Adaptive crystals of homothiacalix[4]arene capable of molecular recognition, with preferential uptake of benzene over cyclohexane
Renny Maria
Losus
 a,
Sem
Bleus
a,
Sem
Bleus
 b,
Volodymyr
Bon
b,
Volodymyr
Bon
 c,
Stefan
Kaskel
c,
Stefan
Kaskel
 c,
Wim
Dehaen
c,
Wim
Dehaen
 b and
Liliana
Dobrzańska
b and
Liliana
Dobrzańska
 *a
*a
aFaculty of Chemistry, Nicolaus Copernicus University in Toruń, Gagarina 7, 87-100 Toruń, Poland. E-mail: lianger@umk.pl
bDepartment of Chemistry, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium
cFaculty of Chemistry & Food Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden, Germany
First published on 7th November 2025
Abstract
Unique responsive and selective behaviour of homothiacalix[4]arene crystals is reported. A benzene disolvate adopting the 1,3-alternate conformation (HTCA-s1,3) can be converted to a guest-free non-porous phase (HTCA-a1,3) upon heating, with the host molecules retaining the same 1,3-alternate conformation. The process is reversible, with non-porous apohost crystals ‘opening up’ upon exposure to benzene vapour to reaccommodate the solvent molecules, as confirmed by SCXRD and sorption studies monitored in situ via PXRD analysis. The gate opening mechanism is selective, preventing cyclohexane molecules from passing through, which creates the opportunity to apply this system, for example, in benzene capture/separation. Furthermore, with a higher energy boost, the crystal of the HTCA-a1,3 apohost undergoes a significant conformational change, resulting in HTCA-a1,2, an apohost in which the host molecules adopt the 1,2-alternate conformation.
Porous materials are highly recognized in materials science, as their sorption properties are desired in many chemical processes, involving areas such as petrochemistry, mining, and pharmaceuticals while also facilitating greener, more energy-efficient chemistry.1,2 Established porous materials like zeolites, metal–organic frameworks (MOFs),2,3 and emerging covalent organic frameworks (COFs)4 are widely studied. However, porous organic molecular crystals are less explored in this regard, even though they offer distinct advantages, including, among others, an easier synthetic pathway, as well as processability.5
Calixarenes have been extensively studied as host molecules in solution and the solid state for many decades due to their facile synthesis, well-defined and adaptable cavities, strong guest-binding capabilities, and structural flexibility.6 The latter is an essential feature for fine-tuning their guest-binding properties in solution and arises from conformational adaptability, which, among others, can be controlled by regulating the rotation of aryl rings through the macrocyclic cavity, incorporating specific bridging groups, or increasing the number of aryl rings.7 The macrocyclic cavity can be expanded during synthesis by increasing the ring size, replacing conventional methylene (–CH2–) linkers with extended/more flexible versions, including (–CH2–S–S–CH2–, –CH2–S–CH2–, –CH2–O–CH2–, etc.).8,9 The flexibility of calixarenes in the solid state has not been studied as broadly as in solution, and revealing host adaptivity in single-crystals of these macrocycles upon triggering is still exceptional.9–11 Two of our earlier reports on conformational changes taking place in the crystal unity of calixarenes, revealed for homodithiacalixarene and its selena analogue, are still the only examples presenting such molecular rearrangements for this group of compounds.10,11 The potential of structural adaptability renders crystalline material based on calixarenes promising for future applications in gas storage, molecular separation, or sensing.
Chemical separation has significantly advanced over the past century,12 with modern technologies such as adsorption-based separation providing a more energy-efficient alternative to conventional methods like distillation, which accounts for 10–15% of global energy consumption.13 The separation of benzene from cyclohexane remains a major challenge in the chemical industry due to their nearly identical boiling points, with a difference of just 0.6 K (353.23 K for benzene and 353.85 K for cyclohexane), and their tendency to form azeotropes. Six-membered cyclic alkanes and alkenes, particularly cyclohexane, are commonly produced via benzene hydrogenation, making their efficient separation crucial for industrial applications. The predominant industrial methods for benzene/cyclohexane separation are extractive and azeotropic distillation,14 though there are some reports on applying materials based on molecular crystals for such separations.15
Previously, we reported macrocyclisation protocols for obtaining homothiacalix[n]arenes, including, among others, the synthetic procedure for the homothiacalix[4]arene (HTCA, Scheme S1) presented here, accompanied by basic structural studies which confirmed formation of the desired product, and showed that non-cone conformations (1,3-alt and 1,2-alt) are preferred in solution.8b Herein, we reveal distinct conformational conversions of HTCA via single-crystal analyses.
Moreover, we show that these crystals show a high affinity towards particular guest molecules, indicating that crystalline materials based on calixarenes could find further applications in sorption-based separation. To the best of our knowledge, this is the first report on the potential application of crystalline calixarene material in a vapour separation process of benzene/cyclohexane with preferential uptake of benzene, as well as the first time that such a complex transition path takes place in organic molecular crystals (Fig. S1 and S2).
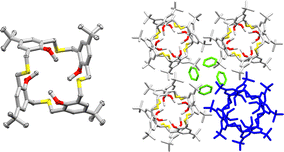
Crystals of HTCA were obtained from benzene as a disolvate that crystallizes in the I41/a space group of a tetragonal system. The asymmetric unit contains two crystallographically independent quarters of the host molecule, as the complete molecules are situated around a 4-fold rotoinversion axis, and one benzene molecule. Both calixarene molecules adopt a distorted 1,3-alternate conformation with all the methoxy groups, as well as the S-atoms, tilted inwards toward the macrocyclic ring, which is stabilized in this position via weak C–H—S intramolecular interactions, with D—A distances ranging from 3.66 to 3.86 Å. The two molecules which are not related by symmetry show the same conformation (RMSD = 0.155 Å), but differ in the intermolecular interactions formed, namely one of these molecules is involved in host–guest interactions. The calixarene molecules form columns along the crystallographic c-axis, consisting of alternating, crystallographically independent host molecules connected by weak hydrogen bonds, involving O-atoms from all methoxy groups and CH2-groups from the macrocyclic linker, such as C2–H2B—O24 and C30–H30B—O9. The columns are interconnected with those adjacent via C13–H13B—O24 hydrogen bonding, involving a tert-butyl group and one of the methoxy groups, to form a 3D assembly (Table S1).
The benzene molecules occupy the interstitial space formed between the columns of the host molecules (Fig. 1) and interact, as mentioned above, only with one of the two calixarene molecules via a C35–H35—O9 hydrogen bond with a donor–acceptor distance of 3.52 Å. As the interstitial space is rather extended, leading to the formation of channels along the c axis, it seemed that removal of the solvent molecules from the crystal could be feasible.
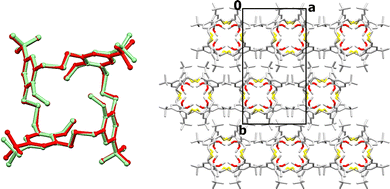
Thermal analyses (TGA and DTA; Fig. S3) of HTCA-s1,3 revealed that desolvation takes place just below 100 °C and is connected with a phase transition, whereas decomposition begins at 260 °C. Consequently, the crystal was heated at 100 °C for 3 min, and gratifyingly, it did not lose its integrity, allowing for SCXRD data to be collected (Fig. S4 and Movie S1). This revealed that the solvent molecules were removed from the crystal upon heating, yielding a densely packed apohost phase (HTCA-a1,3). Removal of the solvent molecules induces changes in the crystal symmetry, which is lowered to orthorhombic (space group Pccn). This apohost crystal shows the presence of half a calixarene molecule in the asymmetric unit, as the host molecule is located around a 2-fold axis. The host molecules in general retain the same conformation as in the solvated phase (RMSD = 0.246 Å), but their shift and rotation eliminate the voids that were created upon desolvation (Fig. 2).
As in the solvated phase, the molecules are stacked in columns along the c axis which, as before, are stabilized via C–H—O interactions, involving likewise the methoxy groups and the macrocyclic linkers C17–H17B—O9 and C2–H2A—O24.
The columns are interconnected along the a axis via weak C29–H29D—O9 interactions between a tert-butyl group and one of the methoxy groups, resulting in layers. Lastly, the C13–H13B—O24 interactions could be perceived as forces which extend the structure in the third dimension.
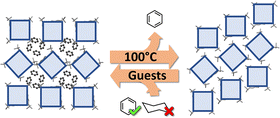
Interestingly, the process is reversible and exposing a non-porous crystal of HTCA-a1,3 to benzene vapour returns it to its initial, solvated phase (Fig. 3).
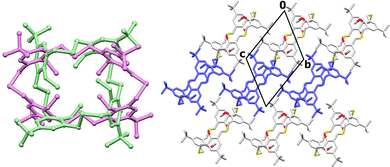
The presented course of adaptive porosity differs from the one we reported earlier for homodithia- and homodiselenacalixarene, where the apohost, with longer linkers connecting the aromatic units, was going through reversible conformational changes upon heating/exposure to vapour.10,11 Moreover, the molecular flexibility is not restricted to this transformation. Heating a HTCA-a1,3 crystal at a higher temperature of 130 °C (vs. 100 °C previously) or applying a high vacuum leads to the formation of a new apohost of altered conformation, namely HTCA-a1,2 (Fig. 4). This process is not reversible upon exposure of the crystal to solvent vapour, but the initial HTCA-a1,3 phase can be recovered via recrystallization from benzene.
The symmetry of the HTCA-a1,2 undergoes further changes. The new phase belongs to the centrosymmetric space group of a triclinic system. The asymmetric unit consists of half a HTCA molecule adopting the 1,2-alternate conformation, with other part being generated by an inversion centre. The molecule adopts a rectangular shape compared to the square-like architecture of the HTCA-a1,3 apohost (Fig. 4). This is caused by a change in orientation of two opposing S-atoms, which are pointing outside of the macrocyclic ring with a distance between these S-atoms of 13.4 Å.
The distance between the S-atoms pointing towards the macrocyclic ring almost does not change (6.0 Å vs. 6.1 Å in HTCA-a1,3). As previously, all the methoxy groups are pointing towards the macrocyclic ring, and they are stabilized in this position via weak interactions, after rotation of two aromatic rings in order to adopt a 1,2-alternate conformation.
Zooming in on the structure shows that the methoxy groups are stabilized (as in HTCA-a1,3) by intramolecular C–H—S hydrogen bonds (C8–H8C—S29 and C23–H23C—S29), drawing the methoxy groups (O7, O22) into the macrocyclic cavity. The two methoxy groups containing O22 are further stabilized by newly formed intermolecular C–H—O interactions, involving the tert-butyl groups from adjacent molecules (e.g. C10A–H10F—O22, C11A–H11F—O22, C12–H12D—O22) as well as –CH2 group originating from the macrocyclic ring (C28–H28B—O22). Consequently, the molecules form layers, which are further stabilized via weak C–H—π interactions (C13–H13A—Cg1) (Table S2), forming a three-dimensional supramolecular framework. The HTCA-a1,2 phase is a polymorphic variation of the structure of HTCA adopting the 1,2-alternate conformation we reported earlier,8b which was obtained via vapour diffusion of methanol into chloroform solutions of calixarenes. The unit cell parameters of these two forms vary significantly because of differences in packing and hydrogen bond patterns.
The transformation of HTCA-a1,3 to HTCA-a1,2 indicates that reducing the macrocyclic ring size, compared with homodithiacalixarene, does not hamper the flexibility in the crystal unity, as with an extra energy boost, the phenyl rings can rotate in a collaborative way, yielding a phase which contains another set of intermolecular interactions, despite the constraints imposed by the crystal (Tables S1 and S2).
We analysed the response of the HTCA-a1,3 apohost towards various solvents. Exposure of HTCA-a1,3 to THF, pyridine or 1,4-dioxane led to the formation of phases which are isostructural with the benzene disolvate (Table S3). Interestingly, the crystal did not exhibit any response towards toluene or cyclohexane.
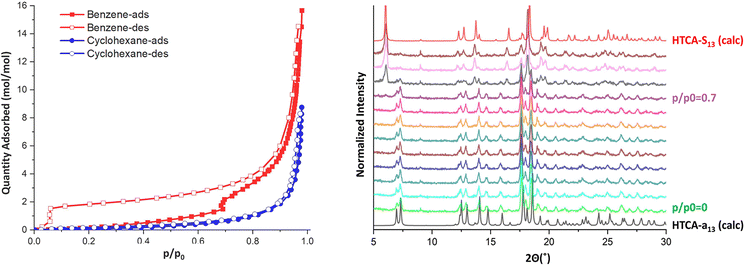
To verify the different responsivity towards benzene and cyclohexane, the corresponding isotherms were measured (Fig. 5) after confirming the homogeneity of the bulk sample by PXRD studies (Fig. S5). The benzene isotherm clearly shows the gate opening event as a step in the adsorption branch at p/p0 = 0.7, whereas the reverse transformation is visible on the desorption branch at p/p0 = 0.03. The steep step in the isotherm at p/p0 > 0.9 can be explained by the adsorption of the fluid in the interparticle voids, followed by the condensation. The isotherm collected for cyclohexane vapour confirmed the lack of responsivity of the apohost to this guest molecule.
In situ PXRD studies confirmed that the observed gating mechanism on the isotherms corresponds well with the phases isolated during the single-crystal to single-crystal transformations. The resulting PXRD patterns match well with the simulated patterns for HTCA-a1,3 at p/p0 = 0, and the solvated phase HTCA-s1,3 at p/p0 > 0.7 (Fig. 5).
To gain further insight into the adsorption process of benzene molecules, a time-resolved solid-vapour adsorption experiment was conducted and monitored via NMR (solution). As expected, the single-component adsorption of benzene occurred, reaching maximum adsorption after 22 hours, while the single-component adsorption of cyclohexane confirmed once again a lack of response of the host molecules to such molecular geometry (Fig. S6 and S7).
In summary, a series of structural transformations taking place in a single-crystal of homothiacalix[4]arene was revealed, as well as the selective sorption behaviour and adaptability of these crystals in response to a particular trigger. The crystals open their pores upon exposure to benzene vapour, but stay closed upon exposure to cyclohexane. The geometry of the guest molecule as well as its predisposition to form host–guest interactions is key to changing a non-porous host crystal into an environment that welcomes guests. This study points out that a molecular structure determined for a calixarene or by extension, most likely for any macrocyclic host–guest system, could merely be a particular snapshot of a medium in transition, a fact which should be taken into consideration by chemists who might base conclusions on such a fleeting, one-time results. Moreover, the selectivity of the calixarene crystals during the vapour adsorption makes these macrocycles a promising group of compounds for follow-up studies towards applications in molecular separation and sensing.
R. M. L. and L. D. would like to thank the National Science Centre – Poland for grant no. 2014/14/E/ST5/00611. S. B. and W. D. acknowledge the KU Leuven for postdoctoral fellowship PDMT2/24/051.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included in the supplementary information (SI). Supplementary information: experimental methods, crystallographic details, hydrogen bonding parameters, thermal analysis, micrographs and a movie presenting the removal of solvent molecules from HTCA-s1,3 upon heating, PXRD patterns, results of single-component time-dependent solid-vapor sorption experiments. See DOI: https://doi.org/10.1039/d5cc05862g.CCDC 2468996–2468998 contain the supplementary crystallographic data for this paper.16a–c
References
- (a) K. Liu, Y. Liu, Y. Wu, J. Liu, Q. Shuai, L. Huang, Z. Hu and Y. Yamauchi, Coord. Chem. Rev., 2025, 522, 216199 CrossRef CAS; (b) T. Kumeria, ACS Biomater. Sci. Eng., 2022, 8, 4025–4027 CrossRef CAS PubMed; (c) L. Yang, S. Qian, X. Wang, X. Cui, B. Chen and H. Xing, Chem. Soc. Rev., 2020, 49, 5359–5406 RSC; (d) X. Zhang, X. Wang, F. Gao, Y. Chen, H. Liu, P. Zhou, Z. Kang, Y. Wang and W. Fan, Mater. Adv., 2024, 5, 3135–3157 RSC.
- (a) B. Saboorizadeh, R. Zare-Dorabei, M. Safavi and V. Safarifard, Langmuir, 2024, 40, 22477–22503 CrossRef CAS PubMed; (b) Y. Wu and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2021, 60, 18930–18949 CrossRef CAS.
- (a) K. Wen, J. Zhou, T. Ke, J. Li, Y. Jin, Q. Zhang, Z. Zhang, Z. Bao, Q. Ren and Q. Yang, Angew. Chem., Int. Ed., 2025, 64, e202500519 CrossRef CAS PubMed; (b) I. Senkovska, V. Bon, A. Mosberger, Y. Wang and S. Kaskel, Adv. Mater., 2025, 2414724 CrossRef.
- Y. Song, Q. Sun, B. Aguila and S. Ma, Adv. Sci., 2019, 6, 1801410 CrossRef PubMed.
- (a) E. Martínez-Ahumada, D. He, V. Berryman, A. López-Olvera, M. Hernandez, V. Jancik, V. Martis, M. A. Vera, E. Lima, D. J. Parker, A. I. Cooper, I. A. Ibarra and M. Liu, Angew. Chem., Int. Ed., 2021, 60, 17556–17563 CrossRef PubMed; (b) N. B. McKeown, J. Mater. Chem., 2010, 20, 10588 RSC; (c) K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064–2072 CrossRef CAS PubMed; (d) S. Tashiro and M. Shionoya, Acc. Chem. Res., 2020, 53, 632–643 CrossRef CAS PubMed; (e) X.-N. Han, Y. Han and C.-F. Chen, Chem. Soc. Rev., 2023, 52, 3265–3298 RSC.
- (a) Y. Pan, X. Hu and D. Guo, Angew. Chem., Int. Ed., 2021, 60, 2768–2794 CrossRef CAS PubMed; (b) S. Kumar, S. Chawla and M. C. Zou, J. Inclusion Phenom. Macrocyclic Chem., 2017, 88, 129–158 CrossRef CAS; (c) P. M. Marcos and M. N. Berberan-Santos, Sensors, 2024, 24, 7181 CrossRef CAS PubMed; (d) Y. Zhou, K. Jie, R. Zhao and F. Huang, Adv. Mater., 2020, 32, 1904824 CrossRef CAS.
- (a) C. D. Gutsche and L. J. Bauer, J. Am. Chem. Soc., 1985, 107, 6052–6059 CrossRef CAS; (b) C. D. Gutsche, Acc. Chem. Res., 1983, 16, 161–170 CrossRef CAS; (c) V. Guérineau, M. Rollet, S. Viel, B. Lepoittevin, L. Costa, P. Saint-Aguet, R. Laurent, P. Roger, D. Gigmes, C. Martini and V. Huc, Nat. Commun., 2019, 10, 113 CrossRef PubMed.
- (a) B. Dhawan and C. D. Gutsche, J. Org. Chem., 1983, 48, 1536–1539 CrossRef CAS; (b) J. Thomas, K. Van Hecke, K. Robeyns, W. Van Rossom, M. P. Sonawane, L. Van Meervelt, M. Smet, W. Maes and W. Dehaen, Chem. – Eur. J., 2011, 17, 10339–10349 CrossRef CAS PubMed.
- (a) P. K. Thallapally, L. Dobrzańska, T. R. Gingrich, T. B. Wirsig, L. J. Barbour and J. L. Atwood, Angew. Chem., Int. Ed., 2006, 45, 6506–6509 CrossRef CAS PubMed; (b) S. J. Dalgarno, P. K. Thallapally, L. J. Barbour and J. L. Atwood, Chem. Soc. Rev., 2007, 36, 236 RSC; (c) P. K. Thallapally, B. Peter McGrail, S. J. Dalgarno, H. T. Schaef, J. Tian and J. L. Atwood, Nat. Mater., 2008, 7, 146–150 CrossRef CAS PubMed; (d) J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000–1002 CrossRef CAS PubMed; (e) N. Morohashi and T. Hattori, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 261–277 CrossRef CAS.
- J. Thomas, G. Reekmans, P. Adriaensens, L. Van Meervelt, M. Smet, W. Maes, W. Dehaen and L. Dobrzańska, Angew. Chem., Int. Ed., 2013, 52, 10237–10240 CrossRef CAS PubMed.
- J. Thomas, L. Dobrzańska, L. Van Meervelt, M. A. Quevedo, K. Woźniak, M. Stachowicz, M. Smet, W. Maes and W. Dehaen, Chem. – Eur. J., 2016, 22, 979–987 CrossRef CAS PubMed.
- (a) Q. Shi, J. C. Gonçalves, A. F. P. Ferreira and A. E. Rodrigues, Chem. Eng. Process, 2021, 169, 108603 CrossRef CAS; (b) Z. Bao and B. Chen, Chem. Bio. Eng., 2025, 2, 68–70 CrossRef CAS PubMed; (c) W. Liu, Y. Yang, L. Guo, J. Di, C. Hon Lau, M. V. Bermeshev and L. Shao, Chem. Eng. J., 2024, 498, 155569 CrossRef CAS; (d) Z. Chen, Z. Li, J. Chen, P. Kallem, F. Banat and H. Qiu, J. Environ. Chem. Eng., 2022, 10, 107104 CrossRef CAS; (e) C. Y. Chuah, H. Lee and T.-H. Bae, Chem. Eng. J., 2022, 430, 132654 CrossRef CAS.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- (a) J. Qin, Q. Ye, X. Xiong and N. Li, Ind. Eng. Chem. Res., 2013, 52, 10754–10766 CrossRef CAS; (b) J. P. Garcia Villaluenga and A. Tabe-Mohammadi, J. Membr. Sci., 2000, 169, 159–174 CrossRef CAS.
- (a) T. Pausch, S. Clopot, D. N. Jordan, O. Weingart, C. Janiak and B. M. Schmidt, Angew. Chem., Int. Ed., 2024, 63, e202418877 CrossRef CAS PubMed; (b) Z. Xu, W. Yang, H. Liu, S. Jiang and A. C.-H. Sue, JACS Au, 2024, 4, 3475–3483 CrossRef CAS; (c) F. Zeng, L. Cheng, W.-J. Zhang, L.-L. Tang and X.-F. Wang, Org. Chem. Front., 2022, 9, 3307–3311 RSC; (d) D. Wang and Y. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202217903 CrossRef CAS; (e) J. Zhou, G. Yu, Q. Li, M. Wang and F. Huang, J. Am. Chem. Soc., 2020, 142, 2228–2232 CrossRef CAS PubMed; (f) W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994–3999 CrossRef CAS PubMed; (g) B. Moosa, L. O. Alimi, W. Lin, A. Fakim, P. M. Bhatt, M. Eddaoudi and N. M. Khashab, Angew. Chem., Int. Ed., 2023, 62, e202311555 CrossRef CAS PubMed.
- (a) CCDC 2468996: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2nw61t; (b) CCDC 2468997: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2nw62v; (c) CCDC 2468998: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2nw63w.
| This journal is © The Royal Society of Chemistry 2025 |