Open Access Article
Open Access ArticleSynergistic self-seeding in one-dimension: a route to patchy and block comicelles with uniform and controllable length†
Jiangping
Xu
 ac,
Hang
Zhou
a,
Qing
Yu
a,
Gerald
Guerin
a,
Ian
Manners
b and
Mitchell A.
Winnik
ac,
Hang
Zhou
a,
Qing
Yu
a,
Gerald
Guerin
a,
Ian
Manners
b and
Mitchell A.
Winnik
 *a
*a
aDepartment of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON M5S 3H6, Canada. E-mail: mwinnik@chem.utoronto.ca
bSchool of Chemistry, University of Bristol, Bristol, BS8 1TS, UK
cKey Laboratory of Material Chemistry for Energy Conversion and Storage, Ministry of Education, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Wuhan 430074, China
First published on 4th January 2019
Abstract
Self-seeding is a process unique to polymer crystals, which consist of regions of different chain packing order and different crystallinity. Here we report the synergistic self-seeding behaviour of pairs of core-crystalline block copolymer (BCP) micelle fragments and show how this strategy can be employed to control the morphology of these BCP comicelles. Each micelle fragment has a critical dissolution temperature (Tc), and unimers of each BCP have a characteristic epitaxial growth rate. The Tc value affects the dissolution sequence of the fragments upon heating, while the unimer growth rate affects the growth sequence upon cooling. By carefully choosing micelle fragments having different Tc values as well as growth rates, we could prepare patchy comicelles and block comicelles with uniform and controllable length. This synergistic self-seeding strategy is a simple yet effective route to control both length and morphology of core-crystalline comicelles
Introduction
Cylindrical micelles of block copolymers (BCPs) have important applications in drug delivery,1–3 epoxy resin toughening,4 and templates for hybrid materials.5 Other applications may be possible if one could control their core/corona structure as well as their length. Various groups have examined AB/AC diblock copolymer blends and ABC triblock copolymers as ways to obtain different core or corona structures.6–11 These micelle structures can be manipulated by changing the blend ratio, the block ratio or the self-assembly protocol. However, it is a challenge to control the micelle length, and cylindrical micelles with broad length distributions are usually obtained.12Recently, crystallization-driven self-assembly (CDSA) has become a powerful strategy to control both the length and the structure of cylindrical micelles.13–24 Like an initiator of living chain polymerization of monomers, a seed micelle with a crystalline core can direct the epitaxial deposition of the newly added BCP unimers (seeded-growth). The length of the cylindrical micelles can be controlled precisely by changing the unimer-to-seed ratio.16,20 Moreover, one can manipulate the crystallization kinetics to control the morphology of cylindrical micelles.25–28 For example, in the seeded-growth process, a slow crystallization rate leads to linear micelles, while a rapid crystallization rate can lead to branched micelles.25 In addition, by sequential addition of unimers bearing different corona chains, block comicelles can be obtained,19,20 while simultaneous addition of pairs of different unimers leads to patchy micelles characterized by phase-segregation into nano-sized local patches.23,27,28
We are interested in developing methodologies to construct well-defined morphologies of cylindrical micelles by manipulating their crystallization behaviour. Several years ago, we discovered that “self-seeding” of BCP micelle crystallites was an effective means to control micelle length.14,29,30 Self-seeding is a phenomenon unique to polymer crystals, which consist of regions of different chain packing order and different crystallinity, and thus a broad range of melting temperatures.31,32 Upon heating, the least crystalline domains are the first to melt or dissolve, while the most crystalline domains are the last to survive. These surviving nuclei serve as initiators to direct the crystal growth upon cooling.32 Our initial examples involved annealing solutions of polyferrocenyldimethylsilane (PFS)-containing BCP micelle fragments (length: ∼50 nm) above an onset temperature, followed by cooling to room temperature.29,30 Uniform rod-like micelles were formed, of which the length was sensitive to the annealing temperature (Ta). This strategy has also been extended to other BCPs with other crystallizable blocks, such as polycaprolactone,21,33 poly(3-hexylthiophene)14 and oligo(p-phenylenevinylene),34 and BCPs with a liquid crystalline block.35
Although self-seeding is effective for controlling the length of cylindrical micelles, it has not been used for morphology control. Control over morphology in a self-seeding experiment requires an understanding of the factors that affect both the dissolution step upon heating and the growth rate upon cooling. Here we examine the self-seeding behaviour of mixtures of micelle fragments and use kinetic studies of seeded growth to model the regrowth step. Based upon this understanding of the dissolution and regrowth steps, we designed experiments on mixtures of micelle fragments that allow us to control both the length and morphology of the micelles obtained.
Results and discussion
We began by examining the self-seeding behaviour of each type of micelle fragment (Fig. 1). These include three PFS-b-P2VP samples (P2VP = poly(2-vinylpyridine)): PFS17-b-P2VP170 (L0 = 43 nm), PFS25-b-P2VP330 (L0 = 49 nm), and PFS35-b-P2VP400 (L0 = 52 nm) (Chart S1 and Fig. S1 in ESI†). Typically, aliquots (0.5 mL) of a micelle fragment solution (0.02 mg mL−1) in 2-propanol were heated at different temperatures for 30 min and then cooled to room temperature and aged for 1 day (see ESI†). We recently showed that self-seeding is characterized by a critical dissolution temperature (Tc) corresponding to the temperature at which the initial average micelle length (L0) doubled upon cooling (to 2L0).36 When the annealing temperature Ta > Tc, the average length of the micelles (Ln) increased sharply as a function of temperature (Fig. 1 and S2–S6†). These PFS-b-P2VP samples showed very different Tc values (Fig. 1b): for PFS17-b-P2VP170, Tc = 35 °C; for PFS25-b-P2VP330, Tc = 55 °C; and for PFS35-b-P2VP400, Tc = 67 °C. The difference in Tc may be attributed to the difference in PFS length, as the increase in PFS length will decrease its solubility upon heating.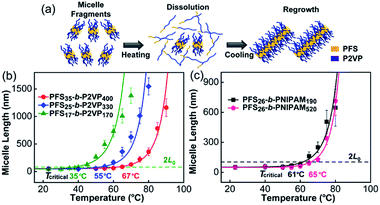 | ||
| Fig. 1 Self-seeding behaviour of single kinds of micelle fragments. (a) A schematic diagram of the self-seeding process. (b) and (c) show the increase in micelle length as a function of annealing temperature. The fitted curves were calculated as described in ref. 36. | ||
Then we examined the self-seeding behaviour of PFS26-b-PNIPAM190 (PNIPAM = poly(N-isopropylacrylamide), L0 = 52 nm) and PFS26-b-PNIPAM520 (L0 = 50 nm) fragments. As shown in Fig. 1c, for PFS26-b-PNIPAM190, Tc = 61 °C; while for PFS26-b-PNIPAM520, Tc = 65 °C. Although the lengths of PNIPAM block are very different, the Tc of these two fragments are similar, confirming that the length of PFS block plays a more important role in determining Tc. It is worth noting that there is an upper limit of the annealing temperature for each self-seeding experiment. When the fragments were heated at a temperature above this limit, branched micelles were formed as the solution cooled (Fig. S2–S6†). The formation of branched structures under these conditions suggests a different dissolution-regrowth mechanism, a topic now under investigation.
On the basis of the above results, we designed self-seeding experiments on micelle fragment mixtures. We initially investigated the self-seeding behaviour of pairs of micelle fragments with similar Tc values, i.e., PFS26-b-PNIPAM190 (Tc = 61 °C) and PFS35-b-P2VP400 (Tc = 67 °C). After annealing this mixture at 40 °C (i.e., Ta < Tc), both fragments could be clearly distinguished in TEM images (Fig. 2a and S7a†), as the PFS35-b-P2VP400 micelles look darker and thicker than PFS26-b-PNIPAM190 micelles. After annealing at 60 °C, some tadpole-like micelles could be observed (Fig. 2b and S7b†) with a thicker PFS35-b-P2VP400 head and a thin PFS26-b-PNIPAM190 tail. When Ta = 70 °C, patchy micelles were obtained, characterized by local microphase separation between P2VP and PNIPAM chains (Fig. 2c and S7c†). Further increase in Ta resulted in longer patchy comicelles (Fig. 2d–f, S7d and e†). Therefore, the length of the patchy comicelles could be controlled by Ta in the range of 70 to 90 °C (Fig. 2f and S8†).
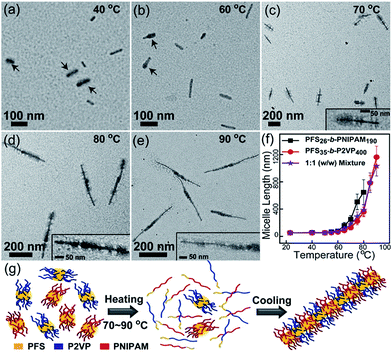 | ||
Fig. 2 Self-seeding behaviour of a PFS26-b-PNIPAM190 and PFS35-b-P2VP400 micelle fragment mixture (mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (a–e) TEM images of the micelles obtained by annealing the mixture at 40 to 90 °C. Arrows in (a) indicate PFS35-b-P2VP400 fragments. Arrows in (b) indicate tadpole-like comicelles. The insets in (c–e) show magnified TEM images. (f) shows the change in comicelle length as a function of Ta. (g) is a schematic diagram of the self-seeding process of the mixture. These structures are further confirmed by selectively staining the P2VP chains with PtNPs (see Fig. S7†). 1). (a–e) TEM images of the micelles obtained by annealing the mixture at 40 to 90 °C. Arrows in (a) indicate PFS35-b-P2VP400 fragments. Arrows in (b) indicate tadpole-like comicelles. The insets in (c–e) show magnified TEM images. (f) shows the change in comicelle length as a function of Ta. (g) is a schematic diagram of the self-seeding process of the mixture. These structures are further confirmed by selectively staining the P2VP chains with PtNPs (see Fig. S7†). | ||
Since the Tc values of PFS26-b-PNIPAM190 and PFS35-b-P2VP400 fragments are similar, about half of both fragments simultaneously dissolve into unimers upon heating to Tc, while the rest survive (Fig. S9 and Table S1†). Upon cooling to room temperature, both unimers add competitively to the surviving fragments. In this step, the growth rates of unimers dominate the morphology of the comicelles. In our previous work, we demonstrated that similar epitaxial growth rates resulted in the formation of patchy comicelles, while significantly dissimilar growth rates led to block comicelles. We also showed that the epitaxial growth rates of PFS26-b-PNIPAM190 and PFS35-b-P2VP400 were similar.28 Therefore, in the cooling stage of the self-seeding process for this mixture, both unimers, which come from the dissolution of micelle fragments, add to the surviving seeds at similar rates, resulting in the formation of patchy comicelles (Fig. 2c–e). At higher Ta, more micelle fragments dissolved, leading to fewer seeds and more unimers, and thus longer patchy comicelles.
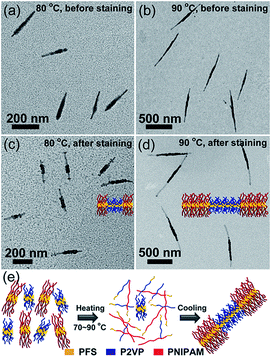
We then examined the self-seeding behaviour of fragments having similar Tc values but very different unimer epitaxial growth rates (Fig. 3). PFS26-b-PNIPAM520 (Tc = 65 °C) and PFS35-b-P2VP400 (Tc = 67 °C) micelle fragments have similar Tc values, but the growth rate of PFS26-b-PNIPAM520 unimer is much slower than that of PFS35-b-P2VP400 unimer.28 After annealing the solution at 60 °C, both micelle fragments could still be distinguished, and their average length (Ln = 56 nm) did not change significantly (Fig. S10a†). However, when the mixture was annealed at 70 to 90 °C, comicelles with a thick middle block flanked by thin tips could be observed (70 °C: Ln = 81 nm, Fig. S10b;† 80 °C: Ln = 210 nm, Fig. 3a; 90 °C: Ln = 731 nm, Fig. 3b). After selectively staining the P2VP chains with platinum nanoparticles (PtNPs), symmetric triblock comicelles with PFS35-b-P2VP400 as the middle block and PFS26-b-PNIPAM520 as the end blocks could be clearly distinguished (Fig. 3c–d and S10f–h†).
Upon heating above Tc, both PFS35-b-P2VP400 and PFS26-b-PNIPAM520 fragments dissolved into unimers, which then regrew onto the surviving seeds upon cooling. Since the epitaxial growth rate of PFS26-b-PNIPAM520 is much slower than that of PFS35-b-P2VP400,28 most of PFS35-b-P2VP400 added onto the seed micelles before PFS26-b-PNIPAM520. After consumption of PFS35-b-P2VP400, the growth of PFS26-b-PNIPAM520 dominated the growth process, resulting in symmetric BAB block comicelles with PFS35-b-P2VP400 as the A blocks and PFS26-b-PNIPAM520 as the B blocks.
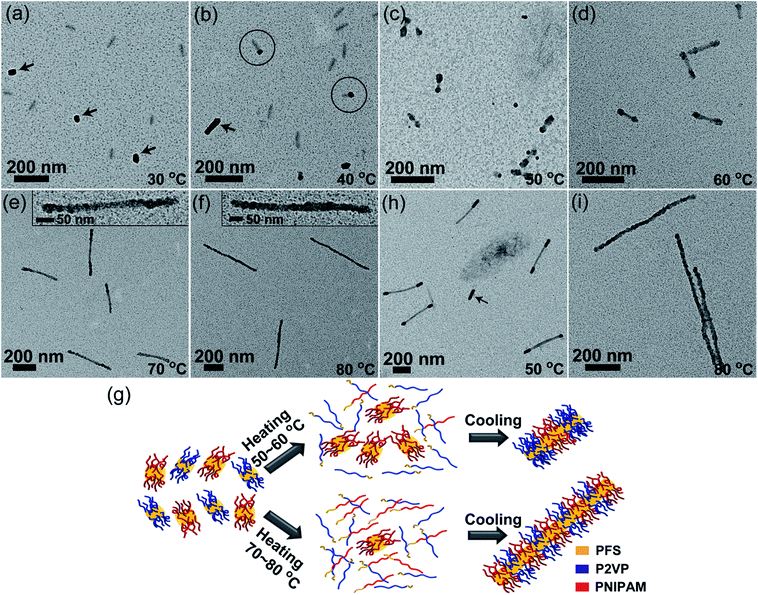
Then we carried out self-seeding experiments with mixed fragments having very different Tc, i.e., PFS17-b-P2VP170 (L0 = 43 nm, Tc = 35 °C) and PFS26-b-PNIPAM190 fragments (L0 = 52 nm, Tc = 61 °C). These two BCP unimers have similar epitaxial growth rates (Fig. S11–S13†). The large difference in Tc values (ΔTc = 26 °C) makes it possible to manipulate the dissolution sequence of fragments by carefully controlling Ta. Fig. 4 shows the morphology evolution of comicelles as Ta was increased. No changes could be observed at 30 °C (Fig. 4a). When Ta = 40 °C, there are a small fraction of matchstick-like comicelles with a PFS17-b-P2VP170 head (∼28 nm) and a PFS26-b-PNIPAM190 stick (∼55 nm) (Fig. 4b), along with PFS17-b-P2VP170 and PFS26-b-PNIPAM190 homomicelles. After annealing at 50 and 60 °C, ABA triblock comicelles were formed with PFS26-b-PNIPAM190 as the central B block, flanked by PFS17-b-P2VP170 A blocks (Fig. 4c and d). Interestingly, further increasing Ta to 70–80 °C led to patchy comicelles rather than block comicelles (Fig. 4e and f).
Due to the large difference in Tc value, the PFS17-b-P2VP170 fragments begin to dissolve at lower temperatures. For example at 40 °C, only 57% of PFS17-b-P2VP170 fragments survive, but 96% of PFS26-b-PNIPAM190 fragments survive (Fig. S9† and Table S1†). Upon cooling, the PFS17-b-P2VP170 unimer adds to both PFS17-b-P2VP170 and PFS26-b-PNIPAM190 fragments, leading to the formation of longer PFS17-b-P2VP170 homomicelles (∼79 nm) and heterogeneous PFS17-b-P2VP170/PFS26-b-PNIPAM190 matchstick-like comicelles (Fig. 4b). Although the length of PFS17-b-P2VP170 homomicelles increased from 43 nm to 79 nm, the length of PFS26-b-PNIPAM190 micelles did not change (∼54 nm, Fig. S14†). For Ta = 50 °C, 96% of PFS26-b-PNIPAM190 fragments survive, but only 16% of PFS17-b-P2VP170 fragments persist; whereas at 60 °C, 66% of PFS26-b-PNIPAM190 fragments survive, but only 6% of PFS17-b-P2VP170 fragments remain (Fig. S9† and Table S1†). Therefore, most PFS17-b-P2VP170 fragments dissolve to give unimers at 50–60 °C, which then add to the surviving PFS26-b-PNIPAM190 fragments upon cooling, resulting in the formation of block comicelles (Fig. 4g). At higher Ta, both PFS17-b-P2VP170 and PFS26-b-PNIPAM190 fragments dissolve to form unimers. For example at 80 °C, all of the PFS17-b-P2VP170 fragments dissolve while 8% of PFS26-b-PNIPAM190 fragments survive. After cooling to room temperature, both unimers, which have similar growth rates, simultaneously add to the surviving PFS26-b-PNIPAM190 seeds, resulting in the formation of patchy comicelles (Fig. 4g).
In order to make the block comicelles shown in Fig. 4c and d more distinguishable, we conducted a self-seeding experiment at 50 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of PFS26-b-PNIPAM190 micelles (Ln = 290 nm, Fig. S15†) and PFS17-b-P2VP170 fragments (L0 = 43 nm). As shown in Fig. 4h and S16,† ABA triblock comicelles can be clearly visualized, accompanied by a few PFS17-b-P2VP170 homomicelles formed by epitaxial growth on surviving PFS17-b-P2VP170 seeds. Upon heating to 80 °C, most of the long and short micelles dissolved. The competitive growth of both unimers on the surviving seeds led to the formation of patchy comicelles (Fig. 4i).
1 mixture of PFS26-b-PNIPAM190 micelles (Ln = 290 nm, Fig. S15†) and PFS17-b-P2VP170 fragments (L0 = 43 nm). As shown in Fig. 4h and S16,† ABA triblock comicelles can be clearly visualized, accompanied by a few PFS17-b-P2VP170 homomicelles formed by epitaxial growth on surviving PFS17-b-P2VP170 seeds. Upon heating to 80 °C, most of the long and short micelles dissolved. The competitive growth of both unimers on the surviving seeds led to the formation of patchy comicelles (Fig. 4i).
Conclusions
In summary, we found that the morphology of core-crystalline cylindrical micelles could be controlled by manipulating the synergistic self-seeding behaviour of micelle fragment mixtures. The morphology of the comicelles is dependent on (1) the dissolution sequence of micelle fragments, and (2) the epitaxial growth rate of the corresponding unimers. By blending two kinds of micelle fragments with similar Tc values and similar growth rates, patchy micelles with controllable length can be obtained by self-seeding. If the fragments have similar Tc values but very different growth rates, block comicelles are obtained. On the other hand, if micelle fragments with very different Tc are blended, the morphology of comicelles can be controlled by varying the annealing temperature. At lower temperatures, block comicelles are obtained; whereas at higher temperatures, patchy comicelles are formed. Thus, by carefully controlling the dissolution and growth sequence of micelle fragments, both the length and morphology of micelles can be varied. This synergistic self-seeding strategy not only offers a convenient route for preparing comicelles with controllable morphology and uniform length, but also provides a platform for studying the synergistic self-seeding behaviour of polymer seed crystallites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Toronto authors thank NSERC Canada for their support of this research.Notes and references
- Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko and D. E. Discher, Nat. Nanotechnol., 2007, 2, 249–255 CrossRef CAS.
- Z. Deng, S. Yuan, R. X. Xu, H. Liang and S. Liu, Angew. Chem., Int. Ed., 2018, 57, 8896–8900 CrossRef CAS.
- X. Hu, S. Zhai, G. Liu, D. Xing, H. Liang and S. Liu, Adv. Mater., 2018, 30, 1706307 CrossRef PubMed.
- J. M. Dean, N. E. Verghese, H. Q. Pham and F. S. Bates, Macromolecules, 2003, 36, 9267–9270 CrossRef CAS.
- J. Yuan and A. H. Müller, Polymer, 2010, 51, 4015–4036 CrossRef CAS.
- D. J. Pochan, J. Zhu, K. Zhang, K. L. Wooley, C. Miesch and T. Emrick, Soft Matter, 2011, 7, 2500–2506 RSC.
- J. Dupont, G. Liu, K. i. Niihara, R. Kimoto and H. Jinnai, Angew. Chem., Int. Ed., 2009, 48, 6144–6147 CrossRef CAS PubMed.
- H. Cui, Z. Chen, S. Zhong, K. L. Wooley and D. J. Pochan, Science, 2007, 317, 647–650 CrossRef CAS PubMed.
- H. Gröschel, A. Walther, T. I. Löbling, F. H. Schacher, H. Schmalz and A. H. Müller, Nature, 2013, 503, 247–251 CrossRef PubMed.
- A. M. Oliver, J. Gwyther, M. A. Winnik and I. Manners, Macromolecules, 2018, 51, 222–231 CrossRef CAS.
- D. J. Lunn, J. R. Finnegan and I. Manners, Chem. Sci., 2015, 6, 3663–3673 RSC.
- Y. Mai and A. Eisenberg, Chem. Soc. Rev., 2012, 41, 5969–5985 RSC.
- N. Petzetakis, A. P. Dove and R. K. O'Reilly, Chem. Sci., 2011, 2, 955–960 RSC.
- J. Qian, X. Li, D. J. Lunn, J. Gwyther, Z. M. Hudson, E. Kynaston, P. A. Rupar, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2014, 136, 4121–4124 CrossRef CAS PubMed.
- B. Fan, R.-Y. Wang, X.-Y. Wang, J.-T. Xu, B.-Y. Du and Z.-Q. Fan, Macromolecules, 2017, 50, 2006–2015 CrossRef CAS.
- J. B. Gilroy, T. Gädt, G. R. Whittell, L. Chabanne, J. M. Mitchels, R. M. Richardson, M. A. Winnik and I. Manners, Nat. Chem., 2010, 2, 566–570 CrossRef CAS PubMed.
- W. N. He and J. T. Xu, Prog. Polym. Sci., 2012, 37, 1350–1400 CrossRef CAS.
- H. Qiu, Y. Gao, C. E. Boott, O. E. Gould, R. L. Harniman, M. J. Miles, S. E. Webb, M. A. Winnik and I. Manners, Science, 2016, 352, 697–701 CrossRef CAS PubMed.
- X. Wang, G. Guerin, H. Wang, Y. Wang, I. Manners and M. A. Winnik, Science, 2007, 317, 644–647 CrossRef CAS PubMed.
- J. Schmelz, A. E. Schedl, C. Steinlein, I. Manners and H. Schmalz, J. Am. Chem. Soc., 2012, 134, 14217–14225 CrossRef CAS PubMed.
- M. C. Arno, M. Inam, Z. Coe, G. Cambridge, L. J. Macdougall, R. Keogh, A. P. Dove and R. K. O'Reilly, J. Am. Chem. Soc., 2017, 139, 16980–16985 CrossRef CAS PubMed.
- D. Tao, C. Feng, Y. Cui, X. Yang, I. Manners, M. A. Winnik and X. Huang, J. Am. Chem. Soc., 2017, 139, 7136–7139 CrossRef CAS PubMed.
- A. Nazemi, X. He, L. R. MacFarlane, R. L. Harniman, M.-S. Hsiao, M. A. Winnik, C. F. Faul and I. Manners, J. Am. Chem. Soc., 2017, 139, 4409–4417 CrossRef CAS PubMed.
- M. Inam, G. Cambridge, A. Pitto-Barry, Z. P. Laker, N. R. Wilson, R. T. Mathers, A. P. Dove and R. K. O'Reilly, Chem. Sci., 2017, 8, 4223–4230 RSC.
- H. Qiu, Y. Gao, V. A. Du, R. Harniman, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2015, 137, 2375–2385 CrossRef CAS PubMed.
- G. Cambridge, G. Guerin, I. Manners and M. A. Winnik, Macromol. Rapid Commun., 2010, 31, 934–938 CrossRef CAS PubMed.
- J. R. Finnegan, D. J. Lunn, O. E. C. Gould, Z. M. Hudson, G. R. Whittell, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2014, 136, 13835–13844 CrossRef CAS PubMed.
- J. Xu, H. Zhou, Q. Yu, I. Manners and M. A. Winnik, J. Am. Chem. Soc., 2018, 140, 2619–2628 CrossRef CAS PubMed.
- J. Qian, Y. Lu, A. Chia, M. Zhang, P. A. Rupar, N. Gunari, G. C. Walker, G. Cambridge, F. He, G. Guerin, I. Manners and M. A. Winnik, ACS Nano, 2013, 7, 3754–3766 CrossRef CAS PubMed.
- J. S. Qian, G. Guerin, Y. J. Lu, G. Cambridge, I. Manners and M. A. Winnik, Angew. Chem., Int. Ed., 2011, 50, 1622–1625 CrossRef CAS.
- D. Blundell, A. Keller and A. Kovacs, J. Polym. Sci., Part B: Polym. Lett., 1966, 4, 481–486 CrossRef CAS.
- J. Xu, Y. Ma, W. Hu, M. Rehahn and G. Reiter, Nat. Mater., 2009, 8, 348–353 CrossRef CAS PubMed.
- W. N. He, B. Zhou, J.-T. Xu, B.-Y. Du and Z.-Q. Fan, Macromolecules, 2012, 45, 9768–9778 CrossRef CAS.
- D. Tao, C. Feng, Y. Lu, Y. Cui, X. Yang, I. Manners, M. A. Winnik and X. Huang, Macromolecules, 2018, 51, 2065–2075 CrossRef CAS.
- X. Li, B. Jin, Y. Gao, D. W. Hayward, M. A. Winnik, Y. Luo and I. Manners, Angew. Chem., Int. Ed., 2016, 55, 11392–11396 CrossRef CAS PubMed.
- G. Guerin, P. A. Rupar, I. Manners and M. A. Winnik, Nat. Commun., 2018, 9, 1158 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, self-seeding kinetics, TEM images of stained micelles, additional figures of the patchy and block comicelles (Fig. S1–S16), and fraction of surviving fragments at different temperatures (Table S1). See DOI: 10.1039/c8sc04705g |
| This journal is © The Royal Society of Chemistry 2019 |