubMed.
- F. Full, A. Artigas, K. Wiegand, D. Volland, K. Szkodzińska, Y. Coquerel and A. Nowak-Król, Controllable 1,4-Palladium Aryl to Aryl Migration in Fused Systems─Application to the Synthesis of Azaborole Multihelicenes, J. Am. Chem. Soc., 2024, 146, 29245–29254 CrossRef CAS PubMed.
- W. D. Petrykowski, N. Vanthuyne, C. Naim, F. Bertocchi, Y. M. Poronik, A. Ciesielski, M. K. Cyrański, F. Terenziani, D. Jacquemin and D. T. Gryko, Double helicene possessing B–N dative bonds built on 1,4-dihydropyrrolo[3,2-b]pyrrole core, Chem. Sci., 2025, 16, 8338–8345 RSC.
- V. F. Pais, M. M. Alcaide, R. López-Rodríguez, D. Collado, F. Nájera, E. Pérez-Inestrosa, E. Álvarez, J. M. Lassaletta, R. Fernández, A. Ros and U. Pischel, Strongly Emissive and Photostable Four-Coordinate Organoboron N,C Chelates and Their Use in Fluorescence Microscopy, Chem.–Eur. J., 2015, 21, 15369–15376 CrossRef CAS PubMed.
- Z. Domínguez, R. López-Rodríguez, E. Álvarez, S. Abbate, G. Longhi, U. Pischel and A. Ros, Azabora[5]helicene Charge-Transfer Dyes Show Efficient and Spectrally Variable Circularly Polarized Luminescence, Chem.–Eur. J., 2018, 24, 12660–12668 CrossRef PubMed.
- D. L. Crossley, I. A. Cade, E. R. Clark, A. Escande, M. J. Humphries, S. M. King, I. Vitorica-Yrezabal, M. J. Ingleson and M. L. Turner, Enhancing electron affinity and tuning band gap in donor–acceptor organic semiconductors by benzothiadiazole directed C–H borylation, Chem. Sci., 2015, 6, 5144–5151 Search PubMed.
- A. Haque, R. A. Al-Balushi, P. R. Raithby and M. S. Khan, Recent Advances in π-Conjugated N^C-Chelate Organoboron Materials, Molecules, 2020, 25, 2645 CrossRef CAS PubMed.
- A. C. Murali, P. Nayak and K. Venkatasubbaiah, Recent advances in the synthesis of luminescent tetra-coordinated boron compounds, Dalton Trans., 2022, 51, 5751–5771 RSC.
- N. Ishida, T. Moriya, T. Goya and M. Murakami, Synthesis of Pyridine−Borane Complexes via Electrophilic Aromatic Borylation, J. Org. Chem., 2010, 75, 8709–8712 CrossRef CAS PubMed.
- E. Vander Donckt, J. Nasielski, J. Greenleaf and J. Birks, Fluorescence of the helicenes, Chem. Phys. Lett., 1968, 2, 409–410 CrossRef CAS.
- K. Dhbaibi, L. Favereau, M. Srebro-Hooper, M. Jean, N. Vanthuyne, F. Zinna, B. Jamoussi, L. Di Bari, J. Autschbach and J. Crassous, Exciton coupling in diketopyrrolopyrrole–helicene derivatives leads to red and near-infrared circularly polarized luminescence, Chem. Sci., 2018, 9, 735–742 RSC.
- R. Bouvier, R. Durand, L. Favereau, M. Srebro-Hooper, V. Dorcet, T. Roisnel, N. Vanthuyne, Y. Vesga, J. Donnelly, F. Hernandez, J. Autschbach, Y. Trolez and J. Crassous, Helicenes Grafted with 1,1,4,4-Tetracyanobutadiene Moieties: π-Helical Push–Pull Systems with Strong Electronic Circular Dichroism and Two-Photon Absorption, Chem.–Eur. J., 2018, 24, 14484–14494 CrossRef CAS PubMed.
- F. Zhang, E. Michail, F. Saal, A. M. Krause and P. Ravat, Stereospecific Synthesis and Photophysical Properties of Propeller-Shaped C90H48 PAH, Chem.–Eur. J., 2019, 25, 16241–16245 CrossRef CAS PubMed.
- J. A. Schellman, Circular dichroism and optical rotation, Chem. Rev., 1975, 75, 323–331 CrossRef CAS.
- H. Kubo, T. Hirose, T. Nakashima, T. Kawai, J.-y. Hasegawa and K. Matsuda, Tuning Transition Electric and Magnetic Dipole Moments: [7]Helicenes Showing Intense Circularly Polarized Luminescence, J. Phys. Chem. Lett., 2021, 12, 686–695 CrossRef CAS PubMed.
- (a) CCDC 2488475: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2pjgd3; (b) CCDC 2488477: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2pjgg5.
| This journal is © The Royal Society of Chemistry 2026 |