Recent advances in BiVO4-based heterojunction photocatalysts for energy and environmental applications
Yajie
Bai
*a,
Zhenyuan
Fang
c,
Yuning
Fang
a,
Chenxiao
Lin
 a,
Hongye
Bai
a,
Hongye
Bai
 b and
Weiqiang
Fan
b and
Weiqiang
Fan
 *b
*b
aCollege of New Energy, Ningbo University of Technology, Ningbo, 315336, P. R. China. E-mail: byj@nbut.edu.cn
bSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, 212013, P. R. China. E-mail: fwq4993329@ujs.edu.cn
cSchool of Chemistry, Beihang University, Beijing, 100191, P. R. China
First published on 12th March 2025
Abstract
Photocatalytic technology offers a promising solution by efficiently converting solar energy into chemical energy and addressing environmental pollution. BiVO4 is a promising semiconductor photocatalytic material due to its narrow band gap, good visible light response, and non-toxicity. Recently, there has been significant interest in developing BiVO4-based heterojunction photocatalysts to overcome the challenges of rapid recombination rate of photogenerated charge carriers and insufficient electron transport capacity in pure BiVO4. However, a comprehensive and systematic summary of the role of BiVO4-based heterojunction catalysts in improving photocatalytic performance is lacking. This review covers the mechanisms, challenges, and classification of BiVO4-based heterojunction photocatalysis. It also summarizes recent advancements in using these photocatalysts for energy and environmental applications, such as water splitting, nitrogen fixation, carbon dioxide reduction, pollutant degradation, etc. Perspectives on the existing challenges, potential solutions, and prospects of BiVO4-based heterojunction photocatalysts are outlined, aiming to offer valuable insights to accelerate their commercialization as high-performance photocatalysts.
1. Introduction
Rapid industrial development and continuous global population growth have accelerated energy shortages and environmental pollution.1 Moreover, global industrialization has driven excessive consumption of traditional fossil fuels, such as oil, coal, and natural gas, leading to significant harmful waste emissions that threaten the ecological environment essential for human survival.2 Therefore, it is crucial to develop environmentally friendly and renewable green technologies for both energy production and environmental remediation, addressing the growing challenges of energy shortages and environmental pollution.3 In this case, photocatalytic technology has garnered significant attention as an effective solution to energy and environmental challenges due to its cost-effectiveness, eco-friendliness, mild reaction conditions, and lack of secondary pollution.4,5 This technology enables the photocatalytic processes of water splitting,6 nitrogen fixation,7 and carbon dioxide (CO2) reduction,8 converting renewable solar energy into valuable, storable chemical energy. Furthermore, photocatalysts can directly harness solar energy to activate semiconductor materials, generating reactive species that effectively remove harmful and toxic pollutants.9,10 Since 1972, after Fujishima and Honda discovered the water-splitting phenomenon using TiO2 photocatalysis,11 numerous semiconductor materials have been developed and widely applied in various photocatalytic reactions. Despite their advantages, photocatalytic reactions face practical application challenges due to the intrinsic limitations of photocatalysts, including low efficiency and instability from rapid recombination of photogenerated charge carriers.12,13 Therefore, designing efficient and sustainable semiconductor photocatalysts is crucial.TiO2 is an outstanding photocatalyst due to its high photocatalytic activity, excellent chemical stability, non-toxicity, and cost-effectiveness.14,15 However, conventional anatase-phase TiO2 photocatalysts are active only under ultraviolet (UV) light (wavelength < 387 nm) due to their wide bandgap of 3.2 eV.16,17 Since visible light dominates the solar spectrum, while UV light accounts for less than 4%,18 developing visible-light-responsive photocatalysts is crucial for efficient solar energy utilization. In this case, BiVO4 has emerged as a promising photocatalytic semiconductor material due to its favorable response to visible light.19 BiVO4 has significant potential in various catalytic applications, including water splitting, organic pollutant degradation, CO2 reduction, and nitrate reduction.20–22 However, BiVO4 faces several challenges like low photogenerated charge separation efficiency, slow water oxidation kinetics, and rapid recombination of photogenerated electron–hole pairs.23,24 These challenges limit its practical applications, making the development of efficient BiVO4-based photocatalysts a critical research focus. To enhance the performance of BiVO4, researchers have employed various techniques, including ion doping, crystal facet regulation, morphology control, and microporous/mesoporous structures construction.25–27 Among these strategies, developing BiVO4-based heterojunction catalysts by combining BiVO4 with semiconductors that possess complementary properties and matching energy levels has proven effective in improving photocatalytic performance.28,29 BiVO4-based heterojunction catalysts enhance charge separation efficiency, reduce the recombination rate of photogenerated electron–hole pairs, and introduce new properties through synergistic effects with other components.30,31 Rational design of BiVO4-based heterojunction catalysts presents a promising approach for developing efficient visible-light-driven photocatalysts, thereby advancing environmental and energy applications. Although BiVO4-based heterojunction catalysts have been developed for photocatalysis, there is a lack of a comprehensive and systematic summary of their role in enhancing photocatalytic performance.
Herein, we provide a comprehensive overview of the photocatalytic mechanism, design strategies and types of BiVO4-based heterojunction photocatalysts. BiVO4-based heterojunction photocatalysts can be categorized into five types based on the mechanisms of separation and transport of photogenerated electron–hole pairs: traditional heterojunctions (type I, type II, and type III), Schottky heterojunctions, Z-scheme heterojunctions, and S-scheme heterojunctions. The applications of BiVO4-based heterojunction photocatalysts in energy and environmental processes, including water splitting, nitrogen fixation, CO2 reduction, H2O2 synthesis, pollutant degradation, HCHO removal, and bacterial inactivation, are summarized. This work also highlights the advantages of BiVO4-based heterojunctions in photocatalytic processes, focusing on their construction methods and efficient photogenerated electron–hole separation and transfer mechanisms. It is expected to provide valuable guidance for developing highly efficient heterojunction photocatalysts and to inspire advancements in practical applications across various fields.
2. Fundamentals of photocatalysis
2.1 Mechanisms of photocatalytic activity in BiVO4-based heterojunctions
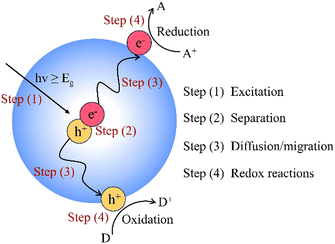
Photocatalytic processes involve a complex reaction mechanism with several complementary and indispensable steps, as shown in Fig. 1. Firstly, light absorption by the semiconductor is a crucial step in photocatalytic reactions. When the photocatalyst is irradiated with photons of energy equal to or greater than its bandgap (Eg), electrons (e−) in the valence band (VB) are excited and transition to the conduction band (CB), forming photogenerated electron–hole pairs (h+). Subsequently, these photogenerated charge carriers separate and migrate to the surface or interface of the semiconductor, facilitating redox reactions and generating radicals such as ˙OH, ˙O2−, and H+. During this process, the recombination of electron–hole pairs can reduce photocatalytic efficiency, as it leads to energy loss in the form of heat instead of driving the reaction. Therefore, it is crucial for the photogenerated electron–hole pairs to selectively transfer to the surface of the photocatalyst to initiate redox reactions, rather than recombine and produce waste heat. Additionally, the adsorption of reactants and desorption of products are also crucial for continuous photocatalytic reaction.32,33 Improving these processes is essential for enhancing photocatalytic performance. Optimizing photocatalyst design and operational conditions can effectively increase the separation efficiency of photogenerated charge carriers, promote the efficient adsorption of reactants, and accelerate product desorption, leading to more efficient photocatalytic reactions.2.2 Challenges of photocatalysis
Despite the significant potential of photocatalytic technologies, their practical implementation still faces several challenges: (1) slow reaction kinetics: photocatalytic processes involve several steps, including excitation, separation, transport, and surface reaction of photogenerated charge carriers. These steps occur at a slow rate, resulting in low efficiency, especially in complex reactions like CO2 and NO3− reduction, where multiple interdependent steps further slow the reaction rate.34 (2) Wide bandgap limiting light absorption: the performance of photocatalysts relies on effective light capture, charge separation, and charge transport. Many commonly used photocatalysts, such as TiO2, have wide bandgaps, limiting their absorption in the visible light range and reducing overall photocatalytic efficiency.35 (3) High recombination rates of photogenerated electrons and holes: photogenerated electrons and holes must be effectively separated and transported to the catalytic surface for redox reactions. However, due to inefficient charge separation and transport, most photogenerated charge carriers recombine, reducing catalytic efficiency and potentially leading to catalyst degradation. Therefore, developing efficient and stable photocatalysts remains a critical challenge in the field.362.3 Advantages of BiVO4-based heterojunction photocatalysts
To address the challenges of photocatalysts, the development of advanced photocatalysts, such as BiVO4-based heterojunction photocatalysts has gained significant attention due to their favorable response to visible light. The main advantages of BiVO4-based heterojunction catalysts are listed as follows: (1) improved reaction kinetics. BiVO4-based heterojunctions can provide more active sites and increase the surface area available for reactions, reducing reaction barriers and accelerating rate-limiting steps. Optimized charge distribution at the interface of BiVO4-based heterojunctions facilitates the adsorption and activation of reactants, enhancing the overall reaction kinetics.37 (2) Expanded light absorption spectrum. Combining BiVO4 with other semiconductors of different bandgaps broadens the light absorption spectrum, enabling efficient absorption of both UV and visible light, thus enhancing overall photocatalytic efficiency.38 (3) Enhanced charge separation and transport efficiency. The interfaces of BiVO4-based heterojunctions can create built-in electric fields that promote effective separation of photogenerated electrons and holes, reducing recombination. When the band structures of the two materials are aligned, electrons can be directed to one material, while holes migrate to the other, further improving charge utilization. Additionally, BiVO4-based heterojunctions can provide multiple pathways for electron transport, accelerating charge migration and enhancing charge separation and transport efficiency.39 By enhancing catalytic activity, optimizing light absorption, and improving charge separation and transport efficiency, BiVO4-based heterojunctions offer a practical solution to the challenges faced by photocatalytic technologies. Rational design and synthesis of BiVO4-based heterojunctions hold great promise for significantly improving the performance and practical applicability of photocatalytic systems.3. Classification of BiVO4-based heterojunction photocatalysts
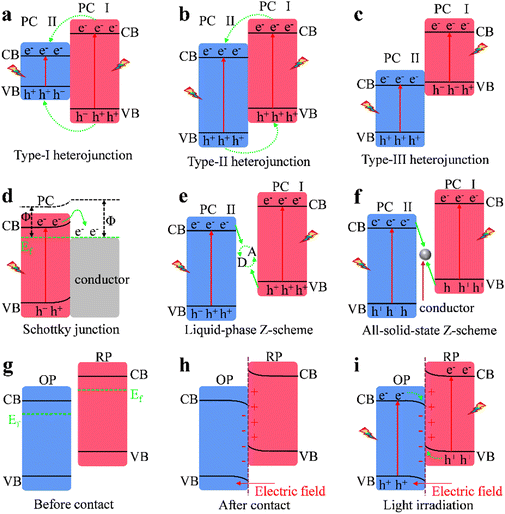
BiVO4 forms a heterojunction with semiconductors that have compatible energy band structures, enabling rapid separation of electrons and holes in the valence band and conduction band. This prevents recombination of electron–hole pairs and enhances the photocatalytic efficiency of BiVO4. According to the various charge separation mechanisms (Fig. 2), BiVO4-based heterojunctions are categorized into: (1) traditional heterojunctions (type I, type II, and type III), (2) Schottky heterojunctions, (3) Z-scheme heterojunctions, and (4) S-scheme heterojunctions.3.1 Traditional heterojunctions
Type I heterojunction photocatalysts consist of two semiconductors: semiconductor A, with an Eg fully within the Eg of semiconductor B, as illustrated in Fig. 2a.40,41 Under illumination, photogenerated electrons transfer from the CB of semiconductor B to the CB of semiconductor A due to the potential difference, while photogenerated holes migrate from the VB of semiconductor B to the VB of semiconductor A. Photogenerated holes and electrons in semiconductor A participate in the redox reactions of photocatalysis.42,43 This charge transfer mechanism may extend the lifetimes of photogenerated charges.44 However, in type I heterojunctions, photogenerated electrons and holes transfer and accumulate in the same semiconductor, leading to insufficient spatial separation of the charge carriers. This lowers the potential of charge carriers for redox reactions, making the redox processes thermodynamically unfavorable.5,45 The limited spatial separation results in a high probability of recombination, which diminishes the overall photocatalytic efficiency. Additionally, the energy band alignment in type I heterojunctions can be influenced by factors such as temperature and material defects, further complicating the charge transfer dynamics.In type II heterojunction photocatalysts, the conduction and VB positions of semiconductors A and B are staggered, as shown in Fig. 2b.46,47 Under light excitation, photogenerated electrons migrate from the higher energy CB of semiconductor B to the lower energy CB of semiconductor A, enriching in A. Simultaneously, photogenerated holes migrate from the VB of semiconductor A to the VB of semiconductor B, enriching in B, thereby achieving spatial separation of charge carriers.48 This process significantly reduces the electron–hole pair recombination and extends the lifetime of the electrons.49,50 The staggered band alignment in type II heterojunctions not only facilitates effective charge separation but also enhances the driving force for redox reactions, as the energy levels of the charge carriers are more favorable for participating in photocatalytic processes. However, this charge transfer mechanism has limitations. Similar to type I heterojunctions, the photogenerated charge carriers have lower redox potentials, reducing their redox capability in photocatalytic reactions.50 Furthermore, photogenerated electron accumulation in the CB of semiconductor A inhibits electron injection from semiconductor B, slowing the migration of the photogenerated charge carriers.51 This accumulation can lead to a saturation effect, where the rate of charge transfer becomes limited by the available states in the conduction band. Additionally, energy losses due to non-radiative recombination and thermalization further limit the effectiveness of photocatalytic reactions,52 making type II heterojunction photocatalysts challenging in terms of thermodynamics, kinetics, and energy efficiency. Type III heterojunctions are characterized by a broken band alignment between the two semiconductors that exhibit no overlap in their Eg (Fig. 2c).53 This configuration allows for the combination of various semiconductors, including those with strong redox abilities and broad light-response ranges. In type III heterojunctions, the lack of overlap in energy bands creates unique charge transfer pathways that can potentially enhance photocatalytic activity by allowing for the utilization of a wider range of light wavelengths. This is particularly advantageous for applications requiring high energy efficiency across different spectral regions. These features give type III heterojunctions the potential to overcome the trade-offs between light response range and redox potential in type II heterojunctions.54 However, high energy barriers hinder charge transfer and separation across the bands, posing challenges for photocatalysis and photothermal catalysis applications.55 The presence of these energy barriers can lead to inefficient charge carrier dynamics, where the photogenerated electrons and holes may recombine before they can participate in catalytic reactions. This recombination can significantly reduce the overall efficiency of the photocatalytic process. Research on the catalytic applications of type III heterojunctions remains limited. Unlocking the potential of type III heterojunctions requires suitable conductive media to overcome the high energy barriers that inhibit charge transfer.53 Effective pairing of photogenerated holes in the VB of semiconductor B with photogenerated electrons in the CB of semiconductor A could significantly enhance their photocatalytic and photothermal catalytic performance. Moreover, the design of type III heterojunctions should consider the alignment of energy levels not only at the interface but also the overall electronic structure of the materials involved, as this can influence the efficiency of charge separation and transfer.
3.2 Schottky heterojunction
A Schottky heterojunction forms between a semiconductor and a metal with different Fermi levels (Fig. 2d), producing a Schottky barrier in semiconductor–metal nanocomposites.56,57 The work function difference drives charge exchange at the interface.58 This charge exchange is crucial as it establishes an electric field at the junction, which influences the movement of charge carriers. In an n-type semiconductor–metal Schottky heterojunction, electrons flow from the semiconductor to the metal upon contact until their Fermi levels equilibrate.59 This process not only results in the formation of a positively charged region in the semiconductor but also alters the energy band structure significantly. Due to the limited density of free electrons, a positively charged region forms in the semiconductor, causing its energy bands to bend upward and creating a Schottky barrier at the metal–semiconductor interface. The height of this Schottky barrier is determined by the difference in work function between the metal and the semiconductor, which plays a critical role in dictating the efficiency of charge carrier separation and transport. The movement of carriers across the Schottky heterojunction is driven by the Fermi level difference between the semiconductor and the metal.60 Under illumination, photogenerated holes in the semiconductor are driven by the internal electric field (IEF) toward the metal, where it acts as a hole trap and an active site for photo-oxidation, enhancing charge carrier separation. This trapping mechanism is essential as it prevents the recombination of photogenerated holes and electrons, thereby increasing the availability of reactive holes for oxidation reactions. The efficiency of this process is influenced by factors such as the thickness of the Schottky barrier and the interface quality, which can affect the rate of charge transfer. The Schottky barrier prevents photogenerated electron migration to the metal.61 This structure promotes continuous migration of photogenerated electrons under light excitation, enhancing photogenerated charge separation, facilitating hole participation, and improving photocatalytic performance.623.3 Z-scheme heterojunctions
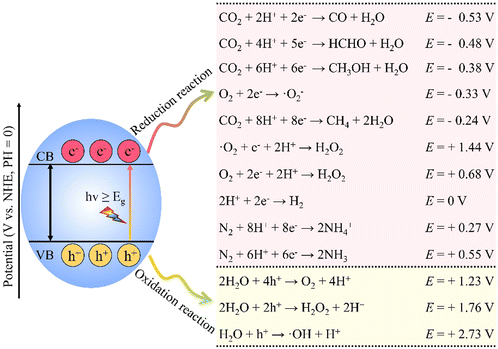
Despite the excellent electron–hole separation efficiency of the aforementioned heterojunctions, their overall redox capability remains limited because oxidation and reduction occur on semiconductors with relatively low oxidation and reduction potentials, respectively. This limitation arises from the intrinsic bandgap energies of the semiconductors used, which dictate their ability to participate in redox reactions. The energy levels of the conduction band (CB) and valence band (VB) must be appropriately aligned with the redox potentials of the target reactions to achieve effective charge transfer. The “Z-scheme” photocatalytic system is a type of photocatalytic mechanism that mimics natural photosynthesis, where two semiconductors are coupled to facilitate the separation and transfer of photogenerated charge carriers. In this system, one semiconductor acts as an electron donor while the other serves as an electron acceptor, allowing for efficient charge separation and redox reactions. To address these limitations, Bard et al. proposed constructing a traditional Z-scheme photocatalytic system (also known as a liquid-phase heterojunction).63 Inspired by the electron transfer pathways in natural photosynthetic systems,64 the ideal charge transfer mechanism achieves efficient photogenerated charge carrier separation while maintaining efficient redox capability.65 However, forming liquid-phase Z-scheme heterojunctions is constrained by the need to couple specific electron acceptor/donor (A/D) pairs, as illustrated in Fig. 2e.66 The selection of these A/D pairs is critical, as it directly influences the thermodynamic feasibility of the redox reactions. The alignment of the energy levels of the acceptors and donors with the band positions of the photocatalysts determines the efficiency of charge transfer and the overall photocatalytic performance. CB electrons from PC II, with a lower band position, are captured by acceptor A and reduced to donor D.67 Similarly, VB holes in PC I are captured by donor D and oxidized to acceptor A, completing the cycle.68 Due to the higher electron potential in PC I, its electrons more readily participate in the reduction reaction of A, while holes in PC II more easily participate in the oxidation reaction of D.69 The traditional Z-scheme photocatalytic system is becoming less favored due to its complex structure, high uncertainty, and limited applicability. To provide a more comprehensive understanding of the photogenerated carrier dynamics in Z-type and S-type heterojunctions, we have included an analysis of the carrier migration pathways and their interactions with the semiconductor interfaces. These challenges include difficulties in optimizing the interface between the semiconductor and the liquid phase, which can lead to mass transfer limitations and reduced reaction rates. In Z-type heterojunctions, the spatial separation of charge carriers is crucial for enhancing photocatalytic efficiency. The dynamics of photogenerated carriers involve their migration from the conduction band of one semiconductor to the valence band of another, facilitated by the energy level alignment. This process is influenced by factors such as the thickness of the semiconductor layers and the presence of defects that can trap carriers, thus affecting their mobility. To improve charge transfer and broaden applications, Tada et al. proposed the all-solid-state Z-scheme photocatalytic system in 2006.70 This system uses two distinct semiconductors and a metal conductor as an electron mediator.71 The introduction of a metal conductor not only facilitates electron transfer but also helps to stabilize the charge carriers, reducing the likelihood of recombination. In this configuration, the dynamics of charge carriers are enhanced by the rapid electron transfer through the metal conductor, which minimizes the distance that electrons must migrate, thereby reducing recombination losses. This configuration allows for a more efficient spatial separation of charge carriers compared to traditional liquid-phase systems. This heterojunction overcomes the limitation of traditional liquid-phase Z-scheme systems by shortening electron migration distance which could enhance photocatalytic performance.72 As shown in Fig. 2f, the all-solid-state Z-scheme photocatalyst consists of two semiconductors and an electron mediator.73 Under illumination, electrons in the VB of semiconductor II are excited to the CB and transferred through the electron mediator to the VB of semiconductor I, where they recombine with holes.74 This process is driven by the internal electric field established at the junctions, which promotes the directional flow of charge carriers. The dynamics of this charge transfer are further influenced by the band alignment between the semiconductors, which dictates the energy barriers for carrier migration. A favourable alignment can enhance the efficiency of charge separation and reduce the likelihood of recombination. The efficiency of this charge transfer is influenced by factors such as the band alignment between the semiconductors and the properties of the electron mediator. The remaining reducing electrons in PC II and oxidizing holes in PC I participate in reduction and oxidation reactions, achieving spatial separation of electrons and holes and high redox potentials.75 However, in practice, photogenerated carriers with strong redox capabilities tend to associate with specific electron acceptor/donor pairs in liquid-phase Z-scheme heterojunctions or with metal conductors in all-solid-state Z-scheme heterojunctions. This association can lead to limitations in the versatility of the photocatalytic systems, as the choice of materials and their compatibility with the desired redox reactions are crucial for optimizing performance. A deeper understanding of the carrier dynamics, including the rates of charge transfer and the effects of various environmental conditions, is essential for advancing the design of more efficient photocatalysts.3.4 S-scheme heterojunction
In 2019, Yu and colleagues reported the S-type heterojunction,76 which improves redox capabilities while maintaining high activity, clarifying carrier transfer mechanisms in photocatalytic reactions. The S-type heterojunction represents a significant advancement in photocatalytic design, as it effectively utilizes the distinct redox potentials of two semiconductors to enhance overall photocatalytic efficiency. As illustrated in Fig. 2g, the S-type heterojunction consists of two semiconductors: an oxidative photocatalyst (OP) and a reductive photocatalyst (RP). RP has a more negative CB position and smaller work function than OP.77 As shown in Fig. 2h, due to its higher Fermi level, electrons in RP transfer spontaneously to OP upon contact, which results in a positive charge on RP and causes its energy bands to bend upward.50,78 This upward bending of energy bands in RP indicates a depletion of electrons, which is critical for establishing a driving force for electron transfer. The energy band alignment between RP and OP is crucial, as it dictates the efficiency of charge separation and the subsequent redox reactions. When the Fermi level equilibrium is reached between RP and OP, the interface near OP becomes negatively charged due to electron acquisition, causing its energy bands to bend downward.79 Consequently, an IEF forms at the interface, directed from RP to OP.80 To provide a more comprehensive understanding of the photogenerated carrier dynamics in S-type heterojunctions, we have included an analysis of the carrier migration pathways and their interactions with the semiconductor interfaces. Under illumination, as depicted in Fig. 2i, the IEF and Coulombic interactions cause electrons in the CB of OP and holes in the VB of RP to recombine at the contact interface, promoting carrier separation and eliminating non-useful charge carriers.81 This recombination process is essential for enhancing the photocatalytic activity, as it ensures that the photogenerated carriers are effectively utilized in redox reactions rather than recombining and losing their energy. The dynamics of these carriers involve their movement through the semiconductor layers, influenced by the band structure and the established IEF. The efficiency of this process is contingent upon the alignment of energy levels and the distance over which carriers must migrate. Photogenerated carriers with stronger redox capabilities are retained for photocatalytic reactions.82 Coulombic repulsion and the IEF direction prevent electron transfer from the CB of RP to that of OP, suppressing undesirable electron transfer and reverse reactions.83 This mechanism of charge separation is particularly advantageous because it minimizes the chances of recombination losses, which are often a significant limitation in traditional photocatalytic systems. The strategic design of the S-type heterojunction thus allows for enhanced charge carrier dynamics, leading to improved photocatalytic performance. Thus, the S-scheme heterojunction facilitates the separation and transfer of light-induced carriers while maintaining robust redox capabilities. By leveraging the unique properties of both OP and RP, the S-type heterojunction not only enhances the efficiency of charge separation but also optimizes the redox potential for various photocatalytic applications, making it a promising approach for future photocatalyst development. Understanding these physicochemical principles is essential for further advancements in the field of photocatalysis.4. Applications of BiVO4-based heterojunction photocatalysts
Semiconductor photocatalytic technology has significant promise for energy conversion and environmental remediation by efficiently transforming solar energy into chemical energy.84,85 Sunlight-driven photocatalytic reactions occur under mild conditions, making them well-suited for green and sustainable development.86 However, traditional semiconductor materials have limited photocatalytic performance due to their low energy conversion efficiency and poor visible light responsiveness, restricting their practical applications. Recently, BiVO4-based heterojunction photocatalysts have emerged as a research focus due to their superior performance.87,88 As shown in Fig. 3, photogenerated electrons and holes migrate to the surface of the photocatalyst, driving surface reduction and oxidation reactions, respectively. These catalysts have achieved significant advancements in photocatalytic water splitting, nitrogen cycling, CO2 reduction, pollutant degradation, HCHO removal, and bacterial inactivation. By rationally designing heterojunction structures, the separation and transport efficiency of photogenerated charges can be significantly enhanced, improving photocatalytic activity and endowing BiVO4-based catalysts a competitive advantage edge in photocatalytic technology. This section will provide an overview of BiVO4-based heterojunctions in photocatalytic applications, highlighting their advancements in energy conversion and environmental remediation, and their critical role in addressing challenges in photocatalytic technologies.4.1 Energy conversion applications
BiVO4-based heterojunctions exhibit remarkable multifunctionality in photocatalytic energy conversion applications, including water splitting,89 nitrogen fixation,90 CO2 reduction,91 and hydrogen peroxide (H2O2) synthesis.92 These capabilities highlight their significance in advancing energy conversion technologies. By harnessing solar energy, these materials are crucial for sustainable energy production and environmental remediation, addressing global energy and environmental challenges.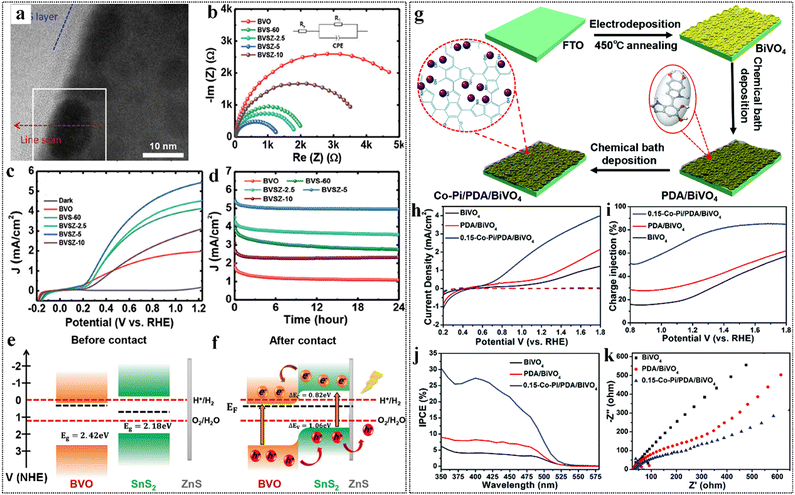 | ||
| Fig. 4 (a) A BFTEM cross-sectional image for a BiVO4 film with embedded SnS2 nanoparticles and a ZnS coating layer. (b) EIS Nyquist plots, (c) LSV curves, and (d) stability curves for the BVO, BVS-60, BVSZ-2.5, BVSZ-5, and BVSZ-10 photoanodes. (e) and (f) Band diagrams of a BVO/SnS2/ZnS photoanode before and after contact. Reproduced from ref. 96 with permission from Wiley, copyright 2024. (g) Synthesis of Co–Pi/PDA/BiVO4. (h) J–V plots of BiVO4, PDA/BiVO4, and 0.15-Co–Pi/PDA/BiVO4. (i) Charge injection efficiencies. (j) IPCE spectra. (k) PEIS plots of BiVO4, PDA/BiVO4, and 0.15-Co–Pi/PDA/BiVO4. Reproduced from ref. 98 with permission from Royal Society of Chemistry, copyright 2019. | ||
Our research team has introduced a novel strategy to overcome these limitations by achieving constrained growth of Co–Pi co-catalysts on BiVO4 using a polydopamine matrix (Fig. 4g).98 This approach enhances co-catalyst anchoring and dispersion while facilitating effective organic–inorganic interactions, resulting in a photocurrent density of 2.47 mA cm−2 (1.23 V vs. RHE), approximately 7 times higher than that of bare BiVO4 (Fig. 4h). Additionally, the charge injection efficiency improved from 25% to 75% (Fig. 4i), achieving a 27% incident photon-to-current efficiency (IPCE) at 400 nm (Fig. 4j). In comparison to previous studies, our method not only focuses on surface passivation and interface enhancement but also promotes the ordered growth of Co–Pi, which is critical for improving charge separation and stability. This dual approach offers new insights for efficient photocatalytic water splitting, addressing both performance and longevity concerns that have been raised in the literature (Fig. 4k). A summary of the water-splitting performance of BiVO4-based heterojunction photocatalysts is presented in Table 1.99–114
| Photoelectrode design | Light source (W Xe-lamp) | Electrolyte used | ABPE (%) | IPCE | Photocurrent density (mA cm−2) (1.23 VRHE) | Ref. |
|---|---|---|---|---|---|---|
| BiVO4/ZnIn2S4 | 300 | 0.2 M Na2SO4 | — | — | 0.42 | 99 |
| BiVO4/C3N4 | 300 | 0.5 M KBi | 2.48 | 85% at 380–460 nm | 5.89 | 100 |
| CuMTZ/BiVO4 | 300 | 1 M KPi | 1.04 | 92.5% at 400 nm | 3.32 | 101 |
| BiVO4/BiOBr | 300 | 0.5 M Na2SO4 | 0.26 | 51% at 340 nm | 2.69 | 102 |
| MW:BiVO4@CFO/TANF | 300 | 0.1 M Na2SO4 | 1.35 | 45.9% at 380 nm | 6.03 | 103 |
| OVs BiVO4/Bi2MoO6 | 300 | 0.5 M Na2SO4 | 0.12 | — | 0.34 | 104 |
| CdSe/BiVO4 | 300 | 0.5 M Na2SO4 | — | — | 0.155 | 105 |
| BiVO4/CuBi2O4 | 300 | 0.2 M PBS | — | 12% at 350–500 nm | 1.15 | 106 |
| N-WO3/BiVO4 | 500 | 0.5 M Na2SO4 | — | — | 0.78 | 107 |
| NiMoO4/BiVO4/Sn:WO3 | 150 | 1 M KPi | 0.52 | 38.3% at 450 nm | 2.06 | 108 |
| g-C3N4/ThO2@BiVO4 | 300 | 0.5 M Na2SO4 | 0.44 | — | 0.45 | 109 |
| BVO/BiVO4 | 300 | 0.5 M KBi | 2.0 | 60% at 470![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm nm |
3.40 | 110 |
| N-CFO/BiVO4 | 300 | 0.1 M KBi | — | 87% at 370![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm nm |
4.83 | 111 |
| CNODs/BiVO4 ODs | 300 | 0.05 M Na2SO4 | 0.24 | — | 2.2 | 112 |
| BiVO4/In2O3/FeNi | 300 | 0.5 M Na2SO4 | 0.95 | — | 4.00 | 113 |
| BiVO4–Co-MOF | 300 | 0.5 M Na2SO4 | — | — | 2.32 | 114 |
A comparative analysis of existing literature reveals varying conclusions regarding the stability and efficiency of BiVO4-based heterojunctions. For instance, Chen et al. fabricated a BiVO4 photoanode with an MgO interlayer, achieving significant improvements in photocurrent density and PEC conversion efficiency (Fig. 5a and b).120 However, they noted that while the MgO interlayer reduced surface defects, the long-term stability of the heterojunction under operational conditions remains a concern (Fig. 5c–f). In contrast, our group's investigation into the CoFeMnO/BiVO4 heterojunction demonstrated a signal recovery time of 9.1 picoseconds, indicating enhanced electron dynamics and stability during photocatalytic reactions (Fig. 5g and h).121 This suggests that while various strategies can improve performance, the stability of the heterojunctions under continuous operation is crucial for practical applications.
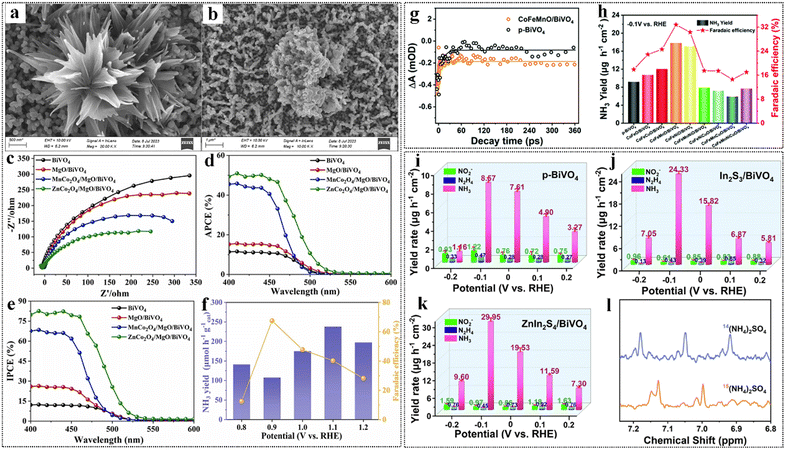 | ||
| Fig. 5 (a) SEM images of MnCo2O4/MgO/BiVO4 and (b) ZnCo2O4/MgO/BiVO4. (c) EIS, (d) APCE and (e) IPCE diagrams of BiVO4, MgO/BiVO4, MCo2O4/BiVO4 (M = Zn, Mn) and MCo2O4/MgO/BiVO4 (M = Zn, Mn). (f) Ammonia formation rates (the average value on 2 h reaction) and corresponding faradaic efficiencies of ZnCo2O4/MgO/BiVO4. Reproduced from ref. 120 with permission from Elsevier, copyright 2024. (g) Characteristic dynamics of p-BiVO4 and CoFeMnO/BiVO4 probed at 480 nm. (h) Yield of NH3 and faradaic efficiency (A-MXOY/p-BiVO4) at −0.1 V vs. RHE. Reproduced from ref. 121 with permission from Royal Society of Chemistry, copyright 2022. Yield rates of (i) p-BiVO4, (j) In2S3/BiVO4 and (k) ZnIn2S4/BiVO4 under different voltages. (l) Qualitative experiments of 14NO3− and 15NO3− isotopes. Reproduced from ref. 122 with permission from Elsevier, copyright 2022. | ||
In comparing our findings with those of other research teams, it is evident that the introduction of structural modifications, such as the incorporation of FLPs, significantly enhances catalytic performance and selectivity (Fig. 5i–k).122 While some studies have focused on optimizing the electronic properties of BiVO4-based heterojunctions, our research highlights the importance of both structural and electronic enhancements to achieve superior stability and efficiency in NH3 synthesis. We validated the NH3 evolution process using isotopic 1H nuclear magnetic resonance spectroscopy with 15NO3− as the nitrogen source, ensuring the reliability of our results (Fig. 5l). This research elucidates the critical role of FLPs in enhancing selective chemical adsorption and catalytic kinetics, broadening the application prospects of FLP active sites in PEC-NITRR. The nitrogen fixation performance of BiVO4-based heterojunction photocatalysts are listed in Table 2.90,120,123–133
| Photoelectrode design | Source of N | Light source (W Xe-lamp) | NH3 yield | Testing method | Ref. |
|---|---|---|---|---|---|
| Cs3MoxSbyBr9/BiVO4 | N2 | 300 | 300 ± 5 μmol h−1 g−1 | Indophenol blue method | 123 |
| Bi/BiVO4 | N2 | 300 | 167.74 μmol h−1 g−1 | Nessler's reagent | 124 |
| BiVO4/CuPc | NO3− | 300 | 12.98 μg h−1 cm−2 | Nessler's reagent | 125 |
| Coupling BiVO4–RuO2 with PPV–Cu | NO3− | 300 | — | Indophenol blue method | 126 |
| CeO2–C/BiVO4 | NO3− | 300 | 21.81 μg h−1 cm−2 | Nessler's reagent | 127 |
| Fe2+Cu2+Fe3+LDH/BiVO4 | NO3− | 300 | 13.8 μg h−1 cm−2 | Nessler's reagent | 128 |
| Ru/BiVO4–VO | N2 | 300 | 3.88 μmol h−1 g−1 | Nessler's reagent | 129 |
| ZnCo2O4/MgO/BiVO4 | N2 | 300 | 35.52 μmol h−1 g−1 | Indophenol blue method | 120 |
| BiVO4/VS–MoS2 | N2 | 300 | 132.8 mmol h−1 g−1 | Indophenol blue method | 90 |
| VO–BP–BiVO4 | N2 | 300 | 208 μmol h−1 g−1 | Nessler's reagent | 130 |
| Co3O4/BiVO4 | N2 | 300 | 35 μmol h−1 g−1 | Nessler's reagent | 131 |
| BiVO4@MXene | N2 | 300 | 27.25 μg h−1 cm−2 | Indophenol blue method | 132 |
| BiVO4/VS/ZnIn2S4 | N2 | 300 | 80.6 μmol h−1 g−1 | Nessler's reagent | 133 |
A comparative analysis of existing literature highlights varying approaches and conclusions regarding the stability and efficiency of BiVO4-based heterojunctions. For instance, Ahmadi et al. synthesized BiVO4/TiO2 photocatalysts with varying molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to evaluate their performance in CO2 reduction.135 Their findings indicated that the heterojunction structure significantly improved optical performance and electron–hole separation efficiency (Fig. 6a–d). However, while the BVT2 sample achieved the highest methane yield, the long-term stability of these photocatalysts under continuous operation was not thoroughly addressed. In contrast, Zhao et al. developed an S-type heterojunction (Zn-MOF/BVON) that exhibited a 22-fold increase in light activity compared to previously reported BiVO4 nanosheets.136 This enhancement was attributed to improved charge transfer and separation, but the authors also emphasized the importance of stability in practical applications, suggesting that the size-matched Zn-MOF/BVON structure not only facilitates efficient charge separation but also provides abundant catalytic active sites, which may contribute to enhanced stability over time (Fig. 6e and f).
4) to evaluate their performance in CO2 reduction.135 Their findings indicated that the heterojunction structure significantly improved optical performance and electron–hole separation efficiency (Fig. 6a–d). However, while the BVT2 sample achieved the highest methane yield, the long-term stability of these photocatalysts under continuous operation was not thoroughly addressed. In contrast, Zhao et al. developed an S-type heterojunction (Zn-MOF/BVON) that exhibited a 22-fold increase in light activity compared to previously reported BiVO4 nanosheets.136 This enhancement was attributed to improved charge transfer and separation, but the authors also emphasized the importance of stability in practical applications, suggesting that the size-matched Zn-MOF/BVON structure not only facilitates efficient charge separation but also provides abundant catalytic active sites, which may contribute to enhanced stability over time (Fig. 6e and f).
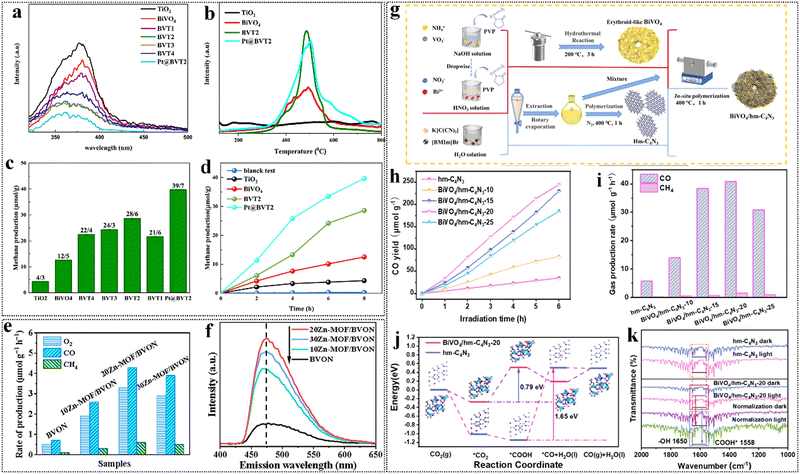 | ||
| Fig. 6 (a) Photoluminescence spectra of the synthesized photocatalysts. (b) CO2-TPD results of TiO2, BiVO6, BVT2 and Pt@BVT2 from 30 to 800 °C. (c) CH4 yield over different photo-catalysts after 8 h of reaction. (d) CH4 yield over different photo-catalysts during 8 h of reaction. Reproduced from ref. 135 with permission from Elsevier, copyright 2024. (e) Photocatalytic activities for CO2 conversion of BVON and xZn-MOF/BVON heterojunctions under visible-light irradiation. (f) FS related to the formed ˙OH amount. Reproduced from ref. 136 with permission from Elsevier, copyright 2022. (g) Illustration for the preparation of erythroid-like BiVO4/hm-C4N3 hybrids. (h) and (i) Time evolutions of CO and average gas production rates over different catalysts. (j) The free energy diagrams of CO2 reduction to CO for hm-C4N3 (blue line) and BiVO4/hm-C4N3 composite (red line) based on DFT calculation. (k) In situ DRIFTS spectra of CO2 photoconversion over different samples. Reproduced from ref. 137 with permission from Elsevier, copyright 2021. | ||
Wu et al. developed a layered, red blood cell-like BiVO4/hm-C4N3 direct Z-type heterojunction using template induction and in situ polymerization (Fig. 6g).137 This Z-type system effectively integrates CO2 capture, activation, and charge transfer, resulting in outstanding catalytic performance. It converts CO2 and H2O into CO and O2 without requiring sacrificial agents or co-catalysts. The optimized BiVO4/hm-C4N3 catalyst achieved a CO generation rate of up to 40.8 μmol g−1 h−1 with over 97% selectivity (Fig. 6a and i). In comparing these studies, it is evident that while different strategies have been employed to enhance photocatalytic performance, the stability of the BiVO4-based heterojunctions remains a critical factor for their practical application. For example, the DFT calculations and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analyses conducted by Wu et al. revealed that BiVO4 facilitates proton replenishment through H2O oxidation, which may contribute to the stability of the photocatalytic process (Fig. 6j and k). This indicates that understanding the underlying mechanisms that enhance both performance and stability is essential for advancing the field of photocatalytic CO2 reduction. This research presents an innovative strategy for developing organic semiconductor-based photocatalysts, paving the way for advancements in artificial photosynthesis. A comparison of CO2 reduction performances of BiVO4-based heterojunction photocatalysts is presented in Table 3.135,138–148
| Photoelectrode design | Mass of sample | Light source | Reduction products | Testing time (h) | Yield | Ref. |
|---|---|---|---|---|---|---|
| tBi2S3/BiVO4–E | 30 mg | 300 W Xe-lamp | CH3OH | 10 | 290.45 μmol g−1 h−1 | 138 |
| Fe3O4/BiVO4 | 20 mg | 20 W LED light (λ = 415 nm) | CH3OH | 12 | 371 mmol g−1 | 139 |
| CuTcPc/BiVO4 | 20 mg | 300 W Xe-lamp | CO | 3 | 42 μmol g−1 h−1 | 140 |
| BiVO4|Au|Eu2Ti4 | Film catalyst | 300 W Xe-lamp (λ ≤ 500 nm) | CO | 5 | 0.38 μmol m−2 h−1 | 141 |
| BiVO4@PCN-224(Cu) | 10 mg | 300 W Xe-lamp | CO | 1 | 70.5 μmol g−1 h−1 | 142 |
| Au–BiVO4/P-CN | 5 mg | 300 W Xe-lamp | CO | 1 | 20.87 μmol g−1 h−1 | 143 |
| BiOBr/BiVO4 | Film catalyst | 300 W Xe-lamp | CO | 6 | 95.27 mmol g−1 h−1 | 144 |
| BiVO4/TiO2 | 0.1 g | UV Hg 8 W pen-ray lamp | CO | 7 | 1.6 μmol gcat−1 | 135 |
| Pt@BiVO4/2TiO2 | 100 mg | 250 W mercury vapor lamp | CH4 | 8 | 39.7 μmol g−1 | 145 |
| Cu1–TiO2/BiVO4-4 | 30 mg | 300 W Xe-lamp | CO | 1 | 17.33 μmol gcat−1 h−1 | 146 |
| CsPbBr3/BiVO4 | 8 mg | 300 W Xe-lamp | CO | 4 | 41.02 μmol g−1 h−1 | 147 |
| BiVO4/Bi19Cl3S27 | 15 mg | 300 W Xe-lamp | CO | 4 | 80.94 μmol g−1 | 148 |
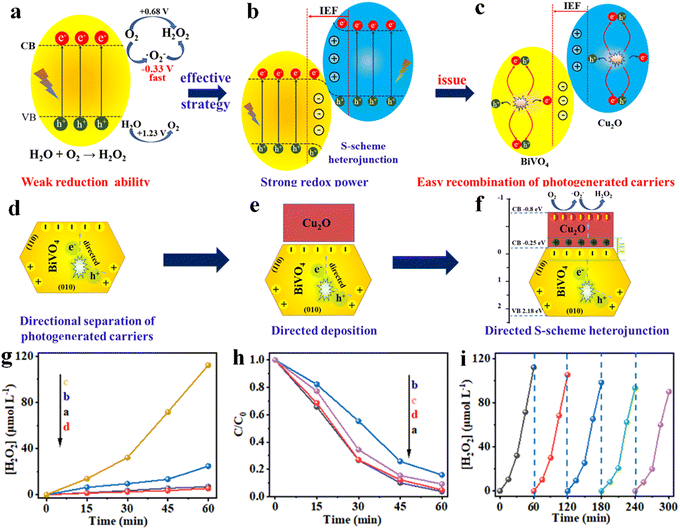 | ||
| Fig. 7 (a)–(f) Graphical illustration showing the design of the S-scheme heterojunction for highly active H2O2 production. (g) Photocatalytic H2O2 production and (h) time-dependent curves of photocatalytic H2O2 decomposition of various samples. (i) Cycle test for the MnOOH/BiVO4/Cu2O photocatalyst. Reproduced from ref. 149 with permission from Royal Society of Chemistry, copyright 2024. | ||
This study demonstrates the potential of S-type heterojunctions for efficient H2O2 synthesis and large-scale production.
4.2 Environmental remediation applications
BiVO4-based heterojunctions exhibit significant versatility in photocatalytic environmental remediation applications, including pollutant degradation,150 formaldehyde (HCHO) removal,151 and bacterial inactivation,152 underscoring their importance in environmental remediation and the chemical industry.OVs are critical active sites in photocatalytic reactions, but their role in S-type heterojunctions requires further exploration.
Su et al. constructed an S-type BiVO4-s/g-C3N4 photocatalyst through interface modulation.155 The Fermi level difference between BiVO4 and g-C3N4 induces charge redistribution at the heterojunction interface, creating an IEF that enhances the transport and separation of photogenerated charge carriers. The introduction of OVs generates defect states within the Eg of BiVO4, forming indirect recombination energy levels that mediate carrier recombination. Experimental results showed that the optimal sample enhanced photocatalytic efficiencies for degrading rhodamine B (RhB) and tetracycline (TC) by 10.7 (Fig. 8a) and 11.8 times (Fig. 8b), respectively, while the hydrogen production rate increased to 558 μmol g−1 h−1 (Fig. 8c). This study highlights the importance of oxygen vacancies in heterojunction photocatalysis, providing insights for optimizing photocatalyst performance in environmental remediation and energy conversion.
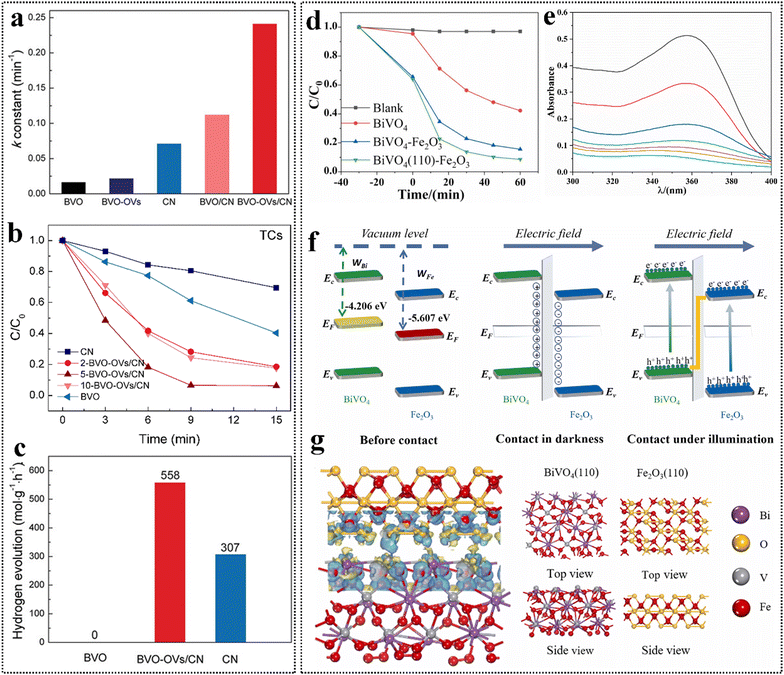 | ||
| Fig. 8 (a) Corresponding first-order kinetic k constant of BVO, BVO-OVs, CN, BVO/CN, and BVO-OVs/CN. (b) Photodegradation on TC pollutant of BVO, CN and BVO-OVs/CN with different BVO-OVs and CN ratios. (c) Comparison of photocatalytic hydrogen evolution rate. Reproduced from ref. 155 with permission from Wiley, copyright 2024. (d) Photocatalytic degradation of tetracycline by different samples. (e) Curve of absorbance with tetracycline concentration during the degradation of tetracycline by BiVO4 (110)–Fe2O3. (f) Schematic diagram of the formation process of the electron transfer mechanism of Z-scheme heterojunctions. (g) Charge density difference diagram of BiVO4 (110)–Fe2O3. Reproduced from ref. 156 with permission from Elsevier, copyright 2023. | ||
The combined strategy of crystal facet engineering and Z-type heterojunction shows promise for addressing internal losses and carrier deficiencies in photocatalytic processes. Fu et al. selectively grew FeO nanoparticles on the (110) crystal facet of BiVO4, creating a BiVO4 (110)–FeO Z-type heterojunction for efficient TC degradation.156 The study systematically examined the effects of catalyst dosage, pH, and temperature on degradation performance, revealing excellent catalytic activity, structural stability, and good reusability. In contrast, other studies have indicated that variations in pH can significantly alter the charge carrier dynamics and, consequently, the photocatalytic efficiency, emphasizing the need for a comprehensive understanding of these parameters across different systems. Under 60 min of visible light irradiation, the TC (15 mg L−1) removal rates were 57.7% (Fig. 8d), 81.7%, and 91.5% (Fig. 8e) for the single-phase catalyst, non-directionally loaded BiVO4–FeO, and directionally loaded BiVO4(110)–FeO Z-type heterojunction (0.3 g L−1), respectively. PEC testing and DFT calculations confirmed that the built-in electric field from BiVO4 (110) to FeO(110) in the Z-type heterojunction facilitates effective separation and transport of photogenerated charge carriers, significantly enhancing photocatalytic degradation performance (Fig. 8f and g). The structures of intermediate products and their degradation pathways could provide valuable insights into photocatalytic reaction mechanisms. Comparative studies have shown that the efficiency of Z-type heterojunctions can vary widely based on the choice of co-catalyst and the specific structural characteristics of the heterojunction, underscoring the importance of tailored design in optimizing photocatalytic systems. A comparison of pollutant degradation performances of BiVO4-based heterojunction photocatalysts is presented in Table 4.28,31,157–167
| Photoelectrode design | Pollutant | Pollutant concentration | Catalyst dose (mg) | Time (min) | Light source | Photodegradation efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu2O/BiVO4 | Bisphenol A | 20 mg L−1 | 30 | 30 | 300 W Xe-lamp | 65 | 31 |
| AgFeO2–BiVO4 | Ciprofloxacin | 10 mg L−1 | 100 | 30 | 300 W Xe-lamp | 89.9 | 157 |
| AgI/Cu–BiVO4 | Tetracycline | 20 mg L−1 | 30 | 30 | 300 W Xe-lamp | 97.6 | 28 |
| xNiCo2O4/BiVO4 | Rhodamine-B | 20 ppm | 50 | 150 | 450 W high-pressure mercury vapour lamp | 91 | 158 |
| F-BiVO4/g-C3N4/CdS | Ciprofloxacin | 20 mg L−1 | 50 | 30 | 500 W Xe-lamp | 98 | 159 |
| BiVO4/PANI | p-Nitrophenol | 20 mg L−1 | 50 | 20 | 500 W tungsten halogen lamps | 98.25 | 160 |
| BiVO4/CoPc | Tetracycline | 10 mg L−1 | 20 | 10 | 300 W Xe-lamp | 76 | 161 |
| BiVO4–NiFe2O4 | Crystal violet dye | 10 mg L−1 | 100 | 60 | 250 W mixed metal halide lamp | 95.65 | 162 |
| Bi2WO6/BiVO4 | Rhodamine-B | 10 mg L−1 | 100 | 100 | 19 W blue LED | 100 | 163 |
| Fe/BiOCl/BiVO4 | Ciprofloxacin | 10 mg L−1 | 50 | 75 | 300 W Xe-lamp | 100 | 164 |
| g-C3N5/BiVO4/CoFe–LDH-30% | Norfloxacin | 10 mg L−1 | 30 | 60 | 300 W Xe-lamp | 95.3 | 165 |
| Co–BiVO4/Bi2O3 | Chlortetracycline hydrochloride | 30 mg L−1 | 30 | 30 | 300 W Xe-lamp | 90.5 | 166 |
| KBr-g-C3N4/t-BiVO4/m-BiVO4 | Ciprofloxacin | 10 mg L−1 | 40 | 240 | 300 W Xe-lamp | 95.3 | 167 |
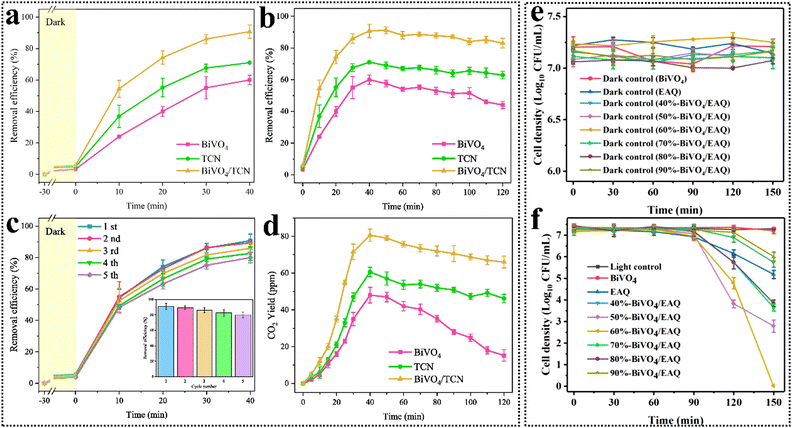 | ||
| Fig. 9 Removal efficiency of HCHO by BiVO4, TCN, and BiVO4/TCN under light irradiation for (a) 40 min, and (b) 120 min. (c) Removal efficiency of HCHO with cycling test experiments by BiVO4/TCN. (d) CO2 yield under prolonged irradiation by BiVO4, TCN, and BiVO4/TCN during photocatalytic degradation of HCHO. Reproduced from ref. 169 with permission from Elsevier, copyright 2023. Effects of various photocatalysts on the survival cell density of S. flexneri HL with and without light irradiation ((e) in the dark and (f) under light irradiation). Reproduced from ref. 170 with permission from Elsevier, copyright 2023. | ||
5. Conclusions and perspectives
Photocatalysis is a highly innovative method for combating the growing issues of fossil fuel exhaustion and environmental contamination. Recent studies have highlighted the significant potential of BiVO4-based heterojunction photocatalysts in both theoretical design and practical applications. Rational construction of heterojunctions is effective in achieving improved photocatalytic performance. Traditional heterojunctions and Schottky heterojunctions often show poor separation of photogenerated electrons and holes, or weak redox ability, resulting in limited photocatalytic efficiency. In contrast, Z-scheme heterojunctions and S-scheme heterojunctions are designed to enhance charge separation and maintain strong redox ability of catalysts, improving photocatalytic performance. Additionally, the diverse applications of BiVO4-based heterojunction photocatalysts in energy and environmental fields, including water splitting, nitrogen fixation, CO2 reduction, H2O2 synthesis, pollutant degradation, HCHO removal, and bacterial inactivation, demonstrate their wide-ranging potential. Future research could focus on optimizing photocatalyst structure and developing multifunctional catalysts that enhance activity through doping and co-catalyst integration. These photocatalysts demonstrate exceptional flexibility and stability in complex reaction systems, overcoming the limitations of single-reaction scenarios. Designing effective heterojunctions and improving reaction kinetics could enhance BiVO4-based photocatalysts for energy conversion and environmental remediation. In view of this, we present some prospects for the development of BiVO4-based heterojunction photocatalysts.(1) Although BiVO4-based heterojunction nanomaterials have been widely used for photocatalysis, challenges such as slow reaction kinetics must still be overcome to improve photocatalytic efficiency and stability. Combining BiVO4 with other semiconductors (e.g., PbS, WO3) to form novel heterojunctions is expected to improve charge separation, reduce recombination rates and enhance overall performance.
(2) To further optimize charge transport in BiVO4-based heterojunction catalysts, nanostructured BiVO4 (e.g., nanorods, nanosheets, or porous frameworks) can be fabricated to shorten the charge carrier diffusion length and increase the surface area for reactions. Additionally, introducing dopants (e.g., Mo, W) or creating oxygen vacancies in the BiVO4 lattice can enhance charge mobility and create localized energy states that facilitate charge separation and transport.
(3) Z-scheme and S-scheme heterojunctions enhance the separation and transfer of light-induced carriers while maintaining robust redox capabilities. Thus, these architectures can be explored in-depth, including the principles of excitation, transport, and recombination of photogenerated charge carriers, as well as the mechanism of photocatalytic reduction reactions.
(4) Given the complexity and diversity of heterojunction catalysts, designing and preparing a BiVO4-based heterojunction with a controlled structure is challenging, and an understanding of the relationship between structure and performance is not yet sufficiently deep. Thus, integrating advanced tools such as artificial intelligence and DFT calculation can be well applied to deeply understand the mechanism of photocatalytic reactions and predict the performance of BiVO4-based heterojunction catalysts.
(5) Advanced characterization techniques, such as high-resolution electron microscopy, and spectroscopic analysis, are suggested to better understand the mechanisms of BiVO4-based heterojunctions and provide critical insights into material structures, defects, and charge transfer processes, aiding in performance optimization.
Data availability
The data that support the findings of this study are available from the corresponding author, FWQ, upon reasonable request.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors are grateful for the Start-up Foundation offered by Ningbo University of Technology (2023KQ039) and the National Natural Science Foundation of China (22075112).Notes and references
- T. Zhang, L. Zhou, G. Chen, S. Xia and M. Qiu, Chem. Commun., 2023, 59, 1517–1520 RSC.
- C. Yue, L. Chen, H. Zhang, J. Huang, H. Jiang, H. Li and S. Yang, Environ. Sci.: Water Res. Technol., 2023, 9, 669–695 RSC.
- T. F. Qahtan, T. O. Owolabi, O. E. Olubi and A. Hezam, Coord. Chem. Rev., 2024, 514, 215839 CrossRef CAS.
- Z. Wang, X. Yue and Q. Xiang, Coord. Chem. Rev., 2024, 504, 215674 CrossRef CAS.
- D. Zu, H. Wei, Z. Lin, X. Bai, M. N. A. S. Ivan, Y. H. Tsang and H. Huang, Adv. Funct. Mater., 2024, 2408213 CrossRef CAS.
- Y. Zhang, X. Wu, Z.-H. Wang, Y. Peng, Y. Liu, S. Yang, C. Sun, X. Xu, X. Zhang, J. Kang, S.-H. Wei, P. F. Liu, S. Dai and H. G. Yang, J. Am. Chem. Soc., 2024, 146, 6618–6627 CrossRef CAS PubMed.
- P. Li, R. Wu, P. Li, S. Gao, Z. Qin, X. Song, W. Sun, Z. Hua, Q. Wang and S. Chen, Adv. Sci., 2024, 2408829 CrossRef CAS PubMed.
- A. M. Sadanandan, J.-H. Yang, V. Devtade, G. Singh, N. Panangattu Dharmarajan, M. Fawaz, J. Mee Lee, E. Tavakkoli, C.-H. Jeon, P. Kumar and A. Vinu, Prog. Mater. Sci., 2024, 142, 101242 CrossRef CAS.
- C. Chen, X. Zhang, E. Liu, J. Xu, J. Sun and H. Shi, J. Mater. Sci. Technol., 2024, 198, 1–11 CrossRef CAS.
- X. Hu, Z. Zhang, P. Lu, Y. Zhou, Y. Zhou, Y. Bai and J. Yao, Water Res., 2024, 260, 121936 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- X. Xin, Y. Zhang, R. Wang, Y. Wang, P. Guo and X. Li, Nat. Commun., 2023, 14, 1759 CrossRef CAS PubMed.
- Q. Wu, J. Cao, X. Wang, Y. Liu, Y. Zhao, H. Wang, Y. Liu, H. Huang, F. Liao, M. Shao and Z. Kang, Nat. Commun., 2021, 12, 483 CrossRef CAS PubMed.
- G. N. Schrauzer and T. D. Guth, J. Am. Chem. Soc., 1977, 99, 7189–7193 CrossRef CAS.
- W. Zhao, Y. Li and W. Shen, Chem. Commun., 2021, 57, 6838–6850 RSC.
- F. Xu, H. Tan, J. Fan, B. Cheng, J. Yu and J. Xu, Solar RRL, 2021, 5, 2000571 CrossRef CAS.
- G. Liao, X. Tao and B. Fang, Matter, 2022, 5, 377–379 CrossRef CAS.
- Y. Yang, J. Liu, M. Gu, B. Cheng, L. Wang and J. Yu, Appl. Catal., B, 2023, 333, 122780 CrossRef CAS.
- G. Zhang, Y. Meng, B. Xie, Z. Ni, H. Lu and S. Xia, Appl. Catal., B, 2021, 296, 120379 CrossRef CAS.
- B. Liu, X. Wang, Y. Zhang, K. Wan, L. Xu, S. Ma, R. Zhao, S. Wang and W. Huang, Adv. Energy Mater., 2024, 2403835 CrossRef.
- Y. Li, B. Bai, M. Zhu, J. Li, Q. Mei and Q. Wang, J. Environ. Chem. Eng., 2024, 12, 113079 CrossRef CAS.
- Y. Bai, Z. Fang, M. Zhai, X. Jiang, J. Li, H. Bai and W. Fan, Chem. Commun., 2024, 60, 6027–6030 RSC.
- G. Zhao, J. Ding, F. Zhou, X. Chen, L. Wei, Q. Gao, K. Wang and Q. Zhao, Chem. Eng. J., 2021, 405, 126704 CrossRef CAS.
- C. Cheng, B. He, J. Fan, B. Cheng, S. Cao and J. Yu, Adv. Mater., 2021, 33, 2100317 CrossRef CAS PubMed.
- J. Gu, C. Ban, J. Meng, Q. Li, X. Long, X. Zhou, N. Liu and Z. Li, Appl. Surf. Sci., 2023, 611, 155575 CrossRef CAS.
- X. Qi, X. Zhu, J. Wu, Q. Wu, X. Li and M. Gu, Mater. Res. Bull., 2014, 59, 435–441 CrossRef CAS.
- J. Zhang, Y. Huang, X. Lu, J. Yang and Y. Tong, ACS Sustainable Chem. Eng., 2021, 9, 8306–8314 CrossRef CAS.
- J. Li, D. Wang, S. Zhao, R. Ma, J. Guo, Z. Li, D. Wang, Y. Xuan and L. Wang, Appl. Catal., B, 2024, 351, 124007 CrossRef CAS.
- W. Xie, Z. Yu, H. Huang, R. Jiang, S. Yao, J. Huang, Y. Hou, S. Yin, R. Mo and C. Wu, J. Colloid Interface Sci., 2024, 665, 977–987 CrossRef CAS PubMed.
- U. Kumar, C.-X. Yang, Z.-Y. Deng, C.-E. Lin, B. C. Yadav and C.-H. Wu, Sen. Actuators, B, 2024, 403, 135144 CrossRef CAS.
- C. Bai, W. Guo, Q. Liu, G. Li, S. Guo and R. Chen, Appl. Catal., B, 2024, 344, 123606 CrossRef CAS.
- F. E. Osterloh, Chem. Mater., 2008, 20, 35–54 CrossRef CAS.
- A. Kubacka, M. Fernández-García and G. Colón, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- T. F. Qahtan, T. O. Owolabi, O. E. Olubi and A. Hezam, Coord. Chem. Rev., 2023, 492, 215276 CrossRef CAS.
- F. Bhom and Y. M. Isa, Glob. Chall., 2024, 8, 2400134 CrossRef PubMed.
- M. A. Ahmed and A. A. Mohamed, RSC Adv., 2023, 13, 421–439 RSC.
- H. Wang, X. Gao, Y. Xie, E. Guo, H. Bai, F. Jiang, Q. Li and H. Yue, Adv. Energy Mater., 2024, 14, 2400493 CrossRef CAS.
- C. Hong, S. Oh, V. K. Dat, S. Pak, S. Cha, K.-H. Ko, G.-M. Choi, T. Low, S.-H. Oh and J.-H. Kim, Light: Sci. Appl., 2023, 12, 280 CrossRef CAS PubMed.
- C. Cui, X. Xu, X. Zhao, N. Xi, M. Li, X. Wang, Y. Sang, X. Yu, H. Liu and J. Wang, Nano Energy, 2024, 126, 109632 CrossRef CAS.
- X. Rong, Y. Mao, J. Xu, X. Zhang, L. Zhang, X. Zhou, F. Qiu and Z. Wu, Catal. Commun., 2018, 116, 16–19 Search PubMed.
- J. Fan, M. Zuo, Z. Ding, Z. Zhao, J. Liu and B. Sun, Chem. Eng. J., 2020, 396, 125263 CrossRef CAS.
- X. Meng, S. Wang, C. Zhang, C. Dong, R. Li, B. Li, Q. Wang and Y. Ding, ACS Catal., 2022, 12, 10115–10126 Search PubMed.
- V. Devthade, A. Gupta and S. S. Umare, ACS Appl. Nano Mater., 2018, 1, 5581–5588 CrossRef CAS.
- Y. Zhu, T. Wan, X. Wen, D. Chu and Y. Jiang, Appl. Catal., B, 2019, 244, 814–822 CrossRef CAS.
- K. Afroz, M. Moniruddin, N. Bakranov, S. Kudaibergenov and N. Nuraje, J. Mater. Chem. A, 2018, 6, 21696–21718 RSC.
- Y. Fang, Y. Ma and X. Wang, Chin. J. Catal., 2018, 39, 438–445 CrossRef CAS.
- J. S. Chang, Y. W. Phuan, M. N. Chong and J. D. Ocon, J. Ind. Eng. Chem., 2020, 83, 303–314 CrossRef CAS.
- Z. Zheng, X. Zu, Y. Zhang and W. Zhou, Mater. Today Phys., 2020, 15, 100262 CrossRef.
- H. Wang, X. Yuan, H. Wang, X. Chen, Z. Wu, L. Jiang, W. Xiong and G. Zeng, Appl. Catal., B, 2016, 193, 36–46 CrossRef CAS.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6, 1543–1559 CAS.
- C. Pan, Z. Mao, X. Yuan, H. Zhang, L. Mei and X. Ji, Adv. Sci., 2022, 9, 2105747 CrossRef PubMed.
- I. Ahmad, S. Shukrullah, M. Y. Naz, E. Ahmed, M. Ahmad, A. J. Obaidullah, A. Alkhouri, A. Mahal and Y. Y. Ghadi, Mater. Sci. Semicond. Process., 2024, 172, 108088 CrossRef CAS.
- P. K. Srivastava, Y. Hassan, Y. Gebredingle, J. Jung, B. Kang, W. J. Yoo, B. Singh and C. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 8266–8275 CrossRef CAS PubMed.
- H. Heng, Q. Gan, P. Meng and X. Liu, J. Alloys Compd., 2017, 696, 51–59 Search PubMed.
- J. Li, J. Chen, H. Fang, X. Guo and Z. Rui, Ind. Eng. Chem. Res., 2021, 60, 8420–8429 CrossRef CAS.
- J. Lin, T. Sun, M. Li, J. Yang, J. Shen, Z. Zhang, Y. Wang, X. Zhang and X. Wang, J. Catal., 2019, 372, 8–18 CrossRef CAS.
- Z. Ni, Y. Sun, Y. Zhang and F. Dong, Appl. Surf. Sci., 2016, 365, 314–335 CrossRef CAS.
- X. Wang, C. Zhang, J. Du, X. Dong, S. Jian, L. Yan, Z. Gu and Y. Zhao, ACS Nano, 2019, 13, 5947–5958 CrossRef CAS PubMed.
- Y. Liu, H. Zeng, Y. Chai, R. Yuan and H. Liu, Chem. Commun., 2019, 55, 13729–13732 RSC.
- M. Chang, M. Wang, M. Shu, Y. Zhao, B. Ding, S. Huang, Z. Hou, G. Han and J. Lin, Acta Biomater., 2019, 99, 295–306 CrossRef CAS PubMed.
- X. Wu, Y. Zhang, K. Wang, S. Zhang, X. Qu, L. Shi and F. Du, J. Hazard. Mater., 2020, 393, 122408 CrossRef CAS PubMed.
- A. Kumar, P. Choudhary, A. Kumar, P. H. C. Camargo and V. Krishnan, Small, 2022, 18, 2101638 Search PubMed.
- B. P. Mishra and K. Parida, J. Mater. Chem. A, 2021, 9, 10039–10080 RSC.
- Y. Wang, X. Shang, J. Shen, Z. Zhang, D. Wang, J. Lin, J. C. S. Wu, X. Fu, X. Wang and C. Li, Nat. Commun., 2020, 11, 3043 Search PubMed.
- Q. Xu, S. Wageh, A. A. Al-Ghamdi and X. Li, J. Mater. Sci. Technol., 2022, 124, 171–173 CrossRef.
- Q. Wang, T. Hisatomi, Y. Suzuki, Z. Pan, J. Seo, M. Katayama, T. Minegishi, H. Nishiyama, T. Takata, K. Seki, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2017, 139, 1675–1683 Search PubMed.
- Q. Wang, J. Warnan, S. Rodríguez-Jiménez, J. J. Leung, S. Kalathil, V. Andrei, K. Domen and E. Reisner, Nat. Energy, 2020, 5, 703–710 CrossRef CAS.
- X. Wu, G. Chen, L. Li, J. Wang and G. Wang, J. Mater. Sci. Technol., 2023, 167, 184–204 CrossRef CAS.
- K. Qi, B. Cheng, J. Yu and W. Ho, Chin. J. Catal., 2017, 38, 1936–1955 CrossRef CAS.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, Nat. Mater., 2006, 5, 782–786 CrossRef CAS PubMed.
- X. Zhou, Y. Xian, Z. Li, C. Yujie, J. Luo, X. Ning, X. Fan, Y. Zhong and X. Zhou, Sep. Purif. Technol., 2025, 357, 130117 CrossRef CAS.
- P. Wen, Y. Sun, H. Li, Z. Liang, H. Wu, J. Zhang, H. Zeng, S. M. Geyer and L. Jiang, Appl. Catal., B, 2020, 263, 118180 CrossRef CAS.
- D. Huang, S. Chen, G. Zeng, X. Gong, C. Zhou, M. Cheng, W. Xue, X. Yan and J. Li, Coord. Chem. Rev., 2019, 385, 44–80 CrossRef CAS.
- F. Chen, Y. Wei, M. Ren, S. Sun, S. Ghosh and R. V. Kumar, ChemCatChem, 2024, 16, e202301492 CrossRef CAS.
- P. Zhou, J. Yu and M. Jaroniec, Adv. Mater., 2014, 26, 4920–4935 CrossRef CAS PubMed.
- J. Fu, Q. Xu, J. Low, C. Jiang and J. Yu, Appl. Catal., B, 2019, 243, 556–565 CrossRef CAS.
- J. Qu, D. Zhang, Y. Li, P. Wang, Y. Mao, T. Zhang, S. Zhan and Y. Li, Appl. Catal., B, 2024, 340, 123246 CrossRef CAS.
- K. Qi, C. Imparato, O. Almjasheva, A. Khataee and W. Zheng, J. Colloid Interface Sci., 2024, 675, 150–191 CrossRef CAS PubMed.
- D. Zu, Y. Ying, Q. Wei, P. Xiong, M. S. Ahmed, Z. Lin, M. M. J. Li, M. Li, Z. Xu and G. J. A. C. I. E. Chen, Angew. Chem. Int. Ed., 2024, 63, 202405756 CrossRef PubMed.
- M. Li, Z. Hu, D. Liu, Y. Liang, S. Liu, B. Wang, C. Niu, D. Xu, J. Li and B. Han, Chem. Eng. J., 2024, 495, 53519 Search PubMed.
- B. Borjigin, L. Ding, C. Liu, H. Li and X. Wang, Chem. Eng. J., 2024, 485, 149995 CrossRef CAS.
- L. Yuan, P. Du, L. Yin, J. Yao, J. Wang and C. Liu, Nanoscale, 2024, 16, 5487–5503 RSC.
- J. Zhou, B. Gao, D. Wu, C. Tian, H. Ran, W. Chen, Q. Huang, W. Zhang, F. Qi and N. J. A. F. M. Zhang, Adv. Funct. Mater., 2024, 34, 2308411 CrossRef CAS.
- H. Su, H. Yin, R. Wang, Y. Wang, W. Orbell, Y. Peng and J. Li, Appl. Catal., B, 2024, 348, 123683 CrossRef CAS.
- M. A. Khan, S. Mutahir, I. Shaheen, Y. Qunhui, M. Bououdina and M. Humayun, Coord. Chem. Rev., 2025, 522, 216227 CrossRef CAS.
- C. Wang, Y. Ding, Y. Wang, Z. Xie, Z. Zeng, X. Li and Y. H. Ng, Carbon Energy, 2024, 6, e500 CrossRef CAS.
- D. Pradhan, S. K. Biswal, R. Singhal, P. k Panda and S. K. Dash, Surf. Interfaces, 2024, 52, 104954 CrossRef CAS.
- Y. Liu, C. Xing, Z. Yao, Q. Deng, T. Liang, S. Zhang, J. Pan, Z. Yu, T. Xie, R. Li and Y. Hou, J. Colloid Interface Sci., 2025, 677, 1095–1106 CrossRef CAS PubMed.
- Z. Wei, Y. Zhu, W. Guo, J. Liu, W. Fang, Z. Jiang and W. Shangguan, Appl. Catal., B, 2020, 266, 118664 CrossRef CAS.
- H.-Y. Luo, Z.-L. Liu, M.-R. Zhang, Y.-F. Mu and M. Zhang, Nanoscale Adv., 2024, 6, 1781–1789 RSC.
- J. Bian, L. Sun, Z. Zhang, Z. Li, M. Chu, X. Li, D. Tang and L. Jing, ACS Sustainable Chem. Eng., 2021, 9, 2400–2408 CrossRef CAS.
- S. Shi, Y. Song, Y. Jiao, D. Jin, Z. Li, H. Xie, L. Gao, L. Sun and J. Hou, Nano Lett., 2024, 24, 6051–6060 CrossRef CAS PubMed.
- Y. An, C. Lin, C. Dong, R. Wang, J. Hao, J. Miao, X. Fan, Y. Min and K. Zhang, ACS Energy Lett., 2024, 9, 1415–1422 CrossRef CAS.
- S. Li, J. Liu, H. Wu, X. Zhang, Y. Guo, B. Kang and L. Zhao, Int. J. Hydrogen Energy, 2024, 84, 739–758 CrossRef CAS.
- Y. Qi, B. Zhang, G. Zhang, Z. Zheng, T. Xie, S. Chen, G. Ma, C. Li, K. Domen and F. Zhang, Joule, 2024, 8, 193–203 CrossRef CAS.
- S. Chaudhary, M. A. Hassan, M.-J. Kim, W.-G. Jung, J.-S. Ha, W.-J. Moon, S.-W. Ryu and B.-J. Kim, Small Methods, 2024, 2400794 Search PubMed.
- R. M. Sánchez-Albores, O. Reyes-Vallejo, F. Pola-Albores, A. Fernández-Madrigal, A. López-López and E. Ríos-Valdovinos, J. Mater. Sci.: Mater. Electron., 2024, 35, 1538 CrossRef.
- Y. Gao, W. Fan, K. Qu, F. Wang, P. Guan, D. Xu, H. Bai and W. Shi, New J. Chem., 2019, 43, 8160–8167 RSC.
- L. Li, Z. Zhang, D. Fang and D. Yang, Inorg. Chem. Commun., 2024, 169, 112971 CrossRef CAS.
- S. Xia, F. Xu and B. Weng, J. Mater. Chem. A, 2024, 12, 4635–4642 RSC.
- J.-W. Ji, L.-J. Zhang, H.-C. Wang, Y.-X. Duan, X.-Z. Yue, J.-H. Chen and S.-S. Yi, Chem. Eng. J., 2024, 484, 149597 CrossRef CAS.
- L. Shuai, L. Tian, X. Huang, J. Dou, J. Yu and X. Chen, Int. J. Hydrogen Energy, 2024, 88, 19–28 CrossRef CAS.
- L. Wang, L. Ding, W. Zhai, S. Chu, J. Li, G. He, B. Wang and Z. Jiao, Appl. Surf. Sci., 2024, 660, 159963 CrossRef CAS.
- L. Liu, M. Ruan, C. Wang and Z. Liu, ACS Appl. Electron. Mater., 2024, 6, 4772–4782 CrossRef CAS.
- Q. Li, H. Chang, G. Gao, H. Jian, W. Liu, H. Jia, J. Xue, H. Liu and Q. Shen, Sep. Purif. Technol., 2025, 353, 128444 CrossRef CAS.
- A. Song, Y. Zhang, Z. Li and J. Hu, Mater. Sci. Eng. B, 2024, 302, 117241 CrossRef CAS.
- D. Li, S. Tian, Q. Qian, C. Gao, H. Shen and F. Han, Ind. Eng. Chem. Res., 2024, 63, 13180–13188 CrossRef CAS.
- H. T. Htet, Y. Jung, Y. Kim and S. Lee, ACS Appl. Mater. Interfaces, 2024, 16, 52383–52392 CrossRef CAS PubMed.
- N. A. Mohamed, A. F. Ismail, T. S. Kiong and M. A. Mat Teridi, Int. J. Hydrogen Energy, 2024, 59, 1063–1079 CrossRef CAS.
- Z. Xie, D. Chen, J. Zhai, Y. Huang and H. Ji, Appl. Catal., B, 2023, 334, 122865 CrossRef CAS.
- J. Lin, X. Han, S. Liu, Y. Lv, X. Li, Y. Zhao, Y. Li, L. Wang and S. Zhu, Appl. Catal., B, 2023, 320, 121947 CrossRef CAS.
- J. Li, Y. Wang, K. Sun, C. Cui, G. Zhong, W. Li, X. Yang, S. Liu, Z. Xing and M. Ma, Next Energy, 2024, 2, 100056 CrossRef.
- D. Yin, X. Ning, Q. Zhang, P. Du and X. Lu, J. Colloid Interface Sci., 2023, 646, 238–244 CrossRef CAS PubMed.
- D. Wang, J. Gu, H. Wang, M. Liu, Y. Liu and X. Zhang, Appl. Surf. Sci., 2023, 619, 156710 CrossRef CAS.
- S. Shen, R. Chen, X. Li, J. Wang, S. Yu, J. Li and F. Dong, Environ. Sci. Technol., 2024, 58, 7653–7661 CrossRef CAS PubMed.
- C. Zhang, J. Li, R. Chen, S. Shen, J. Wang, Y. Sun and F. Dong, ACS Catal., 2024, 14, 15721–15742 CrossRef CAS.
- M. A. Mushtaq, A. Kumar, G. Yasin, M. Tabish, M. Arif, S. Ajmal, W. Raza, S. Naseem, J. Zhao, P. Li, H. G. Ali, S. Ji and D. Yan, Small, 2024, 20, 2310431 CrossRef CAS PubMed.
- Z. Shen, F. Li, L. Guo, X. Zhang, Y. Wang, Y. Wang, X. Jian, X. Gao, Z. Wang, R. Li, C. Fan and J. Liu, Appl. Catal., B, 2024, 346, 123732 CrossRef CAS.
- Y. Zheng, E. Wang, J. Zhou and Z. Sun, ACS Mater. Lett., 2024, 6, 3572–3601 CrossRef CAS.
- K. Chen, R. Wang, Q. Mei, F. Ding, H. Liu, G. Yang, B. Bai and Q. Wang, Appl. Catal., B, 2024, 344, 123670 CrossRef CAS.
- F. Wang, Q. Ding, Y. Bai, H. Bai, S. Wang and W. Fan, Inorg. Chem. Front., 2022, 9, 805–813 RSC.
- F. Wang, Q. Ding, J. Ding, Y. Bai, H. Bai and W. Fan, Chem. Eng. J., 2022, 450, 138260 CrossRef CAS.
- Z.-L. Liu, H.-Y. Luo, M.-R. Zhang, Y.-F. Mu, F.-Q. Bai, M. Zhang and T.-B. Lu, Chem. Eng. J., 2024, 491, 151913 CrossRef CAS.
- G. Xia, Y. Meng, Q. Fu, Z. Huang, B. Xie, Z. Ni and S. Xia, Chem. Eng. J., 2024, 498, 155849 CrossRef CAS.
- Y. Bai, S. Gao, W. Xie, Z. Fang, H. Bai and W. Fan, Int. J. Hydrogen Energy, 2023, 48, 10882–10890 CrossRef CAS.
- F. Hong, X. Su, Y. Fang, X. He and B. Shan, J. Am. Chem. Soc., 2024, 146, 25200–25210 CrossRef CAS PubMed.
- H. Bai, F. Wang, Q. Ding, W. Xie, H. Li, G. Zheng and W. Fan, Inorg. Chem., 2023, 62, 2394–2403 CrossRef CAS PubMed.
- Y. Bai, Z. Fang, Y. Lei, L. Liu, H. Zhao, H. Bai, W. Fan and W. Shi, Green Energy Environ., 2024, 9, 1112–1121 CrossRef CAS.
- G. Ren, Z. Zhao, Z. Li, Z. Zhang and X. Meng, J. Catal., 2023, 428, 115147 CrossRef CAS.
- R. Zha, C. Li, L. He and M. Zhang, J. Colloid Interface Sci., 2022, 628, 378–388 CrossRef CAS PubMed.
- Y. J. Yuan, N. Lu, L. Bao, R. Tang, F. G. Zhang, J. Guan, H. D. Wang, Q. Y. Liu, Q. Cheng, Z. T. Yu and Z. Zou, ACS Nano, 2022, 16, 12174–12184 CrossRef CAS PubMed.
- D. Zhang, S. Yang, X. Fang, H. Li, X. Chen and D. Yan, Chin. Chem. Lett., 2022, 33, 4669–4674 CrossRef CAS.
- G. Zhang, X. Yuan, B. Xie, Y. Meng, Z. Ni and S. Xia, Chem. Eng. J., 2022, 433, 133670 CrossRef CAS.
- C. Zhu, J. Cheng, H. Lin, Z. Yang, Y. Huang, F. Lv, H. Bai and S. Wang, J. Am. Chem. Soc., 2024, 146, 24832–24841 CrossRef CAS PubMed.
- M. Ahmadi, S. M. Alavi and A. Larimi, Surf. Interfaces, 2024, 45, 103908 CrossRef CAS.
- Z. Zhao, J. Bian, L. Zhao, H. Wu, S. Xu, L. Sun, Z. Li, Z. Zhang and L. Jing, Chin. J. Catal., 2022, 43, 1331–1340 CrossRef CAS.
- J. Wu, L. Xiong, Y. Hu, Y. Yang, X. Zhang, T. Wang, Z. Tang, A. Sun, Y. Zhou, J. Shen and Z. Zou, Appl. Catal., B, 2021, 295, 120277 CrossRef CAS.
- Y. Deng, J. Wang, J. Wang, W. Wang, H. Zhang and S. Yin, Chem. Eng. J., 2024, 496, 153963 CrossRef CAS.
- N. Saini, S. Saini, S. Majumder, K. S. Campbell and S. L. Jain, Sustainable Energy Fuels, 2024, 8, 1750–1760 RSC.
- Z. He, Y. Liu, Z. Li, S. Xu, Z. Li, J. Bian and L. Jing, Appl. Catal., B, 2024, 355, 124207 CrossRef CAS.
- R. Chen, Q. Wang, G. Gao, L. Wu and J. Luo, ACS Catal., 2024, 14, 12234–12241 CrossRef CAS.
- M. Xie, Q. Xu, T. Lv and H. Liu, J. Alloys Compd., 2024, 977, 173342 CrossRef CAS.
- X. Liu and Z. Lou, Appl. Surf. Sci., 2025, 680, 161328 CrossRef CAS.
- Z. Li, B. Liu, X. Zhang, C. Zhang, Y. Bai, J. Liu, Y. Wang, S. Yang, R. Li and C. Fan, Sustainable Energy Fuels, 2024, 8, 262–271 RSC.
- V. Liapun, M. B. Hanif, M. Sihor, X. Vislocka, S. Pandiaraj, V. K. Unnikrishnan, G. K. Thirunavukkarasu, M. F. Edelmannová, M. Reli, O. Monfort, K. Kočí and M. Motola, Chemosphere, 2023, 337, 139397 CrossRef CAS PubMed.
- C. Chen, M. Wu, Y. Xu, C. Ma, M. Song and G. Jiang, J. Am. Chem. Soc., 2024, 146, 9163–9171 CrossRef CAS PubMed.
- P. Tan, R. Ren, L. Yang, M. Zhang, H. Zhai, H. Liu, J. Chen and J. Pan, J. Mol. Liq., 2024, 393, 123644 CrossRef CAS.
- B. Huang, X. Fu, K. Wang, L. Wang, H. Zhang, Z. Liu, B. Liu and J. Li, Adv. Powder Mater., 2024, 3, 100140 CrossRef.
- J. Huang, H. Shi, X. Wang, P. Wang, F. Chen and H. Yu, Catal. Sci. Technol., 2024, 14, 2514–2521 RSC.
- Y. Gao, F. Liu, X. Chi, Y. Tian, Z. Zhu, R. Guan and J. Song, Appl. Surf. Sci., 2022, 603, 154416 CrossRef CAS.
- Z. Jiang, T. Wang, J. Wang, T. Yu, C. Kong, Z. Yang and H. Zhu, Sep. Purif. Technol., 2025, 353, 128581 CrossRef CAS.
- S. Bao, Z. Wang, J. Zhang and B. Tian, ACS Appl. Nano Mater., 2020, 3, 8604–8617 CrossRef CAS.
- Z. Ciğeroğlu, N. El Messaoudi, Z. M. Şenol, G. Başkan, J. Georgin and S. Gubernat, Mater. Today Sustainability, 2024, 26, 100735 CrossRef.
- J. Yang, S. Tian, Z. Song, Y. Hao and M. Lu, Coord. Chem. Rev., 2025, 523, 216257 CrossRef CAS.
- K. Su, L. Zheng, M. Liu, J. Gao, Z. Shi, C. Chen, Y. Li, J. He and M. Peng, Small, 2024, 2405551 CrossRef CAS PubMed.
- Q. Fu, Y. Meng, Y. Yao, H. Shen, B. Xie, Z. Ni and S. Xia, J. Environ. Chem. Eng., 2023, 11, 111060 CrossRef CAS.
- Q. Ma, J. Ren, X. Sun, X. Chen, G. Liu, S. Wang and H. Yang, Appl. Surf. Sci., 2025, 679, 161275 CrossRef CAS.
- N. D. Kolhe, L. S. Walekar, A. N. Kadam, M. A. Kulkarni, H. A. Parbat, M. Misra, B. J. Lokhande, S. W. Lee, V. Patil, D. Mhamane and M. G. Mali, Chemosphere, 2024, 352, 141353 CrossRef CAS PubMed.
- Z. Yang, J. Yang, L. Li, W. Cao, J. Zhang, H. Zhao and L. Wang, Appl. Surf. Sci., 2024, 672, 160738 CrossRef CAS.
- T. Jiteshwaran, B. Janani, A. Syed, A. M. Elgorban, I. Abid, L. S. Wong and S. S. Khan, Surf. Interfaces, 2024, 49, 104361 CrossRef CAS.
- Z. Long, X. Zheng and H. Shi, Sci. Chin. Mater., 2024, 67, 550–561 CrossRef CAS.
- R. C. Ghaware, N. B. Birajdar, G. S. Kamble and S. S. Kolekar, Langmuir, 2024, 40, 14426–14439 CrossRef CAS PubMed.
- S. J. Segovia-Sandoval, E. Mendoza-Mendoza, A. Jacobo-Azuara, B. A. Jiménez-López and A. C. Hernández-Arteaga, Environ. Sci. Pollut. Res., 2023, 31, 39945–39960 CrossRef PubMed.
- G. Yu, Q. Sun, Y. Yang, S. Chen, Y. Long, Y. Li, S. Ge and D. Zheng, Prog. Nat. Sci.: Mater. Int., 2024, 34, 290–303 CrossRef CAS.
- E. Lee, G. Jagan, J. U. Choi, B. Cha, Y. Yoon, K. Saravanakumar and C. M. Park, Chem. Eng. J., 2024, 494, 152961 CrossRef CAS.
- Y. Gao, Y. Hu, F. Liu, Y. Tian, D. Zeng, T. Shen, H. Yuan, R. Guan and J. Song, Ceram. Int., 2024, 50, 5643–5656 CrossRef CAS.
- P. Chen, X. Ou, C. Xia, K. Wang, M. Zhang, M. Wei and Y. Wang, Sep. Purif. Technol., 2024, 349, 127866 CrossRef CAS.
- S. Suresh and T. J. Bandosz, Carbon, 2018, 137, 207–221 CrossRef CAS.
- Y.-W. Li, S.-Z. Li, M.-B. Zhao and W.-L. Ma, Sep. Purif. Technol., 2023, 327, 124966 CrossRef CAS.
- Z. Lin, S. Ye, Y. Xu, X. Lin, Z. Qin, J. Bao and H. Peng, Chem. Eng. J., 2023, 453, 139747 CrossRef CAS.
- E. Belykh, T. Maystrenko, I. Velegzhaninov, M. Tavleeva, E. Rasova and A. Rybak, J. Microorganisms, 2024, 12, 733 CrossRef CAS PubMed.
- B. Xue, L. Tian, Y. Liu, L. Peng, W. Iqbal, L. Li and Y. Mao, Environ. Sci. Ecotechnology, 2024, 21, 100390 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |