Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced luminescence in 1D corrugated lead bromides via reduced flexibility of trivalent cations†
Chang-Chun
Fan
 *a,
Zi-Han
Fang
a,
Shu-Lin
Jiao
b,
Jing-Xue
Yu
a,
Cheng-Dong
Liu
*a,
Zi-Han
Fang
a,
Shu-Lin
Jiao
b,
Jing-Xue
Yu
a,
Cheng-Dong
Liu
 b,
Wei
Wang
b,
Wei
Wang
 *b,
Ming-Liang
Jin
b,
Bei-Dou
Liang
*b,
Ming-Liang
Jin
b,
Bei-Dou
Liang
 b and
Xiang-Bin
Han
b and
Xiang-Bin
Han
 *b
*b
aSchool of Materials Engineering, Jinling Institute of Technology, Nanjing 211169, China. E-mail: fccchem@163.com
bJiangsu Key Laboratory for Science and Applications of Molecular Ferroelectrics and School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China. E-mail: wangwei96@seu.edu.cn; hanxiangbin.edu@gmail.com
First published on 19th February 2025
Abstract
Hybrid metal halides (HMHs) have attracted considerable attention from researchers exploring broadband luminescence materials due to their low cost and excellent photophysical properties. Although lots of structures have been developed by researchers based on monovalent and bivalent cations, the importance of trivalent cations in the design of low-dimensional HMH materials with broadband luminescence has been overlooked. In our study, we obtained two new 1D corrugated structures (MPEA)PbBr5·H2O (MPEA is 4-methyl-1-piperazineethanammonium) and (PEA)2Pb2Br10·H2O (PEA is 1-piperazineethanammonium) based on trivalent cations, which can efficiently emit yellow-white light emission with CIE color coordinates of (0.35, 0.42) and (0.42, 0.47) at room temperature with a photoluminescence quantum yield of 0.6% and 10.9%, respectively. Our research underscores the advantages of utilizing low flexible trivalent cations in the development of HMHs with outstanding broadband emission performance and provides novel insight into the design of advanced solid-state luminescent materials.
Introduction
Hybrid metal halides (HMHs) with excellent photophysical properties,1,2 bandgap tunability,3 physical stimulus-response behavior,4 and low cost have shown great competitiveness in the field of optoelectronics, such as solar cells,5 light emitting diodes,6 non-volatile memories,7 lasers,8,9 photodetectors,10 and multiferroic devices,11 and attracted extensive attention from relevant researchers. After nearly a decade of development, researchers have developed a series of HMH semiconductor materials with different structural dimensionalities (quasi-3D, quasi-2D, 2D, 1D, and 0D) using 3D MAPbI3 as a template,12–18 which has greatly contributed to the development of HMH semiconductors. Particularly, in the field of solid-state luminescence, HMHs can achieve broadband emission of a single component based on the mechanism of self-trapping excitons,19,20 which makes them the strongest contenders for rare-earth-based solid-state luminescent materials and mixtures of phosphors.21–23 And, more interesting is that the organic composition, halides and structural dimensionality of HMHs can also be targeted to tune the emission color and polarization state of light.24–27It has been evidenced that the highly localized charge generated by the strong quantum and dielectric confinement effects of HMHs with low structural dimensionality results in structures with higher exciton binding energy and photoluminescence quantum yield (PLQY) than classical 3D HMHs (MAPbX3).28–30 Therefore, 1D HMHs could be an ideal platform for highly efficient broadband luminescent materials. However, in terms of structure synthesis, organic cations with large structural size or steric resistance are often used,4,30–33 and there is still a lack of effective strategies to guide the synthesis of corner- or edge-sharing 1D chain structures of metal halide octahedra. Structurally, the flexibility and steric hindrance of organic cations and their interaction with the inorganic framework are important factors affecting the arrangement of inorganic octahedra and dimensionality of HMHs. When the configuration of the cation changes from a highly flexible chain to a less flexible ring, the inorganic anion octahedron will also change the distortion degree and connection mode to adapt to the shape of the cation, further affecting the photophysical properties of the material.34,35 Multivalent alicyclic cations with certain rigidity, which can not only reduce the dimensionality of the inorganic framework through abundant H⋯X (X = Cl−, Br−, I−) interactions, but also provide support for inorganic chains through alicyclic rings with relative rigidity, can be ideal template molecules for the synthesis of 1D materials.
In this work, we successfully synthesized two new 1D corrugated structures (PEA)2Pb2Br10·H2O (PEA is 1-piperazineethanammonium) and (MPEA)PbBr5·H2O (MPEA is 4-methyl-1-piperazineethanammonium) by reducing the flexibility of trivalent cations with broadband luminescence emission. In particular, the compound (PEA)2Pb2Br10·H2O with adjacent 1D inorganic chains stacked vertically features room-temperature yellowish white-light emission, a high PLQY value of 10.9%, CIE color coordinates of (0.42, 0.47), and correlated color temperatures (CCTs) of 3550 K (Scheme 1). Our research highlights the advantage of trivalent alicyclic cations in the construction of 1D luminescent materials and provides insight into novel broadband optoelectronic devices.
 | ||
| Scheme 1 Illustration of the design of 1D corrugated lead bromide hybrids with broadband luminescence by reducing the flexibility of trivalent cations. | ||
Results and discussion
Synthesis
Crystal structure
(DTA)PbBr5·H2O crystallizes in the centrosymmetric space group P21/c at 298 K, and the cell parameters are: a = 10.5210(17) Å, b = 10.7415(16) Å, c = 14.898(2) Å, α = γ = 90°, and β = 110.020(6)° (Table S1†). The asymmetric unit contains one DTA cation, one Pb ion, five Br ions, and one H2O molecule (Fig. 1a), the [PbBr6] octahedra are completely isolated and surrounded by DTA and H2O molecules through H⋯Br interactions (lengths ranging from 2.45 to 2.69 Å), and the crystal adopts a typical zero-dimensional structure (Fig. 1b and c, and Table S2†). The (DTA)PbBr5·H2O crystals are air and heat stable below 344 K; above that, the crystals first lose crystal water and then gradually decompose (Fig. S2a†).By cyclization, the highly flexible DTA cation transformed into a less flexible PEA cation, and (PEA)2Pb2Br10·H2O (Fig. 1a) adopts a 1D non-typical corrugated lead-bromide framework (Fig. 1d–f). It crystallizes in the centrosymmetric space group P21/c at 298 K, and the cell parameters are: a = 13.8538(3) Å, b = 17.5673(3) Å, c = 13.8398(3) Å, α = γ = 90°, and β = 96.191(2)° (Fig. 1 and Table S1†). The 1D chain is composed of corner-sharing [PbBr6] octahedra. The octahedra are arranged in a corrugated pattern in the (101) direction, with a pocket in the middle of the three adjacent octahedra stabilized by H⋯Br interactions among cations, H2O, and bromine ions (Fig. 1d and f and Table S2†). The H2O molecule is in the pocket formed by the octahedron, not between inorganic chains, which results in a 43 K increase in the thermal stability of the crystal compared to (DTA)PbBr5·H2O (Fig. S2b†). It also differs from the general 1D structure in crystal stacking, where the adjacent inorganic chains are not stacked in parallel, but almost vertically. This could improve the performance of materials based on the self-trapping exciton luminescence mechanism. In contrast to (PEA)2Pb2Br10·H2O, (MPEA)PbBr5·H2O retains a similar corrugated inorganic framework (Fig. 1g–i), but the H2O molecules move from the pocket within the inorganic chain to the inorganic interchain, greatly reducing its structural thermal stability.
In order to further evaluate the influence of cation changes on the microstructure of the inorganic framework, we studied the distortion of [PbBr6]. Two parameters, Δd and σ2, reflecting the bond length distortion and bond angle variance, respectively, were calculated using the following expressions
where do and di are the average and individual Pb–Br bond distance, respectively, and θi is the angle of the individual Br–Pb–Br angle. In the (DTA)PbBr5·H2O structure synthesized from flexible cations, the Pb–Br bond lengths vary between 2.921 and 3.109 Å with a distortion parameter Δd of 4.3 × 10−4 (Fig. 2a), which is comparable to 1D chiral HMHs (R/S-3-OHPD)PbI3 (ref. 36) and 2D chiral ferroelectric (R/S-3AMP)PbBr4.37 At the same time, the bond angles vary between 84.36 and 95.81° with a distortion parameter σ2 of 16.1° (ref. 2) (Fig. 2b and Table S3†). In the (PEA)PbBr5·H2O structure synthesized from rigid cations, Δd and σ2 are approximately doubled compared to those of (DTA)PbBr5·H2O, reaching 9.4 × 10−4 and 29.0° (ref. 2) (Fig. 2d and e and Table S3†), respectively. The increase of cation rigidity not only changes the dimensionality of the inorganic anion skeleton, but also greatly increases the degree of distortion of the inorganic octahedron. Hirshfeld surface analysis also showed that the enhancement of cation rigidity enhanced the H⋯Br interaction among organic cations, H2O and inorganic anions, and weakened the interaction between cation molecules (Fig. 2c and f and S3 and S4†), which was consistent with the change of the structural dimensionality (Fig. 1b and e). After further methylation of the secondary amine end of the PEA cation to the tertiary amine, it is found that although the inorganic framework maintains the 1D corrugated structure, the distortion of the [PbBr6] and H⋯Br interaction is greatly reduced (Fig. 2g–i and S5†), which is comparable to the 0D structure. This suggests that rigid cations help improve the structural dimensionality of HMHs, while tertiary amines are not a good template for increasing the interaction of cations with inorganic frameworks.
 | ||
| Fig. 2 Distorted [PbBr6] octahedra and Hirschfeld surface of the cations and H2O in (DTA)PbBr5·H2O (a–c), (PEA)2Pb2Br10·H2O (d–f), and (MPEA)2Pb2Br10·H2O (g–i) at 298 K. | ||
Semiconducting and photoluminescence properties
The photophysical properties of the targeted compounds were characterized by using ultraviolet-visible diffuse reflectance spectroscopy and steady-state time-resolved PL spectroscopy. The band gap estimated by the Kubelka–Munk function is 3.57 eV for (DTA)PbBr5·H2O, 3.22 eV for (PEA)2Pb2Br10·H2O, and 3.19 eV for (MPEA)PbBr5·H2O (Fig. 3a and b). There is a significant redshift in the band gap (Fig. 3b), which is consistent with the changes in structural dimensionality and [PbBr6] octahedral distortion (Fig. 2).27 | ||
| Fig. 3 UV-vis diffuse-reflectance spectra (a) and bandgaps derived from the Kubelka–Munk function (b) of (DTA)PbBr5·H2O, (PEA)2Pb2Br10·H2O, and (MPEA)2Pb2Br10·H2O. | ||
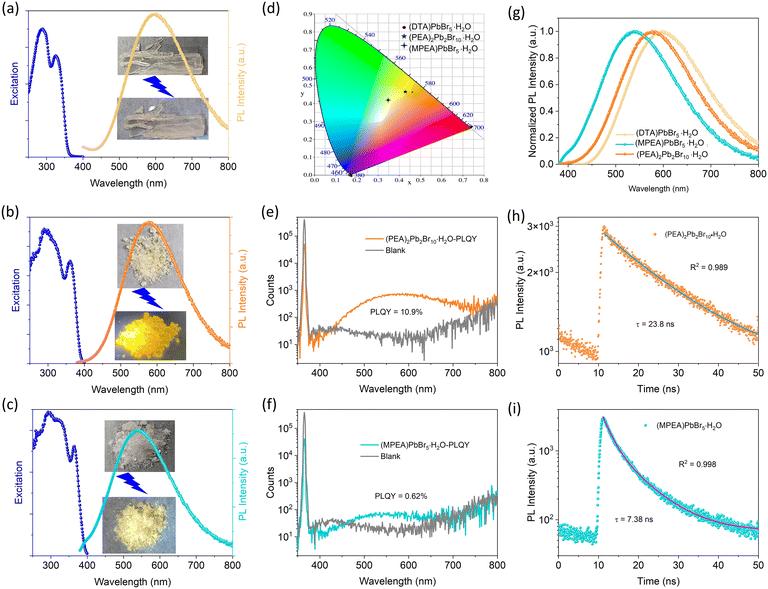
Consequently, the PL properties of the targeted compounds were studied, and all the compounds exhibit broadband emission from the STE mechanism.38 Under 325 nm excitation, (DTA)PbBr5·H2O shows broadband orange emission, which is invisible to the naked eye and corresponds to a weak quantum yield (Fig. 4a and S6†). (PEA)2Pb2Br10·H2O emits brighter yellowish light that covers the visible light range with a full width at half-maximum (FWHM) exceeding 184 nm (Fig. 4b), under 365 nm excitation. The international chromaticity coordinates of (0.42, 0.47) represent yellow emission (Fig. 4d). The PLQY and average luminescence lifetime are 10.9% and 23.8 ns, respectively (Fig. 4e and h), which are much higher than those of 1D chiral HMH (R/S-3-OHPD)PbI3.36 (MPEA)PbBr5·H2O emits yellowish white-light with CIE coordinates of (0.35, 0.42) (Fig. 4d), but the brightness, PLQY, and average luminescence lifetime are significantly reduced (Fig. 4c, f and i). From the above PL data, we found that the emission wavelength of the 1D structure is blue-shifted compared with that of the 0D structure (Fig. 4g), and the vertical stacking between chains in the 1D structure has better optical performance than the parallel stacking. In addition, since the structure contains a solvent of crystallization, there may be a phenomenon of fluorescence changes caused by the loss of solvent of crystallization.39–41 We further investigated the fluorescence properties after the dehydration of the structure. (PEA)2Pb2Br10·H2O shows a slight redshift in the fluorescence spectrum (4 nm) after the loss of water of crystallization, with a decrease in PLQY of 1.36% (Fig. S4a–c†). Meanwhile (MPEA)PbBr5·H2O shows a large redshift in the fluorescence spectrum of 77 nm after the loss of water of crystallization, with an increase in PLQY of 0.32% (Fig. S4e and f†). The above phenomena further indicate that the fluorescence properties of the materials originate from the inorganic backbone. In addition, the crystals can return to the initial state by regaining water molecules after losing them (Fig. S8†), showing potential applications in the field of chemical sensors and solid-state luminescent materials.
Conclusions
In conclusion, we constructed two new 1D corrugated structures (PEA)2Pb2Br10·H2O and (MPEA)PbBr5·H2O with broadband emission by the strategy of reducing the flexibility of trivalent cations. In particular, the compound (PEA)2Pb2Br10·H2O with inorganic chains alternately stacked vertically along the b direction features room-temperature yellow-white light emission and a high photoluminescence quantum yield (PLQY) value of 10.9%, which make it a promising light emitter in optoelectronic devices. Our research highlights the advantage of less flexible multi-valent cations in the construction of 1D luminescent materials and a new platform for fundamental studies of the structure–property relationships in broadband luminescent systems.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Chang-Chun Fan: conceptualization, data curation, funding acquisition, formal analysis, methodology, validation and writing – original draft; Zi-Han Fan: data curation and investigation; Shu-Lin Jiao: investigation and methodology; Jing-Xue Yu: supervision; Cheng-Dong Liu: data curation and methodology; Wei Wang: investigation and writing – review & editing; Ming-Liang Jin: formal analysis; Bei-Dou Liang: visualization and formal analysis; Xiang-Bin Han: conceptualization, funding acquisition, resources, supervision and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Talent Introduction Project of Jinling Institute of Technology (JIT-B-202414) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20230811).References
- B. R. Sutherland and E. H. Sargent, Nat. Photonics, 2016, 10, 295–302 CrossRef CAS
.
- J. Lim, M. T. Hörantner, N. Sakai, J. M. Ball, S. Mahesh, N. K. Noel, Y.-H. Lin, J. B. Patel, D. P. McMeekin, M. B. Johnston, B. Wenger and H. J. Snaith, Energy Environ. Sci., 2019, 12, 169–176 RSC
.
- H. Y. Ye, W. Q. Liao, C. L. Hu, Y. Zhang, Y. M. You, J. G. Mao, P. F. Li and R. G. Xiong, Adv. Mater., 2016, 28, 2579–2586 CrossRef CAS PubMed
.
- B. D. Liang, C. C. Fan, C. D. Liu, C. Y. Chai, X. B. Han and W. Zhang, Nat. Commun., 2022, 13, 6599 CrossRef CAS PubMed
.
- S. Li, Y. Jiang, J. Xu, D. Wang, Z. Ding, T. Zhu, B. Chen, Y. Yang, M. Wei, R. Guo, Y. Hou, Y. Chen, C. Sun, K. Wei, S. M. H. Qaid, H. Lu, H. Tan, D. Di, J. Chen, M. Grätzel, E. H. Sargent and M. Yuan, Nature, 2024, 635, 82–88 CrossRef CAS PubMed
.
- Y. H. Kim, Y. X. Zhai, H. P. Lu, X. Pan, C. X. Xiao, E. A. Gaulding, S. P. Harvey, J. J. Berry, Z. V. Vardeny, J. M. Luther and M. C. Beard, Science, 2021, 371, 1129–1133 CrossRef CAS PubMed
.
- H. Xu, F. Sun, E. Li, W. Guo, L. Hua, R. Wang, W. Li, J. Chu, W. Liu, J. Luo and Z. Sun, Adv. Mater., 2024, 36, 2414339 Search PubMed
.
- J. Cheng, G. Yi, Z. Zhang, Y. Long, H. Zeng, L. Huang, G. Zou and Z. Lin, Angew. Chem., Int. Ed., 2024, 63, e202318385 CrossRef CAS PubMed
.
- H. Zhu, Y. Fu, F. Meng, X. Wu, Z. Gong, Q. Ding, M. V. Gustafsson, M. T. Trinh, S. Jin and X. Y. Zhu, Nat. Mater., 2015, 14, 636–642 CrossRef CAS PubMed
.
- J. Feng, C. Gong, H. Gao, W. Wen, Y. Gong, X. Jiang, B. Zhang, Y. Wu, Y. Wu, H. Fu, L. Jiang and X. Zhang, Nat. Electron., 2018, 1, 404–410 CrossRef CAS
.
- M.-L. Jin, X.-B. Han, C.-D. Liu, C.-Y. Chai, C.-Q. Jing, W. Wang, C.-C. Fan, J.-M. Zhang and W. Zhang, J. Am. Chem. Soc., 2024, 146, 6336–6344 CrossRef CAS PubMed
.
- X. Li, J. M. Hoffman and M. G. Kanatzidis, Chem. Rev., 2021, 121, 2230–2291 CrossRef CAS PubMed
.
- P. Siwach, P. Sikarwar, J. S. Halpati and A. K. Chandiran, J. Mater. Chem. A, 2022, 10, 8719–8738 RSC
.
- H. P. Wang, S. Li, X. Liu, Z. Shi, X. Fang and J. H. He, Adv. Mater., 2020, 32, 2003309 Search PubMed
.
- C.-C. Fan, C.-D. Liu, B.-D. Liang, T.-Y. Ju, W. Wang, M.-L. Jin, C.-Y. Chai and W. Zhang, Chem. Sci., 2024, 15, 11374–11381 RSC
.
- W. Wang, C.-D. Liu, C.-C. Fan and W. Zhang, Chem. Sci., 2024, 15, 18455–18462 RSC
.
- C.-C. Fan, B.-D. Liang, C.-D. Liu, C.-Y. Chai, X.-B. Han and W. Zhang, Inorg. Chem. Front., 2022, 9, 6404–6411 RSC
.
- W. Wang, C.-D. Liu, C.-C. Fan, X.-B. Fu, C.-Q. Jing, M.-L. Jin, Y.-M. You and W. Zhang, J. Am. Chem. Soc., 2024, 146, 9272–9284 CrossRef CAS PubMed
.
- L. N. Quan, J. Kang, C.-Z. Ning and P. Yang, Chem. Rev., 2019, 119, 9153–9169 CrossRef CAS PubMed
.
- X.-B. Han, C.-Q. Jing, H.-Y. Zu and W. Zhang, J. Am. Chem. Soc., 2022, 144, 18595–18606 CrossRef CAS PubMed
.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef
.
- J. Zhang, L. Wang, A. Zhong, G. Huang, F. Wu, D. Li, M. Teng, J. Wang and D. Han, Dyes Pigm., 2019, 162, 590–598 CrossRef CAS
.
- Z. Liu, X. Li, W. Dai, J.-J. Liu and M.-J. Lin, Coord. Chem. Rev., 2025, 526, 216350 CrossRef CAS
.
- C. Y. Chai, Q. K. Zhang, C. Q. Jing, X. B. Han, C. D. Liu, B. D. Liang, C. C. Fan, Z. Chen, X. W. Lei, A. Stroppa, R. O. Agbaoye, G. A. Adebayo, C. F. Zhang and W. Zhang, Adv. Opt. Mater., 2022, 11, 2201996 CrossRef
.
- K. H. Jin, Y. Zhang, K. J. Li, M. E. Sun, X. Y. Dong, Q. L. Wang and S. Q. Zang, Angew. Chem., Int. Ed., 2022, 61, e202205317 CrossRef CAS PubMed
.
- H. Y. Zu, X. B. Han, C. C. Fan, B. D. Liang and W. Zhang, lnorg. Chem, 2022, 61, 17738–17745 CrossRef CAS PubMed
.
- H.-Y. Zu, C.-C. Fan, C.-D. Liu, C.-Q. Jing, C.-Y. Chai, B.-D. Liang, X.-B. Han and W. Zhang, Chem. Mater., 2023, 35, 5854–5863 CrossRef CAS
.
- J. Zhou, M. Li, L. Ning, R. Zhang, M. S. Molokeev, J. Zhao, S. Yang, K. Han and Z. Xia, J. Phys. Chem. Lett., 2019, 10, 1337–1341 CrossRef CAS PubMed
.
- G.-E. Wang, G. Xu, M.-S. Wang, L.-Z. Cai, W.-H. Li and G.-C. Guo, Chem. Sci., 2015, 6, 7222–7226 RSC
.
- L. Mao, P. Guo, M. Kepenekian, I. Hadar, C. Katan, J. Even, R. D. Schaller, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 13078–13088 CrossRef CAS PubMed
.
- V. Gomez, O. Fuhr and M. Ruben, CrystEngComm, 2016, 18, 8207–8219 RSC
.
- R. Chen, C. Sun, X. Cheng, Y. Lin, J. Zhou, J. Yin, B. B. Cui and L. Mao, Inorg. Chem., 2023, 62, 9722–9731 CrossRef CAS PubMed
.
- C.-C. Gao, X.-N. Tang, J.-L. Miao, Y. Li, C. Li and G.-N. Liu, CrystEngComm, 2023, 25, 6556–6567 RSC
.
- A. Yangui, R. Roccanova, Y. T. Wu, M. H. Du and B. Saparov, J. Phys. Chem. C, 2019, 123, 22470–22477 CrossRef CAS
.
- Y. L. Zeng, X. Q. Huang, C. R. Huang, H. Zhang, F. Wang and Z. X. Wang, Angew. Chem., Int. Ed., 2021, 60, 10730–10735 CrossRef CAS PubMed
.
- M. L. Jin, X. B. Han, C. D. Liu, B. D. Liang, C. Q. Jing, W. Wang, C. C. Fan, T. Y. Ju, J. M. Zhang and W. Zhang, Adv. Funct. Mater., 2024, 34, 2408120 CrossRef CAS
.
- C. C. Fan, X. B. Han, B. D. Liang, C. Shi, L. P. Miao, C. Y. Chai, C. D. Liu, Q. Ye and W. Zhang, Adv. Mater., 2022, 34, 2204119 CrossRef CAS PubMed
.
- W. Wang, C.-D. Liu, X.-B. Han, C.-Q. Jing, C.-Y. Chai, C.-C. Fan, M.-L. Jin, J.-M. Zhang and W. Zhang, ACS Mater. Lett., 2024, 6, 203–211 CrossRef CAS
.
- S. A. Adonin, M. E. Rakhmanova, A. I. Smolentsev, I. V. Korolkov, M. N. Sokolov and V. P. Fedin, New J. Chem., 2015, 39, 5529–5533 RSC
.
- S. A. Adonin, M. N. Sokolov, M. E. Rakhmanova, A. I. Smolentsev, I. V. Korolkov, S. G. Kozlova and V. P. Fedin, Inorg. Chem. Commun., 2015, 54, 89–91 CrossRef CAS
.
- D. Yan, G. Fan, Y. Guan, Q. Meng, C. Li and J. Wang, Phys. Chem. Chem. Phys., 2013, 15, 19845–19852 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Fig S1–S8 and Table S1–S3. CCDC 2404396 and 2404397. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ce00003c |
| This journal is © The Royal Society of Chemistry 2025 |