Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling crystallisation with audible low frequency sound
Yasmin C. S.
Figueiredo
a,
Thiago G.
Tabuti
a,
Thaisa B. F.
Moraes
a,
Julian
Ticona-Chambi
 b,
Rafael G.
Candido
a,
Silvia L.
Cuffini
b,
Marcelo
S. Martins
b,
Rafael G.
Candido
a,
Silvia L.
Cuffini
b,
Marcelo
S. Martins
 d,
Walter L. M.
Tupinambá
d,
Eduardo R.
Triboni
d,
Walter L. M.
Tupinambá
d,
Eduardo R.
Triboni
 *a and
Jonathan W.
Steed
*a and
Jonathan W.
Steed
 *c
*c
aLaboratório Nanotecnologia e Engenharia de Processos-- NEP, Universidade de São Paulo, CEP 12602-810, Lorena, SP, Brazil. E-mail: tribonier@up.br
bPós-graduação em Engenharia e Ciência de Materiais, Instituto de Ciência e Tecnologia (ICT), Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil
cDepartment of Chemistry, Durham University, South Road, Durham DH1 3LE, UK. E-mail: jon.steed@durham.ac.uk
dDepartamento de Mecânica – Laboratório de Estruturas, Universidade Estadual Paulista, UNESP/FEG – Campus de Guaratinguetá, Brazil
First published on 29th September 2025
Abstract
We show that low-frequency audible sound drives the crystallisation of distinct solid forms of organic compounds in solution crystallisation. In contrast to silent crystallisation, ROY and anthranilic acid crystallized as distinct polymorphs, concomitant forms, and with novel morphologies resulting in preferred orientation depending on the sound frequency and amplitude.
Audible low frequency sound (ALFS) in the range of 20–20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz is not expected to interfere directly with matter at atomic or molecular levels due to its long wavelengths. The usual ALFS outcomes are the occurrence of patterns over liquid surfaces and from materials which are spread upon plates, termed Chladni figures or Faraday instability.1,2 However, there is evidence that ALFS can promote rearrangements and assemblies of molecules in solution,3,4 regulate biological processes, and enhance the rate of crystallisation of proteins.5,6 In addition, Wang and coworkers found a significant influence of ALFS on the redox-reaction of viologen molecules forming spatio-temporal patterns related to oxidation or reduction process.7 Closely related studies have captured acoustic sounds derived from solution crystallisations and applying acoustic levitation to evaluate crystal growth.8,9 ALFS differs from ultrasound because it is not expected to give rise to acoustic cavitation. Ultrasonic waves of frequencies between 20 kHz and 100 kHz and at sufficient amplitude can form and collapse microscopic bubbles within liquids,10 delivering high thermal energy and shock waves which may provide sonochemically induced transformations as well as impact the assembly of molecules and macromolecules.11,12 Hence, ultrasound has resulted in accelerated crystallization, controlled size distribution and morphology, and polymorphic outcome.13–17 There are many crystallisation techniques such as antisolvent crystallisation, vapor diffusion, melt crystallisation, evaporation, cooling and rapid evaporation.18,19 All of these approaches at a certain point create a supersaturation domain whereby molecules may nucleate and grow to bulk crystals. Upon crystallisation molecules may pack in distinct arrangements and conformations giving rise to the occurrence of polymorphs whose physicochemical properties such as melting point, solubility, electrical conductivity and colour can be significantly different from one another.18–20 Such a structure–property relationship brings about the need for exhaustive screening looking for desired features, particularly for active pharmaceutical ingredients which have particular requirements for solubility, processing characteristics, and bioavailability. Herein, we have conceived an unprecedented ALFS crystallisation (sound crystallisation) technique based on the premise that ALFS passing through the crystallising solution may perturb and drive all stages of crystallisation, since it is an out-of-equilibrium process. To test this hypothesis we chose the popular 5-methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile (ROY) as a model because it provides a range of colours and specific morphologies for each of its fourteen polymorphs including yellow prisms (Y), red prisms (R), orange needles (ON), orange plates (OP), yellow needles (YN), orange-red plates (ORP), and red plates (RP),21–25Fig. 1A. The seven most commonly accessible ROY polymorphs are stable at room temperature and crystallise from solvent-based processes, which leads to the occurrence of concomitant polymorphism.26–28 A homemade acoustic apparatus comprising wave-generator, sub-woofer, amplifier, and an acrylic plate was built up for performing the ROY sound crystallisations. Crystallisations were performed in ethanol (EtOH) and isopropanol (i-Prop) and were found to give rise to concomitant forms, preferred orientation, and unexpected polymorphs depending upon the ALFS setup and the interplay of frequency, amplitude and solvent (see SI, Section SI-1 for experimental and sound standardisation details). In this work typical solvent-based crystallisation is termed ‘silent crystallisation’ and was carried out as a control in order to compare with crystallisation under ALFS conditions (‘sound crystallisation’). Additionally, we extended the ALFS setup to crystallise anthranilic acid (AA) in water, giving rise to the unexpected form I in this solvent. To the best of our knowledge this is the first report of the use of ALFS for controlling the crystal characteristics of organic compounds and, hence, we anticipate that sound crystallisation may offer a new tool in solid form screening and control and may serve as key technique in process industries dealing with molecular crystallisation.
000 Hz is not expected to interfere directly with matter at atomic or molecular levels due to its long wavelengths. The usual ALFS outcomes are the occurrence of patterns over liquid surfaces and from materials which are spread upon plates, termed Chladni figures or Faraday instability.1,2 However, there is evidence that ALFS can promote rearrangements and assemblies of molecules in solution,3,4 regulate biological processes, and enhance the rate of crystallisation of proteins.5,6 In addition, Wang and coworkers found a significant influence of ALFS on the redox-reaction of viologen molecules forming spatio-temporal patterns related to oxidation or reduction process.7 Closely related studies have captured acoustic sounds derived from solution crystallisations and applying acoustic levitation to evaluate crystal growth.8,9 ALFS differs from ultrasound because it is not expected to give rise to acoustic cavitation. Ultrasonic waves of frequencies between 20 kHz and 100 kHz and at sufficient amplitude can form and collapse microscopic bubbles within liquids,10 delivering high thermal energy and shock waves which may provide sonochemically induced transformations as well as impact the assembly of molecules and macromolecules.11,12 Hence, ultrasound has resulted in accelerated crystallization, controlled size distribution and morphology, and polymorphic outcome.13–17 There are many crystallisation techniques such as antisolvent crystallisation, vapor diffusion, melt crystallisation, evaporation, cooling and rapid evaporation.18,19 All of these approaches at a certain point create a supersaturation domain whereby molecules may nucleate and grow to bulk crystals. Upon crystallisation molecules may pack in distinct arrangements and conformations giving rise to the occurrence of polymorphs whose physicochemical properties such as melting point, solubility, electrical conductivity and colour can be significantly different from one another.18–20 Such a structure–property relationship brings about the need for exhaustive screening looking for desired features, particularly for active pharmaceutical ingredients which have particular requirements for solubility, processing characteristics, and bioavailability. Herein, we have conceived an unprecedented ALFS crystallisation (sound crystallisation) technique based on the premise that ALFS passing through the crystallising solution may perturb and drive all stages of crystallisation, since it is an out-of-equilibrium process. To test this hypothesis we chose the popular 5-methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile (ROY) as a model because it provides a range of colours and specific morphologies for each of its fourteen polymorphs including yellow prisms (Y), red prisms (R), orange needles (ON), orange plates (OP), yellow needles (YN), orange-red plates (ORP), and red plates (RP),21–25Fig. 1A. The seven most commonly accessible ROY polymorphs are stable at room temperature and crystallise from solvent-based processes, which leads to the occurrence of concomitant polymorphism.26–28 A homemade acoustic apparatus comprising wave-generator, sub-woofer, amplifier, and an acrylic plate was built up for performing the ROY sound crystallisations. Crystallisations were performed in ethanol (EtOH) and isopropanol (i-Prop) and were found to give rise to concomitant forms, preferred orientation, and unexpected polymorphs depending upon the ALFS setup and the interplay of frequency, amplitude and solvent (see SI, Section SI-1 for experimental and sound standardisation details). In this work typical solvent-based crystallisation is termed ‘silent crystallisation’ and was carried out as a control in order to compare with crystallisation under ALFS conditions (‘sound crystallisation’). Additionally, we extended the ALFS setup to crystallise anthranilic acid (AA) in water, giving rise to the unexpected form I in this solvent. To the best of our knowledge this is the first report of the use of ALFS for controlling the crystal characteristics of organic compounds and, hence, we anticipate that sound crystallisation may offer a new tool in solid form screening and control and may serve as key technique in process industries dealing with molecular crystallisation.
The silent ROY crystallisations in both EtOH and i-Prop gave rise to crystals with the same powdery appearance as the purchased material, Fig. 1B, and the powder X-ray diffraction (PXRD) patterns showed it to be the Y form (monoclinic, space group P21/n – CSD refcode QAXMEH01,29 SI-2). This polymorph has a torsion angle (θ) of 104.7° and its structure is dominated by π–π stacking of the nitrophenyl and thiophenyl substituents. This polymorph is the most stable polymorph in the solid state at room temperature.30 Moreover, the Y polymorph is expected to nucleate in alcohols like MeOH, EtOH and i-Prop as well as the YN and OP ROY forms.27,29 Sound crystallisation significantly modifies the crystal morphologies and colours of ROY samples depending upon the acoustic setup and whether the crystallisation vessel is either placed on the plate or simply hung on the subwoofer. This suspended arrangement is simply influenced by the acoustic field passing through it, whereas that settled on the plate must be seen as a mechanically coupled acoustic crystallisation because the whole system, i.e. vessel wall, plate, and subwoofer, is interconnected and vibrates as the sound parameters change. The suspended arrangement gave rise to the same Y polymorph as the silent procedures but with different crystal colours and preferred orientation depending mainly upon the solvent, but not significantly on the ALFS parameters. Dark yellow prisms (Fig. 2A, Y1) came out from ethanol while fibre-like structures were obtained from i-Prop (Fig. 2B, Y2) regardless of the amplitude or frequency used. Thermal analysis of these two solids shows very similar melting onset temperatures, 110.1 °C and 110.7 °C, respectively, for the prismatic- and fibre-like ROY, Fig. 2A and B; this difference is within experimental error. The pictures in Fig. 2 highlight the distinct appearance of the Y1 and Y2 ROY morphologies, and Fig. 3 shows the diffractogram of these solids after grinding. An SEM image of this needle-like structure is shown in detail (Fig. SI-3B). Combining ALFS and isopropanol, therefore, an unexpected morphology and hence preferred orientation arises for the Y form whose usual habit is prismatic. The morphology of the sound-crystallized ROY resembles the YN form (triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) – CSD QAXMEH04)29 but PXRD analysis confirms it to be the thermodynamically stable Y polymorph (QAXMEH01). Hence, the ALFS induces a preferential growth of one of the facets of the Y crystal resulting in (010) preferred orientation (SI-3).
– CSD QAXMEH04)29 but PXRD analysis confirms it to be the thermodynamically stable Y polymorph (QAXMEH01). Hence, the ALFS induces a preferential growth of one of the facets of the Y crystal resulting in (010) preferred orientation (SI-3).
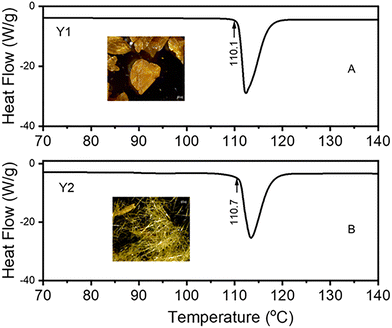 | ||
| Fig. 2 DSC thermograms showing the melting onset of ROY crystals (polymorph Y) produced using ALFS crystallisation in suspended mode (A) Y1 and (B) Y2. | ||
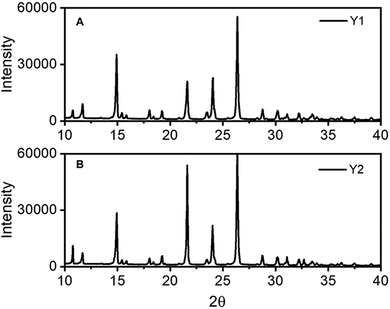 | ||
| Fig. 3 PXRD of the ROY obtained by suspended ALFS crystallisation; (A) Y1 from EtOH, and (B) Y2 from i-Prop. | ||
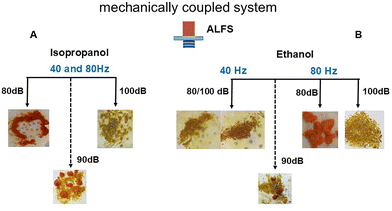
On the other hand, the mechanically coupled crystallisations were more sensitive to the ALFS parameters and to the solvents as well, providing a variety of ROY forms, Fig. 4A and B. In i-Prop at varying intensities of 80 dB, 90 dB, and 100 dB, and at frequencies of both 40 and 80 Hz, three different solid forms or mixtures were obtained, respectively: ON form (monoclinic, P21/c, CSD QAXMEH),29 ON and Y concomitant forms, and the Y polymorph. In EtOH, however, distinct frequencies led to different results. At 40 Hz only the Y polymorph is formed when using 80 dB and 100 dB, whereas at 80 Hz the low amplitude delivers predominantly the ON polymorph while the high amplitude one the Y one. Fig. 5 depicts the diffractograms of pure ROY polymorphs ON and Y. The ON form is unexpected when crystallising in alcohols from which the most common form is the OP polymorph.25 However, the most significant outcome is the selective crystallisation of ROY polymorphs as the amplitude is changed, and in ethanol different frequencies of 80 or 40 Hz nucleate distinct polymorphs. The tendency is at low intensity 80 dB the ON form is preferred over any other while at 100 dB the Y form is prevalent. Notably, at 90 dB, for all the ALFS parameters the two ON and Y forms always solidified out, hence resulting in consistent concomitant polymorphism (see PXRD and pictures of these solid forms in Section SI-4). These outcomes demonstrate a significant impact of ALFS on crystallisation result, whether just passing the wave through the solution or mechanically coupling it.
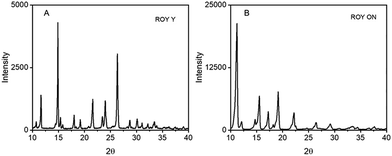 | ||
| Fig. 5 Diffractograms of the ROY, (A) Y and (B) ON polymorphs obtained at different ALFS parameters. | ||
In order to rule our extraneous factors we measured the amount of evaporation and cooling rate of the solvents, as well as determined the possible water sorption under both ALFS and silent conditions for 60 min (SI-5). Fig. SI-5 shows temperatures obtained at different frequencies and amplitudes at 80 and 100 dB, and those for silent conditions. In all cases, the starting temperatures at 60 °C decrease by approximately half of their initial value after 10 min then drops smoothly to room temperature (at 22 °C), but the solutions remain around 2° to 4 °C warmer under ALFS than in silent mode. The solvent loss by evaporation averaged 0.7 mL for both silent and ALFS crystallisation systems and with no significant water uptake by Karl Fischer titration, indicating the same supersaturation regime during the crystallisation processes. Given that ROY crystallisation begins in a range of 5 to 10 min, and taking into account distinct results obtained by changing the ALFS parameters, we can rule out differences in the cooling, evaporation rate, and water absorption on the ALFS crystallisation results.
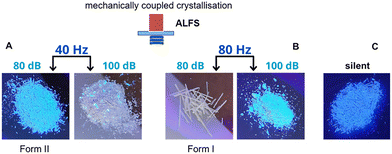
Anthranilic acid (AA, 2-aminobenzoic acid) has three known polymorphs.30,31 Forms I and II (CSD refcodes AMBACO07 and AMBACO05)32,33 are orthorhombic structures in space groups P21cn and Pbca, while polymorph III adopts P21/c (CSD AMBACO08).34 Form I is a zwitterionic polymorph with one neutral and one ionic hydrogen bond in the asymmetric unit (Z′ = 2), whereas forms II and III have the centrosymmetric carboxylic acid dimer synthon (Z′ = 1).31 Form I has been reported to crystallise from ethanol and methanol, and form II in protic polar and aprotic dipolar solvents as water, methanol, ethanol, acetic acid, acetonitrile and nitrobenzene mainly by using slow evaporation, whereas thermal techniques such as sublimation and melt crystallisation have been the best way to prepare form III.30,31 Recently, Bag and coworkers obtained the kinetically crystallised form I from water by a fast evaporation technique that avoids conversion into the most stable form II.30 We attempted to prepare AA polymorphs by mechanically coupled ALFS crystallisation in water at varying intensities of 80 dB and 100 dB, and at frequencies of 40 and 80 Hz. Single and concomitant crystallisation of forms I and II was observed depending on the ALFS configuration, and were confirmed by PXRD, Fig. 6. In addition, these polymorphs can be easily identified by their intrinsic solid-state fluorescence since form I exhibits a bright blueish colour while form II is off-white when exciting at λ = 365 nm, Fig. 7. Variation of the ALFS parameters provides distinct and opposite results. At 40 Hz, crystallisations performed at low (80 dB) and high (100 dB) amplitudes give rise, respectively, to form II and to the occurrence of concomitant polymorphs, with form I being quite prevalent. Conversely, at 80 Hz, form I crystallises out at low amplitude while form II is formed at high amplitude, with single polymorphs obtained in each case. Silent crystallisation in water leads to the expected form II.32,33 Therefore, beyond driving a selective occurrence of two anthranilic acid polymorphs, sound crystallisation is allowing the crystallisation of the metastable form I in water.
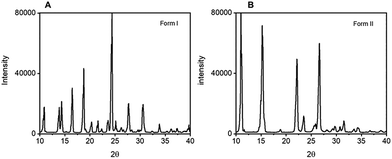 | ||
| Fig. 6 PXRD of the AA crystals obtained by sound crystallisation; (A) diffractogram of the form I and (B) of the form II.30 | ||
Audible low frequency sound has a very significant effect on the solvent crystallisation of ROY and anthranilic acid and is likely to have a significant influence on the crystallisation outcomes of many substances including those of pharmaceutical interest. The results of both the suspended and mechanically coupled crystallisations gave rise to different polymorphs, influenced preferred orientation and resulted in concomitant crystallisation of multiple forms depending on the ALFS amplitude and frequency as well as the solvent. In the ROY crystallisation system, polymorphs ON and Y selectively crystallised out from ethanol and isopropanol, and an unusual needle-like morphology of Y was obtained in preference to the prismatic one in the suspended ALFS arrangement, resulting in pronounced preferred orientation. In the anthranilic acid system, both the forms I and II were selectively formed in water, hence, giving rise to the unexpected formation of the less stable form I which does not usually crystallise from water due to its conversion to the most stable form II. These results highlight the promising application of the ALFS on such out-of-equilibrium process although it remains very difficult yet to predict in advance which ALFS parameters will result in the crystallisation of a particular polymorph. Nevertheless, these results indicate that ALFS represents a potentially significant new crystallisation tool in the polymorph screening arsenal.
We thank the undergraduate Jonas Luis Massuchini de Carvalho from EEL-USP for the starting studies with ROY crystallisation supported by USP fellowship. The São Paulo Research Foundation – FAPESP grants 2021/01509-0, 2022/06346-5, 2024/09719-2 (ERT), 2019/27157-3 (TBFM). CAPES grant 88887.847777/2023-00 (TGT) and FINEP grant 012323700 (RGC).
Conflicts of interest
There are no conflicts to declare.Data availability
Underlying research data for this paper is available from https://doi.org/10.15128/r28k71nh14w.Supplementary information (SI): experimental details, PXRD pattern of ROY preferred orientation, author information. See DOI: https://doi.org/10.1039/d5cc04356e.
Notes and references
- V. Zhou, K. Sariola, V. Latifi and V. Liimatainen, Nat. Commun., 2016, 7(1), 12764 CrossRef PubMed.
- M. T. Westra, D. J. Binks and W. Van De Water, J. Fluid Mech., 2003, 496, 1 CrossRef.
- A. Tsuda, Y. Nagamine, R. Watanabe, Y. Nagatani, N. Ishii and T. Aida, Nat. Chem., 2010, 2, 977 CrossRef CAS PubMed.
- Y. S. Fukushima, J. Motoyanagi and A. Tsuda, Chem. Commun., 2015, 51, 2790 RSC.
- C. Y. Zhang, Y. Wang, R. Schubert, Y. Liu, M. Y. Wang, D. Chen and D. C. Yin, Cryst. Growth Des., 2016, 16, 705 CrossRef CAS.
- C. Y. Zhang, J. Liu, M. Y. Wang, W. J. Liu, N. Jia, C. Q. Yang, M. L. Hu, Y. Liu, X. Y. Ye, R. B. Zhou and D. C. Yin, Cryst. Growth Des., 2018, 19, 258 CrossRef.
- I. Hwang, R. D. Mukhopadhyay, P. Dhasaiyan, S. Choi, S. Y. Kim, Y. H. Ko and K. Kim, Nat. Chem., 2020, 12, 808 CrossRef CAS PubMed.
- S. B. Gregersen, M. J. Povey, M. D. Andersen, M. Hammershøj, M. Rappolt, A. Sadeghpour and L. Wiking, Eur. J. Lipid Sci. Technol., 2016, 118, 1257 CrossRef CAS.
- W. J. Xie, C. D. Cao, Y. J. Lü and B. Wei, Phys. Rev. E, 2002, 66, 061601 CrossRef CAS PubMed.
- K. S. Suslick, Science, 1990, 247, 1439 CrossRef CAS PubMed.
- W. Chen, P. Dai, C. Hong, C. Zheng, W. Wang and X. Yan, Cryst. Eng. Commun., 2018, 20, 2989 RSC.
- J. Liu, G. Song, Y. Yuan, L. Zhou, D. Wang, T. Yuan and J. Gong, Ultrason. Sonochem., 2022, 86, 106025 CrossRef CAS PubMed.
- A. Kozell, A. Solomonov and U. Shimanovich, FEBS Lett., 2023, 597, 3013 CrossRef CAS PubMed.
- K. A. Ramisetty, A. B. Pandit and P. R. Gogate, Ind. Eng. Chem. Res., 2013, 52, 17573 CrossRef CAS.
- Q. Zhao, L. Yang, C. Yao and G. Chen, Ind. Eng. Chem. Res., 2023, 62, 20083 CrossRef CAS.
- P. R. Birkin, J. J. Youngs, T. T. Truscott and S. Martini, Phys. Chem. Chem. Phys., 2022, 24, 11552 RSC.
- J. R. Sander, B. W. Zeiger and K. S. Suslick, Ultrason. Sonochem., 2014, 21, 1908 CrossRef CAS PubMed.
- A. Myerson, Handbook of Industrial Crystallisation, Butterworth-Heinemann, 2022 Search PubMed.
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, John Wiley & Sons, 2022 Search PubMed.
- J. Bernstein, Polymorphism in Molecular Crystals, International Union of Crystal, 2020 Search PubMed.
- A. J. Alvarez, A. Singh and A. S. Myerson, Cryst. Growth Des., 2009, 9, 4181 CrossRef CAS.
- A. Nangia, Acc. Chem. Res., 2008, 41, 595 CrossRef CAS PubMed.
- S. Chen, I. A. Guzei and L. Yu, J. Am. Chem. Soc., 2005, 127, 9881 CrossRef CAS PubMed.
- A. Levesque, T. Maris and J. D. Wuest, J. Am. Chem. Soc., 2020, 142, 11873 CrossRef CAS PubMed.
- G. J. Beran, I. J. Sugden, C. Greenwell, D. H. Bowskill, C. C. Pantelides and C. S. Adjiman, Chem. Sci., 2022, 13, 1288 RSC.
- A. R. Tyler, R. Ragbirsingh, C. J. McMonagle, P. G. Waddell, S. E. Heaps, J. W. Steed and M. R. Probert, Chem, 2020, 6, 1755 CAS.
- T. Gnutzmann, Y. Nguyen Thi, K. Rademann and F. Emmerling, Cryst. Growth Des., 2014, 14, 6445 CrossRef CAS.
- P. T. Cardew and R. J. Davey, Cryst. Growth Des., 2019, 19, 5798 CrossRef CAS.
- L. Yu, Acc. Chem. Res., 2010, 43, 1257 CrossRef CAS PubMed.
- M. Tan, A. G. Shtukenberg, S. Zhu, W. Xu, E. Dooryhee, S. M. Nichols and Q. Zhu, Faraday Discuss., 2018, 211, 477 RSC.
- L. Yu, G. A. Stephenson, C. A. Mitchell, C. A. Bunnell, S. V. Snorek, J. J. Bowyer and S. R. Byrn, J. Am. Chem. Soc., 2000, 122, 585 CrossRef CAS.
- P. P. Bag and C. M. Reddy, Cryst. Growth Des., 2012, 12, 2740 CrossRef CAS.
- S. S. Kumar and A. Nangia, Cryst. Growth Des., 2014, 14, 1865 CrossRef CAS.
- C. J. Brown and M. Ehrenberg, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1985, 41, 441 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |