Photoinduced EDA complex triggered difluoroalkylation of quinoxalin-2(1H)-ones with unactivated alkenes and fluoroalkyl bromides
Huanhuan
Cui
a,
Xiulin
Qiao
a,
Tiesheng
Shi
 a,
Wei
Wei
b,
Huilan
Yue
a,
Wei
Wei
b,
Huilan
Yue
 b,
Zuli
Wang
d,
Zhuoming
Ma
*a and
Zi
Yang
b,
Zuli
Wang
d,
Zhuoming
Ma
*a and
Zi
Yang
 *c
*c
aCollege of Chemistry, Chemical Engineering and Material Science, Zaozhuang University, Zaozhuang, 277160, Shandong, China. E-mail: mazhuoming@uzz.edu.cn
bQinghai Provincial Key Laboratory of Tibetan Medicine Research and CAS Key Laboratory of Tibetan Medicine Research, Northwest Institute of Plateau Biology, Qinghai 810008, China
cHunan Provincial University Key Laboratory of the Fundamental and Clinical Research on Functional Nucleic Acid, First Clinical College, Changsha Medical University, Changsha 410219, China. E-mail: yangziycy@163.com
dNational Engineering Research Center of Low-Carbon Processing and Utilization of Forest Biomass, Nanjing Forestry University, Nanjing 210037, China
First published on 13th October 2025
Abstract
A metal-free photo-induced three-component difluoroalkylation reaction between quinoxalin-2(1H)-ones, unactivated alkenes and fluoroalkyl bromides via an EDA strategy has been reported. This reaction was initiated by the photochemistry of electron donor acceptor (EDA) complexes formed by TMEDA and fluoroalkyl bromide. A variety of 3-difluoroalkylated quinoxalin-2(1H)-ones with diverse functional groups could be accessed under mild conditions.
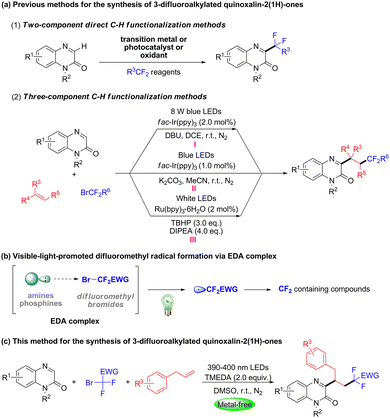
Difluoroalkylated compounds have remarkably important applications in agrochemicals, pharmaceuticals and materials science1 due to their unique chemical and biological properties.2 Meanwhile, quinoxalin-2(1H)-ones are a crucial class of heterocyclic units, endowed with significant pharmaceutical activities, such as aldose reductase inhibitory activity, antitumor activity, antimicrobial activity, antibacterial activity, and antihistaminic activity.3 In particular, 3-difluoroalkylated quinoxalin-2(1H)-ones are a fascinating class of compounds commonly featuring privileged pharmacophores.4 To date, several significant achievements for accessing 3-difluoroalkylated quinoxalin-2(1H)-ones have been reported by employing a transition metal catalyzed or photo-catalyzed direct two component C3–H functionalization strategy (Scheme 1a, (1)).5
 | ||
| Scheme 1 Strategies for the synthesis of 3-difluoroalkylated quinoxalin-2(1H)-ones and EDA strategies to activate difluoroalkyl bromides. | ||
Over recent decades, the direct difunctionalization of alkenes has been one of the most attractive strategies to install two functional groups simultaneously into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond via a one pot procedure.6 Recently, some three-component difunctionalization strategies have been utilized in synthesizing 3-difluoroalkylated quinoxaline-2(1H)-ones. For example, in 2022, the Wang group reported a photo-induced three-component difluoromethylative heteroarylation of unactivated alkenes, quinoxaline-2(1H)-ones and ethyl 2-bromo-2,2-difluoroacetate by using fac-Ir(ppy)3 as a photocatalyst (Scheme 1a, (I)).7 In 2023, the Zhang group reported a fac-Ir(ppy)3 catalyzed three-component protocol for the synthesis of difluorobenzylated quinoxalin-2(1H)-ones (Scheme 1a, (II)).8 In 2023, the Singh group unveiled a similar Ru(bpy)3Cl2 catalyzed three-component reaction of quinoxalin-2(1H)-ones by employing TBHP as the oxidant (Scheme 1a, (III)).9 These methods require the addition of photocatalyst or oxidant, which limit their wide application in pharmaceutical synthesis. Therefore, the development of metal-free and operationally convenient strategies for the synthesis of 3-difluoroalkyl-containing quinoxalin-2(1H)-ones via photo-induced three-component reactions has attracted great interest from chemists.
C bond via a one pot procedure.6 Recently, some three-component difunctionalization strategies have been utilized in synthesizing 3-difluoroalkylated quinoxaline-2(1H)-ones. For example, in 2022, the Wang group reported a photo-induced three-component difluoromethylative heteroarylation of unactivated alkenes, quinoxaline-2(1H)-ones and ethyl 2-bromo-2,2-difluoroacetate by using fac-Ir(ppy)3 as a photocatalyst (Scheme 1a, (I)).7 In 2023, the Zhang group reported a fac-Ir(ppy)3 catalyzed three-component protocol for the synthesis of difluorobenzylated quinoxalin-2(1H)-ones (Scheme 1a, (II)).8 In 2023, the Singh group unveiled a similar Ru(bpy)3Cl2 catalyzed three-component reaction of quinoxalin-2(1H)-ones by employing TBHP as the oxidant (Scheme 1a, (III)).9 These methods require the addition of photocatalyst or oxidant, which limit their wide application in pharmaceutical synthesis. Therefore, the development of metal-free and operationally convenient strategies for the synthesis of 3-difluoroalkyl-containing quinoxalin-2(1H)-ones via photo-induced three-component reactions has attracted great interest from chemists.
Photo-induced EDA complexes have been recognized as a powerful and versatile strategy for radical reactions in the absence of extra photocatalysts.10 In particular, harnessing the EDA complexes constructed from difluoromethyl bromides and amines/phosphines under visible light irradiation could generate the difluoroalkyl radical (Scheme 1b).11 This strategy provides an efficient and simple new method for the synthesis of difluoride compounds. With our interests in photochemical reactions and the development of simple and mild methods for synthesizing diverse alkylated heteroaryl compounds,12 herein, we wish to report a photo-induced three-component difluoroalkylation of unactivated alkenes with quinoxalin-2(1H)-ones and fluoroalkyl bromides via an EDA complex strategy (Scheme 1c).
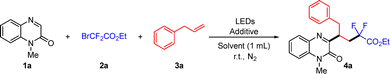
Our investigation was started from using 1-methylquinoxalin-2(1H)-one (1a), ethyl bromodifluoroacetate (2a), and allylbenzene (3a) as the model substrates to explore this reaction (Table 1). The desired product 4a could be obtained in 41% yield by using 2.0 equivalents of TMEDA under the irradiation of blue LEDs (460–475 nm) (entry 1). Then, we tested different electron donors such as HE, DIPEA, PMDETA, DBU and Et3N, which resulted in lower yields of 4a (entries 2–6). Increasing TMEDA loading to 3.0 equivalents or 4.0 equivalents gave a lower yield (entries 7 and 8). We then tested different solvents, and the results showed that DMSO was the best solvent (Table S1, SI). Next, we tried to replace the light source using purple light (390–400 nm), and the desired product 4a was increased to 83% (entry 9). Shortening the reaction time to 24 hours, the yield did not decrease (entry 10). When the molar ratio of 1a, 2a and 3a was changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the yield of the desired product 4a was improved to 86% (entry 11 and 12). Moreover, no desired product was observed without TMEDA or visible-light irradiation, which illustrated that both of them are necessary for the transformation (entry 13 and 14). When the model reaction was carried out under air, only a trace amount of product was detected (entry 15).
2, the yield of the desired product 4a was improved to 86% (entry 11 and 12). Moreover, no desired product was observed without TMEDA or visible-light irradiation, which illustrated that both of them are necessary for the transformation (entry 13 and 14). When the model reaction was carried out under air, only a trace amount of product was detected (entry 15).
| Entry | Additive | Light source (nm) | Yieldb (%) |
|---|---|---|---|
a
1a (0.1 mmol), 2a (0.3 mmol), 3a (0.3 mmol), additive (2.0–4.0 equiv.), DMSO (1 mL), N2, room temperature, and 48 h.
b Isolated yields based on 1a.
c 24 h.
d
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1 3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
e
1a 3.
e
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1 3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
f No TMEDA.
g No light.
h Under air. 2.
f No TMEDA.
g No light.
h Under air.
|
|||
| 1 | TMEDA (2.0 equiv.) | 460–475 | 41% |
| 2 | HE (2.0 equiv.) | 460–475 | N.R. |
| 3 | DIPEA (2.0 equiv.) | 460–475 | Trace |
| 4 | PMDETA (2.0 equiv.) | 460–475 | Trace |
| 5 | DBU (2.0 equiv.) | 460–475 | N.R. |
| 6 | Et3N (2.0 equiv.) | 460–475 | <10% |
| 7 | TMEDA (3.0 equiv.) | 460–475 | 25% |
| 8 | TMEDA (4.0 equiv.) | 460–475 | 23% |
| 9 | TMEDA (2.0 equiv.) | 390–400 | 83% |
| 10c | TMEDA (2.0 equiv.) | 390–400 | 83% |
| 11d | TMEDA (2.0 equiv.) | 390–400 | 82% |
| 12e | TMEDA (2.0 equiv.) | 390–400 | 86% |
| 13f | — | 390–400 | N.R. |
| 14g | TMEDA (2.0 equiv.) | — | N.R. |
| 15h | TMEDA (2.0 equiv.) | 390–400 | Trace |
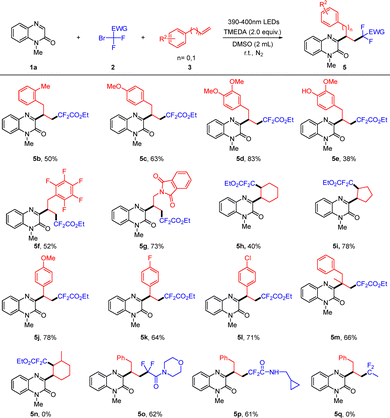
Having identified the optimal conditions, we investigated the substrate scope of various quinoxalin-2(1H)-ones (Scheme 2). Quinoxalinones bearing either electron-donating groups or electron-withdrawing groups could proceed smoothly to give the products 4b–4i in moderate to good yields. Furthermore, a series of the 6,7-disubstituted quinoxalin-2(1H)-ones were tested for this reaction and the desired products were obtained in moderate yields (4j–4k). It is worth noting that N-free protected quinoxalinone was also compatible with this reaction system, generating the desired product 4l in 47% yield. Besides, some N-substituted substrates of quinoxalin-2(1H)-ones including N-ethyl, N-esteryl, N-propargyl, N-benzyl, N-phenethyl and N-cyanomethyl groups also exhibited good applicability (4m–4r). Nevertheless, when other heterocycles such as quinoxaline, 1-(1-methyl-1H-indol-3-yl)ethan-1-one, 1-(1H-pyrrol-3-yl)ethan-1-one, 3,5-dimethyl-1H-pyrazole, 1,3-dimethylpyrimidine-2,4(1H,3H)-dione, and 2H-chromen-2-one were utilized in this reaction system, none of the desired products were observed (see the SI).
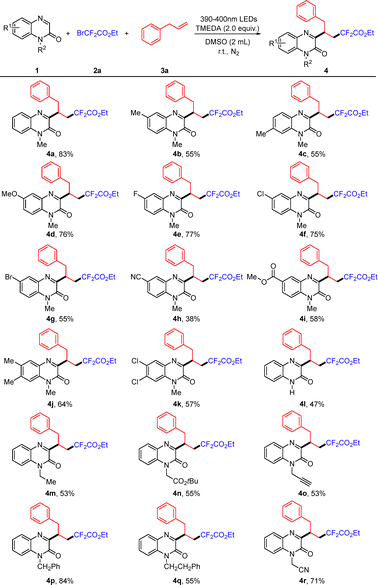 | ||
Scheme 2 Substrate scope of quinoxalin-2(1H)-ones.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), 3a (0.4 mmol), TMEDA (0.4 mmol), DMSO (2.0 mL), room temperature, N2, and 24 h. Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), 3a (0.4 mmol), TMEDA (0.4 mmol), DMSO (2.0 mL), room temperature, N2, and 24 h. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield. Isolated yield. | ||
After examining the substrate scope of quinoxalin-2(1H)-ones, a series of difluoroalkyl bromides 2 and unactivated alkenes 3 were then explored (Scheme 3). A variety of allylbenzenes with electron-donating groups and electron-withdrawing groups on the phenyl ring all worked well, giving the desired products (5b–5f). Notably, the allylbenzene containing free hydroxyl group was well tolerated, leading to the desired product 5e in 38% yield. When 3-phthalamido-substituted propylene was used as the substrate, the corresponding product 5g could be obtained in 73% yield. Other cycloalkenes such as cyclohexene and cyclopentene were also suitable for this reaction, affording the corresponding products 5h and 5i in 40% and 78% yields. When aromatic alkenes such as 4-methoxystyrene, 4-fluorostyrene and 4-chlorostyrene were used in this reaction system, the corresponding products 5j–5l could be obtained in 64–78% yields. The impact of the steric hindrance was also investigated in this reaction. When more sterically demanding (2-methylallyl)benzene was used as a substrate, the desired product 5m was obtained in 66% yield. Nevertheless, when 3-methylcyclohex-1-ene was employed in this reaction procedure, none of desired product 5n was observed. Unfortunately, none of the desired product was detected when propargyl benzene was used as the substrate under the standard conditions. Finally, various bromodifluoroacetamides were subjected to reaction with 1a and 3a under the standard conditions, affording the desired products 5o and 5p in moderate yields. When 1-bromo-1,1-difluoroethane was used in this reaction, the desired product 5q was not obtained. We speculate that the presence of a EWG at the alpha -position of the BrCF2 functionality is necessary for promoting this reaction.13
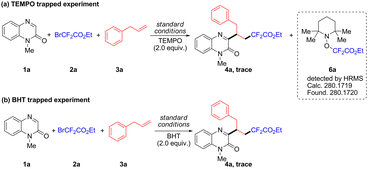
To gain insight into the possible reaction mechanism, several control experiments were conducted. Firstly, when TEMPO and BHT were added into the reaction system, the formation of 4a was inhibited (Scheme 4). Meanwhile, the radical adduct 6a was detected by HRMS analysis (Fig. S1, SI). The above results indicated that this procedure might proceed via a radical pathway. Next, light on/off experiments were performed; the results demonstrated that the constant light irradiation was necessary for this transformation (Fig. S2, SI). To further explore whether this reaction was mediated by the EDA complex, UV-vis analysis of 2a and TMEDA was carried out. When 2a and TMEDA were mixed in DMSO, the formation of a mixed solution was confirmed by the appearance of a yellow colour and an obvious red shift in the absorption spectrum (Fig. S3, SI). Meanwhile, the 19F NMR titration experiments performed with 2a and TMEDA demonstrated that the 19F NMR signal distinctly moved downfield when the ratios of TMEDA increased (Fig. S4, SI). These results suggested the formation of an EDA complex between 2a and TMEDA.11d In order to prove the synthetic applicability of this reaction, a scale-up experiment was carried out between 1-methylquinoxalin-2(1H)-one 1a, ethyl bromodifluoroacetate 2a and allylbenzene 3a, and the corresponding product 4a was obtained in 76% yield (SI).
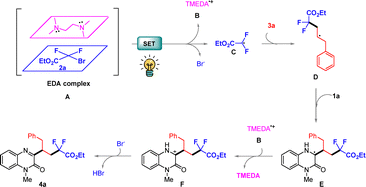
On the basis of previous literature reports and the above experimental results,14,15 a possible mechanism was illustrated as shown in Scheme 5. Firstly, this reaction commences with the formation of an electron donor–acceptor (EDA) complex between compound 2a and TMEDA. Upon irradiation with visible light, the EDA complex undergoes a SET process, generating radical cationic B, difluoroalkyl radical C and releasing a bromide anion. Next, difluoroalkyl radical C undergoes radical addition with alkene 3a to generate the intermediate D. Then, the alkyl radical D couples with quinoxalin-2(1H)-one 1a to produce carbon radical intermediate E. E proceeds through the SET oxidative process by a radical cationic B to give carbon cation intermediate F. Finally, the deprotonation of intermediate F affords the desired product 4a.
In summary, we have developed a visible-light-induced EDA complex that promotes a three-component difluoroalkylation reaction of quinoxalin-2(1H)-ones, unactive alkenes and difluoroalkyl bromides. Various 3-difluoroalkylated quinoxalin-2(1H)-ones could be efficiently obtained with good functional group tolerance under the mild conditions. Mechanistic control experiments indicated that the reaction was triggered by photoactivation through the generation of an EDA complex between TMEDA and BrCF2CO2Et. It provided a green and efficient strategy to construct difluoroalkylated quinoxalinone derivatives from simple small molecules.
We are grateful to the Natural Science Foundation of Shandong Province (ZR2024QB249) for the financial support.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: general synthetic experimental details, characterization data, and NMR spectra. See DOI: https://doi.org/10.1039/d5cc04758g.Notes and references
- (a) D. O’Hagan, Chem. Soc. Rev., 2008, 37, 308–319 RSC; (b) C. Ni and J. Hu, Chem. Soc. Rev., 2016, 45, 5441–5454 RSC; (c) K. Yamamoto, J. Li, J. A. O. Garber, J. D. Rolfes, G. B. Boursalian, J. C. Borghs, C. Genicot, J. Jacq, M. van Gastel, F. Neese and T. Ritter, Nature, 2018, 554, 511–514 CrossRef CAS.
- (a) N. J. Carter and G. M. Keating, Drugs, 2007, 67, 2277–2288 CrossRef CAS PubMed; (b) J. Wang, M. Sanchez-Rosello, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (c) Z. Feng, Y. L. Xiao and X. Zhang, Acc. Chem. Res., 2018, 51, 2264–2278 CrossRef CAS; (d) Y. Zhu, J. Han, J. Wang, N. Shibata, M. Sodeoka, V. A. Soloshonok, J. A. S. Coelho and F. D. Toste, Chem. Rev., 2018, 118, 3887–3964 CrossRef CAS PubMed.
- (a) H. M. Refaat, A. A. Moneer and O. M. Khalil, Arch. Pharmacal Res., 2004, 27, 1093–1098 CrossRef CAS PubMed; (b) B. Wu, Y. Yang, X. Qin, S. Zhang, C. Jing, C. Zhu and B. Ma, ChemMedChem., 2013, 8, 1913–1917 CrossRef CAS PubMed; (c) L. L. Shi, W. Hu, J. F. Wu, H. Y. Zhou, H. Zhou and X. Li, Mini-Rev. Med. Chem., 2018, 18, 392–413 CrossRef CAS PubMed; (d) Q. M. Ke, G. B. Yan, J. Yu and X. Wu, Org. Biomol. Chem., 2019, 17, 5863–5881 RSC.
- (a) W. W. Wang, T. H. Zhu and J. Wu, Org. Chem. Front., 2023, 10, 5375–5382 RSC; (b) R. Shaw, N. Sihag, H. Bhartiya and M. R. Yadav, Org. Chem. Front., 2024, 11, 954–1014 RSC.
- (a) G. F. Hong, J. W. Yuan, J. H. Fu, G. Y. Pan, Z. W. Wang, L. R. Yang, Y. M. Xiao, P. Mao and X. M. Zhang, Org. Chem. Front., 2019, 6, 1173–1182 RSC; (b) C. Jin, X. Zhuang, B. Sun, D. Li and R. Zhu, Asian J. Org. Chem., 2019, 8, 1490–1494 CrossRef CAS; (c) L. Wang, H. Liu, F. Li, J. Zhao, H. Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 2354–2359 CrossRef CAS; (d) Z. Wei, S. Qi, Y. Xu, H. Liu, J. Wu, H. Li, C. Xia and G. Duan, Adv. Synth. Catal., 2019, 361, 5490–5498 CrossRef CAS.
- (a) H. Jiang and A. Studer, Chem. Soc. Rev., 2020, 49, 1790–1811 RSC; (b) Y. Q. Li, D. Wu, H. G. Cheng and G. Y. Yin, Angew. Chem., Int. Ed., 2020, 59, 7990–8003 CrossRef CAS; (c) Q. Feng, J. Hao, Y. Hu, R. Fu, W. Wei and D. Yi, Chin. Chem. Lett., 2025, 36, 110582 CrossRef CAS; (d) X.-M. Chen, L. Song, J. Pan, F. Zeng, Y. Xie, W. Wei and D. Yi, Chin. Chem. Lett., 2024, 35, 110112 CrossRef CAS; (e) Y. Wang, J. Wang, C. Chen, L. Song, Z.-L. Wang, W. Wei and D. Yi, Chem. Commun., 2025, 61, 10812–10815 RSC.
- Y. R. Yuan, L. Li, X. Bu, X. Wang, R. Sun, M. D. Zhou and H. Wang, Asian J. Org. Chem., 2022, 11, e202200139 CrossRef CAS.
- L. Shen, J. W. Yuan, B. Zhang, S. Y. Song, L. R. Yang, Y. M. Xiao, S. R. Zhang and L. B. Qu, Z. Naturforsch., 2023, 78, 245–250 CrossRef CAS.
- T. Singh, S. R. Nasireddy, G. C. Upreti, S. Arora and A. P. Singh, Org. Lett., 2023, 25, 5558–5562 CrossRef CAS.
- (a) C. G. S. Lima, T. d M. Lima, M. Duarte, I. D. Jurberg and M. W. Paixão, ACS Catal., 2016, 6, 1389–1407 CrossRef CAS; (b) L. M. Kammer, S. O. Badir, R.-M. Hu and G. A. Molander, Chem. Sci., 2021, 12, 5450–5457 RSC; (c) G.-Z. Wang, M.-C. Fu, B. Zhao and R. Shang, Sci. China. Chem., 2021, 64, 439–444 CrossRef CAS; (d) Y.-M. Tian, E. Hofmann, W. Silva, X. Pu, D. Touraud, R. M. Gschwind, W. Kunz and B. König, Angew. Chem., Int. Ed., 2023, 62, e202218775 CrossRef CAS PubMed; (e) M. Huang, G. Wang, H. Li, Z. Zou, X. Jia, G. Karotsis, Y. Pan, W. Zhang, J. Ma and Y. Wang, Green Chem., 2024, 27, 413–419 RSC.
- (a) D. M. Whalley, H. A. Duong and M. F. Greaney, Chem. – Eur. J., 2019, 25, 1927–1930 CrossRef CAS PubMed; (b) G. Zhao, S. Lim, D. G. Musaev and M.-Y. Ngai, J. Am. Chem. Soc., 2023, 145, 8275–8284 CrossRef CAS; (c) S. P. Zhang, J. X. Lan, M. L. Yang, J. Y. Cao, Y. C. Zhang and W. C. Yang, Org. Lett., 2024, 26, 9990–9995 CrossRef CAS PubMed; (d) S. J. Song, C. Luo, G. Wang, J. J. Guo, Z. Chen and J. J. Li, Chem. Commun., 2024, 60, 11323–11326 RSC; (e) K. Mondal and M. Baidya, Adv. Synth. Catal., 2024, 366, 148–153 CrossRef CAS.
- (a) Y. Lv, H. Ding, J. You, W. Wei and D. Yi, Chin. Chem. Lett., 2024, 35, 109107 CrossRef CAS; (b) Z. Wang, N. Meng, Y. Lv, W. Wei, H. Yue and G. Zhong, Chin. Chem. Lett., 2023, 34, 107599 CrossRef CAS; (c) Z. Yang, C. Liu, J. Lei, Y. Zhou, X. Gao and Y. Li, Chem. Commun., 2022, 58, 13483–13486 RSC; (d) C. Liu, Q. Dai, Y. Li, C. Huang, L. Guo and Z. Yang, J. Org. Chem., 2023, 88, 7281–7289 CrossRef CAS PubMed; (e) Z. Yang, Y. Zhou, H. Li, J. Lei, P. Bing, B. He and Y. Li, Asian J. Org. Chem., 2022, 11, e202100656 CrossRef CAS; (f) Z. Yang, J. Luo, W. Zhang, J. Lei, C. Liu and Y. Li, Org. Biomol. Chem., 2025, 23, 323–327 RSC.
- G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, J. Am. Chem. Soc., 2020, 142, 5461–5476 CrossRef CAS PubMed.
- (a) A. Carrër, J. D. Brion, S. Messaoudi and M. Alami, Org. Lett., 2013, 15, 5606–5609 CrossRef PubMed; (b) K.-J. Li, Y.-Y. Jiang, K. Xu, C.-C. Zeng and B.-G. Sun, Green Chem., 2019, 21, 4412–4421 RSC; (c) Z.-Z. Xie, Y. Zheng, K. Tang, J.-P. Guan, C.-P. Yuan, J.-A. Xiao, H.-Y. Xiang, K. Chen, X.-Q. Chen and H. Yang, Org. Lett., 2021, 23, 9474–9479 CrossRef CAS PubMed.
- (a) W. F. Wang, T. Liu, Y. L. Cheng and Q. H. Song, Org. Biomol. Chem., 2024, 22, 805–810 RSC; (b) J.-C. Hou, J. Jiang, Y.-C. Wen, Y.-Y. Zeng, Y.-H. Lu, J.-S. Wang, L.-J. Ou and W.-M. He, J. Org. Chem., 2024, 89, 6117–6125 CrossRef CAS PubMed; (c) Y.-D. Xu, Y.-M. Xing, H.-T. Ji, L.-J. Ou, W.-B. He, J. Peng, J.-S. Wang, J. Jiang and W.-M. He, J. Org. Chem., 2024, 89, 17701–17707 CrossRef CAS PubMed; (d) J. H. Ji, K. J. Li, X. Z. Zhou, C. Xu, D. Zhang and W. Shao, Chem. Commun., 2025, 61, 6767–6770 RSC.
| This journal is © The Royal Society of Chemistry 2025 |