 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancing DTT assays for reactive oxygen species detection in atmospheric particulate matter: key factors and methodological insights
W. P. D.
Vimukthi
 ,
Shenghong
Dong
,
Chi
Yang
,
Shenghong
Dong
,
Chi
Yang
 ,
Yanlin
Zhang
,
Gulinasahan
Baikeri
,
Ting
Lou
,
Fuyang
Deng
,
Ziyang
Li
and
Fang
Cao
*
,
Yanlin
Zhang
,
Gulinasahan
Baikeri
,
Ting
Lou
,
Fuyang
Deng
,
Ziyang
Li
and
Fang
Cao
*
School of Ecology and Applied Meteorology, Nanjing University of Information Science and Technology, Nanjing 210044, China. E-mail: caofang@nuist.edu.cn; caofangle@163.com
First published on 27th May 2025
Abstract
Accurate detection of reactive oxygen species (ROS) in atmospheric particulate matter (PM) is essential for assessing the oxidative potential (OP) of airborne pollutants and their associated health risks. While multiple methods exist for ROS detection, inconsistencies in assay conditions often lead to variable outcomes, limiting cross-study comparability. This review systematically evaluates key methodological factors affecting the dithiothreitol (DTT) assay, one of the most widely used techniques for measuring the OP of PM. Critical parameters—including intrinsic assay variables such as initial DTT concentration and incubation conditions, as well as extrinsic factors such as light exposure and metal–organic interactions—are analyzed to identify sources of variability. To improve sensitivity and reliability, this study proposes standardized protocols, the incorporation of positive controls, and methodological refinements. By addressing these challenges, this review enhances the accuracy of ROS detection and contributes to a more comprehensive understanding of the OP of PM, with significant implications for environmental monitoring and public health.
Environmental significanceThis review highlights the critical role of reactive oxygen species (ROS) in determining the oxidative potential (OP) of particulate matter (PM), a key indicator of air pollution toxicity. By identifying and addressing methodological inconsistencies in ROS detection assays, this work underscores the need for standardized protocols to ensure accurate assessments of air pollution's health impacts. These insights contribute to advancing atmospheric science, improving air quality standards, and guiding targeted regulatory measures to mitigate oxidative stress-related health risks and environmental damage. |
1. Introduction
Atmospheric Particulate Matter (PM) poses significant risks to both human health and environmental systems, primarily due to its ability to generate ROS, which contribute to oxidative stress and tissue damage.1,2 Exposure to ROS from PM has been linked to various adverse health outcomes, including respiratory and cardiovascular diseases, due to its role in inducing cellular damage and inflammation.3–5 Beyond health effects, ROS also play a critical role in atmospheric chemistry, particularly in the formation of secondary organic aerosols (SOAs), which contribute to air quality degradation.6,7 Accurate detection of ROS is essential for assessing the OP of PM and understanding its broader health and environmental implications.Various analytical techniques have been developed to measure ROS in PM, with the DTT assay emerging as one of the most widely used methods.8–10 The DTT assay provides a quantitative measure of the oxidative potential of PM by assessing its capacity to deplete DTT, a thiol-based reducing agent. This method is favored due to its simplicity, cost-effectiveness, and reproducibility. However, significant variations in experimental protocols—such as differences in initial DTT concentration, incubation conditions, light exposure, and metal–organic interactions—compromise its reliability and hinder cross-study comparisons.11,12 Understanding these inconsistencies is essential for improving the accuracy and standardization of ROS detection.13–16
The OP measured by the DTT assay is influenced by multiple PM components, including soluble metals (e.g., copper and manganese)14,17 and organic compounds like water-soluble organic carbon (WSOC),18,19 oxidized polycyclic aromatic hydrocarbons (PAHs) such as quinones,20,21 and humic-like substances (HULIS).22,23 While these components strongly correlate with DTT oxidation, their interactions introduce complexities that can obscure causal relationships.24–27 For instance, recent research suggests that metal ions not only drive direct oxidative reactions but also interact with organic compounds, further complicating ROS generation mechanisms.7,18,28
Despite its widespread use, the DTT assay presents several methodological challenges. The OP of PM can be expressed in two formats: volume-normalized DTT activity (DTTv), which is relevant for human exposure assessments, and mass-normalized DTT activity (DTTm), which provides insight into the intrinsic oxidative properties of PM components.29,30 However, achieving reliable ROS measurements can be challenging due to practical factors like light exposure and the complex chemical nature of PM as illustrated in Fig. 1.
Unlike previous reviews, this study systematically evaluates key methodological factors affecting the DTT assay and proposes improvements to enhance its sensitivity and accuracy. By identifying sources of variability—such as light-induced ROS formation and metal–organic interactions—and recommending standardized protocols and the inclusion of positive controls, this review aims to improve the reliability of ROS detection. These refinements will contribute to a more accurate assessment of the oxidative potential of PM, advancing both environmental monitoring and health risk evaluation strategies.
2. Overview of ROS detection techniques for particulate matter
The detection of ROS in PM is essential for evaluating the OP of atmospheric pollutants. ROS detection techniques can be broadly classified into fluorescence-based techniques and spectrometric methods, each with distinct advantages and limitations, as summarized in Table 1.39–41 Although multiple techniques exist, this review primarily focuses on the DTT assay, while briefly discussing alternative ROS detection methods to provide context.| Method | Advantages | Limitations | Reference |
|---|---|---|---|
| DCFH (dichlorofluorescein) assay | High sensitivity; rapid detection; adaptability | Cross-reactivity with multiple ROS types; sensitivity to pH and reagent concentration | 31–33 |
| DTT assay (chemical OP assay) | Quantitative OP measurement; established protocols | Sensitivity to metal ions and reaction conditions | 17,34–36 |
| EPR spectroscopy | Specific ROS identification | Requires specialized equipment and stabilization agents | 37 |
| Chemiluminescence techniques | High sensitivity for specific applications | Limited use in PM studies; specialized reagents required | 38 |
2.1. Fluorescence-based techniques
Fluorescence-based methods are widely used for detecting particle-bound ROS due to their high sensitivity, rapid response, and adaptability. These techniques utilize chemical probes that emit fluorescence upon reacting with ROS, enabling quantitative analysis.42,43 The most commonly used probe is dichlorofluorescin (DCFH), a non-fluorescent molecule that becomes fluorescent (DCF) upon oxidation by ROS. The DCFH assay involves mixing DCFH with horseradish peroxidase (HRP) in a sodium phosphate buffer, followed by sonication to extract ROS from PM samples. The fluorescence intensity is then measured and converted to hydrogen peroxide (H2O2) concentration using a calibration curve.44–46Alternative particle-bound ROS detection techniques vary in reagents, concentrations, mixing procedures, and fluorescence measurement approaches. Commonly used reagents include 9-(1,1,3,3,tetramethylisoindolin-2-yloxyl-5-ethynyl)-10-(phenylethynyl)anthracene (BPEAnit), aminophenyl fluorescamine (APF), and 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red). APF is selective for hydroxyl radicals, while Amplex Red is primarily used for detecting H2O2.47–49 However, fluorescence techniques can be limited by cross-reactivity with multiple ROS types, which can reduce specificity.49 Additionally, the efficacy of these methods is influenced by experimental factors, such as pH, reagent concentration, and extraction methods.50
Thus, while fluorescence techniques provide a sensitive approach for ROS detection, their reliability depends on the careful selection of probes and optimized experimental conditions.
2.2. Spectrometric methods
Spectrometric methods measure ROS by detecting changes in light absorption or emission, providing precise quantitative data.51 These techniques include chemiluminescence, UV-visible spectrophotometry, and electron paramagnetic resonance (EPR) spectroscopy.40,41While spectrometric methods are precise and valuable for quantitative data, they are sensitive to factors like the choice of extraction solvent, incubation time, and presence of metal ions, which can complicate cross-study comparisons.55–57
2.3. Selecting an appropriate ROS detection method
Selecting an appropriate ROS detection method depends on study-specific factors, including the required sensitivity, specificity, equipment availability, and the intended level of ROS identification.11,58,59 Among spectrometric methods, the DTT assay stands out for its reproducibility and applicability in PM research. While EPR spectroscopy offers high specificity for ROS analysis, its complexity and resource-intensive nature limit its routine use in OP assessments. Fluorescence-based techniques, although highly sensitive, primarily serve studies requiring rapid ROS detection rather than comprehensive OP analysis. Given these considerations, this review primarily focuses on the DTT assay as a robust and widely applied method for evaluating the OP of PM.3. Key methodological considerations of the DTT assay in ROS detection
The DTT assay is widely used to measure the OP of PM by assessing its ability to deplete DTT, a thiol-based reducing agent. However, the reliability of this assay is influenced by several internal and external factors, including initial DTT concentration, incubation conditions, light exposure, and metal–organic interactions. Addressing these factors is crucial for improving assay consistency and cross-study comparability.3.1. Internal assay variables and their influence on ROS detection
A clear understanding of how DTT functions as a reducing agent is essential for optimizing its use in OP measurements. Key factors such as initial DTT concentration, incubation conditions, and assay standardization significantly affect the reproducibility and accuracy of results.Fig. 2 illustrates the two-step process involved in the DTT assay, a method for assessing the OP of PM. In Step A, DTT functions as a reducing agent, mimicking the production of ROS by transferring electrons from DTT to oxygen, which leads to the formation of superoxide anions and hydrogen peroxide. This reaction is driven by redox-active compounds in PM, such as quinones, and can generate additional ·OH in the presence of transition metals like Fe and Cu through Fenton-like reactions. During this process, DTT is converted to its disulfide form (DTT-disulfide).10,13,14,60
 | ||
| Fig. 2 (A) Reduction of O2 by DTT, leading to the formation of ROS, with PM acting as a catalyst. (B) Reaction of DTT with DTNB, resulting in the formation of the colored product 2-nitro-5-thiobenzoic acid (TNB), which is measured spectrophotometrically. Adapted from Ayres et al., 2008;62 Rattanavaraha et al., 2011;27 Visentin., 2016;63 and Thomson 2022.60 | ||
In Step B, any remaining DTT that hasn't reacted yet quickly binds with DTNB, producing DTT-disulfide and a yellow compound called TNB. TNB has a strong molar extinction coefficient of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 M−1 cm−1 at 412 nm, which makes it measurable by UV-visible spectrophotometry to determine the rate at which DTT is consumed—indicating the oxidative potential of the PM.10,60 The DTT consumption rate (σDTT), is calculated from the linear slope of DTT depletion and serves as an OP indicator. Essentially, the rate of ROS production by a PM sample is inferred from the DTT consumption rate, which is directly proportional to the concentration of redox-active compounds in the sample.8,11,13,64Eqn (1) expresses the rate of DTT consumption in nmol min−1.
150 M−1 cm−1 at 412 nm, which makes it measurable by UV-visible spectrophotometry to determine the rate at which DTT is consumed—indicating the oxidative potential of the PM.10,60 The DTT consumption rate (σDTT), is calculated from the linear slope of DTT depletion and serves as an OP indicator. Essentially, the rate of ROS production by a PM sample is inferred from the DTT consumption rate, which is directly proportional to the concentration of redox-active compounds in the sample.8,11,13,64Eqn (1) expresses the rate of DTT consumption in nmol min−1.
 | (1) |
In the literature, DTT oxidative potential (OPDTT) is typically reported using two standard units. The first, DTTv (nmol DTT min−1 m−3), measures the rate of DTT consumption per minute per unit volume of sampled air, making it particularly relevant for evaluating human exposure. The second, DTTm (nmol DTT min−1 μg−1), normalizes the DTT consumption rate by the mass of particulate matter in the reaction, providing insight into the particulate matter's intrinsic oxidative potential, as shown in eqn (2) and (3).65–67
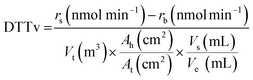 | (2) |
 | (3) |
The DTT assay is widely applied in aerosol studies, including both primary and secondary organic aerosols (SOAs).6,11,12,26 However, non-catalytic pathways also contribute to total OP measurements, requiring careful interpretation.11,26,36
Setting optimal incubation conditions for DTT assays also presents challenges, as studies vary widely in their approach. Extracted PM samples are usually incubated with buffer solutions (pH 7–7.4) and DTT at 37 °C for a set period, after which the reaction is halted by adding TCA. While some studies use a fixed incubation time (30–60 minutes) before measuring absorbance to quantify DTT consumption,10 others continuously monitor DTT depletion at multiple points to calculate the slope and intercept, giving a more accurate measurement of the DTT depletion rate.11,34,71–73 Evidence suggests that DTT consumption may not remain linear throughout the incubation, and using a fitted slope can provide a more reliable metric for OP.12,13 However, some samples reach a plateau or show reduced DTT consumption over time, likely due to the depletion of catalytically active species, interference from DTT-inhibiting compounds, or secondary reactions.8
To ensure accuracy, it's essential to measure DTT consumption within the linear range, which depends on the sample's composition and incubation conditions.11 Charrier and Anastasio recommend limiting incubation times to this range to avoid inaccuracies, especially when non-catalytic DTT-reactive species are present. Keeping DTT loss within 20% of its initial concentration helps maintain linearity, and assays are often conducted under pseudo-first-order conditions, where less than 50% of DTT is consumed during the reaction.14 Proper control of initial DTT concentration and incubation conditions is crucial to avoid bias. A summary of methods used in past studies is presented in Table 2. The variability in OP values across studies highlights the need for standardized protocols to improve the consistency and comparability of results11 (see Section 3.1.3).
| [DTT]0 (μM) | Method | Withdrawal (mL) | Incubation time (min) | Reaction time (min) | Detection instrument | Reference | Primary findings/effectiveness |
|---|---|---|---|---|---|---|---|
| a [DTT]0 is the initial DTT concentration in the reaction tube in μM. | |||||||
| 100 | 37 °C, incubator | 0.5 | 15–90 | Variable (unspecified) | UV-vis spectrophotometer | (Cho et al., 2005)8 | Requires linear rate of DTT consumption; % CV <15% for accuracy |
| 20 | 37 °C, incubator | — | 30 | Fixed with incubation period | UV-vis spectrophotometer | (Li et al., 2009)10 | Fast DTT-DTNB reaction; no quenching needed; simplifies the procedure |
| 100 | 37 °C, dry bath | 0.5 | Variable (unspecified) | Variable (unspecified) | UV-vis spectrophotometer | (Charrier and Anastasio, 2012)14 | Followed the DTT assay method of Cho et al. (2005); improved stability with Chelex 100 resin; DTT loss <20%; EDTA is not recommended in DTT assay |
| 100 | 37 °C, incubator, shaken continuously | 0.1 | 0, 4, 13, 23, 32, 41 | Fixed with incubation period | LWCC + UV-vis spectrometer | (V. Verma et al., 2014)74 | Followed the DTT assay method of Cho et al. (2005); automated system; consistent results; analyses one sample per hour |
| 100 | 37 °C, incubator, shaken continuously | 0.1 | 4, 13, 23, 30, 41 | Fixed with incubation period | LWCC + UV-vis spectrometer | (T. Fang et al., 2015)13 | Semi-automated system; good agreement with manual methods; seasonal variability |
| 20 | 37 °C, dry bath | 0.1 | 90 | Fixed with incubation period | UV-vis spectrophotometer | (Y. Ma et al., 2018)75 | Followed the DTT assay method of Li et al. (2009) and Lin and Yu (2011); DTT activity linearly proportional to HULIS WS mass concentration |
| 100 | 37 °C, incubator | 0.1 | 3, 15, 27, 39, 51 | Fixed with incubation period | LWCC + UV-vis spectrometer | (Yu et al., 2018)72 | Measured DTT consumption and OH generation; interactions of metals with DTT |
| 100 | 37 °C, incubator | 0.5 | 0, 10, 20, 30 | Fixed with incubation period | UV-vis spectrometer | (J. Wang et al., 2019)36 | DTT activity varies diurnally and seasonally; important for exposure level studies |
| 100 | 37 °C, water bath | 0.2 | 0, 5, 10, 20, 30, 45, 60 | Fixed with incubation period | Microplate reader (visible light) | (Lu et al., 2019)67 | DTT oxidation results correlated with ROS levels, seasonal variations observed |
| 100 | 37 °C, incubator | 0.2 | 0–30 (every 10 min) | Fixed with incubation period | UV-vis spectrometer | (Lin and Yu, 2019)12 | Metal concentration affects DTT consumption rates differently; emphasized importance of standardized DTT assay methods for accurate comparisons |
| 100 | 37 °C, incubator | 0.1 | Variable | Fixed with incubation period | UV-vis spectrophotometer | (H. Jiang et al., 2020)26 | DTT consumption rate increases with initial concentrations; second-order reaction with non-catalytic species |
| 100 | 37 °C, incubator | 0.1 | 0, 4, 13, 23, 31, 41 | Fixed with incubation period | Online spectrometer | (D. Gao et al., 2020)2 | DTT assay measures water-soluble and total oxidative potential; seasonal effects |
| 100 | 37 °C, water bath | 0.1 | 0, 4, 13, 23, 32, 41 | Fixed with incubation period | UV-vis spectrophotometer | (Y. Wang, et al., 2020)76 | Seasonal DTTv levels highest in winter; long-range transport impacts oxidation |
| 100 | 37 °C, water bath | 1 | 0, 15, 30, 45, 60 | Fixed with incubation period | Multifunctional microplate reader | (Ma et al., 2021)77 | Seasonal variations in oxidative potential; transition metals impact winter months |
| 1000 | 37 °C, incubator | 1 | 0, 10, 20, 30, 40 | Fixed with incubation period | LWCC | (Wu et al., 2022)64 | Improved online ROS analyzer; optimized system reduced DTT consumption rate by 44%; daytime ROS higher |
| Factors | Impact on ROS detection | Recommended control |
|---|---|---|
| Light exposure | Enhances ROS generation through photo-oxidation, potentially leading to elevated DTT depletion rates6 | Store DTT and DTNB solutions in amber glass and perform assays under controlled lighting conditions |
| Incubation temperature | Higher temperatures (e.g., 37 °C) improve consistency in ROS detection10 | Maintain the incubation temperature at 37 °C to align with physiologically relevant conditions |
| pH levels | ROS generation is pH-sensitive, with phosphate buffers at pH 7.4 offering optimized reaction conditions14 | Use standardized buffer solutions (pH 7.4 for phosphate, pH 8.9 for tris) across studies |
| Incubation time | Prolonged incubation may result in non-linear DTT consumption, affecting OP values26 | Limit the incubation time to 30–60 minutes, ensuring DTT consumption remains within a linear range |
| Mixing techniques | Ultrasonic water77/dry14 baths or shaking incubators8 can introduce variability in assay results | Standardize mixing techniques to avoid deviations in DTT consumption and assay outcomes |
| Chelating agents | Chelating agents like EDTA may suppress DTT activity by binding metal ions essential for the reaction, leading to an underestimation of ROS production14 | As an alternative, Chelex resin can be used to remove trace metals without inhibiting DTT reactivity12,76 |
| Standardized calibration | To ensure comparability across studies, it is critical to maintain a consistent initial DTT concentration, initial PM concentration, and reaction linearity | Maintain an initial DTT concentration of 0.1 mM,8 limit PM concentrations to 5–20 μg mL−1,11 and ensure reaction linearity by capping DTT consumption at less than 90% |
While previous reviews, including that of Jiang et al. (2020), have highlighted the need for standardization in the DTT assay, this study builds upon their framework by incorporating specific methodological refinements. For instance, controlling light exposure to prevent photo-oxidation is critical, and using amber glassware for DTT and DTNB storage can mitigate this risk. Additionally, maintaining an incubation temperature of 37 °C ensures consistency in DTT consumption, aligning with physiologically relevant conditions.
A key contribution of this study is the introduction of positive controls, such as ferro-ammonium oxalate (FAO)11 and quinones like 1,4-naphthoquinone,60 to enhance assay validation. These controls mimic the redox cycling of ROS under ambient conditions, making them particularly suitable for air quality studies. The inclusion of quinones, which naturally occur in atmospheric PM, strengthens the environmental relevance of the assay and adds a novel dimension to its standardization.
Incorporating these standardization practices, alongside the use of tailored positive controls, will significantly enhance the reproducibility and utility of the DTT assay in environmental and health-related research. These improvements will lead to a more reliable understanding of the oxidative potential of particulate matter and its implications for human health and atmospheric processes.
Additionally, PM components such as transition metals and organic compounds can enhance or inhibit ROS generation through catalytic or non-catalytic pathways. Transition metals, for instance, can catalyze reactions that lead to higher ROS levels, thus impacting the measured OP. Organic particulates, especially highly oxidized compounds, may interact with DTT and complicate result interpretation. The following section examines external factors on ROS measurements, including light exposure and chemical influences.
3.2. Influence of external factors on ROS generation and detection
3.2.1.1. Mechanism of ROS generation through photo-oxidation. Airborne PM can undergo photo-oxidation reactions, which play a major role in ROS generation.6,82,83 These reactions involve interactions between organic compounds,6 transition metals,83 and molecular oxygen (O2) with ultraviolet (UV) light exposure acting as a primary driver.84 UV radiation triggers complex photochemical reactions in organic aerosols (OAs), where organic molecules absorb light, causing their electrons to reach higher energy states. In these excited states, the organic compounds can react with O2, leading to the formation of ROS like singlet oxygen (1O2), O2−, and H2O2, as highlighted by Jiang and Jang.6
Additionally, photo-oxidation of specific aromatic compounds (ACs) including catechol (CAT), phthalic acid (PA), and 4,4′-oxydibenzoic acid (4,4′-OBA) has been shown to significantly enhance ROS production, as demonstrated in a study by Hu et al., 2023. Their research found that longer light exposure increases ROS concentration, often following zero-order reaction kinetics for certain ACs.82 This light-induced ROS generation substantially contributes to the oxidative capacity of atmospheric PM, influencing both environmental processes and human health.
Research by Jiang and Jang also highlights the impact of photo-oxidation of iron species in PM on ROS generation. Sunlight drives the photochemical reduction of Fe(III) to Fe(II), which readily participates in Fenton reactions, generating highly reactive ·OH when combined with H2O2. This transformation is more active during daylight hours due to sunlight exposure, leading to higher daytime ROS production compared to nighttime.83
3.2.1.2. Role of light exposure in ROS detection. The detection of ROS, particularly through the DTT assay, is significantly impacted by light exposure.6,85 The DTT assay is commonly used to measure the oxidative potential of PM by assessing the rate of DTT consumption, which indicates ROS generation.11,13,14,64 Research by Steven Thomson (2022) has shown that the photodegradation of chemicals involved in the DTT assay leads to higher DTT depletion rates. Samples shielded from light displayed a much lower DTT depletion rate (0.385 ± 0.014 nmol DTT min−1) compared to samples exposed to light (0.714 ± 0.34 nmol DTT min−1). To address this issue, researchers recommend using amber glass bottles and flasks for storing and preparing DTT and DTNB solutions.60
Under photo-oxidative conditions, the generation of ROS from aromatic compounds (ACs) also increases. Hu et al. found that ROS concentration from catechol (CAT) and phthalic acid (PA) gradually rises with extended light exposure, whereas 4,4′-oxydibenzoic acid (4,4′-OBA) maintains a more stable ROS concentration after an initial spike.82 This suggests that the photoreaction products formed during light exposure are more effective in inducing ROS than the original compounds, emphasizing the need to factor in light exposure duration in ROS detection methods.
Additionally, light exposure influences the OP of OAs, particularly during their aging process.6,85 Research by Jiang and Jang on freshly emitted wood smoke particles demonstrates a linear rise in DTT consumption over time, indicating that catalytic processes primarily drive the oxidative potential. However, prolonged sunlight exposure reduces the oxidative potential as particulate oxidizers, like quinones and organic hydroperoxides (OHP), begin to degrade, causing a shift from catalytic to noncatalytic ROS generation processes. This effect is particularly observed in SOAs formed via photo-oxidation of hydrocarbons, where DTT consumption varies based on particle aging.6
Further laboratory studies, including those by Hu et al. (2023), have shown that UV irradiation can raise soluble Fe(II) levels in PM, leading to increased ROS concentrations. This underscores the critical role of light exposure in interpreting ROS detection outcomes, as oxidative processes under UV light can produce marked differences in ROS levels.82 Recognizing the dynamics of light exposure is thus essential for accurately interpreting ROS data and ensuring that experimental conditions align with realistic atmospheric scenarios.
3.2.1.3. Effect of light on transition metals and organic compound interactions. The interaction between transition metals (TMs) and organic compounds under light exposure plays a pivotal role in ROS generation and detection in atmospheric aerosols.83,86 Transition metals such as iron (Fe) and copper (Cu) are key in catalyzing ROS formation via Fenton-like reactions.1 UV radiation enhances the redox cycling of these metals, promoting their interactions with organic compounds, which leads to the production of reactive ·OH from H2O2.82,87,88 For instance, research by Xiaoyu Hu has shown that catechol (CAT) combined with Fe(II) and Cu(II) under light exposure has a synergistic effect that substantially boosts ROS generation. However, the interactions between aromatic compounds (ACs) and transition metals can also show antagonistic effects. For example, Mn(II) initially enhances ROS production but later stages show a decline, highlighting the complexity of these interactions.82
Additionally, the photo-oxidation of organic compounds releases reactive intermediates that further interact with transition metals, forming a network of reactions that impact the oxidative potential of PM. Organic ligands within PM can stabilize metal ions in their reduced states, aiding in the production of soluble Fe(II) under UV light.1,6,27,83,89 Conversely, some organic compounds may scavenge ROS, potentially lowering sensitivity in assays like the DTT assay.28 Chelating agents, such as diethylenetriaminepentaacetic acid (DTPA), influence these interactions, adding complexity to ROS detection.6
3.2.2.1. Metal ions as catalysts and interferences in ROS detection. Metal ions are key catalysts in generating ROS, yet they can also interfere with various ROS detection methods28. Transition metals, particularly Fe and Cu, play a major role in ROS generation due to their redox cycling ability.90 The Fenton reaction, in which H2O2 reacts with ferrous (Fe2+) to produce highly reactive ·OH, is a key pathway for ROS formation in both environmental and biological contexts.1,72 Other metals like manganese (Mn) and vanadium(V) also contribute significantly to ROS generation.72,83
The solubility of metal ions further influences their catalytic activity, as only soluble metals participate effectively in redox reactions, making metal solubility in PM a critical factor in evaluating the oxidative potential.14 Environmental factors, such as pH, affect metal solubility, with acidic conditions increasing metal mobility and reactivity, thereby enhancing ROS production.14,58 For instance, adding metal chelators to PM can significantly reduce ROS generation, underscoring the vital role of soluble metal ions in the oxidative potential of PM across various assays.
While early research suggested that DTT oxidation was less sensitive to metals, recent findings reveal that transition metals—including Cu, Mn, and zinc (Zn)—significantly influence DTT depletion in ambient PM2.5.91 Zn though not redox-active, binds strongly to DTT, consuming it without undergoing redox cycling.91 Numerous studies show correlations between DTT depletion and concentrations of Fe, Cu, Mn, and Zn, highlighting the central role these metals play in oxidative potential assays.9,24 Consequently, metals, rather than organic species like quinones, often serve as the primary contributors to DTT oxidation in PM samples.
However, metal ions can also interfere with ROS measurement assays, such as the DTT assay. Metal contamination in reagents and samples may increase DTT depletion rates, leading to an overestimation of the oxidative potential. To reduce these interferences, chelation strategies, such as using EDTA or Chelex 100 resin, can remove trace metals from solutions.14 It is essential to note, however, that while EDTA can reduce background DTT loss, it may also suppress responses from both metals and organic species, complicating the assessment of their contributions to ROS generation.14
3.2.2.2. Influence of organic species on ROS generation. The organic content of PM significantly influences ROS detection. Organic carbon (OC) and WSOC have been widely studied, with numerous global studies demonstrating a strong correlation between these components and OPDTT across different seasons.7,8,92 In the Los Angeles basin, 88% of the variability in volume-normalized OPDTT for quasi-ultrafine ambient PM can be attributed to WSOC, water-insoluble organic carbon (WIOC), elemental carbon, and hopanes. Similarly, OAs contribute to 60% of the OPDTT of water-soluble PM2.5 in the southeastern United States, where the hydrophobic fractions of both water-soluble and water-insoluble organic aerosols significantly contribute to mass-normalized OPDTT.7,56,58,93
PAHs, such as phenanthrene and pyrene, are major contributors to OPDTT. Upon oxidation, these PAHs form DTT-active quinones, including phenanthraquinone (PQN), 1,2-naphthoquinone (1,2-NQ), and 1,4-naphthoquinone (1,4-NQ), with PQN being the most reactive, and participate in redox reactions that generate ROS, including O2− and H2O2.8,14,20,21,61 These quinones can bind to soot particles, and black carbon coated with 1,4-NQ has been shown to significantly increase mass-normalized OPDTT compared to untreated black carbon21,94,95 Methanol extracts of PM often exhibit higher OPDTT than water-soluble PM extracts, as methanol—a less polar solvent—extracts both hydrophilic and hydrophobic organic species. Nevertheless, water-soluble organic PM components remain essential contributors to OPDTT, likely due to their greater biological availability.7,27,55,56,58
Interactions among organic species further influence OPDTT. Nitrogen-containing bases, such as pyridine and imidazole (commonly found in HULIS), enhance OPDTT in the presence of quinones by facilitating hydrogen atom transfer during ROS generation.96,97 High molecular weight organic compounds with multiple reactive functional groups are also abundant in ambient PM and may impact DTT consumption, although their exact effects remain incompletely understood.39,98 These observations underscore the importance of understanding the complex interactions between organic species and ROS detection probes to accurately assess the OP of PM.
Additionally, the chemical aging of organic species in PM enhances their redox activity. Photochemical aging, especially in urban environments where sunlight and atmospheric conditions promote SOA formation, converts less reactive organic compounds into more reactive forms like quinones.58 Aged aerosols exhibit higher oxidative potential than fresh OA, highlighting the critical role of aging processes in determining the oxidative capacity of PM.6,99 Certain organic compounds can undergo photochemical reactions in the presence of sunlight, leading to the generation of ROS such as H2O2 and organic peroxides.6,82,83,100 Compounds like humic and fulvic acids can also interact with metal ions, affecting metal reactivity.6 The presence of these organic species enhances ROS generation through various mechanisms, including the formation of metal–organic complexes.11
3.2.2.3. Effect of metal–organic complexes on ROS generation in PM. Metal–organic complexes in PM are essential in modulating ROS generation, either by enhancing or inhibiting ROS production through several mechanisms. These complexes can improve the solubility and reactivity of metal ions, making them more available for redox reactions that generate ROS.1,18 For example, Wei et al. demonstrated that organic ligands stabilize metals in their more reactive reduced forms, like Fe(II), which participate in ROS production via Fenton-like reactions. In ambient PM, 70–90% of water-soluble Fe and Cu is often complexed with organic compounds, illustrating the considerable role these complexes play in driving ROS generation. Additionally, the interaction of Fe with organic species such as Suwannee River fulvic acid (SRFA) enhances the formation of ·OH, emphasizing the synergistic effects of metal–organic interactions on ROS production.1
Research also indicates that interactions between soluble metals—such as Fe, Cu, and Mn—and quinones can result in both synergistic and antagonistic effects in OP assays. For instance, Fe and Cu enhance the oxidative effects of quinones in assays measuring OPDTT in surrogate lung fluid (SLF).58,61,101,102 However, the impact of these interactions can vary depending on the specific metal–organic combination. Soluble Mn, for example, shows a synergistic effect with quinones in OPDTT but an antagonistic effect on ·OH generation.72 Similarly, Fe displays additive and synergistic effects with quinones in both DTT consumption and hydroxyl radical production, whereas Cu exhibits antagonistic effects in both processes.72
Furthermore, WSOC enhances the solubility of metals such as Fe through complexation, increasing the oxidative potential of PM, particularly in the presence of Fe.18 Understanding these interactions is crucial for developing mitigation strategies that consider both total metal and organic concentrations as well as conditions that promote their interactions. Reducing such interactions could significantly lower the oxidative potential of PM in polluted areas, positively impacting public health.
Additionally, DTT itself can form stable complexes—both polymeric and monomeric—with metals such as Zn(II), Cd(II), Pb(II), Ni(II), and Cu(I), adding complexity to DTT consumption in PM samples.103 These interactions underscore the intricate role of metal–organic complexes in ROS modulation and highlight the need for further research to better understand these synergistic and antagonistic effects across different OP assays.
3.2.3.4. Practical implications and mitigation strategies. Understanding the role of metal–organic complexes in ROS generation has important implications for policy and pollution control. Identifying key contributors to ROS formation, such as metal–organic complexes, could help shape targeted strategies for reducing pollution. For example, emissions from sources that release both OA and transition metals—such as industrial operations and vehicle exhaust—could be managed more effectively to lower the oxidative potential of PM, thereby reducing related health risks.58 Incorporating this understanding into air quality regulations could lead to standards that address not only individual pollutants but also the interactions that amplify ROS production and oxidative stress in exposed populations.
4. Conclusion and future directions
The DTT assay, a widely used method for assessing the OP of PM, faces challenges in standardization and is influenced by external variables such as light exposure and metal–organic interactions. To advance ROS detection in environmental and health research, focused efforts are needed in several key areas:• Standardization of protocols: establishing standardized procedures for DTT assays, including consistent initial DTT concentration, PM mass concentration, incubation time, and avoiding the use of EDTA—will reduce variability in ROS measurements. Standardization would enable accurate comparisons across studies, enhancing the consistency of OP data and making the DTT assay more adaptable to real-world environmental monitoring.
• Optimization of detection methods: future research should prioritize refining and enhancing the specificity of both fluorescence-based and spectrometric ROS detection methods. Improving probe selectivity for distinct ROS types (e.g., ·OH, H2O2) will increase measurement precision across varied environmental conditions and pollution profiles.
•ROS generation through photo-oxidation: light exposure, particularly under UV and sunlight, significantly drives ROS generation via photo-oxidative processes. Future studies could examine the effects of photo-oxidative mechanisms on ROS production in PM under different atmospheric conditions, such as high-oxygen versus anaerobic environments, to mirror real-world conditions. Standardizing procedures for managing light exposure during ROS measurements will improve data accuracy and deepen our understanding of how sunlight impacts the oxidative potential in different geographies and seasons.
• Impact of metal–organic interactions: the interaction of metal ions with organic compounds in PM is central to understanding ROS formation. Investigating these interactions under different emission scenarios (e.g., areas with high metal emissions or organic pollutants) could provide insights into how specific sources contribute to oxidative stress in the environment.
This review underscores the importance of refining DTT assays to enhance the consistency and reliability of oxidative potential measurements. By integrating these standardized approaches into public health frameworks, researchers can better assess PM toxicity and mitigate associated risks.
5. Policy and public health implications
The insights derived from this review have significant implications for both public health and air quality management. Improved ROS detection methods offer a pathway toward more precise air quality standards that reflect the OP of PM, shifting the regulatory focus from total particle mass to oxidative activity—a parameter more directly associated with health risks.• Targeted regulatory frameworks: the influence of light exposure and metal–organic interactions on ROS generation suggests the need for region-specific regulations, particularly in areas with high levels of industrial emissions or intense sunlight. Regulations could prioritize control measures for key ROS-generating components, especially transition metals and organic compounds known to drive ROS production in sunlight.
• Enhanced risk assessments: by integrating oxidative potential into public health risk assessment frameworks, regulatory agencies can better understand and address the nuanced health risks associated with PM exposure. This is especially relevant for vulnerable populations such as children, the elderly, and individuals with pre-existing health conditions, who are more susceptible to ROS-induced oxidative stress.
• Research-driven policy development: expanding our understanding of ROS sources and measurement techniques will enable more data-informed policy decisions. For instance, enforcing stricter emission standards in high-risk areas and promoting cleaner technologies could reduce public health burdens linked to oxidative stress and respiratory diseases.
Incorporating oxidative potential into air quality policies, alongside traditional pollutant metrics, would enable more effective public health strategies and regulatory frameworks that address the complex chemistry behind PM toxicity. This integration represents a progressive step toward policies that mitigate the multifaceted impacts of air pollution on public health.
Data availability
No new data were generated or analyzed in this study. The review synthesizes information from previously published studies, as cited in the text.Author contributions
The contribution of the authors is summarized as follows: conceptualization, C. Y. and V. W. P. D.; resources, Y. Z. and F. C.; data curation, C. Y.; writing—original draft preparation, C. Y. and V. W. P. D.; supervision, C. Y., F. C., S. D., and Y. Z.; writing—review, V. W. P. D.; editing, G. B., T. L., F. D., and Z. L.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors wish to express their sincere gratitude to Nanjing University of Information Science & Technology (NUIST), Nanjing, China for providing the facilities and support necessary for the completion of this work. This work was supported by the National Natural Science Foundation of China (No. 42273087).References
- J. Wei, et al., Complexation of Iron and Copper in Ambient Particulate Matter and Its Effect on the Oxidative Potential Measured in a Surrogate Lung Fluid, Environ. Sci. Technol., 2019, 53(3), 1661–1671, DOI:10.1021/acs.est.8b05731.
- D. Gao, et al., Ambient particulate matter oxidative potential: chemical determinants, associated health effects, and strategies for risk management, Free Radicals Biol. Med., 2020, 151, 7–25, DOI:10.1016/j.freeradbiomed.2020.04.028.
- W. Yang and S. T. Omaye, Air pollutants, oxidative stress and human health, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2009, 674(1–2), 45–54, DOI:10.1016/j.mrgentox.2008.10.005.
- D.-R. Mitrea, et al., Oxidative Stress Produced by Urban Atmospheric Nanoparticles, in Nanomaterials – Toxicity, Human Health and Environment, ed. S. Clichici, A. Filip and G. M. Do Nascimento, IntechOpen, 2020, https://www.intechopen.com/books/nanomaterials-toxicity-human-health-and-environment/oxidative-stress-produced-by-urban-atmospheric-nanoparticles, accessed: 21 September 2024 Search PubMed.
- R. J. Delfino, et al., Air pollution and circulating biomarkers of oxidative stress, Air Qual., Atmos. Health, 2011, 4(1), 37–52, DOI:10.1007/s11869-010-0095-2.
- H. Jiang and M. Jang, Dynamic Oxidative Potential of Atmospheric Organic Aerosol under Ambient Sunlight, Environ. Sci. Technol., 2018, 52(13), 7496–7504, DOI:10.1021/acs.est.8b00148.
- V. Verma, et al., Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5, Environ. Sci. Technol., 2015, 49(7), 4646–4656, DOI:10.1021/es505577w.
- A. K. Cho, et al., Redox activity of airborne particulate matter at different sites in the Los Angeles Basin, Environ. Res., 2005, 99(1), 40–47, DOI:10.1016/j.envres.2005.01.003.
- L. Ntziachristos, et al., Relationship between redox activity and chemical speciation of size-fractionated particulate matter, Part. Fibre Toxicol., 2007, 4(1), 5, DOI:10.1186/1743-8977-4-5.
- Q. Li, et al., Oxidant generation and toxicity enhancement of aged-diesel exhaust, Atmos. Environ., 2009, 43(5), 1037–1042, DOI:10.1016/j.atmosenv.2008.11.018.
- H. Jiang, et al., Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols, Atmosphere, 2019, 10(10), 571, DOI:10.3390/atmos10100571.
- M. Lin and J. Z. Yu, Dithiothreitol (DTT) concentration effect and its implications on the applicability of DTT assay to evaluate the oxidative potential of atmospheric aerosol samples, Environ. Pollut., 2019, 251, 938–944, DOI:10.1016/j.envpol.2019.05.074.
- T. Fang, et al., A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE), Atmos. Meas. Tech., 2015, 8(1), 471–482, DOI:10.5194/amt-8-471-2015.
- J. G. Charrier and C. Anastasio, On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals, Atmos. Chem. Phys., 2012, 12(19), 9321–9333, DOI:10.5194/acp-12-9321-2012.
- Y. Fujitani, et al., Comparison of Oxidative Abilities of PM2.5 Collected at Traffic and Residential Sites in Japan. Contribution of Transition Metals and Primary and Secondary Aerosols, Aerosol Air Qual. Res., 2017, 17(2), 574–587, DOI:10.4209/aaqr.2016.07.0291.
- Y. Lyu, et al., Particle Size Distributions of Oxidative Potential of Lung-Deposited Particles: Assessing Contributions from Quinones and Water-Soluble Metals, Environ. Sci. Technol., 2018, 52(11), 6592–6600, DOI:10.1021/acs.est.7b06686.
- J. Yalamanchili, et al., Precipitation of aqueous transition metals in particulate matter during the dithiothreitol (DTT) oxidative potential assay, Environ. Sci.:Processes Impacts, 2022, 24(5), 762–772, 10.1039/d2em00005a.
- Y. Wang, et al., On the Relative Contribution of Iron and Organic Compounds, and Their Interaction in Cellular Oxidative Potential of Ambient PM2.5, Environ. Sci. Technol. Lett., 2022, 9(8), 680–686, DOI:10.1021/acs.estlett.2c00316.
- W. Yuan, et al., Measurement Report: Oxidation Potential of Water-Soluble Aerosol Components in the Southern and Northern of Beijing, 2024, available https://egusphere.copernicus.org/preprints/2024/egusphere-2024-680/, accessed: 29 October 2024 Search PubMed.
- M. Y. Chung, et al., Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts, Environ. Sci. Technol., 2006, 40(16), 4880–4886, DOI:10.1021/es0515957.
- R. D. McWhinney, et al., Filterable Redox Cycling Activity: A Comparison between Diesel Exhaust Particles and Secondary Organic Aerosol Constituents, Environ. Sci. Technol., 2013, 47(7), 3362–3369, DOI:10.1021/es304676x.
- J. Dou, et al., Reactive Oxygen Species Production Mediated by Humic-like Substances in Atmospheric Aerosols: Enhancement Effects by Pyridine, Imidazole, and Their Derivatives, Environ. Sci. Technol., 2015, 49(11), 6457–6465, DOI:10.1021/es5059378.
- V. Verma, et al., Fractionating ambient humic-like substances (HULIS) for their reactive oxygen species activity – assessing the importance of quinones and atmospheric aging, Atmos. Environ., 2015, 120, 351–359, DOI:10.1016/j.atmosenv.2015.09.010.
- S. Hu, et al., Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles-Long Beach harbor, Atmos. Chem. Phys., 2008, 8(21), 6439–6451, DOI:10.5194/acp-8-6439-2008.
- V. Verma, et al., Physicochemical and Toxicological Profiles of Particulate Matter in Los Angeles during the October 2007 Southern California Wildfires, Environ. Sci. Technol., 2009, 43(3), 954–960, DOI:10.1021/es8021667.
- H. Jiang, et al., Role of functional groups in reaction kinetics of dithiothreitol with secondary organic aerosols, Environ. Pollut., 2020, 263, 114402, DOI:10.1016/j.envpol.2020.114402.
- W. Rattanavaraha, et al., The reactive oxidant potential of different types of aged atmospheric particles: An outdoor chamber study, Atmos. Environ., 2011, 45(23), 3848–3855, DOI:10.1016/j.atmosenv.2011.04.002.
- M. Lin and J. Z. Yu, Effect of metal-organic interactions on the oxidative potential of mixtures of atmospheric humic-like substances and copper/manganese as investigated by the dithiothreitol assay, Sci. Total Environ., 2019, 697, 134012 CrossRef CAS PubMed , available https://pubmed.ncbi.nlm.nih.gov/31476503/.
- A. Saffari, et al., Global Perspective on the Oxidative Potential of Airborne Particulate Matter: A Synthesis of Research Findings, Environ. Sci. Technol., 2014, 48(13), 7576–7583, DOI:10.1021/es500937x.
- S. J. Campbell, et al., Iron and Copper Alter the Oxidative Potential of Secondary Organic Aerosol: Insights from Online Measurements and Model Development, Environ. Sci. Technol., 2023, 57(36), 13546–13558, DOI:10.1021/acs.est.3c01975.
- S. Duanghathaipornsuk, et al., Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications, Biosensors, 2021, 11(2), 30, DOI:10.3390/bios11020030.
- A. Gomes, et al., Fluorescence probes used for detection of reactive oxygen species, J. Biochem. Biophys. Methods, 2005, 65(2–3), 45–80, DOI:10.1016/j.jbbm.2005.10.003.
- D. Yu, et al., Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation, Sens. Actuators, B, 2021, 339, 129878, DOI:10.1016/j.snb.2021.129878.
- D. Gao, et al., A method for measuring total aerosol oxidative potential (OP) with the dithiothreitol (DTT) assay and comparisons between an urban and roadside site of water-soluble and total OP, Atmos. Meas. Tech., 2017, 10(8), 2821–2835, DOI:10.5194/amt-10-2821-2017.
- K. E. Berg, et al., High-throughput, semi-automated dithiothreitol (DTT) assays for oxidative potential of fine particulate matter, Atmos. Environ., 2020, 222, 117132, DOI:10.1016/j.atmosenv.2019.117132.
- J. Wang, et al., Temporal variation of oxidative potential of water-soluble components of ambient PM2.5 measured by dithiothreitol (DTT) assay, Sci. Total Environ., 2019, 649, 969–978, DOI:10.1016/j.scitotenv.2018.08.375.
- S. Suzen, et al., Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique, Molecules, 2017, 22(1), 181, DOI:10.3390/molecules22010181.
- B. Zomer, et al., Chemiluminescent reductive acridinium triggering (CRAT)—mechanism and applications, Anal. Bioanal. Chem., 2011, 401(9), 2945–2954, DOI:10.1007/s00216-011-5342-3.
- J. T. Bates, et al., Reactive Oxygen Species Generation Linked to Sources of Atmospheric Particulate Matter and Cardiorespiratory Effects, Environ. Sci. Technol., 2015, 49(22), 13605–13612, DOI:10.1021/acs.est.5b02967.
- L. Rao, et al., Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review, Processes, 2020, 8(11), 1410, DOI:10.3390/pr8111410.
- J. Herman, et al., Emerging technologies for optical spectral detection of reactive oxygen species, Anal. Bioanal. Chem., 2018, 410(24), 6079–6095, DOI:10.1007/s00216-018-1233-1.
- L. Zuo and T. L. Clanton, Detection of reactive oxygen and nitrogen species in tissues using redox-sensitive fluorescent probes, in Methods Enzymol., vol. 352, Elsevier, 2002, pp. 307–325, available https://linkinghub.elsevier.com/retrieve/pii/S0076687902520280, accessed: 22 September 2024 Search PubMed.
- H.-S. Wang, Development of fluorescent and luminescent probes for reactive oxygen species, TrAC, Trends Anal. Chem., 2016, 85, 181–202, DOI:10.1016/j.trac.2016.09.006.
- P. Venkatachari, et al., Measurement of Particle-Bound Reactive Oxygen Species in Rubidoux Aerosols, J. Atmos. Chem., 2005, 50(1), 49–58, DOI:10.1007/s10874-005-1662-z.
- P. Wardman, Use of the Dichlorofluorescein Assay to Measure “Reactive Oxygen Species”, Radiat. Res., 2008, 170(3), 406–407, DOI:10.1667/RR1439a.1.
- J. M. Antonini, et al., Freshly generated stainless steel welding fume induces greater lung inflammation in rats as compared to aged fume, Toxicol. Lett., 1998, 98(1–2), 77–86, DOI:10.1016/S0378-4274(98)00103-9.
- B. Miljevic, et al., Oxidative Potential of Logwood and Pellet Burning Particles Assessed by a Novel Profluorescent Nitroxide Probe, Environ. Sci. Technol., 2010, 44(17), 6601–6607, DOI:10.1021/es100963y.
- P. Venkatachari and P. K. Hopke, Development and Laboratory Testing of an Automated Monitor for the Measurement of Atmospheric Particle-Bound Reactive Oxygen Species (ROS), Aerosol Sci. Technol., 2008, 42(8), 629–635, DOI:10.1080/02786820802227345.
- C. A. Cohn, et al., Comparison of fluorescence-based techniques for the quantification of particle-induced hydroxyl radicals, Part. Fibre Toxicol., 2008, 5(1), 2, DOI:10.1186/1743-8977-5-2.
- W. Huang, et al., Optimization of the Measurement of Particle-Bound Reactive Oxygen Species with 2′,7′-dichlorofluorescin (DCFH), Water, Air, Soil Pollut., 2016, 227(5), 164, DOI:10.1007/s11270-016-2860-9.
- C. P. Rubio and J. J. Cerón, Spectrophotometric assays for evaluation of Reactive Oxygen Species (ROS) in serum: general concepts and applications in dogs and humans, BMC Vet. Res., 2021, 17(1), 226, DOI:10.1186/s12917-021-02924-8.
- K. E. Berg, et al., Electrochemical dithiothreitol assay for large-scale particulate matter studies, Aerosol Sci. Technol., 2019, 53(3), 268–275, DOI:10.1080/02786826.2018.1560391.
- B. Hellack, et al., Intrinsic hydroxyl radical generation measurements directly from sampled filters as a metric for the oxidative potential of ambient particulate matter, J. Aerosol Sci., 2014, 72, 47–55, DOI:10.1016/j.jaerosci.2014.02.003.
- W. He, et al., Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species, J. Food Drug Anal., 2014, 22(1), 49–63, DOI:10.1016/j.jfda.2014.01.004.
- A. Yang, et al., Measurement of the oxidative potential of PM2.5 and its constituents: The effect of extraction solvent and filter type, Atmos. Environ., 2014, 83, 35–42, DOI:10.1016/j.atmosenv.2013.10.049.
- V. Verma, et al., Contribution of Water-Soluble and Insoluble Components and Their Hydrophobic/Hydrophilic Subfractions to the Reactive Oxygen Species-Generating Potential of Fine Ambient Aerosols, Environ. Sci. Technol., 2012, 46(20), 11384–11392, DOI:10.1021/es302484r.
- K. J. Bein and A. S. Wexler, Compositional variance in extracted particulate matter using different filter extraction techniques, Atmos. Environ., 2015, 107, 24–34, DOI:10.1016/j.atmosenv.2015.02.026.
- J. T. Bates, et al., Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects, Environ. Sci. Technol., 2019, 53(8), 4003–4019, DOI:10.1021/acs.est.8b03430.
- T. Fang, et al., Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays, Atmos. Chem. Phys., 2016, 16(6), 3865–3879, DOI:10.5194/acp-16-3865-2016.
- S. Thomson, How Does the Composition of Atmospheric Aerosol Particles Affect PM2.5 Toxicity?, PhD thesis, University of Birmingham, 2022, available, http://etheses.bham.ac.uk/id/eprint/12442.
- Q. Xiong, et al., Rethinking Dithiothreitol-Based Particulate Matter Oxidative Potential: Measuring Dithiothreitol Consumption versus Reactive Oxygen Species Generation, Environ. Sci. Technol., 2017, 6507–6514, 51, DOI:10.1021/acs.est.7b01272.
- J. G. Ayres, et al., Evaluating the Toxicity of Airborne Particulate Matter and Nanoparticles by Measuring Oxidative Stress Potential—A Workshop Report and Consensus Statement, Inhalation Toxicol., 2008, 20(1), 75–99, DOI:10.1080/08958370701665517.
- M. Visentin, et al., Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays, Environ. Pollut., 2016, 72–79, DOI:10.1016/j.envpol.2016.09.047.
- J. Wu et al. , Development, characterization, and application of an improved online reactive oxygen species analyzer based on the Monitor for AeRosols and Gases in ambient Air (MARGA), 2022, available: https://amt.copernicus.org/articles/15/2623/2022/, accessed: 11 Oct. 2023 Search PubMed.
- Z. Liu, et al., Characteristics of PM2.5 mass concentrations and chemical species in urban and background areas of China: emerging results from the CARE-China network, Atmos. Chem. Phys., 2018, 18(12), 8849–8871, DOI:10.5194/acp-18-8849-2018.
- S. Yu, et al., Characteristics and oxidative potential of atmospheric PM2.5 in Beijing: Source apportionment and seasonal variation, Sci. Total Environ., 2019, 650, 277–287, DOI:10.1016/j.scitotenv.2018.09.021.
- S. Lu, et al., A characterization of HULIS-C and the oxidative potential of HULIS and HULIS-Fe(II) mixture in PM2.5 during hazy and non-hazy days in Shanghai, Atmos. Environ., 2019, 219, 117058, DOI:10.1016/j.atmosenv.2019.117058.
- A. J. Kramer, et al., Assessing the oxidative potential of isoprene-derived epoxides and secondary organic aerosol, Atmos. Environ., 2016, 130, 211–218, DOI:10.1016/j.atmosenv.2015.10.018.
- J. Y. Chen, et al., Characterization of electrophilicity and oxidative potential of atmospheric carbonyls, Environ. Sci.:Processes Impacts, 2019, 21(5), 856–866, 10.1039/C9EM00033J.
- R. D. McWhinney, et al., Naphthalene SOA: redox activity and naphthoquinone gas–particle partitioning, Atmos. Chem. Phys., 2013, 13(19), 9731–9744, DOI:10.5194/acp-13-9731-2013.
- C. Nishita-Hara, et al., Dithiothreitol-Measured Oxidative Potential of Size-Segregated Particulate Matter in Fukuoka, Japan: Effects of Asian Dust Events, GeoHealth, 2019, 3(6), 160–173, DOI:10.1029/2019GH000189.
- H. Yu, et al., Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay, Environ. Sci. Technol., 2018, 52(4), 2261–2270, DOI:10.1021/acs.est.7b04261.
- Y. Kuang, et al., Comparison of light absorption and oxidative potential of biodiesel/diesel and chemicals/diesel blends soot particles, J. Environ. Sci., 2020, 87, 184–193, DOI:10.1016/j.jes.2019.06.014.
- V. Verma, et al., Reactive oxygen species associated with water-soluble PM2.5; in the southeastern United States: spatiotemporal trends and source apportionment, Atmos. Chem. Phys., 2014, 14(23), 12915–12930, DOI:10.5194/acp-14-12915-2014.
- Y. Ma, et al., Sources and oxidative potential of water-soluble humic-like substances (HULIS_WS) in fine particulate matter (PM2.5) in Beijing, Atmos. Chem. Phys., 2018, 18(8), 5607–5617, DOI:10.5194/acp-18-5607-2018.
- Y. Wang, et al., Study on the oxidation potential of the water-soluble components of ambient PM2.5 over Xi’an, China: Pollution levels, source apportionment and transport pathways, Environ. Int., 2020, 136, 105515, DOI:10.1016/j.envint.2020.105515.
- X. Ma, et al., The Relative Contributions of Different Chemical Components to the Oxidative Potential of Ambient Fine Particles in Nanjing Area, Int. J. Environ. Res. Public Health, 2021, 18(6), 2789, DOI:10.3390/ijerph18062789.
- J. G. Charrier, et al., Hydrogen Peroxide Formation in a Surrogate Lung Fluid by Transition Metals and Quinones Present in Particulate Matter, Environ. Sci. Technol., 2014, 48(12), 7010–7017, DOI:10.1021/es501011w.
- M. Kervinen, et al., Lucigenin and coelenterazine as superoxide probes in mitochondrial and bacterial membranes, Anal. Biochem., 2004, 324(1), 45–51, DOI:10.1016/j.ab.2003.09.004.
- P. Zhang, et al., NADPH oxidase contributes to coronary endothelial dysfunction in the failing heart, Am. J. Physiol.: Heart Circ. Physiol., 2009, 296(3), H840–H846, DOI:10.1152/ajpheart.00519.2008.
- J. Tao, et al., Chemical composition of PM2.5 at an urban site of Chengdu in southwestern China, Adv. Atmos. Sci., 2013, 30(4), 1070–1084, DOI:10.1007/s00376-012-2168-7.
- X. Hu, et al., Effects of Photo-Oxidation and Transition Metals on the Formation of Reactive Oxygen Species from Aromatic Compounds Using Spectroscopic Method, 2023, available https://www.ssrn.com/abstract=4618940, accessed: 22 September 2024 Search PubMed.
- S. Y. Jiang, et al., Photo-oxidation of particle phase iron species dominates the generation of reactive oxygen species in secondary aerosol, Sci. Total Environ., 2020, 723, 137994, DOI:10.1016/j.scitotenv.2020.137994.
- N. Li, et al., The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles, Free Radicals Biol. Med., 2008, 44(9), 1689–1699, DOI:10.1016/j.freeradbiomed.2008.01.028.
- H. Jiang, et al., Oxidative potential of secondary organic aerosols produced from photooxidation of different hydrocarbons using outdoor chamber under ambient sunlight, Atmos. Environ., 2016, 131, 382–389, DOI:10.1016/j.atmosenv.2016.02.016.
- B. J. Majestic, et al., Application of synchrotron radiation for measurement of iron red-ox speciation in atmospherically processed aerosols, Atmos. Chem. Phys., 2007, 7(10), 2475–2487, DOI:10.5194/acp-7-2475-2007.
- Y. Gao, et al., Photocatalytic Degradation and Antibacterial Properties of Fe3+-Doped Alkalized Carbon Nitride, Nanomaterials, 2020, 10(9), 1751, DOI:10.3390/nano10091751.
- H. Tong, et al., Reactive Oxygen Species Formed by Secondary Organic Aerosols in Water and Surrogate Lung Fluid, Environ. Sci. Technol., 2018, acs.est.8b03695, DOI:10.1021/acs.est.8b03695.
- J. Huang, et al., Fe(II) Redox Chemistry in the Environment, Chem. Rev., 2021, 121(13), 8161–8233, DOI:10.1021/acs.chemrev.0c01286.
- P. S. J. Lakey, et al., Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract, Sci. Rep., 2016, 6(1), 32916, DOI:10.1038/srep32916.
- P. Lin and J. Z. Yu, Generation of Reactive Oxygen Species Mediated by Humic-like Substances in Atmospheric Aerosols, Environ. Sci. Technol., 2011, 45(24), 10362–10368, DOI:10.1021/es2028229.
- H. A. Jeng, Chemical composition of ambient particulate matter and redox activity, Environ. Monit. Assess., 2010, 169(1–4), 597–606, DOI:10.1007/s10661-009-1199-8.
- A. Saffari, et al., Seasonal and spatial variation in dithiothreitol (DTT) activity of quasi-ultrafine particles in the Los Angeles Basin and its association with chemical species, J. Environ. Sci. Health, Part A, 2014, 49(4), 441–451, DOI:10.1080/10934529.2014.854677.
- P. Pant, et al., The PM 10 fraction of road dust in the UK and India: Characterization, source profiles and oxidative potential, Sci. Total Environ., 2015, 530–531, 445–452, DOI:10.1016/j.scitotenv.2015.05.084.
- M. Antiñolo, et al., Connecting the oxidation of soot to its redox cycling abilities, Nat. Commun., 2015, 6(1), 6812, DOI:10.1038/ncomms7812.
- Q. Liu, et al., Oxidative Potential and Inflammatory Impacts of Source Apportioned Ambient Air Pollution in Beijing, Environ. Sci. Technol., 2014, 48(21), 12920–12929, DOI:10.1021/es5029876.
- M. H. Secrest, et al., The oxidative potential of PM2.5 exposures from indoor and outdoor sources in rural China, Sci. Total Environ., 2016, 571, 1477–1489, DOI:10.1016/j.scitotenv.2016.06.231.
- H. Shen, et al., Generation of hydrogen peroxide from San Joaquin Valley particles in a cell-free solution, Atmos. Chem. Phys., 2011, 11(2), 753–765, DOI:10.5194/acp-11-753-2011.
- B. Zhao, et al., Quantifying the effect of organic aerosol aging and intermediate-volatility emissions on regional-scale aerosol pollution in China, Sci. Rep., 2016, 6(1), 28815, DOI:10.1038/srep28815.
- M. C. Pietrogrande, et al., Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy, Atmosphere, 2019, 10(10), 626, DOI:10.3390/atmos10100626.
- Y. Li, et al., Interactive Enhancements of Ascorbic Acid and Iron in Hydroxyl Radical Generation in Quinone Redox Cycling, Environ. Sci. Technol., 2012, 46(18), 10302–10309, DOI:10.1021/es301834r.
- J. G. Charrier and C. Anastasio, Rates of Hydroxyl Radical Production from Transition Metals and Quinones in a Surrogate Lung Fluid, Environ. Sci. Technol., 2015, 49(15), 9317–9325, DOI:10.1021/acs.est.5b01606.
- A. Kreȩżel, et al., Coordination of heavy metals by dithiothreitol, a commonly used thiol group protectant, J. Inorg. Biochem., 2001, 84(1–2), 77–88, DOI:10.1016/S0162-0134(00)00212-9.
| This journal is © The Royal Society of Chemistry 2025 |

