Recent advances in organic stimuli-responsive tunable circularly polarized luminescence materials
Mengran
Liu†
a,
Chenfei
Yang†
a,
Shuyu
Li
*a,
Xiaotao
Zhang
 *a and
Wenping
Hu
b
*a and
Wenping
Hu
b
aKey Laboratory of Organic Integrated Circuits Ministry of Education, Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Institute of Molecular Aggregation Science, Tianjin University, Tianjin 300072, China. E-mail: zhangxt@tju.edu.cn
bKey Laboratory of Organic Integrated Circuits Ministry of Education, Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin 300072, China
First published on 11th June 2025
Abstract
Circularly polarized luminescence (CPL) materials have manifested extensive application prospects in domains owing to their distinctive chiral optical properties, such as three-dimensional display, information encryption and optical sensing. However, conventional CPL materials with fixed emission wavelengths and polarization directions struggle to meet the growing demand for multifunctional materials. In recent years, organic stimuli-responsive tunable CPL materials have attracted significant attention due to their reversible and dynamic properties. These materials can realize the dynamic regulation of the emission wavelength, polarization direction or luminescence efficiency under external stimuli (light, temperature, pH, ions, etc). This review systematically summarizes the design principles, regulatory mechanisms, and potential applications of organic stimuli-responsive tunable CPL materials with different types of stimuli.
1. Introduction
Chirality is one of the fundamental attributes in nature, extensively present in molecules, materials and even life systems.1–6 Its essence is that an object or molecule cannot be completely superimposed on its mirror image. As early as the middle of the 19th century, Louis Pasteur first quantitatively confirmed the existence of molecular chirality by chiral resolution of tartaric acid crystals, opening a new era of scientific research on molecular chirality. Up to now, the research surrounding chiral molecules has achieved remarkable progress in numerous aspects, such as synthesis and resolution, structural characterization, and functional exploration.6–12Chiral molecules have numerous distinctive optical properties, such as optical rotatory dispersion (ORD), circular dichroism (CD), and circularly polarized luminescence (CPL). CPL is an optical phenomenon of polarized emission resulting from material chirality, which is manifested as the ability of chiral materials to directly generate circularly polarized light under excitation.13–16 In contrast to the conventional method of generating circularly polarized light that requiring the polarizer, this avoids energy loss and wavelength dependence. In addition, different polarization direction makes it have more application scenarios and stronger application potential compared to traditional fluorescence emission, including 3D display,17,18 anti-counterfeiting encryption,19,20 biomedical imaging,21,22 asymmetric catalysis,23,24 and so on.
The luminescence dissymmetry factor (glum) and the photoluminescence luminescence efficiency (PLQY) are the primary indicators for measuring the performance of CPL materials. The intensity and sign of the circularly polarized light are described by glum, which is defined in glum = 2(IL − IR)/(IL + IR), where IL and IR represent the intensity of left- and right-handed circularly polarized light. Organic materials are emerging as ideal systems for developing high-performance CPL materials due to the remarkable designability of molecular structure, flexible regulation of excited states, and outstanding solution processability.25–34
Recently, researchers have successfully developed multiple types of high-performance organic CPL materials through molecular engineering. Chiral binaphthyl and helicene derivatives are representative CPL materials which have a wide range of applications in optoelectronic devices due to the rigid molecular frameworks and high PLQY.35–40 Nevertheless, the further development is restricted by the moderate glum. Supramolecular self-assembly strategies have emerged as a powerful and reliable solution to address this limitation. This bottom-up fabrication technique leverages non-covalent interactions, including π–π stacking, hydrogen bonding, and halogen bonding, to form aggregates with supramolecular chirality, which exhibits significantly enhanced glum.41–48 Remarkably, through the “sergeants-and-soldiers” principle, this assembly mechanism can effectively transfer molecular chirality from a limited number of chiral “sergeant” molecules to achiral “soldier” luminescent molecules, thereby inducing chirality in the entire system. This approach not only circumvents the synthetic challenges associated with chiral systems but also provides outstanding flexibility in selecting various achiral luminescent molecules for CPL material preparation, substantially expanding the scope of available material systems.49–55
While significant progress has been achieved in the molecular design and synthesis of organic CPL materials, current research remains focused on optimizing luminescence performance of CPL materials.56,57 Although these CPL materials exhibit excellent performance in single-functional scenarios, their fixed emission wavelengths and polarization directions are hard to accommodate the dynamic requirements in complex environments. For example, in contrast to common optical labels, the anti-counterfeiting performance of dynamic tunable optical labels has been significantly enhanced. Stimuli-responsive CPL systems are emerging as a transformative frontier which address the inherent limitations of conventional materials through their dynamic and reversible responsiveness to external stimuli (light, thermal, pH, ionic, etc.).58–60 This type of materials not only retains the intrinsic properties of CPL but also can dynamically regulate properties such as luminescence color, luminescence intensity or polarization direction through external stimuli, achieving the multifunctionality of CPL materials.
In this review, we briefly summarize the design principles, performance modulation mechanisms and potential applications of organic stimuli-responsive CPL materials in recent years. Particular emphasis is placed on the achievement of the control over the color, intensity, and sign of circularly polarized light. Furthermore, this review discusses the current challenges faced by these materials and offers insights into its future development.
2. Light-responsive
Light-responsive CPL materials have emerged as prominent research, owing to their unique non-contact characteristics, remote control capabilities, and precisely tunable properties. Compared to other stimuli, light stimulation not only prevents surface contamination and structural damage to materials, but also exhibits rapid response capabilities and excellent reversibility. More importantly, the diverse kind of molecular photoswitches and their facile chemical modifiability provide vast opportunities for developing novel light-responsive CPL materials. Through rational molecular design, performance parameters such as emission wavelength and glum can be precisely modulated.The primary approach for the preparation of light-responsive CPL materials is to introduce light-responsive groups. By introducing photochromic groups, the molecular response ability to light stimulation can be significantly enhanced. The fundamental mechanism of photochromism lies in the light-driven reversible chemical transformations of the material, with typical processes including reversible ring-opening/ring-closing transitions and cis/trans isomerization. Additionally, the phenomenon of photo-induced molecular dimerization/dissociation or radical generation can also lead to photochromism. Spiropyrans (SP) and their derivatives are notable photochromic compounds that exhibit excellent light-responsive performance, owing to the transformation between the opening/closing ring state under ultraviolet (UV)-visible light irradiation.61–65
Han et al. prepared light-responsive supramolecular co-gels LG/SP-COOH and DG/SP-COOH by non-chiral SP dyes (SP-COOH) and N,N′-bis(octadecyl)-L/D-glutamic diamide (LG/DG) (Fig. 1a and b).66 The LG/SP-COOH in ethanol solvent showed no detectable CPL signal before UV light irradiation (Fig. 1c). Upon irradiation with 365 nm UV light for 2 minutes, the closed ring state of SP-COOH could be converted to the open ring state of merocyanine (MC), with the system turning purple and showing red circularly polarized light emission (Fig. 1d). After 5 minutes of exposure to visible light, the purple gel faded and reverted to the initial white and non-fluorescent state, thereby achieving a reversible switch transformation of CPL. This switching process demonstrated excellent repeatability and fatigue resistance (Fig. 1e). Huang et al. reported a copolymer poly(DPCH-co-SPCH) PDS, which included vibration-induced emission (VIE) derivatives (DPCH) and SP derivatives (SPCH).67 This copolymer was doped with chiral helical polymers substituted polyacetylenes (R/S-PSA) to generate tunable CPL signals (Fig. 1f). DPCH exhibited typical VIE properties, showing different emission behaviors in solid and solution states. Specifically, it displayed orange-red emission in solution while emitting blue light in the solid state. Furthermore, owing to the photochromic property of SP, SPCH was capable of generating a pronounced absorption band at 580 nm upon UV light irradiation, which exhibited a strong overlap with the blue and red emissions of DPCH. Consequently, the transformation of SPCH under UV irradiation could serve as an energy acceptor and establish Förster resonance energy transfer (FRET) between DPCHs, with a variation in fluorescence color. The CPL emission peak at 500 nm gradually disappeared and a new CPL signal was observed at 650 nm in the solid state (Fig. 1g). For the solution state, its red emission gradually attenuated and redshifted (Fig. 1h). A logic gate system was successfully constructed based on the reversible switching of light-responsive CPL signals in different states in hybrid polymer materials. The reversibility of the photochromic processes enabled the designed logic gate to exhibit excellent repeatability through multiple cycles.
 | ||
| Fig. 1 Light-responsive CPL materials based on SP derivatives. (a) Molecular structures of the chiral gelators LG/DG and SP-COOH dye, and the illustration of the formation of co-assembly of SP-COOH with LG/DG and switchable CPL of the co-gel. (b) Reversible transformation between the purple gel with red emission and non-luminescent white gel. (c) CPL spectra of the LG/SP-COOH co-gel before UV irradiation. (d) CPL spectra of LG/SP-COOH and DG/SP-COOH co-gel after UV irradiation. (e) |glum| spectra at 650 nm of LG/SP-COOH upon alternating irradiation of UV and visible light. Reproduced with permission from ref. 66. Copyright 2022, American Chemical Society. (f) The structures of the copolymer PDS and illustration of the FRET between DPCH and SPCH, and induction of CPL doped with chiral helical polymers (R)/(S)-PSA. The CPL spectra changes of the mixtures in the solid state (g) and solution state (h) upon 365 nm light irradiation. Reproduced with permission from ref. 67. Copyright 2021, Wiley-VCH. | ||
Diarylethenes and its derivatives constitute another class of photoactive molecules, which can regulate CPL via reversible Z/E isomerization.68–70 Fu et al. developed a pyridinethiazole acrylonitrile-cholesterol derivative (Z-PTC) which exhibited reversible Z/E isomerization upon exposure to 454 nm or 365 nm light and heating at 343 K.71 This reversible isomerization process could be repeated for at least 10 cycles. Nevertheless, this derivative will be induced a cyclization reaction after prolonged exposure to UV light (Fig. 2a). Moreover, Z-PTC was able to self-assemble into supramolecular gels (SG). Under 454 nm light irradiation and subsequent heating, SG underwent reversible morphological changes from helical nanofibers to fusiform nanostructures, along with CPL on/off switching. Z-PTC forms a supramolecular polymer (SP1) through coordination with Ag+, where the metal coordination significantly enhanced the chiral properties. Under 454 nm light irradiation, SP1 underwent Z/E isomerization, accompanied by a reversal of the CPL signal from positive to negative and a morphological transformation from nanotubes to nanospheres. This transformation could be reversed by heating at 343 K (Fig. 2b). The researchers constructed a multi-mode message encryption system based on these properties, which realized dynamic encoding and decoding through light and heat treatment (Fig. 2c). Xue et al. designed a pair of enantiomeric pyrene-appended cyclohexanediamide derivatives S,S/R,R-CHPA with a V-shaped conformation.72 Due to the presence of the vinylene groups, the fluorescence shifts from blue-white to deep blue under 365 nm irradiation, accompanied by the inversion of the CPL signal (Fig. 2d). Additionally, Hashimoto and co-works investigated a helical tetrathiazole derivative with pyrene fluorophores, whose helical chirality was precisely controlled by chiral phenylalanine spacers.73 In the folded state, the two pyrene units adopted a chiral stacking configuration, forming an excimer that generates the strong CPL signal. Upon UV irradiation, the molecular switch underwent a reversible transformation from the open form to the closed form, accompanied by significant fluorescence quenching of the pyrene excimer and disappearance of the CPL signal. The system could be reset to its initial state with restored CPL emission through visible-light irradiation. This switching behavior demonstrated excellent reversibility over multiple cycles (Fig. 2e).
 | ||
| Fig. 2 Light-responsive CPL materials based on diarylethene derivatives. (a) Reaction pathways for the formation of E-PTC and C-PTC from Z-PTC under 454 and 365 nm light irradiation. (b) Schematic representation of light-responsive CPL activity in supramolecular polymers and metal–organic supramolecular polymers. (c) Principle design of information encryption involving solvent-induced emission, time-dependent PL color change and CPL inversion. Reproduced with permission from ref. 71. Copyright 2024, American Chemical Society. (d) Schematic illustration of the chemical structure, color and CPL spectra changes of S,S-CHPA after 365 nm light irradiation. Reproduced with permission from ref. 72. Copyright 2022, Wiley-VCH. (e) Schematic illustration of the chiral pyrene stack and reversible changes of CPL spectra. Reproduced with permission from ref. 73. Copyright 2016, the Royal Society of Chemistry. | ||
Liquid crystal has emerged as a significant research direction for the light-responsive CPL materials due to its highly ordered molecular arrangement and dynamically adjustable chiral characteristics.74–79 Through the incorporation of appropriate chiral dopants, liquid crystals can be induced to form organized helical superstructures with long-range orientational, which significantly enhances the efficiency of intermolecular chiral transfer compared to conventional systems. These helical supramolecular structures endow liquid crystal CPL materials with remarkable performance characteristics. Notably, by changing the type of chiral dopant, the helical pitch, chirality, or optical bandgap of liquid crystal CPL materials can be controlled by external stimuli to enable dynamic regulation of CPL signals.80–82
Li et al. developed blue-phase liquid crystals (BPLCs) incorporating halogen-bonded light-driven fluorescent molecular switches (HB-switch 1 and HB-switch 2).83 Notably, the BPLC incorporating HB-switch 1 achieved reversible inversion of CPL signals due to the Z/E photoisomerization of HB-switch 1 under irradiation with UV and visible light (Fig. 3a). Yuan et al. synthesized non-chiral liquid crystal block copolymer (LC-BCP) films with cyanostilbene side groups and induced the helical assembly through chiral molecules (R/S-DINBPA) as chiral sources.84 Moreover, due to the dynamic covalent bonds of cyanodibenzyl groups, the system exhibited the reversible CPL color change from cyan to blue under the alternating irradiation of UV light 365 nm and 254 nm (Fig. 3b).
 | ||
| Fig. 3 Light-responsive CPL materials based on liquid crystal system. (a) Schematic diagram of assembly structure of the halogen bonded switches (including HB switches 1 and 2), and the inversion of the light/thermally responsive CPL signal due to phase transition in a system of BPLCs doped with HB switch 1. Reproduced with permission from ref. 83. Copyright 2024, Wiley-VCH. (b) Illustration of axial chiral molecules inducing helical assembly of liquid crystal block copolymers and the regulation of CPL under UV irradiation. Reproduced with permission from ref. 84. Copyright 2025, Wiley-VCH. (c) Schematic illustration of CPL signal inversion enabled by dichroism modulation in CLC. (d) CPL spectra of CLC-KG at the initial state and after 365 nm light irradiation. (e) CPL spectra of CLC-KGNR-0.5 at the initial state and after 365 nm light irradiation. Reproduced with permission from ref. 85. Copyright 2024, Wiley-VCH. | ||
The traditional regulation of CPL signals typically required the modification of the chiral superstructure or helical arrangement of liquid crystals. However, Li et al. managed to achieve the chiral inversion of CPL signals without altering the superstructure of cholesteric liquid crystals (CLCs).85 CLC system was introduced by chiral dopants and light responsive achiral negative dichroic dyes (KG), which synthesized by the perylene diimide derivative and α-cyanostyrene. The isomerization of the KG side chain led to the disorder of the helical arrangement of the CLC under the UV light irradiation, thereby significantly reducing the intensity of the CPL signal (Fig. 3d). Furthermore, the system of light-driven CPL sign inversion was achieved by doping KG and positive dichroic dyes (NR) as dopants at the 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 weight ratio (Fig. 3c). After the isomerization of the KG dye under UV light irradiation, its dichroism decreased and the positive dichroism of NR was not influenced by UV light. Prior to UV exposure, the CPL signal direction of the CLC system was dominated by negative dichroism of KG. Following irradiation, the diminished dichroism of KG allowed positive dichroism of NR to become the predominant contributor to the optical activity of system. This enabled the inversion of the CPL signal while maintaining the helical structure of the liquid crystal unaltered (Fig. 3e). By taking advantage of the light-driven CPL sign inversion characteristic of this material, a multidimensional information encryption model with a higher security level was designed.
0.5 weight ratio (Fig. 3c). After the isomerization of the KG dye under UV light irradiation, its dichroism decreased and the positive dichroism of NR was not influenced by UV light. Prior to UV exposure, the CPL signal direction of the CLC system was dominated by negative dichroism of KG. Following irradiation, the diminished dichroism of KG allowed positive dichroism of NR to become the predominant contributor to the optical activity of system. This enabled the inversion of the CPL signal while maintaining the helical structure of the liquid crystal unaltered (Fig. 3e). By taking advantage of the light-driven CPL sign inversion characteristic of this material, a multidimensional information encryption model with a higher security level was designed.
In addition to SP and diarylethene systems, the distinctive light-responsiveness of azobenzene structures has also been employed in the inversion of CPL signal.86,87 Kang et al. designed and fabricated a kind of CPL molecular switch, (S,R,S)-switch 1 and (R,R,R)-switch 2, based on azoarene (Fig. 4a).88 These two molecules demonstrated reversible trans/cis isomerization when irradiated with 365 nm UV light and 520 nm green light in solvent and liquid crystal media. Compared with switch 2, liquid crystal which was doped with switch 1 exhibited the inversion and off of CPL signal under the irradiation with 365 nm UV and 520 nm green light (Fig. 4b). Meanwhile, the fluorescence intensity of the system remained essentially unchanged during this process. The researches constructed a CPL optical Morse code system for multilevel message encryption by using this light-responsive liquid crystal material. In contrast to the conventional approach of introducing photochromic molecules, the inversion of CPL signals can also be accomplished by inducing the generation of free radicals through light illumination. Ji et al. developed a CPL gel, which was self-assembled from the C3-symmetric triarylamine derivative TPA-Ala in chloroform.89 Under UV light irradiation, an emission color change from blue to cyan was observed, which was attributed to the photo-induced radicals of TPA-Ala. Continuous UV irradiation over 120 min led to the formation of a photo-stationary state with an inverted CPL signal compared to the original TPA-Ala gel. It could keep stable luminescence intensity after 10 days of aging in dark or 3 days under visible light. Additionally, transient white luminescence and CPL were achieved by doping the guest dye rhodamine B (Fig. 4c).
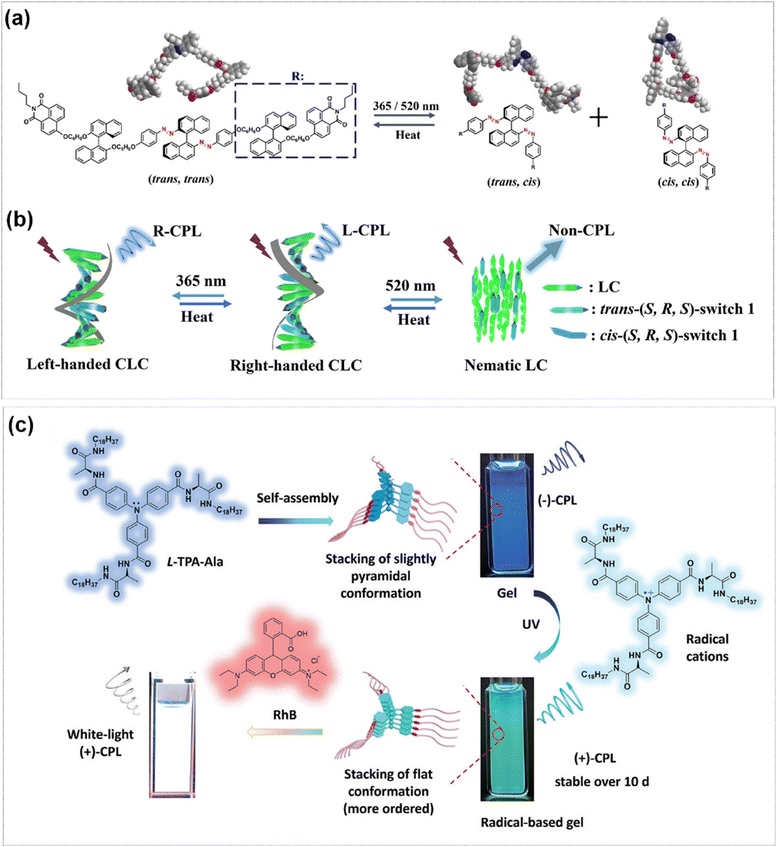 | ||
| Fig. 4 (a) Trans/cis reversible isomerization process of (S,R,S)-switch 1. (b) Changes in the structure of the CLC system under the light-/thermal stimuli. Reproduced with permission from ref. 88. Copyright 2023, Wiley-VCH. (c) Schematic representation of photo-induced radicals with emission color change and CPL inversion. Reproduced with permission from ref. 89. Copyright 2025, Wiley-VCH. | ||
3. Temperature-responsive
Temperature-responsive CPL materials are a type of functional materials that can precisely regulate the CPL signal through temperature variations. These materials not only demonstrate high reversibility and precise controllability but also possess excellent environmental adaptability. Through gradient temperature changes, the CPL emission of such materials can be finely regulated, while conversely, real-time temperature monitoring can be achieved by detecting variations in their CPL signals.The CPL signal can attain dynamic controllability in terms of direction, intensity, or wavelength under temperature variations through reversible transformation of helical structures, regulation of intermolecular hydrogen bonds, and adjustment of dynamic conjugated systems. Wang et al. synthesized two novel poly(3,5-diamide substituted phenylacetylene)s bearing L- or D-alanine residues with a long alkyl chain (sP-2C11 and rP-2C11) which could regulate CPL via the reversible transformation between cis–cisoid (cc) helix and cis–transoid (ct) helix structures (Fig. 5a).90 In toluene solutions, the CPL intensity of sP-2C11 still existed even at 80 °C, suggesting the thermal stability of cc helix in toluene (Fig. 5b). However, in the CHCl3 solution, the CPL signal vanished when the temperature decreased to 0 °C, along with the transformation of the cc helical structure to the ct helical structure. Upon rising temperature to 30 °C, the initial signal of CPL was recovered, demonstrating outstanding reversibility without the occurrence of obvious decline after multiple cycles (Fig. 5c and d). This temperature responsive helical transition could be attributed to the competitive relationship between intramolecular hydrogen bonding and solvent–polymer chain interactions. The cc helical structure remained stable at elevated temperatures due to the prevailing strength of intramolecular hydrogen bonding over CHCl3–polymer chain interactions. Conversely, at lower temperatures, the predominance of CHCl3–polymer chain interactions induced the transition of cc to ct structure. Murata et al. prepared chiral bisimidazolyl 1,1′-bi-2-naphthol dimethyl, which showed dual stimuli-responsive turn-on luminescence through two distinct mechanisms (Fig. 5e).91 Under mechanical stimulation, the weakly emissive crystals developed blue-violet emission with significantly enhanced intensity due to imidazolyl hydrogen bond cleavage, though without CPL emission (Fig. 5f). Upon thermal stimulation, the crystals underwent thermal demethylation to generate compounds possessing excited-state intramolecular proton transfer (ESIPT) characteristics, manifesting orange emission. Remarkably, the crystals demonstrated CPL signals arising from configurational changes following thermal treatment (Fig. 5g).
 | ||
| Fig. 5 (a) Schematic diagram of conformational transitions in stimuli response between a contracted cc helix and a stretched ct helix, corresponding energy levels of ground and lower excited states, and fluorescence photographs. The green and black dotted arrows represent intramolecular hydrogen bonds and intermolecular interactions between polymer chains and solvent molecules, respectively. And the structures of polymer sP-2C11 and rP-2C11. CPL intensity of sP-2C11 in toluene (b) and CHCl3 (c) at distinct temperature. (d) CPL intensity of sP-2C11 reversibly switches with alternative temperature. Reproduced with permission from ref. 90. Copyright 2023, Wiley-VCH. (e) Photographs and schematic diagram of mechano-responsive and thermo-responsive luminescence of (S)-1. Normalized CPL and fluorescence spectra of ground (f) and heated (g) (S)-1 (blue) and (R)-1 (red). Reproduced with permission from ref. 91. Copyright 2024, Wiley-VCH. | ||
Organic crystals exhibited a natural 2D chiroptical response due to the coupling between the linear birefringence (LB) induced by anisotropy and the fluorescence linear polarization induced by the electric transition dipole moment (μ). Ji et al. fabricated a supramolecular microcrystal (DCMC), which asymmetric intramolecular charge transfer (ICT) dye molecules was utilized as the luminescent unit (DSP) and β-cyclodextrin (β-CD) was adopted as the host (Fig. 6a).92 The microcrystal demonstrated significant 2D chiral activity, generating CP laser with a high glum of 1.78. The DCMC could enable the generation of tunable CPL signal based on the thermo-responsivity of its remarkable LB index (Δn). With the increase of temperature, the electron cloud could be redistributed on the ICT dye molecules within the β-CD cavity, resulting in a more polar and anisotropic molecular configuration (Fig. 6b). This further induced a remarkable change in the refractive LB index, possibly leading to phase modulation and generating CP lasers with different polarization states (Fig. 6c). The glum of this system varied sinusoidally with temperature, being capable of dynamically switching between positive and negative values and remaining stable and reversible during multiple temperature cycles (Fig. 6d and e).
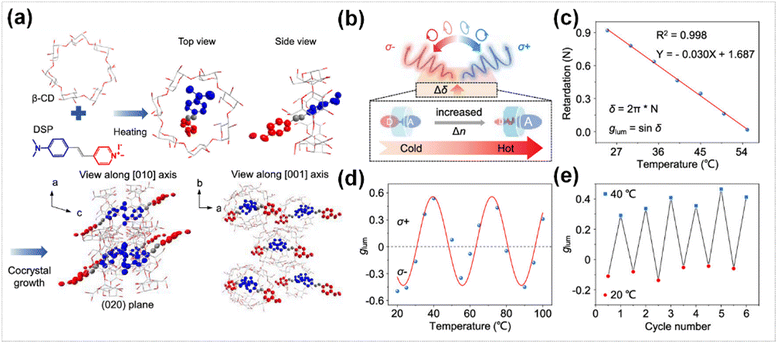 | ||
| Fig. 6 (a) Schematic for the inclusion of DSP in β-CD and the resulted single-crystal structures. (b) Schematic diagram of laser polarization state changes at different temperatures. (c) Experimental data and fitting results of temperature variation of delay (N) at 579 nm wavelength using the Soleil–Babinet compensator. (d) glum of DCMC after lasing with increased temperatures. (e) Reversible glum switch with cyclic temperature changes. Reproduced with permission from ref. 92. Copyright 2024, American Chemical Society. | ||
Supramolecular systems display distinct advantages in temperature-responsive CPL materials because of their dynamically adjustable intermolecular interactions. Liu et al. discovered that naphthalene-histidine (NIHis) could self-assembled into distinct chiral nanostructures (nanofibers and microbelts) via different self-assembly pathways, which exhibit opposite CPL signals (Fig. 7a).93 Notably, the microbelts exhibited controllable CPL emission characteristics under thermal stimulation. Upon heating the microbelts to 383 K, the yellow emission of the microbelts transformed into yellow-green while the CPL signals vanished (Fig. 7b and d). Both the emission color and the CPL signal could be recovered when the temperature was cooled to 298 K. This emission color and switching could be repeated for at least four cycles, which demonstrated excellent fatigue resistance under alternating heating–cooling treatment environmental conditions (Fig. 7c and e). Zhang et al. synthesized a chiral amphiphilic molecule CPSB-GLU-PEG350 (CGP), which is composed of the aggregation-induced emission (AIE) chromophore (CPSB), chiral glutamic acid group and polyethylene glycol (PEG) thermosensitive segment.94 It could self-assembled into two-dimensional supramolecular nanosheets and achieved the temperature-responsive CPL signal switch. This system showed a strong CPL signal at 3 °C. As the temperature increased to 50 °C, the PEG chains collapsed, resulting in the decrease of the CPL signal. The order–disorder transition could be reversibly cycled multiple times within a specified temperature range, thereby realizing the reversible switching of the CPL signal (Fig. 7f).
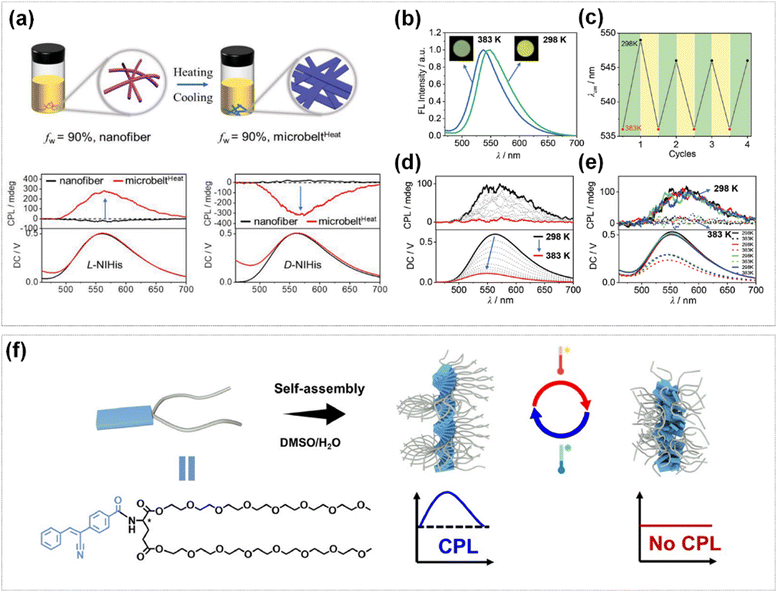 | ||
| Fig. 7 (a) Nanofiber to nanobelt transition by heating and cooling, and CPL spectra of nanofibers (black lines) and microbelts (red lines) of L-NIHis and D-NIHis. (b) The fluorescence spectra of the L-NIHis microbelts at 383 K (blue line) and 298 K (green line). (c) The change of the maximum emission wavelengths of L-NIHis microbelts upon alternating heating (383 K, red dots) and cooling (298 K, dark dots). (d) The CPL spectra of L-NIHis microbelts upon heating from 298 to 383 K. (e) The CPL spectra of L-NIHis microbelts with alternative temperature. Reproduced with permission from ref. 93. Copyright 2024, Wiley-VCH. (f) Schematic illustration for thermo-responsive self-assembly of L/D-CGP for switchable CPL signal. Reproduced with permission from ref. 94. Copyright 2024, Science China Press. | ||
The co-assembly of natural chiral substances and biocompatible materials is a simple approach for the preparation of CPL-active biomaterials. Ding et al. prepared CPL materials through co-assembling carbazole-based biscyanine fluorophores with DNA.95 The achiral cyanine molecules combined with the DNA grooves via electrostatic interaction and the chirality of the DNA molecules could be transferred to the cyanine dyes, which inducing significant CPL (Fig. 8a and b). Switchable structural changes of DNA–cyanine nanocomposites were achieved by alternately changing the temperature between 80 and 25 °C. Due to the thermal control hybridization and dissociation of DNA–cyanine nanocomposites, periodic changes in the intensity of CPL were observed (Fig. 8c).
 | ||
| Fig. 8 (a) Schematic illustration of DNA–biscyanine hybrid CPL active materials. (b) The CPL spectra of D- and L-DNA duplex-biscyanine. (c) Tunable CPL under cyclical thermal treatment. Reproduced with permission from ref. 95. Copyright 2019, American Chemical Society. | ||
The self-assembly of chiral rotaxanes constitutes a novel strategy for the construction of temperature-responsive CPL materials. Liu et al. covalently potted the emitter molecular into a flexible chiral macrocycle as an adaptive chiral [1]rotaxane which could self-assemble two distinct solid-state nanoarchitectures ([DS]A and [DS]B) (Fig. 9a).96 [DS]A demonstrated the thermo-amplified CPL activity which the glum increases from 0.023 to 0.040 (Fig. 9b and d). However, the glum of [DS]B exhibited gradual decline during thermal cycling (Fig. 9c and e). The thermal response property of [DS]A was ascribed to hydrogen bond reconfiguration and ring structure deformation. This mechanism effectively restricted molecular motion and enhanced chiral transfer and the performance of the CPL.
 | ||
| Fig. 9 (a) Schematic representation of structural changes in [DS]A and [DS]B under thermal stimulation. The glum spectra and cycle thermal stimuli tests of [DS]A (b) and (d) and [DS]B (c) and (e). Reproduced with permission from ref. 96. Copyright 2022, Wiley-VCH. | ||
The liquid crystal materials exhibit outstanding thermal stimuli-response capabilities because of their adjustable pitch and optical bandgap under the stimulation of temperature. Zhang et al. employed three non-chiral liquid crystal polymers (LC-P1, LC-P2, and LC-P3) and chiral binaphthyl inducers (R/S-M) for co-assembly.97 The chirality of binaphthyl inducers was transferred to the liquid crystal via π–π stacking, and helical nanofibers were prepared through the supramolecular co-assembly (Fig. 10a). In contrast to the rigid backbone structures of LC-P1 and LC-P2, LC-P3 possessed a more flexible polymer main chain which was more prone to co-assemble with the chiral inducer R/S-M. The CPL emission direction of (R/S-M)0.1-(P3)0.9 which had the strongest emission intensity showed a negative signal at an annealing temperature less than or equal to 95 °C, but a positive signal was observed at an annealing temperature of 100 °C (Fig. 10b and c). The reversed CPL signal was ascribed to the inversion of the (R/S-M)0.1-(P2)0.9 helical nanofibers, since LC-P3 was a more flexible polymer chain and was prone to undergo polymer chain inversion under thermal annealing treatment. And below an annealing temperature of 100 °C, these helical nanocrystals could remain rather stable. The thermal control switching of CPL signals also could be achieved by the selective reflection characteristic of nematic liquid crystal (N*-LC). Akagi et al. fabricated a device capable of optical resolving and thermal chirality switching of CPL by integrating a luminescent conjugated polymer film with a bilayer chiral N*-LC cell.98 This device was composed of two layers of N*-LC units with opposite chirality and each layer was doped with chiral dopants of different molar concentrations. Specifically, the left-handed N*-LC and right-handed N*-LC contained chiral dopants of opposite chirality. At 60 °C, the selective emission band of the left-handed N*-LC overlapped with the photoluminescence band of the polymer, reflecting left-handed CPL and transmitting right-handed CPL. The selective reflection band redshifted when the temperature rose to 127 °C, resulting in the liquid crystal reflecting right-handed CPL and transmitting left-handed CPL, which inverted the CPL signal (Fig. 10d). This system retained excellent reversibility after multiple thermal cycles and had a high glum.
 | ||
| Fig. 10 (a) Diagram of the possible co-assembly route for (R/S-M)0.1-(P3)0.9. (b) The CPL spectra of (S-M)0.1-(P3)0.9 and (c) glum values of (R/S-M)0.1-(P3)0.9 after thermal annealing at distinct temperatures. Reproduced with permission from ref. 97. Copyright 2022, American Chemical Society. (d) Schematic representation of double-layered cell for tunable CPL. Reproduced with permission from ref. 98. Copyright 2017, Wiley-VCH. | ||
4. Acid–base-responsive
Acid–base responsive CPL materials are a kind of intelligent optical materials that can regulate CPL under pH variations. The response mechanism for achieving the change of the CPL signal is mainly based on the molecular configuration changes and supramolecular assembly or disassembly. Through incorporating pH-sensitive functional groups (amino or carboxyl groups), these materials can achieve highly selective and reversible CPL signal regulation over a specific pH range. Notably, acid–base stimulation operates without requiring external energy input, as the modulation is solely driven by alterations in the local pH environment.As classically axial materials, 1,1′-binaphthalene-2,2′-diol (BINOL) is widely used in many chiroptical applications.99–103 Zhou et al. fabricated a series of dyes with the property of CPL by introducing the BINOL group into the thiazolothiazole (TTz) core structure (Fig. 11a).104 The color and emission spectra of (S)-OEt-OH exhibited remarkable alterations under the exposure in the HCl vapor (Fig. 11b and c). The fluorescence transformed from blue-green to orange-red and the CPL signal was inverted. The color and emission characteristics of this dye were capable of being restored to the initial state by exposing in NH3 vapor (Fig. 11d). This acid–base responsive CPL switching performance was attributed by the electron transfer of the dye molecule upon protonation.
 | ||
| Fig. 11 (a) Chemical structures of DTTz and BINOL-based TTz dyes. (b) Normalized emission spectra of (S)-OEt-OH in contact with HCl and NH3 vapor. (c) The photograph of (S)-OEt-OH in contact with HCl and NH3 vapor under visible and UV light. (d) CPL spectra of (S)-OEt-OH in contact with HCl and NH3 vapor. Reproduced with permission from ref. 104. Copyright 2022, Elsevier. (e) Chemical structure of S-1 and R-1, and changes in structure and CPL with different stimuli response. Reproduced with permission from ref. 110. Copyright 2024, the Royal Society of Chemistry. (f) Chemical structure of S-Rhodol-TPE and mechanism of CPL switching under acid–base stimulation. Reproduced with permission from ref. 111. Copyright 2025, Wiley-VCH. | ||
Flexible organic crystals are progressively emerging as a frontier research direction in the domain of optoelectronic materials due to their ability to transmit light and respond to various stimuli. An important class of organic materials, including Schiff bases,105–107 diarylethenes,108 and azobenzenes,109 can exhibit photochromic or acidochromic properties in the crystalline state, with the Schiff bases formed from salicylaldehydes and amines being particularly representative. Pan et al. designed and synthesized a pair of single-component stimuli-responsive flexible organic crystals composed of chiral Schiff base molecules, S-,R-4-methyl-2-(((1phenylethyl)imino)methyl)phenol (S-,R-1), which demonstrated significant acid and light responsive CPL (Fig. 11e).110 Under the action of acid vapor, the color of the CPL was switched from yellow to green because of the protonation of the imine groups induced by acid fumigation which inhibited ESIPT. Through heating or exposure to air, both the fluorescence color and CPL signal could be restored to their initial states. This multi-stimuli-responsive CPL flexible organic crystal exhibited potential application value in optical sensing and tunable optical storage.
Sun et al. designed and synthesized a novel acid-responsive CPL switch molecule S-Rhodol-TPE through the covalent connection of tetraphenylethylene (TPE), acid-responsive luminophore (Rhodol) and chiral aniline.111 This molecule successfully integrated the AIE property of TPE, the acid-sensitive fluorescence switch property of Rhodol and the central chirality of chiral aniline. By taking advantage of these properties, when S-Rhodol-TPE was doped in polymethyl methacrylate (PMMA) matrix and acidified, high PLQY and reversible CPL switch ability could be achieved. In addition, based on the FRET effect between TPE and Rhodol, the emission color of S-Rhodol-TPE doped films could be effectively regulated by adjusting the pH value or doping ratio (Fig. 11f).
Stimuli-responsive liquids (SRLs) with luminescent π-conjugated skeletons are important research targets for the development of novel functional soft materials. SRLs can be easily applied to various substrates in a solvent-free manner for use in security labels, sensors, and memory devices. Ikenaga et al. synthesized π-conjugated N-heteroacene framework liquid materials (1S/1R) containing chiral alkyl chains, which were in a liquid state at room temperature.112 Remarkably, under the stimuli of HCl vapor, they could be induced to transform from disordered liquids to ordered chiral self-assembled solids, showing the properties of CPL (Fig. 12a). The chirality feature and the aggregated structure with a preferential orientation of 1S/1R were not observed before exposing in HCl vapor. The protonated π-conjugated frameworks self-assembled and stacked in both parallel and antiparallel manners through dipole–dipole interactions, resulting in the observed CD and CPL signals during the self-assembly process. In addition, the material could revert to its initial liquid state when heating or exposing in air, with the CPL signal being deactivated (Fig. 12b). This exhibited excellent stimuli response and multiple-cycle stability in liquid materials.
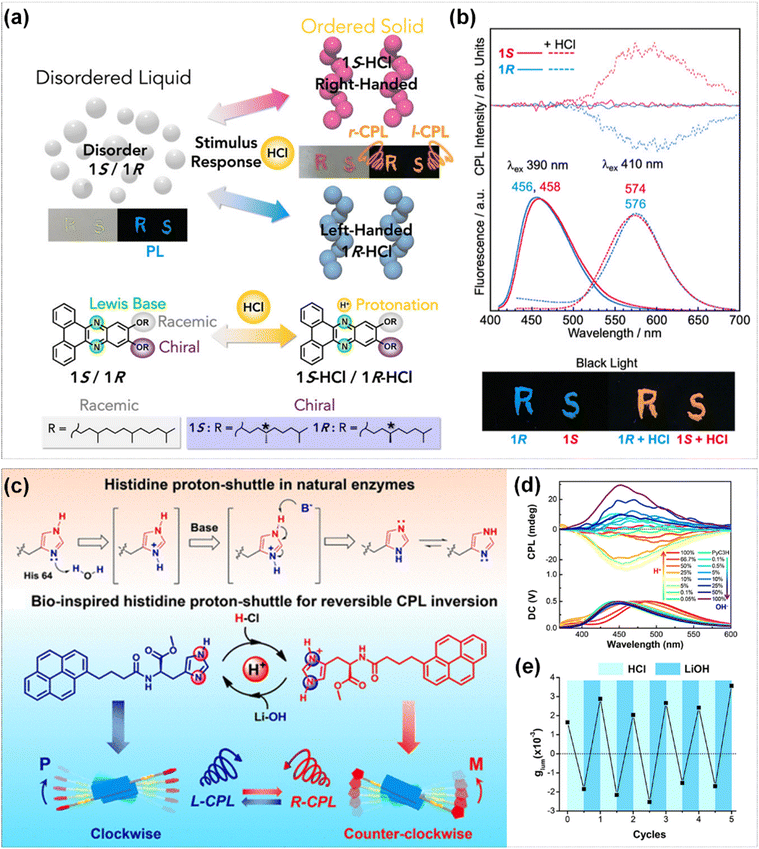 | ||
| Fig. 12 (a) Schematic representation of acid-stimulated transformation of liquid to CPL solid and changes in structure. (b) CPL, fluorescence spectra and fluorescence photographs of 1S and 1R before and after acid stimulation. Reproduced with permission from ref. 112. Copyright 2021, American Chemical Society. (c) Illustration of histidine proton-shuttle in nature enzymes, and structure of the chiral histidine π-gelator PyC3H and its reversible protonation–deprotonation process. (d) CPL spectra of PyC3H gels after adding different proportions of H+ and OH−. (e) Cycles of CPL inversion by alternate addition of HCl and LiOH. Reproduced with permission from ref. 113. Copyright 2020, American Chemical Society. | ||
Histidine is an important basic amino acid. The imidazole group of it contains two nitrogen atoms, which can function as both an acid and a base. This distinctive property contributes to the role of histidine as a proton shuttle in numerous natural enzymes. Based on the proton shuttling mechanism of histidine, Niu et al. designed a pyridine-based modified L-histidine gelator (PyC3H) and discovered that it demonstrated reversible CPL signal inversion by alternative protonation–deprotonation (Fig. 12c).113 Upon the gradual addition of proton acid to the PyC3H gel system, the sign of CPL could be entirely inversed from positive signal to negative signal, along with the supramolecular chirality inversion. The chiral structure and CPL signal could be restored to the initial state when base was introduced into the system (Fig. 12d). This phenomenon was primarily attributed to the protonation of the imidazole group of histidine, which modified the intermolecular hydrogen bond network and π–π stacking mode, resulting in the reversal of the stacking orientation of the pyrene chromophores and consequently inducing the inversion of the CPL signal. Through successive protonation–deprotonation processes, the CPL signal could be switched for at least five cycles which exhibits outstanding fatigue resistance (Fig. 12e).
Moreover, chiral co-assembly strategies are also employed for the construction of acid–base responsive CPL materials. Chen et al. prepared a pair of pH-responsive chiral co-assemblies, P7R3 and P7S3, by co-assembling the polymer PFIQ containing isoquinoline units with the chiral inducer (R/S-5011) (Fig. 13a).114 Under the fumigation of trifluoroacetic acid and annealing treatment, the system accomplished the reversible switching of CPL color from blue to green. Furthermore, the chiral co-assemblies could effectively induce various achiral luminescent molecules to generate intense full-color CPL emissions via CPL energy transfer (CPL-ET), with |glum| all exceeding 0.2 (Fig. 13b). Lin et al. synthesized three pyrene-conjugated histidine derivatives with varied acyl linkers (PyHis, PyC1His, and PyC3His) and the co-assemble systems of different linker length derivatives with 1,2,4,5-tetracyanobenzene (TCNB) exhibited distinct CPL emissions.115 PyC1His/TCNB merely generated a weak CPL signal, whereas PyHis/TCNB and PyC3His/TCNB manifested strong CPL emissions (Fig. 13c). The systems showed tunable CPL emission due to the protonation of the histidine moiety. The PyHis/TCNB system disassembled upon the protonation, leading to the quenching of the CPL signal (Fig. 13d). By contrast, owing to the longer alkyl chain which strengthened the self-assembly capacity, PyC3His/TCNB exhibited a reversed CPL signal through adaptive reassembly (Fig. 13e).
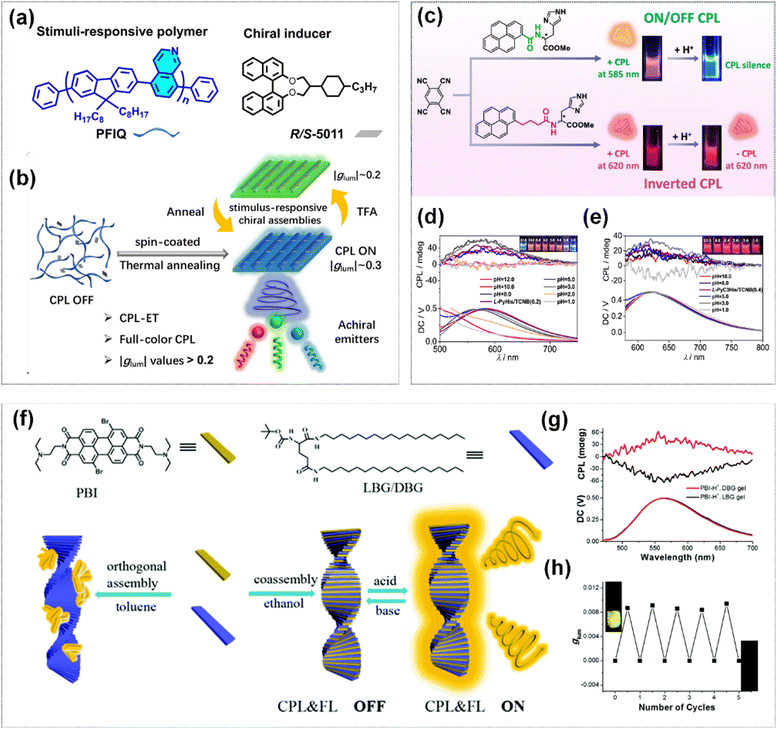 | ||
| Fig. 13 (a) The molecular structures of PFIQ and R/S-5011. (b) Preparation method and CPL signal modulation approaches of chiral stimuli-responsive assemblies, and schematic diagram of full-color CPL-ET. Reproduced with permission from ref. 114. Copyright 2025, Wiley-VCH. (c) Schematic of PyHis/PyC3His and TCNB co-assembly and their tunable CPL emission in response to acid stimulation. CPL spectra of PyHis/TCNB (d) and PyC3His/TCNB (e) under different pH. Reproduced with permission from ref. 115. Copyright 2023, American Chemical Society. (f) Molecular structures of the chiral gelators LBG/DBG and PBI, and illustration of the formation and acid–base stimuli response of co-assembly PBI with LBG/DBG. (g) CPL spectra of protonated co-gel. (h) The changes in glum intensity under the stimulation of the acid–base cycle. Reproduced with permission from ref. 116. Copyright 2018, the Royal Society of Chemistry. | ||
Duan et al. found that the chiral gelators (LBG/DBG) and the achiral perylene bisimide (PBI) could form two distinct assembly patterns in different solvent environments (Fig. 13f).116 The orthogonal assembly occurred in toluene, where PBI only existed in a disordered aggregated state without chiral transfer. However, the chirality transferred from LBG/DBG to PBI occurred in ethanol accompanying with the observation of intense CD signals in the co-assembled gel. In the co-gel state, the fluorescence of PBI was quenched due to the photoinduced electron transfer (PET) effect. Nevertheless, the nitrogen atoms of PBI were protonated in an acidic environment, which inhibited the PET process and significantly enhanced the fluorescence and concurrently generated the CPL signal (Fig. 13g). In an ammonia environment, the signal of CPL could be wiped out. Thus, an acid–base responsive fluorescence and CPL switch could be realized (Fig. 13h).
Liquid crystal materials have emerged as an important platform for the construction of acid–base responsive CPL materials by virtue of their tunable optical band gap. Miao et al. fabricated a biomaterial with dynamic regulation of the properties of CPL by doping the fluorescent dye fluorescein isothiocyanate (FITC) into cellulose nanocrystals (CNC).117 In this system, the enhancement of the CPL signal and the chirality inversion were successfully achieved by adjusting the relative position of the photonic bandgap (PBG) of the CNC film and the fluorescence emission wavelength (Fig. 14a). Upon fumigation treatment with an aqueous solution of HCl, the CPL signal exhibited an inversion from positive to negative (Fig. 14b). Whereas, the CPL intensity of the film was conspicuously enhanced after fumigation treatment under alkaline conditions (Fig. 14c). A chiral liquid crystal system with high optical activity was constructed by using a helical co-assembly strategy, which doping an aldehyde-functionalized fluorescent dye (Rhodol-CHO) and a chiral dopant (S/R811) into a N*-LC.118 This system was highly sensitive to acids, bases and compounds containing primary amino groups (–NH2). It was found that CPL emission can be switched from green-yellow to orange by adjusting the matching relationship between the PBG and the dye emission wavelength (Fig. 14d). Moreover, a prototype application mode of information encryption was explored using this liquid crystal system.
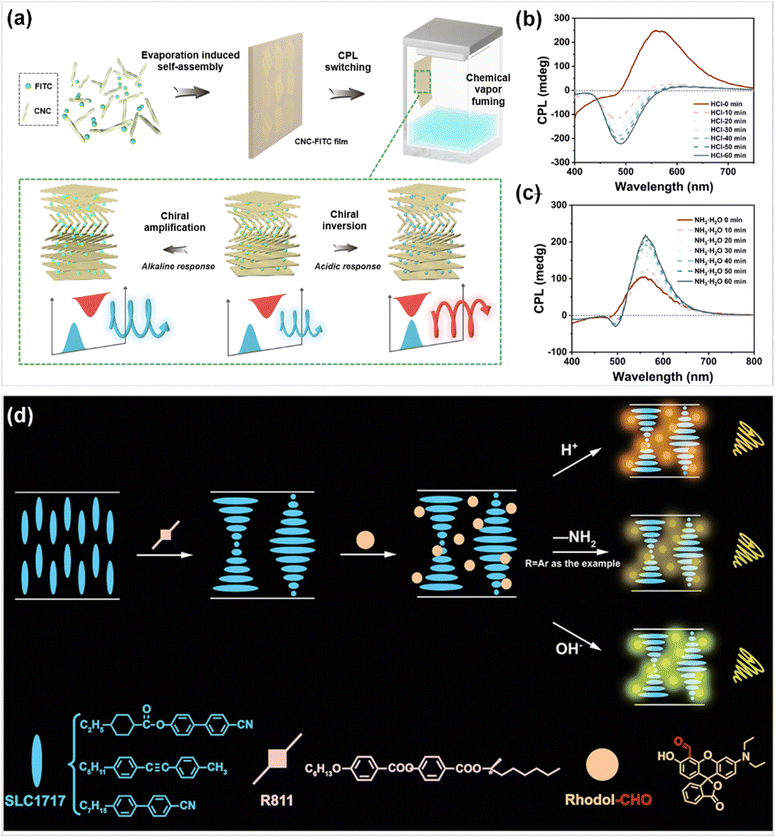 | ||
| Fig. 14 (a) Schematic of acid–base stimulated CPL modulation based on CNC-FITC film. CPL spectra of CNC-FITC film under HCl (b) and NH3·H2O (c) at different time. Reproduced with permission from ref. 117. Copyright 2024, the Royal Society of Chemistry. (d) Schematic diagram of the multiple CPL-regulation chiral doping liquid crystal driven by acid, base, and amines. Reproduced with permission from ref. 118. Copyright 2024, Wiley-VCH. | ||
5. Ion-responsive
Ion-responsive CPL materials demonstrate tunable CPL properties owing to their distinctive ion–molecule interaction mechanisms. The regulation mechanisms can be categorized into two pathways: (i) coordination-induced alterations in intermolecular interactions and packing modes; (ii) ion-triggered chemical reactions that modify molecular structures. This unique response mechanism confers upon these materials both exceptional ion selectivity and remarkable sensitivity, thereby facilitating highly accurate detection of specific target ions.Li et al. successfully accomplished the reversible regulation of the CPL signal of CLCs by regulating the molecular conjugation of chiral binaphthyl dopants in CLCs (Fig. 15a).119 They initially developed two binaphthyl chiral dopants (R/S-1 and R/S-2). The CPL signals were opposite after doping these two dopants, suggesting that the CPL inversion of CLCs could be achieved by modulating the conjugation degree of chiral binaphthyl (Fig. 15b and c). Based on this characteristic, a thioacetal binaphthyl R-2S was developed and served as a mercury-responsive chiral dopant in CLCs. The thioacetal in R-2S was transformed into an aldehyde group after doping mercury ions, which enhanced the molecular conjugation and reversed the CPL signal of CLCs (glum changed from 0.22 to −0.29) (Fig. 15d). This system demonstrated outstanding application potential in the field of information encryption (Fig. 15e). By exploiting the differences in chirality and response to mercury ions of different dopants, three CLC inks were injected into grids following a ‘CORE’ pattern. Only the true message could be detected through a right-handed CPL detector after mercury ions treatment. Fu et al. prepared various metal–organic supramolecular polymers (MOSPs) with multi-color CPL and handedness inversion. These polymers were constructed from the coordination-driven assembly of pyridine–cyanostilbene–cholesterol (PCSPCC) and metal ions (Fig. 15f).120 When PCSPCC molecules self-assemble into a gel in n-BuOH, a negative CPL signal was observed at 524 nm. Upon the addition of Ag+, an opposite CPL signal was observed at the same wavelength. Further, the aggregates exhibited a negative CPL signal and orange-yellow emission after adding Bi3+ and subjecting the mixture to a heating–cooling cycle while a positive orange-yellow CPL emission was observed in the Zn2+ aggregates. Additionally, after ultrasonic processing of the corresponding complexes in n-BuOH, a negative CPL signal with red emission was acquired in the Al3+ aggregates, while a positive red CPL emission was observed in the thermally treated samples (Fig. 15g and h). These findings suggested that the CPL color and sign of MOSPs could be further modulated by regulating the types of metal ions and the treatment approaches, achieving metal-ion-driven CPL optical regulation.
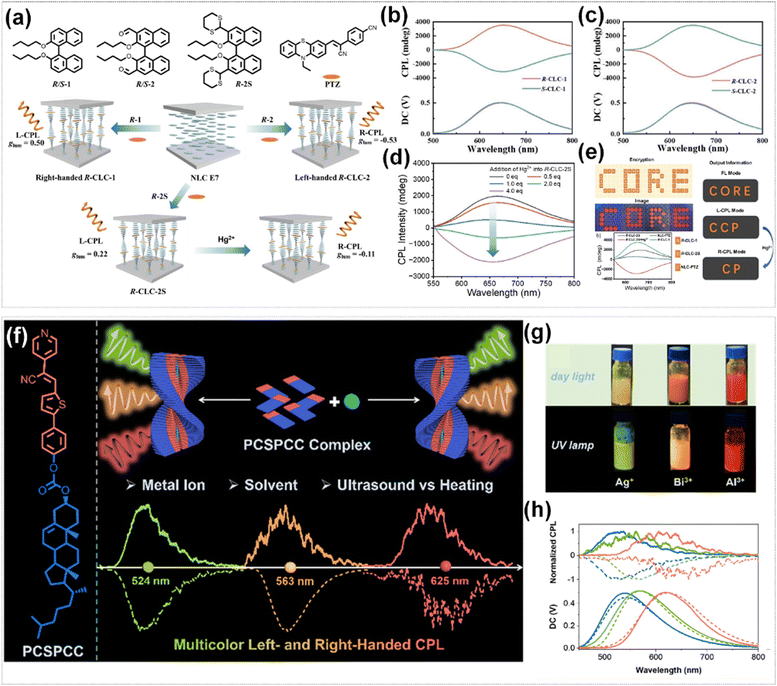 | ||
| Fig. 15 (a) The molecular structures of binaphthyl derivatives and achiral dye phenothiazine derivative (PTZ), and illustration of CPL signals of CLCs with different chiral dopant. CPL spectra of (b) R/S-CLC-1 and (c) R/S-CLC-2. (d) CPL spectra of R-CLC-2S after adding different equivalent Hg ion. (e) The schematic diagram of information coding using the N*LC-PTZ, R-CLC-1 and R-CLC-2S system. Reproduced with permission from ref. 119. Copyright 2025, Wiley-VCH. (f) Schematic illustrations of CPL modulation of MOSPs regulated by the metal ions, solvents, and treatment modes. (g) The photographs of MOSPs with distinct ions under the irradiation of daylight and UV lamp. (h) The normalized CPL spectra of PCSPCC (blue dotted line), PCSPCC + Ag+ (blue solid line), PCSPCC + Bi3+ (green dotted line), PCSPCC + Zn2+ (green solid line), and PCSPCC + Al3+ (red solid line) treated with heating–cooling cycle and PCSPCC + Al3+ (red dotted line) treated with ultrasound in n-BuOH. Reproduced with permission from ref. 120. Copyright 2023, the Royal Society of Chemistry. | ||
The inversion of CPL signal can also be realized by changing the stacking mode through coordination action. Liu et al. designed and synthesized a novel chiral gelator PyHis, which self-assembles into a nanofiber structure with right-handed CPL through supramolecular interactions during the self-assembly process.121 By adding Zn2+ to the system, dynamic structural reconfiguration of the nanofibers could be induced (Fig. 16a). As the molar ratio of Zn2+ to PyHis gradually increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 to 1
128 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18, the nanofibers gradually transformed into nanospheres, and the CPL intensity continuously decreased. When the molar ratio reached 1
18, the nanofibers gradually transformed into nanospheres, and the CPL intensity continuously decreased. When the molar ratio reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or higher, the system completely transformed into a nanosphere structure, and the CPL signal reversed (Fig. 16b). Additionally, the addition of ethylene diamine tetraacetic acid (EDTA) to the system could restore the CPL signal, indicating the controllability of this process (Fig. 16c). The single crystal structure showed that the coordination of Zn2+ significantly alters the molecular packing mode, with the two pyrene units changing from CH–π interactions to π–π interactions. This transformation in molecular arrangement regulated the exciton coupling, ultimately leading to the reversal of the CPL signal. Similarly, Fang et al. fabricated supramolecular CPL hydrogels through the co-assembly of chiral a phenylalanine-derived hydrogelator (LPF) and different achiral coumarin derivatives (G).122 The CPL signal direction of the hydrogel could be effectively controlled by modifying the spatial positions of the nitrogen atom from the ortho position to the meta or para position in the pyridine ring of coumarin derivatives. In addition, the introduction of metal ions could achieve the inversion or weakening of the CPL signal due to the coordination interaction between the metal ions and the pyridine group and alteration of the molecular packing pattern (Fig. 16d).
5 or higher, the system completely transformed into a nanosphere structure, and the CPL signal reversed (Fig. 16b). Additionally, the addition of ethylene diamine tetraacetic acid (EDTA) to the system could restore the CPL signal, indicating the controllability of this process (Fig. 16c). The single crystal structure showed that the coordination of Zn2+ significantly alters the molecular packing mode, with the two pyrene units changing from CH–π interactions to π–π interactions. This transformation in molecular arrangement regulated the exciton coupling, ultimately leading to the reversal of the CPL signal. Similarly, Fang et al. fabricated supramolecular CPL hydrogels through the co-assembly of chiral a phenylalanine-derived hydrogelator (LPF) and different achiral coumarin derivatives (G).122 The CPL signal direction of the hydrogel could be effectively controlled by modifying the spatial positions of the nitrogen atom from the ortho position to the meta or para position in the pyridine ring of coumarin derivatives. In addition, the introduction of metal ions could achieve the inversion or weakening of the CPL signal due to the coordination interaction between the metal ions and the pyridine group and alteration of the molecular packing pattern (Fig. 16d).
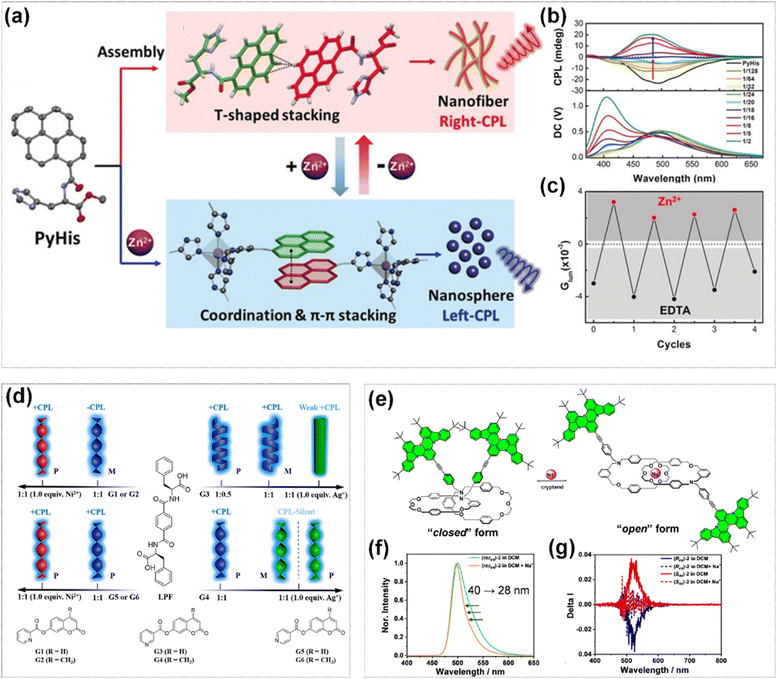 | ||
| Fig. 16 (a) Illustration of inversion of CPL by coordination of Zn2+ and π–π stacking of pyrene. (b) CPL spectra change of gel in presence of different amounts of Zn2+. (c) The inversion of CPL by alternately adding certain amounts of Zn2+ and EDTA. Reproduced with permission from ref. 121. Copyright 2019, Wiley-VCH. (d) Schematic representation of the co-assembly of chiral LPF and achiral fluorescent G and their response to metal ions. Reproduced with permission from ref. 122. Copyright 2019, American Chemical Society. (e) Reversible co-conformation transformation between the “closed” and “open” form upon the addition of Na+ and [2,2,2]cryptand. (f) Normalized emission spectra before and after the addition of Na+. (g) CPL spectra before and after the addition of Na+. Reproduced with permission from ref. 123. Copyright 2025, Wiley-VCH. | ||
Chiral mechanically interlocked molecules have garnered significant attention owing to their potential applications in CPL and ion recognition. Wang et al. successfully constructed circularly polarized multi-resonance thermally activated delayed fluorescence emitters through using topologically chiral [2]catenane as a chiral platform. The introduction of Na+ could realize the dynamic switching of CPL (Fig. 16e).123 In the absence of Na+, topological chiral [2]catenane molecules assumed a “closed” form. At this time, the π–π coupling between the two BN-doped chromophores was relatively strong, manifesting a broad emission spectrum and a strong CPL signal. Upon the introduction of Na+, the ions coordinated with the ethylene glycol groups in the molecule, causing the molecule to transform from “closed” to “open” form. This transformation weakened the coupling between the BN-doped chromophores, resulting in a significantly narrowed emission spectrum and the disappearance of the CPL signal (Fig. 16f and g). Furthermore, by adding chelating agents (such as [2.2.2]cryptand) to trap the Na+, the molecule could revert from the “open” to “closed” and led to the restoration of the broad emission spectrum and CPL signal.
Fu et al. prepared supramolecular polymers with full-color CPL and inverted handedness through the co-assembly of a cholesterol derivative (PVPCC), Zn2+ and fluorescent dyes.124 The co-assembled systems demonstrated anion-induced supramolecular chirality inversion through the exchange of anions such as NO3−, ClO4−, BF4− and Cl− (Fig. 17a). For example, the PVPCC/Zn(NO3)2 aggregate manifested negative CD and right-handed CPL, whereas the system converted to positive CD and left-handed CPL after introducing Cl−, accompanied by the transformation of its microstructure from nanorods to nanofibers (Fig. 17b and c). This was attributed to the strong coordination ability of Cl−, which enabled it to bind with Zn2+ and resulting in the changing of supramolecular interaction and inversion of CPL signals. Different assembly behaviors were observed in the PVPCC/Zn(BF4)2 system. After Cl− was added to the PVPCC/(BF4)2 aggregates, an amplification and redshift of both the CD and CPL signals were achieved (Fig. 17d and e). Correspondingly, the microscopic morphology transformed from nanofiber bundles to nanorods. However, the CD signals were almost unchanged after adding NO3−, ClO4− or BF4− to the PVPCC/ZnCl2 aggregates, further substantiating the strong coordination ability of Cl−.
 | ||
| Fig. 17 (a) The proposed mechanism illustration of the CD and CPL inversion of PVPCC/Zn(NO3)2 and PVPCC/Zn(BF4)2 aggregates induced by the addition of MgCl2. CD and CPL spectra of PVPCC/Zn(NO3)2 (b) and (c) and PVPCC/Zn(BF4)2 (d) and (e) aggregates before and after adding MgCl2 in PX/n-BuOH. Reproduced with permission from ref. 124. Copyright 2024, American Chemical Society. | ||
Xu et al. introduced the typical 9,14-diphenyl-9,14-dihydrodibenzo[a,c]phenazine (DPAC) luminogen with attractive vibration-induced emission (VIE) behavior into [2]rotaxane as a stopper (Fig. 18a).125 Utilizing its unique stimuli responsiveness to anions, the resulting [2]rotaxane (pR/pS-5C[2]R and pR/pS-6C[2]R) exhibited tunable VIE and switchable CPL (Fig. 18b and e). Under the stimuli of anions, trifluoroacetate (CF3COO−) was capable of inducing the macrocycle the planar chiral pillar[5]arene macrocycles (DEP[5]A) to move along the axial chain, modifying the distance of chiral information transmission and enhancing the CPL signal intensity (Fig. 18c and f). The signal of CPL could be switched reversibly through the addition of Na+ to remove CF3COO− (Fig. 18d and g).
 | ||
| Fig. 18 (a) The chemical structures and representation of the CPL switching system based on anion-induced motions of the chiral wheels. CPL spectra of pR/pS-5C[2]R (b) and pR/pS-6C[2]R (e), and pR/pS-5C[2]R upon the addition (c) or removal of CF3COO− (d), and pR/pS-6C[2]R upon the addition (f) or removal of CF3COO− (g). Reproduced with permission from ref. 125. Copyright 2024, Wiley-VCH. | ||
The CPL signal inversion can be realized by modifying the intermolecular packing and interactions through specific chemical reactions. The CPL probe (PTZ-D/L) compound was fabricated through the covalent combination of chiral amino acid-based lipid and achiral phenothiazine derivatives. This compound self-assembled to form an organic gel with the characteristics of reaction-based tunable CPL probe (Fig. 19a).126 After adding ClO− to the organic gel, the intensity of the fluorescence band at 625 nm gradually decreased, while that at 498 nm gradually increased (Fig. 19b), and the negative CPL signal of the organic gel gradually reversed to a positive signal (Fig. 19c). This phenomenon was ascribed to the sulfur atom on the phenothiazine moiety was oxidized to sulfoxide first, and then to sulphone with more hypochlorite, leading to the transformation of π–π stacking and intermolecular interaction. Cheng et al. employed pentylamine- substituted cholesteryl naphthalimide (PNC) and cholesteryl coumarin (CC) derivatives the energy transfer donor and acceptor respectively to co-assemble into vesicles and nanohelices under the solvent strategy which realized the CPL transition from green to red depending on the molar fraction.127 The stilbene structure of CC was capable of undergoing nucleophilic addition with SO32−. Upon the addition of Na2SO3, the π-conjugated structure of the system was disrupted, thereby blocking energy transfer and restoring the CPL signal to the original luminescent properties of PNC (Fig. 19d).
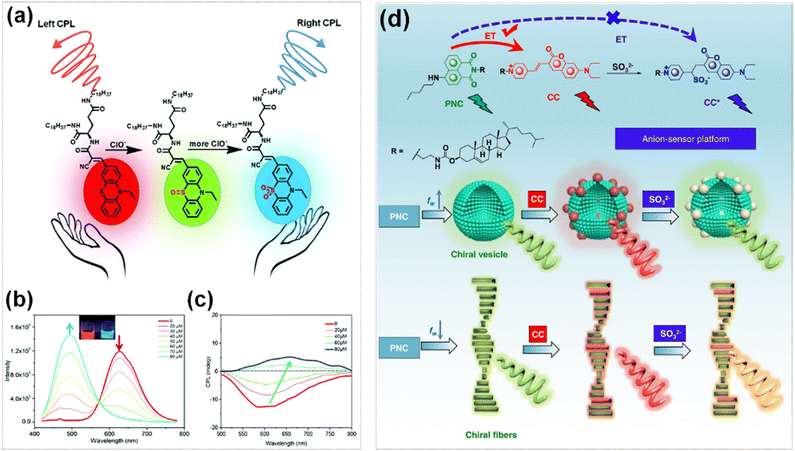 | ||
| Fig. 19 (a) Chemical structure of PTZ-D and the proposed switchable CPL mechanism with adding ClO−. (b) Fluorescence spectral changes of PTZ-D with different concentrations of ClO−. (c) CPL spectral changes of PTZ-D with different concentrations of ClO−. Reproduced with permission from ref. 126. Copyright 2019, the Royal Society of Chemistry. (d) Molecular structure of PNC and CC as well as the anion-responsive energy transfer effect in vesicle and helical fiber structures mediated by water fraction (fw) (energy transfer (ET), pentylamine-substituted cholesteryl naphthalimide (PNC), cholesteryl coumarin (CC)). Reproduced with permission from ref. 127. Copyright 2021, Springer Nature. | ||
6. Others
6.1. Magnetic-responsive CPL materials
Magnetic-responsive CPL materials make use of magnetic fields to modulate the electronic structure and the optical rotational properties of the molecules in order to achieve a dynamic modulation of chiral signals. Toda et al. investigated the magnetic circularly polarized luminescence (MCPL) of various polycyclic aromatic hydrocarbons unsubstituted or carrying electron-withdrawing carboxylic acid groups under magnetic fields (Fig. 20a).128 In both N-up and S-up magnetic field geometries, the MCPL spectra of these aromatic molecules exhibited mirror symmetry. For instance, the MCPL sign of phenanthrene (PHE) was negative in the N-up magnetic field direction and positive in the S-up direction (Fig. 20b). In contrast to the distinct MCPL of PHE, anthracene (ANT) did not show a significant MCPL signal, which might be due to the fact that the presence of a bent or curved structure is more conducive to enhancing MCPL compared to a linear structure (Fig. 20c). Additionally, the MCPL sign was significantly influenced by the position around the substituent. In the PHE derivatives in DMSO with the N-up magnetic field direction, PHE and PHE-2 manifested as negative signals, while PHE-3 presented as positive signals (Fig. 20d and e). The diamagnetic non-chiral pyrene and phenanthrene derivatives substituted with electron-donating hydroxyl/methoxy and electron-withdrawing carboxylic acid groups also displayed remarkable MCPL signals in a 1.6 T Faraday geometry magnetic field (Fig. 20f).129 Specifically, electron-donating groups enhanced the electron cloud density of the molecule to elevate the MCPL signal intensity, whereas electron-withdrawing groups reduced the electron cloud density to weaken the MCPL signal intensity and lead to the inversion of the signal. Furthermore, the position of the substituents exerted a significant influence on the MCPL sign. For example, substituents at the 1- and 2-positions of pyrene molecules generated completely opposite MCPL signs (Fig. 20g–j). | ||
| Fig. 20 (a) Chemical structures of ANT and PHE without or with substituents at two different peripheral positions (ANT-1, ANT-2, PHE-2, and PHE-3). (b) MCPL spectra at H0 = 1.6 T of PHE (b), ANT (c), PHE-2 (d), PHE-3 (e) (red and blue lines denote N-up and S-up geometries, respectively), CPL spectra at H0 = 0.0 T (green line), and the corresponding PL spectra. Reproduced with permission from ref. 128. Copyright 2021, the Royal Society of Chemistry. (f) Structure of pyrene substituted with electron donating and electron withdrawing groups. (g) MCPL and PL spectra of the 1a (black), 1b (red), and 1c (blue) luminophores. (h) The value of gMCPL of 1a (black), 1b (red), and 1c (blue). (i) MCPL and PL spectra of the 2a (black), 2b (red), and 2c (blue) luminophores. (j) The value of gMCPL of 2a (black), 2b (red), and 2c (blue). Reproduced with permission from ref. 129. Copyright 2021, the Royal Society of Chemistry. | ||
6.2. Mechanical-responsive CPL materials
Mechanical responsive CPL materials can dynamically regulate the CPL signal by altering the chiral structure of the materials through external mechanical stimuli. CNC is capable of self-assembling into chiral helical structures and achieving CPL emissions of multiple colors through doping with different dyes.130–132 Xu et al. fabricated two types of luminescent CNC shape-memory polymers (CNC-SMPs) composite films with red emission (RE-SMP) and green emission (GE-SMP) by combining CNC with SMP and adding luminescent monomers.133 The CPL emission could be switched reversibly by treating the luminescent CNC-SMPs with hot-pressing and recovery by heating to change the PBG of CNC. The distinct R-CPL emission was observed in the unpressed RE-SMP. However, the CPL nearly vanished after pressing with 70 N. In contrast, the uncompressed GE-SMP was CPL-inactive, while the pressed GE-SMP generated CPL activity (Fig. 21a). By doping the two luminescent monomers, the CPL emission of CNC-SMP could be switched from red to green. The uncompressed RE-GE-SMP exhibited red emission. As the applied pressure increased, the blue-shift of the CD and CPL peaks was observed (Fig. 21b and c). The green emission was observed after compressing the polymer under 87 N. By controlling the pressure in different areas of the composite film, the film can display different patterns through the clear color contrast. | ||
| Fig. 21 (a) Schematic representations of the chemical structure of CNCs and chiral nematic structure of CNC film. (b) Normalized CD and emission spectra of unpressed and pressed RE-GE-SMP. (c) CPL spectra of unpressed and pressed RE-GE-SMP. Reproduced with permission from ref. 133. Copyright 2023, Wiley-VCH. (d) Structure of chiral diamine-linked bispyrene derivative and photographs of the MCL of (R,R)-1. Normalized CPL and FL spectra of 1C (e) and 1A (f) for (R,R)- (red and black) and (S,S)- (blue and violet) samples. Reproduced with permission from ref. 134. Copyright 2021, Wiley-VCH. (g) Structure of the tetraphenylethene core with four L- or D-alanine branch side chains. (h) Time-dependent CPL spectra of L-1 (blue line) and D-1 (red line) with 1000 rpm in THF. (i) Plot of timedependent glum factor of L-1 and D-1 with 1000 rpm at 455 nm in THF; (a) 1000, (b) 600, (c) 200, (d) 0 rpm for L-1 (positive signals) and D-1 (negative signals), respectively. Reproduced with permission from ref. 135. Copyright 2019, Wiley-VCH. | ||
In typical organic mechanochromic luminescence (MCL) molecules, the emission color can be switched by grinding the powder sample, and the original color can be restored by heating or exposure to solvents. Ito et al. synthesized a chiral diamine-linked bispyrene derivative (R,R-1) which exhibited a rare MCL behavior in response to two mechanical stimuli (Fig. 21d).134R,R-1 demonstrated intense blue-green CPL emission in the amorphous state (Fig. 21f). The material underwent a transformation from the amorphous state to the crystalline state after ultrasonic treatment, accompanied by weakened CPL intensity (Fig. 21e). The CPL signal was restored through the materials revert from the crystalline state to the amorphous state under grinding. (S,S-1) showed the identical MCL behavior.
Lee et al. designed chiral molecules (L-1 and D-1) with tetraphenylethylene as the core (Fig. 21g).135 By introducing mechanical energy through stirring, the intramolecular hydrogen bonds were disrupted while the formation of intermolecular hydrogen bonds were promoted, thereby driving the rapid polymerization of the monomers and generating chiral supramolecular polymers. The rotational speed exerted a remarkable influence on the CPL signal. The CPL signal was weak at lower stirring speeds. However, the CPL signal was significantly enhanced and the polymerization lag time was shortened at higher stirring speeds. Additionally, the CPL signal gradually intensified and eventually stabilized as the stirring time increased (Fig. 21h and i).
Polyvinyl alcohol (PVA) is a typical amorphous material without metal. It can be stretched into an ordered configuration to induce chirality in the film. Wang et al. constructed PVA films doped with different dyes which could generate controllable CPL signals via stretching the films (Fig. 22a).136 Through doping molecules with different emission colors and types, full-color CPL and long afterglow CPL could be achieved. Moreover, the direction of CPL could be controlled by adjusting the stretching direction (Fig. 22b–d).
 | ||
| Fig. 22 (a) Diagram of stretched film to produce CPL. (b) CPL signal of rhodamine B film after stretching in different direction. (c) CPL signal of a sodium fluorescein film after stretching in different directions. (d) CPL signal of a luminol film after stretching in different directions. Reproduced with permission from ref. 136. Copyright 2024, Wiley-VCH. | ||
7. Conclusions
Organic stimuli-responsive tunable CPL materials have garnered significant attention due to their broad application potential. This review summarizes the design principles, performance regulation strategies and potential applications of such materials and focus on the dynamic regulation of CPL signals by external stimuli such as light, temperature, pH, and ions.Despite the remarkable advancements achieved in the organic stimuli-responsive CPL research, some challenges still persist: (1) the PLQY and glum value of CPL materials remain to be enhanced to satisfy the practical demands of high-performance optoelectronic devices. (2) Current researches mainly focus on single-stimuli response systems, while the researches on CPL materials with multi-stimuli cooperative regulation are relatively limited, making it difficult to achieve more complex information storage and regulation. (3) The long-term stability, processability, and environmental adaptability of organic stimuli-responsive CPL materials still require further optimization to accommodate the requirements of diverse application scenarios.
In the design of organic stimuli-responsive tunable CPL materials, it is requisite to collaboratively integrate crucial elements such as chiral molecules, luminescent groups, stimuli-responsive components, so as to realize the unification of efficient CPL and dynamic tunability. Self-assembly strategies provide a straightforward and effective solution for this. Through the rational design of non-covalent interaction, such as hydrogen bonds and π–π interactions, each component can be integrated into a supramolecular self-assembly system. Supramolecular self-assembly is capable of achieving the transfer and amplification of chirality from the molecular scale to the supramolecular assembly scale via the synergy of non-covalent interactions. Moreover, the introduction of external stimuli-responsive groups or dynamic non-covalent interactions enables the controllable modulation of molecular configurations or packing patterns, resulting in the tunable CPL. The preparation of multi-stimuli-responsive CPL materials is expected to be realized by introducing several different stimuli-responsive groups into a single molecular/supramolecular system, or by constructing composite systems with different stimuli-responsive materials. For example, composite systems can be constructed by introducing the light-responsive molecules to temperature-responsive liquid crystals. Utilizing the configurational changes of light-responsive molecules upon illumination and the variation of the selective reflection band of liquid crystals at different temperatures, multi-stimuli-responsive CPL regulation can be realized. In addition, protective layers can be introduced to materials through approaches such as polymer doping to enhance the stability and environmental adaptability of the materials while ensuring their performance.
Through the optimization of molecular design and the synergistic regulation of multiple stimuli, organic stimuli-responsive tunable CPL materials will develop towards greater efficiency and multi-functionality in the future, and expand their applications in information encryption, optical storage, and biosensing. This review aims to provide researchers with systematic design ideas and novel application, and to offer inspiration for cross-disciplinary research in related fields.
Data availability
Data availability does not apply to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Key R&D Program (2022YFA1204401), the National Natural Science Foundation of China (52473239, 52403301, 52121002, U21A6002).Notes and references
- J. R. Cronin and S. Pizzarello, Science, 1997, 275, 951 CrossRef CAS
.
- S. Zhang, Y. Zheng, H. An, B. Aguila, C. Yang, Y. Dong, W. Xie, P. Cheng, Z. Zhang, Y. Chen and S. Ma, Angew. Chem., Int. Ed., 2018, 57, 16754–16759 CrossRef CAS PubMed
.
- Q. Gao, L. Tan, Z. Wen, D. Fan, J. Hui and P. Wang, Nano Res., 2023, 16, 11107–11124 CrossRef
.
- C. Chu, Y. Wang and Y. Ma, J. Am. Chem. Soc., 2024, 146, 35339–35346 CrossRef CAS PubMed
.
- T. Li, D. Niu, L. Ji, Q. Li, B. Guan, H. Wang, G. Ouyang and M. Liu, Nat. Commun., 2025, 16, 1698 CrossRef CAS PubMed
.
- T. Lou, X. Wang, J. Li, W. Wang, P. Han, S. Yu, C. Fan, C. Zhou and H. Ruan, ACS Nano, 2025, 19, 7767–7783 CrossRef CAS PubMed
.
- W. Jiang, X. Yi and M. D. McKee, Mater. Horiz., 2019, 6, 1974–1990 RSC
.
- W. Fan, T. Matsuno, Y. Han, X. Wang, Q. Zhou, H. Isobe and J. Wu, J. Am. Chem. Soc., 2021, 143, 15924–15929 CrossRef CAS
.
- H. Wu, Y. Wang, L. Đorđević, P. Kundu, S. Bhunia, A. X.-Y. Chen, L. Feng, D. Shen, W. Liu, L. Zhang, B. Song, G. Wu, B. Liu, M. Y. Yang, Y. Yang, C. L. Stern, S. I. Stupp, W. A. Goddard III, W. Hu and J. F. Stoddart, Nature, 2025, 637, 347–353 CrossRef CAS PubMed
.
- Y. Qiu, X. Wei, J. W. Y. Lam, Z. Qiu and B. Z. Tang, ACS Nano, 2025, 19, 229–280 CrossRef CAS PubMed
.
- M. Lu, P. Li, X. Dong, Z. Jiang, S. Ren, J. Yao, H. Dong and Y. S. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202408619 CrossRef CAS PubMed
.
- K. Chen, Y. Liu, Z. Wang, S. Hu, Y. Zhao, W. Wang, G. Liu, Z. Wang and W. Jiang, J. Am. Chem. Soc., 2024, 146, 13499–13508 CrossRef CAS PubMed
.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2020, 32, 1900110 CrossRef CAS
.
- Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light: Sci. Appl., 2021, 10, 76 CrossRef CAS
.
- H. Zhong, X. Gao, B. Zhao and J. Deng, Acc. Chem. Res., 2024, 57, 1188–1201 Search PubMed
.
- N. Liang, C. Cao, Z. Xie, J. Liu, Y. Feng and C. Yao, Mater. Today, 2024, 75, 309–333 Search PubMed
.
- M. Zhang, Q. Guo, Z. Li, Y. Zhou, S. Zhao, Z. Tong, Y. Wang, G. Li, S. Jin, M. Zhu, T. Zhuang and S. H. Yu, Sci. Adv., 2023, 9, eadi9944 Search PubMed
.
- X. Zhan, F. F. Xu, Z. Zhou, Y. Yan, J. Yao and Y. Zhao, Adv. Mater., 2021, 33, 2104418 Search PubMed
.
- L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2021, 5, 109 CrossRef CAS PubMed
.
- Q. Wang, J. Bao, Y. Zhang, Y. Wang, D. Qiu, J. Yang, J. Zhang, H. Gao, Y. Wu, H. Dong, H. Yang and Z. Wei, Adv. Mater., 2024, 36, 2312396 CrossRef CAS PubMed
.
- V. Sharma, M. Crne, J. O. Park and M. Srinivasarao, Science, 2009, 325, 449 CrossRef CAS PubMed
.
- Y. Zhou, C. Lu, Z. Lu, Z. Guo, C. Ye, V. Tsukruk and R. Xiong, Small, 2023, 19, 2303064 Search PubMed
.
- C. He and Y. Li, Chin. Chem. Lett., 2023, 34, 108077 Search PubMed
.
- R. Richardson, M. Baud, C. Weston, H. Rzepa, M. Kuimova and M. Fuchter, Chem. Sci., 2015, 6, 3853–3862 RSC
.
- X. Zou, N. Gan, Y. Gao, L. Gu and W. Huang, Angew. Chem., Int. Ed., 2025, 64, e202417906 CrossRef CAS PubMed
.
- J. L. Greenfield, J. Wade, J. R. Brandt, X. Shi, T. J. Penfold and M. J. Fuchter, Chem. Sci., 2021, 12, 8589–8602 Search PubMed
.
- J.-M. Teng and C.-F. Chen, Adv. Opt. Mater., 2023, 11, 2300550 CrossRef CAS
.
- Y. Wang, D. Niu, G. Ouyang and M. Liu, Nat. Commun., 2022, 13, 1710 CrossRef CAS PubMed
.
- P. Shen, S. Jiao, Z. Zhuang, X. Dong, S. Song, J. Li, B. Z. Tang and Z. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202407605 Search PubMed
.
- W. Shang, Y. Wang, X. Zhu, T. Liang, C. Du, J. Xiang and M. Liu, J. Am. Chem. Soc., 2023, 145, 27639–27649 CrossRef CAS PubMed
.
- X. Pan, A. Zheng, X. Yu, Q. Di, L. Li, P. Duan, K. Ye, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202203938 CrossRef CAS PubMed
.
- Q. Wang, L. Yuan, C. Qu, T. Huang, X. Song, Y. Xu, Y.-X. Zheng and Y. Wang, Adv. Mater., 2023, 35, 2305125 CrossRef CAS
.
- L. Chen, P. Zou, J. Chen, L. Xu, B. Z. Tang and Z. Zhao, Nat. Commun., 2025, 16, 1656 CrossRef CAS
.
- C. Yang, S. Fu, S. Li, F. Li, Y. Su, T. Li, H. Liu, X. Zhang and W. Hu, Adv. Opt. Mater., 2025, 13, 2402522 CrossRef CAS
.
- X.-Z. Wang, S. Xing, X. Xiao, L. Yuan, Z.-Y. Hou and Y.-X. Zheng, Adv. Funct. Mater., 2025, 35, 2412044 Search PubMed
.
- P. Zhao, W.-C. Guo, H.-Y. Lu and C.-F. Chen, Angew. Chem., Int. Ed., 2025, 64, e202424918 CrossRef CAS PubMed
.
- Y.-F. Wang, X. Liu, Y. Zhu, M. Li and C.-F. Chen, J. Mater. Chem. C, 2022, 10, 4805 RSC
.
- Z. Ye, H. Wu, Y. Xu, T. Hua, G. Chen, Z. Chen, X. Yin, M. Huang, K. Xu, X. Song, Z. Huang, X. Lv, J. Miao, X. Cao and C. Yang, Adv. Mater., 2024, 36, 2308314 Search PubMed
.
- L. Dang, W. Xu, S. Qiu, Y. Yu, Z. Ma, L. Yue, H. Su, C. Li and H. Wang, Org. Lett., 2024, 26, 10141–10145 CrossRef CAS
.
- M. Schnitzlein, K. Shoyama and F. Würthner, Chem. Sci., 2024, 15, 2984–2989 RSC
.
- J. Han, J. You, X. Li, P. Duan and M. Liu, Adv. Mater., 2017, 29, 1606503 CrossRef PubMed
.
- Y. Sun, Y. Jiang, J. Jiang, T. Li and M. Liu, Chin. Chem. Lett., 2024, 35, 108409 Search PubMed
.
- R. Liu, Z. Feng, X. Yan, Y. Lv, J. Wei, J. Hao and Z. Yang, J. Am. Chem. Soc., 2023, 145, 17274–17283 Search PubMed
.
- J. Han, D. Yang, X. Jin, Y. Jiang, M. Liu and P. Duan, Angew. Chem., Int. Ed., 2019, 58, 7013–7019 CrossRef CAS
.
- Y. Yang, C. Yang, X. Zhu, L. Zhang and M. Liu, Chem. Commun., 2024, 60, 6631–6634 RSC
.
- H. Zhang, J. Han, X. Jin and P. Duan, Angew. Chem., Int. Ed., 2021, 60, 4575–4580 CrossRef CAS
.
- S. Zheng, J. Han, X. Jin, Q. Ye, J. Zhou, P. Duan and M. Liu, Angew. Chem., Int. Ed., 2021, 60, 22711–22716 CrossRef CAS PubMed
.
- S. Ghorai, S. Show and A. Das, Angew. Chem., Int. Ed., 2025, e202500879 CAS
.
- Y.-C. Tao, Z.-Q. Li, J. Yao and Y.-W. Zhong, Cryst. Growth Des., 2024, 24, 9126–9132 CrossRef CAS
.
- L. Wei, S. Guo, B. Zhang, B. Jiang, Y. Wang, Z. Liu, Y. Xu, Y. Gong, Y. Liu and W. Z. Yuan, Adv. Funct. Mater., 2024, 34, 2409681 Search PubMed
.
- X. Wang, B. Zhao and J. Deng, Adv. Mater., 2023, 35, 2304405 CrossRef CAS
.
- J. Liu, Z.-P. Song, J. Wei, J.-J. Wu, M.-Z. Wang, J.-G. Li, Y. Ma, B.-X. Li, Y.-Q. Lu and Q. Zhao, Adv. Mater., 2024, 36, 2306834 CrossRef CAS
.
- C. Du, Z. Li, X. Zhu, G. Ouyang and M. Liu, Nat. Nanotechnol., 2022, 17, 1294–1302 Search PubMed
.
- F. Wang, C. Shen, F. Gan, G. Zhang and H. Qiu, CCS Chem., 2023, 5, 1592–1601 Search PubMed
.
- L. Meng, D.-X. Ma, Z.-Q. Li, T. Liang, Z. Chen and Y.-W. Zhong, Adv. Sci., 2025, 12, 2500789 Search PubMed
.
- B.-H. Liu, Y. Zong, N. Liu and Z.-Q. Wu, Sci. China: Chem., 2024, 67, 3247–3257 Search PubMed
.
- L. Yuan, Y.-P. Zhang and Y.-X. Zheng, Sci. China: Chem., 2024, 67, 1097–1116 CrossRef CAS
.
- X.-M. Chen, S. Zhang, X. Chen and Q. Li, ChemPhotoChem, 2022, 6, e202100256 CrossRef CAS
.
- L. Zhang, H.-X. Wang, S. Li and M. Liu, Chem. Soc. Rev., 2020, 49, 9095–9120 Search PubMed
.
- J.-L. Ma, Q. Peng and C.-H. Zhao, Chem. – Eur. J., 2019, 25, 15441–15454 Search PubMed
.
- J. Sheng, J. Perego, S. Bracco, W. Czepa, W. Danowski, S. Krause, P. Sozzani, A. Ciesielski, A. Comotti and B. L. Feringa, Adv. Mater., 2024, 36, 2305783 CrossRef CAS
.
- Z. He, Y. Li, H. Wu, Y. Yang, Y. Chen, J. Zhu, Q. Li and G. Jiang, ACS Appl. Mater. Interfaces, 2022, 14, 48133–48142 CrossRef CAS PubMed
.
- L. Qu, X. Xu, J. Song, D. Wu, L. Wang, W. Zhou, X. Zhou and H. Xiang, Dyes Pigm., 2020, 181, 108597 CrossRef CAS
.
- M. Du and C. Li, Adv. Mater., 2024, 36, 2408484 CrossRef CAS PubMed
.
- L. Wang, W. Xiong, H. Tang and D. Cao, J. Mater. Chem. C, 2019, 7, 9102–9111 RSC
.
- D. Han and T. Jiao, Langmuir, 2022, 38, 13668–13673 CrossRef CAS PubMed
.
- Z. Huang, Y. Hu, X. Jin, Y. Zhao, J. Su and X. Ma, Adv. Opt. Mater., 2021, 9, 2100135 CrossRef CAS
.
- Z. Li, Y.-J. Hu, K. Zhang, Y. Zhang, Q.-Q. Hu, X.-J. Zhang, X.-K. Zhang and Y.-P. Zhu, Dyes Pigm., 2020, 182, 108686 Search PubMed
.
- G. Liu, J. Leng, Q. Zhou, Z. Deng, L. Shi, C. Fan, X. Xu and M.-P. Song, Dyes Pigm., 2022, 203, 110361 CrossRef CAS
.
- T. Hao, Y. Yang, W. Liang, C. Fan, X. Wang, W. Wu, X. Chen, H. Fu, H. Chen and C. Yang, Chem. Sci., 2021, 12, 2614–2622 Search PubMed
.
- K. Fu, D.-H. Qu and G. Liu, J. Am. Chem. Soc., 2024, 146, 33832–33844 CrossRef CAS PubMed
.
- C. Xue, L. Xu, H.-X. Wang, T. Li and M. Liu, ChemPhotoChem, 2022, 6, e202100255 CrossRef CAS
.
- Y. Hashimoto, T. Nakashima, D. Shimizu and T. Kawai, Chem. Commun., 2016, 52, 5171–5174 RSC
.
- F. Shi, F. Han, W. Zhang, Y. Yang and H. Li, Dyes Pigm., 2024, 222, 111910 Search PubMed
.
- Y. Li, Y. Liu and D. Luo, J. Mater. Chem. C, 2019, 7, 15166–15170 RSC
.
- A. Kappelt and M. Giese, Chem. – Eur. J., 2020, 26, 13347–13351 Search PubMed
.
- L. D. C. Castro, J. Lub, O. N. Oliveira and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2025, 64, e202413559 CrossRef PubMed
.
- I. Bala, J. T. Plank, B. Balamut, D. Henry, A. R. Lippert and I. Aprahamian, Nat. Chem., 2024, 16, 2084–2090 CrossRef CAS PubMed
.
- Z. Zheng, H. Hu, Z. Zhang, M. Li, D.-H. Qu, H. Tian, W.-H. Zhu, B. L. Feringa and B. Liu, Nat. Photonics, 2022, 16, 226–234 CrossRef CAS
.
- J. Gao, Y. Tang, D. Martella, J. Guo, D. S. Wiersma and Q. Li, Responsive Mater., 2023, 1, e20230008 CrossRef
.
- Y. Wu, M. Li, Z.-G. Zheng, Z.-Q. Yu and W.-H. Zhu, J. Am. Chem. Soc., 2023, 145, 12951–12966 CrossRef CAS PubMed
.
- J. Liu, Z.-P. Song, L.-Y. Sun, B.-X. Li, Y.-Q. Lu and Q. Li, Responsive Mater., 2023, 1, e20230005 CrossRef
.
- S. Li, J. Wang, M. Tian, X. Meng, J. Wang and J. Guo, Angew. Chem., Int. Ed., 2024, 63, e202405615 CrossRef CAS PubMed
.
- J. Yuan, X. He, L. Chen, X. Lu and Q. Lu, Angew. Chem., Int. Ed., 2025, 64, e202419924 Search PubMed
.
- Y. Li, Y. Chen, J. Luo, Y. Quan and Y. Cheng, Adv. Mater., 2024, 36, 2312331 CrossRef CAS PubMed
.
- B. Sezginab and T. Hegmann, J. Mater. Chem. C, 2022, 10, 18120–18126 RSC
.
- J. Li, C. Liu, J. Wang, C. Liu, C. Zhao, J. Ren, H. Huang, Y. Wang, Q. Zhang, Y. J. Dappe, R. J. Nichols and L. Yang, Nanoscale, 2025, 17, 2147 RSC
.
- W. Kang, Y. Tang, X. Meng, S. Lin, X. Zhang, J. Guo and Q. Li, Angew. Chem., Int. Ed., 2023, 62, e202311486 CrossRef CAS PubMed
.
- L. Ji, J. Li, T. Meng, Z. Li, H. Zhu, G. Ouyang and M. Liu, Small Methods, 2025, 9, 2400824 CrossRef CAS PubMed
.
- S. Wang, X. Sun, H. Zeng, J. Zhang and X. Wan, Chem. – Eur. J., 2023, 29, e202301080 CrossRef CAS PubMed
.
- H. Murata, S. Suzuki, K. Terakubo, Y. Imai and S. Ito, Chem. – Asian J., 2024, 19, e202400293 Search PubMed
.
- S. Ji, M. Zeng, X. Zhan, H. Liu, Y. Zhou, K. Wang, Y. Yan, J. Yao and Y. S. Zhao, J. Am. Chem. Soc., 2024, 146, 22583–22589 Search PubMed
.
- C. Zhao, Y. Wang, Y. Jiang, N. Wu, H. Wang, T. Li, G. Ouyang and M. Liu, Adv. Mater., 2024, 36, 2403329 Search PubMed
.
- M. Pan, G. Zhang, H. Ma, X. Cheng, J. Li and W. Zhang, Sci. China: Chem., 2024, 67, 2362–2372 Search PubMed
.
- Q. Jiang, X. Xu, P.-A. Yin, K. Ma, Y. Zhen, P. Duan, Q. Peng, W.-Q. Chen and B. Ding, J. Am. Chem. Soc., 2019, 141, 9490–9494 Search PubMed
.
- X. Song, X. Zhu, S. Qiu, W. Tian and M. Liu, Angew. Chem., Int. Ed., 2022, 61, e202208574 CrossRef CAS PubMed
.
- Y. Zhang, H. Li, Z. Geng, W.-H. Zheng, Y. Quan and Y. Cheng, ACS Nano, 2022, 16, 3173–3181 Search PubMed
.
- J. Yan, F. Ota, B. A. San Jose and K. Akagi, Adv. Funct. Mater., 2017, 27, 1604529 Search PubMed
.
- S. Feuillastre, M. Pauton, L. Gao, A. Desmarchelier, A. J. Riives, D. Prim, D. Tondelier, B. Geffroy, G. Muller, G. Clavier and G. Pieters, J. Am. Chem. Soc., 2016, 138, 3990–3993 CrossRef CAS PubMed
.
- Z.-G. Wu, H.-B. Han, Z.-P. Yan, X.-F. Luo, Y. Wang, Y.-X. Zheng, J.-L. Zuo and Y. Pan, Adv. Mater., 2019, 31, 1900524 CrossRef PubMed
.
- Y.-F. Wang, X. Liu, Y. Zhu, M. Li and C.-F. Chen, J. Mater. Chem. C, 2022, 10, 4805–4812 RSC
.
- Z.-P. Yan, T.-T. Liu, R. Wu, X. Liang, Z.-Q. Li, L. Zhou, Y.-X. Zheng and J.-L. Zuo, Adv. Funct. Mater., 2021, 31, 2103875 CrossRef CAS
.
- W. Huang, C. Fu, Z. Liang, K. Zhou and Z. He, Angew. Chem., Int. Ed., 2022, 61, e202202977 CrossRef CAS PubMed
.
- W. Zhou, D. Wu, H. Xiao, J. Song, L. Qu, L. Wang, X. Zhou, Z.-X. Xu and H. Xiang, Dyes Pigm., 2022, 197, 109906 CrossRef CAS
.
- W. Wu, K. Chen, X. Zhang, T. Wang, S. Li, H. Zhao, L. Zhou, X. Huang and H. Hao, Chem. – Eur. J., 2023, 29, e202202598 CrossRef CAS PubMed
.
- W. Zhong, J. Zhang, Y. Lin, S. Li, Y. Yang, W.-J. Wang, C. Si, F. E. Kühn, Z. Zhao, X.-M. Cai and B. Z. Tang, Chem. Sci., 2024, 15, 3920–3927 RSC
.
- Y.-Y. Tang, J.-C. Liu, Y.-L. Zeng, H. Peng, X.-Q. Huang, M.-J. Yang and R.-G. Xiong, J. Am. Chem. Soc., 2021, 143, 13816–13823 Search PubMed
.
- P. Jin, J. Xue, L. Sun, J. Huang, J. Zhang and H. Tian, Nat. Commun., 2019, 10, 4232 CrossRef PubMed
.
- M. Yamauchi, K. Yokoyama, N. Aratani, H. Yamada and S. Masuo, Angew. Chem., Int. Ed., 2019, 58, 14173–14178 CrossRef CAS PubMed
.
- X. Pan, L. Lan and H. Zhang, Chem. Sci., 2024, 15, 17444–17452 RSC
.
- J. Sun, M. Han, G. Yang, Y. Wang, W. Fang, A. Shi, C. Xiang, J. Wang, T. Zhang and H. Wang, Adv. Opt. Mater., 2025, 13, 2402080 Search PubMed
.
- A. Ikenaga, Y. Akiyama, T. Ishiyama, M. Gon, K. Tanaka, Y. Chujo and K. Isoda, ACS Appl. Mater. Interfaces, 2021, 13, 47127–47133 CrossRef CAS PubMed
.
- D. Niu, L. Ji, G. Ouyang and M. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 18148–18156 CrossRef CAS PubMed
.
- W.-L. Zhao, W.-C. Guo, K.-K. Tan, Z.-X. Yu, M. Li and C.-F. Chen, Angew. Chem., Int. Ed., 2025, 64, e202416863 CrossRef CAS PubMed
.
- X. Lin, G. Ouyang and M. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 19741–19749 CrossRef CAS PubMed
.
- D. Han, J. Han, S. Huo, Z. Qu, T. Jiao, M. Liu and P. Duan, Chem. Commun., 2018, 54, 5630–5633 RSC
.
- Q. Miao, X. Wang, B. Jin, Y. Chen, Y. Zhou, R. Xiong, X. Meng and C. Ye, J. Mater. Chem. C, 2024, 12, 4861–4869 RSC
.
- J. Li, G. Yang, A. Shi, D. Han, M. Han, C. Xiang, T. Zhang and H. Wang, Adv. Opt. Mater., 2024, 12, 2400156 CrossRef CAS
.
- L. Li, P. Jiang, X. Zhang and Y. Li, Angew. Chem., Int. Ed., 2025, 64, e202417149 CrossRef CAS PubMed
.
- K. Fu and G. Liu, Chem. Commun., 2023, 59, 13751–13754 RSC
.
- D. Niu, Y. Jiang, L. Ji, G. Ouyang and M. Liu, Angew. Chem., Int. Ed., 2019, 58, 5946–5950 CrossRef CAS PubMed
.
- F. Wang, W. Ji, P. Yang and C.-L. Feng, ACS Nano, 2019, 13, 7281–7290 CrossRef CAS PubMed
.
- Y. Wang, W.-L. Zhao, Z. Gao, C. Qu, X. Li, Y. Jiang, L. Hu, X.-Q. Wang, M. Li, W. Wang, C.-F. Chen and H.-B. Yang, Angew. Chem., Int. Ed., 2025, 64, e202417458 CrossRef CAS PubMed
.
- K. Fu and G. Liu, ACS Nano, 2024, 18, 2279–2289 CrossRef CAS PubMed
.
- W.-T. Xu, X. Li, P. Wu, W.-J. Li, Y. Wang, X.-Q. Xu, X.-Q. Wang, J. Chen, H.-B. Yang and W. Wang, Angew. Chem., Int. Ed., 2024, 63, e202319502 CrossRef CAS PubMed
.
- J. Gong, M. Yu, C. Wang, J. Tan, S. Wang, S. Zhao, Z. Zhao, A. Qin, B. Tang and X. Zhang, Chem. Commun., 2019, 55, 10768–10771 RSC
.
- Q. Cheng, A. Hao and P. Xing, Nat. Commun., 2021, 12, 6320 CrossRef CAS PubMed
.
- H. Toda, N. Hara, M. Fujiki and Y. Imai, RSC Adv., 2021, 11, 1581–1585 RSC
.
- N. Hara, M. Kitahara, T. Sugimura, H. Toda, M. Shizuma, A. Ito, M. Miyasaka, M. Fujiki and Y. Imai, Phys. Chem. Chem. Phys., 2021, 23, 8236–8240 Search PubMed
.
- S. Jia, B. Yang, Y. Xie, T. Tao, J. Du, L. Yu, Y. Zhang, J. Zhang, W. Tang and J. Gong, Adv. Funct. Mater., 2024, 34, 2410206 CrossRef CAS
.
- H. Yu, B. Zhao, J. Guo, K. Pan and J. Deng, J. Mater. Chem. C, 2020, 8, 1459–1465 RSC
.
- D. Lu, X. Yu, S. Zhu and Y. Xu, Cellulose, 2023, 30, 8325–8338 CrossRef CAS
.
- M. Xu, Z. Xu, M. A. Soto, Y.-T. Xu, W. Y. Hamad and M. J. MacLachlan, Adv. Mater., 2023, 35, 2301060 CrossRef CAS PubMed
.
- S. Ito, R. Sekine, M. Munakata, M. Asami, T. Tachikawa, D. Kaji, K. Mishima and Y. Imai, ChemPhotoChem, 2021, 5, 920–925 CrossRef CAS
.
- S. Lee, K. Y. Kim, S. H. Jung, J. H. Lee, M. Yamada, R. Sethy, T. Kawai and J. H. Jung, Angew. Chem., Int. Ed., 2019, 58, 18878–18882 CrossRef CAS PubMed
.
- T. Wang, S. Sun, X. Jiang and X. Ma, Adv. Opt. Mater., 2024, 12, 2301770 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |




